Abstract
OBJECTIVE:
To examine whether uterine cancer symptoms differ between Black and White patients and how this may influence their stage at diagnosis.
METHODS:
Using the Surveillance, Epidemiology and End Results-Medicare database, we identified 2,328 Black and 21,774 White patients with uterine cancer in 2008–2017. Their symptoms in the 18 months before diagnosis were categorized as postmenopausal bleeding (PMB) alone, PMB together with other symptoms (e.g., abdominal/pelvic pain, bloating), non-PMB symptoms alone, or no symptoms. Stage at diagnosis was dichotomized as advanced (i.e., regional/distant) versus localized. The association between race and stage was analyzed using regression models incrementally adjusting for symptoms and other patient characteristics.
RESULTS:
A larger proportion of Black than White patients experienced PMB together with other symptoms (63.1% versus 58.0%) or experienced non-PMB symptoms alone (13.1% versus 9.4%) (p<0.001). Black patients had a higher risk of advanced-stage diagnosis than White patients (45.0% versus 30.3%, unadjusted RR=1.52, 95% CI: 1.44–1.59). Adjusting for Black-White differences in symptoms attenuated the RR to 1.46 (95% CI: 1.39–1.53). Compared to PMB symptoms alone, having additional non-PMB symptoms (RR=1.21, 95% CI: 1.15–1.26) and having non-PMB symptoms alone (RR=1.99, 95% CI: 1.88–2.10) were associated with increased risk of advanced-stage diagnosis. Further adjusting for histology and other patient characteristics reduced Black-White disparity in advanced-stage diagnosis to 1.08 (95% CI: 1.03–1.14) but symptoms remained significantly associated with stage at diagnosis.
CONCLUSIONS:
Having non-PMB symptoms was associated with more advanced stage at diagnosis. Non-PMB symptoms were more common among Black than White patients, which might hinder symptom recognition/evaluation.
INTRODUCTION
With 822,388 women living with uterine cancer and 66,200 new diagnoses each year, uterine cancer is the most common gynecologic malignancy in the United States [1, 2]. While the five-year relative survival for patients diagnosed at localized stage is 94.9%, it reduces substantially to 69.8% and 18.4% for those diagnosed at regional and distant stage, respectively [1]. Therefore, early diagnosis is crucial for prognosis. There is unfortunately a large racial gap in stage at diagnosis: 55% of Black patients with uterine cancer are diagnosed at localized stage, whereas approximately two-thirds of patients from all other racial and ethnic groups are diagnosed at localized stage (e.g., 69% of White patients) [3]. This disparity has been identified as the primary driver for a nearly two-fold Black-White difference in the uterine cancer mortality rate (Black: 9.0 deaths per 100,000 women; White: 4.6 deaths per 100,000 women) [3, 4].
The reasons for this disparity in stage at diagnosis remain poorly understood. Since there is no routine screening for uterine cancer, one potential mechanism is difference in symptomatology experienced by Black and White patients, which can affect symptom recognition, reporting, and evaluation. Most uterine cancers occur in postmenopausal women [1] with abnormal vaginal bleeding being the most common symptom [5]. However, some patients have no clear symptoms or present with symptoms that are less specific to uterine cancer (e.g., abdominal pain and weight loss) [6–9]. Prior research on other gynecologic cancers suggests that patients with non-specific symptoms tend to have delayed diagnostic evaluation and more advanced stage at diagnosis [10]. It is possible that Black patients with uterine cancer may experience non-specific symptoms more often than White patients, thus hindering timely recognition and diagnosis. In addition, since non-specific symptoms may appear less alarming, their care may be viewed as more discretionary and hence more subject to the impact of sociocultural barriers such as limited health care access and mistrust of the health care system among Black patients and potential implicit bias among clinicians [11, 12].
This study aimed to compare symptoms experienced by Black and White patients with uterine cancer and test the hypothesis that their difference in symptomatology may in part mediate racial disparity in stage at diagnosis. While doing so, we also examined differences in symptoms by histologic subtype to further inform the inter-relationship among race, histology, and symptoms in influencing stage at diagnosis.
MATERIALS AND METHODS
Data Source and Study Sample
This study used the Surveillance, Epidemiology and End Results (SEER)-Medicare database. SEER-Medicare data provide information on sociodemographic characteristics and detailed tumor characteristics of patients with cancer, along with their linked Medicare claims [13]. Our data included patients from the following tumor registries: Connecticut, Detroit, Atlanta, Greater Georgia, Rural Georgia, San Francisco-Oakland, San Jose-Monterey, Greater California, Hawaii, Idaho, Iowa, Kentucky, Los Angeles, Louisiana, Massachusetts, New Mexico, New Jersey, New York, Seattle-Puget Sound, and Utah. The Columbia University Institutional Review Board determined this study as exempt because it only involved secondary analysis of a limited data set.
