Abstract
Background:
Intra-articular corticosteroid injections (IACS) are interventions which provide pain relief in knee osteoarthritis (OA). It remains unclear whether IACS have a deleterious effect on knee cartilage structure.
Purpose:
To estimate the effect of IACS on cartilage structure in patients with knee OA, using joint space width (JSW) (in radiographic studies), and cartilage thickness (in magnetic resonance imaging).
Materials and methods:
A literature search was performed to identify randomized control trials and observational studies published from inception to June 15, 2022. Studies were included if patients received IACS for knee OA, with a control arm. Given the different metrics used in reporting continuous variable outcomes among studies, pooled estimates for cartilage thickness change were assessed using standardized mean differences (defined as the difference between the means of the groups divided by a within-group standard deviation) to odds ratio transformation. Sensitivity analyses were conducted based on outcome metric, imaging modality, and number of injections.
Results:
Six studies (1437 participants) were identified. The estimated effect of IACS on cartilage structure revealed greater odds of cartilage structure worsening (Odds Ratio (OR): 2.01, 95% Confidence Interval (CI): 1.18,3.44). Sensitivity analyses revealed similar trends, with significant results for singular injections with preference to JSW (OR: 2.44, 95%CI: 1.23,4.82), radiographic outcomes with preference to KL grade (OR: 2.03, 95%CI: 1.01,4.10), binary outcomes with preference to KL grade (OR: 2.93, 95%CI: 1.18,7.25) and quantitative measures (Standardized Mean Differences (SMD): −0.34, 95%CI: −0.66, −0.02)
Conclusions:
IACS use may contribute to imaging features of knee cartilage loss. Further studies are warranted to investigate the underlying pathogenesis.
Keywords: Osteoarthritis, Intra-articular, Corticosteroid, Cartilage, Joint Space
Introduction
Osteoarthritis (OA) is the most common form of arthritis, with knee OA accounting for an estimated 80% of OA burden worldwide1. Global knee OA prevalence and incidence were recently estimated to be 16%2. Knee OA is multifactorial, caused by biomechanical, biochemical, and metabolic derangements, ultimately leading to joint failure3. In the absence of disease modifying OA drugs, pharmacological treatment for the disease has been aimed at pain management and inflammatory control so far4.
Intra-articular injection is a common treatment choice that is considered a minimally invasive “bridging” intervention to delay surgical knee replacement in patients with advanced knee OA4. Particularly, intra-articular corticosteroid injections (IACS) are used for their anti-inflammatory and analgesic properties. IACS are strongly recommended by the American College of Rheumatology for short term pain relief (3–4 weeks) in patients with knee OA5, conditionally recommended by the Osteoarthritis Research Society International for short term pain relief6 and moderately recommended by the American Academy of Orthopedic Surgeons for IACS in knee OA treatment7.
A main emerging concern of IACS usage is its deleterious effects on knee cartilage structure including accelerated articular cartilage loss4. Studies have shown time and dose dependent adverse effects of high-dose corticosteroids on human and animal knee cartilage structure in vitro and in vivo8. In addition to accelerated cartilage loss, other potentially negative outcomes have been reported albeit causality to date has not been shown. Such safety events included accelerated OA progression, subchondral insufficiency fractures, complications of pre-existing osteonecrosis, and rapid joint destruction including bone loss9,10. In addition, some studies have shown increased loss of joint space width (JSW) in patients receiving IACS11.
The purpose of this systematic review and meta-analysis was to synthesize an estimate of longitudinal knee cartilage loss based on either direct assessment using quantitative MRI or using radiographic loss in JSW as a surrogate for cartilage loss and meniscal changes in patients with knee OA who received IACS, compared to patients with knee OA who received placebo or no IACS.
Materials and Methods
Search Strategy and Study Selection
A comprehensive literature search was conducted to find eligible studies in the PubMed, Embase, and Cochrane Library databases (see Appendix 1 for search terms). No date restrictions were applied to include all published studies up to June 15, 2022. Peer-reviewed, original research on the effect of IACS on cartilage thickness and joint space width in patients with knee OA were identified. Screening of articles was performed independently by two authors (H.I. and A.K.). Randomized control trials and observational studies were screened for final eligibility based on the following inclusion criteria: 1) published as original research, 2) full-text was available in English, 3) intervention arm participants (or cases for observational studies) had received at least a single intra-articular administration of a corticosteroid agent in the knee joint, 4) no or placebo injections were administered in the control arm participants, 5) cartilage thickness or JSW were used as outcome measures for structural changes in cartilage, 6) participants of the study met the American College of Rheumatology’s criteria for Knee Osteoarthritis or presented with KL grade ≥ 2. Exclusion criteria included 1) animal or cadaveric studies, 2) studies assessing non-cartilage measures (e.g., synovial thickness), 3) studies with the binary outcome but with insufficient data to reconstruct a 2×2 table (Not applicable to quantitative studies e.g., JSW in mm, cartilage thickness in mm), 4) studies that used data from the same overarching study (unless there was a different sub-sampling technique), and 5) studies that used a modality other than MRI or X-ray (such as ultrasound). In case of studies that used the same data from the same overarching study we chose the study that was the first to be published as an original research paper. Final eligibility of studies, study selection, and data extraction were reviewed by S.D (an attending musculoskeletal radiologist with 12 years of clinical experience).
Data Extraction
Data extraction was performed by two authors independently (H.I. and A.K.). The baseline clinical characteristics include age, gender, body mass index, time from diagnosis of OA, Kellgren-Lawrence (KL) grade, Western Ontario and McMaster Universities Osteoarthritis Index score, and non-steroidal anti-inflammatory drugs use. Data extracted on interventions included number of IACS injections performed and time intervals between administrations. The longitudinal outcome measurements extracted included follow-up time, imaging intervals, imaging modality, change in JSW or number of patients with change in JSW, change in cartilage volume and/or thickness (in the total, medial, or lateral compartment), and change in KL grade (available in one study). Changes in JSW and cartilage thickness were defined in both quantitative and qualitative (binary) terms in different studies whereas KL grade progression was defined in binary terms in one study. For our primary cumulative synthesis, in studies that reported both quantitative and qualitative measurements, quantitative measurements were preferentially used. In studies with quantitative measurements reporting changes in both cartilage thickness and JSW, measurements of change in cartilage thickness were preferentially used due to their higher sensitivity regarding change over time (offered using MRI). In one study, cartilage thickness data was compartment-based, in which case, quantitative measurements of JSW loss were used after failed correspondence with the author. Preferential use of other measurements was explored in our sensitivity analyses (see below).
