Abstract
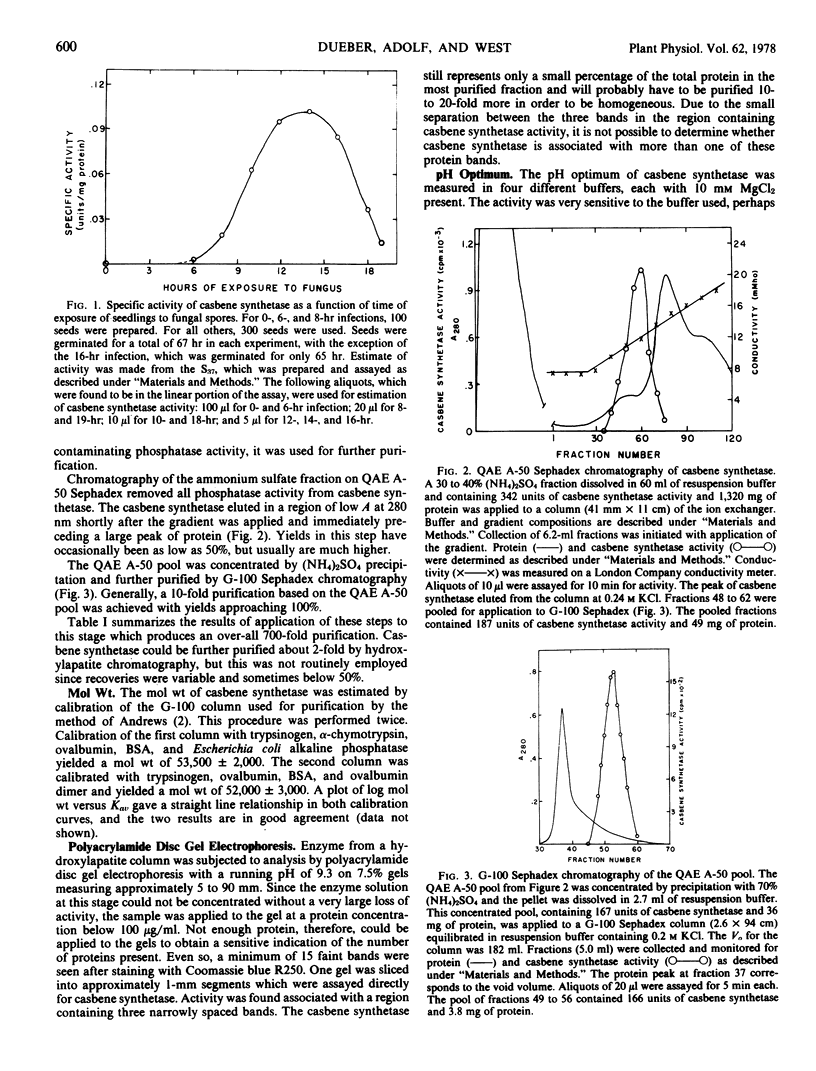
Casbene synthetase from 67-hour seedlings of Ricinis communis L. which had been treated with Rhizopus stolonifer spores was purified 700-fold by a combination of ammonium sulfate fractionation, QAE A-50 Sephadex chromatography, and G-100 Sephadex chromatography. Polyacrylamide disc gel electrophoresis revealed that the purified fraction was heterogeneous. No casbene synthetase was detected in extracts of seedlings which had not been exposed to the fungal spores; maximum activity was obtained from seedlings 14 hours after exposure to spores.