Our sample included non-Hispanic Black and non-Hispanic White patients 66 years of age or older with uterine cancer diagnosed in 2008–2017. Diagnosis of uterine cancer was based on International Classification of Diseases Oncology 3rd edition (ICD-O-3) site code C54.0-C54.9 and C55.9 with malignant behavior. Patients were eligible if they further met the following criteria: 1) uterine cancer was confirmed by positive histology, 2) uterine cancer was not diagnosed on autopsy or death certificate only, 3) did not have a history of other cancer prior to uterine cancer diagnosis (to minimize confounding in symptoms), 4) had consistent month of birth in SEER and in Medicare (difference ≤3 months), and 5) had continuous enrollment in Medicare Parts A and B fee-for-service plans in the 18 months before uterine cancer diagnosis (to capture a complete record of pre-diagnosis symptoms). Since prior research showed that about 90% uterine cancer patients were diagnosed within one year after symptom presentation [14, 15], we chose an 18-month continuous enrollment period to enhance our ability to capture symptom onset.
As SEER data only tracked the year and month of diagnosis (without the day of diagnosis), we determined the date of diagnosis by additionally using information from the first Medicare claim that had a diagnosis code of uterine cancer. To enhance accuracy in determining diagnosis date, we excluded patients whose month of uterine cancer diagnosis documented in SEER and the month of first Medicare claim with a diagnosis code of uterine cancer differed by more than 1 month of each other. We then defined the date of uterine cancer diagnosis as: 1) the date of first Medicare claim with a diagnosis code of uterine cancer if its month was the same as SEER-documented month of diagnosis, 2) the first day of the SEER-documented month of diagnosis if SEER-documented month of diagnosis was 1 month after the month of the first Medicare claim with a diagnosis code of uterine cancer, or 3) the last day of the SEER-documented month of diagnosis if SEER-documented month of diagnosis was 1 month before the month of the first Medicare claim with a diagnosis code of uterine cancer.
Uterine Cancer Stage at Diagnosis
For each patient, we categorized their uterine cancer stage at diagnosis as advanced versus localized stage. This was determined using SEER summary stage which included localized, regional, distant, and unknown stage. We dichotomized these categories into advanced stage (i.e., regional or distant stage) versus localized stage. Patients with unknown stage were included in descriptive analysis but were excluded from regression analysis when stage was examined as an outcome variable.
Uterine Cancer Symptoms
For each patient, we categorized them into four mutually exclusive groups based on their symptoms: postmenopausal bleeding (PMB) alone, PMB in conjunction with non-PMB symptoms, non-PMB symptoms alone, or no symptoms. Since all patients in our sample were 66 years or older, we accepted all diagnosis codes related to abnormal uterine bleeding on their Medicare claims in the 18 months before through the date of uterine cancer diagnosis as indicative of PMB (see Supplementary Table S1 for relevant International Classification of Diseases [ICD] codes). Non-PMB symptoms were also identified based on diagnosis codes on Medicare claims in the 18 months before through the date of uterine cancer diagnosis and they included: abdominal/pelvic pain, change in bowel habits, constipation, rectal bleeding/blood in stool, intestinal obstruction, bloating, abnormal weight loss, early satiety or nausea/vomiting, vaginal discharge, fatigue, and anemia (see Supplementary Table S1). As a patient might experience two or more of these symptoms, we also measured the number of non-PMB symptoms experienced by each patient, which has been shown to delay the diagnosis of gynecologic cancer [16]. Patients who did not have any of the above symptoms documented were considered as having no symptoms.
Race and Ethnicity
Race and ethnicity categories in SEER-Medicare data included non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian/Pacific Islander, non-Hispanic American Indian/Alaska Native, and non-Hispanic unknown [17]. Race and ethnicity in SEER were determined by the reporting facility based on all information sources available at the facility (e.g., medical record, physician and nursing notes) with enhancement through additional information from states and other sources as well as a surname and birthplace-based estimation algorithm for Hispanic classification [18, 19]. Because stage at diagnosis for Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native patients was similar to White patients (i.e., they did not experience disparities) [3], we limited this study to non-Hispanic White and non-Hispanic Black patients to focus on understanding Black-White differences in symptomatology and stage at diagnosis. For simplicity, hereinafter we referred to them as White and Black patients for short and referred to their difference as racial difference.
