Abstract
Type 2 diabetes mellitus (T2DM) is recognized as a serious public health concern with a considerable impact on human life, long-term health expenditures, and substantial health losses. In this context, the use of dietary polyphenols to prevent and manage T2DM is widely documented. These dietary compounds exert their beneficial effects through several actions, including the protection of pancreatic islet β-cell, the antioxidant capacities of these molecules, their effects on insulin secretion and actions, the regulation of intestinal microbiota, and their contribution to ameliorate diabetic complications, particularly those of vascular origin. In the present review, we intend to highlight these multifaceted actions and the molecular mechanisms by which these plant-derived secondary metabolites exert their beneficial effects on type 2 diabetes patients.
Keywords: Polyphenols, Antioxidants, Oxidative stress, Type 2 diabetes mellitus, Health benefits
Core Tip: At present, a compelling body of evidence suggests that dietary polyphenols may represent an important alternative source to the management of type 2 diabetes mellitus due to their multifaceted actions on glucose homeostasis as well as in attenuating many diabetes complications raised because of the hyperglycemic condition. Additionally, new data derived from either clinical trials or meta-analyses have started to figure out the usefulness of these bioactive compounds thus providing solid clinical shreds of evidence.
INTRODUCTION
Diabetes mellitus (DM) is a heterogeneous group of chronic metabolic disorders characterized by hyperglycemia resulting from defects of insulin action, insulin secretion, or both[1]. This metabolic disease is a global health issue, which has been increasing from time to time and it is now considered as one of the most important disorders worldwide. According to International Diabetes Federation, 10.5% of adults of the world population are currently living with diabetes and this alarming indicator is predicted to rise to 11.3 % (643 million people) by 2030 and to 12.2 % (783 million) by 2045[2].
Noteworthy, a considerable proportion of the world's burden of diabetes is caused by type 2 DM (T2DM). In this regard, T2DM is recognized as a serious public health concern with a considerable impact on human life and health expenditures[3]. The onset and progression of T2DM are determined by a complex pathophysiological basis where oxidative stress is a crucial contributor not only involved in the disease development but also to diabetes complications, particularly those associated with both microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular complications (ischemic heart disease, peripheral vascular disease, and cerebrovascular disease[4].
Acute or chronic hyperglycemia upregulates reactive oxygen species (ROS) production in the mitochondrial electron transfer chain. This excessive production of superoxide mediates the downregulation of glyceraldehyde-3-phosphate dehydrogenase levels, which in turn activates the major pro-oxidative pathways involved in the pathogenesis of diabetes complications, such as the activation of protein kinase C, the polyol and hexosamine pathways, the formation of advanced glycation end products productions (AGEs), as well as the increased expression of the receptor for AGEs[5-7]. On the other hand, antioxidant mechanisms are diminished in diabetic patients, which may further augment oxidative stress[8-10].
During the last few years, compelling shreds of evidence have shed light on the usefulness of dietary antioxidants as an alternative option in the treatment of T2DM, considering both the adverse effects conferred by conventional pharmacological treatments as well as the enormous economic burden that lifelong treatments place on patients[11].
In this regard, dietary polyphenols have emerged as an option to manage T2DM[12]. These compounds are one of the most abundant secondary plant metabolites, which are grouped into four major families, flavonoids, ligands, stilbenes, and phenolic acids, and are widely found in fruits, vegetables, nuts, cereals, and in many beverages such as tea, coffee, and red wines. These bioactive phytochemicals can reach and act at several cellular compartment levels including cellular membranes by binding to the bilayer interface or by interacting with the hydrophobic fatty acid tails[13].
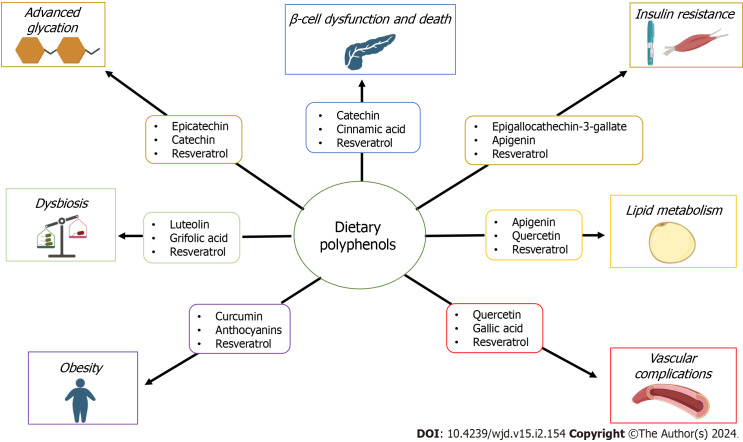
A growing body of experimental and clinical evidence supports the protective role of these compounds on several human diseases through their antioxidant activity and diverse molecular mechanisms[14-18] (Figure 1). This review aims to highlight the roles of this large and heterogeneous family of secondary metabolites of plants containing phenol rings, on pancreatic islet β-cell functioning and promotion of insulin production and signaling, protection against micro-and microvascular complications, protection against the progression of T2DM-associated obesity, management of dyslipidemia and gut microbiome dysbiosis. In addition, the capacity of polyphenols to reduce both the formation of advanced glycation products and their pathologic consequences is also addressed.
Figure 1.
Some polyphenols for which there is documented information about their beneficial properties in the management of the main alterations observed in type 2 diabetes mellitus.
LITERATURE SEARCH
The literature search was conducted using Medline/PubMed, Embase, Cochrane, and RCA, databases. Search terms included “type-2 diabetes mellitus”, “prediabetes”, “polyphenols”, “natural antioxidants”, “oxidative stress” and “abnormal glucose homeostasis”. Articles published between January 2013 to March 2023 and additional publications were retrieved by snowballing. Exclusion criteria included T1DM (autoimmune β-cell destruction), gestational DM, pancreatogenic diabetes, drug-induced diabetes, and the monogenic diabetes syndromes.
Β-CELL DYSFUNCTION AND DEATH
Currently, both clinical and experimental data support that during the development of T2DM, there is not only a progressive deterioration in β-cell functioning but also a marked reduction of the β-cell mass in the pancreatic islets of Langerhans[19-21]. Many factors such as the glucotoxicity associated with the hyperglycemic state, the oxidative and endoplasmic reticulum stresses, as well as the lipotoxicity due to chronic exposure to saturated free fatty acids, are crucial elements in decreased β-cell functioning and, eventually in β cell death through apoptosis[19,22,23].
Hyperglycemia is a crucial factor in the onset of oxidative stress in T2DM and it even correlates with the progression of disease[24]. Additionally, β-cells are very susceptible to oxidative damage, because of their low antioxidant capacity[25,26], and consequently, oxidative stress is a very important contributor to the impairment of β-cell functioning[23,27,28]. Furthermore, oxidative stress mediates the permeabilization of mitochondrial membranes, and consequently the release of cytochrome C and thus β-cell death by apoptosis[29].
Based on their antioxidant activities polyphenols are major regulators of oxidative stress and consequently the improvement of mitochondrial functions. At present, compelling pieces of evidence support that many metabolic disorders such as type 2 diabetes, are associated with impaired mitochondrial function such as diminished oxidative capacity and antioxidant defense, mainly due to the onset of an oxidative stress condition[30,31].
Oxidative stress condition is established by the imbalance between the production of ROS and antioxidant defense mechanisms, and where the detrimental ROS activities exceed the antioxidant capacities of the cell. Mitochondrial dysfunction is defined by several features including a diminished mitochondrial biogenesis, an altered membrane potential, a decrease in the mitochondrial number as well as by an altered activity of oxidative proteins due to the accumulation of ROS in cells and tissue[32,33].
Polyphenols can not only exert powerful antioxidant actions and thus protect against oxidative stress[34], they have additional capacities to modulate crucial pathways in mitochondrial functionality such as mitochondrial biogenesis, mitochondrial membrane potential, ATP synthesis, intra-mitochondrial oxidative status, and apoptosis cell death[35-38]. Cocoa catechins can also improve insulin secretion by increasing the expression of some genes involved in mitochondrial respiration[39].
