SUMMARY
The inability of antibodies to penetrate the blood-brain barrier (BBB) is a key limitation to their use in diverse applications. One promising strategy is to deliver IgGs using a bispecific BBB shuttle, which involves fusing an IgG to a second affinity ligand that engages a cerebrovascular endothelial target and facilitates transport across the BBB. Nearly all prior efforts have focused on shuttles that target transferrin receptor (TfR-1) despite inherent delivery and safety challenges. Here we report bispecific antibody shuttles that engage CD98hc, the heavy chain of the large neutral amino acid transporter (LAT1), and efficiently transport IgGs into the brain. Notably, CD98hc shuttles lead to much longer-lived brain retention of IgGs than TfR-1 shuttles while enabling more specific targeting due to limited CD98hc engagement in the brain parenchyma, which we demonstrate for IgGs that either agonize a neuronal receptor (TrkB) or target other endogenous cell-surface proteins on neurons and astrocytes.
Keywords: 4F2, 4F2hc, SLC3A2, LAT1, transferrin receptor, blood-brain barrier, BBB, cerebrovascular, endothelium, neuron, astrocyte, TrkB, BDNF, neurotrophin
Graphical Abstract

eTOC blurb
Although the blood-brain barrier largely inhibits brain entry of biologics, Pornnoppadol et al. demonstrated a bispecific shuttle targeting CD98hc for transport of IgGs into the brain and demonstrated it displays much longer-lived brain retention than transferrin receptor shuttles while selectively targeting different brain cell types.
INTRODUCTION
The brain is highly vascularized, and its hundreds of miles of blood vessels provide an incredible opportunity to, in principle, deliver any molecule of interest to every brain region. However, in practice, the blood-brain barrier (BBB) strictly regulates molecular transport into the parenchyma, only allowing entry to select nutrients and other biomolecules needed for essential cellular functions1,2. Most biologics, ranging from small peptides to large proteins like IgGs, are largely excluded by the BBB. Even in rare cases where specific IgGs accumulate at levels greater than non-specific uptake (~0.01–0.1% of injected dose), the enhanced levels are modest and the transport mechanism(s) are typically unclear, making it difficult to extrapolate from animal studies to human trials3,4.
While many strategies for brain delivery of IgGs have been proposed, the leading approach is to use an affinity-based shuttle, which engages a cerebrovascular target on the luminal surface of endothelial cells and facilitates transport across the BBB5–8. After nearly two decades of research, the first therapeutics using this strategy only recently entered clinical trials9. This slow progress may be partially attributed to the nearly exclusive focus on the transferrin receptor (TfR-1) as the prototypical endothelial target10–12 and several challenges specific to this iron-transport protein13. First, TfR-1 expression is not exclusively endothelial and is, in fact, used as a marker of immature erythrocytes13. Second, binding of antibodies and other affinity ligands to TfR-1 can result in lysosomal degradation of both the receptor and bound antibody, leading to decreased receptor levels and suppression of BBB shuttling7,8. Even more complex, the process appears to depend on the affinity, valency, and epitope of TfR-1 antibodies, and conflicting experimental results have confounded attempts to define these relationships4,7,13,14. As a result, there is no consensus as to the optimal construct for maximizing overall brain uptake, parenchymal delivery, and retention of shuttled cargo in the brain.
Given these potential issues with TfR-1 mediated shuttling, there continues to be great interest in identifying other BBB receptors that may enable greater parenchyma delivery or different kinetics of BBB transport and brain retention. One of the most promising targets is CD98hc, the heavy chain of the large neutral amino acid transporter (LAT1)15,16. CD98hc is highly expressed on both mouse and human brain endothelium and is present on both sides of the BBB. A 2016 study demonstrated the potential of CD98hc-mediated antibody shuttling, showing that it was capable of achieving greater brain uptake than that typically seen with TfR-1 shuttles in mice17. A more recent (2022) study investigated CD98hc-mediated brain delivery of IgGs in cynomolgus monkeys18.
These previous studies17,18, while groundbreaking, have several limitations that are the focus of this work. First, the 2016 report only demonstrates brain shuttling of antibody fragments (Fabs) using a so-called ‘1×1 shuttle’ format – i.e., one Fab arm against a brain target (β-secretase) and the other Fab arm against CD98hc17. While useful in terms of its monovalent binding to the cerebrovascular target (e.g., CD98hc)4, this format is incapable of delivering existing IgGs as reformatted CD98hc shuttles. This is critical, as reformatting bivalent IgGs into monovalent Fabs for incorporation into a 1×1 shuttle inevitably results in loss of binding affinity and avidity, which is likely to compromise biological function in the brain parenchyma. While the 2022 study addresses this limitation, the reported CD98hc shuttles cannot be used in mice as they are not cross-reactive with mouse CD98hc18. Second, the investigators in both studies did not publish the CD98hc antibody sequences, preventing validation of their results or use of the reported shuttles for brain delivery of other antibodies. Third, target engagement within the brain was demonstrated for only two antigens (β-secretase and tau), leaving unanswered questions about the range of parenchymal targets that may be accessed via these approaches. Fourth, it was not evaluated in either study if CD98hc shuttles engaged CD98hc in the brain parenchyma without engagement of a primary brain target. This is notable because the widespread uptake of bispecific antibodies into brain cells, as is commonly observed for TfR-1 shuttles7,9,19,20, can limit the ability of bispecific antibodies to selectively bind a second target in the brain parenchyma, such as extracellular targets or specific cell types. Finally, both studies reported little comparison of CD98hc versus TfR-1 shuttles at different doses, such as tracer and saturating doses, limiting understanding of their differences in transport capacity or kinetics.
In this study, we have sought to address each of these limitations. First, we report a 2×1 CD98hc shuttle that efficiently delivers IgGs to the brain while maintaining monovalent engagement of CD98hc via a single-chain antibody (scFv) fused to the C-terminus of one of the two IgG heavy chains. Second, we generated our CD98hc shuttle using an independently validated antibody specific for mouse CD98hc with a publicly available sequence21 to enable others to reproduce our findings and use our technology for brain delivery of other IgGs. Third, we demonstrate parenchymal engagement of multiple endogenous targets in wild-type mice, including a neurotrophin receptor (TrkB) and cell-surface proteins on multiple types of brain cells (e.g., neurons and astrocytes). Fourth, we demonstrate limited CD98hc target engagement in the brain parenchyma and compare this directly to the dissimilar behavior of an equivalent TfR-1 shuttle. Finally, we extensively characterize the kinetics of the CD98hc shuttle, comparing them to an equivalent TfR-1 shuttle both at tracer and therapeutic doses, revealing unique advantages of each transport pathway. Ultimately, we demonstrate that the CD98hc shuttle is capable of efficient delivery of IgGs across the BBB, with remarkable retention within the brain and the ability to access a wide variety of parenchymal targets in a simple and predictable manner.
RESULTS
In vitro characterization of CD98hc brain shuttles
Toward our goal of delivering IgGs to the brain while minimizing IgG modification, we fused a CD98hc single-chain (scFv) antibody to the C-terminus of one of the IgG heavy chains, using a short flexible linker to separate the domains (Fig. 1A). This “2×1 format” (i.e., one single chain antibody attached to a bivalent IgG) was chosen to preserve the affinity and avidity of the IgG being shuttled into the brain parenchyma while mediating monovalent, lower affinity binding to CD98hc. Production of heterodimerized antibody was maximized using knob-into-hole pairing technology and an optimized transfection ratio of the two heavy chain plasmids22,23. The CD98hc-binding scFv was derived from a monoclonal antibody whose sequence has been defined and published (GenBank: OR253074 for CTL-IgG/CD98-scFv)21.
Figure 1. In vitro characterization of IgGs and bispecific antibody shuttles.

(A) Schematic of the IgGs and bispecific antibodies evaluated in this work. (B) SDS-PAGE analysis of the non-heated/non-reduced (-) and heated/reduced (+) antibodies. Bispecific antibodies display three bands due to the knob-into-hole design, with the heaviest band being the knob portion with the CD98hc single-chain (scFv) antibody. (C) Flow cytometry analysis of IgG and bispecific antibody binding to TfR-1 or CD98hc-expressing cells.
For comparison to the CD98hc shuttle, we generated an analogous 2×1 TfR-1 shuttle using a validated, published, and sequence-defined antibody specific for mouse TfR-1 (clone 8D3; GenBank: OR253073 for CTL-IgG/TfR-1-scFv)24. Both shuttles were first synthesized with a non-targeted IgG (i.e., specific for human phosphorylated tau without a target in wild-type mice) to enable study of in vitro and in vivo behavior in the absence of a second target. The shuttles were also engineered with standard Fc mutations to eliminate effector functions25,26. These two shuttles, along with the corresponding non-targeted IgG and two IgGs corresponding to the parental CD98hc and TfR-1 antibodies, were expressed and first purified using Protein A chromatography, which resulted in purification yields of 11–19 mg/L for the IgGs and 5–13 mg/L for the bispecifics. Size-exclusion chromatography (SEC) was also used, as needed, to achieve high purity (>90% monomer by analytical SEC). The proper sizes for the heavy and light chains were also confirmed on a reducing SDS-PAGE gel (Fig. 1B). Notably, this analysis revealed the expected three bands for the 2×1 shuttles, with the two heavy chain bands in roughly equal proportion, indicating a high percentage of heterodimer. We used flow cytometry to confirm that the 2×1 shuttles, with only a single binding site for CD98hc or TfR-1, bound to their targets (expressed on human REN cells) with order-of-magnitude lower affinities (EC50 of 26.2±3.4 nM for CTL-IgG/CD98-scFv and 10.3±1.4 nM for CTL-IgG/TfR-scFv) relative to their corresponding parental IgGs (EC50 of 1.9±0.5 nM for CD98-IgG and 3.1±0.2 nM for TfR-IgG; Fig. 1C). Similar binding results were observed using recombinant TfR-1 and CD98hc ectodomains, as the parental IgGs displayed at least order-of-magnitude lower EC50s than those for the 2x1 shuttles (Table S1).
Antibody engagement of TfR-1 is well known to mediate strong receptor internalization8,27. Therefore, we sought to evaluate if our TfR-1 shuttle reduced cell-surface levels of the receptor and if similar behavior is observed for shuttles targeting CD98hc (Table S2). Using a standard mouse brain endothelial cell line (bEnd.3), we confirmed that both the TfR-1 IgG and shuttle reduced cell-surface levels, which corresponded to 20±3% relative to untreated cells for the IgG and 51±10% for the shuttle. In contrast, the CD98hc IgG and shuttle had more modest impacts on CD98hc cell-surface levels, which corresponded to 75±4% relative to untreated samples for the IgG and 115±2% for the shuttle.
CD98hc shuttles enable long-lived IgG brain delivery
We next evaluated the pharmacokinetics (PK) of the 2×1 shuttles in wild-type mice. Previous studies of TfR-1-targeted antibodies and shuttles have demonstrated greater brain uptake of high affinity, bivalent binders when given at tracer dose and lower affinity, monovalent binders when given at “saturating” doses4,8,17. For the latter case, cerebrovascular TfR-1 is completely bound and there is continued brain uptake over a period of hours to days. With this in mind, we performed dose escalation studies to identify the saturating dose of 2x1 shuttles for both CD98hc and TfR-1 (Table S3). While relatively low doses (0.12 and 0.36 mg/kg) of 2x1 shuttle were not saturating for either target, both targets showed an obvious decrease in brain uptake of radioactive tracer after 1 h at the highest shuttle dose (3.6 mg/kg), indicating at least partial saturation.
Based on these results, we chose 3.6 mg/kg (20 nmol/kg) as the dose for detailed characterization of antibody levels in the brain, blood, and other organs (Fig. 2). An equimolar dose of 3 mg/kg (IgGs) and 3.6 mg/kg (shuttles) was used. Consistent with prior reports4,8,28,29, the TfR-1 shuttle demonstrated greater brain uptake than its bivalent IgG counterpart, reaching a peak concentration of 5.8±0.1 nM after 8 h (Fig. 2A). Despite high initial uptake, the brain concentration of both the TfR-1 shuttle and IgG decreased rapidly with time, falling below 1 nM by 3 days and returning to baseline levels after 5 days. The TfR-1 antibodies showed fast clearance from the blood relative to untargeted IgG (Fig. 2B), presumably due to target-mediated drug disposition. Brain:blood ratios were relatively high, peaking after 8 h at 0.16 for the TfR-1 shuttle and 0.5 for the TfR-1 IgG (Fig. 2C).
Figure 2. Quantitative pharmacokinetic analysis of CD98hc- and TfR-1-mediated antibody brain uptake.
