Abstract
A firm link between endoplasmic reticulum (ER) stress and tumors has been wildly reported. Endoplasmic reticulum oxidoreductase 1 alpha (ERO1α), an ER-resident thiol oxidoreductase, is confirmed to be highly upregulated in various cancer types and associated with a significantly worse prognosis. Of importance, under ER stress, the functional interplay of ERO1α/PDI axis plays a pivotal role to orchestrate proper protein folding and other key processes. Multiple lines of evidence propose ERO1α as an attractive potential target for cancer treatment. However, the unavailability of specific inhibitor for ERO1α, its molecular inter-relatedness with closely related paralog ERO1β and the tightly regulated processes with other members of flavoenzyme family of enzymes, raises several concerns about its clinical translation. Herein, we have provided a detailed description of ERO1α in human cancers and its vulnerability towards the aforementioned concerns. Besides, we have discussed a few key considerations that may improve our understanding about ERO1α in tumors.
Keywords: Cancer, ER stress, ERO1α, PDI, Immune escape, Prognosis, Inhibitor
Introduction
The endoplasmic reticulum (ER) in eukaryotic cells is the largest organelle of interconnected membranes with diverse functions, including protein synthesis, transport and folding, lipid and steroid synthesis, calcium storage and crosstalk with other organelles [1]. The ER is classified as rough ER and smooth ER, depending on the presence of ribosomes. The rough ER is defined by the presence of membrane-bound ribosomes and mainly performs functions associated with the biosynthesis of membrane and secretory proteins, including their proper folding and modification. The smooth ER, where ribosomes are absent, is primarily involved in lipid and steroid synthesis, carbohydrate metabolism, and calcium ion storage [1, 2]. However, there is little evidence that the rough ER is excluded from the functions of the smooth ER. For instance, the rough ER is also involved in calcium homeostasis in the ER [3, 4]. With the assistance of chaperones, nascent unfolded proteins from ribosomes are subjected to the ER quality control mechanisms [5]. Qualified proteins are subsequently packaged into vesicles and trafficked to the Golgi apparatus for further processing, while misfolded proteins are degraded in the cytosol through ER-associated degradation (ERAD). ERAD is a process driven by proteasomes whereby misfolded proteins are retrogradely transferred from the ER to the cytosolic proteasomes through channel proteins in an energy-consuming manner [5, 6].
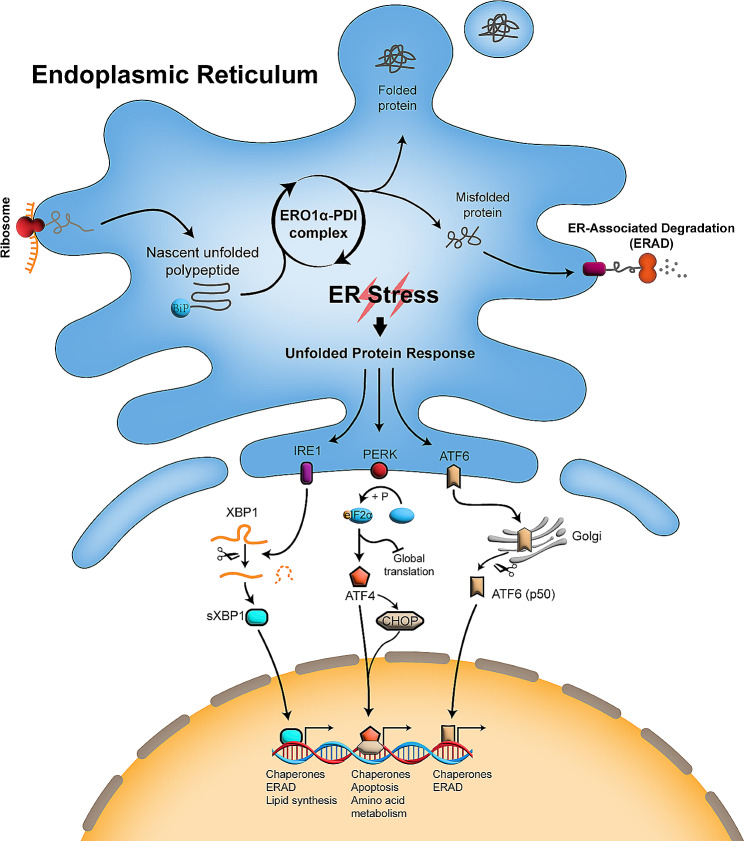
Given the complex and pivotal functions, the ER is strictly and intricately regulated to meet cellular biological activities. Protein homeostasis is a distinctive feature of a properly functioning ER, where protein synthesis is compatible with processing [7]. However, when cells are exposed to stressful conditions such as nutrient shortage, hypoxia, calcium dyshomeostasis, and oxidative stress, the protein-folding capacity of cells is disrupted, leading to the accumulation of unfolded and misfolded proteins in the ER lumen, thereby provoking ER stress (Fig. 1) [8]. In reaction to ER dysfunction, cells initiate an adaptive defense mechanism known as the unfolded protein response (UPR) to reinforce protein folding and degradation capacities, ultimately tackling the ER stress and restoring protein homeostasis [9]. Therefore, the UPR is a protective response by which cells to handle ER stress.
Fig. 1.
The oxidative protein folding in the ER and the unfolded protein response. Nascent polypeptides from ribosomes are oxidatively folded by the ERO1α-PDI complex. Dysfunctional ERO1α-PDI complex results in accumulation of unfolded and misfolded protein, which then arouses the UPR and sends signals to retard translation and facilitate processing and degradation of protein. During the UPR activation, ATF6 is transported to the Golgi apparatus, where it is processed to its activating form ATF6 (p50) and then locates to the nucleus for ERAD-related gene transcription. Activated PERK phosphorylates eIF2α, leading to global translation inhibition but selectively inducing ATF4. AFT4 then enters into the nuclear and activates gene transcription. Of note, facing the overwhelming ER stress, ATF4 can also activate CHOP, a transcription factor then inducing apoptosis through the caspase pathway. Activation of IRE1 induces the splicing of XBP1 and its activating form sXBP1 then goes into the nucleus and initiates the ERAD-related gene transcription. ERO1α, endoplasmic reticulum oxidoreductase 1 alpha; PDI, protein disulfide isomerase; BiP, binding immunoglobulin protein; ERAD, endoplasmic reticulum (ER)-associated degradation; UPR, unfolded protein response; IRE1, inositol requiring enzyme 1; PERK, protein kinase (PKR)-like ER kinase; ATF6, activating transcription factor 6; eIF2α, eukaryotic initiation factor 2α; XBP1, X-box binding protein 1; sXBP1, spliced XBP1; CHOP, C/EBP homologous protein
The UPR is initiated by three transmembrane sensors: inositol requiring enzyme 1 (IRE1), protein kinase (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Fig. 1) [8, 10]. The accumulation of unfolded and misfolded proteins binds more chaperone proteins, such as glucose-regulated protein 78 (GRP78, also known as binding immunoglobin protein (BiP)), resulting in the dissociation of BiP from these three sensors. Upon BiP release, ATF6 is transported to the Golgi apparatus where it is processed into its active form, ATF6 (p50), and subsequently translocated to the nucleus to promote the transcription of chaperone and ERAD-related genes. Dimerization and autophosphorylation occur when PERK and IRE1 decouple from BiP. Activated PERK in turn phosphorylates eukaryotic initiation factor 2α (eIF2α), which leads to transient inhibition of global protein translation to restore abnormal ER but selectively induces activating transcription factor 4 (ATF4). AFT4 then enters the nucleus and activates the transcription of genes related to chaperone, apoptosis and amino acid metabolism. Activation of IRE1 induces the splicing of X-box protein 1(XBP1) mRNA and then the formation of its active form, sXBP1. sXBP1 is translocated to the nucleus to initiate the transcription of genes responsible for chaperone, ERAD and lipid synthesis. Collectively, UPR contributes to restore ER protein homeostasis by retarding general protein translation and increasing the translation of ER resident chaperones and components of the protein degradative machinery to prevent the aggregation of unfolded and misfolded proteins. Moderate ER stress can be dispelled by proper collaboration among the respective UPR branches, therefore maintaining cell survival, however, persistent or severe ER stress eventually induces cell death [11]. Ample evidence supports that unrelievable ER stress leads to cell apoptosis, and two UPR branches, PERK and IRE1, control cell fate under ER stress [10, 12, 13]. In the face of overwhelming ER stress, ATF4, which is selectively activated in the PERK branch, has been shown to induce apoptosis by both inhibiting the anti-apoptotic protein Bcl-2 [14] and promoting the pro-apoptotic proteins BIM [15] and PUMA [16] through the activation of the transcription factor C/EBP homologous protein (CHOP). On the other hand, IRE1 can offset the apoptosis signals from the PERK/ATF4/CHOP branch by degrading apoptosis-dependent components [10]. However, IRE1 has also been revealed to promote apoptosis and autophagy by activating the c-Jun N-terminal kinase (JNK) pathway [17, 18].
ER stress has been documented in most major types of human cancer, especially in solid tumor [19]. Of importance, amounting evidence has shown that ER stress and the subsequent UPR modulate various pro-tumoral properties, including angiogenesis, metabolism, metastasis, and chemoresistance in cancers, while reprogramming the function of immune cells in the tumor microenvironment (TME) [8, 20, 21]. In addition, ER stress has also been identified in cancer stem cells (CSCs) and dormant tumor cells, which are mostly to blame for relapse, contributing to their stemness maintenance, quiescence and chemoresistance [22–24]. Targeting UPR, the adaptive mechanism of ER stress, induces the differentiation of CSCs, increases cell death and sensitivity to chemotherapy in CSCs and dormant tumor cells [23–25]. Overall, adaptation to ER stress confers a survival advantage to tumor cells, but also renders them vulnerable to environmental perturbations. Therefore, targeting ER stress to disturb the adaptive mechanism has emerged as an attractive approach for cancer immunotherapy in recent years [26, 27].
Oxidative protein folding is one of the critical functions of the ER, and both folding efficiency and quality play crucial roles in inducing UPR. Compared to the cytosol, the redox environment in the ER is oxidative, which favors the formation of disulfide bonds. The oxidative environment in the ER is mainly due to the distribution of reduced/oxidized glutathione (GSH), where the glutathione redox potential (EGSH) in the ER is much higher than that of in the cytosol [28, 29]. Endoplasmic reticulum oxidoreductase 1 alpha (ERO1α) has been reported to help maintain the oxidative environment in the ER, as knockout of ERO1α significantly reduced EGSH in the ER [30, 31].
ERO1α (also known as ERO1A or ERO1L) is a flavin adenosine dinucleotide (FAD)-containing ER-resident thiol oxidoreductase responsible for catalyzing disulfide bond formation in nascent polypeptides, working in conjunction with protein disulfide isomerase (PDI) [32]. In recent years, ERO1α has been implicated in various facets of tumor progression, such as tumor growth, angiogenesis, metastasis and chemoresistance, due to its high expression in tumors [33]. Given its function, ERO1α has been reported to promote the oxidative folding of certain tumor-favoring proteins, such as vascular endothelial growth factor (VEGF), programmed cell death ligand-1 (PD-L1), and matrix degrading enzymes (MMPs). In this review, we will delineate the profile of ERO1α in the context of tumors, focusing on four key areas: (1) the structure and function of ERO1α, (2) the expression and regulation of ERO1α, (3) the impact of ERO1α, and (4) targeting ERO1α in tumors.
