Abstract
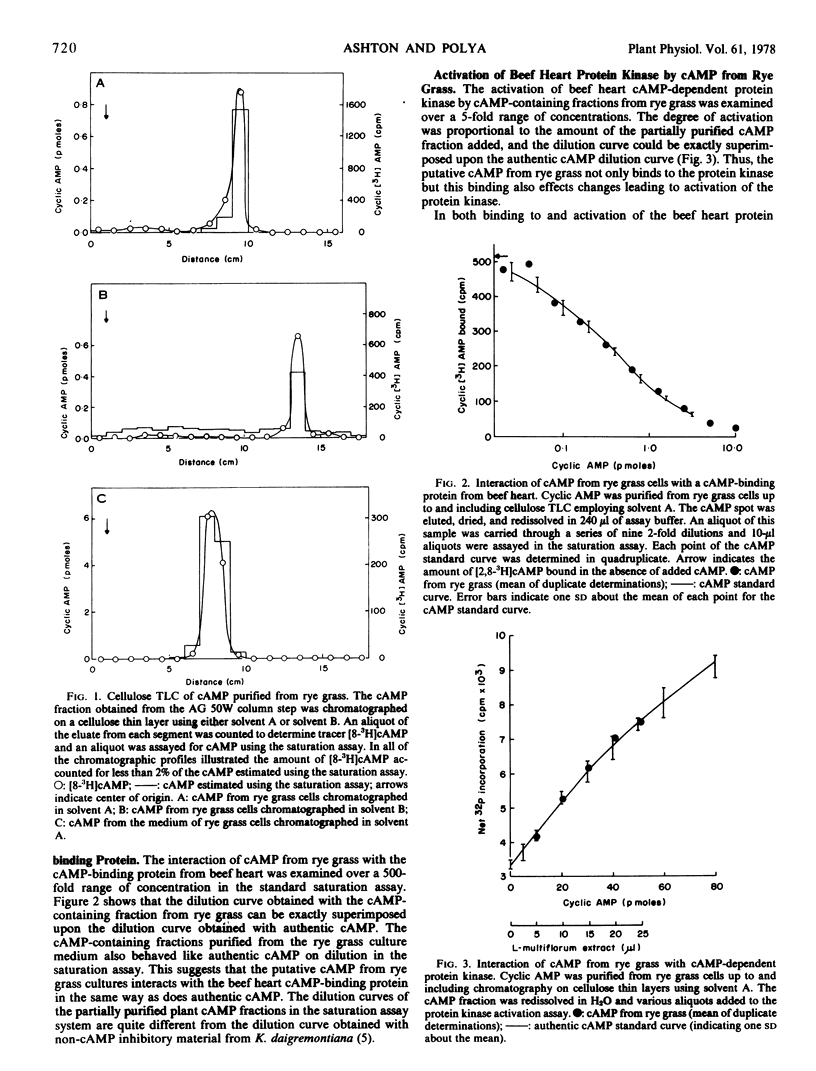
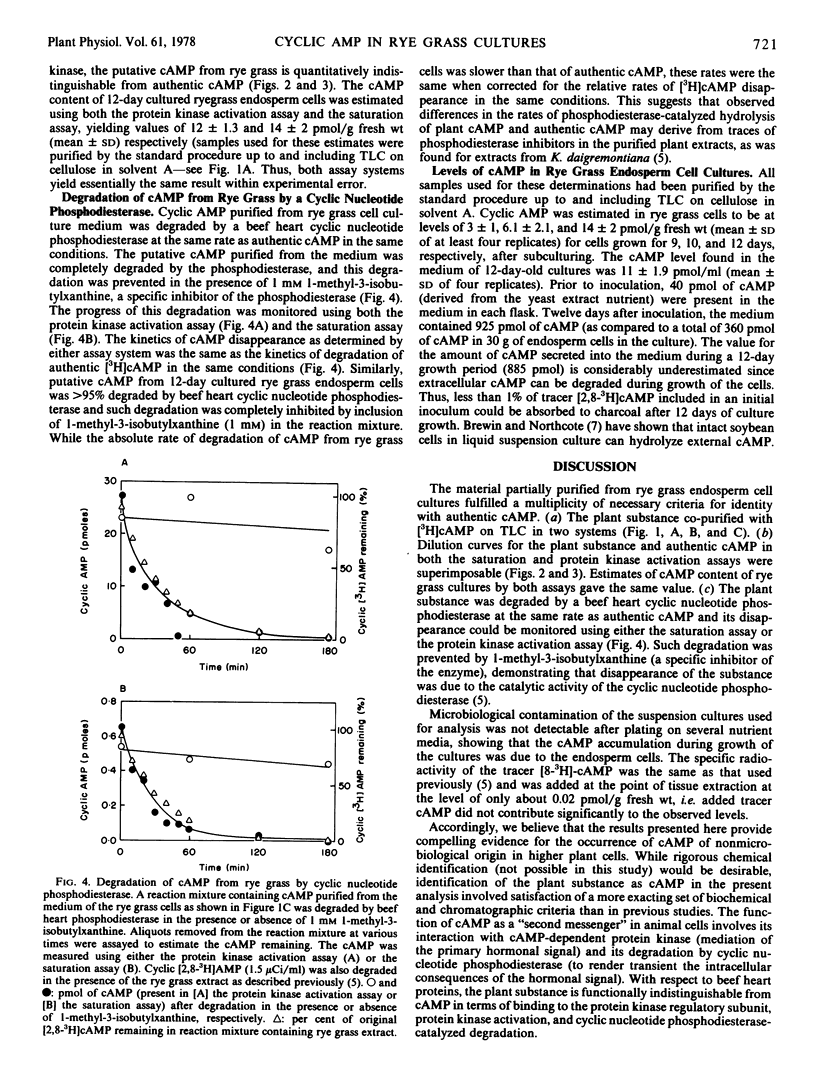
Cyclic adenosine 3′:5′-monophosphate (cAMP) was extensively purified from rye grass (Lolium multiflorum) endosperm cells grown in axenic suspension culture. The cAMP was purified by neutral alumina and anion and cation exchange chromatography. The cAMP was quantitated by means of a radiochemical saturation assay using a beef heart cAMP-binding protein and also by an assay involving activation of beef heart protein kinase. The cAMP levels found (corrected for recovery of tracer cyclic 3′,5′-[8-3H]AMP included from the point of sample extraction) ranged from 2 to 12 pmol/g fresh weight. The material purified from rye grass cultures was indistinguishable from authentic cAMP with respect to chromatography in two cellulose thin layer systems, behavior on dilution in both the saturation and protein kinase activation assays, and rates of degradation by a mammalian cAMP phosphodiesterase. The cAMP from rye grass cultures was completely degraded by a mammalian cAMP phosphodiesterase, and 1-methyl-3-isobutylxanthine inhibited such degradation. The protein kinase activation and saturation assays gave essentially the same values for the cAMP content of axenic rye grass culture extracts. Material satisfying the above criteria for identity with cAMP was also isolated from the culture medium. The increase observed in medium cAMP levels during culture growth provides evidence for the synthesis and secretion of cAMP by rye grass endosperm cells in suspension culture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of adenylate cyclase and phosphodiesterase in muscle, liver and nervous tissues from vertebrates and invertebrates in relation to the control of the concentration of adenosine 3':5'-cyclic monophosphate. Biochem J. 1976 Sep 15;158(3):603–622. doi: 