Abstract
A physical analysis of water movement through elongating soybean (Glycine max L. Merr.) hypocotyls was made to determine why significant water potentials persist in growing tissues even though the external water potentials were zero and transpiration is virtually zero. The analysis was based on a water transport theory modified for growth and assumed that water for growing cells would move through and along the cells in proportion to the conductivity of the various pathways.
Water potentials calculated for individual cells were nearly in local equilibrium with the water potentials of the immediate cell surroundings during growth. However, water potentials calculated for growing tissue were 1.2 to 3.3 bars below the water potential of the vascular supply in those cells farthest from the xylem. Only cells closest to the xylem had water potentials close to that of the vascular supply. Gradients in water potential were steepest close to the xylem because all of the growth-sustaining water had to move through this part of the tissue. Average water potentials calculated for the entire growing region were −0.9 to −2.2 bars depending on the tissue diffusivity.
For comparison with the calculations, average water potentials were measured in elongating soybean hypocotyls using isopiestic thermocouple psychrometers for intact and excised tissue. In plants having virtually no transpiration and growing in Vermiculite with a water potential of −0.1 bar, rapidly growing hypocotyl tissue had water potentials of −1.7 to −2.1 bars when intact and −2.5 bars when excised. In mature, nongrowing hypocotyl tissue, average water potentials were −0.4 bar regardless of whether the tissue was intact or excised.