Distribution, structure and function of ERO1α
ERO1α in mammals was first reported and characterized in 2000, sharing extensive homology with the Saccharomyes cerevisiae ERO1 gene and involved in oxidative protein folding in the ER [34]. In mammals, there are two ERO1 isoforms, ERO1α and ERO1β [34, 35]. ERO1α is expressed ubiquitously in all cell types as its crucial role in oxidative protein folding, whereas ERO1β is selectively expressed in pancreatic and stomach cells, indicating its significance in insulin and glucose metabolism [36]. Of note, in addition to oxidative protein folding, ERO1α is also implicated in various biochemical pathways, such as calcium release and regulation of nicotinamide adenine dinucleotide phosphate oxidase (NOX) activity. ERO1α has been reported to trigger calcium release from the ER to the cytosol or mitochondria via regulating the inositol 1,4,5-triphosphate receptor (IP3R)- and ryanodine receptor (RyR)-induced calcium release [37–39]. Furthermore, the released calcium activates the enzyme calcium/calmodulin-dependent protein kinase II (CaMKII), which in turn induces NOX expression [40]. In addition, an interesting finding is that ERO1α knockout in mammals is not as fatal as in yeast, and mice with ERO1α knockout exhibit a mere retardation in disulfide bond formation [41]. Indeed, it has been demonstrated that ERO1α function in oxidative protein folding can be compensated by other redundant oxidoreductases, such as peroxiredoxin 4 (PRDX4), glutathione peroxidase 8 (GPx8), peroxiredoxin IV (PrxIV) and vitamin K epoxide reductase (VKOR) [42–45].
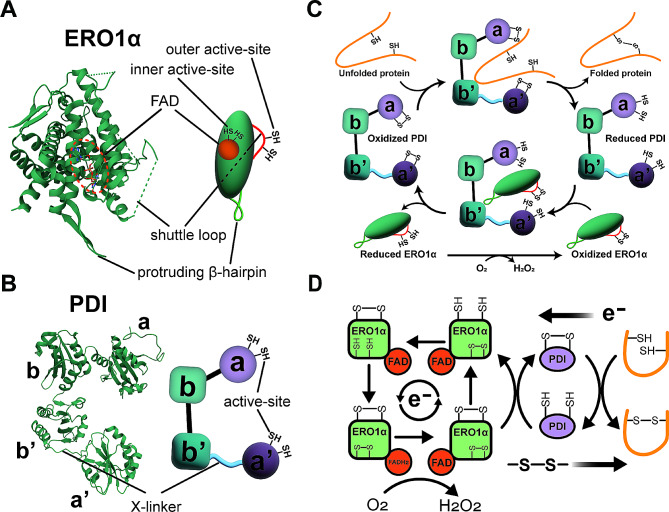
Human ERO1α protein is functionally composed of two regions: a four antiparallel alpha-helices core region containing a binding site for the FAD coenzyme and an adjacent inner active-site, as well as a shuttle loop with an outer active-site (Fig. 2A) [46]. In addition, ERO1α features a protruding β-hairpin responsible for docking with PDI. Human PDI protein consists of two thioredoxin-like redox-active domains (a and a’) and two thioredoxin-like redox-inactive domains (b and b’), and a flexible x-linker between the a’ and b’ domains, in which the b’ domain is the common binding site for polypeptides and ERO1α (Fig. 2B) [47, 48]. The redox state of the PDI a’ domain regulates the affinity of the b’ domain to ERO1α and polypeptides by inducing the spatial rearrangement of the a’ and b’ domains through the conformational change of the x-linker region [49, 50].
Fig. 2.
The crystal structure of human ERO1α and PDI and their working flow. The crystal structure and illustrative diagram of ERO1α (PDB: 3AQH) (A) and PDI (PDB: 4EKZ) (B). (C) The catalytic cycle of the ERO1α-PDI complex. The PDI a’ domain regulates the affinity of PDI to ERO1α and polypeptides by inducing the spatial rearrangement of the a’ and b’ domains through the conformational change of the x-linker region. Oxidized PDI has higher affinity to polypeptides and binds to them via the b’ domain. Oxidizing equivalents are transferred from the active site disulfide bonds of oxidized PDI to the unfolded polypeptides and PDI is therefore reduced. Reduced PDI shows higher affinity to ERO1α. Consequently, polypeptides dissociate from reduced PDI and are displaced by ERO1α. PDI is re-oxidized by ERO1α and then re-enters into a new catalytic cycle. (D) The electron transport chain within the oxidative folding. PDI oxidizes cysteines in nascent polypeptides to form disulfide bonds and accepts electrons from polypeptides, resulting in the reduction of PDI. Electrons from PDI are passed onto ERO1α leading to the reduction of the outer active site of ERO1α and the oxidation of PDI. Oxidized PDI then goes into a new round, while the outer active site of ERO1α shuffles electrons to the inner active site and onto the adjacent FAD coenzyme. FAD is reduced to FADH2 upon accepting electrons. As the ultimate acceptor, molecular oxygen accepts electron from FADH2 with the production of H2O2. ERO1α, endoplasmic reticulum oxidoreductase 1 alpha; PDI, protein disulfide isomerase; FAD, flavin adenosine dinucleotide
ERO1α functions as an exchange center for disulfide bonds and electrons to assist PDI in the de novo disulfide bond formation in nascent polypeptides (Fig. 2C and D). Briefly, disulfide bond formation occurs between the sulfhydryl (-SH) side chains of two cysteine residues of substrate proteins. One sulfhydryl in the substrate attacks a disulfide bond in the active site of PDI, creating a transient mixed-disulfide bond. Another sulfhydryl then initiates a nucleophilic attack on this mixed-disulfide bond, resulting in the formation of an intramolecular disulfide bond within the substrate, leaving PDI in reduced state. Reduced PDI requires re-oxidation to allow another round of disulfide bond formation. ERO1α continuously re-oxidizes PDI and transfers disulfide bonds to PDI by reducing molecular oxygen, making the process sustainable. During the process, electrons flow in the reverse direction with disulfide bonds, and ultimately are accepted by molecular oxygen with the production of H2O2 (Fig. 2D). It has been reported that ERO1α is one of the primarily sources of H2O2, accounting for 25% of the total H2O2 in cells [51, 52]. H2O2 is involved in cell signaling cascades as a secondary messenger and can also be reduced to O2 and H2O through antioxidant enzyme system, such as superoxide dismutase, catalase, and glutathione peroxidase [53, 54].
Notably, in terms of tumors, especially for solid tumors, hypoxia is a prominent characteristic [55]. Hypoxia retards the formation of disulfide bonds, as molecular oxygen acts as a provider of oxidizing equivalents and an acceptor of electrons [56, 57]. However, some hypoxia-induced pro-tumoral proteins, such as VEGF and carbonic anhydrase 9 (CA9), complete disulfide bond formation and traverse through the secretory pathway in anoxic conditions, suggesting that ERO1α can utilize alternative electron acceptors instead of oxygen [56]. In addition, despite the existence of back-up systems of ERO1α, the disulfide bond formation of some pro-tumoral proteins, such as VEGF, PD-L1, is indeed restrained upon ERO1α inhibition, implying the dysfunction of these compensatory mechanisms in tumor cells [58, 59]. Therefore, tumor cells are more dependent on ERO1α than normal cells, thereby providing an excellent opportunity for the use of ERO1α inhibitors. ERO1α inhibition impairs oxidative protein folding in tumor cells, whereas it has a limited effect on normal cells due to the presence of back-up systems [41, 45, 60, 61]. Furthermore, it remains unclear whether the functions of ERO1α beyond disulfide bond formation are substitutable.
ERO1α in tumor landscapes
Expression profile of ERO1α in diverse tumors
All data from different studies were integrated to Table 1. The expression data consist of tumor cell lines (vs. normal cell lines), human tumor tissues (vs. normal or tumor adjacent tissues) and online gene expression databases (Oncomine, GEO, TCGA and GTEx). According to the consolidated result, except prostate cancer, in which ERO1α expression had no significant difference in both tumor tissues and tumor cell lines compared to their normal counterparts, ERO1α expression was up-regulated in bile duct cancer, cervical cancer, lung cancer, pancreatic cancer, breast cancer, liver cancer and gastric cancer. A pan-cancer expression analysis from the Oncomine database revealed up-regulated ERO1α in 10 cancer types while reduced in esophageal cancer, head and neck cancer, and leukemia [64]. Taken together, these data support that ERO1α is highly expressed in the vast majority of tumors, implying a potential role in tumor biology.
Table 1.
Expression profile of ERO1α in tumors
| Cancer Type | Ref. | Data source | Positive group | Normal Control | ERO1A expression |
|---|---|---|---|---|---|
| Bile duct cancer | [62] | Cell line | Tumor cell lines (n=5) | Bile duct epithelial cell line (n=1) | ↑ (Protein-WB) |
| Tissue | Tissue microarray (n=186) | Adjacent normal tissues (n=36) | ↑ (Protein-IHC) | ||
| Cervical cancer | [63] | Tissue | Patient tumor tissues (n=15) | Adjacent normal tissues (n=15) | ↑ (Protein-WB) |
| Tissue | Tissue microarray (n=69) | Normal cervical tissues (n=9) | ↑ (Protein-IHC) | ||
| Lung cancer | [64] | Database | Patient tumor tissues (n=376, Oncomine) | Normal tissues (n=150) | ↑ (mRNA) |
| Database | Patient tumor tissues (n=483, TCGA) | Normal tissues (n=59) | ↑ (mRNA) | ||
| Database | Patient tumor tissues (n=483, TCGA + GTEx) | Normal tissues (n=347) | ↑ (mRNA) | ||
| Database | Patient tumor tissues (n=356, GEO) | Normal biopsies (n=170) | ↑ (mRNA) | ||
| [65] | Tissue | Tissue microarray (n=80) | Adjacent normal tissues (n=80) | ↑ (Protein-IHC) | |
| [66] | Tissue | Patient tumor tissues (n=102) | Adjacent normal tissues (n=102) | ↑ (Protein-IHC) | |
| Cell line | Tumor cell lines (n=4) | Lung epithelial cell line (n=1) | ↑ (Protein-WB) | ||
| [67] | Database | Patient tumor tissues (n=502, TCGA) | Normal tissues (n=49) | ↑ (mRNA) | |
| Pancreatic cancer | [68] | Database | Patient tumor tissues (n=179, TCGA + GTEx) | Normal tissues (n=171) | ↑ (mRNA) |
| Database | Patient tumor tissues (n=96, GEO) | Normal tissues (n=122) | ↑ (mRNA) | ||
| Tissue | Tissue microarray (n=205) | Adjacent normal tissues (n=205) | ↑ (Protein-IHC) | ||
| [69] | Database | Patient tumor tissues (n=145, GEO) | Normal tissues (n=46) | ↑(mRNA) | |
| Tissue | Patient tumor tissues (n=8) | Normal tissues (n=3) | ↑ (mRNA & protein, qPCR & WB) | ||
| Cell line | Tumor cell lines (n=6) | Pancreatic epithelial cell line (n=1) | ↑ (mRNA & protein, qPCR & WB) | ||
| Breast cancer | [70] | Cell line | Tumor cell lines (n=7) | Normal tissue (n=1) | ↑ (mRNA, qPCR) |
| Prostate cancer | [71] | Tissue | Patient tumor tissues (n=12) | Normal tissues (n=6) | NS (protein, WB) |
| Cell line | Tumor cell lines (n=4) | Epithelial prostate cell line (n=1) | NS (protein, WB) | ||
| Liver cancer | [58] | Tissue | Patient tumor tissues (n=114) | Adjacent normal tissues (n=114) | ↑ (mRNA & protein, qPCR & WB & IHC) |
| Database | Patient tumor tissues (n=371, TCGA) | Normal tissues (50) | ↑ (mRNA) | ||
| Cell line | Tumor cell lines (n=5) | Liver cell line (n=1) | ↑ (mRNA & protein, qPCR & WB & IHC) | ||
| Gastric cancer | [72] | Tissue | Patient tumor tissues (n=105) | Adjacent normal tissues (n=105) | ↑ (mRNA & protein, qPCR & WB & IHC) |
Duplicate data in different references was deleted and only one was retained. NS, no significance; GEO, Gene Expression Omnibus; TCGA, The Cancer Genome Atlas Program; GTEx, Genotype-Tissue Expression; WB, Western blot;IHC, Immunohistochemistry; qPCR, quantitative polymerase chain reaction.