Quality Assessment
The Cochrane risk of bias tool for randomized trials (RoB2) was used to assess the methodological quality and risk of bias in four randomized trials and the Newcastle-Ottawa Quality Assessment Scale (NOSGEN) was used to qualitatively assess the two remaining case-control studies with nine being the maximum score. RoB 2’s domains of bias focus on randomized trial design, conduct, and reporting. “Signaling questions” in each domain seek trial features relevant to the risk of bias. Based on signaling questions, an algorithm is generated which proposes a domain-specific risk of bias. The judgment about the risk of bias can be ‘Low’ or ’High’ risk or ‘Some concerns’12.Using RoB2, five general domains including the randomization process, deviations from intended interventions, missing outcome data, measurements of the outcome, and selection of the reported results in the four randomized trials were independently evaluated for risk of bias. The remaining case-control studies were assessed and scored based on NOSGEN’s three general domains including selection (of cases and controls), their comparability, and exposure. Two authors evaluated the RoB2 and NOSGEN domains and scores independently. In case of disagreement, a joint consensus was achieved through consultation.
Statistical Analysis
For each study, data extraction yielded several different outcome measures: numerical cartilage thickness change, numerical JSW change, binary cartilage thickness change, binary JSW change, and binary KL grade change. Log odds ratios were used as the outcome effect-size measure for the cumulative synthesis of all studies and for studies of binary outcomes (Binary JSW or KL grade change). Standardized mean differences were used as the outcome effect-size measure for the synthesis of studies of numerical scale outcomes (Numerical JSW and cartilage thickness change). Statistical transformation of the standardized mean differences to an estimate of log odds ratio assuming logistic distributions was performed for the cumulative synthesis of all identified studies.13–15
The amount of heterogeneity for all syntheses was estimated using the restricted maximum-likelihood estimator. The Cochrane’s Q-test for heterogeneity and the I2 statistic are reported. To account for the heterogeneity test result and provide generalizable results from the six studies with varying sample sizes included in the meta-analysis, a random effects model using a restricted maximum likelihood estimator was used to synthesize the results16,17. Funnel plots of extracted outcome measures were created to evaluate the presence of publication bias. Egger’s funnel plot asymmetry tests were performed, and P-values less than 0.1 indicated statistically significant coefficients. The trim-and-fill method was used to obtain adjusted funnel plots. The analysis was carried out using R (version 4.2.0) and the metafor package (version 3.4.0)
Due to several eligible outcomes being reported by some studies, sensitivity analyses were performed giving preference to one outcome measure over another (e.g., separate analyses for JSW binary change and KL-grade binary change for the same set of studies). In the case of one study (Pelletier et al), compartment-wise cartilage thickness change and cumulative JSW change were reported. The authors were contacted to acquire whole cartilage thickness change, but no response was received. Hence, we chose to report the cumulative JSW loss as the measure of choice at our discretion.
Results
Study Selection
The literature search identified 270 studies from PubMed, 151 studies from Embase, and 281 studies from the Cochrane Library. Twenty-three duplicated studies were excluded, and the remainder of studies were assessed based on the previously described inclusion criteria. Only one study which did not strictly meet the inclusion criterion of presence of KL ≥ 2 was included. In this study participants of the case arm stated a positive response to the following question: ‘During the past 6 months, have you had a treatment with injections of steroids (cortisone, corticosteroids) in either of your knee(s) for your arthritis?’. However, 72.1% of the participants in the case arm of this study and 50.8% of the control arm presented with baseline radiographic KL grade ≥ 2. Forty-one studies which met the inclusion criteria were evaluated at the full text level. Thirty-four of these studies were excluded after full-text evaluations due to use of wrong interventions (n=1), wrong outcome (n=15), lack of control arm (n=4), wrong study design (n=6), wrong language (n=4), or being reports of studies already included in our analysis (n=4). One additional study was excluded in which the association between IACS use and OA outcomes were assessed only using a cross-sectional design, which did not allow the longitudinal assessment of IACS vs. no IACS on the OA outcomes. The resulting six studies containing measurements of joint space width change, cartilage thickness change, or KL grade change in either quantitative or binary metrics were included in our meta-analysis. The exclusion chart demonstrating the literature review as well as the screening and selection of studies is shown in Figure 1.