Resveratrol is known for its remarkable activities in improving pancreatic β-cell function mainly by its effect on sirtuin 1 (SIRT1), a master regulator for β-cell function[40]. Cinnamic acid derivatives can improve the insulin-secreting capacity of β-cells, by raising the levels of intracellular calcium[41]. Noteworthy, compelling pieces of evidence support that the hyperglycemia-associated overexpression of human amylin, also known as islet amyloid polypeptide, can form aggregates to favor amylin fibril formation, and these fibrils evoke the activation of caspases cascade, and thus leading to β-cell death by apoptosis[42,43]. Several polyphenols such as rosmarinic acid, ferulic acid, epigallocatechin gallate, and resveratrol, among many others, can interfere with the formation of fibrillar structures and thus avoid β-cell death[44,45].
INSULIN RESISTANCE
Insulin receptor (IR) is a tyrosine kinase receptor, which is autophosphorylated upon insulin binding and it is expressed in all tissues. The major responders to IR engagement by insulin are the liver, skeletal muscle, and adipose tissue[46]. Upon insulin binding complex signaling is activated including several substrates such as insulin or insulin-like growth factor-1, IR, IR substrate (IRS)-1, and phosphatidylinositol-3 kinase (PI3K)/Akt or ERK kinases. The phosphorylation of IRS1 can recruit PI3K rendering Akt phosphorylated, which in turn can regulate crucial events such as the translocation of glucose transporter-4 (GLUT4) to the cell surface, promoting glycogen synthesis through inhibition of glycogen synthase kinase 3 activity, the induction of protein synthesis via activation of mammalian target of rapamycin and the inhibition of Forkhead transcription factors of the O class (FoxO) transcription factors[47,48].
The inactivation of Akt and activation of FoxO1, through the suppression of IRS1 and IRS2 in different organs following hyperinsulinemia, over-nutrition, and inflammation, represent crucial mechanisms for insulin resistance in humans[49,50]. Compelling shreds of evidence support that oxidative stress is an important contributor to insulin resistance in T2DM[51], and that the overproduction of mitochondrial H2O2[52,53], and the overactivation of NAPDPH oxidase, via angiotensin II/AT1 receptor can mediate skeletal muscle insulin resistance[54-56].
ROS are known to actively participate in several crucial physiological processes at the cellular level such as differentiation, cellular signaling, and phosphorylation/dephosphorylation events among many others[57]. The existence of various endogenous antioxidant systems is responsible for maintaining ROS at the low levels required to contribute to cellular homeostasis[58]. However, the hyperglycemia condition, which is a hallmark of T2DM, is crucial in the acquisition of a dysfunctional state of these antioxidant systems, thus favoring the onset of the oxidative stress condition[59,60]. Thus, this condition is a crucial element in the multifactorial etiology of insulin resistance. Oxidative stress impairs β-cell function, which markedly reduces not only insulin production but also its secretion into the circulation. Additionally, oxidative stress can reduce GLUT-4 gene expression and translocation to the membrane[61-63].
The c Jun-N-terminal kinases (JNKs) is major signal transducer driving the physio-logical response to several cellular stressors, including oxidative stress. Epigallocatechin gallate, the major green tea catechin can protect both the IR and IRS proteins from phosphorylation by JNKs, a crucial event in the onset of insulin resistance[63], as well as by reducing the expression of the negative regulator of IR protein tyrosine phosphatase 1B (PTP1B)[64].
Resveratrol, which is one of the main polyphenolic compounds of red wines, peanuts, and apples, is a potent activator of SIRT1, which is a potent intracellular inhibitor of oxidative stress, and thus attenuates insulin resistance and improves insulin signaling in the skeletal muscle cells[65,66]. Additionally, some polyphenols can also stimulate glucose uptake in both skeletal muscle and adipocytes by translocating GLUT4 to the plasma membrane through an adenosine monophosphate (AMP)-activated protein kinase (AMPK)-dependent pathway[67].
PTP1B is an intracellular enzyme responsible for the deactivation of the IR, resulting in insulin resistance in various tissues[68,69]. Hence, PTP1B has become an important target for controlling insulin resistance and T2DM. In this regard, many polyphenols have inhibitory activity on PTP1B as demonstrated either by screening platforms for detecting the inhibition activity or by Quantitative Structure-Activity Relationship analysis[70,71].
OBESITY
Obesity is the major driving factor of T2DM and it is characterized by chronic low-grade inflammation with permanently increased oxidative stress[72,73]. The onset of a chronic condition of oxidative stress in obesity is supported by different mechanisms implicated in the homeostasis of adipose tissue, which contributes to the development of pathological systemic consequences[74].
On one hand, those associated with increased ROS production such as the adipocytes-associated endoplasmic reticulum stress, a sustained increase of NOX activities, as well as the high level of post-prandial-associated ROS generation, and on the other, the altered antioxidant defenses observed in obese patients[75-78]. In addition to the antioxidant properties of polyphenols, they exert several beneficial effects on obesity far beyond their antioxidant capacity[79], such as the attenuation of obesity-linked inflammation[80-82], the beneficial regulation of several key obesity path-ways such as the modulation of food intake[81], the inhibition of pancreatic lipase[82,83], decreasing lipogenesis by inhibiting both fatty acid synthase activity and the activation of the AMP-AMPK[84,85], and by increasing thermogenesis and mitochondrial biogenesis[86].
Finally, some polyphenols have been reported to mediate the suppression of the conversion of preadipocytes into adipocytes, which can store an excessive lipid load. This polyphenols-mediated suppression of adipocyte differentiation occurs by the regulation of crucial factors such as the CCAAT/enhancer binding protein α, the nuclear receptor peroxisome proliferator-activated receptor γ 1 and 2, (PPARγ1, PPARγ2), and GLUT-4 in mature adipocytes[84,86-88].
DYSBIOSIS
Human gut microbiota is considered a complex microbial ecosystem composed of different microorganisms, including bacteria, archaea, viruses, fungi, and protists, which are involved in the regulation of many physiological processes and numerous diseases[89].
Firmicutes and Bacteroidetes are the main phyla that compose the adult gut flora, regulating the homeostatic production of microbiota-induced metabolites such as butyrate, which have anti-inflammatory and antioxidative properties, and the production of lipopolysaccharide (LPS), which can promote systemic inflammation and insulin resistance through induction of metabolic endotoxemia[90,91].
Growing data raised from both clinical and experimental evidence shows that T2DM patients have an altered gut microbiota, where the Bacterioidetes/Firmicutes ratio of the intestinal flora of diabetic patients significantly differs from non-T2DM adults[92,93]. A crucial consequence of the quantitative change in gut microbiota composition in T2DM patients is the impairment of the expression of gut-microbiota-related metabolites, which have crucial consequences in the metabolic regulation of glucose homeostasis, and insulin sensitivity[93].
Short-chain fatty acids (SCFAs) are considered one of the main microbial metabolites, that have crucial effects on the expression of glucagon-like peptide-1 (GLP-1) and GLP-2 via stimulating G-protein-coupled receptors, thus contributing to improving glucose homeostasis and amplification of insulin sensitivity[94].
Under this dysbiosis condition that affects T2DM patients, structural changes in the intestinal epithelium barrier allow LPS translocation into the bloodstream, resulting in increased plasmatic levels of LPS, which in consequence, activates Toll-like receptor-4 leading to the production of pro-inflammatory mediators, and sustaining low-grade systemic inflammation[95].
This condition known as metabolic endotoxemia induces a significant decrease in bacterial populations which are crucial producers of beneficial gut-derived metabolites such as SCFA, thus supporting the impairment of glucose metabolism and insulin resistance[96,97]. In addition, different studies have demonstrated that specific gut microbiota dysbiosis in mice models of T2DM, induces GLP-1 resistance and consequently, the impairment of GLP1-induced insulin secretion, which is crucial in the acquisition of the insulin resistance condition in diabetic individuals[98].
At present, polyphenols have emerged as novel compounds that could interact with microbiota and exert strong regulatory effects on intestinal bacteria, with subsequent regulation of gut microbiota and its derivate metabolites[99]. These interactions between polyphenols and gut microbiota can positively affect crucial metabolic markers of T2DM, improving systemic inflammation and insulin sensitivity[100,101].
Growing evidence reveals that distinct types of polyphenolic compounds, such as genistein, curcumin, and grifolic acid can increase GLP-1 secretion from L-cells via different mechanisms[102-105]. Besides their effect to directly stimulate GLP-1 secretion, some polyphenols, particularly luteolin, apigenin, and resveratrol may also naturally suppress DPP-IV activity, which potentially increases the half-life of GLP-1, thus stimulating glucose-dependent insulin secretion and regulating glycemia[106,107].