Regulation of ERO1α in tumors
In all cases, the interaction with PDI is the fundamental regulation of ERO1α. ERO1α is tightly controlled by intramolecular disulfide bonds and PDI to maintain an equilibrium between reduced and oxidized PDI, ensuring sustainable oxidative protein folding [73–76]. In the UPR process, ERO1α is regulated by CHOP, an activated downstream transcription factor in the PERK branch [77, 78]. Furthermore, the phosphorylation of Ser145 has been reported to greatly enhance ERO1α oxidase activity [31].
In tumor settings, hypoxia is the leading factor for the regulation of ERO1α. Hypoxia is a distinctive feature of the TME, especially in the case of solid tumors [55]. In adapting to hypoxia, tumor cells evolve into an aggressive phenotype, acquiring invasive and metastatic properties, and crafting an immunosuppressive environment [55]. Importantly, hypoxia also induces ER stress and consequently, the UPR [79, 80]. Hypoxia-induced up-regulation of ERO1α has been demonstrated to depend on hypoxia-inducible factor-1 alpha (HIF-1α) [81], which controls the up-regulated transcription of most downstream genes in adaptive responses to hypoxia, and the ablation of HIF-1α resulted in a complete failure to up-regulate ERO1α under hypoxic condition [82]. In an esophageal cancer study, ERO1α was found to be capable of sensing and being post-translationally regulated by the sulfur amino acid precursor homocysteine [83]. The researchers observed that homocysteine induced the active form of ERO1α, suggesting ERO1α might be regulated by antioxidants or redox-active metabolites in epithelial cells. The affinity of ERO1α to amino acid precursor identifies a potential link between diet, antioxidants, and oxidative protein folding in the ER. The transcription factor nuclear factor IB (NFIB) has previously been shown to facilitate tumorigenesis in several cancer types [84–86]. Federica et al. found that NFIB enhanced angiogenesis in breast cancer via the ERO1α/HIF-1α/VEGF pathway, in which ERO1α was identified as a direct transcriptional target of NFIB through chromatin immunoprecipitation (ChIP) assay [87]. MicroRNAs, a category of small non-coding RNAs that target mRNA and inhibit their expression, have also been shown to down-regulate ERO1α. Li et al. demonstrated that microRNA-144-3p inhibited tumorigenesis in oral squamous cell carcinoma by down-regulating the ERO1α/STAT3 pathway [88]. In addition, down-regulated microRNA-582-5p and microRNA-218-5p in cervical cancer and lung cancer, respectively, have been shown to promote tumor progression via targeting ERO1α [89, 90]. Accumulating evidence has shown that epigenetic modifications play a crucial role in the regulation of gene expression [91]. Using bioinformatic analysis, Liu et al. and Shi et al. found that the promoter methylation of ERO1α was markedly reduced in lung cancer, suggesting that hypomethylation of the promoter relieved transcription inhibition, resulting in the overexpression of ERO1α [64, 67]. In a study focusing on liver cell apoptosis, DNA methyltransferase 1 (DNMT1) and G9a (also known as euchromatic histone-lysine N-methyltransferase 2 (EHMT2)) were shown to be responsible for the reduction of ERO1α by mediating the hypermethylation and H3K9me2 modification of the ERO1α promoter, respectively [92].
Secreted proteins from non-tumor cells can also influence ERO1α in tumor cells. Seungeun et al. reported that tumor associated microphage (TAM) derived C-C chemokine ligand (CCL) 2 induced ERO1α mRNA expression in breast epithelial cells, leading to the upregulation of MMP-9 and an invasive phenotype [93].
Overall, various intrinsic and extrinsic cellular regulators show ability to modulate ERO1α. Indeed, as a vital adaptive mechanism for tumor cells responding to environmental perturbations, the up-regulation of ERO1α contributes to improve the plasticity and survival of tumors. It is worth noting that the current regulatory factors may primarily originate from tumor cells themselves. Nevertheless, the regulatory roles of the interplays between tumor and non-tumor cells, in particular immune cells that modulate these interactions, warrant more attention. Likewise, epigenetic regulation of ERO1α modulation also needs consideration.
ERO1α in mediating cancer progression and immune escape
Biological behavior
Tumor cells evolved from normal ones through precancerous status under the influence of carcinogenic factors [102]. During this process, cells undergo a series of biological events, including initiation, promotion and progression, ultimately acquiring an aggressive phenotype. The malignant potential of tumor cells is typically in vitro assessed by proliferation, migration and invasion assays. The effects of ERO1α on tumor biological behavior across various tumor types have been described in Table 2. In nearly all relevant studies, knockdown (KD) or knockout (KO) of ERO1α impaired the proliferation, migration and invasion of tumor cells, while overexpression (OE) resulted in opposite outcomes. However, one study on breast cancer has shown that knockdown of ERO1α had no significant impact on malignant potential compared to the normal cells. Additionally, two studies demonstrated that ERO1α also inhibited the proliferation of tumor cells by arresting the cell cycle [66, 67].
Table 2.
The implications of ERO1α in tumors
| Cancer type | Ref. | Biological behavior | EMT | Angiogenesis | Xenograft in mice | Prognostic significance (Patients with ERO1A +/high) |
|---|---|---|---|---|---|---|
| Breast cancer | [93] | - | - | - | - | RFS↓ |
| [87] | - | - | CD31(KD↓, OE↑), HUVEC(OE↑) | Lung metastasis (KD↓), OS (KD↓) | - | |
| [70] | KD(NS) | - | - | Tumor growth (KD↓, OE↑), OS (KD↑, OE↓) | - | |
| [94] | - | - | CD31 (KD↓, OE↑) | Tumor growth (KD↓, OE↑) | OS↓, # | |
| [95] | - | - | Tumor growth (KD↓), lung metastasis (KD↓) | DFS↓, OS↓, # | ||
| [96] | KO↓ | - | HUVEC (KO↓) | Tumor growth (KO NS), lung metastasis (KO↓) | OS (NS) | |
| Lung cancer | [97] | - | - | - | - | RFS↓ |
| [64] | - | - | - | - | RFS↓, OS↓, DFS↓ | |
| [65] | KD↓, OE↑ | KD↓, OE↑ | - | Tumor metastasis (KD↓, OE↑) | RFS↓, OS↓ | |
| [67] | KD↓ | - | - | - | OS↓ | |
| [66] | KD↓, OE↑ | - | - | Tumor growth (KO↓) | - | |
| [68] | - | - | - | - | DFS↓, # | |
| Bile duct cancer | [63] | KD↓, OE↑ | KD↓, OE↑ | - | - | DFS (NS), OS↓, # |
| Prostate cancer | [71] | KD↓ | - | - | - | - |
| Pancreatic cancer | [99] | KD↓ | - | - | Tumor growth (EN460↓), | OS↓, DFS ↓ |
| liver metastasis (EN460↓), OS (EN460↓) | ||||||
| [69] | KD↓, OE↑ | - | - | Tumor growth (KD↓, OE↑) | - | |
| [68] | KD↓, OE↑ | - | - | Tumor growth (KD↓, OE↑) | OS↓, # | |
| Gastric cancer | [100] | KD↓ | - | - | - | RFS↓, OS↓ |
| Colon cancer | [101] | KD (NS) | KO↓ | - | Tumor growth (KO↓) | - |
| Liver cancer | [58] | KD↓, OE↑ | KD↓, OE↑ | HUVEC (KD↓, OE↑), CD34 (KD↓, OE↑) | Lung metastasis (KD↓, OE↑) | RFS↓, OS↓ |
| Cervical cancer | [63] | KO↓ | KO↓ | - | Tumor growth (KO↓) | OS↓ |
Duplicate data in different references was deleted and only one was retained. EMT, epithelial–mesenchymal transition; NS, no significance; KD, knockdown; KO, knockout; OE, overexpression; EN460, ERO1α inhibitor; HUVEC, human umbilical vein endothelial cells; OS, overall survival; RFS, recurrence-free survival; DFS, disease-free survival; #, Independent prognostic factor; -, none.
Epithelial-mesenchymal transition (EMT)
EMT is an important feature of tumor cells in pre-metastatic niche [103]. During the EMT, epithelial tumor cells can transform into cells with a mesenchymal phenotype, gradually losing the connection to basement membrane, degrading extracellular matrix (ECM), and increasing invasion abilities. EMT is commonly characterized by the decrease of epithelial cell hallmarks and the increase of mesenchymal cell hallmarks. ERO1α has been shown to promote EMT in lung cancer, liver cancer, colorectal cancer, bile duct cancer and cervical cancer (Table 2). Aside from EMT hallmarks, integrins and MMPs are also crucial molecules in tumor migration, in which integrins are responsible for the adhesion of tumor cells to ECM, while MMPs are major contributors to the degradation of ECM [103, 104]. Hence, the collaboration between integrins and MMPs contributes to the metastatic capacity of tumors. ERO1α has been reported to promote the expression of integrin β1 and MMP2/9 by enhancing the oxidative folding of these proteins [69, 71, 93, 101].
Angiogenesis
Angiogenesis is a pivotal factor for tumor growth, metastasis and colonization, as it supplies nutrients and channels for tumor spread [105]. The ERO1α effects on angiogenesis were in vitro and in vivo investigated by human umbilical vein endothelial cells (HUVEC) migration and tube-formation assay and CD31+/CD34+ staining in human or mouse tumor tissues (Table 2). Studies in breast and liver cancer revealed that ERO1α contributed to promoting the migration and tube formation of HUVEC cells [58, 87, 96], as well as increasing blood vessel density in mouse tumor tissues [58, 87, 94]. Moreover, ERO1α levels were also positively correlated with blood vessel density in human tumor tissues [94]. For the mechanism, current studies indicate that VEGF, a potent angiogenic agent, is the common effector by which ERO1α exerts its pro-angiogenesis role. On the one hand, as a protein with disulfide bonds, VEGF is up-regulated by ERO1α through enhancing its oxidative folding [87, 96]. On the other hand, ERO1α indirectly up-regulates VEGF via HIF-1α [87, 94], which is a well-established mediator in VEGF regulation [106]. H2O2 generated by ERO1α during oxidative folding in the ER freely diffuses into the cytoplasm, where it then stabilizes HIF-1α by inhibiting prolyl hydroxylases (PHDs) [107, 108]. In addition, ERO1α has been reported to modulate VEGF via the S1PR1-STAT3 signaling pathway in liver cancer cells [58], and the deficiency of ERO1α in cervical cancer cells impaired the secretion of VEGF due to N-hyper-glycosylation [109].
In vivo tumorigenesis
Xenograft models of knockdown/knockout or overexpressing ERO1α tumor cells into mice were employed to investigate the in vivo tumorigenesis of ERO1α. Studies showed that silencing ERO1α resulted in retarded tumor growth, metastasis, and ameliorated overall survival (OS), while the overexpression of ERO1α produced opposite results (Table 2). In a study focusing on breast cancer, knockout of ERO1α did not significantly impact tumor growth compared to wild-type (WT) cells, however, it impeded lung metastasis [96].
Immunosuppressive tumor microenvironment (iTME)
TME is a complex ecosystem that contains immune cells, stromal cells, vasculature and ECM. However, immune cells in TME, such as TAM and myeloid-derived suppressor cells (MDSCs), often exhibit an immune-suppressive phenotype due to their “education” through the crosstalk with tumor cells [110]. Current findings indicate that ERO1α has broad and profound influences on TME, contributing to shape an immunosuppressive microenvironment. Analyses demonstrated that ERO1α mRNA levels were negatively correlated with the number of cells that define anti-tumor immunity, such as CD8+ T cells, B cells and natural killer (NK) cells, whereas positively correlated with immunosuppressive cells, including cancer-associated fibroblasts (CAFs), MDSCs and TAMs [64, 111].
