Abstract
Aberrant sperm protamination is linked to sperm dysmorphology and nuclear chromatin condensation. Yet, its effects on sperm cytoplasmic maturation remain largely unexplored. The relationships of protamines, sperm morphology, DNA damage, and cytoplasmic remodeling were illustrated in this study to provide fresh perspectives on the mechanisms of male infertility. A total of 205 infertile males were allocated into 5 groups according to the percentage of spermatozoa exhibiting abnormal morphology within their samples. Sperm concentration, motility, abnormal sperm morphology, cytoplasmic droplets (CDs), and excess residual cytoplasm (ERC) were analyzed according to the World Health Organization manual (2010). Sperm nuclear vacuoles (NVs) were determined by propidium iodide (PI) staining. Sperm protamine expressions (P1 and P2) were detected by western blot. DNA damage was measured by acridine orange test (AOT) to calculate the proportion of sperm with single-strand DNA breaks (SSBs). Our data showed that sperm concentration and motility in infertile males significantly decreased with the severity of abnormal sperm morphology (both P < 0.01). P1 level, P1/P2 ratio, and SSB rate increased with the severity of sperm dysmorphology, whilst the P2 level decreased (all P < 0.01). NVs, CDs, and ERC were more common in males with sperm dysmorphology and positively correlated with the SSB rate (all P < 0.01). The relationships between the SSB rate and the P1/P2 ratio were also significant (P < 0.01). Aberrant protamination may cause sperm dysmorphology and compromise male fertility by impairing sperm’s nucleus and cytoplasm maturation, with the P1/P2 ratio potentially serving as a valuable indicator of sperm quality and male fertility.
Keywords: abnormal sperm morphology, excess residual cytoplasm, male infertility, protamines, sperm DNA damage, sperm nuclear vacuoles
INTRODUCTION
Spermiogenesis is the last stage of spermatogenesis, where the postmeiotic male germ cells finally differentiate into mature spermatozoa. During spermiogenesis, round spermatids go through remarkably complex structural and biochemical changes, compacting DNA in the nucleus and losing most of their cytoplasm.1 An anomaly in these processes can lead to sperm morphological abnormalities and aberrant differentiation.2 Abnormal sperm morphology is usually evaluated based on head shape, nuclear vacuoles, tail structures, and residual cytoplasm. Sperm morphological defects are always mixed and can affect male fertility.3
Sperm head morphology usually correlates with nuclear chromatin condensation,4 which is caused by the histone-to-protamine exchange during spermiogenesis.5 Human spermatozoa express two types of protamines, called protamine 1 (P1) and protamine 2 (P2), and their expressions exhibit an ideal ratio between 0.8 and 1.2.6 In late spermiogenesis, P2 would be replaced by P1, which has greater affinity for DNA and contributes to well-compacted sperm.7 Aberrant protamination, characterized by the alterations of protamine contents and/or ratio, can affect nuclear chromatin compaction,8,9,10 leading to sperm DNA damage,11 which can impair sperm functions and male fertility.12 Furthermore, sperm nuclear vacuoles (NVs) are common within nuclear chromatin, though large NVs are considered a type of sperm head morphological defect.13 Protamine deficiency may conspire to the formation of NVs,14 as NVs may result from insufficient chromatin compaction and can in part reflect the level of sperm DNA damage.15 Therefore, aberrant protamination is supposed to correlate with abnormal sperm morphology and male infertility.16,17
In addition to nuclear changes, sperm’s cytoplasm is extremely reduced during spermiogenesis.18 Most of the spermatid cytoplasm is phagocytosed by the Sertoli cells while a small amount of retention may be found around the midpiece due to the incomplete extrusion.19 When cytoplasmic retentions present in large amounts, they are termed excess residual cytoplasm (ERC) and deemed abnormal sperm morphology,13 which is associated with sperm dysfunction and male infertility.20 Cytoplasmic droplets (CDs) less than one-third the size of the sperm head are considered normal morphological appearances. ERC and poor protamination are two hallmarks of defective spermatozoa,21 whether there is a linkage between them is worthy of further research. It is likely that aberrant protamination is involved in both sperm’s nuclear and cytoplasmic maturation.
The aim of this study was to evaluate the effects of protamination on sperm morphology and male fertility by assessing sperm’s nuclear and cytoplasmic parameters; all the cases were grouped according to the percentage of spermatozoa with abnormal morphology. Semen analysis is the routine physical examination to assess male fertility,13 as infertile males usually display poor sperm parameters.22 However, there are some infertile males in whom no abnormalities can be detected by semen analysis; this is referred to normozoospermic infertility.23 The infertile males with normozoospermia were set as controls in the present study, aiming to illustrate the relationships between protamination and sperm morphology, to provide further insights into the underlying mechanisms of male infertility.
PARTICIPANTS AND METHODS
Study group and semen analysis
All semen samples were obtained from infertile males who came to the Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Longgang Maternity and Child Institute of Shantou University Medical College; Shenzhen, China) for infertility treatment from July 2018 to August 2020. All the included subjects had a history of infertility for more than 12 months and did not receive exogenous hormonal drugs, chemotherapy, or other medicines known for impairing testicular functions within the past 6 months before semen collection. Semen samples were collected by masturbation after 3–7 days of sexual abstinence. The present study was approved by the Research Ethics Committee of Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Approval No. LGFYYXLL-033). Informed consent was obtained from all the participating males.