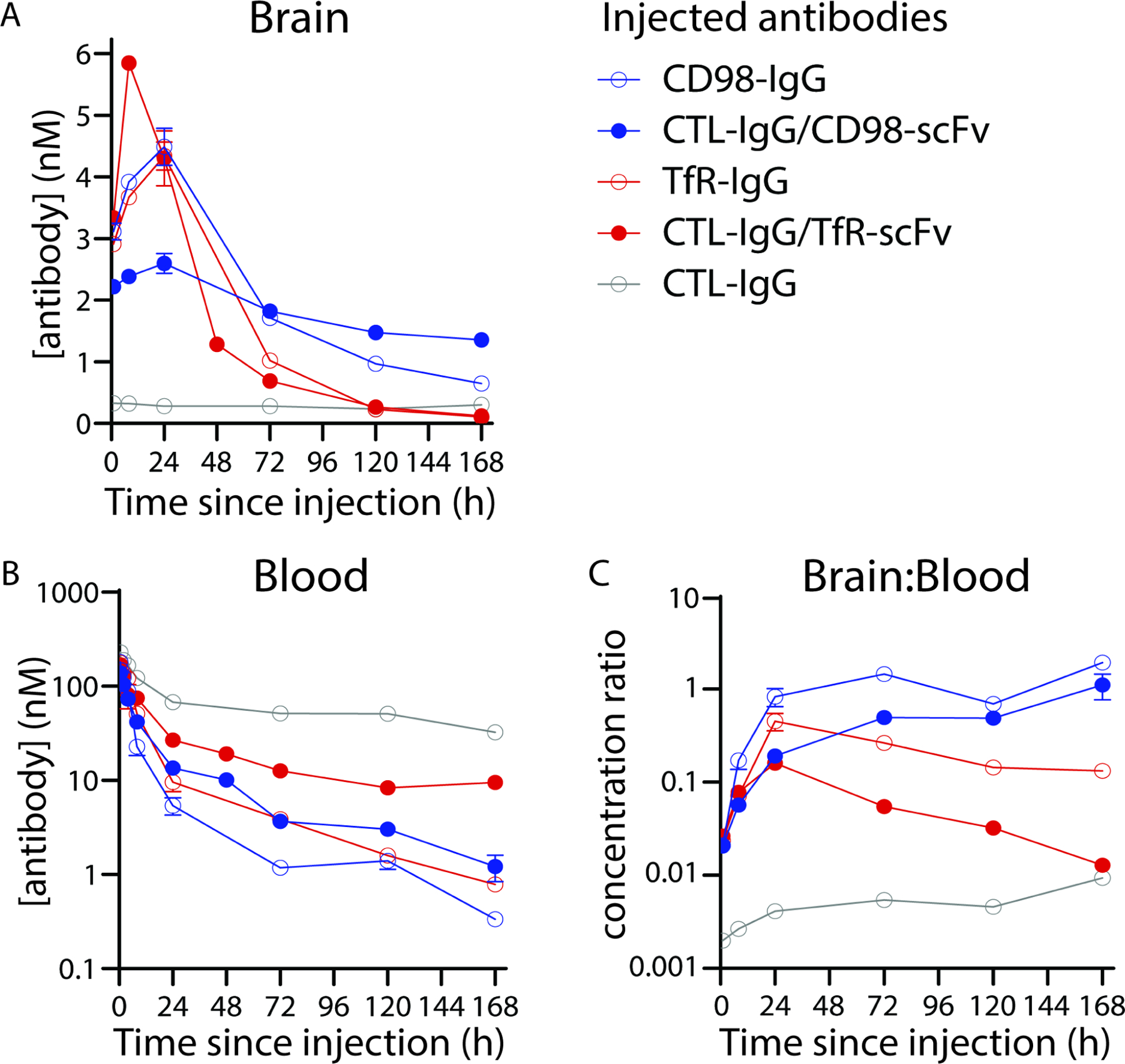
Equimolar doses (20 nmol/kg) of IgGs (3.0 mg/kg) and shuttles (3.6 mg/kg) were radiolabeled with 125I and injected retro-orbitally. Following transcardial perfusion with PBS, concentrations of antibody in the (A) total brain and (B) blood, along with the (C) brain:blood ratios, were determined. n=3–5 mice per antibody group.
Despite the similarity in initial uptake, the CD98hc shuttle displayed distinct brain PK relative to its TfR-1 counterpart (Fig. 2). The peak brain concentration was not achieved until 24 h after administration and, in this case, uptake of the bivalent IgG (4.5±0.6 nM) was greater than that of the shuttle (2.6±0.5 nM). However, this finding was dependent on dose, as we observed the opposite behavior at higher doses, namely 9 mg/kg (60 nmol/kg) for CD98hc IgG and 10.8 mg/kg (60 nmol/kg) for CD98hc shuttle (Table S4). At this higher dose, the CD98hc shuttle reached a higher brain concentration (7.0±0.7 nM) than the CD98hc IgG (5.2±0.4 nM) after 24 h.
Notably, the brain concentration of the CD98hc shuttle remained >1 nM for at least 7 days following a single dose (3.6 mg/kg; Fig. 2). As with TfR-1, blood PK appeared to be driven largely by target-mediated effects, with blood concentrations of both CD98hc IgG and shuttle dropping below 10 nM by 24 h post-injection. The combination of blood clearance and sustained brain concentration resulted in impressive brain:blood ratios, which peaked at 1.1 for the CD98hc shuttle and 1.9 for the CD98hc IgG at 7 days post-injection (Fig. 2C). Remarkably, the brain:blood ratio for the CD98hc shuttle was ~110 times higher than that of the TfR-1 shuttle at this time point, highlighting the distinct brain PK of the two shuttles. A statistical analysis of the antibody brain concentrations is provided in Table S5, and a summary of the PK in other organs is provided in Fig. S1.
Given the potential therapeutic implications of sustained brain delivery for at least one-week post-injection, we sought to confirm the radiotracing results using additional methodologies. First, we injected fluorescently labeled shuttles and IgGs, and evaluated their organ distribution after seven days (Fig. 3). Consistent with the radiotracing data, animals injected with a single dose of CD98hc shuttle and IgG had persistent fluorescent signal in their brains for at least 7 days, whereas no signal was seen in those dosed with TfR-1 shuttle, TfR-1 IgG or control IgG. Of note, the fluorescent signal seen in the lungs of mice injected with control IgG likely reflects residual blood content, given its relatively high blood concentration at 7 days (Fig. 2B) and variable flushing of the pulmonary circulation when animals are transcardially perfused via the left ventricle. Finally, we performed IgG ELISAs on mice injected with either shuttle and observed brain levels that closely matched the radiotracing results, including results at 3 days that were not statistically different and results at 5 days that displayed minor but statistically significant differences (Table S6).
Figure 3. Fluorescent imaging of antibody organ distribution.

Equimolar doses (20 nmol/kg) of IgGs (3.0 mg/kg) and shuttles (3.6 mg/kg) were labeled with Alexa Fluor-647 and injected intravenously into wild-type mice. After 7 d, various organs were harvested and imaged with IVIS spectrum. These experiments were repeated in triplicate (n=3), and representative images are shown.
To investigate the distribution of CD98hc- and TfR-1-targeted antibodies within brain tissue, we first performed classical fractionation of brain homogenate in mice treated with radiolabeled CD98hc and TfR-1 shuttle and IgG. One hour after injection, the radioactive shuttles for both cerebrovascular targets were enriched in the parenchymal fraction (69.0±5.5% and 62.0±2.9%, respectively), whereas the IgGs were relatively evenly split between capillary and parenchymal fractions (47.0±0.8% and 45.3±2.3%, respectively; Table S7). In the original description of the capillary depletion technique30, 3H-albumin was used to quantify the small amount of leakage of radioactive protein from the vascular lumen into the brain parenchymal fraction, which inevitably occurs during organ homogenization. Similarly, we used a radiolabeled control (non-targeted) IgG to assess for this homogenization leak, as well as non-affinity mediated mechanisms of IgG transport (e.g., absorptive-mediated transcytosis) across the BBB. Consistent with previously reported results for 3H-albumin, the majority of radiolabeled control IgG was recovered in the parenchymal fraction (80.7±5.1%). However, it accounted for only ~2% of the parenchymal uptake of the CD98hc shuttle (Table S7), indicating that the vast majority of the CD98hc and TfR-1 mAbs and shuttles that are recovered in the parenchyma arrive there via affinity-mediated transport and not due to technical factors (i.e., homogenization leak) or absorptive-mediated transcytosis.
We next performed fluorescence microscopy on sectioned brain tissue from animals injected with fluorescently labeled shuttles after one to five days post injection (Fig. 4). After one day, both shuttles largely localized to blood vessels, as confirmed by co-localization with ZO-1 staining (Fig. S2). After three days, fluorescent signal from the TfR-1 shuttle was only weakly associated with blood vessels and appeared within various cell types, including both NeuN-positive neurons and other types of NeuN-negative cells (Fig. 4, top). In contrast, the CD98hc shuttle remained largely associated with vessels (Fig. 4, bottom). After five days, fluorescent signal from the TfR-1 shuttle was no longer detectable (Fig. 4, top), consistent with radiotracing (Fig. 2), fluorescence imaging (Fig. 3), and ELISA (Table S6) results, whereas the CD98hc shuttle remained largely associated with vessels (Fig. 4, bottom).
Figure 4. Brain distribution of CD98hc and TfR-1 shuttles delivering a non-targeted IgG.

Brains were harvested at 1, 3 and 5 d after injection (3.6 mg/kg, 20 nmol/kg) of fluorescently labeled shuttles and sectioned for immunostaining. The injected shuttles (green) are shown relative to NeuN+ neurons (red) and nuclei (blue). Scale bars are 50 μm.
Brain delivery of cell-specific and agonist IgGs
We next sought to evaluate the ability of the IgGs shuttled into the brain via CD98hc to engage targets in the brain parenchyma. To enable testing in wild-type mice, we searched for highly validated monoclonal antibodies that target endogenous, cell-surface antigens on different types of mouse brain cells. We identified two candidates, one specific for neurons (clone M6)31 and the other specific for astrocytes and oligodendrocytes (clone M2)32,33, and reformatted the IgGs as 2×1 CD98hc shuttles.
For the M2/CD98hc shuttle specific for astrocytes, we observed little co-localization between the shuttle, which appeared to largely localize with blood vessels, and a marker of astrocytes (glial fibrillary acidic protein, GFAP), after one day (Fig. 5). However, three days after administration, there was overlap between M2/CD98hc shuttle and GFAP staining, and by five days we observed strong colocalization of the two fluorescent signals. Moreover, a 3 mg/kg dose of non-shuttled M2 IgG did not produce detectable fluorescent signal in the brain (Fig. S3).
Figure 5. Brain distribution of a CD98hc-shuttled IgG specific for astrocytes.
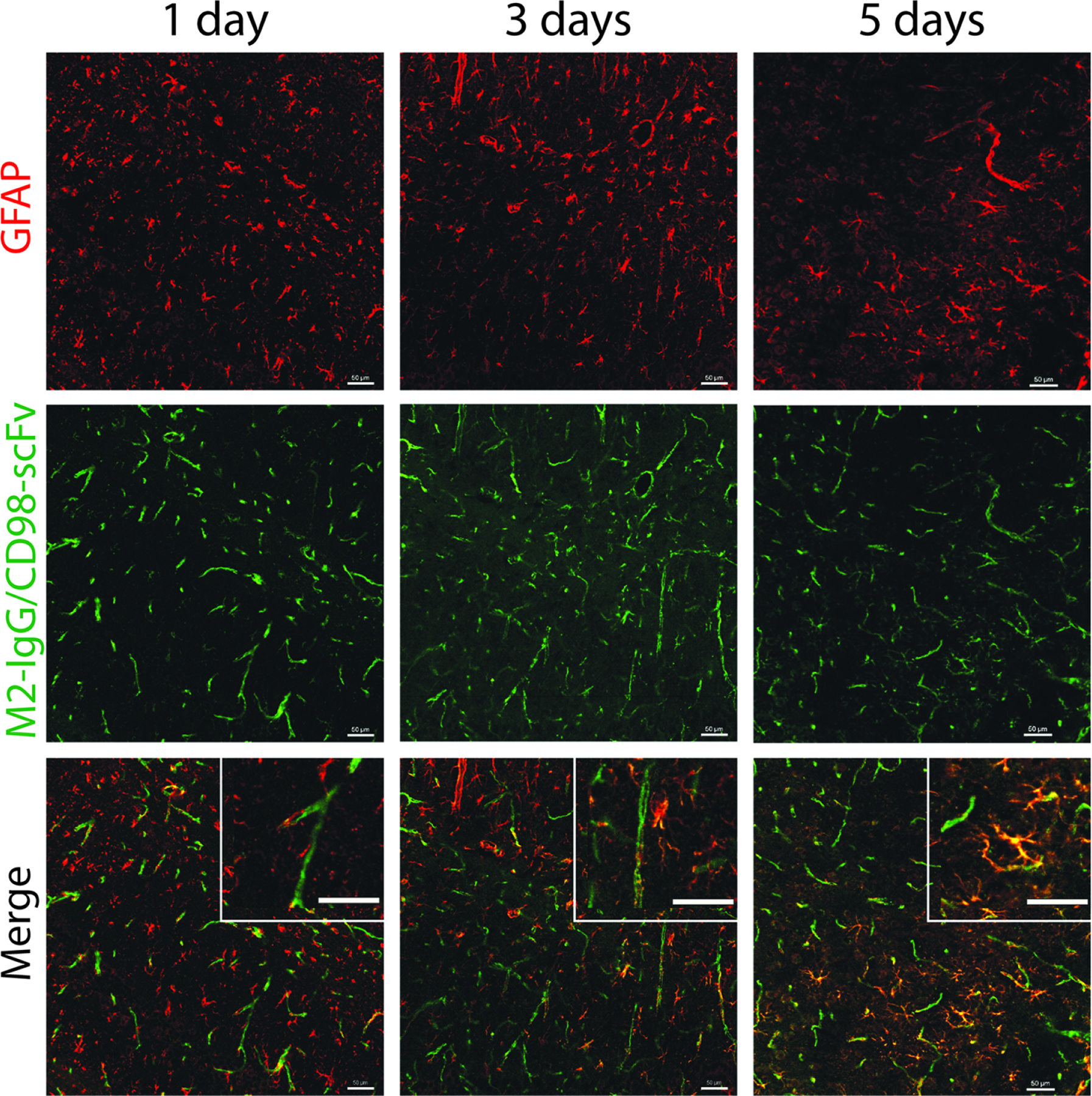
An astrocyte-specific IgG (M2), delivered via a CD98hc shuttle, was labeled with Alexa Fluor-647 and injected into wild-type mice (3.6 mg/kg, 20 nmol/kg). Brains were harvested after 1, 3 and 5 d, and sectioned for immunostaining. Antibody distribution of the shuttle (green) is shown relative to that of GFAP+ astrocytes (red). Scale bars are 50 μm.
