Abstract
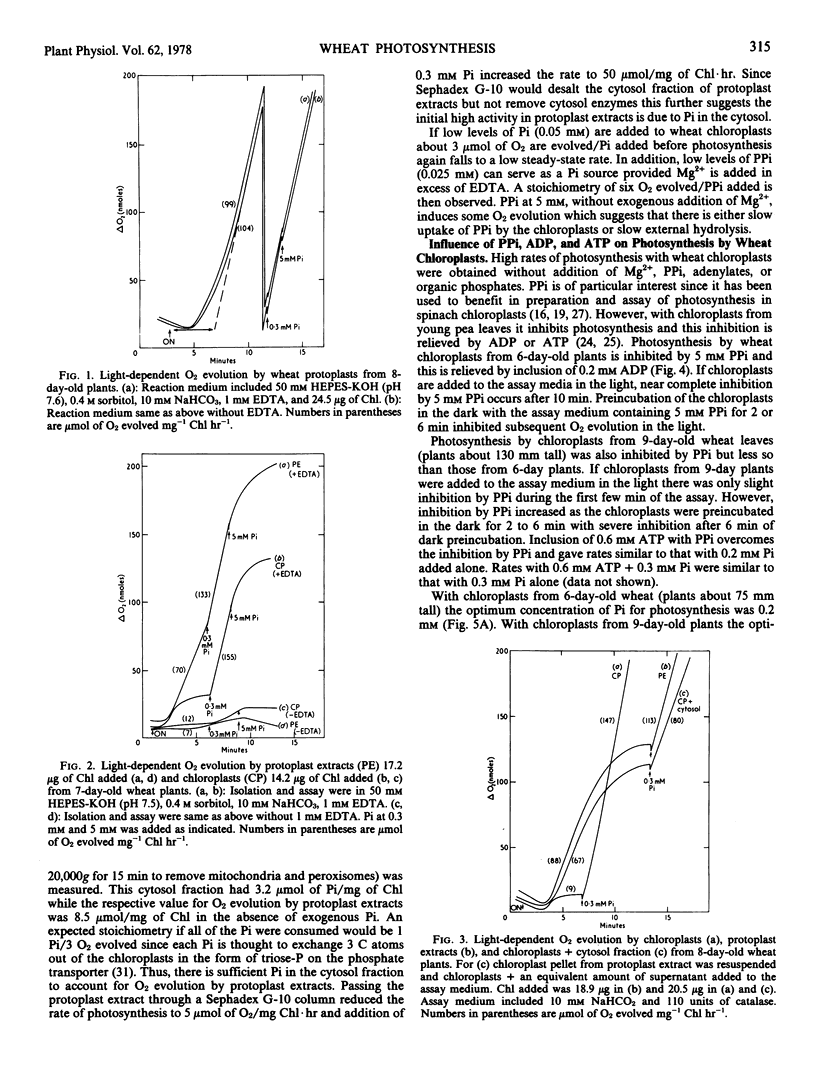
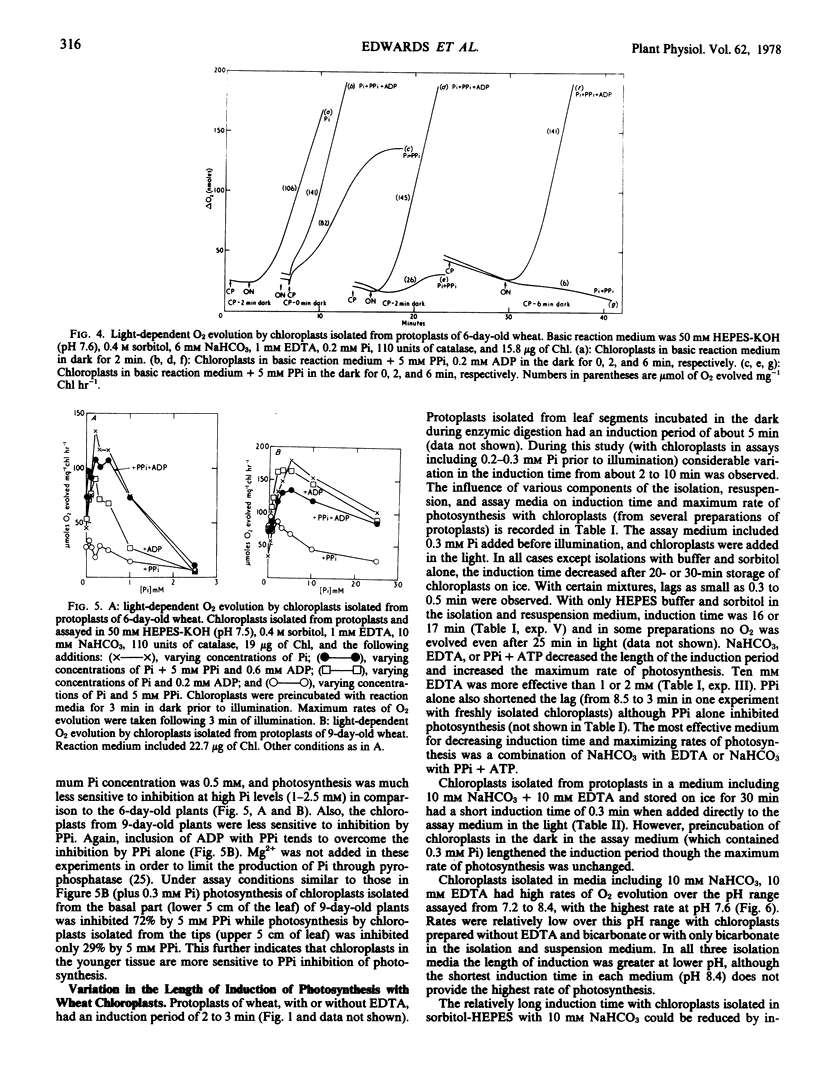
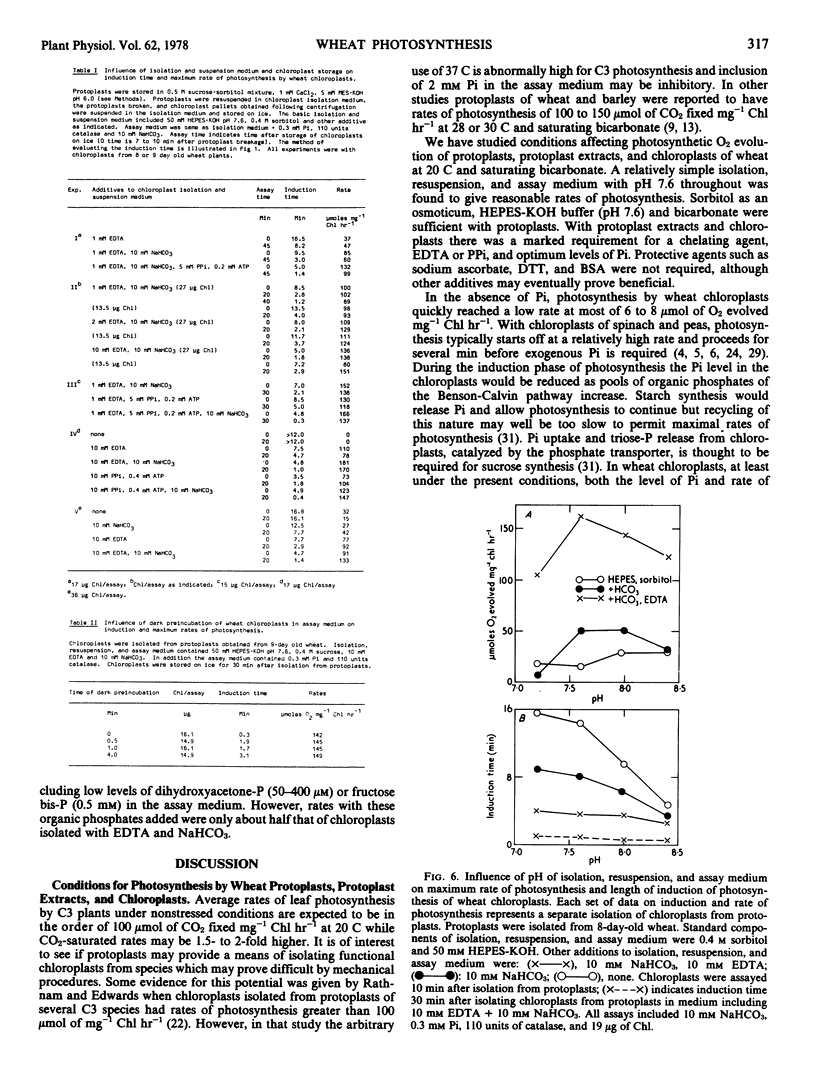
Protoplasts, protoplast extracts (intact chloroplasts plus extrachloroplastic material), and chloroplasts isolated from protoplasts of wheat (Triticum aestivum) have rates of photosynthesis as measured by light-dependent O2 evolution of about 100 to 150 micromoles of O2 per milligram of chlorophyll per hour at 20 C and saturating bicarbonate. The assay conditions sufficient for this activity were 0.4 molar sorbitol, 50 millimolar N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid KOH (pH 7.6), and 10 millimolar NaHCO3 with protoplast, plus a requirement of 1 to 10 millimolar ethylenediaminetetraacetate (EDTA) and 0.2 to 0.5 millimolar inorganic orthophosphate (Pi) with protoplast extracts and chloroplasts. Protoplast extracts evolved approximately 6 micromoles of O2 per milligram of chlorophyll before photosynthesis became largely dependent on exogenous Pi while photosynthesis by chloroplasts had a much stronger dependence on exogenous Pi from the outset.