After liquefaction for 30 min at room temperature, the sperm smears were prepared, and semen analysis was performed according to the World Health Organization manual.13 The wet smears were first observed at 400× magnification (catalog No.16242; Nikon, Tokyo, Japan) to ensure that the sperm was evenly dispersed across the slides and lacked any agglomeration. For each specimen, two duplicate smears were prepared to ensure the accuracy of our observations. Sperm morphology was assessed after the semen smear was stained using the modified Papanicolaou staining method. The stained semen smears were examined at 1000× magnification under oil immersion (catalog No.16242; Nikon), and a minimum of 200 spermatozoa per slide were examined. A total of 177 infertile males were allocated into 4 groups according to the percentage of spermatozoa with abnormal morphology in their semen samples: Group A (abnormal spermatozoa = 100%; n = 20; age range [mean±standard deviation, s.d.]: 29.0–46.0 [36.0 ± 3.9] years); Group B (abnormal spermatozoa >96% and ≤99%; n = 65; age range [mean±s.d.]: 24.0–46.0 [33.0 ± 3.7] years); Group C (abnormal spermatozoa >93% and ≤96%; n = 56; age range [mean±s.d.]: 25.0–40.0 [32.0 ± 3.1] years); Group D (abnormal spermatozoa ≤93%; n = 36; age range [mean±s.d.]: 26.0–37.0 [32.0 ± 2.6] years). There are 28 infertile men with the WHO normal sperm parameters enrolled in the control group (Group E; age range [mean±s.d.]: 24.0–37.0 [32.0 ± 2.7] years).
Assay of sperm CDs and ERC
The semen smear was stained and examined as described in the preceding section. CDs were defined as membrane-bound vesicles on the midpiece at the head-neck junction and being smaller than one-third the sperm head size. ERC is characterized by large amounts of irregularly stained cytoplasm along the midpiece, one-third or more the size of the sperm head. The percentages of CDs and ERC were evaluated from a minimum of 200 spermatozoa in two duplicate smears when assessing sperm morphology.13
Assay of sperm NVs
Our research group had developed a method to evaluate sperm NVs with propidium iodide (PI).24 In the current study, this established method was used to classify sperm nuclei into 14 distinct categories, designated as types “a” to “n”, based on the nuclear shape and the positioning of the vacuole.24 Thereafter, the percentage of each sperm nuclei type was calculated. The quality control of the NVs analysis was determined following the method proposed in the WHO manual.13
Analysis of sperm protamines by western blot
Protamine proteins were entirely procured from sperm specimens, and the protein quantification was carried out via a protein assay kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). The P1 and P2 quantities were ascertained utilizing acid-urea polyacrylamide gel electrophoresis (AU-PAGE; Bio-Rad, Hercules, CA, USA). The separating gel was made with 20% acrylamide, 0.29% bisacrylamide, 2.5 mol l−1 urea, 0.9 mol l−1 acetic acid, 0.33% N,N,N’,N’-Tetramethylethylenediamine (TEMED), and 0.18% ammonium persulfate. Meanwhile, the stacking gel was synthesized with 7.5% acrylamide, 0.11% bisacrylamide, 2.5 mol l−1 urea, 0.27 mol l−1 acetic acid, 0.5% TEMED, and 0.48% ammonium persulfate. Gels were prerun for 1 h at 180 V before protein loading. The isolated proteins were dissolved in a 0.9 mol l−1 glacial acetic buffer. The gels were then hued with a BioSafe Coomassie Blue G-250 solution. Protein bands were detected through electrochemiluminescence (ECL; TransGen Biotech, Beijing, China), and the P1 and P2 bands were semi-quantified with the aid of Bio-Rad Quantity One software (Bio-Rad).
Acridine orange test (AOT)
The compact acridine orange molecule is capable of penetrating dense sperm chromatin, binding with double-strand DNA (native DNA) to emanate a green fluorescence, and stacking on single-strand DNA (denatured DNA) to produce a red fluorescence.25 The quantification of sperm DNA damage in infertile men with varying degrees of abnormal sperm morphology was measured by the proportion of spermatozoa bearing single-strand DNA breaks (SSBs). AOT procedures were executed according to the method described by Tejada et al.26 but with minor adjustments. After the adjustment for sperm count (5 × 107 ml−1), medium-thickness smears were placed on precleaned slides, left to air dry, and subsequently fixed overnight in recently prepared Carnoy’s solution (methanol/glacial acetic acid=3:1 [v/v]), and stained with AO staining solution after another round of air drying (Sigma, Deisenhofen, Germany). Every slide was prepared in duplicate and examined under a fluorescent microscope (DM400B, Leica, Wetzlar, Germany). Spermatozoa with normal, undamaged double-strand DNA exhibited a green fluorescence, whereas those with denatured single-strand DNA displayed a red fluorescence. The percentage of spermatozoa with a red fluorescence was derived by counting a minimum of 200 cells per slide. All readings were carried out in duplicate, and the average value was utilized for further analysis.
Statistical analyses
Statistical analysis was performed using SPSS 21.0 (SPSS, Chicago, IL, USA). Differences among groups were determined by one-way analysis of variance (ANOVA) followed by Tukey multiple-comparison tests. Results were presented as mean±s.d. The sperm parameters were correlated with the rate of SSBs using Spearman’s rank correlation. P < 0.05 was considered statistically significant.
RESULTS
Sperm parameters of infertile males with varying degrees of abnormal sperm morphology
The sperm parameters of infertile males with varying degrees of abnormal sperm morphology are shown in Table 1. With respect to the conventional sperm parameters, significantly lower levels of sperm concentration were observed in Groups A, B, C, and D when compared to that in Group E (all P < 0.01). Significantly lower levels of sperm motility were also observed in Groups A, B, and C (all P < 0.01). However, while sperm concentration and motility showed a significant decline in infertile males with severely abnormal sperm morphology (both P < 0.01), the sperm motility of Groups D and E was not significantly different from that of Group E (both P > 0.05).
Table 1.
