Abstract
Background
Sudden infant death syndrome (SIDS) has been considered to be triggered by a combination of underlying immune dysregulation and infections. The thymus is a crucial lymphatic organ responsible for T cell development in infancy. We hypothesized that an altered thymic immune status may be detectable by intrathymic cytokine profiling in SIDS.
Methods
27 cytokines in protein lysates of thymus tissue and thymus weights were assessed in 26 SIDS cases and 16 infants who died of other reasons.
Results
Seventeen out of 27 cytokines were increased in thymic tissue of SIDS compared to controls without infections, and the most significant discrepancy was in infants younger than 20 weeks. The thymic cytokine profiles in SIDS cases were similar to those in controls with severe infection; however, the magnitude of the cytokine concentration elevation in SIDS was less pronounced, indicating sub-clinical infections in SIDS. In contrast to SIDS, intrathymic cytokine concentrations and thymus weight were increased with age in control children.
Conclusions
Elevated thymic cytokine expression and thymus weight, as well as impaired age-related alterations in SIDS, may be influenced by subclinical infection, which may play a role in initiating SIDS in infants with a compromised immune response.
Impact Statement
Increased thymic weight and cytokine concentration may suggest possible subclinical infection in SIDS.
Elevated thymic weight and cytokine concentration mainly in SIDS cases aged <20 weeks.
Age-related impairment in the thymic weight and cytokine expression may be impaired by subclinical infection in SIDS.
Introduction
Sudden infant death syndrome (SIDS)1 is the leading cause of death among infants aged 1 month to 1 year in developed countries. Triple risk models suggest that a combination of critical developmental stages, vulnerable infants, and exogenous factors are associated with SIDS.2,3 Nevertheless, the exact etiologic mechanism remains unclear. It has been discussed for a long time that an impaired immune response and silent infections may precipitate SIDS.4–12 Some studies implied the existence of hyperinflammation, whilst others suggested immunodeficiency.7,13–17 Therefore, more information is needed to figure out the precise mechanism that may contribute to an impaired immune response in SIDS.
To this goal, the thymus, which is charged with the development of the immune system during infancy,18 comes into view. It is well acknowledged that severe primary immunodeficiency originating from the thymus, such as DiGeorge syndrome,19 can result in a weakened response to common infections, ultimately leading to infant mortality.20 Regarding sudden deaths of infants, an involvement of the thymus was suspected long before the term SIDS was introduced: According to Dally,21 the Austrian forensic pathologist reported an increased weight of the thymus in suddenly deceased infants and coined the term “status lymphaticus”.22 Numerous studies extensively investigated thymic weight, histology, etc. despite divergent outcomes.23–33 Goldwater et al. discovered an increased thymus weight in SIDS cases, which may be the consequence of subclinical infections.27 Several other studies regarding thymus weight observed comparable or contradictory findings.25,26,31,32 An article by Varga et al. indicated that immunohistochemistry (IHC) revealed the presence of thymic involution in SIDS, which may be driven by stress.33 However, Bajanowski et al. reported an absence of deficits of T or B cells in the thymus and other selected lymphatic organs.23
The cytokine network in the thymus is crucial for responding to infection and regulating thymus maturation.34–36 Changes in the cytokine network are expected if the thymus displays functional disorders. However, there is a paucity of research addressing changes in the cytokine network between SIDS and control cases. Since probable alterations in thymic weight and histology have been described earlier in SIDS, we hypothesized that these alterations would be accompanied by changes in cytokine and chemokine profiling. This study employed a multiplex cytokine measurement approach to characterize the thymic cytokine profile, which may aid in reflecting the thymus status and clarifying probable SIDS etiology mechanisms.
Materials and methods
Study participants
The subjects investigated were 42 control and SIDS cases autopsied at the Institute of Legal Medicine, MHH, between 2010 and 2020. Approval was given by the local ethics committee (No. 1211–2011). Based on the cause of death, the cases were divided into three groups: Cases with noninfectious causes of death were the Control- group (n = 11). Control+ cases (n = 5) died from severe infections. In none of the SIDS cases (n = 26), specific causes of death were found following thorough post-mortem and death scene investigations. Tables 1 and 2 provide detailed information on control and SIDS cases. Thymus tissue samples were collected during the autopsy and preserved at -80°C for long-term storage.
Table 1.
Control cases with detailed information.
| Coded case No. | Group | Sex | Age (weeks) | Postmortem interval (days) | Storage period (years) | Thymus weight (g) | Body weight (g) | Ratio of thymus weight to body weight (%) | Height (cm) | BMI (kg/m2) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1-3 | Control– | Female | 1 | 2 | 9 | 14 | 2928 | 0.48 | 50 | 11.71 | Heart defect |
| C2-3 | Control– | Male | 17 | 2 | 9 | 20 | 8000 | 0.25 | 66 | 18.37 | Trauma |
| C6-3 | Control– | Male | 21 | 2 | 9 | 30 | 6100 | 0.49 | 65 | 14.44 | Heart defect |
| C13-3 | Control– | Female | 25 | 2 | 5 | 50 | 8850 | 0.56 | 68 | 19.14 | Metabolic disorder (LCHAD defect) |
| C-S16-3 | Control– | Female | 10 | 1 | 8 | 24 | 3980 | 0.60 | 52 | 14.72 | Trauma & cardiomyopathy |
| C14-3 | Control– | Male | 9 | 2 | 2 | 28 | 5684 | 0.49 | 61 | 15.28 | Trauma |
| C16-3 | Control– | Male | 0 | 1 | 2 | 6 | 2500 | 0.24 | 47 | 11.32 | Death during birth (umbilical knot) |
| C17-3 | Control– | Male | 5 | 3 | 2 | 22 | 5300 | 0.42 | 57 | 16.31 | Trauma |
| C18-3 | Control– | Female | 8 | 2 | 1 | 14 | 4648 | 0.30 | 57 | 14.31 | Shaken impact syndrome |
| C20-3 | Control– | Male | 65 | 3 | 1 | 18 | 9000 | 0.20 | 71 | 17.85 | Trauma |
| C21-3 | Control– | Male | 0 | 2 | 1 | 16 | 2350 | 0.68 | 47 | 10.64 | Premature placental ablation |
| C8-3 | Control+ | Male | 15 | 1 | 9 | 20 | 5600 | 0.36 | 56 | 17.86 | Trauma & aspiration pneumonia |
| C10-3 | Control+ | Male | 23 | 3 | 8 | 48 | 10,800 | 0.44 | 66 | 24.79 | Aspiration pneumonia |
| C3-3 | Control+ | Female | 97 | 2 | 9 | 34 | 12,000 | 0.28 | 87 | 15.85 | Sepsis |
| C7-3 | Control+ | Male | 8 | 1 | 9 | 4 | 2974 | 0.13 | 43 | 16.08 | Sepsis |
| C12-3 | Control+ | Female | 63 | 4 | 8 | 14 | 12,200 | 0.11 | 80 | 19.06 | Sepsis |
The Control- represents control cases without symptoms of infection. The Control+ group represents control cases who died from severe infection.
Table 2.