The M6/CD98hc shuttle that is specific for neurons led to significantly different behavior (Fig. 6). Like the CD98hc-shuttled M2 antibody, a pattern suggestive of blood vessel localization was seen one day after administration, with no overlap with neuronal (NeuN) staining. After three days, the M6/CD98hc shuttle was strongly colocalized with NeuN+ neurons and appeared in a punctate distribution suggestive of cellular internalization. Finally, after five days, fluorescent signal from the shuttle was undetectable (Fig. S4). As with the M2 IgG, injection of non-shuttled M6 IgG produced little detectable fluorescent signal in the brain (Fig. S4).
Figure 6. Brain distribution of a CD98hc-shuttled IgG specific for neurons.

A neuron-specific IgG (M6), delivered via a CD98hc shuttle, was labeled with Alexa Fluor-647 and injected into wild-type mice (3.6 mg/kg, 20 nmol/kg). Brains were harvested after 1 and 3 d, and sectioned for immunostaining. Antibody distribution of the shuttle (green) is shown relative to that of NeuN+ neurons (red). Scale bars are 50 μm.
Finally, we sought to demonstrate the ability of the CD98hc shuttle to deliver an agonist antibody capable of activating an endogenous receptor in the mouse brain. Therefore, we selected an existing agonist IgG specific for the TrkB receptor with a published amino acid sequence, namely 29D734. We reformatted the TrkB IgG as a 2×1 CD98hc shuttle (GenBank: OR253075), and confirmed its ability to recognize and activate TrkB in vitro in a similar manner as the corresponding IgG agonist (Fig. S5).
Next, we intravenously administered the TrkB IgG and shuttle, and evaluated their target engagement (i.e., TrkB receptor activation) using brain immunostaining analysis (Fig. 7). Similar to the M2 and M6 shuttles, the TrkB/CD98hc shuttle was predominantly localized to blood vessels after one day, although some signal was observed within NeuN+ neurons. At this same time point, we observed staining for phospho-Akt (p-Akt), a marker of TrkB signaling (Fig. 7A). A similar pattern was seen after three days, at which point, most of the TrkB/CD98hc shuttle was internalized into NeuN+ neurons. After five days, the shuttle was barely detectable and p-Akt staining was strongly reduced. Similar to the p-Akt immunostaining results, we observed staining for phospho-ERK1/2 (p-ERK1/2), another key signaling molecule downstream of the TrkB receptor, at 1 and 3 days after injection with the TrkB/CD98hc shuttle (Figs. 7B and S6). In contrast, injection of non-shuttled TrkB IgG produced little p-Akt or p-ERK1/2 staining (Fig. S7). These results collectively demonstrate that the CD98hc-shuttled TrkB antibody strongly and specifically activates TrkB in the mouse brain for multiple days.
Figure 7. Brain distribution and TrkB activation of a CD98hc-shuttled agonist IgG.

A TrkB agonist IgG, delivered via a CD98hc shuttle, was labeled with Alexa Fluor-647 and injected into wild-type mice (3.6 mg/kg, 20 nmol/kg). Brains were harvested after 1, 3 and 5 d, and sectioned for immunostaining. Antibody distribution of the shuttle (grey) is shown relative to that of NeuN+ neurons (red) and either p-AKT in (A) or p-ERK1/2 in (B) in green. Scale bars are 50 μm in the main images and 10 μm in the enlarged inset images.
DISCUSSION
The high potency and extreme specificity of therapeutic IgGs have made them both the fastest growing drug class and essential tools for defining the molecular mechanisms of human disease. Despite their remarkable advantages, IgGs have had relatively little impact on the study or treatment of brain disorders, in large part, due to their inability to cross the BBB. Indeed, the recent failure of several, high-profile clinical trials has underscored the limitations of traditional antibody therapeutics for testing specific disease mechanisms within the brain35,36. In the absence of a defined mechanism or kinetics of brain uptake or pharmacodynamic markers of target engagement, these trials have failed not only to achieve their intended clinical outcomes, but also to convincingly test the underlying therapeutic hypothesis37. With this in mind, we have focused on characterizing a BBB transport pathway with a defined molecular mechanism. Our current results represent detailed characterization of the kinetics of brain uptake, parenchymal retention, and target engagement of IgGs shuttled into the brain via CD98hc, with particular focus on the ways in which this transport pathway differs from that of TfR-1.
Perhaps the most remarkable and interesting finding of our work is the sustained brain concentration of IgGs shuttled across the BBB via CD98hc. Our PK data, confirmed by three different methods, indicate IgG concentrations more than half their peak value at one week after CD98hc shuttling, a time point at which TfR-1-shuttled IgG can no longer be detected in the brain. There are a number of plausible mechanisms which may explain the differences in delivery and retention of IgG shuttled by the two pathways. First, fluorescence imaging indicates profound differences in the localization of non-targeted IgG shuttled into the brain via CD98hc versus TfR-1. The former remains associated with, or at least in close proximity to, blood vessels for at least five days post-injection, whereas TfR-1 shuttled IgG appears to be internalized by multiple cell types in the brain parenchyma. These data suggest a relative lack of CD98hc engagement in the brain parenchyma, and conversely, robust TfR-1 engagement, leading to internalization, intercellular catabolism, and a faster reduction in brain levels of the TfR-1 shuttle7,9,19,20. A second potential contributor is internalization and degradation of the cerebrovascular targets (i.e., TfR-1 and CD98hc) in the presence of saturating doses of 2x1 shuttle. This effect, which our in vitro analysis confirmed is far more pronounced for TfR-1 than CD98hc, would result in a steep decline in brain uptake and could explain both the earlier peak and the more rapid decline in brain concentration of the TfR-1 shuttle, relative to its CD98hc counterpart. Finally, there are potential differences in the polarization of CD98hc and TfR-1 on cerebrovascular endothelial cells38–41, which could impact and even bias bidirectional transport across the BBB and contribute to differences in brain accumulation and retention of the CD98hc and TfR-1 shuttles.
Another key aspect of our work is the 2x1 bispecific antibody format, which deserves further consideration. While there are many possible formats for bispecific antibodies42, including those that have been used as BBB shuttles4,8,18,20,28,43–50, the 2x1 format reported previously for TfR-1 shuttles (IgG fused to one single-chain Fab)8 and used in this work (IgG fused to one scFv) is compelling for multiple reasons. First, the two Fab regions of the IgG portion of the shuttle are not modified in any way. This is a distinct advantage because it eliminates the potential for avidity changes and potential changes in Fab affinity and specificity for the shuttled IgG. In comparison, bispecific antibody formats, such as dual-variable-domain (DVD) shuttles, require changes to the Fab regions of the shuttled IgG, including the introduction of linkers between the outer and inner variable regions, which can impact the affinity of both the inner and outer variable domains43,51. Most importantly, our results demonstrate straightforward conversion of existing IgGs (e.g., M2, M6, and 29D7) into 2x1 bispecific antibodies. There are presumably hundreds of similar antibodies with brain targets, developed for diagnostic or research applications, that have never been tested in vivo for their activity in the central nervous system, especially without the need for complex and highly invasive delivery methods.
Beyond its utility as a platform for delivering existing IgGs as reformatted brain shuttles, the other major advantage of the 2x1 format is its monovalent engagement of the BBB target7,49,52. Our data largely support the need for monovalent antibody engagement of TfR-1, which appears to reduce internalization and ultimately results in higher brain concentrations relative to the bivalent TfR-1 IgG. We observed mixed results for CD98hc IgG, which had higher peak brain concentrations than the 2x1 CD98hc shuttle at an intermediate dose (3 mg/kg for IgG and 3.6 mg for shuttle, 20 nmol/kg) and lower concentrations at a higher dose (9 mg/kg for IgG and 10.8 mg for shuttle, 60 nmol/kg). Given that the monovalent 2x1 CD98hc shuttle resulted in extended brain retention relative to its bivalent counterpart, monovalent engagement of CD98hc appears optimal for maximizing IgG brain levels and extended brain retention, assuming a sufficiently high dose is used. This observation will need to be investigated further given that the intrinsic CD98hc antibody affinity and epitope53, in addition to valency, are likely to impact the efficiency of IgG brain shuttling and retention.
It is also important to consider the implications of the unique biodistribution of the CD98hc and TfR-1 shuttles within the brain and between different organs. In the brain, we found that non-targeted CD98hc shuttles do not mediate appreciable cellular uptake in the brain parenchyma, unlike the corresponding TfR-1 shuttles. This potentially enables improved ability of CD98hc shuttles to specifically target different cell types in the brain. However, this increased specificity should be considered in the context of CD98hc- and TfR-1-mediated shuttling of antibodies to the brain versus other organs. In some cases, CD98hc shuttles led to increased non-brain organ uptake, including in the kidney and spleen, relative to TfR-1 shuttles. For certain applications, the reduced delivery of bispecific antibodies to certain non-brain organs may be an advantage for TfR-1 shuttles relative to CD98hc shuttles.
The interaction of CD98hc and TfR-1 shuttles with their cerebrovascular and parenchymal targets and their impact on brain uptake, retention, and target engagement are likely to have profound implications for the many exciting future directions of this work. First, the CD98hc shuttles, which result in limited engagement of CD98hc in the brain parenchyma, could be used in applications that seek to deliver IgGs to the brain without cellular internalization within the brain parenchyma. These applications could include delivery of IgGs that capture soluble factors such as Aβ in Alzheimer’s disease54,55 and VEGF in glioblastoma56,57. These tasks are more complex to perform using TfR-1 shuttles because of TfR-1–mediated shuttle internalization into cells within the brain parenchyma. Second, the ability of the CD98hc-shuttled M2 antibody to enter the brain parenchyma and remain stably associated with astrocytes could be exploited for the brain delivery of conjugated drugs. This may be particularly useful in applications where extracellular brain release of drugs is desired, such as antibiotics58,59 or extended release of drugs into the brain parenchyma after brain localization to minimize systemic side effects, such as various types of toxins used for treating epilepsy60. Third, in contrast, the ability of the CD98hc-shuttled M6 antibody to specifically internalize into neurons could be utilized to deliver conjugated drugs that require intracellular delivery. For example, anti-sense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) need intracellular delivery to evaluate the impact of altering protein expression on normal and disease phenotypes61,62. This would be particularly significant because these agents are typically delivered to the brain by invasive methods (e.g., intrathecal or intracerebroventricular administration)63–66. Fourth, the ability of the CD98hc-shuttled TrkB antibody to activate both AKT and ERK1/2 for at least two days after a single intravenous dose, which is one of the longest periods of in vivo TrkB activation reported to date after a single dose67–69, could be used to evaluate the therapeutic impact of activating TrkB in the context of several acute (e.g., traumatic brain injury and stroke) and chronic (e.g., neurodegenerative) diseases. This is significant because there is preclinical evidence that TrkB activation is neuroprotective70–76 and/or reduction of brain-derived neurotrophic factor (BDNF) or TrkB signaling is linked to neuronal dysfunction77–81. These and other exciting possibilities are expected to simplify fundamental and therapeutic studies of the delivery of antibodies and other agents to the brain.
LIMITATIONS OF THIS STUDY
Our study has some limitations that will need to be addressed in future work. First, our radiotracing data must be interpreted in the context of 125I being a non-residualizing radiolabel – i.e., one that rapidly diffuses out of cells and organs once released from labeled antibody. This impacts the interpretation of the pharmacokinetic results, as a decrease in radioactive signal can reflect either clearance of intact antibody or antibody catabolism and free iodine release. Future work using residualizing labels (e.g., 89Zr) will be needed to differentiate these phenomena and fully define the fate of antibodies shuttled across the BBB by CD98hc and TfR-1. Second, the mechanism of CD98hc-mediated transcytosis remains unclear, as the endogenous function of the LAT1 channel does not necessitate physical transport of cargo across endothelial cells. Third, the epitope of the CD98hc antibody in this study is unknown, and the impact of the antibody epitope on the CD98hc transport mechanism and kinetics will need to be further evaluated in future studies. Finally, the M6 antibody reported in Fig. 6 recognizes GPM6a, which is not entirely specific for neurons, as it is also found on astrocytes and oligodendrocyte progenitor cells.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peter Tessier (ptessier@umich.edu)
Materials Availability
CD98hc sequences used in this study are published in Figure S1. All antibodies used are available upon reasonable request from the lead contact. All sequences of antibodies were verified via Sanger sequencing.
Data and Code Availability
The data reported in this paper will be shared by the lead contact upon request. The sequences of antibodies developed in this study have been deposited in GenBank under accession numbers OR253073-OR253075 and are publicly available. Accession numbers for specific antibodies can also be found in the Key Resources Table.
