Abstract
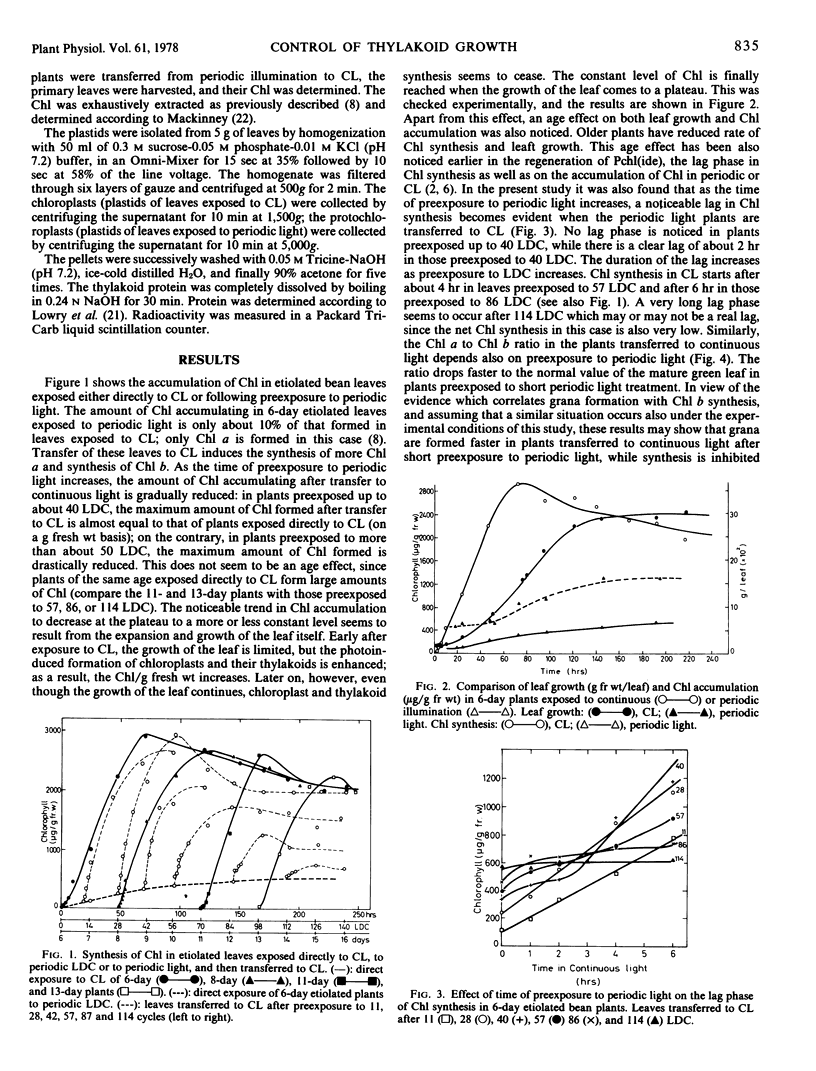
An attempt was made to answer whether the extent of thylakoid growth in Phaseolus vulgaris is controlled by a feedback inhibition mechanism, operating after insertion of all of the necessary components of the mature thylakoid, in the right amounts and ratio, or by parameters independent of the developmental stage of the membrane. This was done by following the growth of thylakoids, as monitored by the rate of chlorophyll accumulation and the rate of thylakoid protein synthesis, in etiolated plants exposed either directly to continuous light (transformation of prolamellar body to mature thylakoid) or first to periodic light and then to continuous light (transformation of prolamellar body to primary thylakoids and then to mature thylakoids). It was found that prolonged etiolation has no effect on the rate of thylakoid synthesis in continuous light. However, prolonged preexposure to periodic light diminishes drastically the rate of new thylakoid synthesis in continuous light. Since the thylakoids formed in the latter case are far from being complete, it seems that thylakoid growth can stop long before all of the necessary components are incorporated. Parameters independent of the developmental stage and composition of the membrane, therefore, seem to control membrane growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akoyunoglou G. A., Siegelman H. W. Protochlorophyllide resynthesis in dark-grown bean leaves. Plant Physiol. 1968 Jan;43(1):66–68. doi: 10.1104/pp.43.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoyunoglou G. Development of the photosystem II unit in plastids of bean leaves greened in periodic light. Arch Biochem Biophys. 1977 Oct;183(2):571–580. doi: 10.1016/0003-9861(77)90392-7. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Photoinduced changes in the chlorophyll a to chlorophyll B ratio in young bean plants. Plant Physiol. 1970 Aug;46(2):247–249. doi: 10.1104/pp.46.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Feleki Z., Akoyunoglou G. Formation of two chlorophyll-protein complexes during greening of etiolated bean leaves. Biochem Biophys Res Commun. 1971 Nov 5;45(3):606–614. doi: 10.1016/0006-291x(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Tsakiris S. Development of the cation-induced stacking capacity during the biogenesis of higher plant thylakoids. Arch Biochem Biophys. 1977 Nov;184(1):307–315. doi: 10.1016/0003-9861(77)90355-1. [DOI] [PubMed] [Google Scholar]
- Beck D. P., Levine R. P. Synthesis of chloroplast membrane polypeptides during synchronous growth of Chlamydomonas reinhardtii. J Cell Biol. 1974 Dec;63(3):759–772. doi: 10.1083/jcb.63.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. 8. Modulation of chloroplast lamellae composition and function induced by discontinuous illumination and inhibition of ribonucleic acid and protein synthesis during greening of Chlamydomonas reinhardi y-1 mutant cells. J Biol Chem. 1972 Jan 10;247(1):122–129. [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. VI. Cooperation between cytoplasmic and chloroplast ribosomes in the synthesis of photosynthetic lamellar proteins during the greening process in a mutant of Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4297–4307. [PubMed] [Google Scholar]
- Goldberg I., Ohad I. Biogenesis of chloroplast membranes. IV. Lipid and pigment changes during synthesis of chloroplast membranes in a mutant of Chlamydomonas reinhardi y-1. J Cell Biol. 1970 Mar;44(3):563–571. doi: 10.1083/jcb.44.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Hoober J. K. Sites of synthesis of chloroplast membrane polypeptides in Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4327–4334. [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]


