Abstract
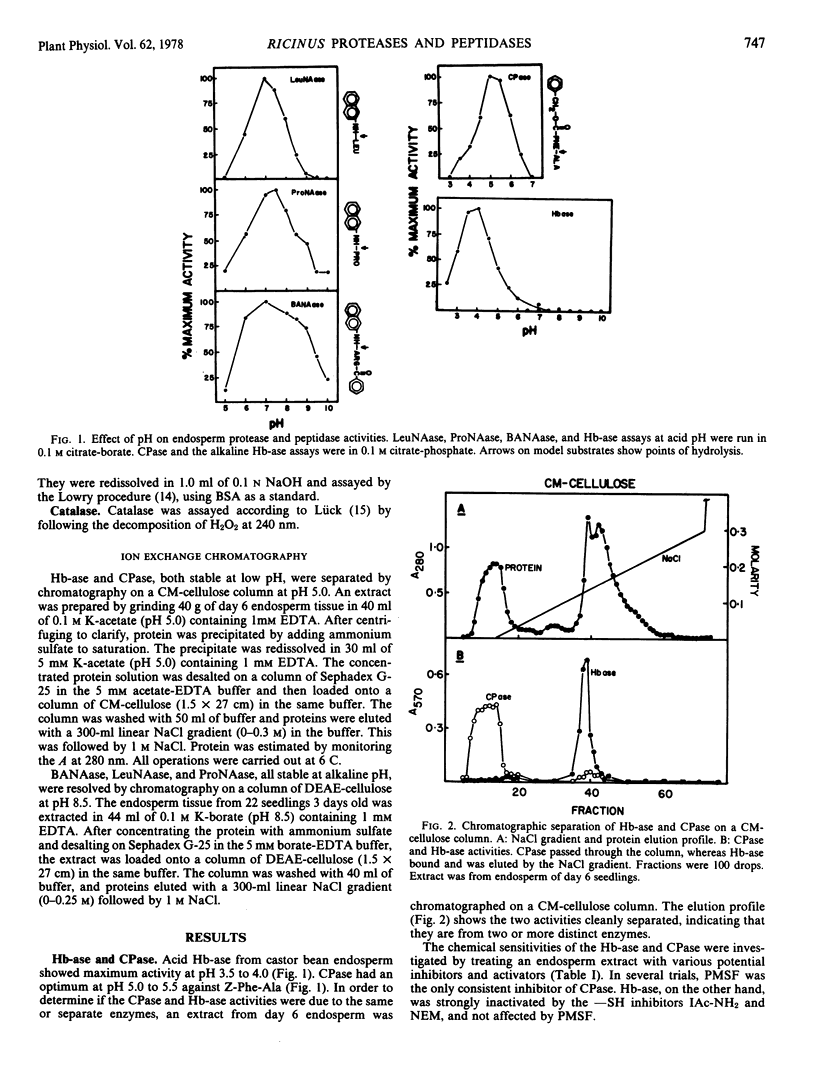
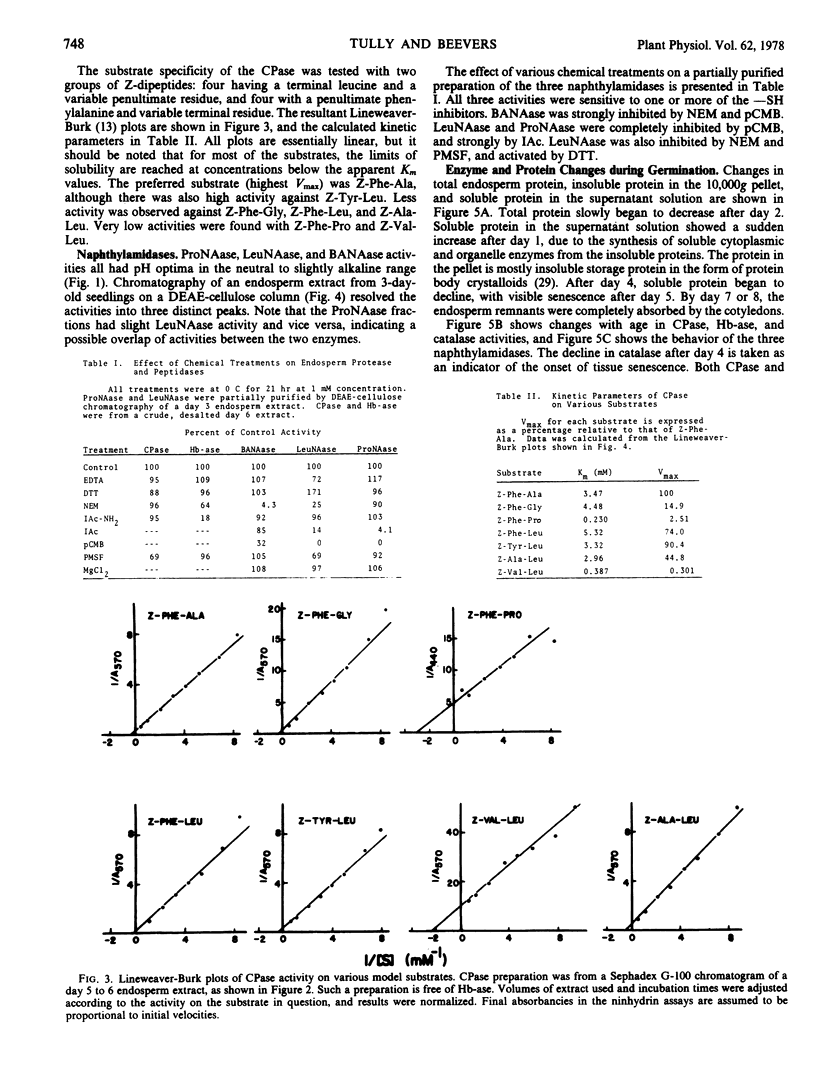
The endosperm of castor bean seeds (Ricinus communis L.) contains two —SH-dependent aminopeptidases, one hydrolyzing l-leucine-β-naphthylamide optimally at pH 7.0, and the other hydrolyzing l-proline-β-naphthylamide optimally at pH 7.5. After germination the endosperm contains in addition an —SH-dependent hemoglobin protease, a serine-dependent carboxypeptidase, and at least two —SH-dependent enzymes hydrolyzing the model substrate α-N-benzoyl-dl-arginine-β-naphthylamide (BANA). The carboxypeptidase is active on a variety of N-carbobenzoxy dipeptides, especially N-carbobenzoxy-L-phenylalanine-l-alanine and N-carbobenzoxy-l-tyrosine-l-leucine. The pH optima for the protease, carboxypeptidase, and BANAase acivities are 3.5 to 4.0, 5.0 to 5.5, and 6 to 8, respectively.
The two aminopeptidases increased about 4-fold in activity during the first 4 days of growth, concurrent with the period of rapid depletion of storage protein. Activities then declined as the endosperm senesced, but were still evident after 6 days. Senescence was complete by day 7 to 8. Hemoglobin protease, carboxypeptidase, and BANAase activities appeared in the endosperm at day 2 to 3, and reached peak activity at day 5 to 6.
The data indicate that the aminopeptidases are involved in the early mobilization of endosperm storage protein, whereas protease, carboxypeptidase, and BANAase may take part in later turnover and/or senescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell J. B., Sparrow L. G. Partial purification and characterization of two Peptide hydrolases from pea seeds. Plant Physiol. 1976 May;57(5):795–798. doi: 10.1104/pp.57.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C. Aminopeptides of pea. Biochem J. 1974 Jul;141(1):113–118. doi: 10.1042/bj1410113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg G. K., Virupaksha T. K. Acid protease from germinated sorghum. 1. Purification and characterization of the enzyme. Eur J Biochem. 1970 Nov;17(1):4–12. doi: 10.1111/j.1432-1033.1970.