Abstract
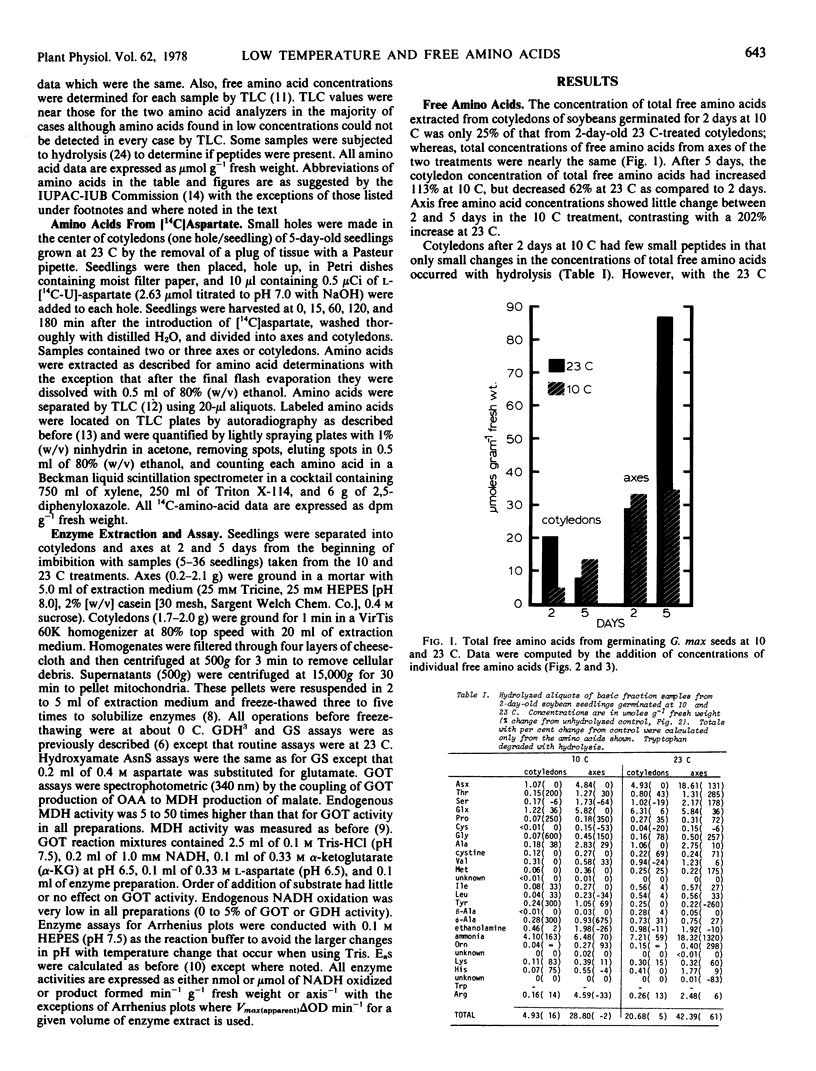
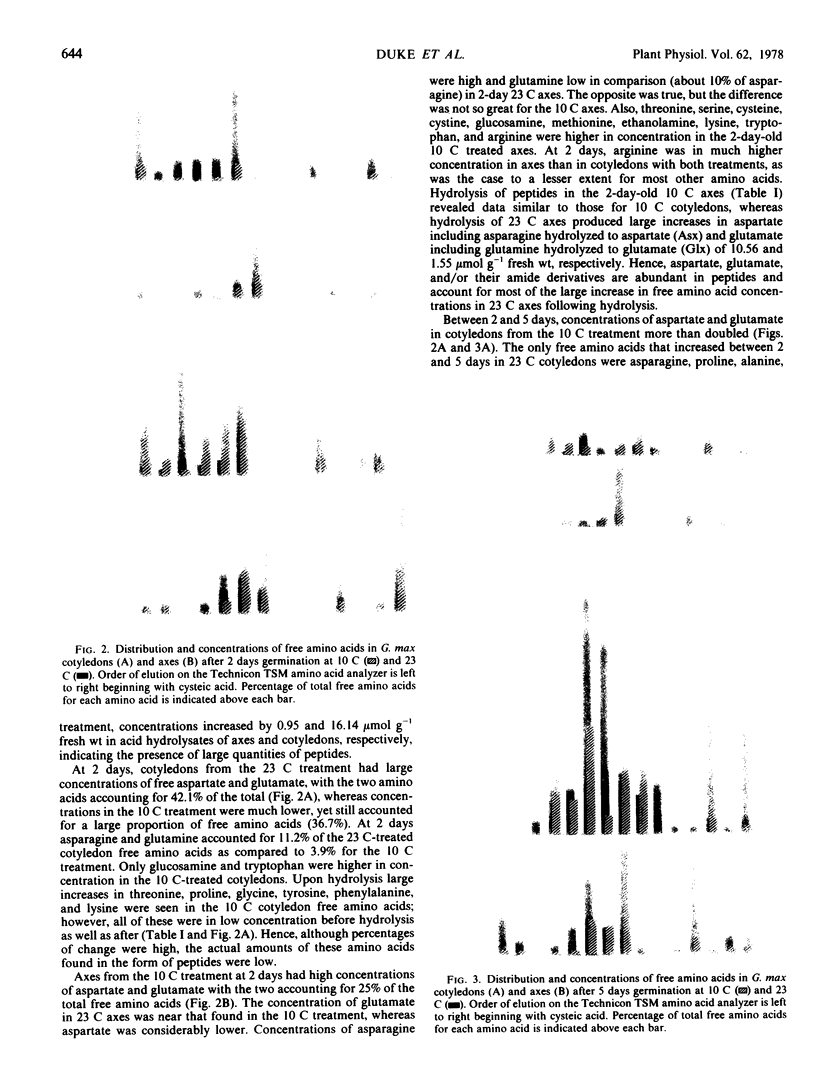
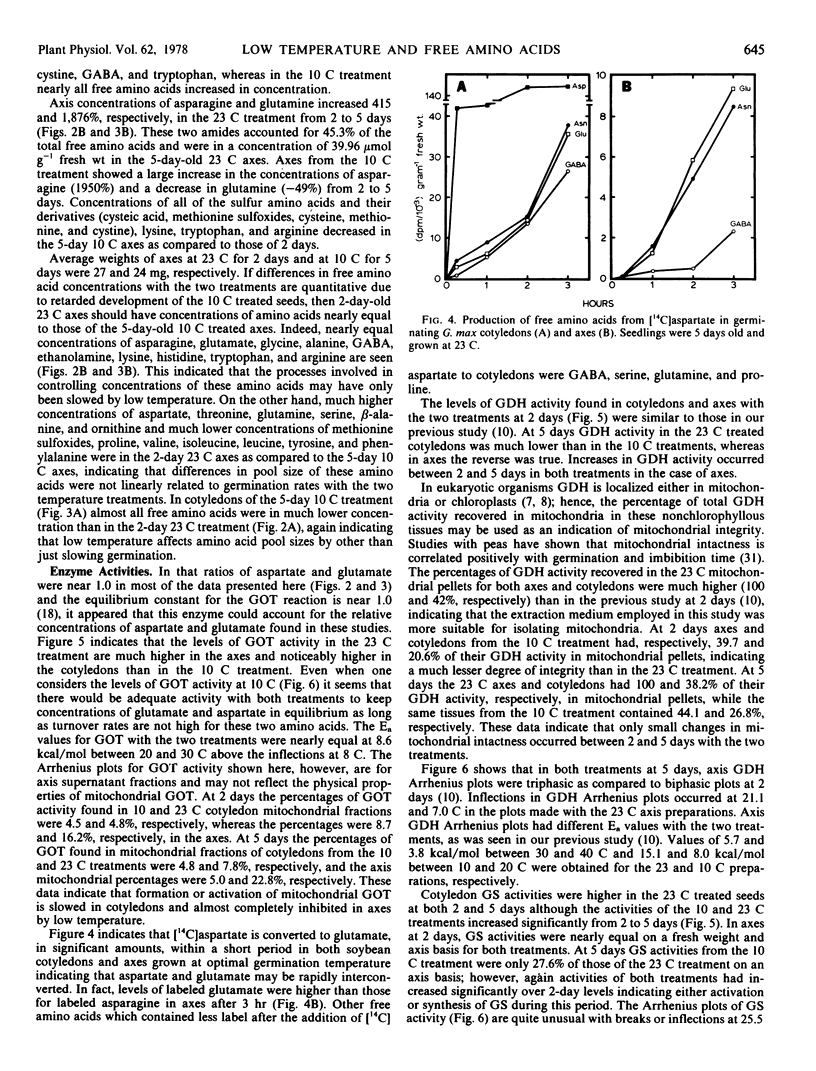
The free amino acid concentrations in cotyledons and axes of soybean (Glycine max [L.] Merr. cv. Wells) seedlings were determined by automated single column analysis after germination at 10 and 23 C. After 5 days germination at 10 C, glutamate and aspartate were in high concentration in both cotyledons and axes (38 and 24% of total free amino acids recovered, respectively), whereas the concentrations of their amide derivatives, asparagine and glutamine, were low in cotyledons (4.4%) and high in axes (21%). In contrast, after 5 days germination at 23 C, asparagine and glutamine accounted for 22 and 45% of total free amino acids in cotyledons and axes respectively, and aspartate and glutamate concentrations were low. The activities of glutamine synthetase and asparagine synthetase were considerably lower in tissues from the 10 C treatment than those from the 23 C treatment.
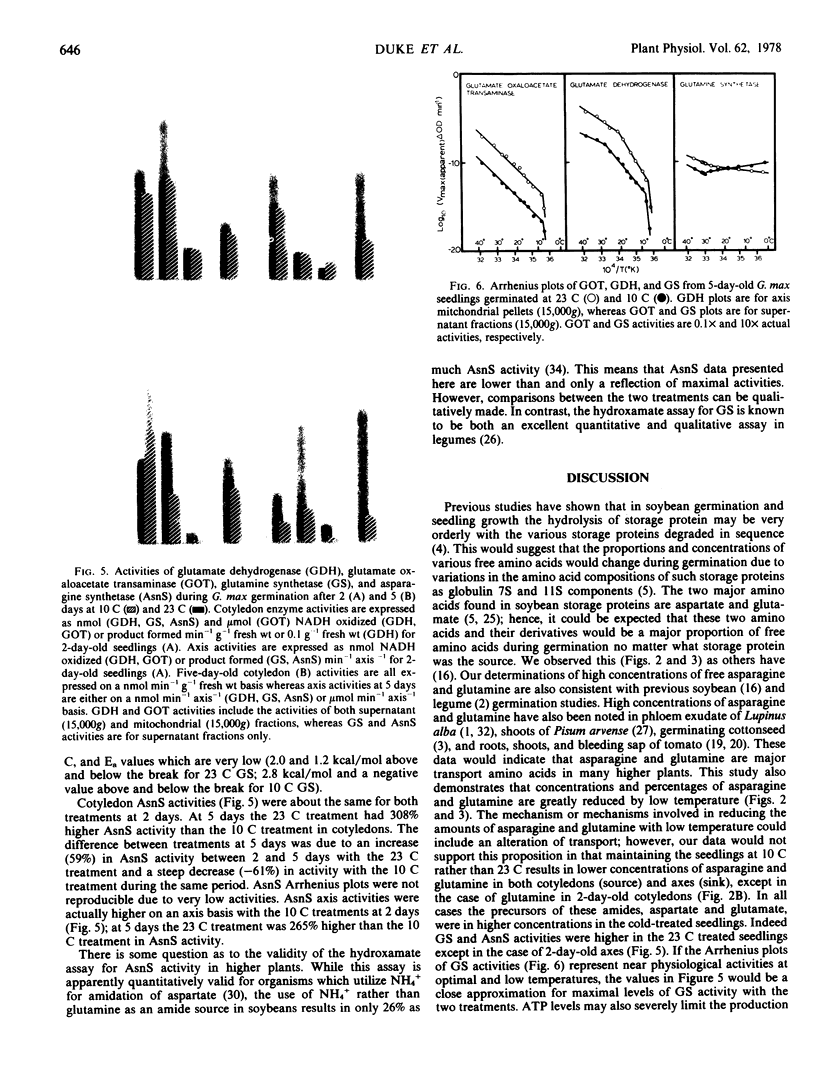