SIDS cases with detailed information.
| Coded case No. | Group | Sex | Age (weeks) | Postmortem interval (days) | Storage period (years) | Thymus weight (g) | Body weight (g) | Ratio of thymus weight to body weight (%) | Height (cm) | BMI (kg/m2) | Autopsy findings | Clinical infection history | Infection duration in history | Sleeping position | Co-sleeping | Maternal smoking | Other risk factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2-3 | SIDS- | Female | 22 | 2 | 10 | 48 | 10,100 | 0.48 | 69 | 21.21 | – | – | – | Prone | No | Yes | – |
| S3-3 | SIDS- | Male | 13 | 1 | 9 | 50 | 6900 | 0.72 | 62 | 17.95 | – | – | – | Prone | No | Unknown | – |
| S7-3 | SIDS- | Female | 3 | 2 | 9 | 24 | 4100 | 0.59 | 55 | 13.55 | – | – | – | Prone | No | Unknown | – |
| S9-3 | SIDS- | Male | 14 | 4 | 9 | 34 | 6400 | 0.53 | 63 | 16.12 | – | – | – | Prone | Yes | Unknown | – |
| S10-3 | SIDS- | Female | 36 | 1 | 9 | 34 | 6400 | 0.53 | 63 | 16.12 | – | – | – | Prone | No | Unknown | Preterm |
| S11-3 | SIDS- | Male | 7 | 2 | 9 | 16 | 5100 | 0.31 | 61 | 13.71 | – | – | – | Back | No | Unknown | Preterm |
| S13-3 | SIDS- | Female | 19 | 2 | 8 | 16 | 3500 | 0.46 | 49 | 14.58 | – | – | – | Prone | No | Unknown | Preterm (26 weeks) |
| S15-3 | SIDS- | Male | 5 | 1 | 8 | 30 | 4200 | 0.71 | 54 | 14.40 | – | – | – | Back | Yes | Unknown | – |
| S18-3 | SIDS- | Male | 9 | 2 | 8 | 28 | 4350 | 0.64 | 54 | 14.92 | – | – | – | Unknown | No | Unknown | – |
| S22-3 | SIDS- | Male | 18 | 2 | 7 | 44 | 6595 | 0.67 | 63 | 16.62 | – | – | – | Prone | No | Unknown | – |
| S23-3 | SIDS- | Male | 12 | 2 | 7 | 30 | 7800 | 0.38 | 65 | 18.46 | – | – | – | Prone | No | Unknown | – |
| S25-3 | SIDS- | Female | 11 | 1 | 6 | 34 | 5720 | 0.59 | 60 | 15.89 | – | – | – | Prone | No | Unknown | – |
| S26-3 | SIDS- | Male | 10 | 2 | 6 | 36 | 6015 | 0.60 | 61 | 16.17 | – | – | – | Back | No | Yes | – |
| S27-3 | SIDS- | Male | 30 | 2 | 6 | 38 | 8960 | 0.42 | 69 | 18.82 | – | – | – | Prone | No | Unknown | – |
| S28-3 | SIDS- | Male | 23 | 4 | 6 | 50 | 8465 | 0.59 | 70 | 17.28 | – | – | – | Back | Yes | Unknown | – |
| S29-3 | SIDS- | Male | 23 | 1 | 6 | 55 | 9350 | 0.59 | 71 | 18.55 | – | – | – | Prone | No | Unknown | – |
| S30-3 | SIDS- | Female | 8 | 2 | 6 | 30 | 4007 | 0.75 | 53 | 14.26 | – | – | – | Prone | No | Unknown | – |
| S32-3 | SIDS+ | Male | 17 | 2 | 3 | 52 | 8000 | 0.65 | 65 | 18.93 | – | – | – | Side | No | Unknown | – |
| S5-3 | SIDS+ | Female | 28 | 2 | 9 | 24 | 7600 | 0.32 | 67 | 16.93 | Otitis media | Otitis media | 2 weeks | Prone | No | Unknown | – |
| S6-3 | SIDS+ | Male | 11 | 2 | 9 | 44 | 5900 | 0.75 | 61 | 15.86 | – | Fever | 5 days | Prone | Yes | Unknown | – |
| S8-3 | SIDS+ | Female | 14 | 2 | 9 | 28 | 6000 | 0.47 | 65 | 14.20 | – | Respiratory infection | 4 weeks | Back | No | Unknown | – |
| S12-3 | SIDS+ | Male | 6 | 3 | 8 | 26 | 4600 | 0.57 | 53 | 16.38 | Tracheobronchitis | Fever | 2 days | Prone | No | Unknown | – |
| S17-3 | SIDS+ | Male | 19 | 2 | 8 | 26 | 6100 | 0.43 | 64 | 14.89 | – | Respiratory infection | 2 weeks | Back | No | Unknown | – |
| S20-3 | SIDS+ | Female | 13 | 1 | 8 | 14 | 4342 | 0.32 | 55 | 14.35 | – | Rota virus | 3 weeks | Back | No | Unknown | – |
| S21-3 | SIDS+ | Male | 16 | 3 | 7 | 36 | 5400 | 0.67 | 60 | 15.00 | Tracheobronchitis | Respiratory infection | 1 week | Prone | Yes | Unknown | – |
| S24-3 | SIDS+ | Male | 29 | 1 | 6 | 36 | 7720 | 0.47 | 72 | 14.89 | Tonsillitis | – | – | Prone | No | Unknown | – |
The SIDS– group represents SIDS cases with no symptoms of infection. The SIDS+ group represents SIDS cases with observed symptoms of infection or a documented history of infection.
Measurement of cytokines using Multiplex arrays
The Bio-Rad cell lysis Kit (#171304011, BioRad, Hercules, CA) was used to extract protein from thymus tissue specimens. To determine the protein concentration, the PierceTM BCA Protein Assay Kit (#23225, Thermo Scientific, Waltham, MA) was used. The concentration was adjusted to 1000 μg/mL with Sample Diluent (BioRad, Hercules, CA).
The concentrations of cytokines were measured in thymus protein lysates containing 50 µg protein using the Bio-Plex ProTM Human Cytokine 27-Plex Assay (#500KCAF0Y, BioRad, Hercules, CA) following the manufacturer’s instructions. “Key biomarkers of inflammation from the TNF superfamily proteins, IFN family proteins, Treg cytokines, and MMPs can be measured using a single multiplex kit.” (https://www.bio-rad.com). These 27 cytokines are classified into four groups according to their biological functions in the inflammatory response (Table 3).
Table 3.
27 cytokines included in the study.
| Cytokine groups | Specific cytokines in each group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pro-inflammatory cytokines | IL-1β | IL-2 | IL-6 | IL-7 | IL-12 (p70) | IL-15 | IL-17 | IFN-γ | TNF-α |
| Pro-inflammatory chemokines | CCL1 (MCP-1) | CCL3 (MIP-1α) | CCL4 (MIP-1β) | CCL5 (RANTES) | CCL11 (Eotaxin) | CXCL10 (IP-10) | CXCL8 (IL-8) | ||
| Anti-inflammatory mediators | IL-1RA | IL-4 | IL-5 | IL-9 | IL-10 | IL-13 | |||
| Growth factors | FGF basic | G-CSF | GM-CSF | PDGF-bb | VEGF | ||||
The Bio-Plex-Manager 6.2 software (BioRad, Hercules, CA) was used to calculate the standard curves and concentrations. If the concentration was below the lower limit of detection (LLOD), it was assigned the value of LLOD, and if it was beyond the upper limit of detection (ULOD), it was assigned the value of ULOD.