Code for curve fitting of binding curves has been deposited in GitHub and can be found at https://github.com/Tessier-Lab-UMich/Data_Fitting (DOI: 10.5281/zenodo.8317352).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
| Antibodies | ||
| CTL-IgG | Bujotzek et al. 2016 | PDB: 5DMG |
| CD98hc IgG | Yasutomo et al. 2013 | N/A |
| TfR-1 IgG | Kissel et al. 1998 | N/A |
| CTL-IgG/CD98-scFv | This paper | Genbank: OR253074 |
| CTL-IgG/TfR-1-scFv | This paper | Genbank: OR253073 |
| M2 | Dr. Carl Lagenaur | N/A |
| M6 | Dr. Carl Lagenaur | N/A |
| 29D7 | Qian et al. 2006 | N/A |
| TrkB-IgG/CD98-scFv | This Paper | Genbank: OR253075 |
| Goat anti-human IgG AlexaFluor 647 | Jackson ImmunoResearch | Cat#109-605-098 |
| Goat anti-human IgG AlexaFluor 488 | Jackson ImmunoResearch | Cat#109-546-170 |
| R17 | BioLegend | Cat#113802 |
| RL388 | BioLegend | Cat#128202 |
| Mouse anti-rat IgG AlexaFluor 647 | BioLegend | Cat#407511 |
| Anti-human Polyvalent Immunoglobulins (G,A,M) | Millipore Sigma | Cat#I1761 |
| Goat anti-human IgG-peroxidase | Millipore Sigma | Cat#A0170 |
| NeuN Monoclonal Antibody (1B7) | Thermo Fisher Scientific | Cat#MA5–33103 |
| GFAP Polyclonal Antibody | Thermo Fisher Scientific | Cat#PA1–10019 |
| Phospho-AKT1 (Ser473) Monoclonal Antibody | Thermo Fisher Scientific | Cat#44–621G |
| Phospho-ERK1/ERK2 (Thr202. Tyr204) Polyclonal Antibody | Thermo Fisher Scientific | Cat#36–8800 |
| ZO-1 Monoclonal Antibody (ZO1–1A12), AlexaFluor 488 | Thermo Fisher Scientific | Cat#339188 |
| Akt (pan) (11E7) Rabbit mAb | Cell Signaling Technology | Cat#4685 |
| P44/42 MAPK (Erk1/2) Antibody | Cell Signaling Technology | Cat#9102 |
| TrkB (80E3) Rabbit mAb | Cell Signaling Technology | Cat#4603 |
| Phospho-Akt (Ser473) (D9E) Rabbit mAb | Cell Signaling Technology | Cat#4060 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody | Cell Signaling Technology | Cat#9101 |
| Phospho-TrkB (Tyr516) Polyclonal Antibody | Thermo Fisher Scientific | Cat#PA5–36695 |
| Anti-rabbit IgG HRP-linked Antibody | Cell Signaling Technology | Cat#7074 |
| Bacterial and virus strains | ||
| DH5α | Thermo Fisher Scientific | Cat#18265017 |
| Biological samples | ||
| None | ||
| Chemicals, peptides, and recombinant proteins | ||
| DMEM media | Corning | Cat#10-013-CV |
| BSA | Thermo Fisher Scientific | Cat#BP9706–100 |
| 0.25% trypsin-EDTA | Gibco | Cat#25200056 |
| Antibiotic-antimycotic | Gibco | Cat#15240062 |
| FBS | Corning | Cat#35-010-CV |
| RPMI media | Corning | Cat#10-040-CV |
| Lipofectamine 2000 | Invitrogen | Cat#11668–027 |
| EcoRI-HF | New England Biolabs | Cat#R3101L |
| XbaI | New England Biolabs | Cat#R0145S |
| Q5 High-Fidelity DNA Polymerase | New England Biolabs | Cat#M0491L |
| NheI-HF | New England Biolabs | Cat#R3131L |
| HindIII-HF | New England Biolabs | Cat#R3104L |
| Quick CIP calf intestinal alkaline phosphatase | New England Biolabs | Cat#M0525L |
| T4 ligase | New England Biolabs | Cat#M0202L |
| F17 media | Thermo Fisher Scientific | Cat#A1383502 |
| Glutamine | Invitrogen | Cat#25030081 |
| Kolliphor | Thermo Fisher Scientific | Cat#NC0917244 |
| G418 | Thermo Fisher Scientific | Cat#10131035 |
| Yeastolate | BD Sciences | Cat#292804 |
| Streptavidin AlexaFluor 647 | Invitrogen | Cat#S32357 |
| Imidazole | ACROS Organics | Cat#12202–5000 |
| Mouse TfR | ACRO Biosystems | Cat#TFR-M524b |
| [125I]NaI | Perkin Elmer | Cat#NEZ033A |
| Pierce iodination reagent | Thermo Fisher Scientific | Cat#28601 |
| Phusion HF PCR MasterMix w/ HF Buffer | New England Biolabs | Cat#M0531S |
| Methanol | Thermo Fisher Scientific | Cat#A421–4 |
| Sodium acetate | Sigma-Aldrich | Cat#S2889–250G |
| Isoflurane | Henry Schein | Cat#66794-017-25 |
| Trypan blue | Gibco | Cat#15250061 |
| Krebs buffer | Thermo Fisher Scientific | Cat#J67591.AP |
| Dextran | Acros | Cat#406271000 |
| RIPA buffer | Thermo Fisher Scientific | Cat#WK333492 |
| 100X Halt Protease Inhibitors | Thermo Fisher Scientific | Cat#1860932 |
| Goat serum | Novus Biologicals | Cat#S13150NOV |
| Tween 20 | Thermo Fisher Scientific | Cat#J20605-AP |
| TMB substrate solution | Thermo Fisher Scientific | Cat#N301 |
| H2SO4 | Thermo Fisher Scientific | Cat#A300–212 |
| Sodium bicarbonate | Sigma-Aldrich | Cat#S6297–250G |
| 4% paraformaldehyde | Thermo Fisher Scientifice | Cat#J19943.K2 |
| Sucrose | Thermo Fisher Scientific | Cat#S5–500 |
| OCT | Thermo Fisher Scientific | Cat#4585 |
| Sodium citrate buffer | Thermo Fisher Scientific | Cat#S279–3 |
| 0.5% Triton-X 100 | Alfa Aesar | Cat#A16046 |
| DAPI | Thermo Fisher Scientific | Cat#YC3861511 |
| Anti-fade reagent | Thermo Fisher Scientific | Cat#P36930 |
| Dry milk | Kroger | N/A |
| Phosphatase and protease inhibitor cocktail 100X | Thermo Fisher Scientific | Cat#P178440 |
| 20% SDS | Dot Scientific Inc. | Cat#DSL23100–500 |
| Trizma base | Sigma-Aldrich | Cat#T1503–1KG |
| HCl | Thermo Fisher Scientific | Cat#SA56–4 |
| β-mercaptoethanol | ACROS Organics | Cat#125472500 |
| Glycerol | Thermo Fisher Scientific | Cat#BP229–1 |
| EDTA | Thermo Fisher Scientific | Cat#O2793–500 |
| Bromophenol blue | Thermo Fisher Scientific | Cat#A18469.09 |
| SignalFire ECL Reagent | Cell Signaling Technology | Cat#6883 |
| Critical commercial assays | ||
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat#23227 |
| Deposited data | ||
| Antibody Sequences | GenBank | OR253073–5 |
| Experimental models: Cell lines | ||
| Human: HEK293–6E | National Research Council (NRC) of Canada | N/A |
| Human: REN-CD98hc | This paper | N/A |
| Human: REN-TfR | This paper | N/A |
| Human: REN-mTrkB | This paper | N/A |
| Mouse: bEnd.3 | ATCC | Cat#CRL-2299 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice | Jackson Laboratory | Strain#000664 |
| Oligonucleotides | ||
| None | ||
| Recombinant DNA | ||
| None | ||
| Software and algorithms | ||
| Prism 9.5.1 | GraphPad | https://www.graphpad.com/updates/prism-951-release-notes |
| Python 3.11.5 | Python Software Foundation | https://www.python.org |
| Data fitting algorithm | This Paper | DOI: 10.5281/zenodo.8317352 |
| Other | ||
| Streptavidin MicroBeads | Miltenyi Biotec | Cat#130-048-101 |
| Streptavidin Dynabeads | Invitrogen | Cat#11047 |
| Protein A magnetic beads | Invitrogen | Cat#88846 |
| QIAquick Gel Extraction Kit | Qiagen | Cat#28704 |
| QIAquick PCR Purification Kit | Qiagen | Cat#28104 |
| QIAprep Spin Miniprep Kit | Qiagen | Cat#27106 |
| Protein A Agarose | Thermo Fisher Scientific | Cat#20333 |
| Centrifuge columns | Thermo Fisher Scientific | Cat#89898 |
| Zeba Spin Desalting Columns | Thermo Fisher Scientific | Cat#89894; Cat#89892; Cat#89890 |
| Invitrogen NuPAGE 10% Bis-Tris, 1.0 mm, Midi Protein GEL (SDS-PAGE) | Thermo Fisher Scientific | Cat#WG1203BOX |
| Superdex 200 Increase 10/300 GL column | GE | Cat#28990944 |
| 96-well plates | VWR | Cat#650261 |
| Protein A Dynabeads | Invitrogen | Cat#10002D |
| Ni-NTA agarose | Qiagen | Cat#30230 |
| Heparin-coated capillary tubes | Thermo Fisher Scientific | Cat#22–3652566 |
| TissueLyser LT | Qiagen | Cat#85600 |
| Immulon 4HBX flat bottom plates | Thermo Fisher Scientific | Cat#3855 |
| 30kDa MW centrifugal filter | Millipore Sigma | Cat#UFC503096 |
| IVIS Lumina S5 | Perkin Elmer | |
| Cryostat | Leica | Cat#NX50 |
| Glass slides | Thermo Fisher Scientific | Cat#1255015 |
| Hydrophobic pen | Vector Laboratories | Cat#H-4000 |
| Coverslip | Thermo Fisher Scientific | Cat#12543D |
| Confocal microscope | Nikon | A1si |
| Flow cytometer, ZE5 cell analyzer | Bio-Rad | Cat#12004278 |
| 6-well plates | Thermo Fisher Scientific | Cat#140675 |
| TLC silica gel 60F254 plates | Millipore Sigma | Cat#105554 |
| 4–12% Bis-Tris SDS-PAGE gel | Thermo Fisher Scientific | Cat#WG1401A |
| MagicMark XP western Protein Standard | Thermo Fisher Scientific | Cat#LC5602 |
| Nitrocellulose membrane | Thermo Fisher Scientific | Cat#IB23001 |
| iBlot 2 Dry Transfer System | Thermo Fisher Scientific | Cat#IB21001 |
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Cell Lines
All cell culture work was performed using standard aseptic technique. All facilities were rated for biosafety level 2 or 3 for cell culturing and experiments, and appropriate safety precautions were observed for the respective biosafety levels. HEK293–6E cells are a human female embryonic kidney cell line licensed from the National Research Council of Canada for the transient expression of protein. The growth media for these cells is F17 media supplemented with Glutamine, Kolliphor, and G418, and they are cultured at 37 °C with 5% CO2 with agitation at 250 RPM. Cells were passaged or transfected after reaching a density of approximately 2 million cells/mL. Transfection was performed as described in the Method Details.
REN cells are a human epithelial cell line (unknown sex) cultured in RPMI media with 10% FBS, 1% Antibiotic-Antimycotic (“anti-anti”), and 200 μg/mL Geneticin at 37 °C with 5% CO2. Production of TfR and CD98hc expressing REN cells and binding assays with REN cells are described in the Method Details.
Mouse brain endothelial cells (bEnd.3, ATCC, CRL-2299, unknown sex) were cultured in DMEM media with 10% FBS and 1% Antibiotic-Antimycotic (“anti-anti”). Receptor level experiments are described in the Method Details.
METHOD DETAILS
Recombinant antibodies.
Antibody sequences are available in GenBank (OR253073-ORF253075). CTL IgG is an anti-phospho-tau IgG (PDB ID: 5DMG), which was generated by rat immunization using phosphorylated tau (pS422)82. CD98hc IgG is an anti-mouse CD98hc IgG generated by rat immunization using mouse bone marrow cells21. TfR-1 IgG (clone 8D3) is an anti-mouse TfR-1 IgG prepared by rat immunization using murine endothelial cells (t.end 1)83. M2 is an anti-mouse astrocyte surface antigen IgG produced by rat immunization using the particulate fraction of mouse cerebellar homogenates32. M6 is an anti-mouse neuronal membrane glycoprotein M6-a IgG generated by rat immunization using mouse cerebellar homogenate31. The variable regions of M2 and M6 hybridomas were sequenced after generating cDNA internally or by GenScript with permission from Dr. Carl Lagenaur, University of Pittsburgh, and University of Heidelberg. 29D7 (TrkB-IgG) is an anti-TrkB IgG produced via mouse immunization using the extracellular domains of human and mouse TrkB84.
Cloning and production of IgGs and bispecific shuttles.