Characteristics of semen samples from infertile males with varying degrees of abnormal sperm morphology
| Parameter | Group A (n=20) | Group B (n=65) | Group C (n=56) | Group D (n=36) | Group E (n=28) |
|---|---|---|---|---|---|
| Age (year) | 36.0±3.9 | 33.0±3.7 | 32.0±3.1 | 32.0±2.6 | 32.0±2.7 |
| Abnormal sperm morphology (%) | 100.0±0 | 97.9±0.8 | 95.3±0.7 | 92.2±0.7 | 89.2±0.9 |
| Sperm concentration (×106 ml−1) | 6.6±4.3** | 21.3±15.4** | 46.4±26.0** | 60.7±26.0** | 89.1±8.4 |
| Sperm motility (%) | 16.4±8.6** | 38.2±10.2** | 57.8±7.7** | 64.6±5.9 | 67.6±4.9 |
| Sperm single-strand DNA breaks (%) | 20.9±8.9** | 13.5±3.4** | 10.9±1.4** | 8.6±1.0 | 6.4±1.2 |
| CDs (%) | 39.3±5.1** | 34.7±2.8** | 26.6±2.3** | 19.2±2.4** | 15.4±2.9 |
| ERC (%) | 35.1±2.0** | 32.7±4.2** | 30.4±4.3** | 29.3±4.6 | 27.3±3.4 |
**P<0.01 (the indicated value vs that of Group E). Data are presented as mean±standard deviation. Group A: abnormal spermatozoa=100%; Group B: abnormal spermatozoa >96% and ≤99%; Group C: abnormal spermatozoa >93% and ≤96%; Group D: abnormal spermatozoa ≤93%; Group E: control group. CDs: cytoplasmic droplets; ERC: excess residual cytoplasm
Based on the results, both CDs and ERC were more common in infertile males with severely abnormal sperm morphology. ERC occurrence was significantly elevated in Groups A, B, and C when compared to Group E (all P < 0.01), though the difference between Groups D and E was not significant (P > 0.05). The percentages of CDs in Groups A, B, C, and D were significantly higher than that in Group E (all P < 0.01).
Sperm SSB rates in infertile males with abnormal sperm morphology
In addition, the sperm SSB rates of Groups A, B, and C were significantly higher than that of Group E (all P < 0.01), while the values between Groups D and E were similar (P > 0.05). The representative images of sperm AOT staining among the groups of infertile males with abnormal sperm morphology are shown in Figure 1.
Figure 1.
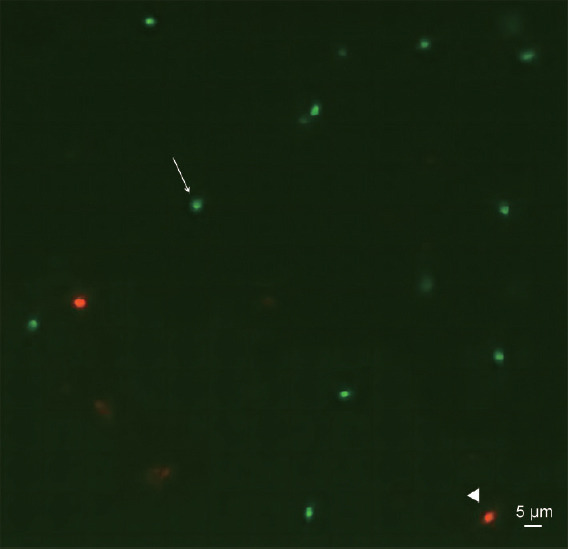
Representative image of sperm DNA denaturation assessed by AOT in the enrolled infertile males. Spermatozoa with double-stranded DNA were green (arrow); those with single-stranded DNA were red (triangle). AOT: acridine orange test.
Correlations between sperm SSBs and sperm parameters in infertile males with abnormal sperm morphology
The results of the correlation analysis between the sperm SSB rate and sperm parameters are expressed in Table 2. Abnormal sperm morphology positively correlated to sperm SSB rate (r = 0.884, P < 0.01). Abnormal sperm morphology was significantly associated with higher percentages of CDs (r = 0.968, P < 0.01) and ERC (r = 0.505, P < 0.01) while negatively correlating to sperm concentration (r = −0.772, P < 0.01) and motility (r = −0.880, P < 0.01). Moreover, the sperm SSB rate positively correlated to the percentages of CDs (r = 0.861, P < 0.01) and ERC (r = 0.403, P < 0.01) whilst negatively correlating to sperm concentration (r = −0.756, P < 0.01) and motility (r = −0.774, P < 0.01).
Table 2.
Correlations between sperm single-strand DNA breaks and sperm parameters in infertile males with abnormal sperm morphology
| Variable | Sperm SSBs | Abnormal morphology (%) | ||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| Abnormal morphology (%) | 0.884 | <0.01** | 1 | - |
| Sperm concentration (×106 ml−1) | −0.756 | <0.01** | −0.772 | <0.01** |
| Motility (%) | −0.774 | <0.01** | −0.880 | <0.01** |
| CDs (%) | 0.861 | <0.01** | 0.968 | <0.01** |
| ERC (%) | 0.403 | <0.01** | 0.505 | <0.01** |
**P<0.01. Data are presented as mean±standard deviation. r: correlation coefficient; SSB: single-strand DNA break; CDs: cytoplasmic droplets; ERC: excess residual cytoplasm; -: no value
The status of sperm NVs in infertile males with varying degrees of abnormal sperm morphology
The characteristics of NVs in infertile males with varying degrees of abnormal sperm morphology are presented in Table 3. NVs showed a variety in number, size, shape, location, and distribution and appeared anywhere within the nucleus, such as the anterior and posterior regions, nuclear edge, or near the neck. In general, the incidences of NVs were significantly higher in infertile males with abnormal sperm morphology when compared to the normozoospermic males. Moreover, the incidences increased with the severity of abnormal sperm morphology. The representative images of NVs in infertile males are shown in Figure 2.