Statistical analysis
The distribution of variables was analyzed with the Shapiro-Wilk (S-W) normality test. A Mann–Whitney U test was used to compare two groups of continuous variables. A Chi-square (x2) test was used to determine the statistical significance of the proportion of males and females in two groups. The age-related correlation was examined using a Spearman’s rank correlation analysis.
To obtain a general understanding of the age-related alterations in the cytokine/chemokine milieu in the human thymus of infants less than 52 weeks, mean-centered scaled concentrations of cytokines, as proposed in our previous study37, were used. To achieve this, we calculated the mean value for each cytokine across all samples and expressed individual results as a percentage of the mean. The sum of all 27 cytokines was then determined for each sample.
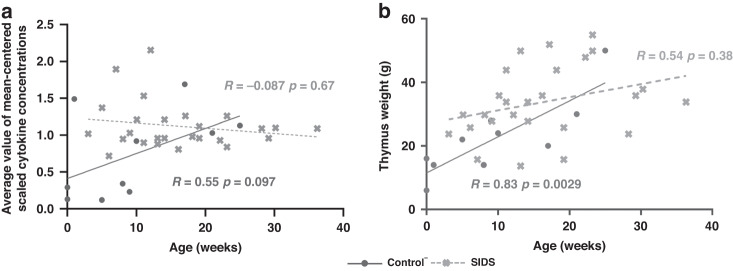
To reduce the influence of age, a confounding variable, an age-layered analysis was performed to calculate the differences in cytokines levels between the two groups. The cutoff age was established based on the intersection of the age-related correlation lines between Control- and SIDS groups (Thymus: 20 weeks based on Fig. 3).
Fig. 3. Age-dependent analyses on the mean-scaled cytokine concentrations and thymic weight between the groups of Control- and SIDS.
a Age-dependent analyses on the mean-scaled cytokine levels. b Age-dependent analysis on the thymic weight. A p value < 0.05 indicates statistical significance. The Control- represents control cases without symptoms of infection.
A p value < 0.05 (two-tailed) indicates statistical significance. All statistical analyses were conducted by SPSS 24.0 (SPSS Inc. Chicago) or Graphpad Prism 9.0 (Graphpad Software, San Diego).
Histological and immunohistochemistry (IHC) studies
A subset of cases was analyzed histopathologically. Thymic tissue of five SIDS and five control cases (age-matched) was formalin-fixed and paraffin-embedded. Analysis of the distribution of immune cells was performed by light microscopy. Therefore, hematoxylin-eosin (HE) staining and IHC of all 10 cases was done, including CD4 and CD8 (both Roche, Basel, Switzerland) for T lymphocytes, CD 138 (Dako, Glostrup, Denmark) for plasma cells and CD68-PGM-1 (Dako, Glostrup, Denmark) for macrophages.
Results
Baseline analysis
To control the confounding factors, an initial baseline analysis was carried out (Table 4). There were significant differences in thymus weight (p = 0.014) and the ratios of thymus weight to body weight (p = 0.004) between the Control-, Control+, and SIDS groups, showing higher thymic weight in SIDS. There were no significant differences between the three groups in age, sex proportion, body weight, body height, or body mass index (BMI).
Table 4.
Baseline analysis of participants.
| Characteristics | Control- (n = 11) | Control+ (n = 5) | SIDS (n = 26) | p value |
|---|---|---|---|---|
| Age in weeks, median (range) | 9 (0–65) | 23 (8–97) | 14 (3–36) | n.s. |
| Male, % | 64% | 60% | 65% | n.s. |
| Thymus weight in grams, median(range) | 20 (6–50) | 20 (4–48) | 34 (14–55) | 0.014 |
| Body weight in grams, median (range) | 5300 (2350–9000) | 10,800 (2974–12,200) | 6058 (3500–10,100) | n.s. |
| Body height in cm, median (range) | 57 (47–68) | 66 (43–87) | 62.5 (49–72) | n.s. |
| Ratios of thymus weight to body weight (%), median (range) | 0.48 (0.20–0.68) | 0.28 (0.11–0.44) | 0.58 (0.31–0.75) | 0.004 |
| Body Mass Index in kg/m2, median (range) | 14.72 (10.64–19.14) | 17.86 (15.85–24.79) | 16 (13.55–21.21) | n.s. |
n.s. represents no significance in statistics. The Control- represents control cases without symptoms of infection. The Control+ group represents control cases who died from severe infection.
Thymic cytokine profiling in controls with severe infection (Control+)
In the next step we compared the cytokine levels in our 3 groups (Table 5). There were 14 elevated cytokines (IL-1 β, -2, -4, -5, -6, -10, CXCL8, CXCL10, CCL1, CCL3, CCL11, FGF basic, G-CSF, and TNF-α) and one decreased ratio of IL-1RA to IL-1β in the Control+ group, compared to the Control- group (Table 5 left).
Table 5.
Statistical differences in the cytokine level of SIDS cases and controls.