In terms of tumor cells, which live in a hypoxic microenvironment, the high expression of ERO1α is essential for tumor survival and progression. On the one hand, the high proliferation rate and crosstalk with non-tumor cells require a strong demand of protein synthesis. Up-regulated ERO1α allows for efficient processing of protein oxidative folding while avoiding the accumulation of immature proteins. On the other hand, the potential ability of ERO1α to utilize alternative electron acceptors instead of oxygen contributes to the protein synthesis of tumor cells under hypoxia. Additionally, it has been reported that ERO1α not only directly promotes the expression of PD-L1 on tumor cells by increasing its oxidative folding, but also indirectly through HIF-1α, thereby inducing T-cell dysfunction [59]. Similarly, Liu et al. found that knockout of ERO1α in tumor cells promoted the infiltration of CD8+ T cells and enhanced responses to anti-PD-1 treatment [97]. Furthermore, studies also showed that ERO1α levels were negatively correlated with the sensitivity to immune checkpoint inhibitors (ICIs) [64, 97, 111]. So far, the relationship between ERO1α and other immune checkpoint pathways, such as T-cell immunoglobulin and mucin domain 3 (TIM-3), lymphocyte activation gene 3 (LAG-3) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4), have not been reported.
For myeloid-derived cells, ERO1α has been reported to improve the chemotaxis of MDSCs. Tsutomu et al. reported that the secretion of chemokines granulocyte colony-stimulating factor (G-CSF) and C-X-C motif chemokine ligand (CXCL) 1/2 from tumor cells were increased as ERO1α enhanced their oxidative folding, resulting in the promotion of recruitment and induction of polymorphonuclear (PMN)-MDSCs [70]. Moreover, ERO1α was found to affect the infiltration and differentiation of monocytes. Silencing ERO1α in tumor cells facilitated monocyte infiltration and their differentiation into dendritic cells (DCs) in pancreatic cancer [112]. MDSCs in TME are known for their potent immune-suppressive activity, whereas DCs activate T cells by taking up and presenting tumor antigens [113]. In addition, analyses indicated that ERO1α had effects on macrophage polarization [64, 111]. Macrophages are typically classified into two representative types according to their function and activation: classically activated (M1) and alternatively activated (M2) macrophages [114]. TAMs, the macrophages in TME, are considered to possess an M2-like phenotype and favor tumor progression [115, 116]. Database analysis showed that ERO1α expression in tumor cells was positively correlated with M2 macrophages while negatively correlated with M1 macrophages [64]. Single-cell RNA-sequencing (scRNA-seq) analysis from ERO1αKO/WT mouse model also demonstrated that ERO1α promoted a phenotype transition of TAMs from M1 to M2 type [111]. However, most of these works are observational studies, and there is a need for a more comprehensive dissection of the role of ERO1α in the infiltration, differentiation, and functional execution of immune cells, as well as the underlying mechanisms.
For T cells, it has been confirmed that ERO1α in tumor cells instigated the dysfunction of CD8+ T cells, which was characterized by increased exhausted markers (Lag3, Havcr2 and Odcd1) and decreased ability of proliferation, degranulation and secretion of inflammatory cytokines [111]. Mechanistically, ERO1α in tumor cells was revealed to promote the transmission of ER stress to T cells, triggering the CHOP-dependent apoptosis and resulting in dysfunction of T cells [97, 111]. In addition, deletion of ER stress in T cells restrained in vivo tumor growth and restored the sensitivity to ICIs [97]. However, besides being transmitted from tumor cells, ER stress can also be induced in T cells when tumor antigens are submitted to T cells, resulting in a huge protein synthesis burden. Katie et al. reported that the ER stress in CD8+ T cells up-regulated ERO1α expression by the PERK/ATF4/CHOP branch of the UPR, in which ERO1α was identified as a key downstream effector of ATF4/CHOP to promote global protein synthesis [117]. Nevertheless, the high level of H2O2 resulting from up-regulated ERO1α overloaded the processing capability of cells, ultimately leading to mitochondrial exhaustion of CD8+ T cells [117]. Of interest, tumor cells and T cells show distinct fates when they encounter up-regulated ERO1α and H2O2. Though, tumor cells possess a more potent antioxidant capacity than T cells [53, 118]. Whether their ability to reduce oxidizing agents, such as H2O2, is sufficient to control cell fate (i.e., die or survive) remains in question [53, 119].
Taken together, the up-regulation of ERO1α is a crucial adaptive mechanism by which tumor cells respond to unfavorable microenvironment. ERO1α contributes to the formation of a tumor-supporting immunosuppressive microenvironment by affecting the recruitment and differentiation of immune cells, triggering the dysfunction of T cells, and regulating the PD-1/PD-L1 pathway.
Glucose metabolism
It is widely known that aerobic glycolysis is the main pathway of energy metabolism in tumor cells (i.e., the Warburg effect), as well as the pentose phosphate pathway (PPP) [120, 121]. ERO1α has been demonstrated to promote aerobic glycolysis in pancreatic cancer and cervical cancer [68, 89]. In the study of pancreatic cancer, ERO1α was found to promote tumor growth via enhancing aerobic glycolysis, whereas inhibition of aerobic glycolysis partially abrogated the supportive effects of ERO1α on tumor growth [68]. Mechanistically, H2O2 was identified as the mediator for the effects of ERO1α on aerobic glycolysis. However, it remains unclear whether ERO1α can directly regulate the aerobic glycolysis process, and warrants further investigation. Aerobic glycolysis supplies abundant metabolic intermediates, such as glucose-6-phosphate (G-6-P) for the PPP to produce reduced nicotinamide adenine dinucleotide phosphate (NADPH) and GSH, two essential reductants for H2O2. Therefore, ERO1α confers upon tumor cells an augmented antioxidant capacity relative to normal cells by promoting aerobic glycolysis. In addition to its impacts on aerobic glycolysis and PPP, further studies to explore the regulatory roles of ERO1α in other antioxidant systems are needed.
Chemoresistance
Hypoxia and ER stress have been well-documented as major contributors to the chemotherapy resistance of tumor cells, by multiple mechanisms including apoptosis inhibition, metabolic rewiring, anti-oxidant defences and drugs efflux [79]. Meanwhile, hypoxia-/ER stress-induced ERO1α also shows contribution to the chemoresistance of tumor cells. In gastric cancer, silencing ERO1α rendered tumor cells more sensitive to 5-Flourouracil (5-FU) and paclitaxel, suggesting a chemoresistance role of ERO1α in tumor cells [100]. In a breast cancer study, ERO1α inhibition was reported to blunt the tumor resistance to paclitaxel by down-regulating UPR [122]. Numerous anti-tumor drugs, including 5-FU and paclitaxel, have been shown to act by inducing lethal ER stress in tumor cells [20, 123, 124]. Mechanically, increased susceptibility of tumor cells to ER stress upon ERO1α inhibition may explain the drug-resistant role of ERO1α [111]. Furthermore, ERO1α was also shown to undermine anti-tumor immunity by inducing PD-L1 on tumor cells [64, 97, 111].
Cell survival
ERO1α was demonstrated to rescue tumor cells from death under ER stress or therapeutic interventions. Ablation of ERO1α resulted in hyper-activation of PERK and an imbalance between IRE1a and PERK, leading to tumor cells apoptosis via the CHOP and Caspase-12 pathways [111]. Similarly, in a colon cancer research, deletion of ERO1α was found to promote tumor apoptosis via the miR-101/EZH2/Wnt/β-catenin pathway [125]. In addition to apoptosis, ERO1α was also associated to immunogenic cell death (ICD). In lung cancer, ERO1α deletion triggered lethal ER stress in tumor cells and promoted host anti-tumor immunity via ICD [111]. However, it is unclear whether ERO1α is related to other tumor cell death modes, such as autophagy, ferroptosis, pyroptosis and necroptosis.
Prognostic significance
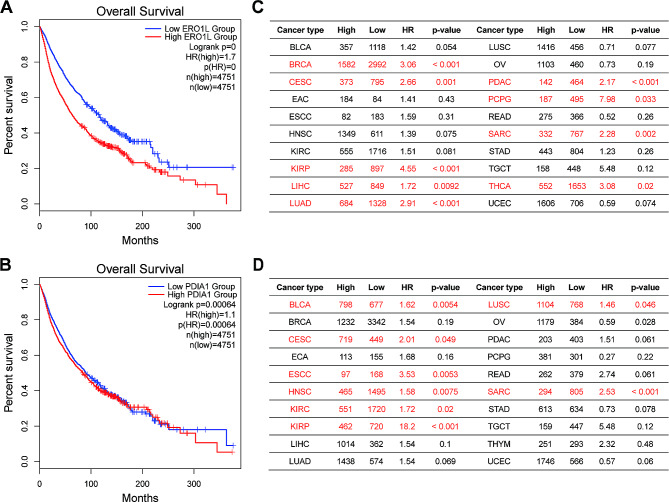
The high expression of ERO1α implies the clinical significance of ERO1α in tumor patients. Data obtained from online databases and clinical follow-up showed that high level of ERO1α in patients was negatively correlated with overall survival (OS), as well as recurrence-free survival (RFS) and disease-free survival (DFS). In addition, results from multivariate Cox regression analysis revealed that high level of ERO1α was also recognized as an independent prognostic factor in breast cancer, lung cancer, pancreatic cancer and bile duct cancer (Table 2). We retrieved prognostic data of ERO1α in tumor patients from public online databases. From the Gene Expression Profiling Interactive Analysis 2 (GEPIA2) database, an integrated result showed that ERO1α expression was negatively associated with patients’ overall survival (OS) with a hazard ratio (HR) of 1.7 (p < 0.0001) across 33 cancer types (Fig. 3A). Specifically, data from the Kaplan-Meier Plotter database showed that ERO1α was identified as an indicator of poor prognosis in 9 out of 20 different cancer types (Fig. 3C). Furthermore, we also analyzed the prognostic value of PDIA1, the canonical member of the PDI family members and the major substrate of ERO1α, in tumor patients and showed similar results to ERO1α (Fig. 3B and D).
Fig. 3.
The pan-cancer prognostic value of ERO1α and PDI. Integrated Kaplan-Meier curves from the GEPIA2 database showing the prognostic effect of ERO1α (A) and PDI (B) expression with patients’ survival across 33 types of cancers. Prognostic analyses from the Kaplan-Meier Plotter database indicating the correlations of ERO1α (C) and PDI (D) with survival in specific cancers. ERO1α, endoplasmic reticulum oxidoreductase 1 alpha; PDIA1: protein disulfide isomerase A1; HR: hazard ratio. BLCA, Bladder urothelial carcinoma; BRCA, Breast invasive carcinoma; CESC, Cervical squamous cell carcinoma and endocervical adenocarcinoma; ECA, Esophageal adenocarcinoma (EAC); ESCC, Esophageal squamous cell carcinoma; HNSC, Head and neck squamous cell carcinoma; KIRC, Kidney renal clear cell carcinoma; KIRP, Kidney renal papillary cell carcinoma; LIHC, Liver hepatocellular carcinoma; LUAD, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; OV, Ovarian serous cystadenocarcinoma; PDAC, Pancreatic ductal adenocarcinoma; PCPG, Pheochromocytoma and paraganglioma; READ, Rectum adenocarcinoma; SARC, Sarcoma; STAD, Stomach adenocarcinoma; TGCT, Testicular germ cell tumors; THCA, Thyroid carcinoma; THYM, Thymoma; UCEC, Uterine corpus endometrial carcinoma
In addition, ERO1α was also included into multi-gene models as a predictor for poor prognosis of tumor patients. Differentially expressed genes (DEGs) between tumor patients and normal individuals were computationally identified and then screened to construct risk score models. In these models, ERO1α was found to be associated with poor prognosis and was proposed for the prognosis prediction in lung cancer [126–128] and pancreatic cancer [129].