Expression vector pTT5 and mammalian cell line (HEK293–6E) were licensed from the National Research Council of Canada (NRC). A bispecific shuttle was generated by first introducing ‘knob-into-hole’ mutations22,23 in the Fc region of human IgG1. Next, genes of either TfR-1 scFv or CD98 scFv were genetically fused via a glycine-serine linker (G4S)3 to the C-terminus of the ‘knob’ chain. The expression vector was digested with appropriate restriction enzymes (New England Biolabs) following the manufacturer’s protocol, and subsequently treated with calf intestinal alkaline phosphatase (New England Biolabs, M0525L). The digested vector was separated by gel electrophoresis (1% agarose). Appropriately sized DNA was excised and purified (Qiagen, 28704). Digested inserts and vectors were ligated with T4 ligase (New England Biolabs, M0202L) and transformed via heat shock into DH5α competent cells. Cells were incubated in the presence of LB media (antibiotic free) for 1 h at 37 °C on an orbital shaker (200 rpm), and then plated on LB plates supplemented with ampicillin (100 μg/mL) overnight at 37 °C. Single colonies were picked, grown in ampicillin-supplemented LB media overnight, miniprepped (Qiagen, 27106), and sequenced by Sanger sequencing.
Expression of antibodies was performed by transfecting 25 mL of mammalian cell culture (2×106 cells/mL) with appropriate plasmids. In each conical tube, 15 μg of total DNA (equal parts knob, hole, and light chains) was mixed with 45 μL of 40 kDa polyethylenimine (1 mg/mL) and 3 mL of F17 media (Thermo Fisher, A1383501) in a sterile hood for 15 min. Next, the mixture was added to the cells and then transferred back to the incubator (37 °C, 5% CO2). After 24 h, 0.75 mL of yeastolate (%20 w/w) (Thermo Fisher, B92804) was added to each tube of transfected cells. The cells were harvested 6 d after transfection and centrifuged at 3500 rpm for 40 min.
The supernatant was filtered (Thermo Fisher, 166–0045) and passed through a pre-packed Protein A agarose (Thermo Fisher, 20333) column. After washing the column with 50 mL of PBS, 20 mL of glycine buffer (pH 3.0) was added to the column to elute the protein. Elution fractions were collected, and immediately buffer exchanged into 20 mM Acetate (pH 5.0) using desalting columns (Thermo Fisher, 89891) and stored at −80 °C. The absorbance (A280) was measured via NanoDrop to determine antibody concentration. Antibodies were run on SDS-PAGE gels as well as analyzed via size exclusion chromatography (SEC) (Superdex 200 Increase 10/300 GL, Cytvia #28990944). Antibodies that were <90% monomer after Protein A purification, as determined by SEC, underwent a second-step purification via fraction collection on SEC until desired purity was achieved.
Generation of mouse TfR and CD98hc expressing cell lines.
Full-length coding sequences for murine TfR-1 (CD71, NCBI reference sequence: NP_035768.1) and CD98hc (NCBI reference sequence: NP_001154885.1) were cloned into the pcDNA 3.1 (+) plasmid between restriction enzyme sites NheI/XbaI and HindIII/EcoRI, respectively. For CD98hc, a FLAG tag was fused to the intracellular N-terminal domain. Plasmids were transfected into the REN cell line using Lipofectamine 2000 per manufacturer protocol85. Cells were selected in media containing 200 μg/mL of Geneticin (Thermo Fisher Scientific) and flow sorted using a MoFlo Astrios cell sorter (Beckman Coulter, Brea, CA) based on binding of TfR1 and CD98hc IgG, respectively.
Flow cytometry binding analysis of TfR-1 and CD98hc shuttles.
TfR-expressing (REN-TfR) and CD98-expressing (REN-CD98) cell lines were grown in RPMI media with 10% FBS, 1% Antibiotic-Antimycotic (“anti-anti”), and 200 μg/mL Geneticin until confluent. Cells were washed with PBS, detached from the flask with 0.25% Trypsin-EDTA, and resuspended in fresh media at a density of 106 cells/mL. Cells were then seeded on a 96-well plate at a density of 105 cells per well. Serial dilutions of antibodies were prepared with cold PBS supplemented with 0.1% BSA (PBSB). Seeded cells were treated with serial dilutions of the antibodies for 1 h at 4 °C on an orbital shaker (200 rpm). After treatment, the plate was centrifuged at 2500 rpm for 4 min and washed with PBSB twice. The cells were then incubated with an anti-human Fc detection antibody AF-647 (1:500; Jackson, 109-605-098) for 4 min on ice. After incubation, the plate was centrifuged at 2500 rpm for 4 min and washed with PBSB twice. The cells were resuspended with 200 μL of PBSB and analyzed on a flow cytometer (Bio-Rad, ZE5 cell analyzer).
Flow cytometry analysis of IgG and bispecific affinity for soluble CD98hc and TfR-1 ectodomains.
Affinities of IgGs and bispecifics were measured by flow cytometry after immobilizing antibodies on the surface of Protein A Dynabeads. Protein A Dynabeads (Invitrogen, 10002D) were washed with 1x PBS supplemented with 0.1% BSA (PBSB) and incubated with antibodies (85 μL at 15 μg/mL) overnight at 4 °C with mild agitation. The beads were then washed with PBSB and incubated with biotinylated CD98hc and TfR-1 ectodomains at a range of concentrations (1250, 250, 50, 10, 2, 0.4, 0.08 and 0.016 nM) such that antigen was present in 10-fold molar excess of immobilized antibodies at 4 °C with mild agitation for approximately 3 h.
CD98hc IgG and CD98hc bispecific antibodies were incubated with mouse CD98hc ectodomain. The CD98hc ectodomain (NP_001154885 residues 139–565 with an N-terminal 6x His tag) was expressed in HEK293–6E cells, as previously described for the production of IgGs and mAbs,86,87 and purified using immobilized metal-affinity chromatography (Qiagen, 30230) and size-exclusion chromatography. Briefly, supernatant containing mouse CD98hc ectodomain was incubated with nickel beads overnight at 4 °C, the beads were washed with 1x PBS and 50 mM imidazole, and ectodomain was eluted from the beads with 500 mM imidazole. The CD98hc ectodomain was then buffer exchanged into 1x PBS, and monomeric CD98hc was further purified by size-exclusion chromatography. Purified CD98hc was aliquoted and stored at −80 °C until use.
TfR IgG and TfR bispecific antibodies were incubated with mouse TfR (ACRO Biosystems, TFR-M524b). The beads were then washed with PBSB and incubated with a 1:1000 dilution of streptavidin Alexa Fluor 647 (Invitrogen, S32357) and 1:1000 dilution of goat anti-human Fc F(‘ab)2 Alexa Fluor 488 (Jackson ImmunoResearch, 109-546-170) on ice for 4 min. Next, the beads were washed once with PBSB and resuspended in PBSB for analysis by flow cytometry using a Bio-Rad ZE5 cell analyzer. The mean fluorescent intensities were recorded and analyzed. Results are reported after subtraction of background signal observed in the absence of antigen (0 nM), subtraction of the lowest binding signal observed (baseline of 0), and normalization to the highest binding signal observed.
Endothelial surface level analysis of TfR-1 and CD98hc.
Mouse brain endothelial cells (bEnd.3, ATCC, CRL-2299) were plated on a 6-well plate at a density of 3x105 cells/well and grown overnight in 2 mL of growth media (DMEM with 10% FBS and 1% anti-anti). Cells were then treated overnight with either 100 nM IgG (TfR or CD98) or 200 nM shuttle (CTL-IgG/TfR- or CD98-scFv) added to the growth media. The next day, media was removed and cells were washed with PBS then treated with 0.25% Trypsin-EDTA for 3–5 min until cells became detached. Cells were then resuspended in 1mL of growth media and transferred to microcentrifuge tubes. Cells pre-treated with anti-mouse TfR-1 antibodies (human constant regions) were incubated with 50 nM of a second TfR-1 antibody for detection (R17, rat anti-mouse antibody; BioLegend, 113802). Cells pre-treated with anti-mouse CD98hc antibodies (human constant regions) were incubated with 10 nM of a second CD98hc antibody for detection (RL388, rat anti-mouse antibody; BioLegend, 128202). Non-treated cells were also incubated with the TfR-1 (R17) and CD98hc (RL388) detection antibodies to determine baseline levels of TfR-1 and CD98hc without antibody-mediated internalization. The incubations with the detection antibodies (R17 and RL388) lasted for 1 h and were performed while rotating at 4 °C. Cells were then twice spun down at 2500 rpm for 4 min and resuspended in PBSB. Following the second wash, cells were incubated for 4 min, on ice, with a secondary (anti-rat) detection antibody labeled with Alexa Fluor-647 (Biolegend, 407511) at a 1:500 dilution. Samples were again twice spun down and washed with PBSB. Cells were resuspended in a final volume of 100–200 μL PBSB and analyzed on a flow cytometer (Bio-Rad, ZE5 cell analyzer).
Animal use and protocol.
Animal studies were conducted following guidance for the care and use of laboratory animals as adopted by the NIH, under protocols PRO00010991 and PRO00009238 approved by the institutional animal care and use committee (IACUC) of the University of Michigan. C57BL/6J male mice (Jackson Laboratory, 000664), aged 8–16 weeks, were used for all animal experiments.
Radiolabeling.
Proteins were directly radioiodinated with [125I]NaI (Perkin Elmer) using Pierce iodination reagent (Thermo Fisher, 28601) and purified using Zeba desalting columns. Radiochemical purity was assessed via thin layer chromatography (TLC) performed using aluminum TLC silica gel 60 F254 plates (Millipore Sigma, 105554) and a 75%:25% mixture of methanol and 1 M sodium acetate (pH 6.8) as a mobile phase, as previously reported88. Radiolabeling efficiency was typically >75% and proteins were only used for radiotracing if free 125I was <5% following purification.
Pharmacokinetic analysis.
For all radiotracing experiments, a tracer dose (~1 μg) of 125I-labeled IgG or bispecific shuttle was added to the appropriate dose of unlabeled protein to achieve approximately 106 cpm on a Wizard2 2470 gamma counter. Prior to injection, mice were weighed, and doses were sterile-filtered and prepared in sterile PBS to a final volume of 110 μL. Mice were sedated with isoflurane (Henry Schein, 66794-017-25) and injected intravenously via the retroorbital plexus. In some experiments, mice were re-sedated briefly (<5 min) at intermediate time points for blood collection using heparin-coated capillary tubes (Thermo Fisher, 22–3652566). At the terminal time point, mice were re-sedated and transcardially perfused with ice-cold PBS. Blood and organs of interest were harvested and weighed prior to gamma counting.
Antibody brain parenchyma concentration analysis.
125I-labeled antibody doses were prepared as described above at concentrations of 3 mg/kg for IgGs (20 nmol/kg) and 3.6 mg/kg for shuttles (20 nmol/kg). One hour after injection, mice were anesthetized with isoflurane and transcardially perfused with ice-cold PBS with 10% trypan blue (Gibco, 15250061). Trypan blue was used to visualize brain vasculature after homogenization. Brains were harvested and homogenized in 1 mL Krebs buffer (Thermo Fisher, J67591.AP) using a TissueLyser LT (Qiagen, 85600) for 5 min at 40 Hz. The resulting homogenate was then added to 9 mL of 18% dextran (Acros, 406271000) in a 15 mL conical tube, shaken vigorously, and then centrifuged at 4300xg in a swinging bucket rotor for 45 min. Capillary depletion was confirmed through visualization of a pellet containing the trypan blue stained vessels while brain parenchyma remained layered above the dextran. Fractions of 1 mL were drawn from the top of the tube and separated into glass vials. The vials were analyzed on the gamma counter and the resulting counts were used to determine the percent of 125I- labeled antibodies in the capillaries and parenchyma.
ELISA for the determination of brain antibody concentration.
Immulon 4HBX flat bottom plates (Thermo Fisher, 3855) were coated with anti-human polyvalent immunoglobulins (Sigma, I1761) in a 1:1000 dilution and stored at 4 °C overnight. Harvested brains were weighed and 1 mL of RIPA buffer, including 10 μL of 100X Halt Protease Inhibitors (Thermo Fisher, 1860932), was added to each brain sample. Brains were homogenized using a Qiagen TissueLyser LT and the resulting homogenate incubated in RIPA buffer overnight at 4 °C. The homogenate was then centrifuged at 14,000xg for 20 min and the supernatant was recovered for ELISA analysis. ELISA plates were washed 4x with 0.5% PBST, blocked for 1 h with 5% goat serum, and then supernatant was added to ELISA plates in serial dilution (100 μL) prepared in 5% goat serum. The corresponding antibody was also prepared in RIPA buffer with protease inhibitors to be used as a standard curve. Samples incubated for 2 h and were then washed 4x with 0.5% PBST. 100 μL of anti-human IgG (Fc specific)-peroxidase antibody produced in goat (Sigma, A0170) was added to each sample in a 1:40,000 dilution of PBS and incubated for 1 h. Plates were washed 4x with 0.5% PBST, incubated for 15 min in TMB Substrate Solution (Thermo Fisher, N301), and quenched with 0.18 M H2SO4. The plates were then analyzed for their absorbance at 450 nm, and concentrations were calculated based on the standard curve and the dilution factor calculated from the initial weight of the brain.
Fluorescence imaging of antibody organ distribution.
Antibodies were first labeled with Alexa Fluor-647 NHS Ester (Thermo Fisher, A20006) following manufacturer’s protocol to achieve a degree of labeling of ~1.0. Briefly, 200 μL of antibody (1 mg/mL) in PBS was mixed with 20 μL of sodium bicarbonate (1 M) and 0.4 μL of dye (10 mg/mL). Purification was performed by centrifuging (1 min, 12000 rpm) the labeled sample in a 30 kDa molecular weight cutoff centrifugal filter (Millipore Sigma, UFC503096) and washing the retained labeled antibody with 200 μL of PBS three times. The absorbances (280 and 650 nm) were measured on a NanoDrop to calculate the degree of labeling.