Photosynthesis by chloroplasts from 6-day-old wheat plants under optimum levels of Pi was similar to that with the addition of 5 millimolar inorganic pyrophosphate (PPi) plus 0.2 millimolar adenosine-5′-diphosphate (ADP). Either PPi or ADP added separately inhibited photosynthesis. When chloroplasts were incubated in the dark for 2 to 6 minutes, photosynthesis was strongly inhibited by 5 millimolar PPi and this inhibiting was relieved by including adenosine-5′-triphosphate (ATP) or ADP (0.2 to 0.6 millimolar). Chloroplasts from 9-day-old wheat leaves were slightly less sensitive to inhibition by PPi and showed little or no inhibition by ADP.
Chloroplasts isolated from protoplasts and assayed with 0.3 millimolar Pi added before illumination have an induction time from less than 1 minute up to 16 minutes depending on the time of the assay after isolation and the components of the medium. In order to obtain maximum rates of photosynthesis and minimum induction time, NaHCO3 and chelating agents, EDTA or PPi (+ATP), are required in the chloroplast isolation, resuspension and assay medium. With these inclusions in the isolation and resuspension medium the induction time decreased rapidly during the first 20 to 30 minutes storage of chloroplasts on ice. Requirements for isolating intact and photosynthetically functional chloroplasts from wheat protoplasts are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Bucke C., Walker D. A., Baldry C. W. Some effects of sugars and sugar phosphates on carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):636–641. doi: 10.1042/bj1010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Oxygen evolution by isolated chloroplasts with carbon dioxide as the hydrogen acceptor. A requirement for orthophosphate or pyrophosphate. Biochim Biophys Acta. 1967 May 9;131(3):594–596. doi: 10.1016/0005-2728(67)90022-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Schwenn J. D., Walker D. A. Inorganic pyrophosphatase and photosynthesis by isolated chloroplasts. II. The controlling influence of orthophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):596–604. doi: 10.1016/0005-2728(73)90219-3. [DOI] [PubMed] [Google Scholar]
- Randall P. J., Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973 Sep;52(3):229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Stimulation of carbon dioxide fixation in isolated pea chloroplasts by catalytic amounts of adenine nucleotides. Plant Physiol. 1976 Aug;58(2):156–162. doi: 10.1104/pp.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic Z. S., Walker D. A. Photosynthesis by isolated pea chloroplasts: some effects of adenylates and inorganic pyrophosphate. Plant Physiol. 1977 Mar;59(3):428–432. doi: 10.1104/pp.59.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