| Cytokines | Cytokine concentration (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control- (n = 11) | Control+ (n = 5) | SIDS (n = 26) | Trends for Control+, compared to Control- | p value | Trends for SIDS, compared to Control- | p value | ||||
| Median | Range (minimum-maximum) | Median | Range (minimum-maximum) | Median | Range (minimum-maximum) | |||||
| IL-1RA/IL-1β | 1419.43 | 237.75–3288.34 | 162.89 | 44.35–266.12 | 363.78 | 184.91–685.21 | ↓ | 0.002 | ↓ | 0.008 |
| IL-1β | 3.37 | 0.6–28.69 | 109.14 | 24.04–420.98 | 20.10 | 10.97–40.86 | ↑ | 0.002 | ↑ | 0.036 |
| IL-1RA | 4778.72 | 1305.88–14194.7 | 11050.49 | 5184.56–18672.52 | 7595.99 | 3576.21–19555.97 | 0.069 | 0.195 | ||
| IL-2 | 4.47 | 1.19–29.12 | 34.31 | 18.66–135.01 | 22.98 | 16.8–31.56 | ↑ | 0.027 | ↑ | 0.017 |
| IL-4 | 0.83 | 0.29–7.18 | 6.42 | 5.13–11.09 | 5.80 | 4.74–7.76 | ↑ | 0.038 | ↑ | 0.023 |
| IL-5 | 30.22 | 6.38–423.64 | 329.94 | 214–524.62 | 231.54 | 164.48–355.55 | ↑ | 0.013 | ↑ | 0.032 |
| IL-6 | 15.09 | 1.31–160.33 | 13701.05 | 137.69–21161.43 | 64.04 | 33.17–367.32 | ↑ | 0.001 | 0.058 | |
| IL-7 | 15.71 | 7.38–24.32 | 20.05 | 8.38–32.71 | 15.91 | 5.84–75 | 0.913 | 0.752 | ||
| CXCL8 (IL-8) | 45.82 | 6.73–330.57 | 2451.54 | 76.07–37479.32 | 76.77 | 41.61–594.02 | ↑ | 0.005 | 0.144 | |
| IL-9 | 31.59 | 4.17–143.75 | 113.58 | 81.34–175.93 | 125.72 | 93.21–205.92 | 0.221 | ↑ | 0.003 | |
| IL-10 | 1.24 | 0.1–4.84 | 4.17 | 3.18–8.82 | 3.35 | 2.37–7.42 | ↑ | 0.013 | ↑ | 0.005 |
| IL-12 (p70) | 2.44 | 0.67–17.21 | 8.54 | 3.78–16.39 | 10.33 | 3.34–22.05 | 0.221 | ↑ | 0.035 | |
| IL-13 | 9.93 | 1.66–38.71 | 10.17 | 3.9–53.01 | 17.68 | 7.01–54 | 0.661 | ↑ | 0.020 | |
| IL-15 | 42.74 | 11.37–190.36 | 134.96 | 121.8–233.72 | 144.60 | 110.96–216.9 | 0.377 | 0.084 | ||
| IL-17 | 9.07 | 2.57–35.04 | 33.17 | 25.74–49.58 | 27.60 | 20.2–37.38 | 0.052 | 0.060 | ||
| CCL11 (Eotaxin) | 2.02 | 0.53–27.7 | 28.52 | 11.98–48.48 | 22.11 | 12.71–63.13 | ↑ | 0.027 | ↑ | 0.002 |
| FGF basic | 188.02 | 35.89–807.75 | 650.49 | 573.89–1257.22 | 712.25 | 286.29–1661.31 | ↑ | 0.013 | ↑ | 0.001 |
| G-CSF | 669.29 | 327.8–946.69 | 17793.88 | 838.68–46018.13 | 810.58 | 627.61–1111.71 | ↑ | 0.002 | ↑ | 0.014 |
| GM-CSF | 2.84 | 0.89–14.9 | 11.43 | 8.63–75.26 | 11.24 | 7.03–16.21 | 0.115 | ↑ | 0.033 | |
| IFN-γ | 5.78 | 2.4–106.81 | 157.93 | 59.91–217.26 | 63.33 | 37.34–94.88 | ↑ | 0.003 | ↑ | 0.032 |
| CXCL10 (IP-10) | 1113.76 | 151.8–4482.72 | 6321.40 | 1532.46–87765.67 | 2733.60 | 1415.57–6949.32 | ↑ | 0.009 | 0.073 | |
| CCL1 (MCP-1) | 76.80 | 10.8–987.27 | 6178.34 | 237.75–24282 | 107.60 | 60.62–833.59 | ↑ | 0.001 | 0.084 | |
| CCL3 (MIP-1α) | 592.95 | 131.09–3149.37 | 577.89 | 296.33–2739.39 | 682.13 | 210.98–2904.2 | 0.827 | 0.370 | ||
| PDGF-bb | 3.01 | 1.54–60.97 | 49.18 | 25.79–85.77 | 47.58 | 27.91–97.68 | 0.052 | ↑ | 0.007 | |
| CCL4 (MIP-1β) | 122.54 | 32.46–494.29 | 390.10 | 157.56–837.52 | 279.54 | 197.46–674.96 | 0.09 | ↑ | 0.018 | |
| CCL5 (RANTES) | 266.10 | 29.08–2195.66 | 662.94 | 508.49–1107.37 | 1549.49 | 604.65–6920.89 | 0.583 | ↑ | 0.002 | |
| TNF-α | 14.31 | 3.4–123.82 | 108.59 | 44.89–326.7 | 67.00 | 49.33–169.33 | ↑ | 0.038 | ↑ | 0.025 |
| VEGF | 99.00 | 35.37–262.66 | 212.64 | 175.56–274.23 | 210.84 | 165.86–348.08 | 0.267 | 0.097 | ||
p value < 0.05 marked in bold. The Mann–Whitney U test was used to compare two groups of continuous variables. The Control- represents control cases without symptoms of infection. The Control+ group represents control cases who died from severe infection.
Thymic cytokines and weight in SIDS
Comparison between the Control- and SIDS groups, on the other hand, revealed statistically significant changes in 17 out of 27 cytokines (IL-1β, -2, -4, -5, -9, -10, -12, -13, CCL4, -5, -11, FGF basic, G-CSF, GM-CSF, IFN-, PDGF-bb, and TNF-α) and the ratio of IL-1RA to IL-1 (Table 5 right). Thus, most cytokines increased in the septic Control+ group were also increased in SIDS. However, at least for most proinflammatory cytokines such as IL-6, IL-8 or TNF- α, the magnitude of these changes in SIDS was lower than that in the Control+ group.
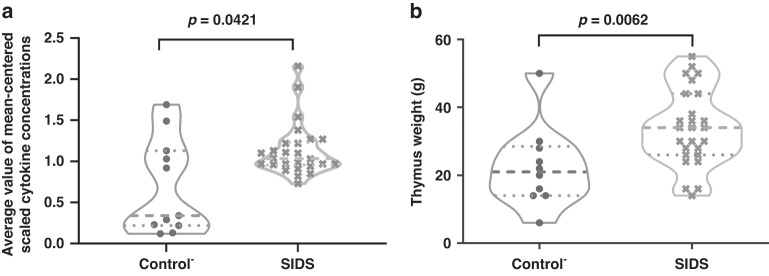
Moreover, normalized averages of all cytokines (p = 0.0421) were higher in the thymus of SIDS compared to those of Control- (Fig. 1a), indicating an activated immune system. Similarly (Fig. 1b), greater thymus weight was seen in SIDS cases (p = 0.0062) compared to Control-.
Fig. 1. Comparison of mean-scaled cytokine concentrations and thymic weight between the groups of Control- and SIDS in violin plots.
a Comparison of mean-scaled cytokine levels. b Comparison of thymic weight. The dash line in each group is the value of 25, 50 (in bold), or 75 percentiles. A p value < 0.05 indicates statistical significance. The Control- represents control cases without symptoms of infection.
For further analysis we concentrated only on the comparison of the SIDS and Control- groups while we did not display results for the septic Control+ group.