C57BL/6J male mice (8–16 weeks old) were intravenously injected with either labeled IgGs (3 mg/kg) or bispecific shuttles (3.6 mg/kg). After 7 days, the mice were sacrificed and transcardial perfusion was performed with 25 mL of ice-cold PBS. Organs including the liver, lung, kidneys, heart, spleen, and brain were removed and transferred to a 6-well plate. Ex vivo imaging of the organs was performed on a fluorescence imager (Perkin Elmer, IVIS Lumina S5).
Brain sectioning and immunofluorescence analysis.
After transcardial perfusion with ice-cold PBS, the mouse brains were removed and submerged in 4% paraformaldehyde (Thermo Fisher, J19943.K2) overnight at 4 °C for fixation. The brains were then transferred to a 30% sucrose solution for 24 h at 4 °C. The whole brains were first divided into two sagittal hemispheres. Next, the right hemispheres were divided coronally in the middle with a razor, placed in a cryomold filled with OTC and transferred to a −80°C freezer. Sections (20 μm) were collected by using a cryostat (Leica, NX50), which were then transferred onto glass slides (Thermo Fisher, 1255015), and left to dry overnight at room temperature.
Immunostaining was performed using standard protocols89,90. A barrier was first drawn around the brain section using a hydrophobic pen (Vector laboratories, H-4000). The section was treated with cold methanol for 5 min and washed with PBS. Antigen retrieval was performed by heating the slides in 10 mM sodium citrate buffer pH 6.0 (Thermo Fisher, S279–3) for 4 min in a microwave and left to cool down for 10 min at room temperature. The sections were washed with PBS and treated with 0.5% Triton-X 100 (Alfa Aesar, A16046) for 10 min. The sections were then blocked with PBS supplemented with 5% goat serum (Novus Biologicals, S13150NOV) and 0.05% Triton-X 100 for 1 h. The sections were washed with PBS and then incubated with primary antibodies (Key Resources Table), prepared in blocking solution using the manufacturer’s suggested dilution, overnight at 4°C. Next, the sections were washed with PBS and incubated with appropriate secondary detection antibodies (Key Resources Table), following manufacturer’s suggested dilutions, for 1 h at room temperature. The sections were washed with PBS and counter stained with DAPI (1:1000) for 4 min. The sections were washed with PBS twice, preserved with an antifade reagent (Thermo Fisher, P36930), and protected with a coverslip (Thermo Fisher, 12543D). Immunofluorescence imaging was performed using a confocal microscope (Nikon, A1si confocal).
In vitro TrkB affinity analysis.
Mouse TrkB-expressing (REN-mTrkB) cells were grown in RPMI media with 10% FBS, 1% anti-anti, and 200 μg/mL Geneticin until confluent. Cells were washed with PBS, detached from the flask with 0.25% trypsin, and resuspended in 2% milk in PBSB to a density of 106 cells/mL. Cells were then seeded on a 96-well plate at a density of 105 cells/well. Serial dilutions of antibodies (TrkB-IgG and TrkB-IgG/CD98-scFv) were prepared with cold PBSB. Seeded cells were treated with serial dilutions of the antibodies for 1 h at 4 °C on an orbital shaker (200 rpm). After treatment, the plate was centrifuged at 2500 rpm for 4 min and washed with PBSB twice. The cells were then incubated with an anti-human Fc detection antibody AF-647 (1:500; Jackson, 109-605-098) for 4 min on ice. After incubation, the plate was centrifuged at 2500 rpm for 4 min and washed with PBSB twice. The cells were resuspended with 200 μL of PBSB and analyzed on a flow cytometer (Bio-Rad, ZE5 cell analyzer).
Western blot analysis.
REN cells expressing mouse TrkB (REN-mTrkB) were seeded into 6-well plates (3×105 cells/well) and grown to confluence in RPMI media with 10% FBS, 1% anti-anti, and 200 μg/mL Geneticin. Once confluent, media was removed, and cells were treated with 1 nM TrkB IgG, 1 nM TrkB shuttle, 10 nM TrkB IgG, and 10 nM TrkB shuttle in RPMI media. Additionally, cells were treated with 1 nM BDNF as a positive control and 10 nM of CTL-IgG as a negative control. Cells were incubated at 37 °C for 15 min. Media was removed and cells were washed with PBS twice. 200 μL of RIPA buffer with phosphatase and protease inhibitors (Thermo Fisher, P178440) and 2% SDS was added to each well and incubated on ice for 30 min. Cells were then scraped from the wells and transferred to a microcentrifuge tube on ice. Samples were boiled at 100 °C for 5 min and centrifuged at max speed (~21000×g) for 5 min. Protein concentration of each sample was determined using a Pierce™ BCA Protein Assay Kit (Thermo Fisher, 23227).
Next, the samples were combined with 4x loading buffer (40 mM Tris-HCl, 0.04% (w/v) β-mercaptoethanol, 40% glycerol, 4 mM EDTA, 0.2 mg/ml bromophenol blue and pH 6.8). 15 μg of each sample was loaded onto a 4–12% Bis-Tris SDS-PAGE gel (Thermo Fisher, WG1401A). Two μL of MagicMark XP Western Protein Standard (Thermo Fisher, LC5602) was loaded with each gel. Proteins were transferred from the SDS-PAGE gels to nitrocellulose membranes (Thermo Fisher, IB23001) using the iBlot 2 Dry Transfer System (Thermo Fisher, IB21001) at 20 V for 1 min followed by 23 V for 4 min and 25 V for 2 min. The blots were then subsequently blocked for 1 h with 5% milk in TBS with 0.5% Tween-20 (TBS-T). The blots were next washed three times for 5 min each with TBS-T. Primary antibodies were diluted in 5% milk in TBS-T and incubated with the membrane rocking overnight at 4 °C. The blots were then washed and incubated for 1 h in goat anti-rabbit HRP conjugated secondary antibody (Cell Signaling Technology, 7074) diluted 1:3000 in TBS-T. The blots were washed three times for 5 min each with TBS-T then incubated in SignalFire ECL Reagent (Cell Signaling Technology, 6883) for 1 min and imaged using a BioRad ChemiDoc imager.
Quantification and Statistical analysis.
All statistical analyses were performed in GraphPad Prism 9.0 using either a two-tailed unpaired t-test (α=0.05) with Welch’s correction or a one-way ANOVA with multiple comparisons using Tukey’s test for significance (α=0.05). Unless otherwise noted, all data presented as the mean ± one S.D. with n = 3–5 replicates.
Supplementary Material
SIGNIFICANCE.
The blood-brain barrier (BBB) is a major impediment to the transport of antibodies and other biologics into the brain parenchyma for many diagnostic and therapeutic applications. Previous methods for penetrating the BBB have focused on designing bispecific antibody shuttles targeting the transferrin receptor (TfR-1) to utilize receptor-mediated transcytosis. However, there are several challenges associated with targeting TfR-1 receptor, including potential lysosomal degradation of the receptor and bound antibody, as well as reticulocyte depletion. Here, bispecific shuttles were designed for targeting an endothelial protein at the BBB involved in amino acid transport (CD98hc) and delivering IgGs to the mouse brain parenchyma, and their brain shuttling performance was compared to that for equivalent shuttles targeting TfR-1. Notably, CD98hc shuttles demonstrated efficient delivery and long-lived brain retention of IgGs for at least seven days, while their TfR-1 shuttle counterparts were undetectable in the brain after five days. The differences in brain pharmacokinetics appear to stem from the fact that TfR-1 targeting leads to uptake of the TfR-1 shuttles into brain cells regardless of whether delivering a targeted IgG, while the CD98hc shuttles result in limited CD98hc engagement in the brain parenchyma except when delivering a targeted IgG. This enables the CD98hc shuttles to selectively target different brain cell types, which is demonstrated in this study for astrocytes and neurons, including in the latter case for an agonist antibody that activates the TrkB receptor. These results demonstrate the potential of CD98hc shuttles for delivering IgGs and other biologics to the brain for diverse applications.
HIGHLIGHTS.
Bispecific antibody shuttles targeting CD98hc result in extended brain retention
CD98hc shuttles delivering untargeted IgGs remain localized to blood vessels
CD98hc shuttles targeting neurons and astrocytes display cell-type specificity
TrkB/CD98hc agonist shuttles demonstrate extended TrkB receptor activation
ACKNOWLEDGEMENTS
We thank members of the Tessier and Greineder labs for their helpful suggestions, Gabriel Corfas for assistance in analyzing the TrkB antibodies, Greg Thurber for suggesting brain delivery of the M2 and M6 antibodies, Richard Keep and Anuska Andjelkovic-Zochowska for advice on brain delivery and immunostaining, and Carl Lagenaur for generating the M2 and M6 hydridomas and providing permission to sequence the corresponding antibodies. This work was supported by the Massey Foundation at the University of Michigan (C.F.G. and P.M.T), National Institutes of Health (R01AG080016 to P.M.T. and C.F.G., RF1AG059723 and R35GM136300 to P.M.T., K08HL130430 to C.F.G., and T32 NS007222 and F32 AG079576 fellowships to M.J.L.), National Science Foundation (CBET 1813963, 1605266 and 1804313 to P.M.T.), BrightFocus Foundation (A2017395S to P.M.T. and A2022050S to P.M.T. and C.F.G), Coins for Alzheimer’s Research Trust (John Trojanowski Memorial Award to P.M.T. and C.F.G.), and the Albert M. Mattocks Chair (to P.M.T).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Daneman R, and Prat A (2015). The blood-brain barrier. Cold Spring Harb Perspect Biol 7, a020412–a020412. 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks WA (2009). Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 9 Suppl 1, S3. 10.1186/1471-2377-9-s1-s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poduslo JF, Curran GL, and Berg CT (1994). Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci U S A 91, 5705–5709. 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, and Dennis MS (2011). Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 3, 84ra44. 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM (2007). Blood-brain barrier delivery. Drug Discov Today 12, 54–61. 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM (2003). Blood-brain barrier genomics and the use of endogenous transporters to cause drug penetration into the brain. Curr Opin Drug Discov Devel 6, 683–691. [PubMed] [Google Scholar]
- 7.Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, et al. (2014). Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med 211, 233–244. 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al. (2014). Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron 81, 49–60. 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Ullman JC, Arguello A, Getz JA, Bhalla A, Mahon CS, Wang J, Giese T, Bedard C, Kim DJ, Blumenfeld JR, et al. (2020). Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci Transl Med 12. 10.1126/scitranslmed.aay1163. [DOI] [PubMed] [Google Scholar]
- 10.Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, and Starzyk RM (1991). Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci U S A 88, 4771–4775. 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardridge WM, Buciak JL, and Friden PM (1991). Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 259, 66–70. [PubMed] [Google Scholar]
- 12.Lee HJ, Engelhardt B, Lesley J, Bickel U, and Pardridge WM (2000). Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292, 1048–1052. [PubMed] [Google Scholar]
- 13.Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, et al. (2013). Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med 5, 183ra157, 181–112. 10.1126/scitranslmed.3005338. [DOI] [PubMed] [Google Scholar]
- 14.Stocki P, Szary J, Rasmussen CLM, Demydchuk M, Northall L, Logan DB, Gauhar A, Thei L, Moos T, Walsh FS, and Rutkowski JL (2021). Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. Faseb j 35, e21172. 10.1096/fj.202001787R. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Wiriyasermkul P, Jin C, Quan L, Ohgaki R, Okuda S, Kusakizako T, Nishizawa T, Oda K, Ishitani R, et al. (2019). Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol 26, 510–517. 10.1038/s41594-019-0237-7. [DOI] [PubMed] [Google Scholar]
- 16.Yan R, Zhao X, Lei J, and Zhou Q (2019). Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130. 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 17.Zuchero Y, Joy Y, Chen X, Bien-Ly N, Bumbaca D, Tong K, Raymond, Gao X, Zhang S, Hoyte K, Luk W, Huntley A, Melanie, et al. (2016). Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 89, 70–82. 10.1016/j.neuron.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Edavettal S, Cejudo-Martin P, Dasgupta B, Yang D, Buschman MD, Domingo D, Van Kolen K, Jaiprasat P, Gordon R, Schutsky K, et al. (2022). Enhanced delivery of antibodies across the blood-brain barrier via TEMs with inherent receptor-mediated phagocytosis. Med (N Y) 10.1016/j.medj.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Haqqani AS, Thom G, Burrell M, Delaney CE, Brunette E, Baumann E, Sodja C, Jezierski A, Webster C, and Stanimirovic DB (2018). Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J Neurochem 146, 735–752. 10.1111/jnc.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, et al. (2020). Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med 12. 10.1126/scitranslmed.aay1359. [DOI] [PubMed] [Google Scholar]
- 21.Yasutomo K (2013) Novel anti-cd98 antibody and use thereof patent application 13/636. [Google Scholar]
- 22.Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, and Carter P (1998). An efficient route to human bispecific IgG. Nat Biotechnol 16, 677–681. 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 23.Ridgway JB, Presta LG, and Carter P (1996). ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 9, 617–621. 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 24.Boado RJ, Zhang Y, Wang Y, and Pardridge WM (2009). Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng 102, 1251–1258. 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, Zuchero YJ, et al. (2017). Effector-attenuating Substitutions That Maintain Antibody Stability and Reduce Toxicity in Mice. J Biol Chem 292, 3900–3908. 10.1074/jbc.M116.767749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, Kubbies M, Klein C, Umaña P, and Mössner E (2016). Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel 29, 457–466. 10.1093/protein/gzw040. [DOI] [PubMed] [Google Scholar]
- 27.Neiveyans M, Melhem R, Arnoult C, Bourquard T, Jarlier M, Busson M, Laroche A, Cerutti M, Pugnière M, Ternant D, et al. (2019). A recycling anti-transferrin receptor-1 monoclonal antibody as an efficient therapy for erythroleukemia through target up-regulation and antibody-dependent cytotoxic effector functions. mAbs 11, 593–605. 10.1080/19420862.2018.1564510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hultqvist G, Syvänen S, Fang XT, Lannfelt L, and Sehlin D (2017). Bivalent brain shuttle increases antibody uptake by monovalent binding to the transferrin receptor. Theranostics 7, 308–318. 10.7150/thno.17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thom G, Burrell M, Haqqani AS, Yogi A, Lessard E, Brunette E, Delaney C, Baumann E, Callaghan D, Rodrigo N, et al. (2018). Enhanced Delivery of Galanin Conjugates to the Brain through Bioengineering of the Anti-Transferrin Receptor Antibody OX26. Mol Pharm 15, 1420–1431. 10.1021/acs.molpharmaceut.7b00937. [DOI] [PubMed] [Google Scholar]
