Abstract
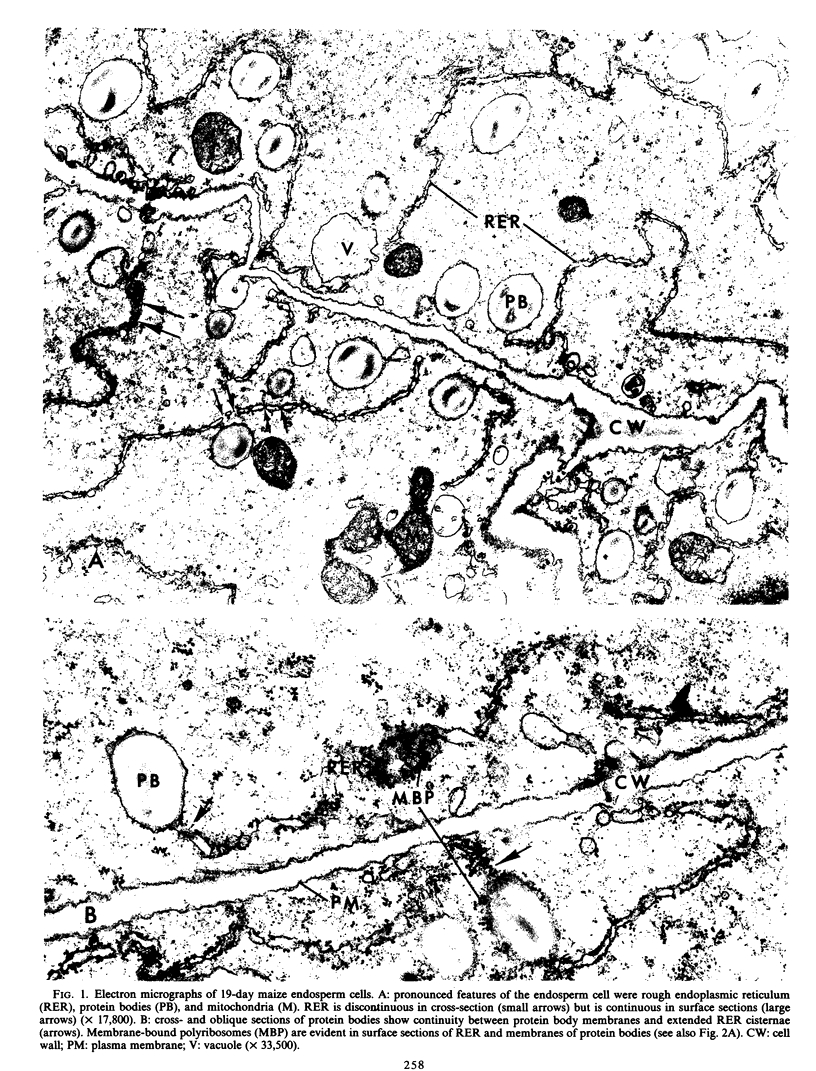
The origin of protein bodies in maize (Zea mays L.) endosperm was investigated to determine whether they are formed as highly differentiated organelles or as protein deposits within the rough endoplasmic reticulum. Electron microscopy of developing maize endosperm cells showed that membranes surrounding protein bodies were continuous with rough endoplasmic reticulum membranes. Membranes of protein bodies and rough endoplasmic reticulum both contained cytochrome c reductase activity indicating a similarity between these membranes. Furthermore, the proportion of alcohol-soluble protein synthesized by polyribosomes isolated from protein body or rough endoplasmic reticulum membranes was similar, and the alcohol-soluble or -insoluble proteins showed identical [14C]leucine labeling. These results demonstrated that protein bodies form simply as deposits within the rough endoplasmic reticulum.
Messenger RNA that directed synthesis of only the smaller molecular weight zein subunit was separated from mRNA that synthesized both subunits by sucrose gradient centrifugation. This result demonstrated that separate but similar sized mRNAs synthesize the major zein components. In vitro translation products of purified mRNAs or polyribosomes were approximately 2,000 daltons larger than native zein proteins, suggesting that the proteins are synthesized as zein precursors. When intact rough endoplasmic reticulum was placed in the in vitro protein synthesis system, proteins corresponding in molecular weight to the native zein proteins were obtained.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Jones R. A., Dalby A., Tsai C. Y. Genetic regulation of storaage protein content in maize endosperm. Biochem Genet. 1976 Aug;14(7-8):641–650. doi: 10.1007/BF00485842. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton R. K., Palk B. A., Raison J. K. Intracellular components associated with protein synthesis in developing wheat endosperm. Biochem J. 1964 Jun;91(3):522–528. doi: 10.1042/bj0910522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton R. K., Raison J. K. The separate incorporation of amino acids into storage and soluble proteins catalysed by two independent systems isolated from developing wheat endosperm. Biochem J. 1964 Jun;91(3):528–539. doi: 10.1042/bj0910528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. R., MACHADO R. D. Studies on the endoplasmic reticulum. IV. Its form and distribution during mitosis in cells of onion root tip. J Biophys Biochem Cytol. 1960 Feb;7:167–180. doi: 10.1083/jcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Functions for polyribosome-membrane interactions in protein synthesis. Biochim Biophys Acta. 1977 Aug 9;472(2):197–236. doi: 10.1016/0304-4157(77)90017-x. [DOI] [PubMed] [Google Scholar]