Table 3.
Characteristics of nuclear vacuoles in infertile males with varying degrees of abnormal sperm morphology
| Variable (%) | Group A (n=20) | Group B (n=65) | Group C (n=56) | Group D (n=36) | Group E (n=28) |
|---|---|---|---|---|---|
| NVs incidence | 95.5±4.0** | 84.8±8.0** | 74.5±7.0** | 61.7±6.8** | 39.0±11.8 |
| Type “a” of sperm | 0.7±0.7** | 3.8±2.5** | 9.6±4.6** | 21.2±5.8** | 41.2±9.0 |
| Type “b” of sperm | 0.6±0.6** | 3.0±2.0** | 6.8±4.2 | 10.3±3.3 | 8.2±4.0 |
| Type “c” of sperm | 0.4±0.6 | 0.5±0.8 | 0.7±1.1 | 0.4±0.5 | 0.4±0.7 |
| Type “d” of sperm | 0.3±0.5 | 1.4±1.3 | 1.5±2.8 | 0.8±1.2 | 1.4±2.2 |
| Type “e” of sperm | 0.7±0.7* | 1.5±2.2 | 1.9±2.5 | 2.7±2.3 | 2.6±1.5 |
| Type “f” of sperm | 0.1±0.3 | 0.3±0.7 | 0.2±0.5 | 0.3±0.6 | 0.3±0.6 |
| Type “g” of sperm | 3.8±3.9** | 11.3±7.0** | 15.8±5.5* | 17.1±4.7 | 19.8±5.9 |
| Type “h” of sperm | 12.5±5.3 | 14.7±6.7** | 15.1±6.4** | 13.7±6.3* | 8.9±4.8 |
| Type “i” of sperm | 2.0±1.5* | 1.7±2.0** | 1.0±1.4 | 0.5±0.9 | 0.5±0.7 |
| Type “j” of sperm | 31.1±5.7** | 24.8±6.6** | 21.3±5.9** | 15.6±4.8** | 7.6±4.8 |
| Type “k” of sperm | 34.9±6.1** | 26.2±6.6** | 19.3±5.8** | 12.9±5.2** | 6.6±5.7 |
| Type “l” of sperm | 0.7±0.9 | 0.8±1.5 | 0.4±0.6 | 0.3±0.5 | 0.3±0.5 |
| Type “m” of sperm | 6.0±3.9** | 5.2±3.5** | 3.4±2.1* | 3.0±1.7 | 1.5±1.6 |
| Type “n” of sperm | 5.6±2.7** | 4.1±2.5** | 2.3±2.1** | 1.3±1.4 | 0.5±0.7 |
*P<0.05; **P<0.01 (the indicated value vs that of Group E). Data are presented as mean±standard deviation. Group A: abnormal spermatozoa=100%; Group B: abnormal spermatozoa >96% and ≤99%; Group C: abnormal spermatozoa >93% and ≤96%; Group D: abnormal spermatozoa ≤93%; Group E: control group. NV: nuclear vacuole
Figure 2.
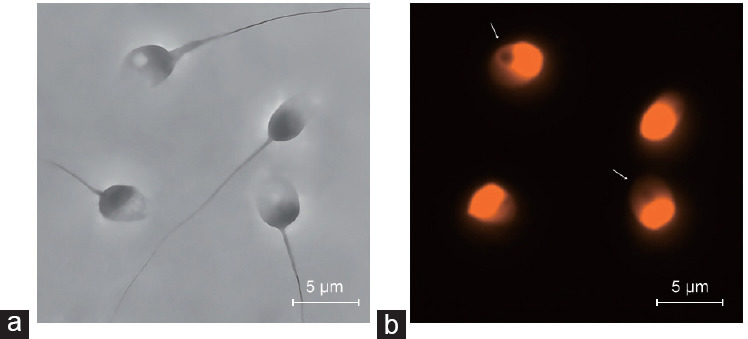
Representative images of NVs in infertile males. (a) Phase contrast photograph of spermatozoa. (b) Fluorescent photograph of spermatozoa stained with PI. The arrows indicate sperm NVs. NV: nuclear vacuole; PI: propidium iodide.
In Groups A, B, C, and D, the incidences of the normal and nonoval nuclear-shaped spermatozoa without vacuoles (types “a” and “g”) were significantly lower when compared to that of Group E, with the lowest level being found in Group A (P < 0.01). In addition, abnormal nuclear-shaped spermatozoa with one large vacuole or ≥2 vacuoles and degenerating nucleus (types “j”, “k”, “m”, and “n”) were more common in Groups A, B, C, and D than in Group E (all P < 0.01). In all the groups, spermatozoa with vacuoles located in the postnuclear regions had very low incidences (types “c”, “d”, “f”, and “l”; all P > 0.05).
Correlations between sperm NVs and sperm parameters in infertile males with varying degrees of abnormal sperm morphology
The results of the correlation analyses of sperm NVs and sperm parameters are presented in Supplementary Table 1. The incidence of sperm NVs positively correlated to abnormal sperm morphology (r = 0.911, P < 0.01), ERC (r = 0.921, P < 0.01), CDs (r = 0.436, P < 0.01), and SSB rate (r = 0.889, P < 0.01).
Supplementary Table 1.
