Abstract
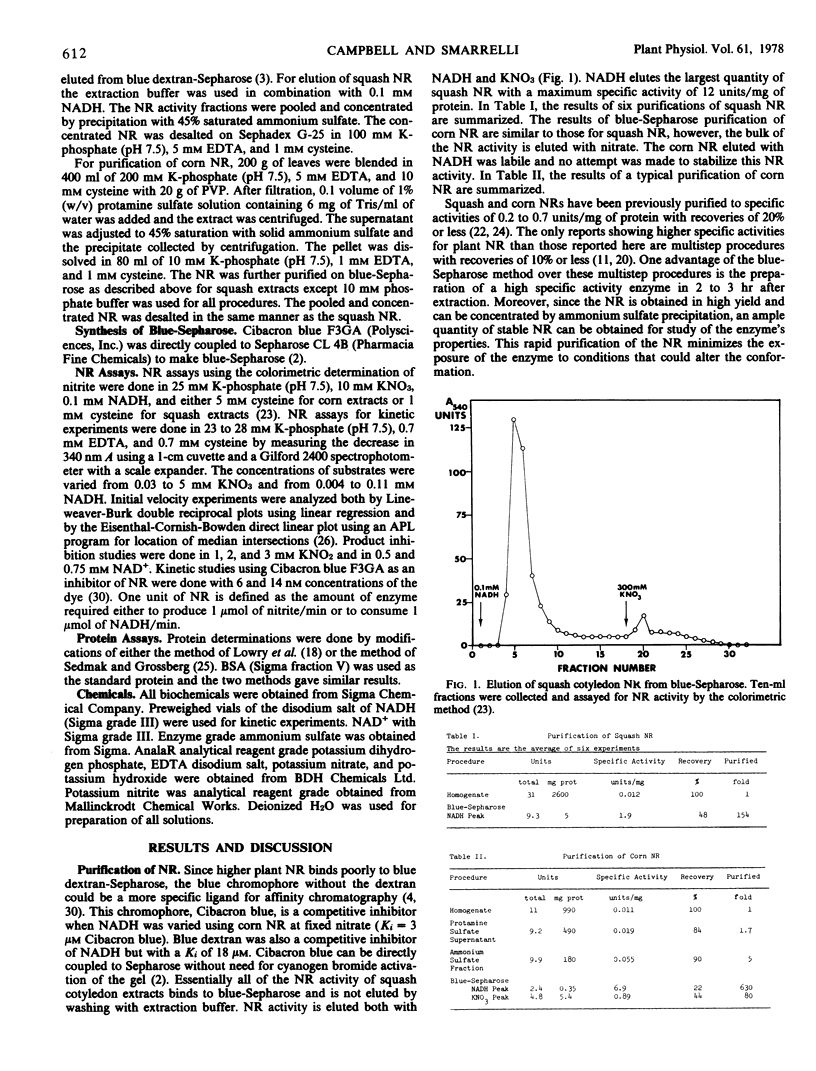
Squash cotyledon (Cucurbita pepo L.) NADH:nitrate reductase (NR) was purified 150-fold with 50% recovery by a single step procedure based on the affinity of the NR for blue-Sepharose. Blue-Sepharose, which is prepared by direct coupling of Cibacron blue to Sepharose, appears to bind squash NR at the NADH site. The NR can be purified in 2 to 3 hours to a specific activity of 2 μmol of NADH oxidized/minute • milligram of protein. Corn (Zea mays L.) leaf NR was also purified to a specific activity of 6.9 μmol of NADH oxidized/minute • milligram of protein using a blue-Sepharose affinity step. The blue-Sepharose method offers the advantages of a rapid purification of plant NR to a high specific activity with reasonable recovery of total activity.