Different studies demonstrate that different doses of oral intake of polyphenols including catechins, and (-)-epigallocatechin-3-gallate, can also favor the increase of different microbial populations of SCFA-producing agents in fecal samples of human patients, thus improving the insulin sensitivity and glucose homeostasis of individuals[108,109].
In addition, other phenolic compounds including chlorogenic and ferulic acid can also act as antidiabetic agents, through significant upregulating of the expression of GLUT4 and PPAR-γ, thus favoring the uptake of 2-deoxyglucose in time- and dose-dependent manner, and improving the pathogenesis of T2DM progression[110-112]. Branched-chain amino acids (BCAAs) include leucine, isoleucine, and valine, which cannot be synthesized de novo by mammalians and consequently, they are acquired either from the diet or gut microbiota. Elevated plasma circulating levels of BCAAs and their ketoacids are associated with insulin resistance in obesity and T2DM[113-117].
Conversely, experimental results have demonstrated that lowering BCAA and branched-chain alpha-keto acid levels is associated with improved insulin sensitivity and reduced fat accumulation in mouse models[118]. Emerging studies have suggested that polyphenol administration may accelerate the catabolism of BCAA, inducing a lowering of circulating BCAA levels, thus improving glucose homeostasis and insulin sensitivity[119].
Additionally, some evidence also supports that intestinal catabolites of polyphenolic compounds by the action of the gut microbiota could act as a strong antiglycative agent[120,121]. In this sense, dietary polyphenolic intake may have a significant positive impact on the generation of glycation products and diabetes-related complications[122,123]. Taken together, those findings suggest that a polyphenols-enriched diet can strongly modulate the dysbiotic changes induced by hyperglycemia, improving the regulation of metabolites that mediate glucose homeostasis and insulin sensitivity in T2DM patients.
VASCULAR COMPLICATIONS
Vascular complications in T2DM are those long-term complications that affect the blood vessel network, and are responsible for most of the morbidity, and required hospitalization in these patients[124]. The vascular complications of diabetes are classified as either microvascular (retinopathy, nephropathy, and neuropathy) or macrovascular, which includes coronary artery, peripheral, and cerebral vascular diseases[125].
At present, a large body of compelling evidence supports that oxidative stress has a key role in the pathogenesis of vascular complications in diabetes[126-128]. As a major regulator of vascular homeostasis, the vascular endothelial cells play crucial roles by controlling vascular tone through a balance between vasodilation and vasoconstriction, fibrinolysis, platelet adhesion and aggregation, leukocyte activation, adhesion, and transmigration, smooth muscle cell proliferation, and modulating the growth of blood vessels[129,130].
The onset of an imbalanced vasodilation and vasoconstriction, elevated ROS, and proinflammatory factors, as well as a reduced nitric oxide (NO) bioavailability, are crucial elements in the onset of the systemic disorder known as endothelial dysfunction[131]. NO is produced in the endothelium by the endothelial NO synthase (eNOS), a Ca2+-calmodulin-dependent enzyme that can convert the L-arginine to NO plus citrulline. By activation of soluble guanylyl cyclase and modulation of cation channels, NO promotes vascular smooth muscle cells relaxation and thus regulates vascular tone. Additionally, NO is a crucial mediator in controlling platelet activation and aggregation[132].
When ROS bioavailability overtakes the antioxidant defenses due to the onset of oxidative stress, superoxide (O2-) rapidly inactivates NO and forms peroxynitrite (ONOO-). It is known that peroxynitrite inactivates prostacyclin synthase thus favoring the deterioration of vascular health due to the vasodilatory, growth-inhibiting, antithrombotic, and antiadhesive effects of prostacyclin. Additionally, peroxynitrite increases the release of prostaglandin H2 and thromboxane A2, which are potent vasoconstrictors, prothrombotic, growth- and adhesion-promoting agents[133-135]. A growing body of data supports the beneficial roles of polyphenols in protecting against endothelial dysfunction induced by oxidative stimuli[136-138].
Of note, some polyphenols, as reported for resveratrol and its derivatives show dual protecting activities, either by the expression of Nox4, a ROS-generating enzyme highly expressed in the endothelium, and by enhancing the expression of two crucial members of the antioxidant defense of the vascular wall, such as glutathione peroxidase 1 and superoxide dismutase 1[139]. Moreover, polyphenols seem to have peroxynitrite-scavenging activity[140]. Furthermore, different reports have demonstrated that some polyphenols such as resveratrol and others derived from strawberry and grape skin and seeds, can promote the phosphorylation of eNOS at Ser1177 by PI3K/Akt pathway, which is essential for NO production[141-143]. In addition, resveratrol is reported to increase both endothelial eNOS mRNA and protein levels[144-146]. This effect seems to be associated with the effects of resveratrol on SIRT1 and FOXO factors[147].
POLYPHENOLS AND ADVANCED GLYCATION
Advanced glycation is one of the major pathways involved in the onset and progression of T2DM complications, particularly those associated with the cardiovascular system[148]. Since the pioneering works of the Vlassara group[149,150], a huge and compelling body of evidence has demonstrated the paramount importance of AGEs in diabetes complications, due to the hyperglycemic condition[151,152].
The formation of AGEs involves the reaction of reducing sugars, such as glucose, with the terminal amino groups of proteins, nucleic acids, or phospholipids to initially form unstable Schiff bases, which evolve towards the formation of more stable compounds called Amadori products, which by a series of complex reaction yield the AGEs. Degradation of both Schiff bases and Amadori products rise to highly reactive short-chain carbonyl compounds, called α-dicarbonyls[153].
These highly reactive compounds can also be formed by hexose autoxidation, as well as by-products of either the glycolytic or polyol pathways and from lipid oxidation. Dicarbolyls can then react non-enzymatically with lysine or arginine residues to produce AGEs[154,155].
The AGEs exert their deleterious effects, either directly by cross-linking of proteins, thus disrupting protein functioning and turn-over[156,157], or indirectly by binding to a signaling receptor for AGE-modified proteins, known as the receptor of advanced glycation end-products (RAGE)[158,159]. Noteworthy, oxidative stress is an important contributor to the formation of endogenous eAGEs, by leading to the increased formation of endogenous reactive aldehydes such as glyoxal, methylglyoxal (MG), and thus favoring the formation of AGEs[160]. Additionally, when AGEs activate RAGE, NADPH oxidase is activated and thus increases ROS levels[161].
At present, compelling evidence derived from experimental and clinical data studies supports the role of different polyphenols as very active inhibitors of the deleterious effects of AGEs, through several mechanisms[162,163]. By their antioxidant activities, polyphenols are potent antiglycation compounds and antiglycation activity strongly correlates with the free radical scavenging activity and antiglycation activity[120], as reported catechins, proanthocyanidins, anthocyanin, stilbenoids, and flavonols[164,165]. Additionally, polyphenols have other properties, which are essential to reduce the formation of AGEs, such as the chelation of transition metal, as reported for chlorogenic and caffeic acids[166,167].
The capacity of trapping dicarbonyl compounds is another crucial activity reported for some polyphenols considering that dicarbonyls are one of the main precursors of AGEs[154], epigallocatechin-3-gallate, resveratrol, catechin, and epicatechin as well as different procyanidins can efficiently trap both glyoxal and MG[162,168,169]. Dicarbonyls are detoxified by the glyoxalase system a highly specific enzyme responsible for the detoxification of dicarbonyl species[170]. Some polyphenols can even stimulate this detoxifying system[171]. Finally, several reports have demonstrated that polyphenols can actively reduce the undesired consequences of the activation of RAGE, either by interfering with receptor signaling as well as by reducing its expression[172-174].
LIPID METABOLISM
T2DM has been widely associated with an increased risk for atherosclerotic cardiovascular disease, which is closely related to raised plasmatic low-density lipoprotein (LDL) levels with important oxidative changes[175], which support diabetic hyperlipidemia and accelerated atherosclerosis, increasing the risk of macrovascular complication and cardiovascular morbidity. Noteworthy, LDL is a highly sensitive molecule to hyperglycemia-induced hyperglycemia damage and modification, making it highly pathogenic and atherogenic[176,177]. Under hyperglycemic conditions, transition metals in the presence of oxygen catalyze the autoxidation of glucose or lipid peroxidation[178]. In addition, excess ROS formation in T2DM patients fuels vascular inflammation and mediates oxidized LDL (ox-LDL) formation, which is considered a hallmark feature of atherosclerotic development due to the crucial induction of atherosclerotic plaque progression and destabilization in T2DM patients[179-181].