Figure 1:
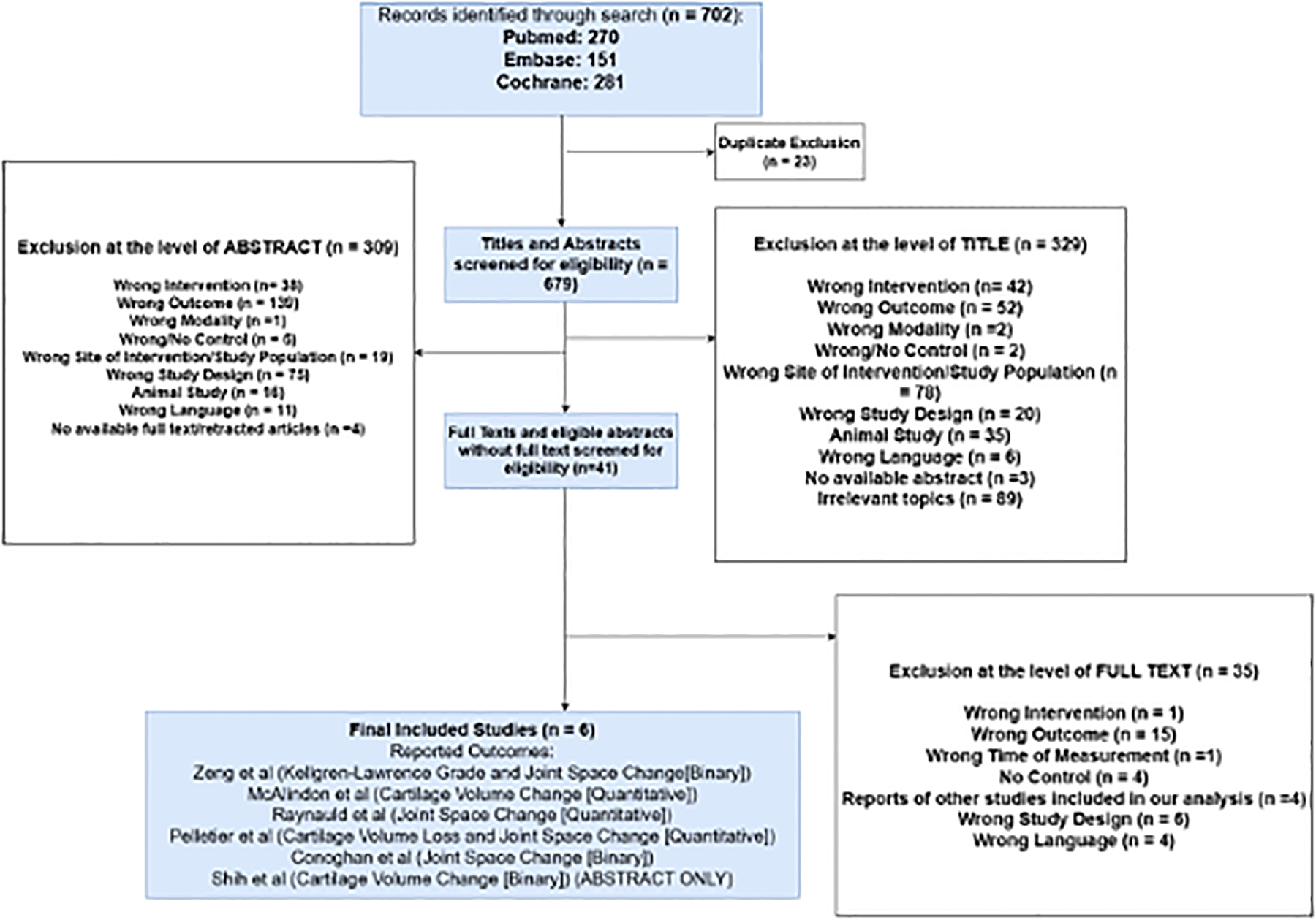
Flowchart summary of literature search and study selection. A more detailed version may be found in Appendix 2.
Study Characteristics and Methodological Quality
A total of six studies were included in the meta-analysis including 1437 participants. Dosage of IACS administration included 40 milligrams(mg) of triamcinolone acetonide, 32 mg of FX006 (a microsphere-based, extended-release triamcinolone acetonide) and 12 and 18 mg of dexamethasone sodium phosphate. The dosage information for IACS is available in Supplementary Table 4. Out of the six studies, three studies measured cartilage thickness changes (derived from MRI) and four studies measured changes in JSW (derived from X-ray). In addition, one study described changes in KL grade in addition to changes in JSW. Three of the studies provided numerical measurement data (e.g., change in cartilage thickness (in mm) or meniscal thickness (mm) or JSW (mm)) and the remaining three studies provided binary measurement data (e.g., JSW or KL grade worsening). Four studies were randomized controlled trials and the other two were case-control studies derived from the Osteoarthritis Initiative (OAI) database. One study was limited to an abstract. The characteristics of the included studies are summarized in Table 1.
Table 1:
Summary characteristics of all studies identified through literature search
| First Author | Year of Publication | Journal | Type of Study | Imaging Modality | Reported Outcomes | Control Subjects (n) | IACS Subjects (n) | Mean Age (years) | Sex (% Female) |
|---|---|---|---|---|---|---|---|---|---|
| Raynauld | 2003 | Arthritis and Rheumatism | Randomized Control Trial | Radiography | JSW (Numerical) | 34 | 34 | 63.1–63.3 | 61–74 |
| McAlindon | 2017 | Journal of the American Medical Association | Randomized Control Trial | Magnetic Resonance Imaging | Cartilage Thickness (Numerical) | 70 | 70 | 57.2–59.1 | 52.9–54.3 |
| Conaghan | 2018 | Journal of Bone and Joint Surgery | Randomized Control Trial | Radiography | JSW (Binary) | 162 | 322 | 62.1 | 83.7 |
| Shih | 2018 | American College of Rheumatology (Abstract) | Randomized Control Trial | Magnetic Resonance Imaging | Cartilage Thickness (Binary) | 22 | 45 | NA | NA |
| Zeng | 2019 | Osteoarthritis and Cartilage | Case-Control | Radiography | JSW (Binary) & KLG(Binary) | 148 (KLG) 104 (JSW) |
536 (KLG) 388 (JSW) |
64.4 (KLG) 63.4–63.8 (JSW) |
64.2–66.8 (KLG) 70.2–73.7 (JSW) |
| Pelletier | 2020 | Scientific Reports | Case-Control | Magnetic Resonance Imaging & Radiography | JSW (Numerical) & Compartment-wise cartilage volume /thickness (Numerical) | 93 | 93 | 66.2–66.6 | 51.6–62.4 |
KLG = Kellgren-Lawrence Grade; JSW = Joints Space Width
Two of the randomized trials had a moderate risk of bias in their deviations from the intended interventions, and one of the trials had a high risk of bias in its deviations from the intended interventions and missing outcome data while also demonstrating a moderate risk of bias in its measurements of the outcome and its selection of the reported results. The results of the RoB 2 tool are shown in Figure 2. Both studies derived from the OAI database were considered to have acceptable methodological quality with NOS scores of 5 but lacked the required quality metrics for their case definition and selection of controls as measured in the NOS selection domain, as well as the information about their ascertainment of exposure and non-response rate as measured in the NOS exposure domain. The results of the NOSGEN tool are shown in Figure 2.
