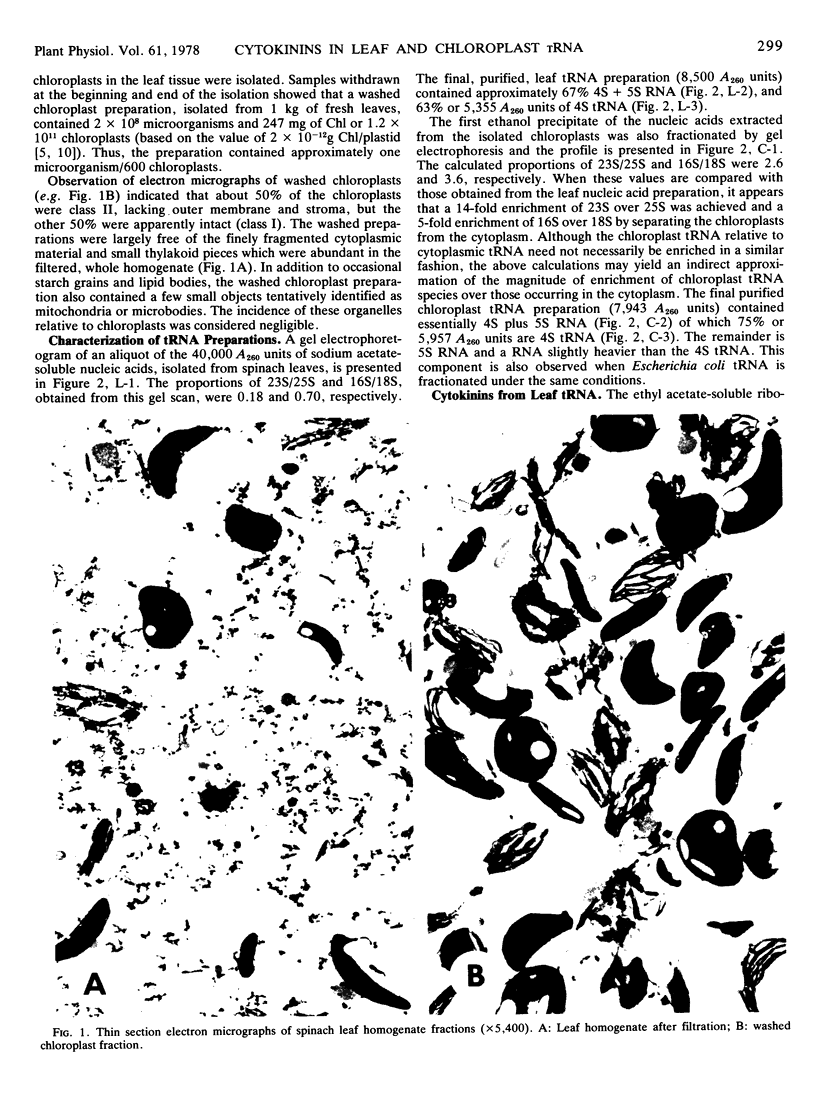
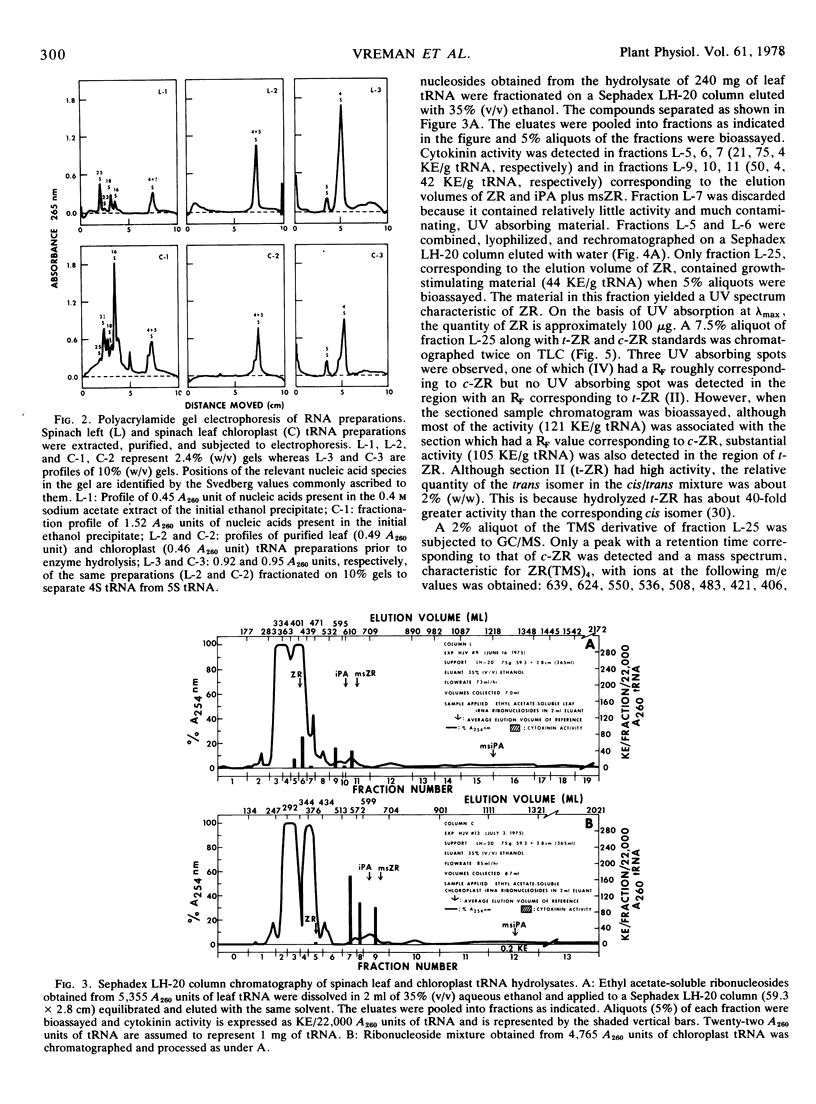
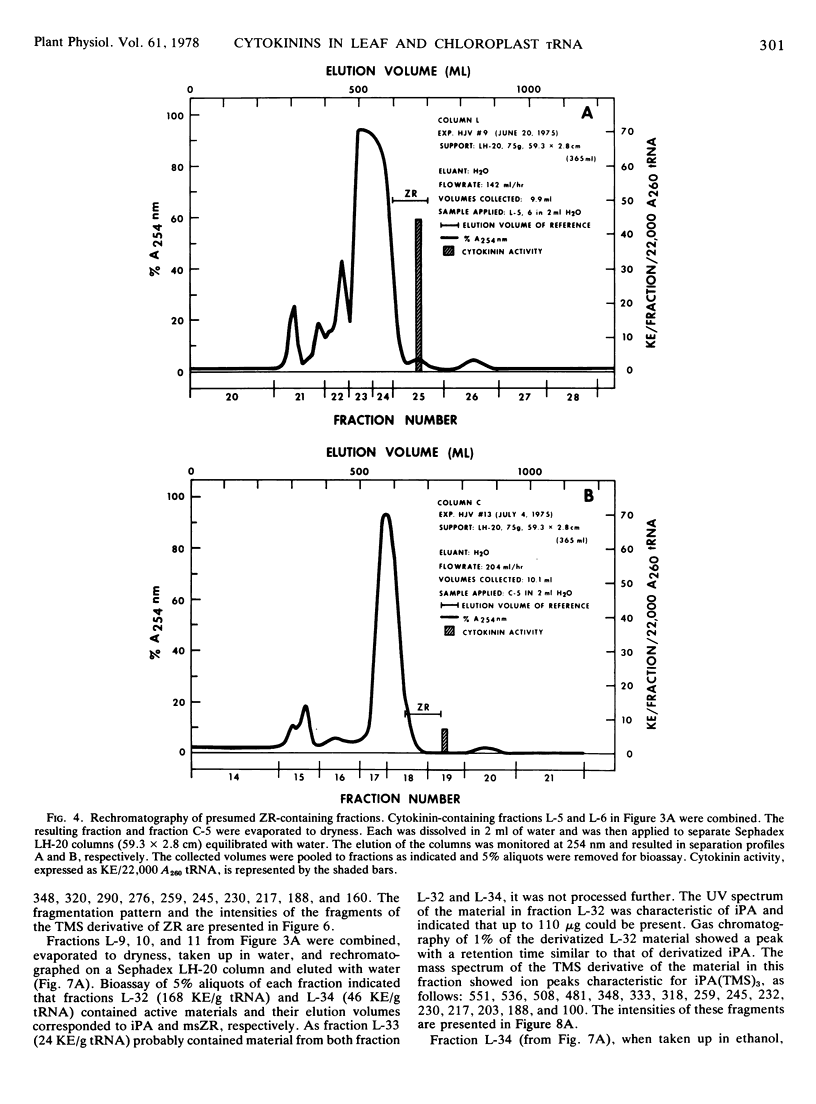
Abstract
Cytokinin-active ribonucleosides have been isolated from tRNA of whole spinach (Spinacia oleracea L.) leaves and isolated spinach chloroplasts. The tRNA from spinach leaf blades contained: 6-(4-hydroxy-3-methyl-2-butenylamino)-9-β-d-ribofuranosylpurine (cis and trans isomers), 6-(3-methyl-2-butenylamino)-9-β-d-ribofuranosylpurine, and 6-(4-hydroxy-3-methyl-2-butenylamino)-2-methylthio-9-β-d -ribofuranosylpurine (cis and trans isomers). A method for isolation of large amounts of intact chloroplasts was developed and subsequently used for the isolation of chloroplast tRNA. The chloroplast tRNA contained 6-(3-methyl-2-butenylamino)-9-β-d-ribofuranosylpurine and 6-(4-hydroxy-3-methyl-2-butenylamino)-2-methylthio-9-β-d -ribofuranosylpurine (the cis isomer only). The structures of these compounds were assigned on the basis of their chromatographic properties and mass spectra of trimethylsilyl derivatives which were identical with those of the corresponding synthetic compounds. The results of this study indicate that ribosylzeatin was present in spinach leaf tRNA, but absent from the purified chloroplast tRNA preparation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D. F., Morris R. O. Quantitative measurement of isoprenoid nuceosides in transfer ribonucleic acid. Biochemistry. 1970 Sep 15;9(19):3701–3705. doi: 10.1021/bi00821a008. [DOI] [PubMed] [Google Scholar]