Besides the different pathways that conflux in activate NADPH oxidase and subsequent ROS production in T2DM patients, the increased expression of ox-LDL also stimulates NADPH oxidase, thus contributing to increment ROS formation and oxidative stress in T2DM patients[182]. In addition, hyperglycemia-mediated mitochondrial ROS production can also promote the nuclear factor kappa-beta-mediated entry of monocytes in atherosclerotic lesions, fueling the inflammation and progression of unstable plaques, and increasing the risk of macrovascular complication in T2DM patients[183], thus, sustaining a vicious cycle that perpetuating ROS production and ox-LDL formation, contributes to the progression of atherosclerosis unstable plaques on DM patients.
In recent years, polyphenols have been postulated to lower lipids through different mechanisms that imply beneficial effects on cardiovascular diseases of T2DM patients[184]. Based on their antioxidant effects, different studies have shown that many polyphenols including resveratrol, apigenin, and some synthetic polyphenol-like molecules can inhibit NAPDH oxidase activity, thus decreasing vascular oxidation and atherogenesis in nondiabetic apolipoprotein (apo) E–deficient mice[185], as well as improve hyperlipidemia and atherosclerosis in diabetic individuals[186].
Resveratrol based on its antioxidant activities can influence lipid metabolism and is considered an important protective compound against LDL oxidation and atherosclerosis progression[187]. In this sense, the free radical scavenging activity of resveratrol has been investigated, revealing that this polyphenol compound can interact with free radicals to form relatively stable free radicals and non-radicals, resulting in inhibition of lipid peroxidation by Fenton reaction products[188,189], which may decrease the progression of accelerated atherosclerosis through inhibition of oxidation in T2DM patients[190,191].
More recently, it was demonstrated that resveratrol can upregulate eNOS expression by increasing cAMP levels, and decreasing ox-LDL-induced oxidative stress in human endothelial cells, leading to a significant improvement of endothelial dysfunction and atherosclerosis in mice[192]. Similar results have been demonstrated for quercetin, an important flavonoid, which has demonstrated protective effects in diabetic individuals through significantly reversed dyslipidemia and hepatic steatosis in diabetic mice, including lowered liver cholesterol and triglycerides contents[193,194]. Taken together, these findings suggest that dietary polyphenols may be crucial in the regulation of dysregulated lipid metabolism through the modulation of antioxidative mechanisms in T2DM patients.
CONCLUSION
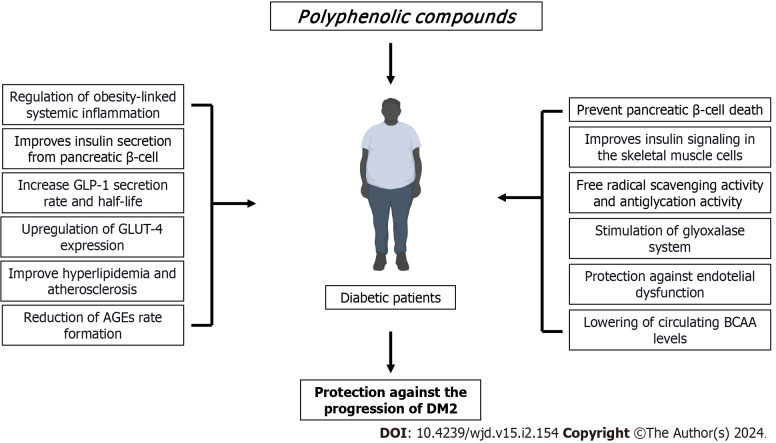
A compelling body of evidence suggests that dietary polyphenols may represent an important alternative to the management of T2DM due to their multifaceted actions on glucose homeostasis as well as by attenuating many diabetes complications raised because of the hyperglycemic condition (Figure 2). Most of the pieces of evidence derived from animals and in vitro studies support these issues. However, new emerging data derived from either clinical trials or meta-analyses have started to figure out the usefulness of these bioactive compounds, and thus providing solid clinical shreds of evidence (Table 1). However, much more research is needed on some topics that may be crucial to explain the current controversial results in some clinical studies. In this regard, a full understanding of the metabolisms and bioavailability, the assessment of dietary intake by measuring urine or blood polyphenol metabolites, duration of exposure, delivery systems that guarantee high stability, as well as more efforts to understand the structure-activity relationship of polyphenols, are crucial elements to be considered in the design and execution of more double-blinded clinical trials.
Figure 2.
Polyphenols have multifaceted actions to support their use in the management of type 2 diabetes mellitus. Due to their positive actions on multiple physiopathological mechanisms which are crucial not only in the onset of type 2 diabetes mellitus (T2DM) by protecting and supporting many functions of β-cells and insulin signaling, but also in those associated with common T2DM complications by improving dyslipidemia profiles, reducing systemic inflammation, dampening the deleterious consequences of the high rate formation of advanced glycation end products production, reducing oxidative stress, as well as by supporting vascular functionality. AGE: Advanced glycation end products production; GLP-1: Glucagon-like peptide-1; GLUT-4: Glucose transporter 4; BCAA: Branched-chain amino acid; DM: Diabetes mellitus.
Table 1.
Clinical trials and meta-analysis studies in the last five years supporting the roles of dietary polyphenols in the management of type 2 diabetes mellitus
|
Type of study
|
Beneficial effects
|
Ref.
|
| Randomized clinical trial | Increased antioxidant capacity and antioxidant gap in T2DM patients | García-Martínez et al[195], 2023 |
| Double-masked, cross-over, dietary intervention trial | Improvement of endothelial function in both healthy individuals and T2DM patients | Bapir et al[196], 2022 |
| Meta-analysis | Improving HbA1c, and insulin levels in T2DM | García-Martínez et al[197], 2021 |
| Randomized, clinical trial | Lowering fasting blood glucose levels in T2DM | Sirvent et al[198], 2022 |
| Systemic review and meta-analysis | Reduction of systolic and diastolic blood pressure and fasting blood glucose levels in T2DM patients | Gu et al[199], 2022 |
| Systematic review and meta-analysis | Reduction of fasting blood glucose and HbA1c levels | Delpino et al[200], 2021 |
| Randomized clinical trial | Improvement of glycemic control by reducing insulin resistance | Mahjabeen et al[201], 2022 |
| Randomized clinical trial | Lowering effects on inflammatory status and oxidative stress biomarkers in diabetic patients | Grabež et al[202], 2022 |
| Randomized clinical trial | Improvement of glycaemia markers | Gómez-Martínez et al[203], 2021 |
| Systematic review and meta-analysis | Improvement of glycemic control and cardiometabolic parameters in patients with T2DM | Abdelhaleem et al[68], 2022 |
| Meta-analysis | Reduction of insulin resistance, HbA1c levels and fasting blood glucose | Delpino and Figueiredo[204], 2022 |
| Meta-analysis | Improvement of glucose control and lowering blood pressure | Nyambuya et al[205], 2020 |
| Randomized clinical trial | Improvement of postprandial dyslipidemia and inflammation following a high-fat dietary challenge in adults with T2D | Davis et al[206], 2020 |
| Meta-analysis | Significant reduction in CRP level in patients with T2D | Hosseini et al[194], 2021 |
| Meta-analysis | Combined effects with anti-diabetic medication to lowering serum glucose levels in individuals with T2D | Raimundo et al[207], 2020 |
| Randomized clinical trial | Improvement of glycemic control and lipid profile | Hoseini et al[208], 2019 |
| Meta-analysis | Lowering fasting blood glucose, HbA1c, and HOMA-IR | Huang et al[209], 2019 |
| Randomized clinical trial | Improvement of lipid profile and lowering serum biomarkers of inflammation | Adibian et al[210], 2019 |
| Randomized clinical trial | Lowering postprandial hyperglycemia and serum biomarkers of inflammation | Schell et al[211], 2019 |
| Randomized clinical trial | Lowering fasting blood glucose and improvement of lipid profile | Mollace et al[212], 2019 |
| Systematic review and meta-analysis | Lowering the risk of T2D | Rienks et al[213], 2018 |
| Randomized clinical trial | Reduction of plasma protein carbonyl content and increasing plasma total antioxidant capacity | Seyyedebrahimi et al[214], 2018 |
T2D: Type 2 diabetes; HbA1c: Glycosylated hemoglobin; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; CRP: C-reactive protein; T2DM: Type 2 diabetes mellitus.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 5, 2023
First decision: December 6, 2023
Article in press: January 19, 2024
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu T, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
Contributor Information
Ileana González, Biomedical Research Laboratories, Faculty of Medicine, Catholic University of Maule, Talca 34600000, Chile.