Figure 2:
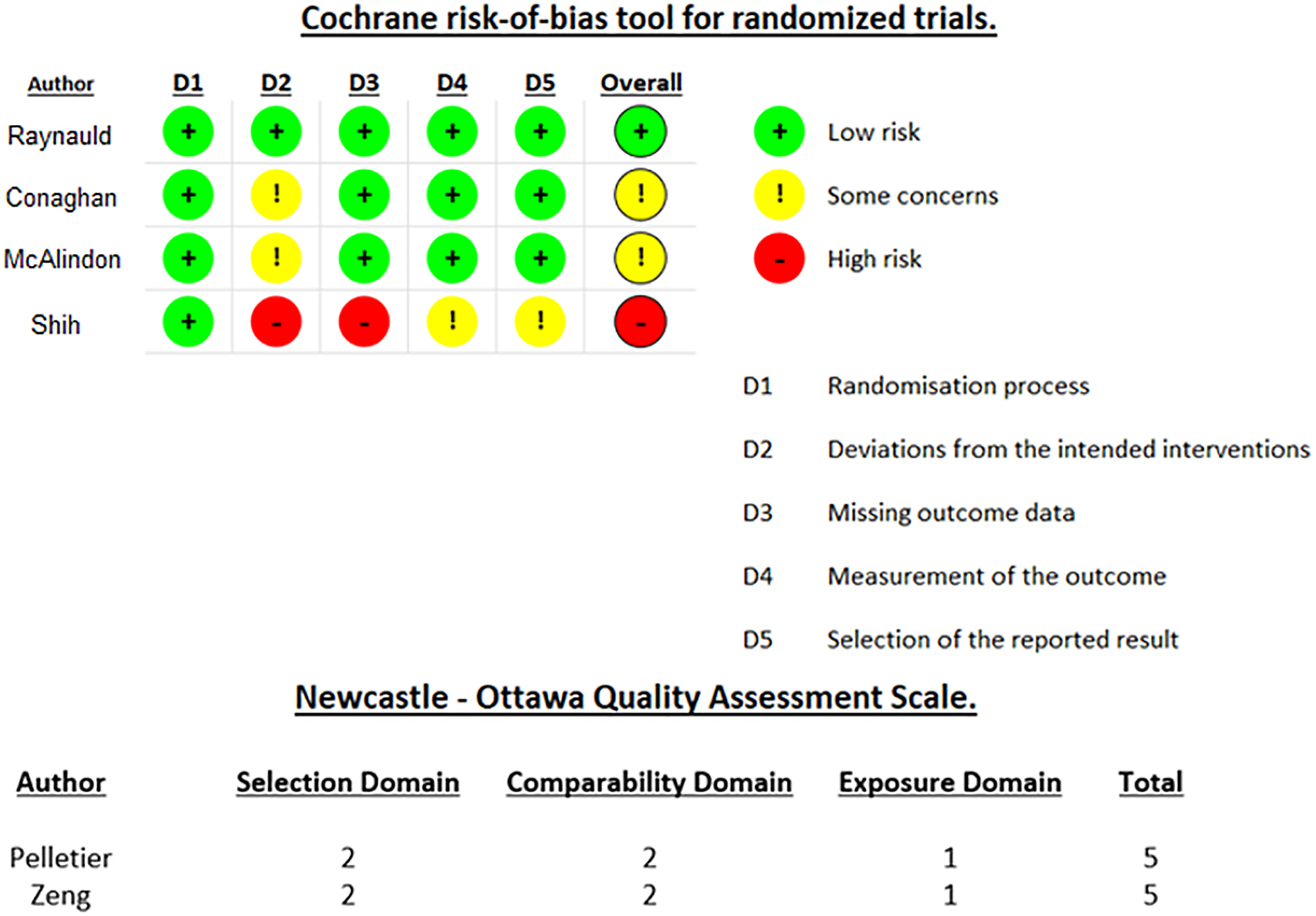
Methodological quality assessment of randomized control trials using the Cochrane Risk of Bias assessment tool for randomized trials and assessment of case-control studies using the Newcastle-Ottawa Quality assessment scale.
Meta-analysis
The effect-size odds-ratio and 95% confidence intervals for knee cartilage structure worsening including all six studies is shown in Table 2. Analysis was stratified into two subgroups to preferentially report two valid outcomes (JSW and KL grade) separately. Forest plots of all included studies with individual study results are provided in Figures 3 and 4 for JSW and KL grade subgroups, respectively.
Table 2:
Summary of random-effects models of all studies identified through literature search. Two subgroups were created, each synthesizing all studies with preference to JSW and KL grade reporting, respectively.
| Analysis | Outcome | Confidence Interval | Cochrane’s Q-test p-value | I2 | τ2 |
|---|---|---|---|---|---|
| All studies, preferentially JSW | 2.01 | 1.18,3.44 | <0.01 | 66.4% | 0.28 |