Other tumoral-favoring effects of ERO1α
In lung cancer, ERO1α promoted IL-6 receptor (IL-6R) secretion by promoting oxidative folding, and increased soluble IL-6R in turn led to the activation of NF-κB [65]. IL-6 and NF-κB are two well-known effectors to be involved in tumor initiation and progression [130, 131]. Since the availability of public databases has allowed researchers to explore different perspectives of cancer biology, one can recognize that the ERO1α protein is also present in tumor-derived exosomes of bladder, liver and squamous cell carcinomas (retrieved from the ExoCarta database). Thus, providing a new avenue to understand the exosome biology behind ERO1α in tumors.
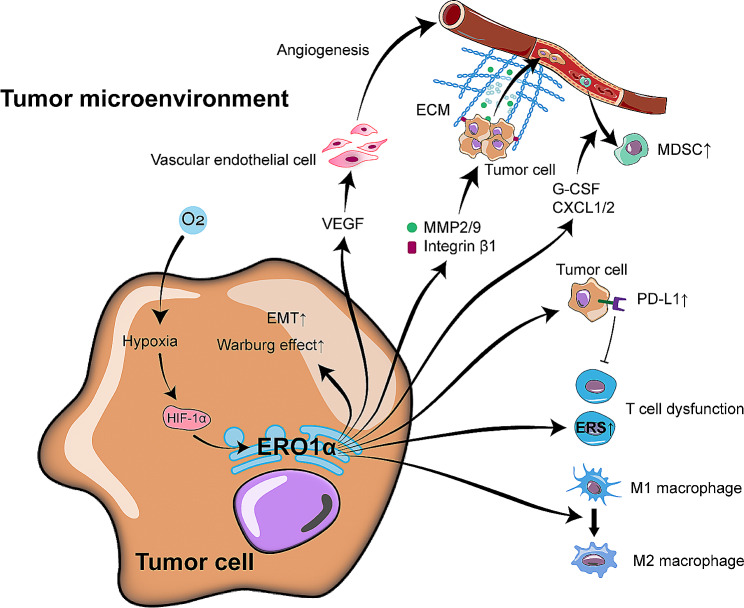
Taken together, ERO1α shows a lot of versatility on both tumor cells and TME (Fig. 4). It not only endows tumor cells with faster growth and aggressive phenotype, but also induces an immunosuppressive TME by improving the angiogenesis, the recruitment and differentiation of immunosuppressive cells, and by causing dysfunction of favorable immune cells. Meanwhile, further investigations to unveil a comprehensive landscape of the effects of ERO1α on immune cells in TME are warranted. The impact of ERO1α on tumor cells primarily hinges on enhanced oxidative protein folding, which can be compensated by other redundant oxidoreductases, as mentioned above [42–44]. However, current in vivo and in vitro experiments did show a significant reduction in the expression of ERO1α target genes, such as VEGF, PD-L1, HIF-1α and MMPs, upon the inhibition of ERO1α, suggesting these compensatory counterparts may be impaired. Therefore, to reveal how tumor cells coordinate ERO1α and its compensatory mechanisms becomes an intriguing avenue of exploration.
Fig. 4.
The regulation and the oncogenic roles of ERO1α on tumors. For the regulation of ERO1α, only hypoxia is shown in this figure. In the TME, hypoxia enhances ERO1α via up-regulating HIF-1α. ERO1α not only endows tumor cells with an aggressive phenotype and promotes aerobic glycolysis of tumor cells, but also contributes to induce an immunosuppressive TME by activating immunosuppressive cells while inhibiting immunocompetent cells. ERO1α, endoplasmic reticulum oxidoreductase 1 alpha; PDI, protein disulfide isomerase; EMT, epithelial-mesenchymal transition; VEGF, vascular endothelial growth factor; MMP, matrix degrading enzyme; ECM, extracellular matrix; MDSC, myeloid-derived suppressor cell; G-CSF, granulocyte colony-stimulating factor; CXCL1/2, C-X-C motif chemokine ligand 1/2. PD-L1, programmed cell death ligand-1
Targeting ERO1α for anti-tumor treatment
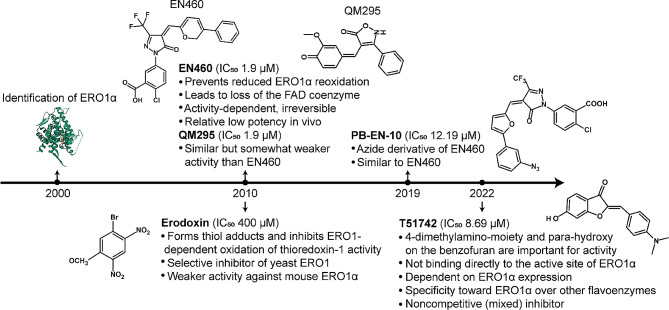
Despite accumulating evidence that ERO1α exerts a profound influence on tumors and would be an attractive target for anti-tumor therapy, few pharmacological inhibitors are available for further validation and none are approved for clinical use. The challenge predominately arises from the highly conserved structure of the FAD cofactor-binding domain across enzymes, suggesting that inhibitors not only recognize the FAD domain in ERO1α, but also other FAD-containing enzymes, such as lysine specific demethylase 1 (LSD1), monoamine oxidases A and B (MAO-A and MAO-B) [132]. To date, several compounds have been reported to target ERO1α in mammals (Fig. 5). EN460 and QM295 stand as the first two identified ERO1α inhibitors through a biochemical high-throughput screen and have been shown to interact with reduced ERO1α and prevent re-oxidation [133]. PB-EN-10 is an azide derivative of EN460 and shows similar effects [132]. Erodoxin, a dinitrobromobenzene compound, acts as a selective inhibitor of yeast ERO1, but has somewhat weaker activity against mouse ERO1α [133, 134]. However, these inhibitors lack selectivity for ERO1α, and indeed, they inhibit other FAD-containing enzymes as well [132]. Recently, Brennan et al. reported a novel ERO1α inhibitor named T151742, a sulfuretin derivative, showing heightened activity (IC50: 8.27µM) compared to EN460 (IC50: 16.46µM) and isozyme specificity for ERO1α as compared to that for ERO1β and no detectable binding to the FAD-containing enzyme LSD-1 [135]. However, further investigations are warranted to determine its in vivo efficacy and safety.
Fig. 5.
Timeline of the ERO1α inhibitors
Given that PDI directly interacts with ERO1α, targeting PDI would also be a viable approach to block oxidative protein folding. In fact, PDI has also been shown to be up-regulated in a variety of cancer types and exhibit pro-tumoral roles [136]. Various chemical inhibitors of PDI have been identified and some of them showed potential anti-tumor effect [137]. However, the presence of over 20 structurally similar PDI homologues in eukaryotes limits the development of specific inhibitors. Considering the inextricable interplay between ERO1α and PDI, a more effective and specific strategy involves developing inhibitors that disrupt the interaction between ERO1α and PDI. Recently, Zhang et al. reported that valine (Val) 101, a hydrophobic residue in the active site-containing loop of ERO1α, is crucial for the recognition of PDI catalytic domain [63]. Mutation of Val101 weaken the activity of ERO1α in oxidative protein folding, and more importantly, impaired tumor progression. This finding not only provides a reliable target site for inhibitor development, but also a paradigm for targeting the ERO1α-PDI interface.
The highly conserved structure of the FAD-binding domain limits the development of ERO1α inhibitors. In recent years, proteolysis-targeting chimera (PROTAC) has been emerged as a novel technology for targeted protein degradation [138, 139]. PROTAC is a bifunctional molecule consists of three domains: a protein of interest (POI) ligand, a E3 ubiquitin ligase ligand, and a linker which covalently interconnects with these two ligands. Upon binding to the target protein, the PROTAC molecule can recruit E3 ubiquitin ligase for protein ubiquitination, which is subjected to proteasome-mediated degradation [140]. Therefore, with respect to ERO1α, the development of PROTAC molecules does not require targeting the active center of ERO1α, but only the ability to specifically recognize ERO1α protein, which would greatly help to avoid off-target effects of the current ERO1α inhibitors. Notably, however, there are also some challenges for PROTAC to be a successful drug development approach [141].
Strategies to disturb UPR or to increase the protein accumulation in the ER are the current approaches in anti-tumor treatment targeting ER stress [26, 27]. For instance, inhibition of the UPR proteins, such as PERK, IRE1 or eIF2α, has been reported to show anti-tumor properties [26]. Tunicamycin, an antibiotic, has been shown to inhibit the N-glycosylation of proteins in the ER, thereby inducing overwhelming ER stress [142]. In addition, proteasome inhibitors, widely used as anti-tumor drugs (especially in hematological tumors), such as bortezomib, have been shown to induce tumor death by inhibiting proteolysis, thereby increasing protein accumulation in the ER and resulting in lethal ER stress [143]. Given that ERO1α is a crucial player in the ER protein homeostasis, synergistic inhibition of ERO1α and other ER stress-inducing targets mentioned above would be a promising approach in anti-tumor treatment. For example, the combined treatment with proteasome inhibitors, which retards the oxidative folding and proteolysis of proteins concurrently, could induce ER stress more efficiently than their single use. Actually, the synergistic effect of this dual inhibition has been in vitro confirmed. ISRIB, a small molecule that inhibits the phosphorylation of eIF2α and removes its inhibition on global protein translation, was found to synergistically interact with the genetic deficiency of ERO1α and to impair breast tumor growth and spread [122]. However, the in vivo availability and utility of the dual inhibition strategy remain unclear, given the current absence of clinically available ERO1α inhibitors. Therefore, the development of highly specific and efficient ERO1α-targeting drugs is a critical objective.
A few key considerations about ERO1α
As mentioned earlier, reports on ERO1α expression as a prognostic indicator in various cancers raise a few important questions: (1) can we target ERO1α without affecting other FAD-containing enzymes, (2) how ERO1α affects the response of cancer immunotherapies, and is there a synergistic effect of the combination treatment with other known ER stress/UPR targeting drugs, (3) ERO1α-PDI interactions have been known for years, and while both are of central importance, it is still difficult to determine which one predominates. Given that both ERO1α and PDI are overexpressed in tumors and their close interplay, can we rule out the possibility that targeting ERO1α might also act by affecting PDI, and how this differs from directly targeting PDI, (4) which cancer immunotherapy approach would benefit from the combined treatment with ERO1α inhibition. Cytokine-induced killer (CIK) cell immunotherapy has been successfully demonstrated to reinforce immune system to fight against tumors due to its attributes such as non-toxic, heterogeneous cell population (T cells, NKT cells and NK cells) and synergistic compatibility with ICIs [144]. Therefore, in our opinion, CIK cell immunotherapy may represent an opportunity in this setting.
Though we mainly focus on cancer, it is worth mentioning that ER-related dysregulation (especially involving ERO1α) has also been found in other diseases such as diabetes [145, 146], neurodegenerative diseases (e.g., Parkinson’s disease [147], Alzheimer’s disease [148, 149], Huntington’s disease [150] and amyotrophic lateral sclerosis [151, 152]), and cardiovascular diseases [153–155]. Therefore, it is important to gain more comprehensive insights into the involvement of disease-specific genetic/epigenetic processes and cellular mechanisms affecting ERO1α in general.
Concluding remarks
ERO1α plays a role for tumor support, and targeting ERO1α holds promise as an antitumor strategy. Besides, the dual characteristics of ERO1α, i.e., flexibility to ER stress in tumors and modulation with immunosuppressive TME, make it a strong candidate for future research on its crucial adaptive mechanisms. Certainly, with the advent of new technologies, the peculiar way of molecular recognition of ERO1α in the cancer landscape is awaited.
Acknowledgements
The author PC would like to acknowledge the support from China Scholarship Council (CSC).