- Burkard G., Guillemaut P., Weil J. H. Comparative studies of the tRNA's and the aminoacyl-tRNA synthetases from the cytoplasm and the chloroplasts of Phaseolus vulgaris. Biochim Biophys Acta. 1970 Nov 12;224(1):184–198. doi: 10.1016/0005-2787(70)90632-5. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Armstrong D. J., Kaminek M., Skoog F., Bock R. M., Hecht S. M., Dammann L. G., Leonard N. J., Occolowitz J. Isolation and identification of four cytokinins from wheat germ transfer ribonucleic acid. Biochemistry. 1970 Apr 28;9(9):1867–1872. doi: 10.1021/bi00811a001. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Skoog F., Leonard N. J. Isolation and identification of cytokinins located in the transfer ribonucleic acid of tobacco callus grown in the presence of 6-benzylaminopurine. Biochemistry. 1971 Jun 8;10(12):2189–2194. doi: 10.1021/bi00788a002. [DOI] [PubMed] [Google Scholar]
- Chapman R. W., Morris R. O., Zaerr J. B. Occurrence of trans-ribosylzeatin in Agrobacterium tumefaciens tRNA. Nature. 1976 Jul 8;262(5564):153–154. doi: 10.1038/262153a0. [DOI] [PubMed] [Google Scholar]
- Fairfield S. A., Barnett W. E. On the similarity between the tRNAs of organelles and prokaryotes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2972–2976. doi: 10.1073/pnas.68.12.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL R. H. ISOLATION OF N6-(AMINOACYL)ADENOSINE FROM YEAST RIBONUCLEIC ACID. Biochemistry. 1964 Jun;3:769–773. doi: 10.1021/bi00894a006. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Csonka L., David H., McLennan B. Cytokinins in the soluble RNA of plant tissues. Science. 1967 Apr 7;156(3771):69–71. doi: 10.1126/science.156.3771.69. [DOI] [PubMed] [Google Scholar]