| All studies, preferentially KL grade | 1.99 | 1.21,3.28 | 0.02 | 63.3% | 0.23 |
Figure 3:
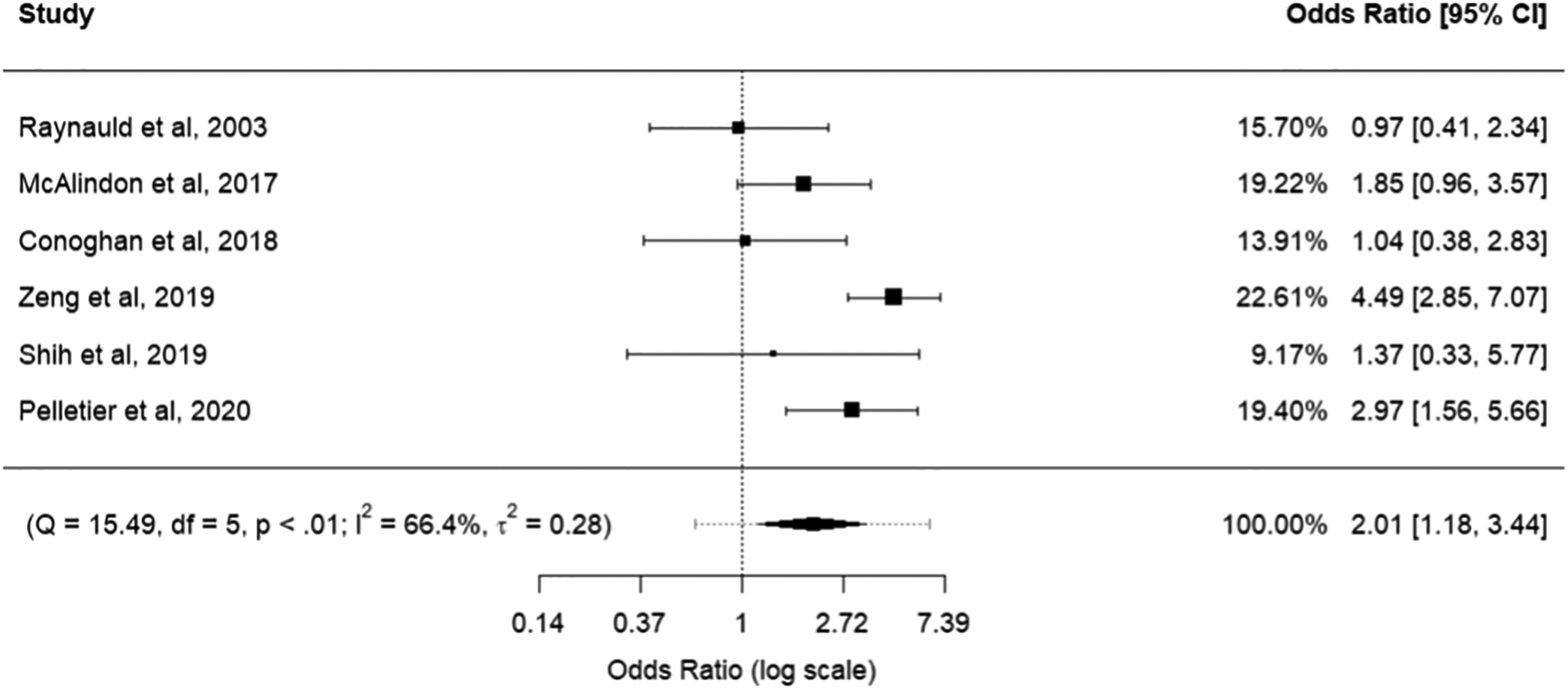
Forest plot for synthesis of all studies identified through literature search. Results from quantitative studies were transformed into standardized mean differences and subsequently into odds ratios for cumulative synthesis with qualitative outcomes. Preference was given to outcomes reporting JSW.
Figure 4:
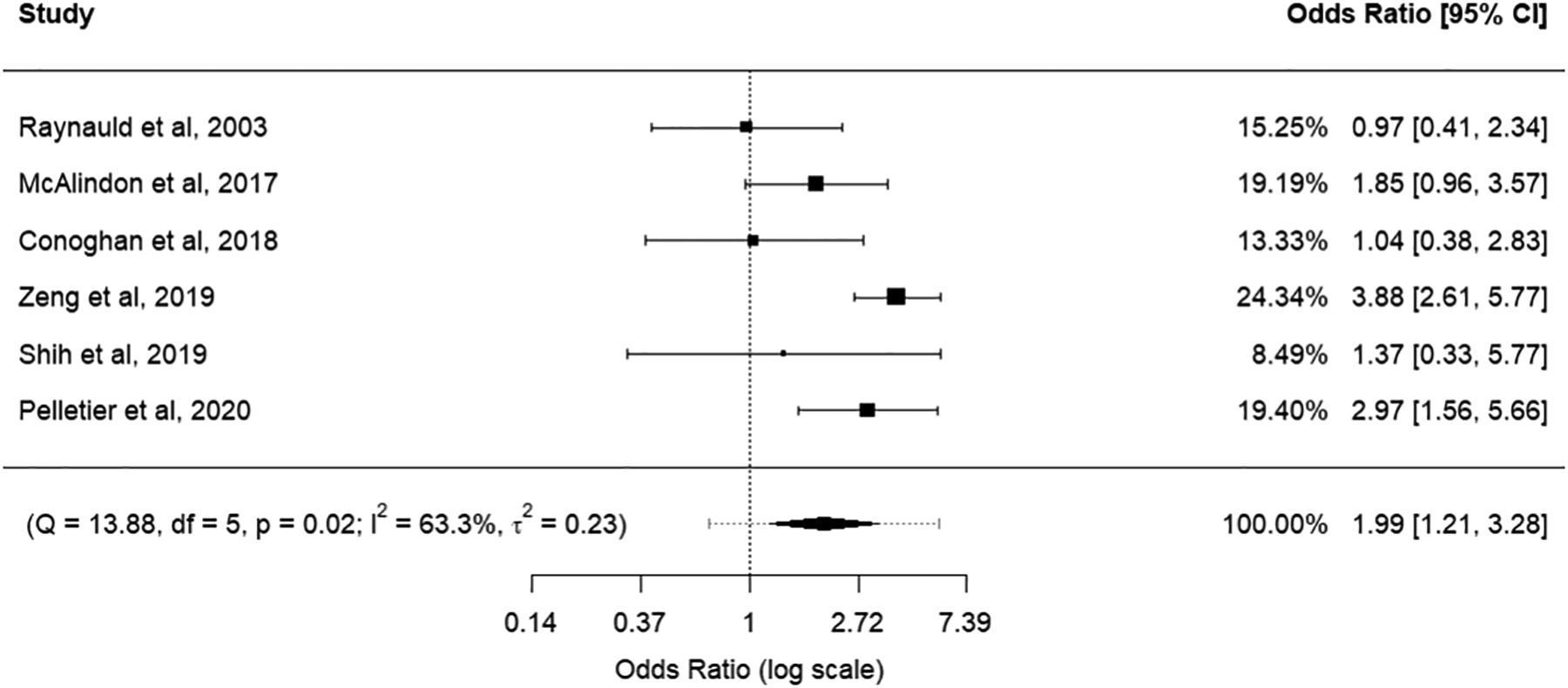
Forest plot for synthesis of all studies identified through literature search. Results from quantitative studies were transformed into standardized mean differences and subsequently into odds ratios for cumulative synthesis with qualitative outcomes. Preference was given to outcomes reporting KL grade.
Overall, effect sizes for the primary syntheses showed a significant odds ratio (OR) signifying knee cartilage structure worsening, both when preferentially considering JSW and KL grade outcomes (OR: 2.01 and 1.99, respectively; 95% Confidence Interval: 1.18,3.44 and 1.21,3.28 respectively).