Abbreviations
- ATF4/6
Activating transcription factor4/6
- CAFs
Cancer-associated fibroblasts
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- CA9
Carbonic anhydrase 9
- CCL
C-C chemokine ligand
- CHIP
Chromatin immunoprecipitation
- CHOP
C/EBP homologous protein
- CIK cell
Cytokine-induced killer cell
- CSCs
Cancer stem cells
- CTLA-4
Cytotoxic T-lymphocyte associated protein 4
- CXCL
C-X-C motif chemokine ligand
- DCs
Dendritic cells
- DEGs
Differentially expressed genes
- DFS
Disease-free survival
- eIF2α
Eukaryotic initiation factor 2α
- DNMT1
DNA methyltransferase 1
- ECM
Extracellular matrix (ECM)
- EGSH
Glutathione reduction potential
- EHMT2
euchromatic histone-lysine N-methyltransferase 2
- EMT
Epithelial-mesenchymal transition
- ERO1α
Endoplasmic reticulum oxidoreductase 1 alpha
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- FAD
Flavin adenosine dinucleotide
- G-6-P
Glucose-6-phosphate
- G-CSF
Granulocyte colony-stimulating factor
- GEO
Gene Expression Omnibus
- GEPIA2
Gene Expression Profiling Interactive Analysis 2
- GPx8
Glutathione peroxidase 8
- GSH
Glutathione
- GTEx
Genotype-Tissue Expression
- HIF-1α
Hypoxia-inducible factor-1 alpha
- HR
Hazard ratio
- HUVEC
Human umbilical vein endothelial cells
- ICD
Immunogenic cell death
- ICIs
Immune checkpoint inhibitors
- IP3R
Inositol 1,4,5-triphosphate receptor
- IRE1
Inositol requiring enzyme 1
- JNK
c-Jun N-terminal kinase
- LAG-3
Lymphocyte activation gene 3
- LSD-1
lysine specific demethylase 1
- MAO-A/B
Monoamine oxidases A/B
- MDSCs
Myeloid-derived suppressor cells
- MMPs
Matrix degrading enzymes
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NFIB
Nuclear factor IB
- NK cells
Natural killer cells
- NOX
NADPH oxidase
- OS
Overall survival
- PDHs
Prolyl hydroxylases
- PD-L1
Programmed cell death ligand-1
- PDI
Protein disulfide isomerase
- PERK
Protein kinase R (PKR)-like endoplasmic reticulum kinase
- PMN-MDSCs
Polymorphonuclear-MDSCs
- POI
Protein of interest
- PPP
Pentose phosphate pathway
- PRDX4
Peroxiredoxin 4
- PROTAC
Proteolysis-targeting chimera
- PrxIV
peroxiredoxin IV
- RFS
Recurrence-free survival
- RyR
Ryanodine receptor
- TAM
Tumor associated macrophage
- TCGA
The cancer genome atlas program
- TIM-3
T-cell immunoglobulin and mucin domain 3
- TME
Tumor microenvironment
- UPR
Unfolded protein response
- VEGF
Vascular endothelial growth factor
- VKOR
vitamin K epoxide reductase
- XBP1
X-box protein 1
- 5-FU
5-Flourouracil
Authors contributions
Writing, original draft preparation: PC, AS; Supervision: HW, IGHS-W.
Funding
Open Access funding enabled and organized by Projekt DEAL and the Open Access Publication Fund of the University of Bonn.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73(1):79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9(12):944–57. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111(2):615–24. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton PD, Airey JA, Sutko JL, Beck CF, Mignery GA, Sudhof TC, Deerinck TJ, Ellisman MH. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J Cell Biol. 1991;113(5):1145–57. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopito RR. ER quality control: the cytoplasmic connection. Cell. 1997;88(4):427–30. doi: 10.1016/S0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- 6.Rao B, Wang Q, Yao D, Xia Y, Li W, Xie Y, Li S, Cao M, Shen Y, Qin A, et al. The cryo-EM structure of the human ERAD retrotranslocation complex. Sci Adv. 2023;9(41):eadi5656. doi: 10.1126/sciadv.adi5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraj GG, Hipp MS, Hartl FU. Functional modules of the Proteostasis Network. Cold Spring Harb Perspect Biol 2020, 12(1). [DOI] [PMC free article] [PubMed]
- 8.Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21(2):71–88. doi: 10.1038/s41568-020-00312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65(6):862–94. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TK, Lawrence DA, Lu M, Tan J, Harnoss JM, Marsters SA, Liu P, Sandoval W, Martin SE, Ashkenazi A. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol Cell. 2018;71(4):629–636e625. doi: 10.1016/j.molcel.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother. 2017;66(8):1069–78. doi: 10.1007/s00262-017-2019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam M, Marsters SA, Ashkenazi A, Walter P. Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. Elife 2020, 9. [DOI] [PMC free article] [PubMed]
- 13.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21(4):1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30(50):16938–48. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–75. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–97. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 20.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in Cancer. Cell. 2017;168(4):692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of Cancer. Trends Cancer. 2016;2(5):252–62. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Liang D, Khoonkari M, Avril T, Chevet E, Kruyt FAE. The unfolded protein response as regulator of cancer stemness and differentiation: mechanisms and implications for cancer therapy. Biochem Pharmacol. 2021;192:114737. doi: 10.1016/j.bcp.2021.114737. [DOI] [PubMed] [Google Scholar]
- 23.Calvo V, Zheng W, Adam-Artigues A, Staschke KA, Huang X, Cheung JF, Nobre AR, Fujisawa S, Liu D, Fumagalli M, et al. A PERK-Specific inhibitor blocks metastatic progression by limiting Integrated stress response-dependent survival of quiescent Cancer cells. Clin Cancer Res. 2023;29(24):5155–72. doi: 10.1158/1078-0432.CCR-23-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–35. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66(3):1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21(2):115–40. doi: 10.1038/s41573-021-00320-3. [DOI] [PubMed] [Google Scholar]
- 27.Salvagno C, Mandula JK, Rodriguez PC, Cubillos-Ruiz JR. Decoding endoplasmic reticulum stress signals in cancer cells and antitumor immunity. Trends Cancer. 2022;8(11):930–43. doi: 10.1016/j.trecan.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugalde JM, Aller I, Kudrjasova L, Schmidt RR, Schlosser M, Homagk M, Fuchs P, Lichtenauer S, Schwarzlander M, Muller-Schussele SJ, et al. Endoplasmic reticulum oxidoreductin provides resilience against reductive stress and hypoxic conditions by mediating luminal redox dynamics. Plant Cell. 2022;34(10):4007–27. doi: 10.1093/plcell/koac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojer K, Riemer J. Balancing oxidative protein folding: the influences of reducing pathways on disulfide bond formation. Biochim Biophys Acta. 2014;1844(8):1383–90. doi: 10.1016/j.bbapap.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Wang CC. Oxidative protein folding fidelity and redoxtasis in the endoplasmic reticulum. Trends Biochem Sci. 2023;48(1):40–52. doi: 10.1016/j.tibs.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zhu Q, Wang X, Yu J, Chen X, Wang J, Wang X, Xiao J, Wang CC, Wang L. Secretory kinase Fam20C tunes endoplasmic reticulum redox state via phosphorylation of Ero1alpha. EMBO J 2018, 37(14). [DOI] [PMC free article] [PubMed]
- 32.Inaba K, Masui S, Iida H, Vavassori S, Sitia R, Suzuki M. Crystal structures of human Ero1alpha reveal the mechanisms of regulated and targeted oxidation of PDI. EMBO J. 2010;29(19):3330–43. doi: 10.1038/emboj.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shergalis AG, Hu S, Bankhead A, Neamati N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol Ther. 2020;210:107525. doi: 10.1016/j.pharmthera.2020.107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem. 2000;275(7):4827–33. doi: 10.1074/jbc.275.7.4827. [DOI] [PubMed] [Google Scholar]
- 35.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275(31):23685–92. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 36.Dias-Gunasekara S, Gubbens J, van Lith M, Dunne C, Williams JA, Kataky R, Scoones D, Lapthorn A, Bulleid NJ, Benham AM. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1beta. J Biol Chem. 2005;280(38):33066–75. doi: 10.1074/jbc.M505023200. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186(6):783–92. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spina A, Guidarelli A, Fiorani M, Varone E, Catalani A, Zito E, Cantoni O. Crosstalk between ERO1alpha and ryanodine receptor in arsenite-dependent mitochondrial ROS formation. Biochem Pharmacol. 2022;198:114973. doi: 10.1016/j.bcp.2022.114973. [DOI] [PubMed] [Google Scholar]
- 39.Bassot A, Chen J, Takahashi-Yamashiro K, Yap MC, Gibhardt CS, Le GNT, Hario S, Nasu Y, Moore J, Gutierrez T, et al. The endoplasmic reticulum kinase PERK interacts with the oxidoreductase ERO1 to metabolically adapt mitochondria. Cell Rep. 2023;42(1):111899. doi: 10.1016/j.celrep.2022.111899. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191(6):1113–25. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zito E, Hansen HG, Yeo GS, Fujii J, Ron D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol Cell. 2012;48(1):39–51. doi: 10.1016/j.molcel.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zito E. PRDX4, an endoplasmic reticulum-localized peroxiredoxin at the crossroads between enzymatic oxidative protein folding and nonenzymatic protein oxidation. Antioxid Redox Signal. 2013;18(13):1666–74. doi: 10.1089/ars.2012.4966. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen VD, Saaranen MJ, Karala AR, Lappi AK, Wang L, Raykhel IB, Alanen HI, Salo KE, Wang CC, Ruddock LW. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J Mol Biol. 2011;406(3):503–15. doi: 10.1016/j.jmb.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 44.Kanemura S, Sofia EF, Hirai N, Okumura M, Kadokura H, Inaba K. Characterization of the endoplasmic reticulum-resident peroxidases GPx7 and GPx8 shows the higher oxidative activity of GPx7 and its linkage to oxidative protein folding. J Biol Chem. 2020;295(36):12772–85. doi: 10.1074/jbc.RA120.013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulleid NJ. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb Perspect Biol 2012, 4(11). [DOI] [PMC free article] [PubMed]
- 46.Araki K, Inaba K. Structure, mechanism, and evolution of Ero1 family enzymes. Antioxid Redox Signal. 2012;16(8):790–9. doi: 10.1089/ars.2011.4418. [DOI] [PubMed] [Google Scholar]
- 47.Matsusaki M, Okuda A, Matsuo K, Gekko K, Masuda T, Naruo Y, Hirose A, Kono K, Tsuchi Y, Urade R. Regulation of plant ER oxidoreductin 1 (ERO1) activity for efficient oxidative protein folding. J Biol Chem. 2019;294(49):18820–35. doi: 10.1074/jbc.RA119.010917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masui S, Vavassori S, Fagioli C, Sitia R, Inaba K. Molecular bases of cyclic and specific disulfide interchange between human ERO1alpha protein and protein-disulfide isomerase (PDI) J Biol Chem. 2011;286(18):16261–71. doi: 10.1074/jbc.M111.231357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serve O, Kamiya Y, Maeno A, Nakano M, Murakami C, Sasakawa H, Yamaguchi Y, Harada T, Kurimoto E, Yagi-Utsumi M, et al. Redox-dependent domain rearrangement of protein disulfide isomerase coupled with exposure of its substrate-binding hydrophobic surface. J Mol Biol. 2010;396(2):361–74. doi: 10.1016/j.jmb.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 50.Nakasako M, Maeno A, Kurimoto E, Harada T, Yamaguchi Y, Oka T, Takayama Y, Iwata A, Kato K. Redox-dependent domain rearrangement of protein disulfide isomerase from a thermophilic fungus. Biochemistry. 2010;49(32):6953–62. doi: 10.1021/bi1006089. [DOI] [PubMed] [Google Scholar]