Impaired age-dependent thymic cytokines and weight in SIDS
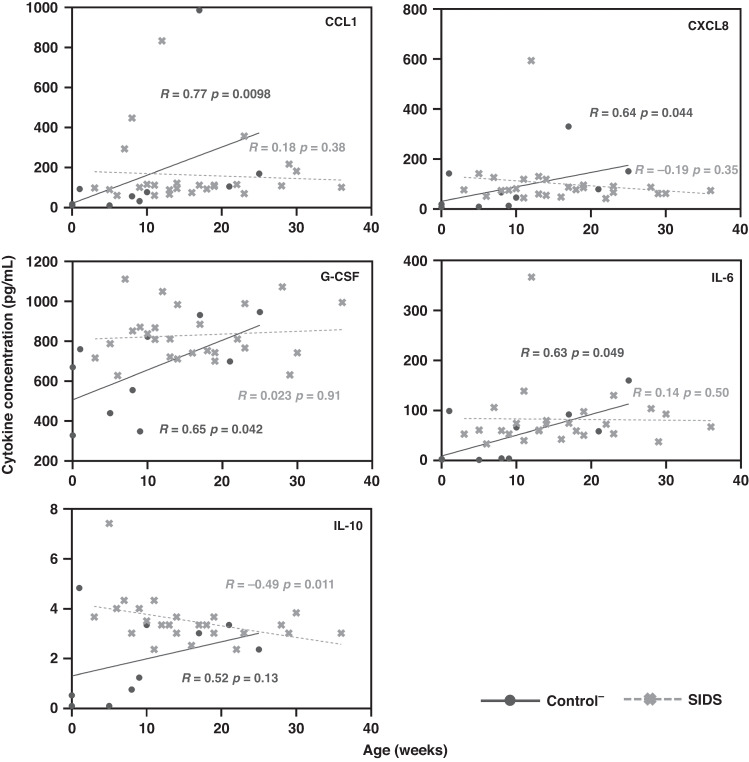
When cytokine concentrations were plotted against age (Fig. 2), four cytokines were positively correlated with age in Control- but not SIDS cases (CCL1, CXCL8, G-CSF, and IL-6). IL-10 was negatively correlated with age in SIDS, but not Control-. When the normalized means for all cytokines were plotted against age (Fig. 3a), a borderline (positive) correlation was found in the Control- group (R = 0.55, p = 0.097), but not in SIDS cases (R = –0.087, p = 0.67). In the Control- group, there was a correlation between thymus weight and age (R = 0.83, p = 0.0029), but not in SIDS (R = 0.54, p = 0.38) (Fig. 3b). However, no statistical association was discovered between the cytokine concentrations and thymus weight (data not shown). To further evaluate the age differences, the Control- and SIDS cases were respectively split into two age groups. As shown in Table 6, elevated cytokine levels in SIDS, compared to Control-, were mainly found in the 0–20 weeks age group but not the group older than 20 weeks.
Fig. 2. Correlation analyses between cytokine concentrations and age in the groups of Control- and SIDS.
Correlations of 5 cytokines (CCL1, CXCL8, G-CSF, IL-6, and IL-10) with age were compared in Control- and SIDS cases. A p value < 0.05 indicates statistical significance. The Control- represents control cases without symptoms of infection.
Table 6.
Statistical differences in the thymic weight and cytokine level of the age subgroups of SIDS cases and controls.
| Cytokines & Thymic weight | 0 <Age≤20 weeks | Age >20 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control- (n = 8) | SIDS (n = 19) | Trends | p value | Control- (n = 3) | SIDS (n = 7) | Trends | p value | |||||
| Cytokine levels in pg/mL& Thymic weight in gram | Cytokine levels in pg/mL& Thymic weight in gram | |||||||||||
| Median | Range (Min.-Max.) | Median | Range (Min.-Max.) | Median | Range (Min.-Max.) | Median | Range (Min.-Max.) | |||||
| Thymic weight | 18 | 6–28 | 30 | 14–52 | ↑ | 0.001 | 30 | 18–50 | 38 | 24–55 | 0.383 | |
| IL-1RA/IL-1β | 1904.52 | 288.67–3288.34 | 349.66 | 184.91–685.21 | ↓ | 0.004 | 499.17 | 237.75–2864.32 | 370.68 | 213.42–619.91 | 0.667 | |
| IL-1β | 2.40 | 0.6–28.69 | 21.62 | 10.97–40.86 | ↑ | 0.034 | 20.10 | 0.76–20.41 | 17.20 | 13.02–27.8 | 1.000 | |
| IL-1RA | 4672.49 | 1305.88–14194.7 | 7340.16 | 3576.21–19555.97 | 0.307 | 4778.72 | 2176.88–10188.13 | 7851.82 | 4646.73–10049.38 | 0.667 | ||
| IL-2 | 3.83 | 1.19–29.12 | 22.98 | 16.8–31.56 | ↑ | 0.016 | 20.82 | 2.22–23.59 | 22.98 | 16.8–24.52 | 0.667 | |
| IL-4 | 0.75 | 0.29–7.18 | 5.77 | 4.74–7.76 | 0.051 | 5.47 | 0.39–5.65 | 5.83 | 4.98–6.3 | 0.117 | ||
| IL-5 | 27.45 | 6.38–423.64 | 232.88 | 164.48–355.55 | ↑ | 0.034 | 208.58 | 21.26–259.52 | 224.81 | 192.19–246.24 | 0.833 | |
| IL-6 | 3.94 | 1.31–99.22 | 60.70 | 33.17–367.32 | 0.075 | 58.53 | 15.09–160.33 | 72.56 | 37.58–130.2 | 0.833 | ||
| IL-7 | 18.47 | 7.38–24.32 | 16.51 | 5.84–75 | 0.979 | 12.44 | 9.22–24.32 | 15.71 | 9.22–34.22 | 0.517 | ||
| CXCL8 (IL-8) | 32.41 | 6.73–330.57 | 80.89 | 44.26–594.02 | 0.075 | 78.71 | 26.58–150.93 | 66.29 | 41.61–90.73 | 0.833 | ||
| IL-9 | 26.04 | 4.17–143.75 | 126.70 | 94.52–205.92 | ↑ | 0.011 | 94.20 | 20.95–125.06 | 120.14 | 93.21–132.93 | 0.267 | |
| IL-10 | 1.00 | 0.1–4.84 | 3.35 | 2.37–7.42 | ↑ | 0.011 | 2.37 | 0.53–3.35 | 3.02 | 2.37–3.84 | 0.383 | |
| IL-12 (p70) | 1.97 | 0.67–13.12 | 10.01 | 3.34–22.05 | ↑ | 0.039 | 6.84 | 1.38–17.21 | 12.29 | 5.98–13.94 | 0.667 | |
| IL-13 | 6.99 | 1.66–38.71 | 17.44 | 7.01–54 | ↑ | 0.013 | 18.91 | 2.26–18.91 | 18.17 | 13.99–29.74 | 1.000 | |
| IL-15 | 38.65 | 11.37–190.36 | 145.23 | 110.96–216.9 | 0.106 | 137.55 | 18.16–150.29 | 134.96 | 113.7–188 | 0.667 | ||
| IL-17 | 7.20 | 2.57–35.04 | 27.60 | 20.2–37.38 | 0.066 | 27.13 | 2.86–28.76 | 27.60 | 24.82–31.31 | 0.517 | ||
| CCL11 (Eotaxin) | 1.83 | 0.53–27.7 | 22.18 | 12.71–63.13 | ↑ | 0.001 | 24.63 | 0.8–27.22 | 21.83 | 14.06–34.5 | 0.667 | |
| FGF basic | 164.89 | 35.89–807.75 | 723.19 | 465.47–1661.31 | ↑ | 0.004 | 477.33 | 80.34–502.06 | 607.05 | 286.29–1069.71 | 0.183 | |
| G-CSF | 612.52 | 327.8–931.95 | 809.67 | 627.61–1111.71 | ↑ | 0.034 | 698.40 | 570.47–946.69 | 811.48 | 631.13–1073.17 | 0.267 | |
| GM-CSF | 2.70 | 0.89–14.9 | 10.33 | 7.03–16.21 | 0.066 | 9.85 | 1.24–12.05 | 11.66 | 9.37–14.16 | 0.267 | ||
| IFN-γ | 5.08 | 2.4–106.81 | 63.33 | 37.34–94.88 | ↑ | 0.045 | 56.49 | 5.78–68.24 | 63.33 | 44.82–75.86 | 0.383 | |
| CXCL10 (IP-10) | 1056.55 | 151.8–4482.72 | 2823.14 | 1415.57–6949.32 | ↑ | 0.029 | 3119.01 | 304.49–3288.69 | 2707.22 | 1495.01–3490.88 | 1.000 | |
| CCL1 (MCP-1) | 44.17 | 10.8–987.27 | 101.04 | 60.62–833.59 | ↑ | 0.011 | 169.91 | 105.77–216.67 | 115.61 | 69.54–357.08 | 1.000 | |
| CCL3 (MIP-1α) | 532.81 | 131.09–3149.37 | 595.80 | 210.98–2904.2 | 0.621 | 592.95 | 132.58–1654.48 | 831.79 | 390.59–1960.21 | 0.517 | ||
| PDGF-bb | 2.38 | 1.54–60.97 | 45.98 | 30.02–97.68 | ↑ | 0.016 | 40.64 | 1.54–44.91 | 50.25 | 27.91–94.43 | 0.267 | |
| CCL4 (MIP-1β) | 92.79 | 32.46–494.29 | 278.56 | 197.46–674.96 | ↑ | 0.019 | 259.95 | 56.83–287.73 | 286.04 | 212.07–314.18 | 0.667 | |
| CCL5 (RANTES) | 199.23 | 29.08–2195.66 | 1556.15 | 604.65–6920.89 | ↑ | 0.003 | 1236.94 | 142.2–1314.73 | 1485.30 | 829.63–2142.64 | 0.183 | |
| TNF-α | 12.33 | 3.4–123.82 | 69.57 | 49.33–169.33 | ↑ | 0.034 | 55.98 | 6.34–78.36 | 64.44 | 58.92–72.87 | 0.517 | |
| VEGF | 94.18 | 35.37–262.66 | 216.21 | 165.86–348.08 | 0.095 | 199.94 | 39.46–243.26 | 189.77 | 166.83–264.33 | 1.000 | ||
p value < 0.05 marked in bold. The Mann–Whitney U test was used to compare two groups of continuous variables. The Control- represents control cases without symptoms of infection.
Immunohistochemistry (IHC) examination on the thymus of SIDS
SIDS and Control- cases consisted of morphologically normal thymic tissue and had the same light microscopic immunoprofile (supplemental Figure S1). Nevertheless, as the slides of the originally deep-frozen tissue was of low quality, these examinations were confined to 10 samples and the conclusions drawn from these are limited.
Discussion