- Hecker L. I., Uziel M., Barnett W. E. Comparative base compositions of chloroplast and cytoplasmic tRNAPhe's from Euglena gracilis. Nucleic Acids Res. 1976 Feb;3(2):371–380. doi: 10.1093/nar/3.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard N. J., Achmatowicz S., Loeppky R. N., Carraway K. L., Grimm W. A., Szweykowska A., Hamzi Q. H., Skoog F. Development of cytokinin activity by rearrangement of 1-substituted adenines to 6-substituted aminopurines: inactivation by N6, 1-cyclization. Proc Natl Acad Sci U S A. 1966 Aug;56(2):709–716. doi: 10.1073/pnas.56.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C., Dure L. S., 3rd The developmental biochemistry of cotton seed embryogenesis and germination. IV. Levels of cytoplasmic and chloroplastic transfer ribonucleic acid species. J Biol Chem. 1972 Dec 25;247(24):7988–7999. [PubMed] [Google Scholar]
- Millard M. M., Scherrer R., Thomas R. S. Surface analysis and depth profile composition of bacterial cells by x-ray photoelectron spectroscopy and oxygen plasma etching. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1209–1217. doi: 10.1016/s0006-291x(76)80259-8. [DOI] [PubMed] [Google Scholar]
- RALPH R. K., BELLAMY A. R. ISOLATION AND PURIFICATION OF UNDEGRADED RIBONUCLEIC ACIDS. Biochim Biophys Acta. 1964 May 18;87:9–16. doi: 10.1016/0926-6550(64)90041-6. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Bock R. M. Subcellular localization of cytokinins in transfer ribonucleic Acid. Plant Physiol. 1977 Apr;59(4):558–563. doi: 10.1104/pp.59.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Cherayil J. D. Unique presence of 2-methylthio-ribosylzeatin in the transfer ribonucleic acid of the bacterium Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1974 Sep 23;60(2):665–672. doi: 10.1016/0006-291x(74)90292-7. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]