Cristian Lindner, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile.
Ivan Schneider, Centre of Primary Attention, South Metropolitan Health Service, Santiago 3830000, Chile.
Erik Diaz, Faculty of Medicine, Catholic University of Maule, Talca 3460000, Chile.
Miguel Angel Morales, Molecular and Clinical Pharmacology Program, Institute of Biomedical Sciences, University of Chile, Santiago 8320000, Chile.
Armando Rojas, Biomedical Research Laboratories, Faculty of Medicine, Catholic University of Maule, Talca 34600000, Chile. arojasr@ucm.cl.
References
- 1.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18:525–539. doi: 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black HS. A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash Mishra A, Nigam M, El Rayess Y, Beyrouthy ME, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasupuleti VR, Arigela CS, Gan SH, Salam SKN, Krishnan KT, Rahman NA, Jeffree MS. A Review on Oxidative Stress, Diabetic Complications, and the Roles of Honey Polyphenols. Oxid Med Cell Longev. 2020;2020:8878172. doi: 10.1155/2020/8878172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golovinskaia O, Wang CK. The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci Hum Wellness. 2023;12:986–1007. [Google Scholar]
- 13.Meleleo D, Avato P, Conforti F, Argentieri MP, Messina G, Cibelli G, Mallamaci R. Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects. Membranes (Basel) 2023;13 doi: 10.3390/membranes13060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomás-Barberán FA, Andrés-Lacueva C. Polyphenols and health: current state and progress. J Agric Food Chem. 2012;60:8773–8775. doi: 10.1021/jf300671j. [DOI] [PubMed] [Google Scholar]
- 16.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, Alshabrmi FM, Palai S, Deb PK, Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol. 2022;13:806470. doi: 10.3389/fphar.2022.806470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White MG, Shaw JA, Taylor R. Type 2 Diabetes: The Pathologic Basis of Reversible β-Cell Dysfunction. Diabetes Care. 2016;39:2080–2088. doi: 10.2337/dc16-0619. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Mari A. β-Cell function in type 2 diabetes. Metabolism. 2014;63:1217–1227. doi: 10.1016/j.metabol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Porte D Jr, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50 Suppl 1:S160–S163. doi: 10.2337/diabetes.50.2007.s160. [DOI] [PubMed] [Google Scholar]
- 23.Cerf ME. Beta Cell Physiological Dynamics and Dysfunctional Transitions in Response to Islet Inflammation in Obesity and Diabetes. Metabolites. 2020;10 doi: 10.3390/metabo10110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatti JS, Sehrawat A, Mishra J, Sidhu IS, Navik U, Khullar N, Kumar S, Bhatti GK, Reddy PH. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184:114–134. doi: 10.1016/j.freeradbiomed.2022.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Drews G, Krippeit-Drews P, Düfer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010;460:703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 26.Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14:130–146. doi: 10.4239/wjd.v14.i3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinić S, Arambašić Jovanović J, Uskoković A, Mihailović M, Grdović N, Tolić A, Rajić J, Đorđević M, Vidaković M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front Endocrinol (Lausanne) 2022;13:1006376. doi: 10.3389/fendo.2022.1006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma ZA. The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β -cell failure. Curr Diabetes Rev. 2012;8:69–75. doi: 10.2174/157339912798829232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2014;1840:1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Kowalczyk P, Sulejczak D, Kleczkowska P, Bukowska-Ośko I, Kucia M, Popiel M, Wietrak E, Kramkowski K, Wrzosek K, Kaczyńska K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222413384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Hu F, Liu F. Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases? Cell Signal. 2011;23:1528–1533. doi: 10.1016/j.cellsig.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh YC, Ho CT, Pan MH. The Role of Mitochondria in Phytochemically Mediated Disease Amelioration. J Agric Food Chem. 2023;71:6775–6788. doi: 10.1021/acs.jafc.2c08921. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval-Acuña C, Ferreira J, Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Chodari L, Dilsiz Aytemir M, Vahedi P, Alipour M, Vahed SZ, Khatibi SMH, Ahmadian E, Ardalan M, Eftekhari A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid Med Cell Longev. 2021;2021:4946711. doi: 10.1155/2021/4946711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhagani H, Nasser SA, Dakroub A, El-Yazbi AF, Eid AA, Kobeissy F, Pintus G, Eid AH. The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21144962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowley TJ 4th, Bitner BF, Ray JD, Lathen DR, Smithson AT, Dallon BW, Plowman CJ, Bikman BT, Hansen JM, Dorenkott MR, Goodrich KM, Ye L, O'Keefe SF, Neilson AP, Tessem JS. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J Nutr Biochem. 2017;49:30–41. doi: 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adisakwattana S, Moonsan P, Yibchok-Anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J Agric Food Chem. 2008;56:7838–7844. doi: 10.1021/jf801208t. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem. 2003;278:52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- 43.Kanatsuka A, Kou S, Makino H. IAPP/amylin and β-cell failure: implication of the risk factors of type 2 diabetes. Diabetol Int. 2018;9:143–157. doi: 10.1007/s13340-018-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sequeira IR, Poppitt SD. Unfolding Novel Mechanisms of Polyphenol Flavonoids for Better Glycaemic Control: Targeting Pancreatic Islet Amyloid Polypeptide (IAPP) Nutrients. 2017;9 doi: 10.3390/nu9070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahboob A, Senevirathne DKL, Paul P, Nabi F, Khan RH, Chaari A. An investigation into the potential action of polyphenols against human Islet Amyloid Polypeptide aggregation in type 2 diabetes. Int J Biol Macromol. 2023;225:318–350. doi: 10.1016/j.ijbiomac.2022.11.038. [DOI] [PubMed] [Google Scholar]
- 46.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saltiel AR. Insulin signaling in health and disease. J Clin Invest. 2021;131 doi: 10.1172/JCI142241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47:R1–10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 49.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wondmkun YT. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. 2020;13:3611–3616. doi: 10.2147/DMSO.S275898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40:257–262. doi: 10.1016/j.bj.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 55.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24097898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 59.Choi SW, Benzie IF, Ma SW, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44:1217–1231. doi: 10.1016/j.freeradbiomed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Qasim N, Arif A, Mahmood R. Hyperglycemia enhances the generation of ROS and RNS that impair antioxidant power and cause oxidative damage in human erythrocytes. Biochem Cell Biol. 2023;101:64–76. doi: 10.1139/bcb-2022-0008. [DOI] [PubMed] [Google Scholar]
- 61.Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020:8609213. doi: 10.1155/2020/8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solinas G, Becattini B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol Metab. 2017;6:174–184. doi: 10.1016/j.molmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mi Y, Zhang W, Tian H, Li R, Huang S, Li X, Qi G, Liu X. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018;9:1510–1523. doi: 10.1039/c7fo01554b. [DOI] [PubMed] [Google Scholar]
- 65.Vlavcheski F, Den Hartogh DJ, Giacca A, Tsiani E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients. 2020;12 doi: 10.3390/nu12040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zin CAJCM, Mohamed WMIW, Khan NAK, Ishak WRW. Effects of Fruit and Vegetable Polyphenols on the Glycemic Control and Metabolic Parameters in Type 2 Diabetes Mellitus: A Review. Prev Nutr Food Sci. 2022;27:257–264. doi: 10.3746/pnf.2022.27.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shahwan M, Alhumaydhi F, Ashraf GM, Hasan PMZ, Shamsi A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int J Biol Macromol. 2022;206:567–579. doi: 10.1016/j.ijbiomac.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Abdelhaleem IA, Brakat AM, Adayel HM, Asla MM, Rizk MA, Aboalfetoh AY. The effects of resveratrol on glycemic control and cardiometabolic parameters in patients with T2DM: A systematic review and meta-analysis. Med Clin (Barc) 2022;158:576–585. doi: 10.1016/j.medcli.2021.06.028. [DOI] [PubMed] [Google Scholar]