The close correspondence between predicted and measured water potentials in growing tissue indicates that significant gradients in water potential are required to move growth-associated water through and around cells over macroscopic distances. The presence of such gradients during growth indicates that cells must have different cell wall and/or osmotic properties at different positions in the tissue in order for organized growth to occur. The mathematical development used in this study represents the philosophy that would have to be followed for the application of contemporary growth theory when significant tissue water potential gradients are present.
Full text
PDF
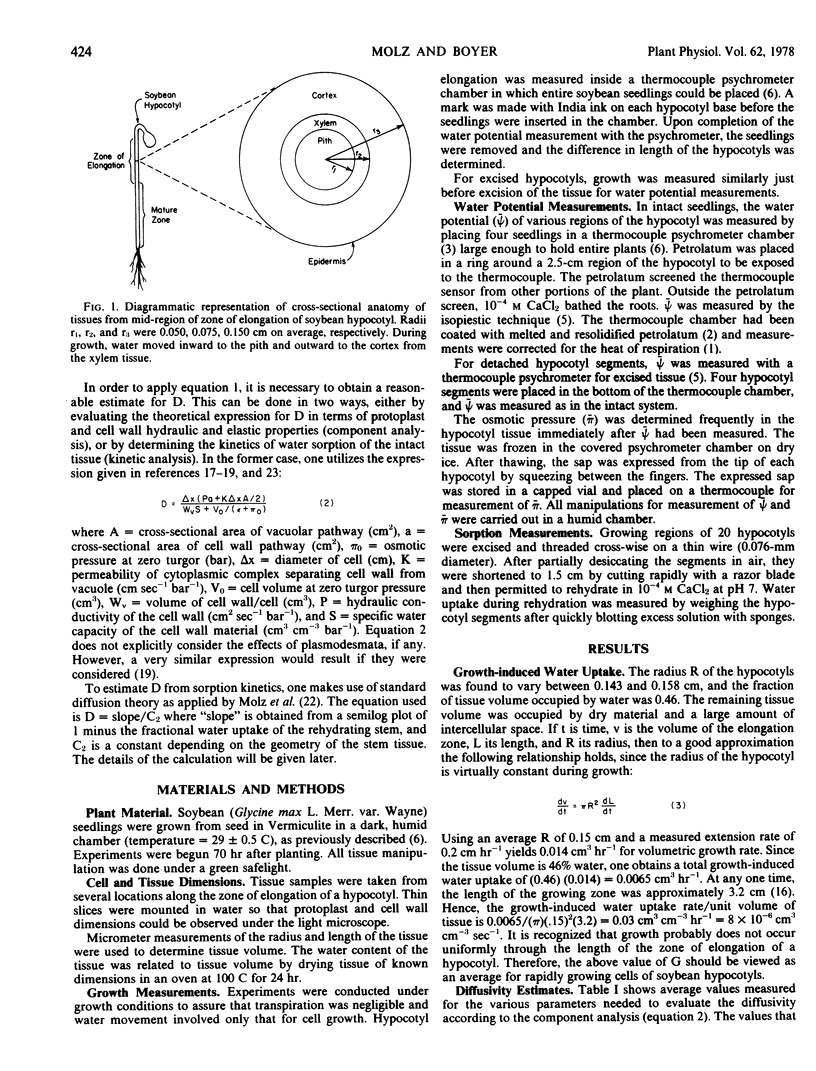
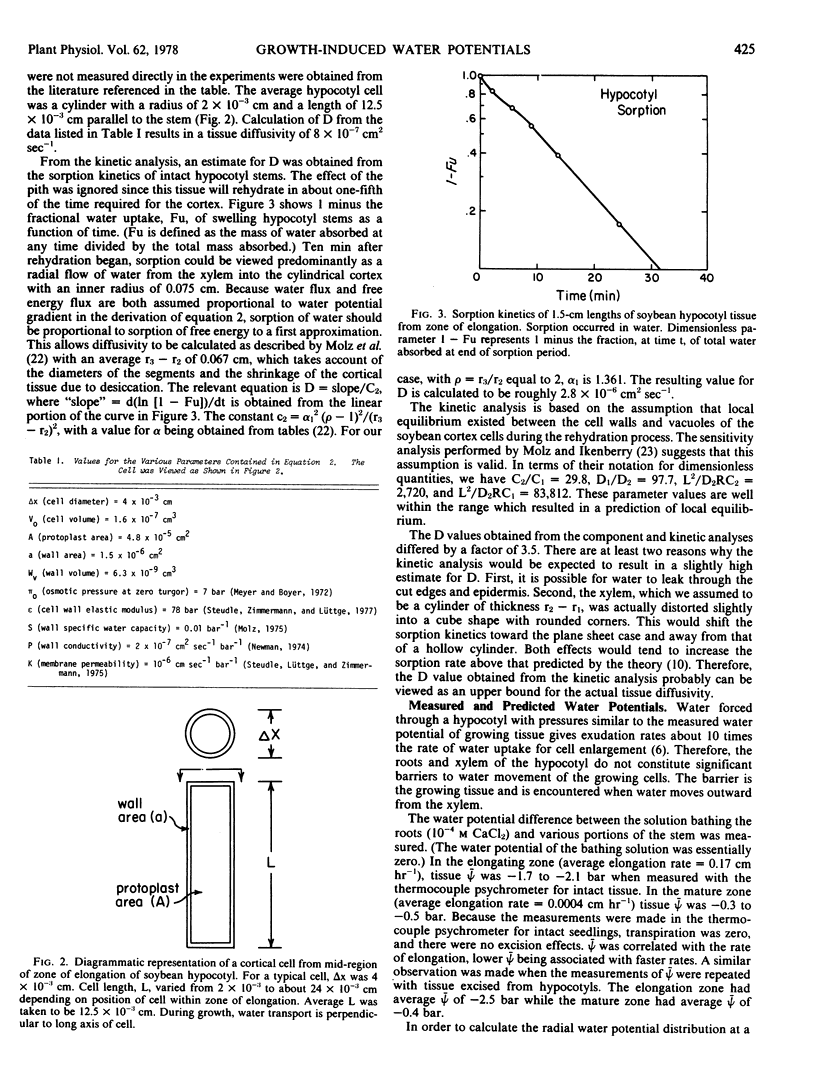




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Erickson R. O., Buggy J. Metabolic and physical control of cell elongation rate: in vivo studies in nitella. Plant Physiol. 1971 Mar;47(3):423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molz F. J. Water transport through plant tissue: the apoplasm and symplasm pathways. J Theor Biol. 1976 Jul 7;59(2):277–292. doi: 10.1016/0022-5193(76)90170-3. [DOI] [PubMed] [Google Scholar]
- Steudle E., Zimmermann U. Effect of turgor pressure and cell size on the wall elasticity of plant cells. Plant Physiol. 1977 Feb;59(2):285–289. doi: 10.1104/pp.59.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