- 51.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164(3):341–6. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9(12):2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 53.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 54.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3(12):1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 55.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levitin F, Lee SCS, Hulme S, Rumantir RA, Wong AS, Meester MR, Koritzinsky M. Oxygen-independent disulfide bond formation in VEGF-A and CA9. J Biol Chem. 2021;296:100505. doi: 10.1016/j.jbc.2021.100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koritzinsky M, Levitin F, van den Beucken T, Rumantir RA, Harding NJ, Chu KC, Boutros PC, Braakman I, Wouters BG. Two phases of disulfide bond formation have differing requirements for oxygen. J Cell Biol. 2013;203(4):615–27. doi: 10.1083/jcb.201307185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S, Yang C, Yu F, Ding W, Hu Y, Cheng F, Zhang F, Guan B, Wang X, Lu L, et al. Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway. Cell Death Dis. 2018;9(11):1105. doi: 10.1038/s41419-018-1134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka T, Kutomi G, Kajiwara T, Kukita K, Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto Y, et al. Cancer-associated oxidoreductase ERO1-α promotes immune escape through up-regulation of PD-L1 in human breast cancer. Oncotarget. 2017;8(15):24706–18. doi: 10.18632/oncotarget.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zito E, Melo EP, Yang Y, Wahlander A, Neubert TA, Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol Cell. 2010;40(5):787–97. doi: 10.1016/j.molcel.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol. 2010;188(6):821–32. doi: 10.1083/jcb.200911086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan W, Wang X, Liu T, Chen L, Han L, Xu J, Jin G, Harada K, Lin Z, Ren X. Expression of endoplasmic reticulum oxidoreductase 1-alpha in cholangiocarcinoma tissues and its effects on the proliferation and migration of cholangiocarcinoma cells. Cancer Manag Res. 2019;11:6727–39. doi: 10.2147/CMAR.S188746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Li T, Zhang L, Shangguan F, Shi G, Wu X, Cui Y, Wang X, Wang X, Liu Y, et al. Targeting the functional interplay between endoplasmic reticulum oxidoreductin-1α and protein disulfide isomerase suppresses the progression of cervical cancer. EBioMedicine. 2019;41:408–19. doi: 10.1016/j.ebiom.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Wang C, Li S, Qu Y, Xue P, Ma Z, Zhang X, Bai H, Wang J. ERO1L is a novel and potential biomarker in lung adenocarcinoma and shapes the Immune-suppressive Tumor Microenvironment. Front Immunol. 2021;12:677169. doi: 10.3389/fimmu.2021.677169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei Y, Zang R, Lu Z, Zhang G, Huang J, Liu C, Wang Z, Mao S, Che Y, Wang X, et al. ERO1L promotes IL6/sIL6R signaling and regulates MUC16 expression to promote CA125 secretion and the metastasis of lung cancer cells. Cell Death Dis. 2020;11(10):853. doi: 10.1038/s41419-020-03067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie J, Liao G, Feng Z, Liu B, Li X, Qiu M. ERO1L promotes the proliferation and metastasis of lung adenocarcinoma via the Wnt2/β-catenin signaling pathway. Mol Carcinog. 2022;61(10):897–909. doi: 10.1002/mc.23441. [DOI] [PubMed] [Google Scholar]
- 67.Shi X, Wu J, Liu Y, Jiang Y, Zhi C, Li J. ERO1L promotes NSCLC development by modulating cell cycle-related molecules. Cell Biol Int. 2020;44(12):2473–84. doi: 10.1002/cbin.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Yang J, Lin C, Liu W, Huo Y, Yang M, Jiang SH, Sun Y, Hua R. Endoplasmic reticulum stress-dependent expression of ERO1L promotes aerobic glycolysis in pancreatic Cancer. Theranostics. 2020;10(18):8400–14. doi: 10.7150/thno.45124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han F, Xu Q, Zhao J, Xiong P, Liu J. ERO1L promotes pancreatic cancer cell progression through activating the Wnt/catenin pathway. J Cell Biochem. 2018;119(11):8996–9005. doi: 10.1002/jcb.27155. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka T, Kajiwara T, Torigoe T, Okamoto Y, Sato N, Tamura Y. Cancer-associated oxidoreductase ERO1-α drives the production of tumor-promoting myeloid-derived suppressor cells via oxidative protein folding. J Immunol. 2015;194(4):2004–10. doi: 10.4049/jimmunol.1402538. [DOI] [PubMed] [Google Scholar]
- 71.Cornelius J, Cavarretta I, Pozzi E, Lavorgna G, Locatelli I, Tempio T, Montorsi F, Mattei A, Sitia R, Salonia A, et al. Endoplasmic reticulum oxidoreductase 1 alpha modulates prostate cancer hallmarks. Transl Androl Urol. 2021;10(3):1110–20. doi: 10.21037/tau-20-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou B, Wang G, Gao S, Chen Y, Jin C, Wang Z, Yang Y, Ma Z, Zhang W, Feng X. Expression of ERO1L in gastric cancer and its association with patient prognosis. Exp Ther Med. 2017;14(3):2298–302. doi: 10.3892/etm.2017.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambers JE, Tavender TJ, Oka OB, Warwood S, Knight D, Bulleid NJ. The reduction potential of the active site disulfides of human protein disulfide isomerase limits oxidation of the enzyme by Ero1alpha. J Biol Chem. 2010;285(38):29200–7. doi: 10.1074/jbc.M110.156596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araki K, Nagata K. Functional in vitro analysis of the ERO1 protein and protein-disulfide isomerase pathway. J Biol Chem. 2011;286(37):32705–12. doi: 10.1074/jbc.M111.227181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Appenzeller-Herzog C, Riemer J, Christensen B, Sorensen ES, Ellgaard L. A novel disulphide switch mechanism in Ero1alpha balances ER oxidation in human cells. EMBO J. 2008;27(22):2977–87. doi: 10.1038/emboj.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker KM, Chakravarthi S, Langton KP, Sheppard AM, Lu H, Bulleid NJ. Low reduction potential of Ero1alpha regulatory disulphides ensures tight control of substrate oxidation. EMBO J. 2008;27(22):2988–97. doi: 10.1038/emboj.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akman M, Belisario DC, Salaroglio IC, Kopecka J, Donadelli M, De Smaele E, Riganti C. Hypoxia, endoplasmic reticulum stress and chemoresistance: dangerous liaisons. J Exp Clin Cancer Res. 2021;40(1):28. doi: 10.1186/s13046-020-01824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartoszewska S, Collawn JF, Bartoszewski R. The Role of the Hypoxia-Related Unfolded Protein Response (UPR) in the Tumor Microenvironment. Cancers (Basel) 2022, 14(19). [DOI] [PMC free article] [PubMed]
- 81.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37(14):4587–602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene. 2005;24(6):1011–20. doi: 10.1038/sj.onc.1208325. [DOI] [PubMed] [Google Scholar]
- 83.Battle DM, Gunasekara SD, Watson GR, Ahmed EM, Saysell CG, Altaf N, Sanusi AL, Munipalle PC, Scoones D, Walker J, et al. Expression of the endoplasmic reticulum oxidoreductase Ero1alpha in gastro-intestinal cancer reveals a link between homocysteine and oxidative protein folding. Antioxid Redox Signal. 2013;19(1):24–35. doi: 10.1089/ars.2012.4651. [DOI] [PubMed] [Google Scholar]
- 84.Moon HG, Hwang KT, Kim JA, Kim HS, Lee MJ, Jung EM, Ko E, Han W, Noh DY. NFIB is a potential target for estrogen receptor-negative breast cancers. Mol Oncol. 2011;5(6):538–44. doi: 10.1016/j.molonc.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Denny SK, Yang D, Chuang CH, Brady JJ, Lim JS, Gruner BM, Chiou SH, Schep AN, Baral J, Hamard C, et al. Nfib Promotes Metastasis through a widespread increase in chromatin accessibility. Cell. 2016;166(2):328–42. doi: 10.1016/j.cell.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495(7439):98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zilli F, Marques Ramos P, Auf der Maur P, Jehanno C, Sethi A, Coissieux MM, Eichlisberger T, Sauteur L, Rouchon A, Bonapace L, et al. The NFIB-ERO1A axis promotes breast cancer metastatic colonization of disseminated tumour cells. EMBO Mol Med. 2021;13(4):e13162. doi: 10.15252/emmm.202013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Li Y, Jiang C, Chen L, Gan N. MicroRNA-144-3p inhibits tumorigenesis of oral squamous cell carcinoma by downregulating ERO1L. J Cancer. 2020;11(3):759–68. doi: 10.7150/jca.33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang D, Li C. circ-ACACA promotes proliferation, invasion, migration and glycolysis of cervical cancer cells by targeting the miR-582-5p/ERO1A signaling axis. Oncol Lett. 2021;22(5):795. doi: 10.3892/ol.2021.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen G, Wang Q, Wang K. MicroRNA-218-5p affects lung adenocarcinoma progression through targeting endoplasmic reticulum oxidoreductase 1 alpha. Bioengineered. 2022;13(4):10061–70. doi: 10.1080/21655979.2022.2063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb) 2010;105(1):4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 92.Shen J, Jiao Y, Ding N, Xie L, Ma S, Zhang H, Yang A, Zhang H, Jiang Y. Homocysteine facilitates endoplasmic reticulum stress and apoptosis of hepatocytes by suppressing ERO1alpha expression via cooperation between DNMT1 and G9a. Cell Biol Int. 2022;46(8):1236–48. doi: 10.1002/cbin.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES, Koh M, Lim HK, Jung J, Park SY, et al. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-α and MMP-9. Cancer Lett. 2018;437:25–34. doi: 10.1016/j.canlet.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka T, Kutomi G, Kajiwara T, Kukita K, Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto Y, et al. Cancer-associated oxidoreductase ERO1-α drives the production of VEGF via oxidative protein folding and regulating the mRNA level. Br J Cancer. 2016;114(11):1227–34. doi: 10.1038/bjc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kutomi G, Tamura Y, Tanaka T, Kajiwara T, Kukita K, Ohmura T, Shima H, Takamaru T, Satomi F, Suzuki Y, et al. Human endoplasmic reticulum oxidoreductin 1-α is a novel predictor for poor prognosis of breast cancer. Cancer Sci. 2013;104(8):1091–6. doi: 10.1111/cas.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varone E, Decio A, Chernorudskiy A, Minoli L, Brunelli L, Ioli F, Piotti A, Pastorelli R, Fratelli M, Gobbi M, et al. The ER stress response mediator ERO1 triggers cancer metastasis by favoring the angiogenic switch in hypoxic conditions. Oncogene. 2021;40(9):1721–36. doi: 10.1038/s41388-021-01659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu L, Li S, Qu Y, Wang J, Fei K, Wang C, Zhang X, Ma Z, Bai H, Wang J. Tumour ERO1A instigates T cell dysfunction by transmission of endoplasmic reticulum stress. J Clin Oncol. 2022;40(16suppl):e14533–3. doi: 10.1200/JCO.2022.40.16_suppl.e14533. [DOI] [Google Scholar]
- 98.Hsu CH, Hsu CW, Hsueh C, Wang CL, Wu YC, Wu CC, Liu CC, Yu JS, Chang YS, Yu CJ. Identification and characterization of potential biomarkers by Quantitative Tissue Proteomics of Primary Lung Adenocarcinoma. Mol Cell Proteom. 2016;15(7):2396–410. doi: 10.1074/mcp.M115.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J, Xu Y, Huo Y, Cai L, Hua R, Zhang J, Chen Z. ERO1L Promotes Hepatic Metastasis through Activating Epithelial-Mesenchymal Transition (EMT) in Pancreatic Cancer. J Immunol Res 2021, 2021:5553425. [DOI] [PMC free article] [PubMed]