Aspartate and glutamate concentrations were nearly equal in all but one sample. Both glutamate oxaloacetate transaminase and glutamate dehydrogenase activities were much higher in axis tissues at 23 C as compared to 10 C. Arrhenius plots of axis glutamate oxaloacetate transaminase and glutamate dehydrogenase activities were biphasic and triphasic, respectively, with energies of activation for both increasing with low temperature. Energies of activation were identical for glutamate oxaloacetate transaminase from 10 and 23 C treatments but much higher for glutamate dehydrogenase from 23 C-treated axes. This indicates a difference in enzyme complement for glutamate dehydrogenase with the two treatments.
Hydrolysis of free amino acid sample (basic fraction) aliquots showed large quantities of peptides in 23 C-treated axes at 2 days, while few or no peptides were found in the 10 C treatment. Amino acid residues most prevalent in peptides were aspartate, threonine, serine, glutamate, and glycine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsimpoolas N., Ekenstam C., Rogers D. A., Meyer E. W. Protein subunits in dormant and germinating soybean seeds. Biochim Biophys Acta. 1968 Sep 10;168(1):122–131. doi: 10.1016/0005-2795(68)90241-9. [DOI] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Mitochondrial Respiration and Several Dehydrogenases during Imbibition and Germination. Plant Physiol. 1977 Nov;60(5):716–722. doi: 10.1104/pp.60.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Haworth C. An improved technique for the analysis of amino acids and related compounds on thin layers of cellulose. II. The quantitative determination of amino acids in protein hydrolysates. J Chromatogr. 1969 Aug 5;43(1):84–92. doi: 10.1016/s0021-9673(00)99169-6. [DOI] [PubMed] [Google Scholar]
- Housley T. L., Peterson D. M., Schrader L. E. Long distance translocation of sucrose, serine, leucine, lysine, and carbon dioxide assimilates: I. Soybean. Plant Physiol. 1977 Feb;59(2):217–220. doi: 10.1104/pp.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L. A., Beevers H. Amino Acid Metabolism in Young Pea Seedlings. Plant Physiol. 1965 May;40(3):424–432. doi: 10.1104/pp.40.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Synthesis and Interconversion of Amino Acids in Developing Cotyledons of Pea (Pisum sativum L.). Plant Physiol. 1977 Sep;60(3):344–348. doi: 10.1104/pp.60.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niece R. L. Automated single-column analysis of amino acids using ascorbic acid as reductant for air-stable ninhydrin. J Chromatogr. 1975 Jan 14;103(1):25–32. doi: 10.1016/s0021-9673(00)83798-x. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- Sato S., Asahi T. Biochemical Properties of Mitochondrial Membrane from Dry Pea Seeds and Changes in the Properties during Imbibition. Plant Physiol. 1975 Dec;56(6):816–820. doi: 10.1104/pp.56.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. M., Guinn G. Chilling injury and changes in adenosine triphosphate of cotton seedlings. Plant Physiol. 1969 Apr;44(4):605–608. doi: 10.1104/pp.44.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Weidner M., Ziemens C. Preadaptation of protein synthesis in wheat seedlings to high temperature. Plant Physiol. 1975 Nov;56(5):590–594. doi: 10.1104/pp.56.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]


