Abstract
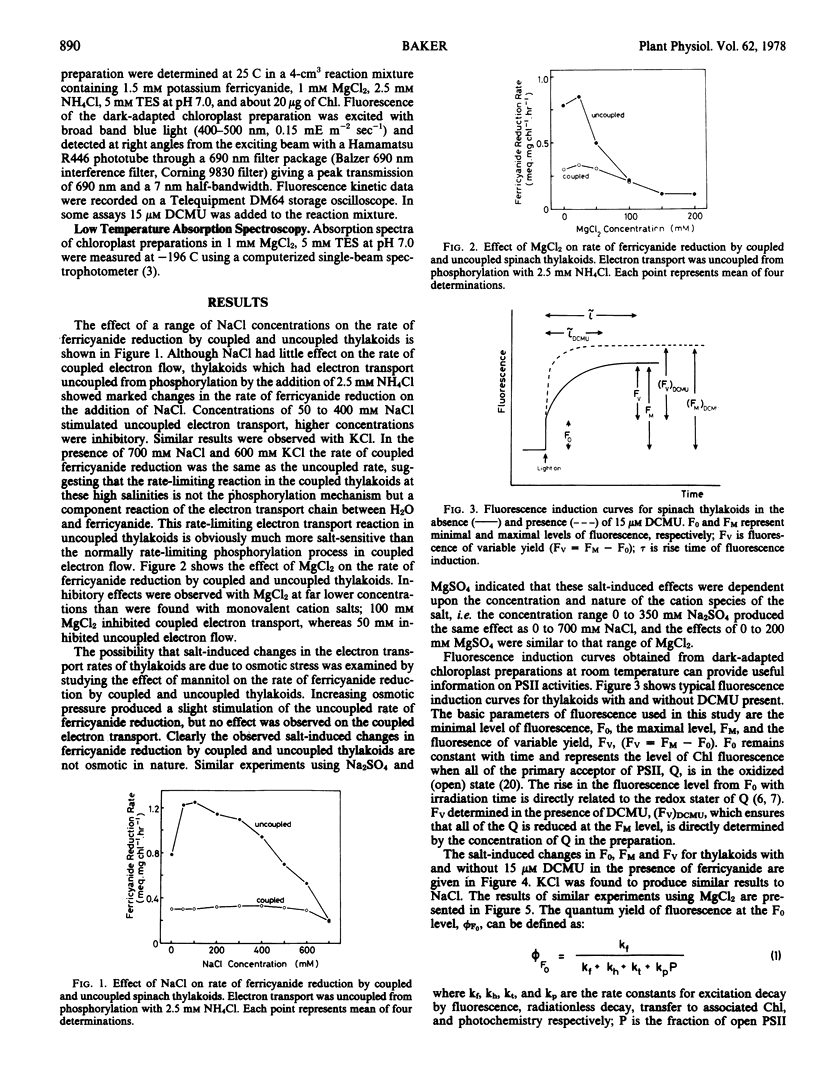
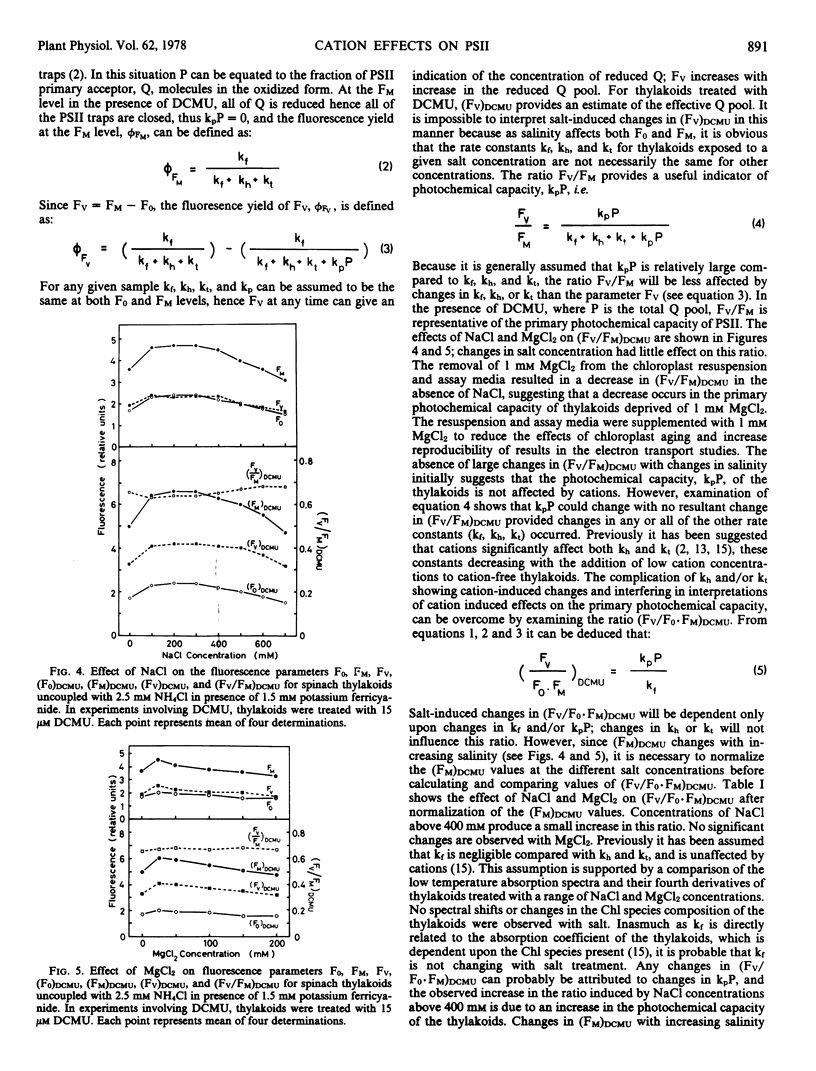
The effects of wide concentration ranges of NaCl, KCl, and MgCl2 on ferricyanide reduction and the fluorescence induction curve of isolated spinach (Spinacia oleracea) chloroplasts were investigated. Concentrations of the monovalent salts above 100 mm and MgCl2 above 25 mm produced a decrease in the rate of ferricyanide reduction by thylakoids uncoupled with 2.5 mm NH4Cl which cannot be attributed to changes in the primary photochemical capacity of photosystem II. Salt-induced decreases in the effective concentration of the secondary electron acceptor of photosystem II, plastoquinone, reduce the capacity for secondary photochemistry of photosystem II and this could contribute to the reduction in ferricyanide reduction by uncoupled thylakoids at high salinities. The rate of ferricyanide reduction by coupled thylakoids is little affected by salinity changes, indicating that the rate-limiting phosphorylation mechanism in electron flow from water to ferricyanide in coupled thylakoids is salt-tolerant, whereas the rate-limiting reaction in uncoupled ferricyanide reduction is considerably affected by salinity changes. Salt-induced changes in the fluorescence induction curve are interpreted in terms of changes in the rate constants for excitation decay by radiationless transitions, exciton transfer from photosystem II chlorophylls to other associated chlorophyll species, and photochemistry.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- Cahen D., Malkin S. Development of Photosystem II Complex during Greening of Chlamydomonas reinhardi y-1. Plant Physiol. 1976 Sep;58(3):257–267. doi: 10.1104/pp.58.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens L. N. 3-(3,4-Dichlorophenyl)-1,1-dimethylurea (DCMU) inhibition of system II and light-induced regulatory changes in energy transfer efficiency. Biophys J. 1972 Jul;12(7):858–863. doi: 10.1016/S0006-3495(72)86129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., Kok B. Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta. 1968 Aug 20;162(2):243–253. doi: 10.1016/0005-2728(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Brown A. P. Nicotinamide cofactors of intact chloroplasts isolated on a sucrose density gradient. Biochim Biophys Acta. 1969 Jan 14;172(1):116–125. doi: 10.1016/0005-2728(69)90096-6. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagendorf A. T., Smith M. Uncoupling Phosphorylation in Spinach Chloroplasts by Absence of Cations. Plant Physiol. 1962 Mar;37(2):135–141. doi: 10.1104/pp.37.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. S. Salts and chloroplast fluorescence. Biochim Biophys Acta. 1975 Jan 31;376(1):180–188. doi: 10.1016/0005-2728(75)90216-9. [DOI] [PubMed] [Google Scholar]
- Malkin S., Siderer Y. The effect of salt concentration on the fluorescence parameters of isolated chloroplasts. Biochim Biophys Acta. 1974 Dec 19;368(3):422–431. doi: 10.1016/0005-2728(74)90187-x. [DOI] [PubMed] [Google Scholar]
- Murakami S., Packer L. The role of cations in the organization of chloroplast membranes. Arch Biochem Biophys. 1971 Sep;146(1):337–347. doi: 10.1016/s0003-9861(71)80072-3. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. Light-induced changes in the ionic content of chloroplasts in Pisum sativum. Biochim Biophys Acta. 1969 Jan 14;172(1):134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G., Isaakidou J., Argoudelis C. Structure dependent control of chlorophyll a excitation density: The role of oxygen. FEBS Lett. 1972 Sep 1;25(1):139–142. doi: 10.1016/0014-5793(72)80471-x. [DOI] [PubMed] [Google Scholar]
- Shavit N., Avron M. The relation of electron transport and photophosphorylation to conformational changes in chloroplasts. Biochim Biophys Acta. 1967 May 9;131(3):516–525. doi: 10.1016/0005-2728(67)90011-4. [DOI] [PubMed] [Google Scholar]