Correlation analyses of sperm nuclear vacuoles and sperm parameters in infertile males with abnormal sperm morphology
| Variable | Outcomes (%) | Correlation to NV (r) |
|---|---|---|
| Abnormal morphology | 95.2±3.4 | 0.911** |
| CDs | 27.6±8.4 | 0.436** |
| ERC | 30.9±4.6 | 0.921** |
| Sperm SSB rate | 11.7±5.2 | 0.889** |
**P<0.01. Data are presented as mean±s.d. r: correlation coefficient; NV: nuclear vacuole; CDs: cytoplasmic droplets; ERC: excess residual cytoplasm; s.d.: standard deviation; SSB: single-strand DNA break
Protamine contents and ratio in infertile males with varying degrees of abnormal sperm morphology
The P1 level, P2 level, and P1/P2 ratio of infertile males with varying degrees of abnormal sperm morphology are shown in Figure 3. The levels of P1 in Groups A, B, and C were significantly higher than that in Group E (all P < 0.01). There was no significant difference in P1 level between Groups D and E (P > 0.05), though P1 in Group D exhibited a numerically higher-level than that in Group E. However, the levels of P2 in Groups A, B, C, and D were all significantly lower than that in Group E (all P < 0.01).
Figure 3.
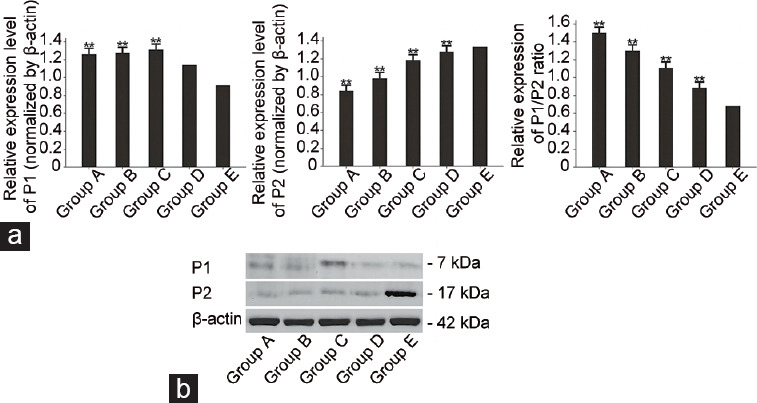
Protamines in infertile males with varying degrees of abnormal sperm morphology. (a) Histograms showed differences between case and control groups for P1, P2, and the P1/P2 ratio. **P < 0.01 (the indicated value vs that of Group E). (b) Representative western blot images showed P1 and P2 proteins in infertile males with different degrees of abnormal sperm morphology. Group A: abnormal spermatozoa=100%; Group B: abnormal spermatozoa >96% and ≤99%; Group C: abnormal spermatozoa >93% and ≤96%; Group D: abnormal spermatozoa ≤93%; Group E: control group. P1: protamine 1; P2: protamine 2.
Correlations among protamines, sperm SSBs, and NVs in infertile males with abnormal sperm morphology
Correlation analyses were conducted to determine the relationships of P1, P2, P1/P2 ratio, sperm SSB rate, and NVs (Supplementary Table 2). P1 expression showed positive correlations with the sperm SSB rate and NVs in infertile males with abnormal sperm morphology (both P < 0.01), with the correlation coefficients corresponding to 0.631 and 0.567, respectively. However, P2 showed negative correlations with the sperm SSB rate (r = −0.847, P < 0.01) and NVs (r = −0.859, P < 0.01). Moreover, the P1/P2 ratio was significantly associated with both sperm SSB rate (r = 0.998, P < 0.01) and NVs (r = 0.968, P < 0.01).
Supplementary Table 2.
Correlation analyses of single-strand DNA breaks, nuclear vacuoles, and sperm protamines in infertile males with abnormal sperm morphology
| Parameter | Correlation to SSB (r) | Correlation to NV (r) |
|---|---|---|
| P1 | 0.631** | 0.567** |
| P2 | −0.847** | −0.859** |
| P1/P2 ratio | 0.998** | 0.968** |
**P<0.01. Data are presented as mean±s.d. r: correlation coefficient, SSB: single-strand DNA break; NV: nuclear vacuole; P1: protamine 1; P2: protamine 2; s.d.: standard deviation
DISCUSSION
Conventional sperm parameters, including sperm concentration, motility, and morphology, can collectively contribute to a greater or reduced pregnancy rate.27 Specifically, abnormal sperm morphology, independently associated with decreased sperm concentration and motility, can potentially impair sperm function and male fertility.28 Nevertheless, the mechanisms correlating abnormal sperm morphology and male infertility remain largely obscure. Protamination of sperm holds a pivotal role in nuclear DNA condensation,29 with the P1/P2 ratio being suggested as a promising biomarker for assessing sperm DNA quality.30 Our study highlighted a significant increase in the P1/P2 ratio, commensurate with the severity of abnormal sperm morphology, within the sperm samples from infertile males. Furthermore, significant correlations were discovered between the P1/P2 ratio and other nuclear and cytoplasmic sperm parameters. These include the sperm SSB rate, NVs, ERC, and CDs. These findings underline our belief that appropriate sperm protamination during spermiogenesis is integral for optimal sperm morphology. Moreover, deviations in sperm protamination may lead to male infertility by influencing not only chromatin condensation but also cytoplasmic maturation.
Sperm with morphology defects have been shown to contain more histones and less protamines than normal sperm.12,16 It seemed paradoxical that in the present study, the P1 levels in the sperm samples of infertile males with abnormal sperm morphology were significantly higher than that of the normozoospermic males, although P2 levels were lower. It is well known that P2 is firstly synthesized as a precursor protein and processed through a series of proteolytic cleavages to a final mature form.31 Mouse models designed to express the P1 gene in excess could result in the premature condensation of nuclear chromatin, abnormal sperm morphogenesis, and incomplete processing of P2.32 Accordingly, the excess P1 in the present study may be responsible for P2 maturation failure and the resulting P2 decrease. Likewise, increased incidences of SSBs and abnormal sperm morphology in infertile males in the study can be caused by aberrant protamination.