- 100.Seol SY, Kim C, Lim JY, Yoon SO, Hong SW, Kim JW, Choi SH, Cho JY. Overexpression of endoplasmic reticulum oxidoreductin 1-α (ERO1L) is Associated with poor prognosis of gastric Cancer. Cancer Res Treat. 2016;48(4):1196–209. doi: 10.4143/crt.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takei N, Yoneda A, Sakai-Sawada K, Kosaka M, Minomi K, Tamura Y. Hypoxia-inducible ERO1α promotes cancer progression through modulation of integrin-β1 modification and signalling in HCT116 colorectal cancer cells. Sci Rep. 2017;7(1):9389. doi: 10.1038/s41598-017-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013;13(7):511–8. doi: 10.1038/nrc3536. [DOI] [PubMed] [Google Scholar]
- 103.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112(4):503–16. doi: 10.1172/JCI200317913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–9. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93(11):1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 107.Kaelin WG., Jr ROS: really involved in oxygen sensing. Cell Metab. 2005;1(6):357–8. doi: 10.1016/j.cmet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 108.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106(3):526–35. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Varone E, Chernorudskiy A, Cherubini A, Cattaneo A, Bachi A, Fumagalli S, Erol G, Gobbi M, Lenardo MJ, Borgese N, et al. ERO1 alpha deficiency impairs angiogenesis by increasing N-glycosylation of a proangiogenic VEGFA. Redox Biol. 2022;56:102455. doi: 10.1016/j.redox.2022.102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–92. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 111.Liu L, Li S, Qu Y, Bai H, Pan X, Wang J, Wang Z, Duan J, Zhong J, Wan R et al. Ablation of ERO1A induces lethal endoplasmic reticulum stress responses and immunogenic cell death to activate anti-tumor immunity. Cell Rep Med 2023:101206. [DOI] [PMC free article] [PubMed]
- 112.Tay A, Lundqvist A, Sze SK. Inhibition of ERO1a and IDO1 improves dendritic cell infiltration into pancreatic ductal adenocarcinoma. Cancer Res. 2023;83(7Supplement):4466–6. doi: 10.1158/1538-7445.AM2023-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu C, Liu Y, Ali NM, Zhang B, Cui X. The role of innate immune cells in the tumor microenvironment and research progress in anti-tumor therapy. Front Immunol. 2022;13:1039260. doi: 10.3389/fimmu.2022.1039260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belgiovine C, D’Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell Mol Life Sci. 2016;73(13):2411–24. doi: 10.1007/s00018-016-2166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 117.Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8(+) T cells. Cancer Immunol Res. 2019;7(3):476–86. doi: 10.1158/2326-6066.CIR-18-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reczek CR, Chandel NS. The two faces of reactive oxygen species in cancer. Annual Rev cancer Biology. 2017;1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808. [DOI] [Google Scholar]
- 119.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 120.Jiang P, Du W, Wu M. Regulation of the pentose phosphate pathway in cancer. Protein Cell. 2014;5(8):592–602. doi: 10.1007/s13238-014-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liberti MV, Locasale JW. The Warburg Effect: how does it Benefit Cancer cells? Trends Biochem Sci. 2016;41(3):211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Varone E, Decio A, Barbera MC, Bolis M, Di Rito L, Pisati F, Giavazzi R, Zito E. Endoplasmic reticulum oxidoreductin 1-alpha deficiency and activation of protein translation synergistically impair breast tumour resilience. Br J Pharmacol. 2022;179(23):5180–95. doi: 10.1111/bph.15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yadunandam AK, Yoon JS, Seong YA, Oh CW, Kim GD. Prospective impact of 5-FU in the induction of endoplasmic reticulum stress, modulation of GRP78 expression and autophagy in Sk-Hep1 cells. Int J Oncol. 2012;41(3):1036–42. doi: 10.3892/ijo.2012.1506. [DOI] [PubMed] [Google Scholar]
- 124.Liao PC, Tan SK, Lieu CH, Jung HK. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem. 2008;104(4):1509–23. doi: 10.1002/jcb.21730. [DOI] [PubMed] [Google Scholar]
- 125.Wang G, Han J, Wang G, Wu X, Huang Y, Wu M, Chen Y. ERO1α mediates endoplasmic reticulum stress-induced apoptosis via microRNA-101/EZH2 axis in colon cancer RKO and HT-29 cells. Hum Cell. 2021;34(3):932–44. doi: 10.1007/s13577-021-00494-3. [DOI] [PubMed] [Google Scholar]
- 126.Liu Z, Zhang K, Zhao Z, Qin Z, Tang H. Prognosis-related autophagy genes in female lung adenocarcinoma. Med (Baltim) 2022;101(1):e28500. doi: 10.1097/MD.0000000000028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo Q, Liu XL, Liu HS, Luo XY, Yuan Y, Ji YM, Liu T, Guo JL, Zhang J. The Risk Model Based on the Three Oxidative Stress-Related Genes Evaluates the Prognosis of LAC Patients. Oxid Med Cell Longev 2022, 2022:4022896. [DOI] [PMC free article] [PubMed] [Retracted]
- 128.Zhu J, Wang M, Hu D. Development of an autophagy-related gene prognostic signature in lung adenocarcinoma and lung squamous cell carcinoma. PeerJ. 2020;8:e8288. doi: 10.7717/peerj.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nie H, Luo C, Liao K, Xu J, Cheng XX, Wang X. Seven glycolysis-related genes predict the prognosis of patients with pancreatic Cancer. Front Cell Dev Biol. 2021;9:647106. doi: 10.3389/fcell.2021.647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–48. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 132.Hayes KE, Batsomboon P, Chen WC, Johnson BD, Becker A, Eschrich S, Yang Y, Robart AR, Dudley GB, Geldenhuys WJ, et al. Inhibition of the FAD containing ER oxidoreductin 1 (Ero1) protein by EN-460 as a strategy for treatment of multiple myeloma. Bioorg Med Chem. 2019;27(8):1479–88. doi: 10.1016/j.bmc.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blais JD, Chin KT, Zito E, Zhang Y, Heldman N, Harding HP, Fass D, Thorpe C, Ron D. A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J Biol Chem. 2010;285(27):20993–1003. doi: 10.1074/jbc.M110.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JL, Toufighi K, Mostafavi S, et al. The genetic landscape of a cell. Science. 2010;327(5964):425–31. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson BD, Kaulagari S, Chen WC, Hayes K, Geldenhuys WJ, Hazlehurst LA. Identification of natural product sulfuretin derivatives as inhibitors for the endoplasmic reticulum redox protein ERO1alpha. ACS Bio Med Chem Au. 2022;2(2):161–70. doi: 10.1021/acsbiomedchemau.1c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rahman NSA, Zahari S, Syafruddin SE, Firdaus-Raih M, Low TY, Mohtar MA. Functions and mechanisms of protein disulfide isomerase family in cancer emergence. Cell Biosci. 2022;12(1):129. doi: 10.1186/s13578-022-00868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Powell LE, Foster PA. Protein disulphide isomerase inhibition as a potential cancer therapeutic strategy. Cancer Med. 2021;10(8):2812–25. doi: 10.1002/cam4.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13(1):50. doi: 10.1186/s13045-020-00885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bekes M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2001;98(15):8554–9. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeng S, Huang W, Zheng X, Liyan C, Zhang Z, Wang J, Shen Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: recent progress and future challenges. Eur J Med Chem. 2021;210:112981. doi: 10.1016/j.ejmech.2020.112981. [DOI] [PubMed] [Google Scholar]
- 142.Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen J, Yang Z, Nie Y, Fan D. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res. 2018;37(1):272. doi: 10.1186/s13046-018-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–33. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma A, Schmidt-Wolf IGH. 30 years of CIK cell therapy: recapitulating the key breakthroughs and future perspective. J Exp Clin Cancer Res. 2021;40(1):388. doi: 10.1186/s13046-021-02184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wright J, Birk J, Haataja L, Liu M, Ramming T, Weiss MA, Appenzeller-Herzog C, Arvan P. Endoplasmic reticulum oxidoreductin-1alpha (Ero1alpha) improves folding and secretion of mutant proinsulin and limits mutant proinsulin-induced endoplasmic reticulum stress. J Biol Chem. 2013;288(43):31010–8. doi: 10.1074/jbc.M113.510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nardai G, Stadler K, Papp E, Korcsmaros T, Jakus J, Csermely P. Diabetic changes in the redox status of the microsomal protein folding machinery. Biochem Biophys Res Commun. 2005;334(3):787–95. doi: 10.1016/j.bbrc.2005.06.172. [DOI] [PubMed] [Google Scholar]
- 147.Lehtonen S, Jaronen M, Vehvilainen P, Lakso M, Rudgalvyte M, Keksa-Goldsteine V, Wong G, Courtney MJ, Koistinaho J, Goldsteins G. Inhibition of excessive oxidative protein folding is protective in MPP(+) toxicity-Induced Parkinson’s Disease models. Antioxid Redox Signal. 2016;25(8):485–97. doi: 10.1089/ars.2015.6402. [DOI] [PubMed] [Google Scholar]
- 148.Xu LR, Liu XL, Chen J, Liang Y. Protein disulfide isomerase interacts with tau protein and inhibits its fibrillization. PLoS ONE. 2013;8(10):e76657. doi: 10.1371/journal.pone.0076657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang K, Liu JQ, Zhong T, Liu XL, Zeng Y, Qiao X, Xie T, Chen Y, Gao YY, Tang B, et al. Phase separation and cytotoxicity of tau are modulated by protein disulfide isomerase and S-nitrosylation of this molecular chaperone. J Mol Biol. 2020;432(7):2141–63. doi: 10.1016/j.jmb.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 150.Zhou X, Li G, Kaplan A, Gaschler MM, Zhang X, Hou Z, Jiang M, Zott R, Cremers S, Stockwell BR, et al. Small molecule modulator of protein disulfide isomerase attenuates mutant huntingtin toxicity and inhibits endoplasmic reticulum stress in a mouse model of Huntington’s disease. Hum Mol Genet. 2018;27(9):1545–55. doi: 10.1093/hmg/ddy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parakh S, Shadfar S, Perri ER, Ragagnin AMG, Piattoni CV, Fogolin MB, Yuan KC, Shahheydari H, Don EK, Thomas CJ et al. The Redox activity of protein disulfide isomerase inhibits ALS phenotypes in Cellular and zebrafish models. iScience 2020, 23(5):101097. [DOI] [PMC free article] [PubMed]
- 152.Woehlbier U, Colombo A, Saaranen MJ, Perez V, Ojeda J, Bustos FJ, Andreu CI, Torres M, Valenzuela V, Medinas DB, et al. ALS-linked protein disulfide isomerase variants cause motor dysfunction. EMBO J. 2016;35(8):845–65. doi: 10.15252/embj.201592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chin KT, Kang G, Qu J, Gardner LB, Coetzee WA, Zito E, Fishman GI, Ron D. The sarcoplasmic reticulum luminal thiol oxidase ERO1 regulates cardiomyocyte excitation-coupled calcium release and response to hemodynamic load. FASEB J. 2011;25(8):2583–91. doi: 10.1096/fj.11-184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang X, Xu H, Hao Y, Zhao L, Cai X, Tian J, Zhang M, Han X, Ma S, Cao J, et al. Endoplasmic reticulum oxidoreductin 1alpha mediates hepatic endoplasmic reticulum stress in homocysteine-induced atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 2014;46(10):902–10. doi: 10.1093/abbs/gmu081. [DOI] [PubMed] [Google Scholar]
- 155.Swiatkowska M, Padula G, Michalec L, Stasiak M, Skurzynski S, Cierniewski CS. Ero1alpha is expressed on blood platelets in association with protein-disulfide isomerase and contributes to redox-controlled remodeling of alphaIIbbeta3. J Biol Chem. 2010;285(39):29874–83. doi: 10.1074/jbc.M109.092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.