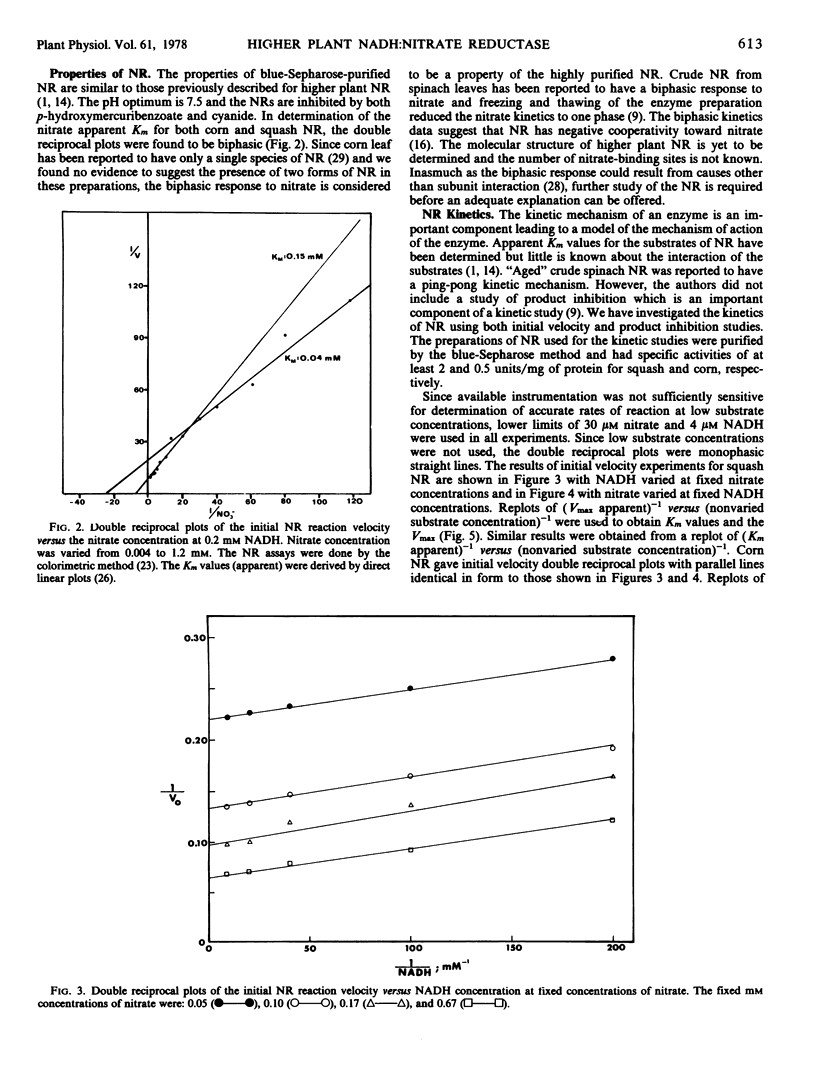
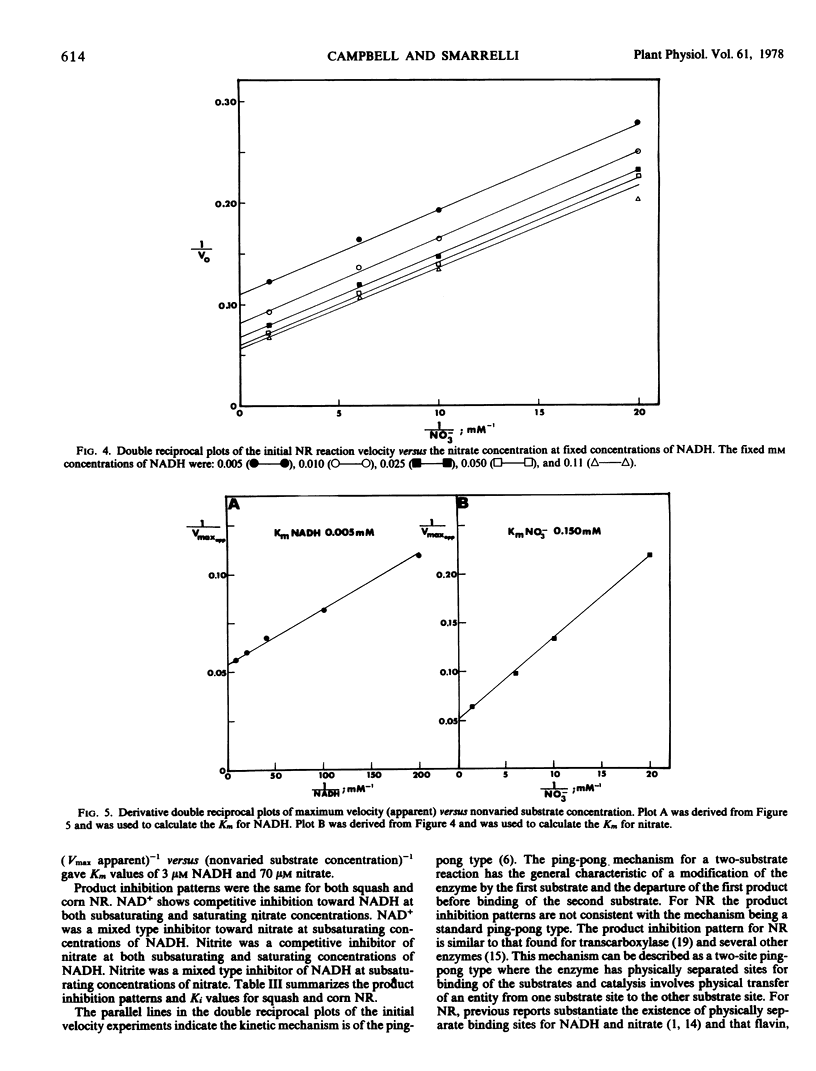
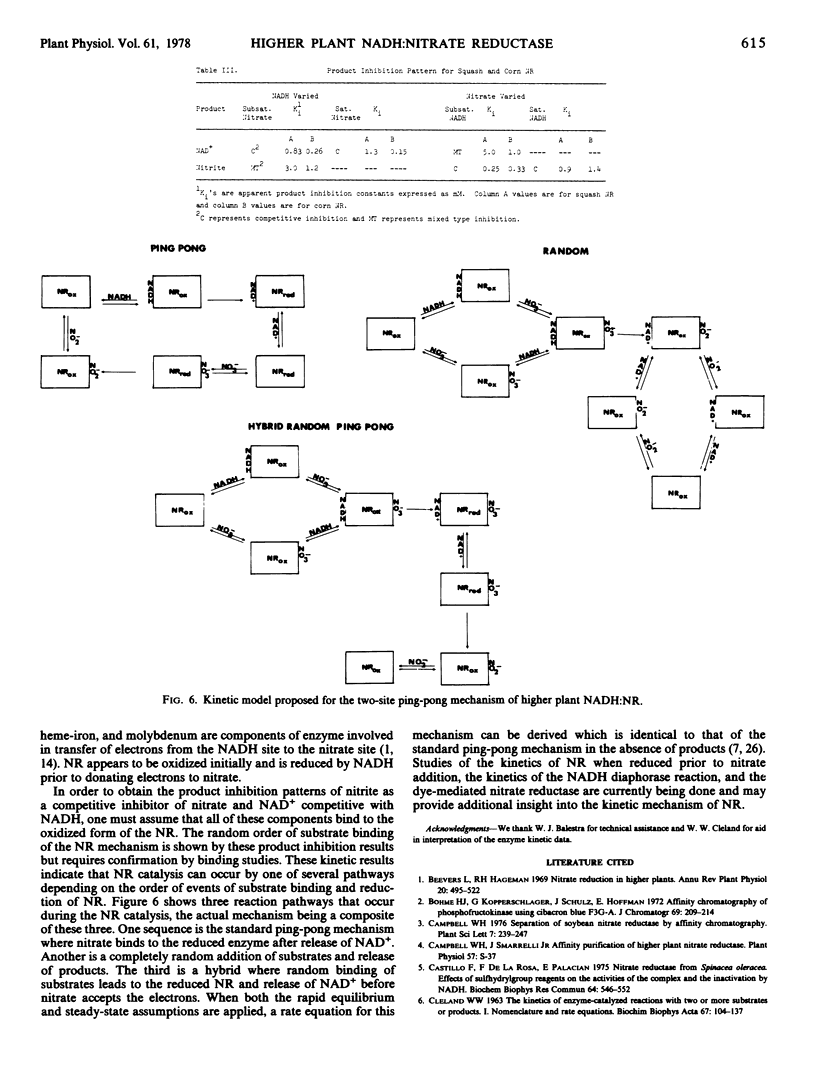
The kinetic mechanism of higher plant NR was investigated using these highly purified squash and corn NR preparations. Based on initial velocity and product inhibition studies utilizing both enzymes, a two-site ping-pong mechanism is proposed for NR. This kinetic mechanism incorporates the concept of the reduced NR transferring electrons from the NADH site to a physically separated nitrate site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Castillo F., de la Rosa F. F., Palacián E. Nitrate reductase from Spinacea oleracea. Effects of sulfhydryl-group reagents on the activities of the complex and the inactivation by NADH. Biochem Biophys Res Commun. 1975 May 19;64(2):546–552. doi: 10.1016/0006-291x(75)90356-3. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Derivation of rate equations for multisite ping-pong mechanisms with ping-pong reactions at one or more sites. J Biol Chem. 1973 Dec 