Visual funnel plot assessment for both subgroups showed missing studies in the bottom right-hand corner of the plot (Figures 5 and 6). Egger’s regression test for funnel plot asymmetry supported the presence of publication bias for both subgroups (p-values <0.01). Trim-and-fill method for bias correction showed an odds ratio of 2.71 (95% Confidence Interval: 1.54–4.77) for the JSW subgroup and 2.89 (95% Confidence Interval: 1.68–4.97) for the KL grade subgroup.
Figure 5:
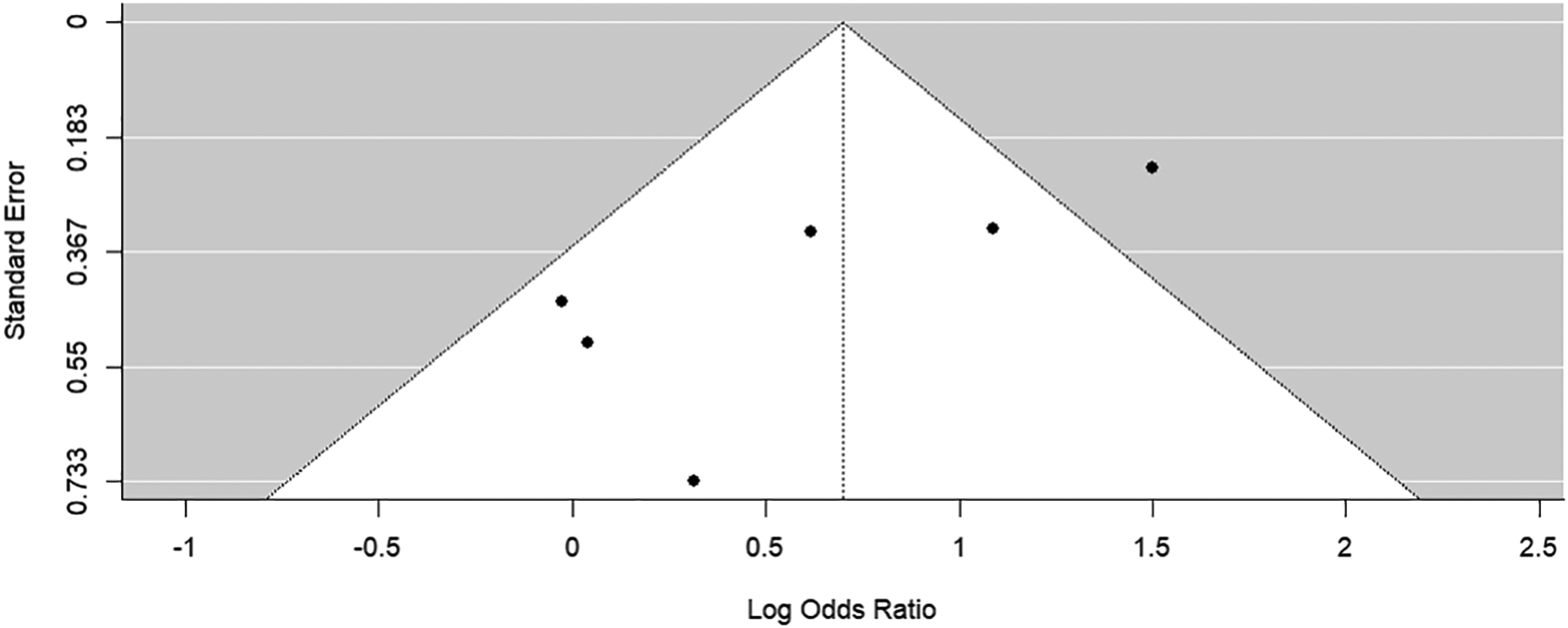
Funnel plot of synthesis of all studies identified through literature search. Preference was given to outcomes reporting JSW.
Figure 6:
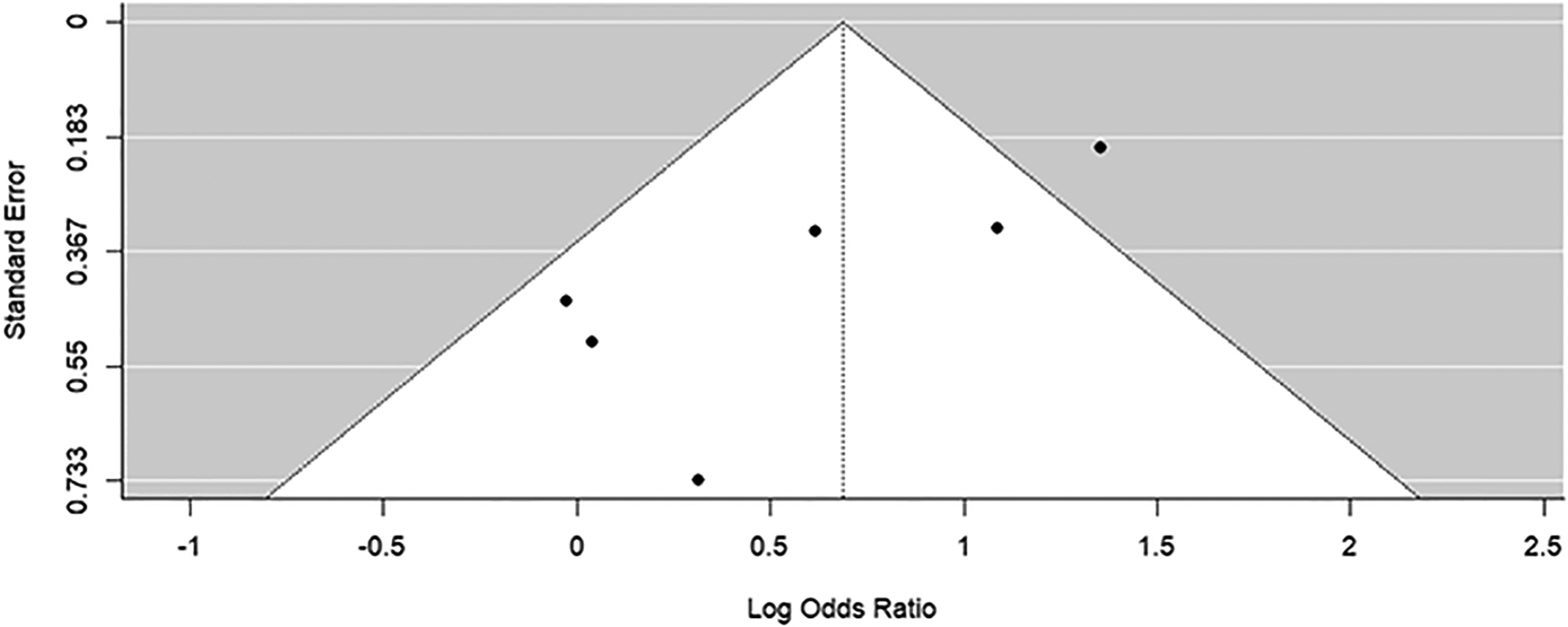
Funnel plot of synthesis of all studies identified through literature search. Preference was given to outcomes reporting KL grade.
