Abstract
Wound-induced ethylene synthesis by subapical stem sections of etiolated Pisum sativum L., cv. Alaska seedlings, as described by Saltveit and Dilley (Plant Physiol 1978 61: 447-450), was half-saturated at 3.6% (v/v) O2 and saturated at about 10% O2. Corresponding values for CO2 production during the same period were 1.1% and 10% O2, respectively. Anaerobiosis stopped all ethylene evolution and delayed the characteristic pattern of wound ethylene synthesis. Exposing tissue to 3.5% CO2 in air in a flow-through system reduced wound ethylene synthesis by 30%. Enhancing gas diffusivity by reducing the total pressure to 130 mm Hg almost doubled the rate of wound ethylene synthesis and this effect was negated by exposure to 250 μl liter−1 propylene. Applied ethylene or propylene stopped wound ethylene synthesis during the period of application, but unlike N2, no lag period was observed upon flushing with air. It is concluded that the characteristic pattern of wound-induced ethylene synthesis resulted from negative feedback control by endogenous ethylene.
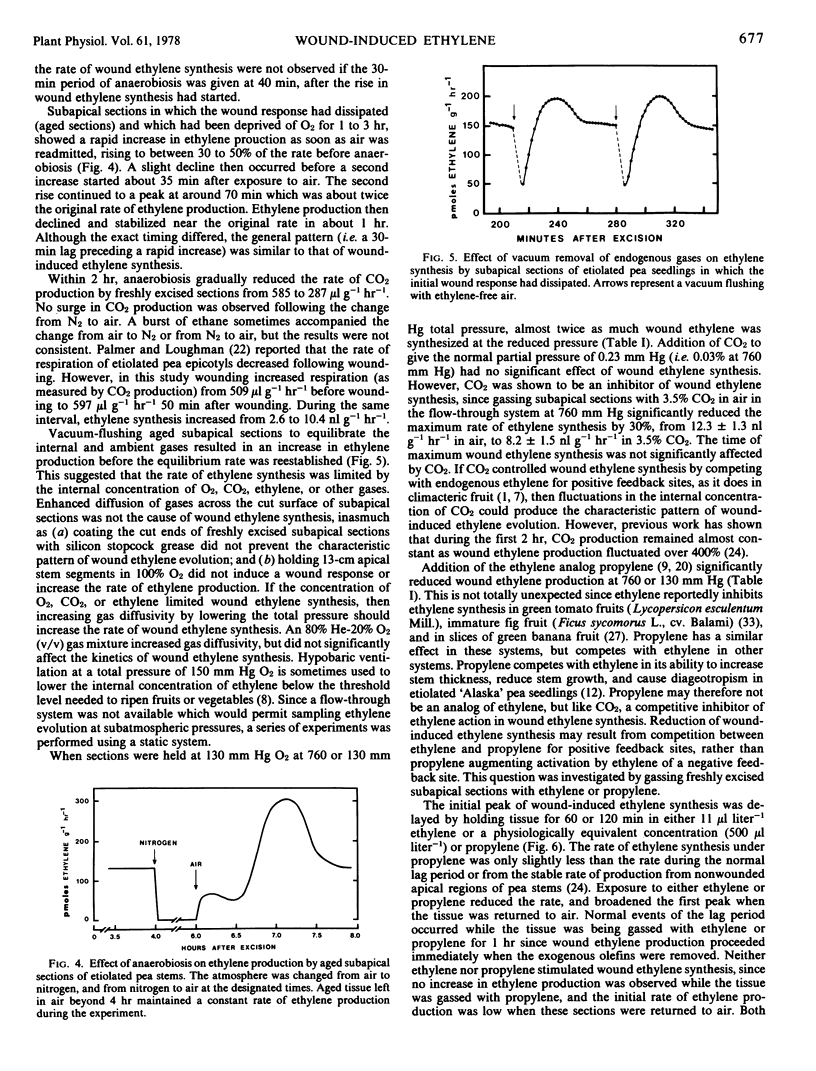
No wound ethylene was produced for 2 hours after excision at 10 or 38 C. Low temperatures prolonged the lag period, but did not prevent induction of the wound response, since tissue held for 2 hours at 10 C produced wound ethylene immediately when warmed to 30 C. In contrast, temperatures above 36 C prevented induction of wound ethylene synthesis, since tissue cooled to 30 C after 1 hour at 40 C required 2 hours before ethylene production returned to normal levels. The activation energy between 15 and 36 C was 12.1 mole kilocalories degree−1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur A. H., Yang S. F., Pratt H. K. Ethylene biosynthesis in fruit tissues. Plant Physiol. 1971 May;47(5):696–699. doi: 10.1104/pp.47.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Fruit storage at subatmospheric pressures. Science. 1966 Jul 15;153(3733):314–315. doi: 10.1126/science.153.3733.314. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene in plant growth. Proc Natl Acad Sci U S A. 1973 Feb;70(2):591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. THE PHYSIOLOGY OF ETHYLENE FORMATION IN APPLES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):335–344. doi: 10.1073/pnas.45.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Rasmussen G. K., Waldon E. S. Ethylene evolution stimulated by chilling in citrus and persea sp. Plant Physiol. 1969 Aug;44(8):1194–1196. doi: 10.1104/pp.44.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollwet H. H., Seeman R. E. Propylene-a competitor of ethylene action. Plant Physiol. 1975 Oct;56(4):552–554. doi: 10.1104/pp.56.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring D. L., Morgan P. W. Ethylene as a Component of the Emanations From Germinating Peanut Seeds and Its Effect on Dormant Virginia-type Seeds. Plant Physiol. 1969 Mar;44(3):326–330. doi: 10.1104/pp.44.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchie E. J., McGlasson W. B., Eaks I. L. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972 May 26;237(5352):235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Saltveit M. E., Dilley D. R. Rapidly Induced Wound Ethylene from Excised Segments of Etiolated Pisum sativum L., cv. Alaska: I. Characterization of the Response. Plant Physiol. 1978 Mar;61(3):447–450. doi: 10.1104/pp.61.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeroni M., Galil J. Autoinhibition of Ethylene Formation in Nonripening Stages of the Fruit of Sycomore Fig (Ficus sycomorus L.). Plant Physiol. 1976 Apr;57(4):647–650. doi: 10.1104/pp.57.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]