25;248(24):8353–8355. [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M. G., Jetschmann K., Völker W. The stereospecificity of nitrate reductase for hydrogen removal from reduced pyridine nucleotides. Biochim Biophys Acta. 1977 May 12;482(1):19–26. doi: 10.1016/0005-2744(77)90349-7. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Krashin S., Riklis E. The use of affinity chromatography for the purification of nitrate reductase. FEBS Lett. 1976 Feb 1;62(1):30–32. doi: 10.1016/0014-5793(76)80009-9. [DOI] [PubMed] [Google Scholar]
- Katiyar S. S., Cleland W. W., Porter J. W. Fatty acid synthetase. A steady state kinetic analysis of the reaction catalyzed by the enzyme from pigeon liver. J Biol Chem. 1975 Apr 10;250(7):2709–2717. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr The role of negative cooperativity and half-of-the-sites reactivity in enzyme regulation. Curr Top Cell Regul. 1976;10:1–40. doi: 10.1016/b978-0-12-152810-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Northrop D. B. Transcarboxylase. VI. Kinetic analysis of the reaction mechanism. J Biol Chem. 1969 Nov 10;244(21):5808–5819. [PubMed] [Google Scholar]
- Palacián E., De la Rosa F., Castillo F., Gómez-Moreno C. Nitrate reductase from Spinacea oleracea. Reversible inactivation by NAD(P)H and by thiols. Arch Biochem Biophys. 1974 Apr 2;161(2):441–447. doi: 10.1016/0003-9861(74)90326-9. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny J. R., Fisher J. R. An alternative to allosterism and cooperativity in the interpretation of enzyme kinetic data. Biochemistry. 1968 Feb;7(2):561–565. doi: 10.1021/bi00842a008. [DOI] [PubMed] [Google Scholar]
- Wells G. N., Hageman R. H. Specificity for nicotinamide adenine dinucleotide by nitrate reductase from leaves. Plant Physiol. 1974 Aug;54(2):136–141. doi: 10.1104/pp.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Applications of blue dextran and Cibacron Blue F3GA in purification and structural studies of nucleotide-requiring enzymes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):816–823. doi: 10.1016/s0006-291x(76)80206-9. [DOI] [PubMed] [Google Scholar]