- 69.Teimouri M, Hosseini H, ArabSadeghabadi Z, Babaei-Khorzoughi R, Gorgani-Firuzjaee S, Meshkani R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J Physiol Biochem. 2022;78:307–322. doi: 10.1007/s13105-021-00860-7. [DOI] [PubMed] [Google Scholar]
- 70.Hussain H, Green IR, Abbas G, Adekenov SM, Hussain W, Ali I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015-2018) Expert Opin Ther Pat. 2019;29:689–702. doi: 10.1080/13543776.2019.1655542. [DOI] [PubMed] [Google Scholar]
- 71.Rath P, Ranjan A, Ghosh A, Chauhan A, Gurnani M, Tuli HS, Habeeballah H, Alkhanani MF, Haque S, Dhama K, Verma NK, Jindal T. Potential Therapeutic Target Protein Tyrosine Phosphatase-1B for Modulation of Insulin Resistance with Polyphenols and Its Quantitative Structure-Activity Relationship. Molecules. 2022;27 doi: 10.3390/molecules27072212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ Framingham Study. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 74.Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. 2008;46:499–505. doi: 10.1515/CCLM.2008.096. [DOI] [PubMed] [Google Scholar]
- 75.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas I, Papademetriou L, Economou M, Stefanadis C. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutr Metab Cardiovasc Dis. 2007;17:590–597. doi: 10.1016/j.numecd.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab Syndr Relat Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aloo SO, Ofosu FK, Kim NH, Kilonzi SM, Oh DH. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants (Basel) 2023;12 doi: 10.3390/antiox12020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zamani-Garmsiri F, Emamgholipour S, Rahmani Fard S, Ghasempour G, Jahangard Ahvazi R, Meshkani R. Polyphenols: Potential anti-inflammatory agents for treatment of metabolic disorders. Phytother Res. 2022;36:415–432. doi: 10.1002/ptr.7329. [DOI] [PubMed] [Google Scholar]
- 81.Badshah H, Ullah I, Kim SE, Kim TH, Lee HY, Kim MO. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides. 2013;47:347–353. doi: 10.1016/j.npep.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Buchholz T, Melzig MF. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015;81:771–783. doi: 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
- 83.Marrelli M, Russo C, Statti G, Argentieri MP, Meleleo D, Mallamaci R, Avato P, Conforti F. Phytochemical and biological characterization of dry outer scales extract from Tropea red onion (Allium cepa L. var. Tropea)-A promising inhibitor of pancreatic lipase. Phytomedicine Plus. 2022;2:100235. [Google Scholar]
- 84.Kim NH, Jegal J, Kim YN, Heo JD, Rho JR, Yang MH, Jeong EJ. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients. 2018;10 doi: 10.3390/nu10111734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocha A, Bolin AP, Cardoso CA, Otton R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr. 2016;55:2231–2244. doi: 10.1007/s00394-015-1033-8. [DOI] [PubMed] [Google Scholar]
- 86.Lee MS, Shin Y, Jung S, Kim Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr Res. 2017;61:1325307. doi: 10.1080/16546628.2017.1325307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valli V, Heilmann K, Danesi F, Bordoni A, Gerhäuser C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid Med Cell Longev. 2018;2018:1617202. doi: 10.1155/2018/1617202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carpéné C, Pejenaute H, Del Moral R, Boulet N, Hijona E, Andrade F, Villanueva-Millán MJ, Aguirre L, Arbones-Mainar JM. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matijašić M, Meštrović T, Paljetak HČ, Perić M, Barešić A, Verbanac D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11 doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 94.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 95.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 96.Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, Serban IL. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM) Nutrients. 2020;12 doi: 10.3390/nu12123719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 98.Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Tercé F, Burcelin R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017;25:1075–1090.e5. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 99.Koudoufio M, Desjardins Y, Feldman F, Spahis S, Delvin E, Levy E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 101.Chen K, Gao Z, Ding Q, Tang C, Zhang H, Zhai T, Xie W, Jin Z, Zhao L, Liu W. Effect of natural polyphenols in Chinese herbal medicine on obesity and diabetes: Interactions among gut microbiota, metabolism, and immunity. Front Nutr. 2022;9:962720. doi: 10.3389/fnut.2022.962720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hara T, Hirasawa A, Sun Q, Sadakane K, Itsubo C, Iga T, Adachi T, Koshimizu TA, Hashimoto T, Asakawa Y, Tsujimoto G. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:247–255. doi: 10.1007/s00210-009-0425-9. [DOI] [PubMed] [Google Scholar]
- 103.Takikawa M, Kurimoto Y, Tsuda T. Curcumin stimulates glucagon-like peptide-1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem Biophys Res Commun. 2013;435:165–170. doi: 10.1016/j.bbrc.2013.04.092. [DOI] [PubMed] [Google Scholar]
- 104.Kato M, Nishikawa S, Ikehata A, Dochi K, Tani T, Takahashi T, Imaizumi A, Tsuda T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600471. [DOI] [PubMed] [Google Scholar]
- 105.Rehman K, Ali MB, Akash MSH. Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses. Biomed Pharmacother. 2019;112:108670. doi: 10.1016/j.biopha.2019.108670. [DOI] [PubMed] [Google Scholar]
- 106.Fan J, Johnson MH, Lila MA, Yousef G, de Mejia EG. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid Based Complement Alternat Med. 2013;2013:479505. doi: 10.1155/2013/479505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Alkhalidy H, Liu D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules. 2021;26 doi: 10.3390/molecules26030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 109.Unno T, Sakuma M, Mitsuhashi S. Effect of dietary supplementation of (-)-epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. J Nutr Sci Vitaminol (Tokyo) 2014;60:213–219. doi: 10.3177/jnsv.60.213. [DOI] [PubMed] [Google Scholar]
- 110.Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16:1119–1126. doi: 10.1016/j.phymed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 111.Prabhakar PK, Doble M. Interaction of phytochemicals with hypoglycemic drugs on glucose uptake in L6 myotubes. Phytomedicine. 2011;18:285–291. doi: 10.1016/j.phymed.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 112.Upadhyay S, Dixit M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid Med Cell Longev. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 114.Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, Hu FB. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2016;39:833–846. doi: 10.2337/dc15-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bragg F, Kartsonaki C, Guo Y, Holmes M, Du H, Yu C, Pei P, Yang L, Jin D, Chen Y, Schmidt D, Avery D, Lv J, Chen J, Clarke R, Hill M, Li L, Millwood I, Chen Z. Circulating Metabolites and the Development of Type 2 Diabetes in Chinese Adults. Diabetes Care. 2022;45:477–480. doi: 10.2337/dc21-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanweert F, Schrauwen P, Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. 2022;12:35. doi: 10.1038/s41387-022-00213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10:350–352. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 118.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, Trimmer JK, Brosnan MJ, Rolph TP, Newgard CB. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5:538–551. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bartova S, Madrid-Gambin F, Fernández L, Carayol J, Meugnier E, Segrestin B, Delage P, Vionnet N, Boizot A, Laville M, Vidal H, Marco S, Hager J, Moco S. Grape polyphenols decrease circulating branched chain amino acids in overfed adults. Front Nutr. 2022;9:998044. doi: 10.3389/fnut.2022.998044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harris CS, Cuerrier A, Lamont E, Haddad PS, Arnason JT, Bennett SA, Johns T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: chemical correlates of in vitro antiglycation activity. Plant Foods Hum Nutr. 2014;69:71–77. doi: 10.1007/s11130-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramkissoon JS, Mahomoodally MF, Ahmed N, Subratty AH. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med. 2013;6:561–569. doi: 10.1016/S1995-7645(13)60097-8. [DOI] [PubMed] [Google Scholar]