The proper condensation of sperm chromatin necessitates an accurate P1/P2 ratio. Unlike P1 or P2 individually, this ratio stands as a more reliable and precise marker for evaluating sperm quality.33 The relative proportion of P1 and P2 in sperm is stringently regulated.34 For fertile males, the ideal P1/P2 ratio falls within a tight range of 0.8–1.2.35 Abnormally high or low P1/P2 ratios are linked to male subfertility and unfavorable pregnancy outcomes.30,36,37 Our findings indicated an upregulated P1/P2 ratio in infertile males who exhibited abnormal sperm morphology. Conversely, a P1/P2 ratio lower than the optimal level was observed in infertile males possessing normal sperm morphology. This suggests that aberrant sperm protamination might be a prevailing defect among infertile males. In addition, the present data exposed a significant increment in the P1/P2 ratio matching the severity of abnormal sperm morphology in infertile males, suggesting the likelihood of protamination playing a pivotal role in the progression of sperm morphogenesis.
Sperm morphology can offer insights into sperm DNA damage.38,39 Recent research has suggested that sperm DNA integrity holds more value as a predictor of male infertility,40,41 given that increased DNA damage can directly compromise sperm functionality.42 These conclusions are further supported by our current findings demonstrating elevated sperm SSB rates in infertile males with a positive correlation to the severity of abnormal sperm morphology. Various mechanisms have been posited to explain sperm DNA fragmentation, with protamination failure emerging as a key factor.43 Our data concurs with this perspective, revealing significant correlations between the sperm SSB rate and both P1 and P2 expressions and, notably, the P1/P2 ratio. In this study, the sperm SSB rate demonstrated a stronger correlation with the P1/P2 ratio than that with P1 or P2 individually, with respective r values of 0.998, 0.638, and −0.846. These results suggest that the P1/P2 ratio could serve as a more sensitive and indicative marker for predicting sperm DNA fragmentation. Infertile males with abnormal sperm morphology exhibited increased P1/P2 ratios and sperm SSB rates in this study. In contrast, normozoospermic infertile males displayed a comparatively low P1/P2 ratio and sperm SSB rate. It is plausible that sperm DNA damage may be responsible for abnormal sperm morphology. Hence, the P1/P2 ratio, which correlates with sperm DNA fragmentation dynamics, can serve as an informative marker of both sperm morphology and male fertility.
NVs are common phenomena within sperm chromatin, yet the mechanisms prompting NVs are unknown, perhaps occurring in the process of spermiogenesis.44 NVs exhibit a diversity in number, size, shape, location, and distribution. They can appear anywhere within the nucleus, including the anterior or posterior region, nuclear edge, or near the neck. In this study, we have classified NVs into 14 distinct types, ranging from mild to severe (type “a”–”n”) based on their unique characteristics. Among these categories, type “a” represents a nucleus with a typical oval shape, devoid of any vacuoles, whereas type “n” symbolizes the most severe form, a degenerating nucleus. Large NVs in spermatozoa are reported to be associated with protamine deficiency and sperm DNA breaks.14,45 Our results are in accordance with these conclusions, revealing that the incidence of sperm NVs had significant positive correlations to the P1/P2 ratio and SSB rate. Moreover, infertile males exhibiting severe sperm morphological abnormalities had a higher proportion of spermatozoa with significant NV abnormalities, such as large NVs (type “j”) and multiple NVs (types “k” and “m”). These data suggest a correlation between the presence of NVs and the process of sperm morphogenesis. The present results reinforce the theory that NVs occur in the process of spermiogenesis. During typical spermiogenesis, histone-to-protamine exchange causes chromatin condensation,29 leading to a highly organized and compact structure. Sperm protamine deficiency leads to a less tightly compact chromatin structure and conspires to the biological basis for NVs formation. However, our data revealed that NVs were more common in infertile males who had an overexpression of P1. Of note, the increased P1 was accompanied by the decreased P2 in these infertile males with abnormal sperm morphology. Although P1 is vital for sperm nuclear compaction,46 its functions should act in synergy with P2.32 Therefore, in comparison to P1 and P2 alone, the appropriate P1/P2 ratio plays a more important role in sperm chromatin condensation.
It is unequivocal that changes in cytoplasmic structure can result in functional deficiencies in sperm. Ideally, substantial cytoplasmic reduction occurs during spermatogenesis. However, instances of cytoplasmic retention may occasionally be observed in ejaculated sperm. CDs and ERC represent forms of these residual cytoplasmic elements. While CDs are typically seen as normal constituents of human spermatozoa, ERC constitutes an abnormal sperm morphology that is often associated with male infertility.47 Nonetheless, the present study found that both CDs and ERC were more frequently observed in infertile males exhibiting abnormal sperm morphology. Moreover, the rate of sperm SSBs displayed an increase corresponding to the presence of both CDs and ERC. It is plausible that there exists a threshold for CDs in infertile males, even though CDs are widely recognized as a physiological phenomenon. When the number of CDs surpasses this threshold, it may collectively contribute to impairing sperm function and DNA integrity, much as ERC does. Importantly, we recently documented a phenomenon of spermatid cytoplasmic retention within the sperm nucleus, terming it “intranuclear cytoplasmic retention (INCR)”.48 INCRs occur within sperm NVs, and males with teratozoospermia appear to possess an increased quantity of INCRs.48 Collectively, it can be reasonably hypothesized that aberrant sperm protamination can influence not only chromatin compaction but also cytoplasmic remodeling. This, in turn, may lead to abnormalities in sperm morphology and DNA damage. Consequently, these males may experience infertility.
