Abstract
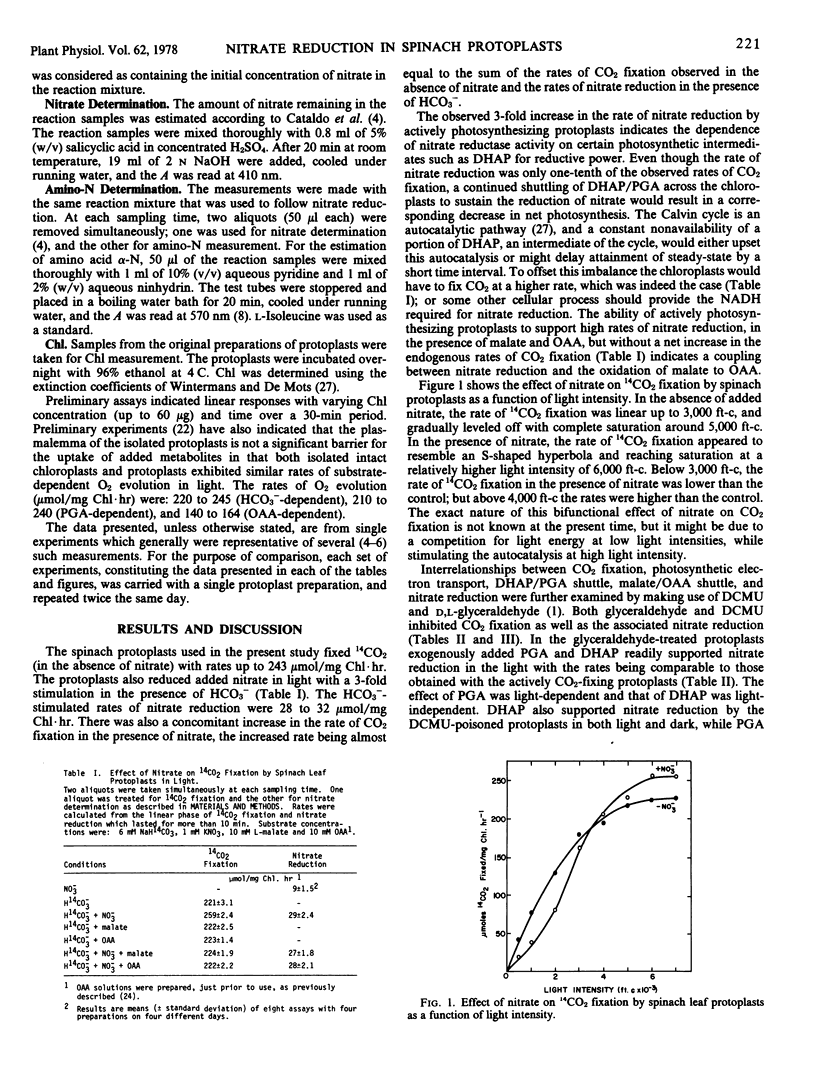
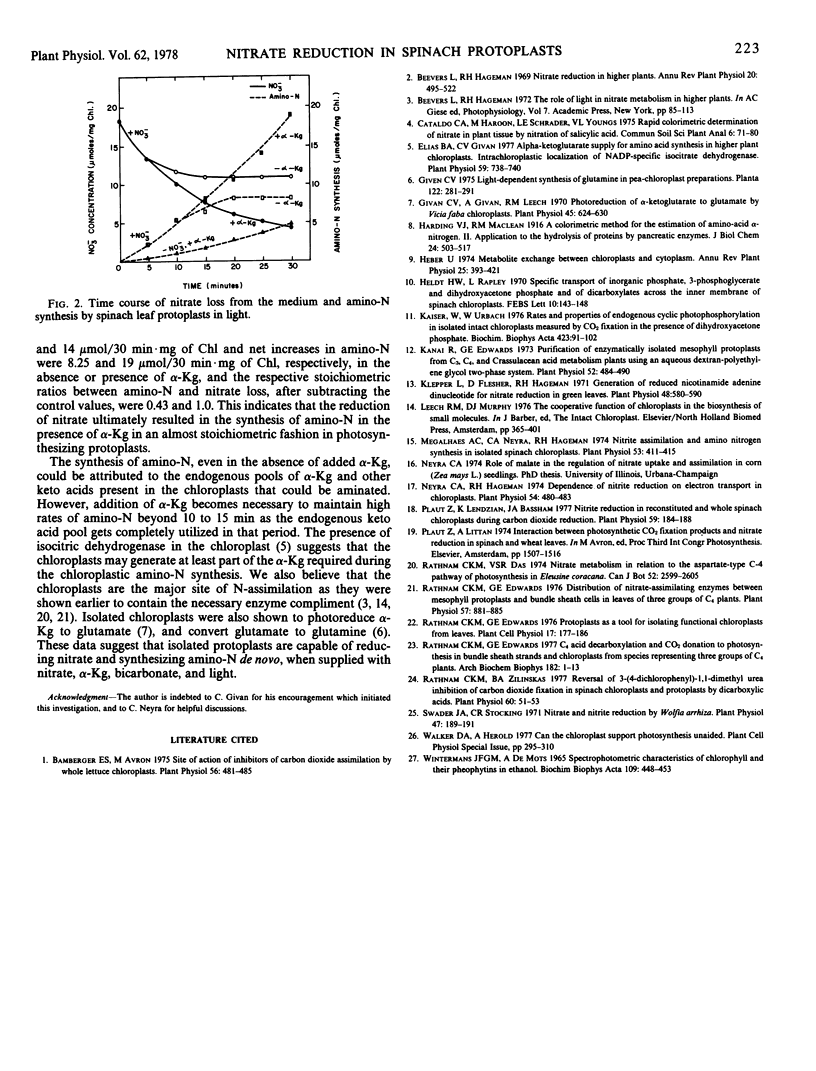
Isolated spinach (Spinacia oleracea L. var. Bloomsdale) leaf protoplasts reduced nitrate at rates of 9 micromoles per milligram chlorophyll per hour in light with a 3- to 4-fold stimulation in the presence of HCO3−. A similar stimulation of nitrate reduction in the absence of CO2 fixation was obtained by the addition of malate, oxaloacetate (OAA), phospho-3-glyceric acid (PGA), or dihydroxyacetone phosphate (DHAP). Stimulation by malate and DHAP was light-independent, while the PGA and OAA effect was light-dependent. Nitrate reduction was found to be coupled to the cytoplasmic oxidation of DHAP or malate. The PGA/DHAP and OAA/malate shuttle across the chloroplast envelope has been demonstrated to support CO2 fixation and/or nitrate reduction. The leaf protoplasts readily assimilated nitrate into amino-N in a stoichiometric relationship.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Hageman R. H. The role of light in nitrate metabolism in higher plants. Photophysiology. 1972;(7):85–113. [PubMed] [Google Scholar]
- Elias B. A., Givan C. V. Alpha-ketoglutarate supply for amino Acid synthesis in higher plant chloroplasts: intrachloroplastic localization of NADP-specific isocitrate dehydrogenase. Plant Physiol. 1977 Apr;59(4):738–740. doi: 10.1104/pp.59.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan C. V., Givan A. L., Leech R. M. Photoreduction of alpha-Ketoglutarate to Glutamate by Vicia faba Chloroplasts. Plant Physiol. 1970 May;45(5):624–630. doi: 10.1104/pp.45.