The thymus plays an essential role in the immune development of infants.18 As the immune system is considered to be involved in the etiology of SIDS, we hypothesized that the morphology and function of the thymus of SIDS infants differ from other deaths. However, earlier morphological SIDS studies reported controversial findings on the thymus.23–28,31,32 We thus performed for the very first time a study on cytokines and other inflammatory mediators in the thymic tissue. We discovered elevated thymic cytokine concentrations and weights in SIDS cases, but as opposed to the controls, the SIDS cases showed no increase of the cytokines with age: Thus, increased concentrations were only detectable before the 20th week of life (and thus in the peak prevalence of SIDS) (Table 6).
The Control+ group who comprised deaths from severe infection had elevated levels of 14 cytokines compared to the Control- group who died without septic diseases (Table 5), including critical pro-inflammatory mediators such as IL-1β, IFN-γ, and TNF-α, which were largely in line with increased cytokine in the cerebrospinal fluid (CSF) of infants that died of viral infections38. Also, in the baseline analysis, the ratio of thymus weight ratio to body weight was decreased in the controls with severe infections (Table 4). These findings, that we considered to be the consequence of glucocorticoids and up-regulated inflammatory mediators,39–41 are in line with previous studies regarding infection-induced thymic atrophy or involution.23,42–45 In the context of our study, we not only used the Control+ group to confirm the reliability of the adopted cytokine measurement method, but also to assess the question whether SIDS – without pathologic anatomical findings of an infection – shows an activation of the immune system similar to that in septic deaths.
When comparing the differences between the SIDS and Control- groups, 17 cytokines, and the thymus weight were increased in SIDS (Table 5). Like for the Control+ group, important pro-inflammatory mediators associated with the infection, including IL-1β, IFN-γ, and TNF-α, were highly expressed. In addition, when compared to Control-, ten significant increased cytokines overlapped between the SIDS and Control+ groups. Altogether, the overlapped cytokine levels were highest in the Control+ group, similar in distribution but mostly of lower magnitude in SIDS infants, and lowest in the Control- group (Table 5). Also, most of these up-regulated cytokines are considered to boost the thymic development, and hence T cell development.34,35 For us, this observation implies that there may be a mild to moderate degree of infection causing increased intrathymic cytokines in SIDS, which in turn induces reactive thymic hyperplasia, as already suggested, e.g. by Goldwater et al.27 The increased thymus weight found in our study (and some, albeit not all, others25,27,32) might be explained by this mechanism. As we know, SIDS cases with a case history of slight infection or postmortem detected upper airway infections are only a fraction of the overall caseload. However, we unexpectedly found no statistically significant differences in thymic cytokine concentrations and thymus weights between SIDS with and without weak infections (data not shown). This just provides the possibility that subtle, easily overlooked, or undetected subclinical infections may be widely prevalent in the SIDS group, causing no obvious differences of cytokines between SIDS cases with or without “observed” infection.
Increasing evidence suggests that transitional, age-dependent disorders (causing a “critical developmental stage” according to the triple risk theory) may be associated with the pathogenesis of SIDS. Our previous publication on the lungs found an impaired age-dependent cytokine network in SIDS, leading to an underlying disturbed immune response state.37 The increasing levels of cytokines during the ageing of healthy infants are considered to be the consequence of a “trained” immune system, due to increasing contacts with microorganisms after birth.46,47 Thus, the normal development of the thymus is accompanied by age-related alterations in cytokine expression and thymic weights.48,49
In this study, thymic weight, and cytokines (e.g., CCL1, CCL8, G-CSF and IL-6) increased with age in infants that died without serious infectious diseases (Control-). In SIDS, on the other hand, we found higher thymic weights and cytokines in infants up to 20 weeks old, but no age-dependent changes. To us, this suggests that infection or other immunologic challenges in SIDS cause elevated cytokine levels during earlier infancy, but also might repress the proper development of the immune system. The higher thymic weight and cytokine levels might thus reflect subclinical infections, mainly present in the first 5 months of life in SIDS (Table 6). Intriguingly, a Canadian study based on data from 900 infant autopsies also demonstrated that the thymus weight of SIDS/ sudden unexpected death syndrome (SUDS) in the infants younger than 25 weeks cases is greater than the counterpart controls’ one.25 Even more so, this age group includes the peak prevalence (2 ~ 4 months) of SIDS. The reasons why SIDS infants are, at a vulnerable period, more prone to succumb to common infections than other infants are still not well known, although several studies tried to interpret a dysfunctional immune status from a genetic viewpoint.6,50–53
Since increased cytokines observed in the thymus of SIDS might be reflected by morphologic changes, such as reactive hyperplasia, age-matched histological examination on composition of thymic immune cells was performed but no significant differences were found between SIDS and Control- groups. Nevertheless, we could not rule out the presence of an altered thymic output in SIDS although this has not been formally shown so far. Thus, it would be interesting to explore whether the altered cytokine and chemokine profile in SIDS thymus tissue would also impair thymic T cell development and the T cell repertoire (TCR) both for CD4 and CD8 T cells. Some further experimental approaches, such as single cell sequencing, quantification of T cell receptor repertoire and TCR excision circles (TRECs), might be needed to investigate cell composition and thymic function, which would assist in understanding the potential role of the thymic output in SIDS.