Covariates
For each patient, we measured their sociodemographic, health, and tumor characteristics. Measures of sociodemographic characteristics included patient age (66–69, 70–74, 75–79, or ≥80 years), marital status (married, unmarried, or unknown), location (metropolitan, urban, or rural), SEER region (eastern, Midwest, or west), and socioeconomic status (SES) index (in quintiles). The SES index was a composite score encompassing information on census tract-level median household income, house value, rent, poverty level, education index, percent working class, and unemployment status from the American Community Survey estimates. Census tracts were categorized into SES quintiles across the entire U.S.. Patients were assigned to these SES quintiles based on their census tract and year of diagnosis [20]. Measures of patients’ health and tumor characteristics included Charlson comorbidities [21], histologic subtype (endometrioid carcinoma, non-endometrioid carcinoma, endometrial carcinoma not otherwise specified, sarcoma, or other), tumor grade (well differentiated, moderately differentiated, poorly differentiated, or unknown), and year of uterine cancer diagnosis. Charlson comorbidities were measured based on diagnosis and procedure codes on patients’ Medicare claims in the 18 months before through the month of uterine cancer diagnosis using algorithm recommended by the National Cancer Institute [21]. Histologic subtype was determined based on ICD-O-3 morphology codes.
Statistical Analysis
Patient characteristics, symptoms, and stage at diagnosis were compared between Black and White patients using Chi-square tests (for categorical variables) and Wilcoxon rank sum tests (for continuous variables). To test our hypothesis that Black-White disparities in stage at diagnosis was in part mediated by symptomatology, we followed the conventional method by Baron and Kenny [22] and assessed A) the relationship between race and stage, B) the relationship between race and symptoms, and C) the relationship between symptoms and stage while controlling for race.
For assessment A, we estimated an unadjusted model with race (Black versus White) being the independent variable and stage at diagnosis (advanced versus localized stage) being the dependent variable (Model 0). Our comparison of symptoms between Black and White patients (described above) helped inform assessment B. For assessment C, we estimated a regression model with stage at diagnosis being the dependent variable and symptom categories (PMB alone [reference group], PMB in conjunction with non-PMB symptoms, non-PMB symptoms alone, or no symptom) being the independent variable, while controlling for race (Model 1). Since patients’ symptoms may be driven by their underlying tumor characteristics, we estimated another model additionally adjusting for histology and grade (Model 2). Finally, to account for other confounding factors, we further added patients’ sociodemographic characteristics, comorbidities, and year of diagnosis as covariates (Model 3).
Since type 2 uterine cancer is more aggressive than type 1 cancer and tends to progress faster, the association between race, symptomatology, and stage at diagnosis may differ by type of uterine cancer. Therefore, we additionally conducted a parallel set of analyses stratified by type 2 versus type 1 uterine cancer. Similar to prior research [23], we grouped non-endometrioid carcinoma, sarcoma, and poorly differentiated endometrioid carcinoma as type 2 uterine cancer and grouped well differentiated and moderately differenced endometrioid carcinoma as type 1 uterine cancer. While we recognized that pure uterine sarcomas differ from other histologic subtypes in symptoms, management and outcomes, given their typically more aggressive nature, we grouped them with other type 2 uterine cancers which also tend to have poor prognosis. This stratified analysis included regression models analogous to Models 0, 1 and 3 as described above without adjustment for histology and grade (which were already accounted for in our definition of type 1 and type 2 uterine cancer strata). We also compared the distribution of symptom categories by histologic subtypes using chi-square tests.
All regression models used a modified Poisson regression with robust variance estimation to produce estimates for relative risk (RR) and 95% confidence interval (CI). All statistical tests were two-sided. A p value below 0.05 was considered statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 2,328 Black and 21,774 White patients met sample eligibility criteria. Black patients with uterine cancer were slightly younger than White patients (age 66–69 years: 30.3% versus 26.2%, p<0.001) (Table 1). A larger proportion of Black than White patients were in the lowest SES index quintile (37.6% versus 8.6%, p<0.001) or had more than two comorbidities (58.4% versus 41.8%, p<0.001). Black patients were also more likely than White patients to be unmarried (48.6% versus 33.7%, p<0.001) or reside in a metropolitan area (92.1% versus 85.5%, p<0.001).
Table 1.