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Garg G. K., Virupaksha T. K. Acid protease from germinated sorghum. 2. Substrate specificity with synthetic peptides and ribonuclease A. Eur J Biochem. 1970 Nov;17(1):13–18. doi: 10.1111/j.1432-1033.1970.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Dure L. S., 3rd The developmental biochemistry of cottonseed embryogenesis and germination. I. Purification and properties of a carboxypeptidase from germinating cotyledons. J Biol Chem. 1972 Aug 25;247(16):5034–5040. [PubMed] [Google Scholar]
- Kolehmainen L., Mikola J. Partial purification and enzymatic properties of an aminopeptidase from barley. Arch Biochem Biophys. 1971 Aug;145(2):633–642. doi: 10.1016/s0003-9861(71)80023-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matoba T., Doi E. Substrate specificity of carboxypeptidase from Watermelon. J Biochem. 1975 Jun;77(6):1297–1303. [PubMed] [Google Scholar]
- Preston K. R., Kruger J. E. Purification and properties of two proteolytic enzymes with carboxypeptidase activity in germinated wheat. Plant Physiol. 1976 Oct;58(4):516–520. doi: 10.1104/pp.58.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sopanen T., Mikola J. Purification and partial characterization of barley leucine aminopeptidase. Plant Physiol. 1975 May;55(5):809–814. doi: 10.1104/pp.55.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P. W., Spencer R. D. Globulin-specific Proteolytic Activity in Germinating Pumpkin Seeds as Detected by a Fluorescence Assay Method. Plant Physiol. 1974 Dec;54(6):925–930. doi: 10.1104/pp.54.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuri K., Mikola J., Enari T. M. Isolation and partial characterization of a carboxypeptidase from barley. Eur J Biochem. 1969 Jan;7(2):193–199. doi: 10.1111/j.1432-1033.1969.tb19591.x. [DOI] [PubMed] [Google Scholar]
- Yomo H., Srinivasan K. Protein Breakdown and Formation of Protease in Attached and Detached Cotyledons of Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):671–673. doi: 10.1104/pp.52.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]