- 30.Triguero D, Buciak J, and Pardridge WM (1990). Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem 54, 1882–1888. 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 31.Lund RD, Chang FL, Hankin MH, and Lagenaur CF (1985). Use of a species-specific antibody for demonstrating mouse neurons transplanted to rat brains. Neurosci Lett 61, 221–226. 10.1016/0304-3940(85)90428-8. [DOI] [PubMed] [Google Scholar]
- 32.Lagenaur C, and Schachner M (1981). Monoclonal antibody (M2) to glial and neuronal cell surfaces. J Supramol Struct Cell Biochem 15, 335–346. 10.1002/jsscb.1981.380150404. [DOI] [PubMed] [Google Scholar]
- 33.Pornnoppadol G, Zhang B, Desai AA, Berardi A, Remmer HA, Tessier PM, and Greineder CF (2021). A hybridoma-derived monoclonal antibody with high homology to the aberrant myeloma light chain. PLOS ONE 16, e0252558. 10.1371/journal.pone.0252558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett EM (2020) Bispecific Antibodies United States. [Google Scholar]
- 35.Lang AE, Siderowf AD, Macklin EA, Poewe W, Brooks DJ, Fernandez HH, Rascol O, Giladi N, Stocchi F, Tanner CM, et al. (2022). Trial of Cinpanemab in Early Parkinson’s Disease. N Engl J Med 387, 408–420. 10.1056/NEJMoa2203395. [DOI] [PubMed] [Google Scholar]
- 36.Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Azulay JP, et al. (2022). Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N Engl J Med 387, 421–432. 10.1056/NEJMoa2202867. [DOI] [PubMed] [Google Scholar]
- 37.Whone A (2022). Monoclonal Antibody Therapy in Parkinson’s Disease - The End? N Engl J Med 387, 466–467. 10.1056/NEJMe2207681. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez del Pino MM, Peterson DR, and Hawkins RA (1995). Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood-brain barrier. J Biol Chem 270, 14913–14918. 10.1074/jbc.270.25.14913. [DOI] [PubMed] [Google Scholar]
- 39.Duelli R, Enerson BE, Gerhart DZ, and Drewes LR (2000). Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab 20, 1557–1562. 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Zaragozá R (2020). Transport of Amino Acids Across the Blood-Brain Barrier. Front Physiol 11, 973. 10.3389/fphys.2020.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huwyler J, and Pardridge WM (1998). Examination of blood-brain barrier transferrin receptor by confocal fluorescent microscopy of unfixed isolated rat brain capillaries. J Neurochem 70, 883–886. 10.1046/j.1471-4159.1998.70020883.x. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann U, and Kontermann RE (2017). The making of bispecific antibodies. mAbs 9, 182–212. 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Do TM, Capdevila C, Pradier L, Blanchard V, Lopez-Grancha M, Schussler N, Steinmetz A, Beninga J, Boulay D, Dugay P, et al. (2020). Tetravalent Bispecific Tandem Antibodies Improve Brain Exposure and Efficacy in an Amyloid Transgenic Mouse Model. Mol Ther Methods Clin Dev 19, 58–77. 10.1016/j.omtm.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boado RJ, Zhang Y, Xia CF, and Pardridge WM (2007). Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood-brain barrier and abeta fibril disaggregation. Bioconjug Chem 18, 447–455. 10.1021/bc060349x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang R, Knox J, Chang J, Derbedrossian A, Vasilevko V, Cribbs D, Boado RJ, Pardridge WM, and Sumbria RK (2017). Blood-Brain Barrier Penetrating Biologic TNF-α Inhibitor for Alzheimer’s Disease. Mol Pharm 14, 2340–2349. 10.1021/acs.molpharmaceut.7b00200. [DOI] [PubMed] [Google Scholar]
- 46.Sonoda H, Morimoto H, Yoden E, Koshimura Y, Kinoshita M, Golovina G, Takagi H, Yamamoto R, Minami K, Mizoguchi A, et al. (2018). A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol Ther 26, 1366–1374. 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster CI, Hatcher J, Burrell M, Thom G, Thornton P, Gurrell I, and Chessell I (2017). Enhanced delivery of IL-1 receptor antagonist to the central nervous system as a novel anti-Transferrin receptor-IL-1RA fusion reverses neuropathic mechanical hypersensitivity. Pain 158, 660–668. 10.1097/j.pain.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou QH, Boado RJ, Lu JZ, Hui EK, and Pardridge WM (2010). Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab Dispos 38, 566–572. 10.1124/dmd.109.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, et al. (2014). Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 6, 261ra154. 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 50.Syvänen S, Hultqvist G, Gustavsson T, Gumucio A, Laudon H, Söderberg L, Ingelsson M, Lannfelt L, and Sehlin D (2018). Efficient clearance of Aβ protofibrils in AβPP-transgenic mice treated with a brain-penetrating bifunctional antibody. Alzheimer’s Research & Therapy 10. 10.1186/s13195-018-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karaoglu Hanzatian D, Schwartz A, Gizatullin F, Erickson J, Deng K, Villanueva R, Stedman C, Harris C, Ghayur T, and Goodearl A (2018). Brain uptake of multivalent and multi-specific DVD-Ig proteins after systemic administration. mAbs 10, 765–777. 10.1080/19420862.2018.1465159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber F, Bohrmann B, Niewoehner J, Fischer JAA, Rueger P, Tiefenthaler G, Moelleken J, Bujotzek A, Brady K, Singer T, et al. (2018). Brain Shuttle Antibody for Alzheimer’s Disease with Attenuated Peripheral Effector Function due to an Inverted Binding Mode. Cell Rep 22, 149–162. 10.1016/j.celrep.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 53.Zuchero YJY, Chen X, Bien-Ly N, Bumbaca D, Tong RK, Gao X, Zhang S, Hoyte K, Luk W, Huntley MA, et al. (2016). Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 89, 70–82. 10.1016/j.neuron.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56. 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 55.Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, Söderberg L, Spens E, Sahlin C, Waara ER, et al. (2015). The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis 43, 575–588. 10.3233/jad-140741. [DOI] [PubMed] [Google Scholar]
- 56.Ghiaseddin A, and Peters KB (2015). Use of bevacizumab in recurrent glioblastoma. CNS Oncol 4, 157–169. 10.2217/cns.15.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castro BA, and Aghi MK (2014). Bevacizumab for glioblastoma: current indications, surgical implications, and future directions. Neurosurg Focus 37, E9. 10.3171/2014.9.focus14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahal JJ Jr. (1972). Treatment of gram-negative bacillary meningitis in adults. Ann Intern Med 77, 295–302. 10.7326/0003-4819-77-2-295. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser AB, and McGee ZA (1975). Aminoglycoside therapy of gram-negative bacillary meningitis. N Engl J Med 293, 1215–1220. 10.1056/nejm197512112932401. [DOI] [PubMed] [Google Scholar]
- 60.Rogawski MA (2009). Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 6, 344–351. 10.1016/j.nurt.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett CF, Krainer AR, and Cleveland DW (2019). Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu Rev Neurosci 42, 385–406. 10.1146/annurev-neuro-070918-050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A, Andresen JL, Manan RS, and Langer R (2021). Nucleic acid delivery for therapeutic applications. Adv Drug Deliv Rev 178, 113834. 10.1016/j.addr.2021.113834. [DOI] [PubMed] [Google Scholar]
- 63.Southwell AL, Skotte NH, Bennett CF, and Hayden MR (2012). Antisense oligonucleotide therapeutics for inherited neurodegenerative diseases. Trends Mol Med 18, 634–643. 10.1016/j.molmed.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Scoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, Hung G, Rigo F, Bennett CF, Otis TS, and Pulst SM (2017). Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 544, 362–366. 10.1038/nature22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rinaldi C, and Wood MJA (2018). Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14, 9–21. 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 66.Schoch KM, and Miller TM (2017). Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron 94, 1056–1070. 10.1016/j.neuron.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Yao H, Xu Y, Hao R, Zhang W, Liu H, Huang Y, Guo W, and Lu B (2020). Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics 10, 6854–6874. 10.7150/thno.44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke E, Stocki P, Sinclair EH, Gauhar A, Fletcher EJR, Krawczun-Rygmaczewska A, Duty S, Walsh FS, Doherty P, and Rutkowski JL (2022). A Single Domain Shark Antibody Targeting the Transferrin Receptor 1 Delivers a TrkB Agonist Antibody to the Brain and Provides Full Neuroprotection in a Mouse Model of Parkinson’s Disease. Pharmaceutics 14, 1335. 10.3390/pharmaceutics14071335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim GS, Cho S, Nelson JW, Zipfel GJ, and Han BH (2014). TrkB Agonist Antibody Pretreatment Enhances Neuronal Survival and Long-Term Sensory Motor Function Following Hypoxic Ischemic Injury in Neonatal Rats. PLoS ONE 9, e88962. 10.1371/journal.pone.0088962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spina MB, Squinto SP, Miller J, Lindsay RM, and Hyman C (1992). Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem 59, 99–106. 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 71.Frim DM, Uhler TA, Galpern WR, Beal MF, Breakefield XO, and Isacson O (1994). Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc Natl Acad Sci U S A 91, 5104–5108. 10.1073/pnas.91.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto Y, Lin Q, Collier TJ, Frim DM, Breakefield XO, and Bohn MC (1995). Astrocytes retrovirally transduced with BDNF elicit behavioral improvement in a rat model of Parkinson’s disease. Brain Res 691, 25–36. 10.1016/0006-8993(95)00596-i. [DOI] [PubMed] [Google Scholar]
- 73.Spina MB, Hyman C, Squinto S, and Lindsay RM (1992). Brain-derived neurotrophic factor protects dopaminergic cells from 6-hydroxydopamine toxicity. Ann N Y Acad Sci 648, 348–350. 10.1111/j.1749-6632.1992.tb24578.x. [DOI] [PubMed] [Google Scholar]
- 74.Tsukahara T, Takeda M, Shimohama S, Ohara O, and Hashimoto N (1995). Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery 37, 733–739; discussion 739–741. 10.1227/00006123-199510000-00018. [DOI] [PubMed] [Google Scholar]
- 75.Jiao SS, Shen LL, Zhu C, Bu XL, Liu YH, Liu CH, Yao XQ, Zhang LL, Zhou HD, Walker DG, et al. (2016). Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl Psychiatry 6, e907. 10.1038/tp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang SS, Wu Z, Liu X, Edgington-Mitchell L, and Ye K (2022). Treating Parkinson’s Disease via Activation of BDNF/TrkB Signaling Pathways and Inhibition of Delta-Secretase. Neurotherapeutics 10.1007/s13311-022-01248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang SS, Zhang Z, Liu X, Manfredsson FP, Benskey MJ, Cao X, Xu J, Sun YE, and Ye K (2017). TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc Natl Acad Sci U S A 114, 10773–10778. 10.1073/pnas.1713969114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcántara S, Frisén J, del Río JA, Soriano E, Barbacid M, and Silos-Santiago I (1997). TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J Neurosci 17, 3623–3633. 10.1523/jneurosci.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montroull LE, Danelon V, Cragnolini AB, and Mascó DH (2019). Loss of TrkB Signaling Due to Status Epilepticus Induces a proBDNF-Dependent Cell Death. Front Cell Neurosci 13, 4. 10.3389/fncel.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin W (2020). Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J Clin Med 9. 10.3390/jcm9010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasegawa Y, Cheng C, Hayashi K, Takemoto Y, and Kim-Mitsuyama S (2020). Anti-apoptotic effects of BDNF-TrkB signaling in the treatment of hemorrhagic stroke. Brain Hemorrhages 1, 124–132. 10.1016/j.hest.2020.04.003. [DOI] [Google Scholar]
- 82.Bujotzek A, Lipsmeier F, Harris SF, Benz J, Kuglstatter A, and Georges G (2016). VH-VL orientation prediction for antibody humanization candidate selection: A case study. mAbs 8, 288–305. 10.1080/19420862.2015.1117720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kissel K, Hamm S, Schulz M, Vecchi A, Garlanda C, and Engelhardt B (1998). Immunohistochemical localization of the murine transferrin receptor (TfR) on blood-tissue barriers using a novel anti-TfR monoclonal antibody. Histochem Cell Biol 110, 63–72. 10.1007/s004180050266. [DOI] [PubMed] [Google Scholar]
- 84.Qian MD, Zhang J, Tan XY, Wood A, Gill D, and Cho S (2006). Novel agonist monoclonal antibodies activate TrkB receptors and demonstrate potent neurotrophic activities. J Neurosci 26, 9394–9403. 10.1523/jneurosci.1118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greineder CF, Chacko A-M, Zaytsev S, Zern BJ, Carnemolla R, Hood ED, Han J, Ding B-S, Esmon CT, and Muzykantov VR (2013). Vascular Immunotargeting to Endothelial Determinant ICAM-1 Enables Optimal Partnering of Recombinant scFv-Thrombomodulin Fusion with Endogenous Cofactor. PLoS ONE 8, e80110. 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makowski EK, Chen H, Lambert M, Bennett EM, Eschmann NS, Zhang Y, Zupancic JM, Desai AA, Smith MD, Lou W, et al. (2022). Reduction of therapeutic antibody self-association using yeast-display selections and machine learning. mAbs 14. 10.1080/19420862.2022.2146629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Makowski EK, Kinnunen PC, Huang J, Wu L, Smith MD, Wang T, Desai AA, Streu CN, Zhang Y, Zupancic JM, et al. (2022). Co-optimization of therapeutic antibody affinity and specificity using machine learning models that generalize to novel mutational space. Nature Communications 13. 10.1038/s41467-022-31457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiseleva RY, Glassman PG, LeForte KM, Walsh LR, Villa CH, Shuvaev VV, Myerson JW, Aprelev PA, Marcos-Contreras OA, Muzykantov VR, and Greineder CF (2020). Bivalent engagement of endothelial surface antigens is critical to prolonged surface targeting and protein delivery in vivo. Faseb j 34, 11577–11593. 10.1096/fj.201902515RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H, and Matise MP (2013). Immunofluorescence staining with frozen mouse or chick embryonic tissue sections. Methods Mol Biol 1018, 175–188. 10.1007/978-1-62703-444-9_17. [DOI] [PubMed] [Google Scholar]
- 90.Fra-Bido S, Walker SA, Innocentin S, and Linterman MA (2021). Optimized immunofluorescence staining protocol for imaging germinal centers in secondary lymphoid tissues of vaccinated mice. STAR Protoc 2, 100499. 10.1016/j.xpro.2021.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper will be shared by the lead contact upon request. The sequences of antibodies developed in this study have been deposited in GenBank under accession numbers OR253073-OR253075 and are publicly available. Accession numbers for specific antibodies can also be found in the Key Resources Table.