Recognizing the necessity for additional research, we acknowledge a key limitation of the current study, namely the lack of controls from a normal fertile population. Regardless, we posit that our findings have directed significant attention toward the synchronous relationship between sperm protamination during spermatogenesis and factors such as sperm morphology, nuclear chromatin condensation, and cytoplasmic maturation. It is worthy of note that deviations in chromatin condensation and/or cytoplasmic architecture may be associated with structurally abnormal spermatozoa, thus potentially implicating male fertility. In contrast, while protamine content is of significance, the P1/P2 ratio furnishes a more reliable measure of abnormal sperm morphology, DNA damage, and male infertility. Overall, our research sheds light on a potential molecular mechanism tied to defective protamination that contributes to male infertility characterized by abnormal sperm morphology.
AUTHOR CONTRIBUTIONS
HJ and CJH contributed to the study conception and design, and have been involved in interpreting the data. HJ collected samples and wrote the article. CH performed experiments and conducted the data analysis. Both authors read and approved the final manuscript.
COMPETING INTERESTS
Both authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Hao SL, Ni FD, Yang WX. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene. 2019;706:201–10. doi: 10.1016/j.gene.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Chemes HE, Rawe VY. The making of abnormal spermatozoa:cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010;341:349–57. doi: 10.1007/s00441-010-1007-3. [DOI] [PubMed] [Google Scholar]
- 3.Sunanda P, Panda B. An illustration of human sperm morphology and their functional ability among different group of subfertile males. Andrology. 2018;6:680–9. doi: 10.1111/andr.12500. [DOI] [PubMed] [Google Scholar]
- 4.Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril. 2011;95:1705–8. doi: 10.1016/j.fertnstert.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Ribas-Maynou J, Benet J. Single and double strand sperm DNA damage:different reproductive effects on male fertility. Genes. 2019;10:105. doi: 10.3390/genes10020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassetti D, Paoli D, Gallo M, D’Ambrosio A, Lombardo F, et al. Protamine-1 and -2 polymorphisms and gene expression in male infertility:an Italian study. J Endocrinol Invest. 2012;35:882–8. doi: 10.3275/8111. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton TR, Simões R, Mendes CM, Goissis MD, Nakajima E, et al. Detection of protamine 2 in bovine spermatozoa and testicles. Andrology. 2019;7:373–81. doi: 10.1111/andr.12610. [DOI] [PubMed] [Google Scholar]
- 8.Hammadeh ME, Hamad MF, Montenarh M, Fischer-Hammadeh C. Protamine contents and P1/P2 ratio in human spermatozoa from smokers and non-smokers. Hum Reprod. 2010;25:2708–20. doi: 10.1093/humrep/deq226. [DOI] [PubMed] [Google Scholar]
- 9.Ribas-Maynou J, García-Peiró A, Martínez-Heredia J, Fernández-Encinas A, Abad C, et al. Nuclear degraded sperm subpopulation is affected by poor chromatin compaction and nuclease activity. Andrologia. 2015;47:286–94. doi: 10.1111/and.12258. [DOI] [PubMed] [Google Scholar]
- 10.Talebi AR, Ghasemzadeh J, Khalili MA, Halvaei I, Fesahat F. Sperm chromatin quality and DNA integrity in partial versus total globozoospermia. Andrologia. 2018;50:e12823. doi: 10.1111/and.12823. [DOI] [PubMed] [Google Scholar]
- 11.Oumaima A, Tesnim A, Zohra H, Amira S, Ines Z, et al. Investigation on the origin of sperm morphological defects:oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ Sci Pollut Res Int. 2018;25:13775–86. doi: 10.1007/s11356-018-1417-4. [DOI] [PubMed] [Google Scholar]
- 12.Eskandari N, Tavalaee M, Zohrabi D, Nasr-Esfahani MH. Association between total globozoospermia and sperm chromatin defects. Andrologia. 2018;50:e12843. doi: 10.1111/and.12843. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization Press; 2010. [Google Scholar]
- 14.Utsuno H, Miyamoto T, Oka K, Shiozawa T. Morphological alterations in protamine-deficient spermatozoa. Hum Reprod. 2014;29:2374–81. doi: 10.1093/humrep/deu225. [DOI] [PubMed] [Google Scholar]
- 15.Franco JG, Jr, Mauri AL, Petersen CG, Massaro FC, Silva LF, et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl. 2012;35:46–51. doi: 10.1111/j.1365-2605.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Xie N, Li Y, Zhang B, Xie D, et al. Teratozoospermia with amorphous sperm head associate with abnormal chromatin condensation in a Chinese family. Syst Biol Reprod Med. 2019;65:61–70. doi: 10.1080/19396368.2018.1543481. [DOI] [PubMed] [Google Scholar]
- 17.Pleuger C, Lehti MS, Dunleavy JE, Fietz D, O'Bryan MK. Haploid male germ cells-the Grand Central Station of protein transport. Hum Reprod Update. 2020;26:474–500. doi: 10.1093/humupd/dmaa004. [DOI] [PubMed] [Google Scholar]
- 18.Sprando RL, Russell LD. Comparative study of cytoplasmic elimination in spermatids of selected mammalian species. Am J Anat. 1987;178:72–80. doi: 10.1002/aja.1001780109. [DOI] [PubMed] [Google Scholar]
- 19.Rago V, Siciliano L, Aquila S, Carpino A. Detection of estrogen receptors ER-alpha and ER-beta in human ejaculated immature spermatozoa with excess residual cytoplasm. Reprod Biol Endocrinol. 2006;4:36. doi: 10.1186/1477-7827-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper TG. Cytoplasmic droplets:the good, the bad or just confusing? Hum Reprod. 2005;20:9–11. doi: 10.1093/humrep/deh555. [DOI] [PubMed] [Google Scholar]
