Abstract
Photosynthetic rate, ribulose 1,5-bisphosphate carboxylase activity, specific leaf weight, and leaf concentrations of carbohydrates, proteins, chlorophyll, and inorganic phosphate were determined periodically from midbloom until maturity in leaves of soybean plants (Glycine max L., var. Hodgson) from which reproductive and vegetative sinks had been removed 32 hours before measurement, or continuously since midbloom.
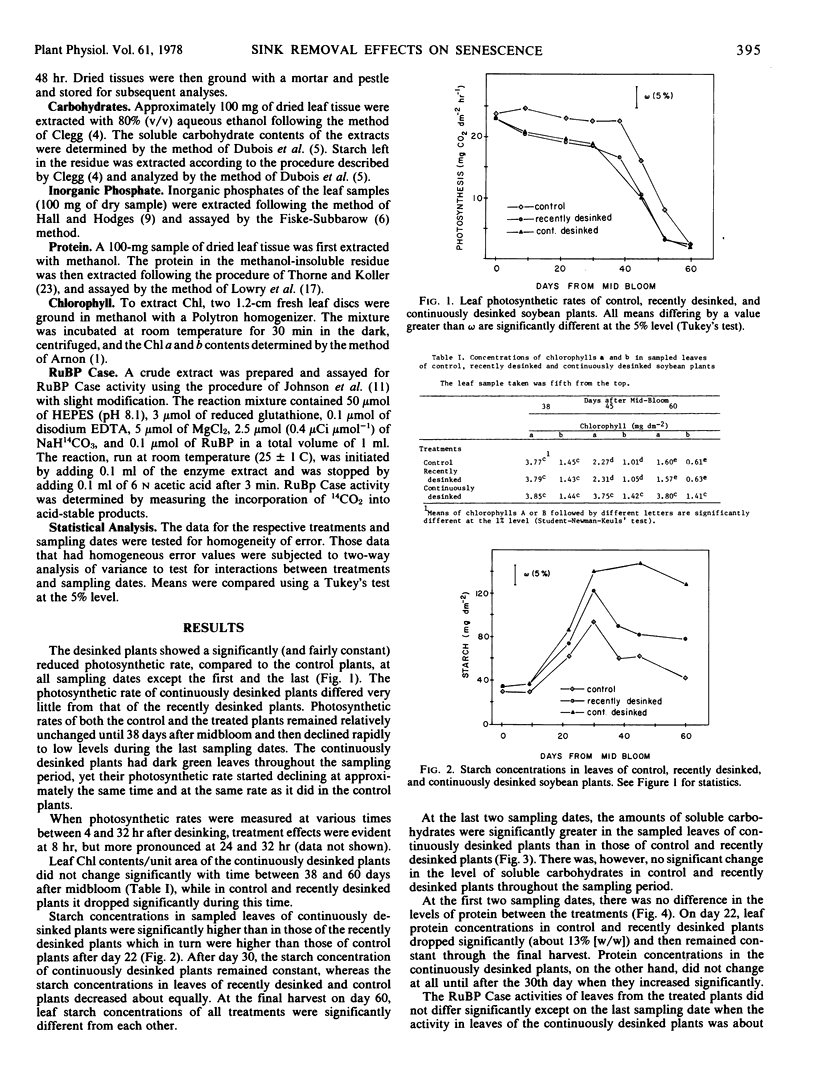
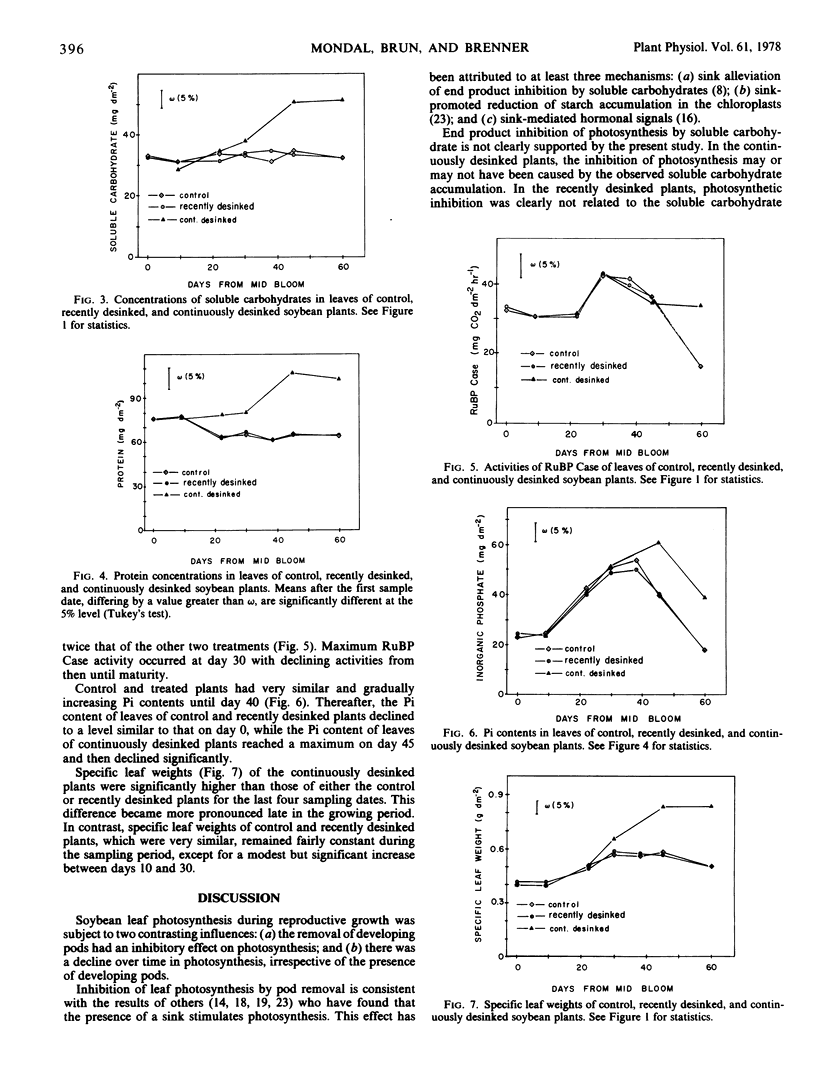
Leaf photosynthesis, measured in the top of the canopy, was partially inhibited by both sink removal treatments. This inhibition was of constant magnitude from midbloom until maturity.
Leaf photosynthesis in the top of the canopy declined from midbloom until maturity in the control as well as in the desinked plants. The decline in photosynthesis was gradual at first, but later became more abrupt. The photosynthetic decline was equally evident in the yellowing leaves of control plants and in the dark green leaves of the continuously desinked plants.
Neither the inhibition of photosynthesis by sink removal nor the decline in photosynthetic rate with time was clearly related to any of the measured traits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. R., Hodges T. K. Phosphorus metabolism of germinating oat seeds. Plant Physiol. 1966 Nov;41(9):1459–1464. doi: 10.1104/pp.41.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Niedergang-Kamien E., Janick J. Experimental Modification of Plant Senescence. Plant Physiol. 1959 Sep;34(5):570–573. doi: 10.1104/pp.34.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger E. D., Koller H. R. Influence of Leaf Starch Concentration on CO(2) Assimilation in Soybean. Plant Physiol. 1976 Apr;57(4):560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Stoddart J. L., Pughe J., Paranjothy K., Wareing P. F. Effects of gibberellin and cytokinins on the activity of photosynthetic enzymes and plastid ribosomal RNA synthesis in Phaseolus vulgaris L. Nature. 1970 Oct 10;228(5267):129–131. doi: 10.1038/228129a0. [DOI] [PubMed] [Google Scholar]