Code for curve fitting of binding curves has been deposited in GitHub and can be found at https://github.com/Tessier-Lab-UMich/Data_Fitting (DOI: 10.5281/zenodo.8317352).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
| Antibodies | ||
| CTL-IgG | Bujotzek et al. 2016 | PDB: 5DMG |
| CD98hc IgG | Yasutomo et al. 2013 | N/A |
| TfR-1 IgG | Kissel et al. 1998 | N/A |
| CTL-IgG/CD98-scFv | This paper | Genbank: OR253074 |
| CTL-IgG/TfR-1-scFv | This paper | Genbank: OR253073 |
| M2 | Dr. Carl Lagenaur | N/A |
| M6 | Dr. Carl Lagenaur | N/A |
| 29D7 | Qian et al. 2006 | N/A |
| TrkB-IgG/CD98-scFv | This Paper | Genbank: OR253075 |
| Goat anti-human IgG AlexaFluor 647 | Jackson ImmunoResearch | Cat#109-605-098 |
| Goat anti-human IgG AlexaFluor 488 | Jackson ImmunoResearch | Cat#109-546-170 |
| R17 | BioLegend | Cat#113802 |
| RL388 | BioLegend | Cat#128202 |
| Mouse anti-rat IgG AlexaFluor 647 | BioLegend | Cat#407511 |
| Anti-human Polyvalent Immunoglobulins (G,A,M) | Millipore Sigma | Cat#I1761 |
| Goat anti-human IgG-peroxidase | Millipore Sigma | Cat#A0170 |
| NeuN Monoclonal Antibody (1B7) | Thermo Fisher Scientific | Cat#MA5–33103 |
| GFAP Polyclonal Antibody | Thermo Fisher Scientific | Cat#PA1–10019 |
| Phospho-AKT1 (Ser473) Monoclonal Antibody | Thermo Fisher Scientific | Cat#44–621G |
| Phospho-ERK1/ERK2 (Thr202. Tyr204) Polyclonal Antibody | Thermo Fisher Scientific | Cat#36–8800 |
| ZO-1 Monoclonal Antibody (ZO1–1A12), AlexaFluor 488 | Thermo Fisher Scientific | Cat#339188 |
| Akt (pan) (11E7) Rabbit mAb | Cell Signaling Technology | Cat#4685 |
| P44/42 MAPK (Erk1/2) Antibody | Cell Signaling Technology | Cat#9102 |
| TrkB (80E3) Rabbit mAb | Cell Signaling Technology | Cat#4603 |
| Phospho-Akt (Ser473) (D9E) Rabbit mAb | Cell Signaling Technology | Cat#4060 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody | Cell Signaling Technology | Cat#9101 |
| Phospho-TrkB (Tyr516) Polyclonal Antibody | Thermo Fisher Scientific | Cat#PA5–36695 |
| Anti-rabbit IgG HRP-linked Antibody | Cell Signaling Technology | Cat#7074 |
| Bacterial and virus strains | ||
| DH5α | Thermo Fisher Scientific | Cat#18265017 |
| Biological samples | ||
| None | ||
| Chemicals, peptides, and recombinant proteins | ||
| DMEM media | Corning | Cat#10-013-CV |
| BSA | Thermo Fisher Scientific | Cat#BP9706–100 |
| 0.25% trypsin-EDTA | Gibco | Cat#25200056 |
| Antibiotic-antimycotic | Gibco | Cat#15240062 |
| FBS | Corning | Cat#35-010-CV |
| RPMI media | Corning | Cat#10-040-CV |
| Lipofectamine 2000 | Invitrogen | Cat#11668–027 |
| EcoRI-HF | New England Biolabs | Cat#R3101L |
| XbaI | New England Biolabs | Cat#R0145S |
| Q5 High-Fidelity DNA Polymerase | New England Biolabs | Cat#M0491L |
| NheI-HF | New England Biolabs | Cat#R3131L |
| HindIII-HF | New England Biolabs | Cat#R3104L |
| Quick CIP calf intestinal alkaline phosphatase | New England Biolabs | Cat#M0525L |
| T4 ligase | New England Biolabs | Cat#M0202L |
| F17 media | Thermo Fisher Scientific | Cat#A1383502 |
| Glutamine | Invitrogen | Cat#25030081 |
| Kolliphor | Thermo Fisher Scientific | Cat#NC0917244 |
| G418 | Thermo Fisher Scientific | Cat#10131035 |
| Yeastolate | BD Sciences | Cat#292804 |
| Streptavidin AlexaFluor 647 | Invitrogen | Cat#S32357 |
| Imidazole | ACROS Organics | Cat#12202–5000 |
| Mouse TfR | ACRO Biosystems | Cat#TFR-M524b |
| [125I]NaI | Perkin Elmer | Cat#NEZ033A |
| Pierce iodination reagent | Thermo Fisher Scientific | Cat#28601 |
| Phusion HF PCR MasterMix w/ HF Buffer | New England Biolabs | Cat#M0531S |
| Methanol | Thermo Fisher Scientific | Cat#A421–4 |
| Sodium acetate | Sigma-Aldrich | Cat#S2889–250G |
| Isoflurane | Henry Schein | Cat#66794-017-25 |
| Trypan blue | Gibco | Cat#15250061 |
| Krebs buffer | Thermo Fisher Scientific | Cat#J67591.AP |
| Dextran | Acros | Cat#406271000 |
| RIPA buffer | Thermo Fisher Scientific | Cat#WK333492 |
| 100X Halt Protease Inhibitors | Thermo Fisher Scientific | Cat#1860932 |
| Goat serum | Novus Biologicals | Cat#S13150NOV |
| Tween 20 | Thermo Fisher Scientific | Cat#J20605-AP |
| TMB substrate solution | Thermo Fisher Scientific | Cat#N301 |
| H2SO4 | Thermo Fisher Scientific | Cat#A300–212 |
| Sodium bicarbonate | Sigma-Aldrich | Cat#S6297–250G |
| 4% paraformaldehyde | Thermo Fisher Scientifice | Cat#J19943.K2 |
| Sucrose | Thermo Fisher Scientific | Cat#S5–500 |
| OCT | Thermo Fisher Scientific | Cat#4585 |
| Sodium citrate buffer | Thermo Fisher Scientific | Cat#S279–3 |
| 0.5% Triton-X 100 | Alfa Aesar | Cat#A16046 |
| DAPI | Thermo Fisher Scientific | Cat#YC3861511 |
| Anti-fade reagent | Thermo Fisher Scientific | Cat#P36930 |
| Dry milk | Kroger | N/A |
| Phosphatase and protease inhibitor cocktail 100X | Thermo Fisher Scientific | Cat#P178440 |
| 20% SDS | Dot Scientific Inc. | Cat#DSL23100–500 |
| Trizma base | Sigma-Aldrich | Cat#T1503–1KG |
| HCl | Thermo Fisher Scientific | Cat#SA56–4 |
| β-mercaptoethanol | ACROS Organics | Cat#125472500 |
| Glycerol | Thermo Fisher Scientific | Cat#BP229–1 |
| EDTA | Thermo Fisher Scientific | Cat#O2793–500 |
| Bromophenol blue | Thermo Fisher Scientific | Cat#A18469.09 |
| SignalFire ECL Reagent | Cell Signaling Technology | Cat#6883 |
| Critical commercial assays | ||
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat#23227 |
| Deposited data | ||
| Antibody Sequences | GenBank | OR253073–5 |
| Experimental models: Cell lines | ||
| Human: HEK293–6E | National Research Council (NRC) of Canada | N/A |
| Human: REN-CD98hc | This paper | N/A |
| Human: REN-TfR | This paper | N/A |
| Human: REN-mTrkB | This paper | N/A |
| Mouse: bEnd.3 | ATCC | Cat#CRL-2299 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice | Jackson Laboratory | Strain#000664 |
| Oligonucleotides | ||
| None | ||
| Recombinant DNA | ||
| None | ||
| Software and algorithms | ||
| Prism 9.5.1 | GraphPad | https://www.graphpad.com/updates/prism-951-release-notes |
| Python 3.11.5 | Python Software Foundation | https://www.python.org |
| Data fitting algorithm | This Paper | DOI: 10.5281/zenodo.8317352 |
| Other | ||
| Streptavidin MicroBeads | Miltenyi Biotec | Cat#130-048-101 |
| Streptavidin Dynabeads | Invitrogen | Cat#11047 |
| Protein A magnetic beads | Invitrogen | Cat#88846 |
| QIAquick Gel Extraction Kit | Qiagen | Cat#28704 |
| QIAquick PCR Purification Kit | Qiagen | Cat#28104 |
| QIAprep Spin Miniprep Kit | Qiagen | Cat#27106 |
| Protein A Agarose | Thermo Fisher Scientific | Cat#20333 |
| Centrifuge columns | Thermo Fisher Scientific | Cat#89898 |
| Zeba Spin Desalting Columns | Thermo Fisher Scientific | Cat#89894; Cat#89892; Cat#89890 |
| Invitrogen NuPAGE 10% Bis-Tris, 1.0 mm, Midi Protein GEL (SDS-PAGE) | Thermo Fisher Scientific | Cat#WG1203BOX |
| Superdex 200 Increase 10/300 GL column | GE | Cat#28990944 |
| 96-well plates | VWR | Cat#650261 |
| Protein A Dynabeads | Invitrogen | Cat#10002D |
| Ni-NTA agarose | Qiagen | Cat#30230 |
| Heparin-coated capillary tubes | Thermo Fisher Scientific | Cat#22–3652566 |
| TissueLyser LT | Qiagen | Cat#85600 |
| Immulon 4HBX flat bottom plates | Thermo Fisher Scientific | Cat#3855 |
| 30kDa MW centrifugal filter | Millipore Sigma | Cat#UFC503096 |
| IVIS Lumina S5 | Perkin Elmer | |
| Cryostat | Leica | Cat#NX50 |
| Glass slides | Thermo Fisher Scientific | Cat#1255015 |
| Hydrophobic pen | Vector Laboratories | Cat#H-4000 |
| Coverslip | Thermo Fisher Scientific | Cat#12543D |
| Confocal microscope | Nikon | A1si |
| Flow cytometer, ZE5 cell analyzer | Bio-Rad | Cat#12004278 |
| 6-well plates | Thermo Fisher Scientific | Cat#140675 |
| TLC silica gel 60F254 plates | Millipore Sigma | Cat#105554 |
| 4–12% Bis-Tris SDS-PAGE gel | Thermo Fisher Scientific | Cat#WG1401A |
| MagicMark XP western Protein Standard | Thermo Fisher Scientific | Cat#LC5602 |
| Nitrocellulose membrane | Thermo Fisher Scientific | Cat#IB23001 |
| iBlot 2 Dry Transfer System | Thermo Fisher Scientific | Cat#IB21001 |