- 21.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 22.Mishra P, Negi MP, Srivastava M, Singh K, Rajender S. Decline in seminal quality in Indian men over the last 37 years. Reprod Biol Endocrinol. 2018;16:103. doi: 10.1186/s12958-018-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pathak UI, Gabrielsen JS, Lipshultz LI. Cutting-edge evaluation of male infertility. TUrol Clin North Am. 2020;47:129–38. doi: 10.1016/j.ucl.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhu WJ, Li J. A simple sperm nuclear vacuole assay with propidium iodide. Andrologia. 2015;47:779–85. doi: 10.1111/and.12328. [DOI] [PubMed] [Google Scholar]
- 25.Küçük N. Sperm DNA and detection of DNA fragmentations in sperm. Turk J Urol. 2018;44:1–5. doi: 10.5152/tud.2018.49321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 1984;42:87–91. doi: 10.1016/s0015-0282(16)47963-x. [DOI] [PubMed] [Google Scholar]
- 27.Manna C, Barbagallo F, Manzo R, Rahman A, Francomano D, et al. Sperm parameters before and after swim-up of a second ejaculate after a short period of abstinence. J Clin Med. 2020;9:1029. doi: 10.3390/jcm9041029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menkveld R, Holleboom CA, Rhemrev JP. Measurement and significance of sperm morphology. Asian J Androl. 2011;13:59–68. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres-Flores U, Hernández-Hernández A. The interplay between replacement and retention of histones in the sperm genome. Front Genet. 2020;11:780. doi: 10.3389/fgene.2020.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amor H, Shelko N, Hamad MF. An additional marker for sperm DNA quality evaluation in spermatozoa of male partners of couples undergoing assisted reproduction technique (IVF/ICSI):protamine ratio. Andrologia. 2019;51:e13400. doi: 10.1111/and.13400. [DOI] [PubMed] [Google Scholar]
- 31.de Mateo S, Ramos L, de Boer P, Meistrich M, Oliva R. Protamine 2 precursors and processing. Protein Pept Lett. 2011;18:778–85. doi: 10.2174/092986611795713998. [DOI] [PubMed] [Google Scholar]
- 32.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 33.Amjad S, Mushtaq S. Protamine 1/protamine 2 mRNA ratio in nonobstructive azoospermic patients. Andrologia. 2021;53:e13936. doi: 10.1111/and.13936. [DOI] [PubMed] [Google Scholar]
- 34.Corzett M, Mazrimas J, Balhorn R. Protamine 1:protamine 2 stoichiometry in the sperm of eutherian mammals. Mol Reprod Dev. 2002;61:519–27. doi: 10.1002/mrd.10105. [DOI] [PubMed] [Google Scholar]
- 35.Francis S, Yelumalai S, Jones C, Coward K. Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum Fertil (Camb) 2014;17:80–9. doi: 10.3109/14647273.2014.915347. [DOI] [PubMed] [Google Scholar]
- 36.de Mateo S, Gázquez C, Guimerà M, Balasch J, Meistrich ML, et al. Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil Steril. 2009;91:715–22. doi: 10.1016/j.fertnstert.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Ni K, Spiess AN, Schuppe HC, Steger K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility:a systematic review and meta-analysis. Andrology. 2016;4:789–99. doi: 10.1111/andr.12216. [DOI] [PubMed] [Google Scholar]
- 38.Jakubik-Uljasz J, Gill K, Rosiak-Gill A, Piasecka M. Relationship between sperm morphology and sperm DNA dispersion. Transl Androl Urol. 2020;9:405–15. doi: 10.21037/tau.2020.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Dai C, Shan G, Chen X, Liu H, et al. Quantitative selection of single human sperm with high DNA integrity for intracytoplasmic sperm injection. Fertil Steril. 2021;116:1308–18. doi: 10.1016/j.fertnstert.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Liffner S, Pehrson I, García-Calvo L, Nedstrand E, Zalavary S, et al. Diagnostics of DNA fragmentation in human spermatozoa:are sperm chromatin structure analysis and sperm chromatin dispersion tests (SCD-HaloSpermG2®) comparable? Andrologia. 2019;51:e13316. doi: 10.1111/and.13316. [DOI] [PubMed] [Google Scholar]
- 41.Dutta S, Henkel R. Comparative analysis of tests used to assess sperm chromatin integrity and DNA fragmentation. Andrologia. 2021;53:e13718. doi: 10.1111/and.13718. [DOI] [PubMed] [Google Scholar]
- 42.Daris B, Goropevsek A, Hojnik N, Vlaisavljević V. Sperm morphological abnormalities as indicators of DNA fragmentation and fertilization in ICSI. Arch Gynecol Obstet. 2010;281:363–7. doi: 10.1007/s00404-009-1140-y. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton T, Assumpção M. Sperm DNA fragmentation:causes and identification. Zygote. 2020;28:1–8. doi: 10.1017/S0967199419000595. [DOI] [PubMed] [Google Scholar]
- 44.Zamboni L. Sperm structure and its relevance to infertility. An electron microscopic study. Arch Pathol Lab Med. 1992;116:325–44. [PubMed] [Google Scholar]
- 45.Pastuszek E, Kiewisz J, Skowronska P, Liss J, Lukaszuk M, et al. An investigation of the potential effect of sperm nuclear vacuoles in human spermatozoa on DNA fragmentation using a neutral and alkaline Comet assay. Andrology. 2017;5:392–8. doi: 10.1111/andr.12324. [DOI] [PubMed] [Google Scholar]
- 46.Iuso D, Czernik M, Toschi P, Fidanza A, Zacchini F, et al. Exogenous expression of human protamine 1 (hPrm1) remodels fibroblast nuclei into spermatid-like structures. Cell Rep. 2015;13:1765–71. doi: 10.1016/j.celrep.2015.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:92. doi: 10.1186/1477-7827-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu WJ. Transmission electron microscopy analysis of the origin and incidence of sperm intranuclear cytoplasmic retention in fertile and teratozoospermia men. Andrology. 2018;6:317–24. doi: 10.1111/andr.12469. [DOI] [PubMed] [Google Scholar]