- 122.Coelho OGL, Ribeiro PVM, Alfenas RCG. Can grape polyphenols affect glycation markers? A systematic review. Crit Rev Food Sci Nutr. 2023;63:1208–1218. doi: 10.1080/10408398.2021.1962796. [DOI] [PubMed] [Google Scholar]
- 123.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 124.Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, Huang L. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8:152. doi: 10.1038/s41392-023-01400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nesto RW. Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med. 2004;116 Suppl 5A:11S–22S. doi: 10.1016/j.amjmed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 126.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 127.Rojas A, Figueroa H, Re L, Morales MA. Oxidative stress at the vascular wall. Mechanistic and pharmacological aspects. Arch Med Res. 2006;37:436–448. doi: 10.1016/j.arcmed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 128.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 129.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 130.Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boulanger CM. Endothelium. Arterioscler Thromb Vasc Biol. 2016;36:e26–e31. doi: 10.1161/ATVBAHA.116.306940. [DOI] [PubMed] [Google Scholar]
- 132.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 133.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 134.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Förstermann U, Xia N, Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 136.Martins TF, Palomino OM, Álvarez-Cilleros D, Martín MA, Ramos S, Goya L. Cocoa Flavanols Protect Human Endothelial Cells from Oxidative Stress. Plant Foods Hum Nutr. 2020;75:161–168. doi: 10.1007/s11130-020-00807-1. [DOI] [PubMed] [Google Scholar]
- 137.Zhou H, Fu B, Xu B, Mi X, Li G, Ma C, Xie J, Li J, Wang Z. Rosmarinic Acid Alleviates the Endothelial Dysfunction Induced by Hydrogen Peroxide in Rat Aortic Rings via Activation of AMPK. Oxid Med Cell Longev. 2017;2017:7091904. doi: 10.1155/2017/7091904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–1114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 139.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60 Suppl 4:111–116. [PubMed] [Google Scholar]
- 140.Alvarez S, Zaobornyj T, Actis-Goretta L, Fraga CG, Boveris A. Polyphenols and red wine as peroxynitrite scavengers: a chemiluminescent assay. Ann N Y Acad Sci. 2002;957:271–273. doi: 10.1111/j.1749-6632.2002.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 141.Liang XX, Wang RY, Guo YZ, Cheng Z, Lv DY, Luo MH, He A, Luo SX, Xia Y. Phosphorylation of Akt at Thr308 regulates p-eNOS Ser1177 during physiological conditions. FEBS Open Bio. 2021;11:1953–1964. doi: 10.1002/2211-5463.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Madeira SV, Auger C, Anselm E, Chataigneau M, Chataigneau T, Soares de Moura R, Schini-Kerth VB. eNOS activation induced by a polyphenol-rich grape skin extract in porcine coronary arteries. J Vasc Res. 2009;46:406–416. doi: 10.1159/000194271. [DOI] [PubMed] [Google Scholar]
- 143.Edirisinghe I, Burton-Freeman B, Tissa Kappagoda C. Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin Sci (Lond) 2008;114:331–337. doi: 10.1042/CS20070264. [DOI] [PubMed] [Google Scholar]
- 144.Wallerath T, Poleo D, Li H, Förstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. J Am Coll Cardiol. 2003;41:471–478. doi: 10.1016/s0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- 145.Wallerath T, Li H, Gödtel-Ambrust U, Schwarz PM, Förstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. 2005;12:97–104. doi: 10.1016/j.niox.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 146.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 147.Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Förstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 149.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 150.Cerami A, Vlassara H, Brownlee M. Role of advanced glycosylation products in complications of diabetes. Diabetes Care. 1988;11 Suppl 1:73–79. [PubMed] [Google Scholar]
- 151.Khalid M, Petroianu G, Adem A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules. 2022;12 doi: 10.3390/biom12040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peppa M, Uribarri J, Vlassara H. Glucose, Advanced Glycation End Products, and Diabetes Complications: What Is New and What Works. Clin Diabetes. 2003;21:186–187. [Google Scholar]
- 153.Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022;11 doi: 10.3390/cells11081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18050984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, Portero-Otin M, Rojas A, Sampaio GR, Wrobel K, Garay-Sevilla ME. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95–108. doi: 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 157.Rojas A, Añazco C, González I, Araya P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis. 2018;39:515–521. doi: 10.1093/carcin/bgy012. [DOI] [PubMed] [Google Scholar]
- 158.Rojas A, Delgado-López F, González I, Pérez-Castro R, Romero J, Rojas I. The receptor for advanced glycation end-products: a complex signaling scenario for a promiscuous receptor. Cell Signal. 2013;25:609–614. doi: 10.1016/j.cellsig.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 159.Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349–364. doi: 10.1146/annurev-med-041316-085215. [DOI] [PubMed] [Google Scholar]
- 160.Moldogazieva NT, Mokhosoev IM, Mel'nikova TI, Porozov YB, Terentiev AA. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2019;2019:3085756. doi: 10.1155/2019/3085756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 162.Yeh WJ, Hsia SM, Lee WH, Wu CH. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J Food Drug Anal. 2017;25:84–92. doi: 10.1016/j.jfda.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khanam A, Ahmad S, Husain A, Rehman S, Farooqui A, Yusuf MA. Glycation and Antioxidants: Hand in the Glove of Antiglycation and Natural Antioxidants. Curr Protein Pept Sci. 2020;21:899–915. doi: 10.2174/1389203721666200210103304. [DOI] [PubMed] [Google Scholar]
- 164.González I, Morales MA, Rojas A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res Int. 2020;129:108843. doi: 10.1016/j.foodres.2019.108843. [DOI] [PubMed] [Google Scholar]
- 165.Sun C, Zhao C, Guven EC, Paoli P, Simal-Gandara J, Ramkumar KM, Wang S, Buleu F, Pah A, Turi V, Damian G, Dragan S, Tomas M, Khan W, Wang M, Delmas D, Portillo MP, Dar P, Chen L, Xiao J. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Frontiers. 2020;1:18–44. [Google Scholar]
- 166.Gugliucci A, Bastos DH, Schulze J, Souza MF. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia. 2009;80:339–344. doi: 10.1016/j.fitote.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 167.Genaro-Mattos TC, Maurício ÂQ, Rettori D, Alonso A, Hermes-Lima M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation--A Chemical Approach. PLoS One. 2015;10:e0129963. doi: 10.1371/journal.pone.0129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bhuiyan MN, Mitsuhashi S, Sigetomi K, Ubukata M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci Biotechnol Biochem. 2017;81:882–890. doi: 10.1080/09168451.2017.1282805. [DOI] [PubMed] [Google Scholar]
- 169.Wang W, Yagiz Y, Buran TJ, Nunes CDN, Gu L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res Int. 2011;44:2666–2673. [Google Scholar]
- 170.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Frandsen J, Narayanasamy P. Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions. Sci Rep. 2017;7:5126. doi: 10.1038/s41598-017-05287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Buttari B, Profumo E, Facchiano F, Ozturk EI, Segoni L, Saso L, Riganò R. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxid Med Cell Longev. 2013;2013:574029. doi: 10.1155/2013/574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kanlaya R, Thongboonkerd V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr Dev Nutr. 2019;3:nzz101. doi: 10.1093/cdn/nzz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dong L, Li Y, Chen Q, Liu Y, Wu Z, Pan D, Yan N, Liu L. Cereal polyphenols inhibition mechanisms on advanced glycation end products and regulation on type 2 diabetes. Crit Rev Food Sci Nutr. 2023:1–19. doi: 10.1080/10408398.2023.2213768. [DOI] [PubMed] [Google Scholar]