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. Rates and properties of endogenous cyclic photophosphorylation of isolated intact chloroplasts measured by CO2 fixation in the presence of dihydroxyacetone phosphate. Biochim Biophys Acta. 1976 Jan 15;423(1):91–102. doi: 10.1016/0005-2728(76)90103-1. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C. A., Hageman R. H. Dependence of nitrite reduction on electron transport chloroplasts. Plant Physiol. 1974 Oct;54(4):480–483. doi: 10.1104/pp.54.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut Z., Lendzian K., Bassham J. A. Nitrite Reduction in Reconstituted and Whole Spinach Chloroplasts during Carbon Dioxide Reduction. Plant Physiol. 1977 Feb;59(2):184–188. doi: 10.1104/pp.59.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Edwards G. E. C4 acid decarboxylation and CO2 donation to photosynthesis in bundle sheath strands and chloroplasts from species representing three groups of C4 plants. Arch Biochem Biophys. 1977 Jul;182(1):1–13. doi: 10.1016/0003-9861(77)90277-6. [DOI] [PubMed] [Google Scholar]
- Rathnam C. K., Edwards G. E. Distribution of Nitrate-assimilating Enzymes between Mesophyll Protoplasts and Bundle Sheath Cells in Leaves of Three Groups of C(4) Plants. Plant Physiol. 1976 Jun;57(6):881–885. doi: 10.1104/pp.57.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Zilinskas B. A. Reversal of 3-(3,4-dichlorophenyl)-1,1-dimethylurea inhibition of carbon dioxide fixation in spinach chloroplasts and protoplasts by dicarboxylic acids. Plant Physiol. 1977 Jul;60(1):51–53. doi: 10.1104/pp.60.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swader J. A., Stocking C. R. Nitrate and Nitrite Reduction by Wolffia arrhiza. Plant Physiol. 1971 Feb;47(2):189–191. doi: 10.1104/pp.47.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