Several sensitivity analyses were performed to account for different outcome measures of reported outcomes (cartilage thickness, JSW, and KL grade), different metrics of outcomes (numerical or binary), different imaging modalities (MRI or radiography), and number of IACS administrations (single or multiple injections). The summary the syntheses conducted using different imaging modalities is reported in Table 3. Additional syntheses are reported in the supplemental material (Supplementary Table 1). Overall, effect sizes of the sensitivity analyses also trended towards signifying knee cartilage structure worsening, with significance in syntheses of quantitative measurements (effect size: −0.34, 95% Confidence Interval: −0.66, −0.02), radiographic outcomes preferentially reporting KL grade (OR: 2.03, 95% Confidence Interval: 1.01, 4.10), binary outcomes with preference to KL grade (OR: 2.93, 95%CI: 1.18,7.25), and singular injection studies (both preferentially reporting JSW or KL grade) (OR: 2.44 and 2.40 respectively; 95% Confidence Interval:1.23,4.82 and 1.30, 4.43 respectively).
Table 3:
Summary of random-effects models of sensitivity analyses conducted based on imaging modality (MRI or radiography)
| Analysis | Outcome | Confidence Interval | Cochrane’s Q-test p-value | I2 | τ2 |
|---|---|---|---|---|---|
| Radiographic outcomes – preferentially JSW | 2.08* | 0.98, 4.45 | <0.01 | 78.3% | 0.45 |
| Radiographic outcomes – preferentially KL grade | 2.03 * | 1.01, 4.10 | <0.01 | 78.3% | 0.45 |
| MRI outcomes | 1.76* | 0.97, 3.20 | 0.71 | 0.0% | 0.00 |
Odds Ratios
The forest plots of all included studies in each synthesis and the respective funnel plots are provided in Supplementary File 1.
Discussion
In this meta-analysis, we summarized the available literature regarding potential adverse effects of IACS injections on either progression of cartilage thickness or JSW loss and found that individuals who received IACS were around twice as likely (OR: 1.99 and 2.01 depending on preferential outcome) to experience deleterious effects on knee cartilage structure than individuals who received no or placebo treatment. Our study also found a significant negative estimate (expressed in standardized mean differences) when synthesizing quantitative reports of changes in JSW and cartilage thickness further demonstrating knee cartilage structure deterioration in the context of IACS injections. These pooled data on the available human studies are in line with findings from a systematic review demonstrating significant cartilage damage with higher doses of corticosteroids in in-vitro animal and human models18.
The primary endpoints of the study were changes in JSW and cartilage thickness and were included in the primary cumulative synthesis, whereas KL grade change data was available in one study in tandem with JSW change and used for sensitivity analysis purposes and an initial subgroup of the primary cumulative synthesis. Interestingly, the worsening of the described endpoints was shown to decrease long-term pain-relief and gain-of-function responses to IACS injections by Maricar et al19. A meta-analysis by Jüni et al. on the effect of IACS on pain, function, and quality of life showed unclear benefits in the short-term and non-existent benefits in the long term and posed that this is partially due to the high risk of bias and low methodological quality20.
Some studies included in our analysis suggested a protective effect of IACS injections against radiological OA outcomes. For instance, Shih et al., demonstrated that long-term use of TLC599 (a liposomal formulation of dexamethasone sodium phosphate) intra-articular injections provide pain-relief, increased function, and protection of articular cartilage with long-term usage. However, these findings are questionable and could raise concern in multiple domains in risk of bias assessment21. McAlindon et al. suggested flaws in the outcome measures of previous studies demonstrating deleterious effects of IACS injections leading to potential biases in reporting treatment complications22. Our meta-analysis attempts to address these concerns by including all such studies that provided well-defined radiographic evidence of change as compared to baseline measurements.
Lack of sensitivity of radiography in the detection of longitudinal knee cartilage structure deterioration in knee OA patients have been raised by several studies, especially in comparison to MRI which can quantitatively measure changes in cartilage morphology and thickness via 3D direct cartilage visualization23,24. To address these concerns, we conducted sensitivity analyses on identified studies based on the used imaging modality (i.e., plain radiograph vs. MRI) and found similar trends in odds ratios as our primary analysis, though some results were found not significant.
Proposed underlying mechanisms of IACS leading to short-term positive outcomes in knee cartilage are related to the steroids’ anti-inflammatory effects, decreasing C-reactive protein and erythrocyte sedimentation rates, and allowing for chondrocyte growth25. Yet factors contributing to the negative effects of IACS injections on knee cartilage structure remain to be investigated in future studies. In-vivo animal models have demonstrated decreased thickness and elasticity of the cartilage as well as inhibited cell maturation and increased fibrillation of the articular surface26. In line with the dose-dependent and time-dependent efficacy of treatment with IACS, the relationships between treatment and negative knee cartilage structure outcomes in knee OA patients may be confounded by both the elevated rate of radiographic knee cartilage volume loss in late stage and symptomatic osteoarthritic knees27. In addition, there may be an increased necessity for receiving injections in individuals with higher pain levels. On the other hand, the analgesic effects of the steroid injections in these patients may accentuate harmful gait patterns due to lack of pain feedback and therefore, lead to accelerated knee articular cartilage deterioration. Our study does not include a risk evaluation regarding potential factors that may or may not increase the likelihood of accelerated progression as defined by our structural outcomes.