- 175.Jonas RA, Crabtree TR, Jennings RS, Marques H, Katz RJ, Chang HJ, Stuijfzand WJ, van Rosendael AR, Choi JH, Doh JH, Her AY, Koo BK, Nam CW, Park HB, Shin SH, Cole J, Gimelli A, Khan MA, Lu B, Gao Y, Nabi F, Nakazato R, Schoepf UJ, Driessen RS, Bom MJ, Thompson RC, Jang JJ, Ridner M, Rowan C, Avelar E, Généreux P, Knaapen P, de Waard GA, Pontone G, Andreini D, Al-Mallah MH, Guglielmo M, Bax JJ, Earls JP, Min JK, Choi AD, Villines TC. Diabetes, Atherosclerosis, and Stenosis by AI. Diabetes Care. 2023;46:416–424. doi: 10.2337/dc21-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W, Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qiu Q, Zhang F, Zhu W, Wu J, Liang M. Copper in Diabetes Mellitus: a Meta-Analysis and Systematic Review of Plasma and Serum Studies. Biol Trace Elem Res. 2017;177:53–63. doi: 10.1007/s12011-016-0877-y. [DOI] [PubMed] [Google Scholar]
- 179.Banerjee J, Mishra N, Damle G, Dhas Y. Beyond LDL-c: The importance of serum oxidized LDL in predicting risk for type 2 diabetes in the middle-aged Asian Indians. Diabetes Metab Syndr. 2019;13:206–213. doi: 10.1016/j.dsx.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 180.Shah MS, Brownlee M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Islam MA, Amin MN, Siddiqui SA, Hossain MP, Sultana F, Kabir MR. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab Syndr. 2019;13:1643–1647. doi: 10.1016/j.dsx.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 182.Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 183.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feldman F, Koudoufio M, Desjardins Y, Spahis S, Delvin E, Levy E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients. 2021;13 doi: 10.3390/nu13020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 186.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 187.Frémont L, Belguendouz L, Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999;64:2511–2521. doi: 10.1016/s0024-3205(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 188.Aviram M, Fuhrman B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann N Y Acad Sci. 2002;957:146–161. doi: 10.1111/j.1749-6632.2002.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 189.Berrougui H, Grenier G, Loued S, Drouin G, Khalil A. A new insight into resveratrol as an atheroprotective compound: inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis. 2009;207:420–427. doi: 10.1016/j.atherosclerosis.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 190.Regnström J, Nilsson J, Tornvall P, Landou C, Hamsten A. Susceptibility to low-density lipoprotein oxidation and coronary atherosclerosis in man. Lancet. 1992;339:1183–1186. doi: 10.1016/0140-6736(92)91129-v. [DOI] [PubMed] [Google Scholar]
- 191.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 192.Li J, Zhong Z, Yuan J, Chen X, Huang Z, Wu Z. Resveratrol improves endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. J Nutr Biochem. 2019;67:63–71. doi: 10.1016/j.jnutbio.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 193.Yang H, Yang T, Heng C, Zhou Y, Jiang Z, Qian X, Du L, Mao S, Yin X, Lu Q. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. 2019;33:3140–3152. doi: 10.1002/ptr.6486. [DOI] [PubMed] [Google Scholar]
- 194.Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: A review. Phytother Res. 2021;35:5352–5364. doi: 10.1002/ptr.7144. [DOI] [PubMed] [Google Scholar]
- 195.García-Martínez BI, Ruiz-Ramos M, Pedraza-Chaverri J, Santiago-Osorio E, Mendoza-Núñez VM. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24087422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bapir M, Untracht GR, Cooke D, McVey JH, Skene SS, Campagnolo P, Whyte MB, Dikaios N, Rodriguez-Mateos A, Sampson DD, Sampson DM, Heiss C. Cocoa flavanol consumption improves lower extremity endothelial function in healthy individuals and people with type 2 diabetes. Food Funct. 2022;13:10439–10448. doi: 10.1039/d2fo02017c. [DOI] [PubMed] [Google Scholar]
- 197.García-Martínez BI, Ruiz-Ramos M, Pedraza-Chaverri J, Santiago-Osorio E, Mendoza-Núñez VM. Hypoglycemic Effect of Resveratrol: A Systematic Review and Meta-Analysis. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sirvent P, Chavanelle V, Otero YF, Bargetto M, Le Joubioux F, Boisseau N, Maugard T, Cazaubiel M, Pereira B, Guigas B, Hadjadj S, Peltier SL, Marette A, Bard JM. TOTUM-63, a plant-based polyphenol-rich extract, improves glycaemic control in subjects with prediabetes or early stage newly-diagnosed type 2 diabetes in a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2022;24:2331–2340. doi: 10.1111/dom.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gu W, Geng J, Zhao H, Li X, Song G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Clin Pract. 2022;2022:9734738. doi: 10.1155/2022/9734738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Delpino FM, Figueiredo LM, Nunes BP. Effects of melatonin supplementation on diabetes: A systematic review and meta-analysis of randomized clinical trials. Clin Nutr. 2021;40:4595–4605. doi: 10.1016/j.clnu.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 201.Mahjabeen W, Khan DA, Mirza SA. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement Ther Med. 2022;66:102819. doi: 10.1016/j.ctim.2022.102819. [DOI] [PubMed] [Google Scholar]
- 202.Grabež M, Škrbić R, Stojiljković MP, Vučić V, Rudić Grujić V, Jakovljević V, Djuric DM, Suručić R, Šavikin K, Bigović D, Vasiljević N. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev Cardiovasc Med. 2022;23:57. doi: 10.31083/j.rcm2302057. [DOI] [PubMed] [Google Scholar]
- 203.Gómez-Martínez S, Díaz-Prieto LE, Vicente Castro I, Jurado C, Iturmendi N, Martín-Ridaura MC, Calle N, Dueñas M, Picón MJ, Marcos A, Nova E. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients. 2021;14 doi: 10.3390/nu14010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Delpino FM, Figueiredo LM. Resveratrol supplementation and type 2 diabetes: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62:4465–4480. doi: 10.1080/10408398.2021.1875980. [DOI] [PubMed] [Google Scholar]
- 205.Nyambuya TM, Nkambule BB, Mazibuko-Mbeje SE, Mxinwa V, Mokgalaboni K, Orlando P, Silvestri S, Louw J, Tiano L, Dludla PV. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules. 2020;25 doi: 10.3390/molecules25235645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Davis DW, Tallent R, Navalta JW, Salazar A, Lyons TJ, Basu A. Effects of Acute Cocoa Supplementation on Postprandial Apolipoproteins, Lipoprotein Subclasses, and Inflammatory Biomarkers in Adults with Type 2 Diabetes after a High-Fat Meal. Nutrients. 2020;12 doi: 10.3390/nu12071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raimundo AF, Félix F, Andrade R, García-Conesa MT, González-Sarrías A, Gilsa-Lopes J, do Ó D, Raimundo A, Ribeiro R, Rodriguez-Mateos A, Santos CN, Schär M, Silva A, Cruz I, Wang B, Pinto P, Menezes R. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: a meta-analysis of randomized controlled human trials. Eur J Nutr. 2020;59:1329–1343. doi: 10.1007/s00394-020-02189-1. [DOI] [PubMed] [Google Scholar]
- 208.Hoseini A, Namazi G, Farrokhian A, Reiner Ž, Aghadavod E, Bahmani F, Asemi Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019;10:6042–6051. doi: 10.1039/c9fo01075k. [DOI] [PubMed] [Google Scholar]
- 209.Huang J, Qin S, Huang L, Tang Y, Ren H, Hu H. Efficacy and safety of Rhizoma curcumea longae with respect to improving the glucose metabolism of patients at risk for cardiovascular disease: a meta-analysis of randomised controlled trials. J Hum Nutr Diet. 2019;32:591–606. doi: 10.1111/jhn.12648. [DOI] [PubMed] [Google Scholar]
- 210.Adibian M, Hodaei H, Nikpayam O, Sohrab G, Hekmatdoost A, Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2019;33:1374–1383. doi: 10.1002/ptr.6328. [DOI] [PubMed] [Google Scholar]
- 211.Schell J, Betts NM, Lyons TJ, Basu A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann Nutr Metab. 2019;74:165–174. doi: 10.1159/000497226. [DOI] [PubMed] [Google Scholar]
- 212.Mollace V, Scicchitano M, Paone S, Casale F, Calandruccio C, Gliozzi M, Musolino V, Carresi C, Maiuolo J, Nucera S, Riva A, Allegrini P, Ronchi M, Petrangolini G, Bombardelli E. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr Metab Immune Disord Drug Targets. 2019;19:136–143. doi: 10.2174/1871530319666181203151513. [DOI] [PubMed] [Google Scholar]
- 213.Rienks J, Barbaresko J, Oluwagbemigun K, Schmid M, Nöthlings U. Polyphenol exposure and risk of type 2 diabetes: dose-response meta-analyses and systematic review of prospective cohort studies. Am J Clin Nutr. 2018;108:49–61. doi: 10.1093/ajcn/nqy083. [DOI] [PubMed] [Google Scholar]
- 214.Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018;55:341–353. doi: 10.1007/s00592-017-1098-3. [DOI] [PubMed] [Google Scholar]