Several of limitations of this study should be mentioned herein. Firstly, a limited number of cases was recruited for this study, which causes reduced statistical test efficacy especially in the subgroup analysis on risk factors like birth weight, preterm birth, family history, maternal risk, etc. The analysis on the association between risk factors and cytokine levels showed no significant results (data not shown). Secondly, a study on postmortem material has to rely on samples from other deceased persons. These are not ideal as controls (healthy infants would be) and the interpretation of our results might be biased by that fact. Finally, it should be kept in mind that postmortem cytokine levels may be affected by postmortem changes (e.g., autolysis and putrefaction) because the postmortem interval between the onset of death and tissue sampling cannot be avoided. Therefore, the possible effects of postmortem changes should be considered when interpreting the relevant data.
Conclusion
In summary, elevated cytokines, as well as thymus weight, and impaired thymic age-dependent changes in SIDS may be influenced by subclinical infections. The increase in thymic cytokine levels and thymic weight were mainly present in SIDS cases under 5 months of age, which overlaps with the high incidence of SIDS onset (2 ~ 4 months), suggesting that subclinical infection might play an important role in the onset of SIDS among young susceptible infants.
Supplementary information
Author contributions
D.Q. performed experiments, carried out the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Preuss, Hagemeier and Vennemann collected the samples, defined cases and controls, helped with the analysis and interpretation of data, and reviewed and revised the manuscript; L.R., K.B., and J.K. performed the parts of experiments, collected the raw data, and reviewed and revised the manuscript; S.N. performed the histopathological analysis, and reviewed and revised the manuscript; C.S.F. contributed to the conceptualization of the study, and critically reviewed and revised the manuscript for important intellectual content; M.K. conceptualized and designed the study, coordinated and supervised data collection, co-drafted the initial manuscript, and critically reviewed and revised the manuscript. All authors approved the final version of this manuscript.
Funding
This study was supported by the German Center of Infection Research DZIF, TTU-IICH 07.003, 07.801,07.821 Hannover-Braunschweig site (C.S.F.), the DFG grant No. FA-483_1 (C.S.F.), the SFB728 project B03 (C.S.F.), and the CSC grant No. 201908440466 (D.Q.). Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-023-02809-6.
References
- 1.Krous HF, et al. Sudden infant death syndrome and unclassified sudden infant deaths: A definitional and diagnostic approach. Pediatrics. 2004;114:234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: The triple-risk model. Neonatology. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 3.Guntheroth WG, Spiers PS. The triple risk hypotheses in sudden infant death syndrome. Pediatrics. 2002;110:e64. doi: 10.1542/peds.110.5.e64. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, C. Vol. 42 1-2 (Blackwell Publishing Ltd Oxford, UK, 2004).
- 5.Blood-Siegfried J. The role of infection and inflammation in sudden infant death syndrome. Immunopharmacol. Immunotoxicol. 2009;31:516–523. doi: 10.3109/08923970902814137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fard D, et al. Candidate gene variants of the immune system and sudden infant death syndrome. Int. J. Leg. Med. 2016;130:1025–1033. doi: 10.1007/s00414-016-1347-y. [DOI] [PubMed] [Google Scholar]
- 7.Ferrante L, Rognum TO, Vege Å, Nygård S, Opdal SH. Altered gene expression and possible immunodeficiency in cases of sudden infant death syndrome. Pediatr. Res. 2016;80:77–84. doi: 10.1038/pr.2016.45. [DOI] [PubMed] [Google Scholar]
- 8.Forsyth KD. Immune and inflammatory responses in sudden infant death syndrome. FEMS Immunol. Med. Microbiol. 1999;25:79–83. doi: 10.1111/j.1574-695X.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldwater PN. Sids, prone sleep position and infection: An overlooked epidemiological link in current sids research? Key evidence for the “Infection Hypothesis”. Med. Hypotheses. 2020;144:110114. doi: 10.1016/j.mehy.2020.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Highet A. An infectious aetiology of sudden infant death syndrome. J. Appl. Microbiol. 2008;105:625–635. doi: 10.1111/j.1365-2672.2008.03747.x. [DOI] [PubMed] [Google Scholar]
- 11.Opdal, S. H. Cytokines, infection, and immunity. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future (eds Duncan, J. R. & Byard, R. W.) 689–710 (University of Adelaide Press, Adelaide, 2018). [PubMed]
- 12.Vege Å, Ole Rognum T. Sudden infant death syndrome. Infect. Inflamm. Responses FEMS Immunol. Med. Microbiol. 2004;42:3–10. doi: 10.1016/j.femsim.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Rognum IJ, et al. Interleukin-6 and the serotonergic system of the medulla oblongata in the sudden infant death syndrome. Acta neuropathologica. 2009;118:519–530. doi: 10.1007/s00401-009-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vege Å, Rognum TO, Ånestad G. Il-6 cerebrospinal fluid levels are related to laryngeal Iga and epithelial Hla-Dr response in sudden infant death syndrome. Pediatr. Res. 1999;45:803–809. doi: 10.1203/00006450-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Vennemann MM, et al. Cytokines and sudden infant death. Int. J. Leg. Med. 2012;126:279–284. doi: 10.1007/s00414-011-0638-6. [DOI] [PubMed] [Google Scholar]
- 16.Howat WJ, Moore IE, Judd M, Roche WR. Pulmonary immunopathology of sudden infant death syndrome. Lancet. 1994;343:1390–1392. doi: 10.1016/s0140-6736(94)92523-2. [DOI] [PubMed] [Google Scholar]
- 17.Forsyth KD, et al. Immunocytologic characterization using monoclonal antibodies of lung lavage cell phenotype in infants who have died from sudden infant death syndrome. Pediatr. Res. 1988;23:187–190. doi: 10.1203/00006450-198802000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Thapa P, Farber DL. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019;29:123–131. doi: 10.1016/j.thorsurg.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan KE. Chromosome 22q11.2 deletion syndrome and digeorge syndrome. Immunol. Rev. 2019;287:186–201. doi: 10.1111/imr.12701. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, et al. Thymic hypoplasia induced by copy number variations contributed to explaining sudden infant death based on forensic autopsies. Forensic Sci. Int. 2022;336:111323. doi: 10.1016/j.forsciint.2022.111323. [DOI] [PubMed] [Google Scholar]