10.1042/bj1580603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Polya G. M. Adenosine 3':5'-cyclic monophosphate in higher plants: Isolation and characterization of adenosine 3':5'-cyclic monophosphate from Kalanchoe and Agave. Biochem J. 1977 Jul 1;165(1):27–32. doi: 10.1042/bj1650027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Polya G. M. Higher-plant cyclic nucleotide phosphodiesterases. Resolution, partial purification and properties of three phosphodiesterases from potato tuber. Biochem J. 1975 Aug;149(2):329–339. doi: 10.1042/bj1490329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Ross C. W. Attempts to detect cyclic adenosine 3':5'-monophosphate in higher plants by three assay methods. Plant Physiol. 1976 Jan;57(1):29–37. doi: 10.1104/pp.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin N. J., Northcote D. H. Partial purification of a cyclic AMP phosphodiesterase from soybean callus. Isolation of a non-dialysable inhibitor. Biochim Biophys Acta. 1973 Aug 17;320(1):104–122. doi: 10.1016/0304-4165(73)90171-2. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Racine-Weisbuch M. S., Delaage M. A. Adenosine 3',5' cyclic monophosphate assay at 10-15 mole level. Anal Biochem. 1973 Dec;56(2):394–407. doi: 10.1016/0003-2697(73)90205-4. [DOI] [PubMed] [Google Scholar]
- Doore B. J., Bashor M. M., Spitzer N., Mawe R. C., Saier M. H., Jr Regulation of adenosine 3' :5'-monophosphate efflux from rat glioma cells in culture*. J Biol Chem. 1975 Jun 10;250(11):4371–4372. [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., O'Toole A. G. Analysis of cyclic 3',5'-adenosine monophosphate and cyclic 3',5'-guanosine monophosphate. Methods Biochem Anal. 1971;20:1–39. doi: 10.1002/9780470110393.ch1. [DOI] [PubMed] [Google Scholar]