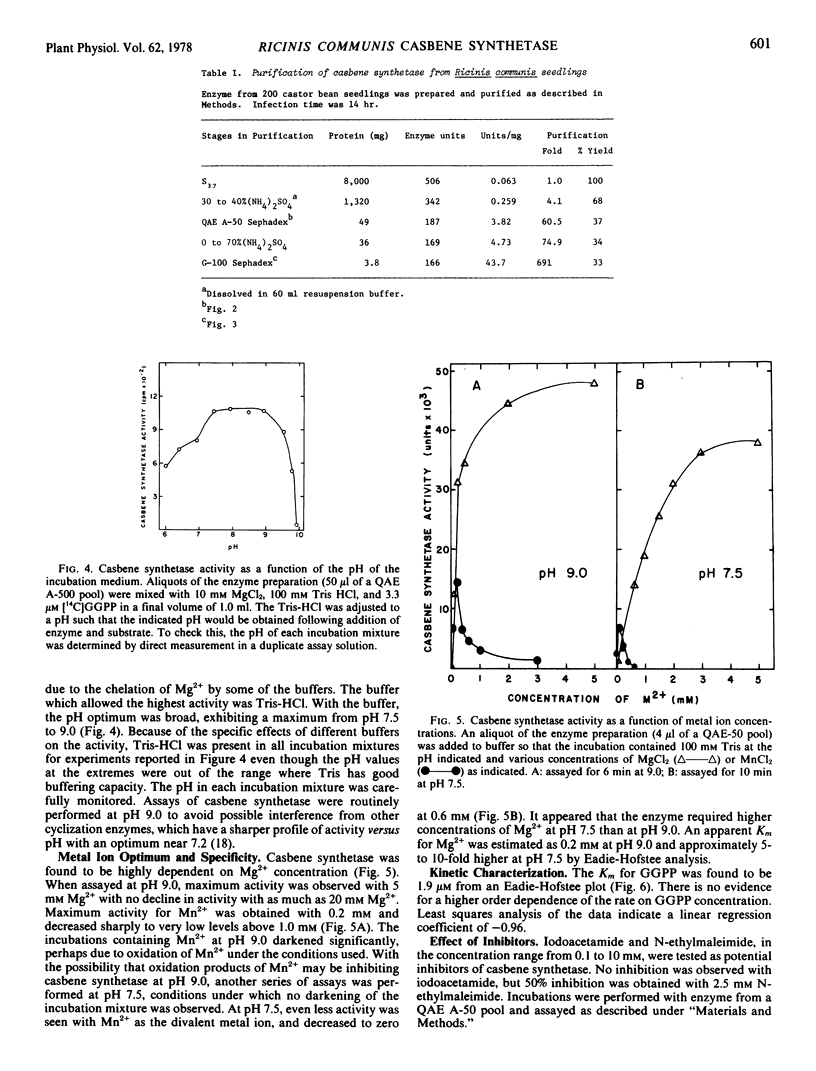
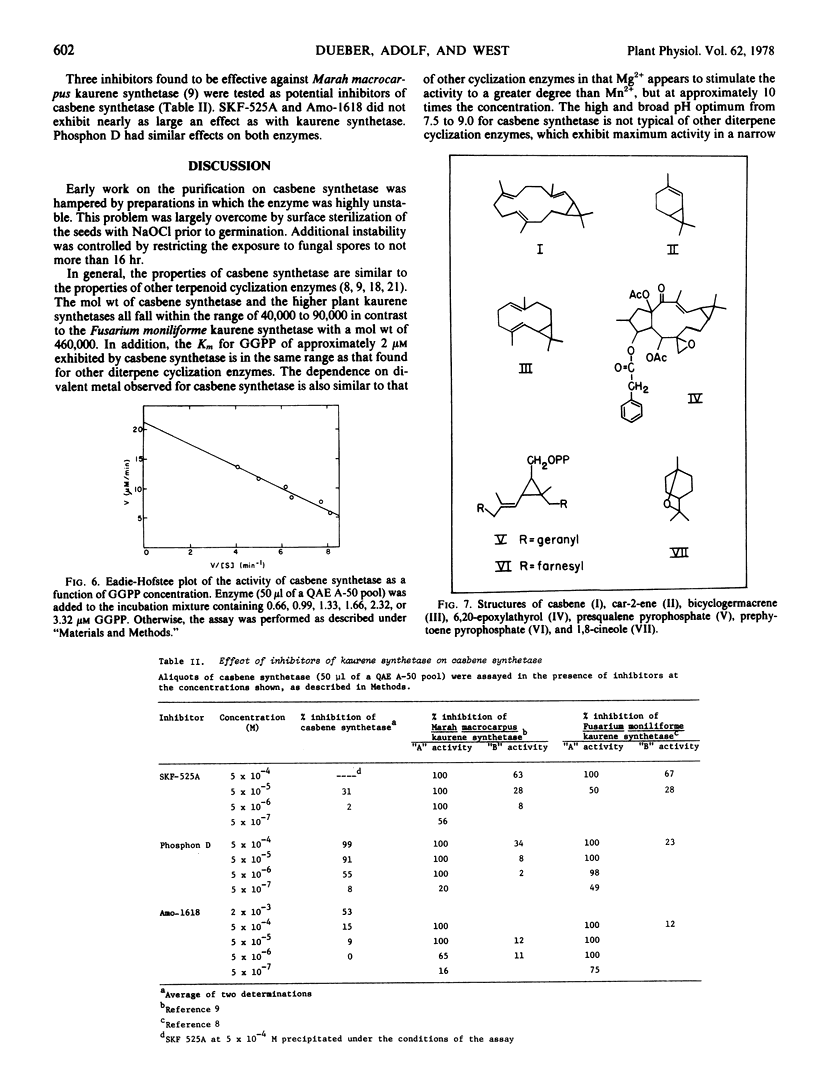
The partially purified enzyme exhibited a broad pH optimum from pH 7.5 to 9.0 with half-maximal activity at pH 6.0 and 9.8. Chromatography on a calibrated Sephadex G-100 column indicated a molecular weight of 53,000 ± 3,000 for casbene synthetase. Concentrations of Mg2+ above 5 mm gave maximal stimulation of the activity. Mn2+ was much less effective and was inhibitory at concentrations above 0.2 mm. The Km for geranyl-geranyl pyrophosphate was estimated as 1.9 μm. The activity was inhibited 50% by 2.5 mm N-ethylmaleimide; 10 mm iodoacetamide was not inhibitory. N,N-Dimethylaminoethyl-2,2-diphenylpentanoate (SKF-525A) and the growth retardant 2′isopropyl-4′-(trimethylammonium chloride)-5′-methylphenyl piperidine-1-carboxylate (Amo-1618) were ineffective inhibitors of casbene synthetase, but the growth retardant tributyl-2, 4-dichlorobenzyl-phosphonium chloride (Phosphon D) at a concentration of 5 μm inhibited the activity by 55%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Croteau R., Karp F. Biosynthesis of monoterpenes: partial purification and characterization of 1,8-cineole synthetase from Salvia officinalis. Arch Biochem Biophys. 1977 Feb;179(1):257–265. doi: 10.1016/0003-9861(77)90110-2. [DOI] [PubMed] [Google Scholar]
- Dugan R. E., Porter J. W. Hog liver squalene synthetase: the partial purification of the particulate enzyme and kinetic analysis of the reaction. Arch Biochem Biophys. 1972 Sep;152(1):28–35. doi: 10.1016/0003-9861(72)90189-0. [DOI] [PubMed] [Google Scholar]
- Fall R. R., West C. A. Purification and properties of kaurene synthetase from Fusarium moniliforme. J Biol Chem. 1971 Nov 25;246(22):6913–6928. [PubMed] [Google Scholar]