Characteristics of non-Hispanic Black and non-Hispanic White patients with uterine cancer
| Characteristic | Non-Hispanic Black (N=2,328) | Non-Hispanic White (N=21,774) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | <0.001 | ||||
| 66–69 | 706 | 30.3 | 5,697 | 26.2 | |
| 70–74 | 747 | 32.1 | 6,512 | 29.9 | |
| 75–79 | 457 | 19.6 | 4,393 | 20.2 | |
| ≥80 | 418 | 18.0 | 5,172 | 23.8 | |
| Stage | <0.001 | ||||
| Localized | 1,149 | 49.4 | 14,357 | 65.9 | |
| Regional | 647 | 27.8 | 4,807 | 22.1 | |
| Distant | 401 | 17.2 | 1,788 | 8.2 | |
| Unknown | 131 | 5.6 | 822 | 3.8 | |
| Histology | <0.001 | ||||
| Endometrioid carcinoma | 930 | 39.9 | 14,565 | 66.9 | |
| Non-endometrioid carcinoma | 928 | 39.9 | 3,720 | 17.1 | |
| Endometrial carcinoma not otherwise specified | 310 | 13.3 | 2,655 | 12.2 | |
| Sarcoma | 91 | 3.9 | 478 | 2.2 | |
| Other | 69 | 3.0 | 356 | 1.6 | |
| Grade | <0.001 | ||||
| Well differentiated | 306 | 13.1 | 5,974 | 27.4 | |
| Moderate differentiated | 298 | 12.8 | 5,000 | 23.0 | |
| Poorly differentiated | 1,095 | 47.0 | 6,130 | 28.2 | |
| Unknown | 629 | 27.0 | 4,670 | 21.4 | |
| Marital status | <0.001 | ||||
| Unmarried | 1,131 | 48.6 | 7,336 | 33.7 | |
| Married | 406 | 17.4 | 6,832 | 31.4 | |
| Unknown | 791 | 34.0 | 7,606 | 34.9 | |
| Urban rural location | <0.001 | ||||
| Metropolitan | 2,144 | 92.1 | 18,625 | 85.5 | |
| Urban | 166 | 7.1 | 2,816 | 12.9 | |
| Rural | 18 | 0.8 | 333 | 1.5 | |
| SEER region | <0.001 | ||||
| Eastern | 1,089 | 46.8 | 10,595 | 48.7 | |
| Midwest | 952 | 40.9 | 5,549 | 25.5 | |
| West | 287 | 12.3 | 5,630 | 25.9 | |
| Socioeconomic status index (quintile) | <0.001 | ||||
| Low | 876 | 37.6 | 1,873 | 8.6 | |
| Medium low | 436 | 18.7 | 2,945 | 13.5 | |
| Medium | 350 | 15.0 | 3,761 | 17.3 | |
| Medium high | 368 | 15.8 | 4,957 | 22.8 | |
| High | 219 | 9.4 | 7,218 | 33.1 | |
| Unknown | 79 | 3.4 | 1,020 | 4.7 | |
| Charlson comorbidities | <0.001 | ||||
| 0 | 455 | 19.5 | 7,080 | 32.5 | |
| 1 | 514 | 22.1 | 5,594 | 25.7 | |
| ≥2 | 1,359 | 58.4 | 9,100 | 41.8 | |
SEER = Surveillance, Epidemiology and End Results.
Overall, 29.4% of patients with uterine cancer experienced PMB alone prior to uterine cancer diagnosis, 58.5% had both PMB and non-PMB symptoms, 9.8% had non-PMB symptoms alone, and 2.3% had no documented symptoms (Table 2). The most common types of non-PMB symptoms were fatigue (32.5%), abdominal/pelvic pain (30.7%), anemia (25.0%), bloating (14.9%), and constipation (10.0%).
Table 2.