- Handa A. K., Johri M. M. Cyclic adenosine 3':5'-monophosphate in moss protonema: a comparison of its levels by protein kinase and gilman assays. Plant Physiol. 1977 Mar;59(3):490–496. doi: 10.1104/pp.59.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler B., Levinstein R. Adenosine 3',5'-cyclic monophosphate in higher plants: assay, distribution and age-dependency. Biochim Biophys Acta. 1974 Mar 20;343(1):156–166. doi: 10.1016/0304-4165(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Lin P. P. Cyclic nucleotides in higher plants? Adv Cyclic Nucleotide Res. 1974;4(0):439–460. [PubMed] [Google Scholar]
- Lin P. P., Varner J. E. Cyclic nucleotide phosphodiesterase in pea seedlings. Biochim Biophys Acta. 1972 Aug 28;276(2):454–474. doi: 10.1016/0005-2744(72)91007-8. [DOI] [PubMed] [Google Scholar]
- Mayer S. E., Stull J. T., Wastila W. B., Thompson B. Assay of cyclic AMP by protein kinase activation. Methods Enzymol. 1974;38:66–73. doi: 10.1016/0076-6879(74)38012-3. [DOI] [PubMed] [Google Scholar]
- Patterson W. D., Hardman J. G., Sutherland E. W. Hydrolysis of guanosine and adenosine 3',5'-monophosphates by rat blood. Biochim Biophys Acta. 1975 Mar 28;384(1):159–167. doi: 10.1016/0005-2744(75)90105-9. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Brownlee A. G., Hynes M. J. Enzymology and genetic regulation of a cyclic nucleotide-binding phosphodiesterase-phosphomonoesterase from Aspergillus nidulans. J Bacteriol. 1975 Nov;124(2):693–703. doi: 10.1128/jb.124.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P., Narayanan A., Pradet A. Evidence for the presence of 3', 5'-cyclic AMP in plant tissues. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1115–1121. doi: 10.1016/0006-291x(73)90580-9. [DOI] [PubMed] [Google Scholar]
- Robertson A., Grutsch J. The role of cyclic AMP in slime mold development. Life Sci. 1974 Sep 15;15(6):1031–1043. doi: 10.1016/s0024-3205(74)80001-9. [DOI] [PubMed] [Google Scholar]