- Frost R. G., West C. A. Properties of Kaurene Synthetase from Marah macrocarpus. Plant Physiol. 1977 Jan;59(1):22–29. doi: 10.1104/pp.59.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustine D. L., Sherwood R. T. Regulation of Phytoalexin Synthesis in Jackbean Callus Cultures: Stimulation of Phenylalanine Ammonia-Lyase and o-Methyltransferase. Plant Physiol. 1978 Feb;61(2):226–230. doi: 10.1104/pp.61.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maudinas B., Bucholtz M. L., Papastephanou C., Katiyar S. S., Briedis A. V., Porter J. W. The partial purification and properties of a phytoene synthesizing enzyme system. Arch Biochem Biophys. 1977 Apr 30;180(2):354–362. doi: 10.1016/0003-9861(77)90049-2. [DOI] [PubMed] [Google Scholar]
- Oba K., Tatematsu H., Yamashita K., Uritani I. Induction of Furano-terpene Production and Formation of the Enzyme System from Mevalonate to Isopentenyl Pyrophosphate in Sweet Potato Root Tissue Injured by Ceratocystis fimbriata and by Toxic Chemicals. Plant Physiol. 1976 Jul;58(1):51–56. doi: 10.1104/pp.58.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Beytia E., Porter J. W. Squalene synthetase. II. Purification and properties of bakers' yeast enzyme. J Biol Chem. 1973 Mar 10;248(5):1848–1855. [PubMed] [Google Scholar]
- Robinson D. R., West C. A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. I. Identification of diterpene hydrocarbons formed from mevalonate. Biochemistry. 1970 Jan 6;9(1):70–79. doi: 10.1021/bi00803a010. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., West C. A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. II. Conversion of geranylgeranyl pyrophosphate into diterpene hydrocarbons and partial purification of the cyclization enzymes. Biochemistry. 1970 Jan 6;9(1):80–89. doi: 10.1021/bi00803a011. [DOI] [PubMed] [Google Scholar]
- Upper C. D., West C. A. Biosynthesis of gibberellins. II. Enzymic cyclization of geranylgeranyl pyrophosphate to kaurene. J Biol Chem. 1967 Jul 25;242(14):3285–3292. [PubMed] [Google Scholar]