In summary, our meta-analysis showed that IACS administration increases the likelihood of knee cartilage structure deterioration as measured by JSW and cartilage thickness (both binary and numerical variables). Given that this meta-analysis included studies investigating MRI-defined cartilage loss and radiographic joint space narrowing and given that JSW may be affected by numerous factors, e.g., swelling and extra fluid in the synovium, this could be considered as a limitation of our results given the available relevant literature. Future trials can be designed to compare the effect of IACS Injection on “MRI-defined Cartilage Loss” vs “Radiographic Joint Space Narrowing”. Further high-quality investigations, including randomized controlled trials are urgently needed to gain further insights in cartilage structure deterioration in knee OA patients undergoing IACS injections to establish guidance for physicians performing IACS by careful selection and screening of candidates prior to intervention. In addition, risk profiles are desirable to avoid potential adverse outcomes including but not limited to cartilage loss and decrease in JSW.
Supplementary Material
Acknowledgement:
The authors have no additional acknowledgements to disclose
Funding:
This research was supported by the NIH National Institute on Aging (NIA) under Award Number P01AG066603 and NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under Award Number R01AR079620-01
Abbreviations:
- OA
Osteoarthritis
- IACS
Intraarticular Corticosteroids
- MRI
Magnetic Resonance Imaging
- JSW
Joint Space Width
- KL
Kellgren- Lawrence
- NOSGEN
Newcastle-Ottawa Quality Assessment Scale
Footnotes
Conflict of Interest:
Ali Guermazi is a shareholder of BICL. He is a consultant to Pfizer, TissueGene, Regeneron, Novartis, AstraZeneca, and Merck-Serono. Frank W Roemer is a shareholder of BICL. He is a consultant to Grünenthal GmbH and Calibr (past 36 months) None of the remaining authors have any conflicting personal or financial relationships that could have influenced the results of this study.
References
- 1.Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29–30. doi: 10.1016/j.eclinm.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. The Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 4.Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA - Journal of the American Medical Association. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020;72(2):149–162. doi: 10.1002/acr.24131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Orthopaedic Surgeons Management of Osteoarthritis of the Knee (Non-Arthroplasty) Evidence-Based Clinical Practice Guideline (3rd Edition). Published August 31, 2021. Accessed July 15, 2022. https://www.aaos.org/oak3cpg [Google Scholar]
- 8.Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop J Sports Med. 2015;3(5):1–7. doi: 10.1177/2325967115581163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guermazi A, Neogi T, Katz JN, et al. Intra-articular Corticosteroid Injections for the Treatment of Hip and Knee Osteoarthritis-related Pain: Considerations and Controversies with a Focus on Imaging— Radiology Scientific Expert Panel. Radiology. 2020;297(3):503–512. doi: 10.1148/radiol.2020200771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps not as safe as we thought? Radiology. 2019;293(3):656–663. doi: 10.1148/radiol.2019190341 [DOI] [PubMed] [Google Scholar]
- 11.Pelletier JP, Raynauld JP, Abram F, Dorais M, Paiement P, Martel-Pelletier J. Intra-articular corticosteroid knee injection induces a reduction in meniscal thickness with no treatment effect on cartilage volume: a case–control study. Sci Rep. 2020;10(1). doi: 10.1038/s41598-020-70064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. doi: 10.1136/BMJ.L4898 [DOI] [PubMed] [Google Scholar]
- 13.Hasselblad V, Hedges L v. Meta-analysis of screening and diagnostic tests. Psychol Bull. 1995;117(1):167–178. doi: 10.1037/0033-2909.117.1.167 [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8(4):448–467. doi: 10.1037/1082-989X.8.4.448 [DOI] [PubMed] [Google Scholar]
- 15.Cox DR and Snell EJ (1989) Analysis of Binary Data. 2nd Edition, Chapman and Hall/CRC, London. - References - Scientific Research Publishing. Accessed December 6, 2022. https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/referencespapers.aspx?referenceid=2052223 [Google Scholar]
- 16.Raudenbush SW Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation; 2009:295–315. [Google Scholar]
- 17.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. Journal of Educational and Behavioral Statistics. 2005;30(3):261–293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- 18.Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop J Sports Med. 2015;3(5):1–7. doi: 10.1177/2325967115581163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maricar N, Parkes MJ, Callaghan MJ, et al. Structural predictors of response to intra-articular steroid injection in symptomatic knee osteoarthritis. Arthritis Res Ther. 2017;19(1):88. doi: 10.1186/s13075-017-1292-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database of Systematic Reviews. 2015;2015(10). doi: 10.1002/14651858.CD005328.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih SF, Brown C, Tai TT, Chuang W. Magnetic Resonance Imaging of Knee Joint Protection Following an Intra-Articular Injection of Lipid-Based Dexamethasone Sodium Phosphate Sustained Release Formulation on Subjects with Knee Osteoarthritis - ACR Meeting Abstracts. Accessed December 19, 2022. https://acrabstracts.org/abstract/magnetic-resonance-imaging-of-knee-joint-protection-following-an-intra-articular-injection-of-lipid-based-dexamethasone-sodium-phosphate-sustained-release-formulation-on-subjects-with-knee-osteoarthri/
- 22.McAlindon TE, Harkey MS, Ward RJ, Hochberg MC, Driban JB. Intra-articular corticosteroid injections in the hip and knee: Perhaps not as dangerous as they want you to believe? Radiology. 2020;295(1):249–250. doi: 10.1148/radiol.2020200050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin S, LaValley MP, Guermazi A, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52(10):3152–3159. doi: 10.1002/art.21296 [DOI] [PubMed] [Google Scholar]
- 24.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. 2011;13(6). doi: 10.1186/ar3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28(7):749–756. doi: 10.1007/s10067-009-1135-x [DOI] [PubMed] [Google Scholar]
- 26.Tung JT, Venta PJ, Caron JP. Inducible nitric oxide expression in equine articular chondrocytes: Effects of antiinflammatory compounds. Osteoarthritis Cartilage. 2002;10(1):5–12. doi: 10.1053/joca.2001.0476 [DOI] [PubMed] [Google Scholar]
- 27.Lahm A, Dabravolski D, Rödig J, Esser J, Erggelet C, Kasch R. Varying development of femoral and tibial subchondral bone tissue and their interaction with articular cartilage during progressing osteoarthritis. Arch Orthop Trauma Surg. 2020;140(12):1919–1930. doi: 10.1007/s00402-020-03480-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