Difference in symptomatology between non-Hispanic Black and non-Hispanic White patients with uterine cancer
| Symptoms | Overall Sample (N=24,102) | Non-Hispanic Black (N=2,328) | Non-Hispanic White (N=21,774) | P Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Symptom | <0.001 | ||||||
| PMB alone | 7,095 | 29.4 | 486 | 20.9 | 6,609 | 30.4 | |
| PMB in conjunction with non-PMB symptoms | 14,099 | 58.5 | 1,468 | 63.1 | 12,631 | 58.0 | |
| Non-PMB symptoms alone | 2,350 | 9.8 | 306 | 13.1 | 2,044 | 9.4 | |
| No symptoms | 558 | 2.3 | 68 | 2.9 | 490 | 2.3 | |
| Type of non-PMB symptom* | |||||||
| Abdominal or pelvic pain | 7,400 | 30.7 | 886 | 38.1 | 6,514 | 29.9 | <0.001 |
| Bloating | 3,590 | 14.9 | 447 | 19.2 | 3,143 | 14.4 | <0.001 |
| Constipation | 2,414 | 10.0 | 307 | 13.2 | 2,107 | 9.7 | <0.001 |
| Rectal bleeding or blood in stool | 1,842 | 7.6 | 225 | 9.7 | 1,617 | 7.4 | <0.001 |
| Intestinal obstruction | 609 | 2.5 | 91 | 3.9 | 518 | 2.4 | <0.001 |
| Change in bowel habits | 567 | 2.4 | 40 | 1.7 | 527 | 2.4 | 0.03 |
| Abnormal weight loss | 1,166 | 4.8 | 181 | 7.8 | 985 | 4.5 | <0.001 |
| Early satiety or nausea/vomiting | 2,349 | 9.7 | 265 | 11.4 | 2,084 | 9.6 | 0.01 |
| Vaginal discharge | 1,287 | 5.3 | 140 | 6.0 | 1,147 | 5.3 | 0.13 |
| Fatigue | 7,841 | 32.5 | 738 | 31.7 | 7,103 | 32.6 | 0.37 |
| Anemia | 6,018 | 25.0 | 910 | 39.1 | 5,108 | 23.5 | <0.001 |
| Number of non-PMB symptoms ≥3* | 5,023 | 20.8 | 684 | 29.4 | 4,339 | 19.9 | <0.001 |
IQR = interquartile range; PMB = postmenopausal bleeding.
A patient could have more than one non-PMB symptom.
When compared by race, a lower proportion of Black patients (20.9%) experienced PMB alone, compared to 30.4% of White patients, whereas a higher proportion of Black patients experienced additional non-PMB symptoms (63.1%) or non-PMB symptoms alone (13.1%), than White patients (58.0% and 9.4%, respectively) (p<0.001) (Table 2). Among the non-PMB symptoms, Black patients were more likely than White patients to experience abdominal/pelvic pain (38.1% versus 29.9%, p<0.001), bloating (19.2% versus 14.4%, p<0.001), constipation (13.2% versus 9.7%, p<0.001), early satiety or nausea/vomiting (11.4% versus 9.6%, p=0.01), or anemia (39.1% versus 23.5%, p<0.001). Black patients also experienced a greater number of non-PMB symptoms than White patients, with 29.4% of Black patients versus 19.9% of White patients having at least three non-PMB symptoms (p<0.001).
When cancer stage was examined, 45.0% of Black patients were diagnosed at advanced stage (regional or distant stage), in comparison to 30.3% of White patients (p<0.001, Table 1), with an unadjusted RR of 1.52 (95% CI: 1.44–1.59) (Model 0 in Table 3). This disparity in stage at diagnosis was reduced to 1.46 (95% CI: 1.39–1.53) after adjusting for patients’ symptoms (Model 1 in Table 3). Compared with patients experiencing PMB symptoms alone, those who additionally experienced non-PMB symptoms (RR=1.21, 95% CI: 1.15–1.26) and those who experienced no symptoms (RR=1.21, 95% CI: 1.07–1.37) had a higher risk of advanced stage at diagnosis. Patients who only experienced non-PMB symptoms had an even higher risk of advanced stage at diagnosis (RR=1.99, 95% CI: 1.88–2.10) (Model 1 in Table 3). These findings are consistent with our hypothesized role of symptoms in partially mediating the relationship between race and stage at diagnosis.
Table 3.
Unadjusted and adjusted racial difference in the likelihood of diagnosis at advanced stage (i.e., regional/distant stage), as opposed to localized stage, in overall sample
| Patient Characteristic | Model 0 (unadjusted) | Model 1 (with adjustment for symptoms) | Model 2 (with adjustmen t for symptoms, histology and grade) | Model 3 (with adjustment for symptoms, histology, grade, and other covariatesa) |
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 1.52 (1.44–1.59)* | 1.46 (1.39–1.53)* | 1.11 (1.061.16)* | 1.08 (1.03–1.14)* |
| Symptoms | ||||
| PMB alone | - | Reference | Reference | Reference |
| PMB in conjunction with non-PMB symptoms | - | 1.21 (1.15–1.26)* | 1.14 (1.091.19)* | 1.11 (1.07–1.16)* |
| Non-PMB symptoms alone | - | 1.99 (1.88–2.10)* | 1.62 (1.541.70)* | 1.58 (1.50–1.66)* |
| No symptoms | - | 1.21 (1.07–1.37)* | 1.14 (1.011.27)* | 1.13 (1.01–1.27)* |
CI = confidence interval; PMB = postmenopausal bleeding; RR = relative risk.
indicate results with p<0.05.
Other covariates included patient age, marital status, Charlson comorbidities, socioeconomic status indicator (quintiles), Surveillance, Epidemiology and End Results (SEER) region, metropolitan/urban/rural location, and year of diagnosis.
