Abstract
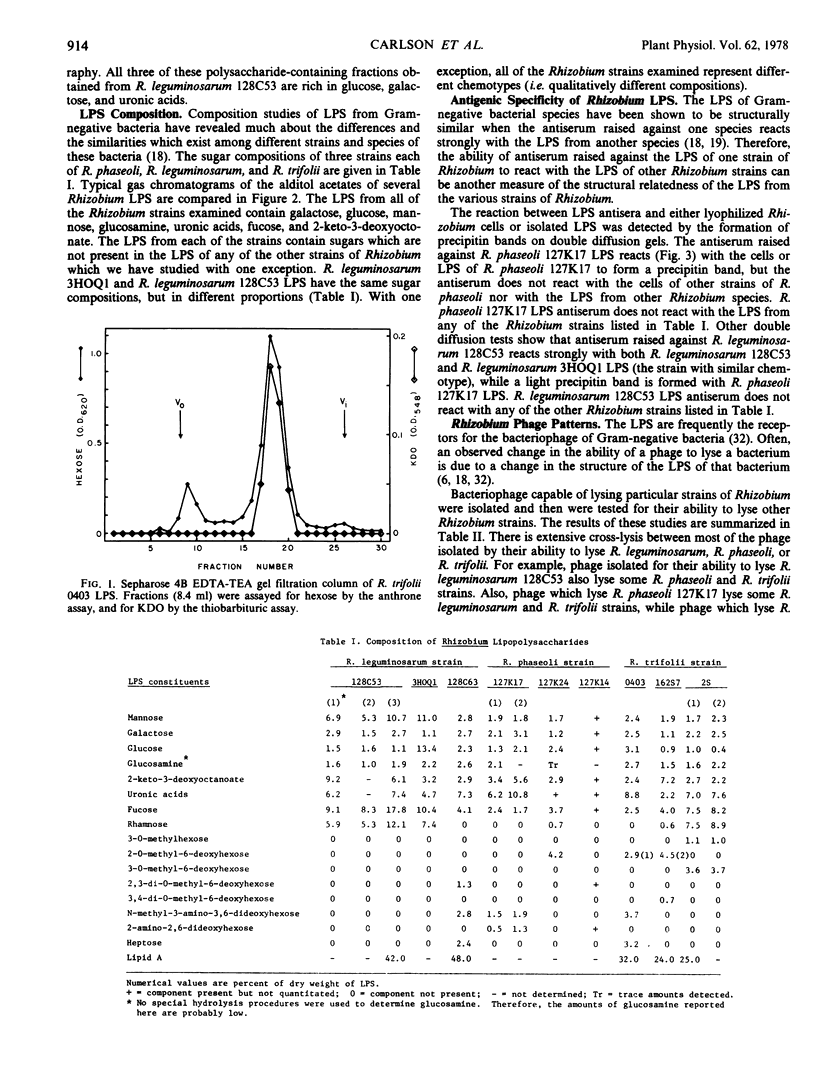
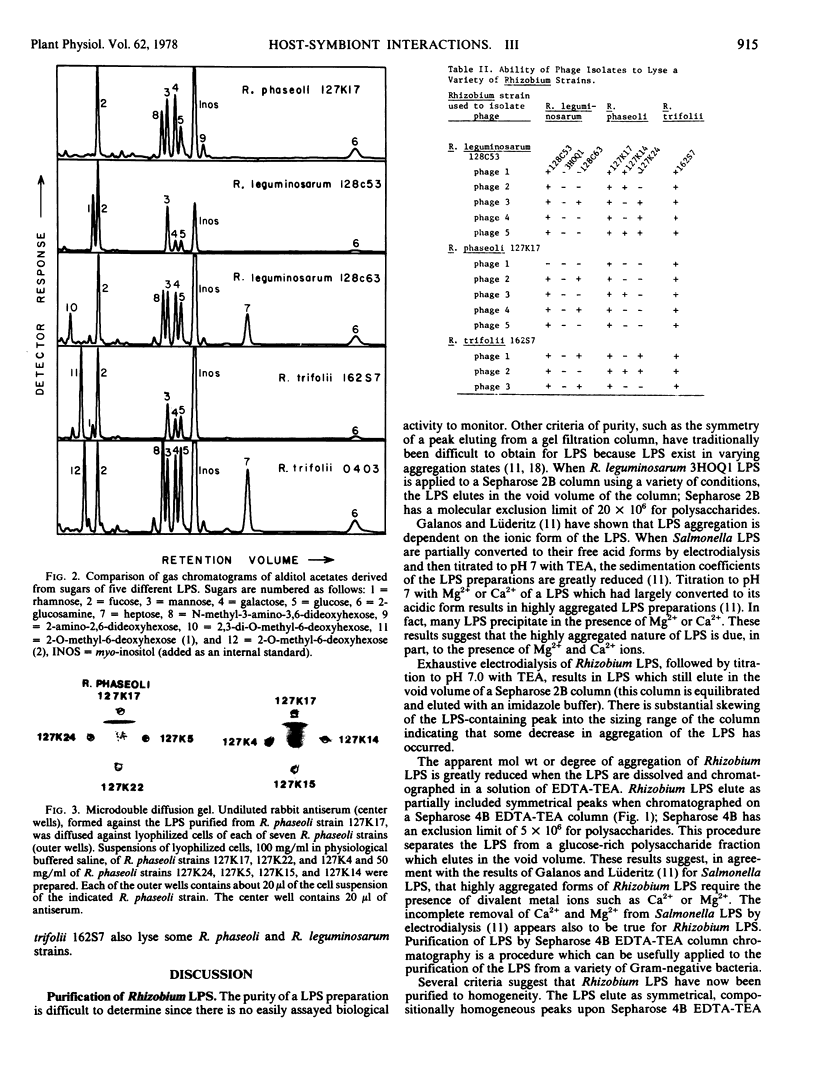
The lipopolysaccharides of three strains each of Rhizobium leguminosarum, R. phaseoli, and trifolii have been purified and partially characterized. The last step in the purification procedure is gel filtration column chromatography using Sepharose 4B with an elution buffer consisting of ethylenediaminetetraacetic acid and triethylamine. Each of the lipopolysaccharides reported in this paper elutes as a symmetrical peak in the partially included volume of this Sepharose 4B column. The ratio of 2-keto-3-deoxyoctonate acid (a sugar which is characteristic of lipopolysaccharides) to hexose is constant throughout the carbohydrate-containing peaks as they elute from the Sepharose 4B. The compositions and immunodominant structures of the purified lipopolysaccharides vary as much among strains of a single Rhizobium species as among the different species of Rhizobium. There is no obvious correlation between the nodulation group to which a Rhizobium belongs and the chemical composition or immunochemistry of the Rhizobium's lipopolysaccharide. There is extensive crosslysis by phage of strains of R. trifolii, R. phaseoli, and R. leguminosarum. This suggests that the receptors for these cross-lysing phage reside either in nonlipopolysaccharide structures or in common structures within the lipopolysaccharide which are not detected by compositional or immunochemical analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bray D., Robbins P. W. Mechanism of epsilon-15 conversion studies with bacteriphage mutants. J Mol Biol. 1967 Dec 28;30(3):457–475. doi: 10.1016/0022-2836(67)90362-2. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Graham T. L., Sequeira L., Huang T. S. Bacterial lipopolysaccharides as inducers of disease resistance in tobacco. Appl Environ Microbiol. 1977 Oct;34(4):424–432. doi: 10.1128/aem.34.4.424-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B. A., Vincent J. M. The effect of calcium nutrition on the production of diffusible antigens by Rhizobium trifolii. J Gen Microbiol. 1965 Oct;41(1):109–118. doi: 10.1099/00221287-41-1-109. [DOI] [PubMed] [Google Scholar]