- 21.Dally A. Status lymphaticus: Sudden death in children from “Visitation of God” to cot death. Med. Hist. 1997;41:70–85. doi: 10.1017/s0025727300062049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paltauf A. Über die Beziehung des Thymus zum plötzlichen Tod. Wien. Klin. Wochenschr. 1889;2:877–881. [Google Scholar]
- 23.Bajanowski T, Ortmann C, Hernandez M, Freislederer A, Brinkmann B. Reaction patterns in selected lymphatic tissues associated with sudden infant death (Sid) Int. J. Leg. Med. 1997;110:63–68. doi: 10.1007/s004140050032. [DOI] [PubMed] [Google Scholar]
- 24.Bonzanigo C, Scheidegger S. [Sudden infant death and hypertrophy of the thymus gland] Schweizerische Rundsch. fur Med. Prax. = Rev. suisse de. Med. Prax. 1983;72:1617–1625. [PubMed] [Google Scholar]
- 25.Evetts AM, Shkrum MJ, Tugaleva E. A new reference source for postmortem body measurements and organ weights in neonates and infants: A statistical analysis based on sudden death classification (Part 2) Am. J. Forensic Med. Pathol. 2018;39:285–303. doi: 10.1097/PAF.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 26.Fracasso T, Vennemann M, Pfeiffer H, Bajanowski T. Organ weights in cases of sudden infant death syndrome: A German study. Am. J. forensic Med. Pathol. 2009;30:231–234. doi: 10.1097/PAF.0b013e318187e0f2. [DOI] [PubMed] [Google Scholar]
- 27.Goldwater, P. N., Kelmanson, I. A. & Little, B. B. Increased thymus weight in sudden infant death syndrome compared to controls: The role of sub-clinical infections. Am. J. Hum. Biol.33, e23528 (2021). [DOI] [PubMed]
- 28.Liebrechts-Akkerman G, et al. Histological findings in unclassified sudden infant death, including sudden infant death syndrome. Pediatr. developmental Pathol. : Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2013;16:168–176. doi: 10.2350/12-10-1262-OA.1. [DOI] [PubMed] [Google Scholar]
- 29.Risse M, Weiler G. [Histologic distribution pattern of thymus hemorrhages in sudden infant death] Beitrage zur. gerichtlichen Med. 1988;46:351–355. [PubMed] [Google Scholar]
- 30.Risse M, Weiler G. Differential diagnosis sids/non-sids on the basis of histological findings of petechial thymus hemorrhages. Am. J. Hum. Biol. Counc. 1989;43:1–7. doi: 10.1016/0379-0738(89)90115-1. [DOI] [PubMed] [Google Scholar]
- 31.Scheimberg I, et al. Weight charts of infants dying of sudden infant death in England. Pediatr. Dev. Pathol. 2014;17:271–277. doi: 10.2350/13-08-1362-OA.1. [DOI] [PubMed] [Google Scholar]
- 32.Siebert JR, Haas JE. Organ weights in sudden infant death syndrome. Pediatr. Pathol. 1994;14:973–985. doi: 10.3109/15513819409037694. [DOI] [PubMed] [Google Scholar]
- 33.Varga I, Bódi I, Mešťanová V, Kováč M, Klein M. Association between histological alterations in the thymus and sudden infant death syndrome. J. forensic Leg. Med. 2018;55:8–13. doi: 10.1016/j.jflm.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Hosokawa, H. & Rothenberg, E. V. Cytokines, transcription factors, and the initiation of T-cell development. Cold Spring Harbor Perspect. Biol.10, a0286211(2018). [DOI] [PMC free article] [PubMed]
- 35.Yarilin AA, Belyakov IM. Cytokines in the thymus: Production and biological effects. Curr. medicinal Chem. 2004;11:447–464. doi: 10.2174/0929867043455972. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnik A, Moore TA. Cytokine production and requirements during T-cell development. Curr. Opin. Immunol. 1995;7:206–213. doi: 10.1016/0952-7915(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 37.Qu D, et al. Pulmonary immune profiling of sids: impaired immune maturation and age-related cytokine imbalance. Pediatr. Res. 2022;93:1239–1249. doi: 10.1038/s41390-022-02203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morichi S, et al. Increased platelet-derived growth factor and cytokine levels in the cerebrospinal fluid of patients of sudden unexpected death with or without viral infection. Indian J. pediatrics. 2021;88:879–884. doi: 10.1007/s12098-020-03588-2. [DOI] [PubMed] [Google Scholar]
- 39.Luo M, Xu L, Qian Z, Sun X. Infection-associated thymic atrophy. Front. Immunol. 2021;12:652538. doi: 10.3389/fimmu.2021.652538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansari AR, Liu H. Acute thymic involution and mechanisms for recovery. Archivum Immunol. et. therapiae experimentalis. 2017;65:401–420. doi: 10.1007/s00005-017-0462-x. [DOI] [PubMed] [Google Scholar]
- 41.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito M, et al. Ultrasound monitoring of thymus involution in septic mice. Ultrasound Med. Biol. 2021;47:769–776. doi: 10.1016/j.ultrasmedbio.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuchler L, et al. Elevated intrathymic sphingosine-1-phosphate promotes thymus involution during sepsis. Mol. Immunol. 2017;90:255–263. doi: 10.1016/j.molimm.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Di Naro E, et al. Fetal thymic involution: A sonographic marker of the fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 2006;194:153–159. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 45.Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PloS one. 2011;6:e17940. doi: 10.1371/journal.pone.0017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Härtel C, et al. Cytokine responses correlate differentially with age in infancy and early childhood. Clin. Exp. Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ygberg S, Nilsson A. The developing immune system—from foetus to toddler. Acta Paediatr. (Oslo, Nor. : 1992) 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 48.Pryce JW, et al. Reference ranges for organ weights of infants at autopsy: Results of >1,000 consecutive cases from a single centre. BMC Clin. Pathol. 2014;14:18. doi: 10.1186/1472-6890-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol. Res. 2000;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 50.Ferrante L, Opdal S, Vege Å, Rognum T. Cytokine gene polymorphisms and sudden infant death syndrome. Acta Paediatr. 2010;99:384–388. doi: 10.1111/j.1651-2227.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 51.Opdal SH, Opstad A, Vege Å, Rognum TO. Il-10 gene polymorphisms are associated with infectious cause of sudden infant death. Hum. Immunol. 2003;64:1183–1189. doi: 10.1016/j.humimm.2003.08.359. [DOI] [PubMed] [Google Scholar]
- 52.Ferrante L, Opdal SH, Vege Å, Rognum TO. Tnf-Α promoter polymorphisms in sudden infant death. Hum. Immunol. 2008;69:368–373. doi: 10.1016/j.humimm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Hafke A, Schürmann P, Rothämel T, Dörk T, Klintschar M. Evidence for an association of interferon gene variants with sudden infant death syndrome. Int. J. Leg. Med. 2019;133:863–869. doi: 10.1007/s00414-018-1974-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.