However, further adjusting for uterine cancer histology and grade substantially reduced the RR of Black-White difference in stage at diagnosis to 1.11 (95% CI: 1.06–1.16) (Model 2 in Table 3). Similarly, the relationship between symptoms and stage at diagnosis was attenuated but remained significant. For instance, for patients who only experienced non-PMB symptoms, their RR of having advanced stage at diagnosis was reduced from 1.99 (95% CI: 1.88–2.10) to 1.62 (95% CI: 1.54–1.70) after adjusting for cancer histology and grade (Models 1 and 2 in Table 3). Additional adjustment for patient sociodemographic characteristics and comorbidities had little impact (Model 4 in Table 3).
Subsequent analysis showed important differences in symptomatology by uterine cancer histology (Figure 1). PMB alone was more common among patients with endometrioid carcinomas (32.3%) than other histologic subtypes (e.g., 24.3% in non-endometrioid carcinoma and 14.1% in sarcoma). In contrast, non-PMB symptoms alone were more common among patients with other histologic subtypes (e.g., 14.2% in non-endometrioid carcinoma and 30.9% in sarcoma) than endometrioid carcinomas (7.0%). Black-White differences in symptoms might be confounded by their differences in the distribution of histologic subtype because a substantially lower proportion of Black than White patients had endometrioid histologic subtype (39.9% versus 66.9%), whereas a higher proportion of Black patients had non-endometrioid carcinoma (39.9% versus 17.1%) or sarcoma (3.9% versus 2.2%) (p<0.001) (Table 1).
Figure 1.
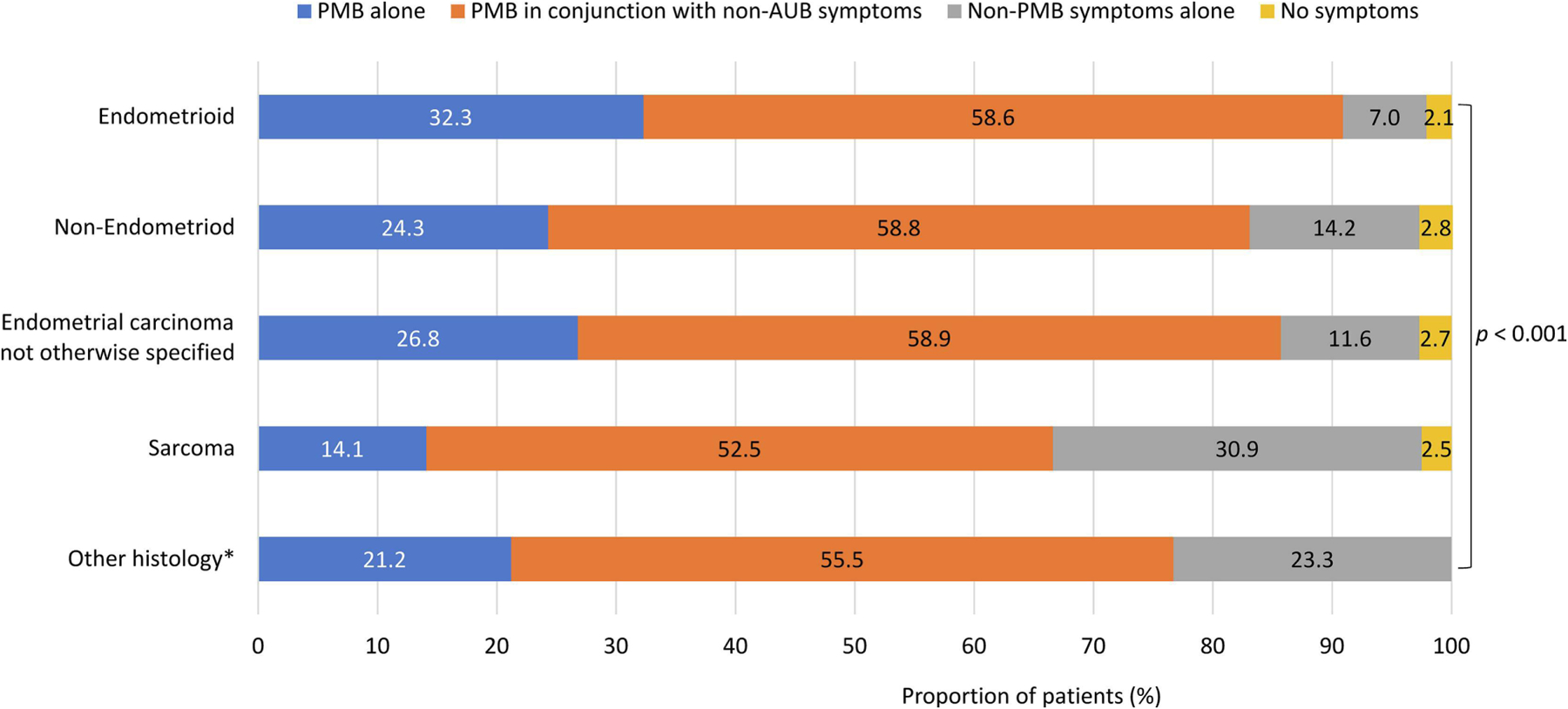
Differences in uterine cancer symptoms by histologic subtype PMB = postmenopausal bleeding.