- Humphrey B., Vincent J. M. The somatic antigens of two strains of Rhizobium trifolii. J Gen Microbiol. 1969 Dec;59(3):411–425. doi: 10.1099/00221287-59-3-411. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planqué K., Kijne J. W. Binding of pea lectins to a glycan type polysaccharide in the cell walls of Rhizobium leguminosarum. FEBS Lett. 1977 Jan 15;73(1):64–66. doi: 10.1016/0014-5793(77)80016-1. [DOI] [PubMed] [Google Scholar]
- Russa R., Lorkiewicz Z. Fatty acids present in the lipopolysaccharide of Rhizobium trifolii. J Bacteriol. 1974 Sep;119(3):771–775. doi: 10.1128/jb.119.3.771-775.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. M., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Salmonella newington. Arch Biochem Biophys. 1974 Jun;162(2):530–535. doi: 10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Koffler H. Bacterial flagella. Adv Microb Physiol. 1971;6:219–339. doi: 10.1016/s0065-2911(08)60070-3. [DOI] [PubMed] [Google Scholar]
- THOMAS L. The physiological disturbances produced by endotoxins. Annu Rev Physiol. 1954;16:467–490. doi: 10.1146/annurev.ph.16.030154.002343. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]
- Wright A., Barzilai N. Isolation and haracterization nonconverting mutants of bacteriophage epsilon 34. J Bacteriol. 1971 Mar;105(3):937–939. doi: 10.1128/jb.105.3.937-939.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac E., Russa R., Lorkiewicz Z. Lipopolysaccharide as receptor for rhizobium phage 1P. J Gen Microbiol. 1975 Oct;90(2):365–367. doi: 10.1099/00221287-90-2-365. [DOI] [PubMed] [Google Scholar]
- Zajac E., Russa R., Lorkiewicz Z. Studies on phage 1P receptors in Rhizobium trifolii and Rhizobium leguminosarum. Acta Microbiol Pol A. 1975;7(4):181–188. [PubMed] [Google Scholar]