* “Other symptoms alone” and “No symptoms” were combined to avoid reporting small cells ≤10.
When stratified by type of uterine cancer, racial disparity in stage at diagnosis was only present among patients with type 2 uterine cancer (including non-endometrioid carcinoma, sarcoma, and poorly differentiated endometrioid carcinoma) but not among patients with type 1 cancer (including well differentiated and moderately differentiated endometrioid carcinoma) (Table 4). Among patients with type 2 uterine cancer, Black patients had a 15% higher risk of being diagnosed at advanced stage than White patients in unadjusted analysis (RR=1.15, 95% CI: 1.10–1.22), which was virtually unchanged after adjusting for symptoms (RR=1.14, 95% CI: 1.08–1.20). However, patients’ symptomatology remained significantly associated with stage at diagnosis regardless of whether they had type 2 (e.g., RR=1.53, 95% CI: 1.43–1.62 for having non-PMB symptoms alone) or type 1 uterine cancer (e.g., RR=1.41, 95% CI: 1.20–1.66 for having non-PMB symptoms alone) (Model 1 in Table 4).
Table 4.
Unadjusted and adjusted racial difference in the likelihood of diagnosis at advanced stage (i.e., regional/distant stage), as opposed to localized stage, stratified by type 1 and type 2 uterine cancer
| Patient Characteristic | Model 0 (unadjusted) | Model 1 (with adjustment for symptoms) | Model 3 (with adjustme nt for symptoms and other covariatesa) |
|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Type 1 uterine cancerb (N=9,835) | |||
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.03 (0.85–1.26) | 1.02 (0.84–1.24) | 0.98 (0.801.21) |
| Symptoms | |||
| PMB alone | - | Reference | Reference |
| PMB in conjunction with non-PMB symptoms | - | 1.13 (1.03–1.25)* | 1.09 (0.99–1.20) |
| Non-PMB symptoms alone | - | 1.41 (1.20–1.66)* | 1.37 (1.171.61)* |
| No symptoms | - | 0.89 (0.64–1.23) | 0.90 (0.65–1.25) |
| Type 2 uterine cancerb (N=7,245) | |||
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.15 (1.10–1.22)* | 1.14 (1.08–1.20)* | 1.12 (1.061.19)* |
| Symptoms | |||
| PMB alone | - | Reference | Reference |
| PMB in conjunction with non-PMB symptoms | - | 1.11 (1.05–1.17)* | 1.09 (1.03–1.15)* |
| Non-PMB symptoms alone | - | 1.53 (1.43–1.62)* | 1.50 (1.401.59)* |
| No symptoms | - | 1.13 (0.98–1.30)* | 1.14 (0.991.31) |
CI = confidence interval; PMB = postmenopausal bleeding; RR = relative risk.
indicate results with p<0.05.
Other covariates included patient age, marital status, Charlson comorbidities, socioeconomic status indicator (quintiles), Surveillance, Epidemiology and End Results (SEER) region, metropolitan/urban/rural location, and year of diagnosis.
Type 1 uterine cancer included well differentiated and moderately differentiated endometrioid carcinoma. Type 2 uterine cancer included non-endometrioid carcinoma, sarcoma, and poorly differentiated endometrioid carcinoma.
DISCUSSION
In this large sample of Medicare beneficiaries with uterine cancer, Black patients were more likely than White patients to be diagnosed at an advanced stage, especially among those with more aggressive type 2 cancer. Having non-PMB symptoms was associated with a higher risk of advanced stage at diagnosis. A larger proportion of Black patients than White patients experienced non-PMB symptoms, which may explain some of their disparity in stage at diagnosis.
Although racial disparity in uterine cancer stage at diagnosis is well-documented in the literature [24], we uniquely demonstrated that such disparity was mainly present among patients with type 2 uterine cancer and not among patients with type 1 cancer. This is not surprising because type 1 uterine cancer includes low grade endometrioid carcinoma which tends to grow slowly and is usually confined within the uterus when diagnosed [6]. In contrast, the more aggressive type 2 uterine cancer progresses faster and is more likely to spread [6]. Therefore, any differences in timeliness of symptom recognition/reporting, care access, or quality of diagnostic evaluation between Black and White patients may lead to a larger inequality in their stage at diagnosis in type 2 uterine cancer. Yet this finding has grave implications. Since type 1 uterine cancer has good survival and accounts for most cases of uterine cancer, providers and patients generally perceive uterine cancer as having good prognosis. However, if racial disparity in stage at diagnosis is mainly among patients with type 2 cancer, the consequences may be more serious than previously appreciated. A recent study by Johnson et al. [23] showed an age-adjusted 5-year survival of 51.6% for type 2 endometrial cancer, compared with 85.3% for type 1 endometrial cancer. The fact that Black patients are more likely than White patients to have the aggressive type 2 disease [24, 25] and that among those who have type 2 cancer, Black patients are more likely than White patients to have advanced stage can place Black patients in double jeopardy.
Identifying and mitigating the reasons for racial disparity in stage at diagnosis are vitally important. In this study, we found that Black patients with uterine cancer were more likely than White patients to experience non-PMB symptoms and that having non-PMB symptoms was associated with a higher risk of advanced stage at diagnosis. Although the mediation effect of Black-White differences in symptomatology on their disparity in stage at diagnosis is modest in magnitude, it revealed a previously untapped area that may help explain some of the disparity. Non-PMB symptoms such as bloating, abdominal pain, and constipation are less specific to uterine cancer and can be attributable to other gynecologic or non-gynecologic conditions. Thus, patients and providers may confuse these symptoms with other etiologies. Such non-specific symptoms are also quite common in the general population. In a random sample of 51,090 women (age ≥20 years) from the Denmark population, 80.3% reported experiencing at least one symptom in the previous 4 weeks that could potentially be indicative of gynecologic cancer and most of those symptoms were not specifically gynecologic (e.g., abdominal pain, bloating, and tiredness) [26]. Hence, non-PMB symptoms may appear less alarming to patients and may desensitize providers’ concern for uterine cancer, hindering cancer diagnosis. This is consistent with evidence from the UK showing that among women with uterine cancer in 2007–2010, the average interval from symptom presentation to diagnosis was 73 days longer for women with vague symptoms than those with more typical symptoms such as PMB [27]. Research on ovarian cancer in the U.S. also showed that patients who presented with gastrointestinal symptoms (as opposed to gynecologic symptoms) or who had more than one symptom tended to experience longer delay in time to diagnosis and were more likely to be diagnosed at later-stage [10, 16]. Therefore, the more common presence of non-PMB symptoms among Black patients may be one of the reasons for their higher risk of advanced stage at diagnosis.
Although our data suggest that histology and grade explained a more substantial portion of the Black-White disparity in stage at diagnosis and might confound some of the racial difference in symptomatology, information on histology and grade is unknown to patients and providers during pre-diagnosis evaluation. In contrast, symptoms are directly observable to both patients and providers and can be acted upon. Appropriate symptom recognition is particularly important for early diagnosis of uterine cancer for which there is no routine screening. Moreover, having non-PMB symptoms remained significantly associated with advanced-stage diagnosis in our data even after accounting for histology. Hence improving patient and provider awareness about uterine cancer symptoms, especially non-PMB symptoms, may not only help attenuate disparity but also promote early diagnosis for all patients. Patients are often unaware of the warning signs of uterine cancer and often attribute symptoms to other diseases, resulting in delayed recognition and reporting of symptoms [11, 28]. This may especially affect Black patients who tend to have more comorbid conditions such as uterine fibroids that may cause similar symptoms [29]. Compared to White patients, Black patients also experience greater barriers to health care access and mistrust of the medical system which can further deter care seeking and symptom reporting [12]. In the meantime, provider education is also important. For example, there is well-documented implicit bias among providers regarding racial differences in pain tolerance and compliance with screening diagnosis [12]. Doll et al. [30] also reported that even among endometrial cancer patients with documented PMB, 90.6% of Black patients and 93.6% of White patients received a guideline-concordant diagnostic procedure. This suggests room for improving diagnostic evaluation and a need to address disparity in evaluation. Our data further suggest that alerting patients and providers of non-typical symptoms of uterine cancer and racial differences in symptom presentation may be beneficial.
Like all research endeavors, this study has several limitations. First, we relied on diagnosis codes to measure symptoms, which may under-capture symptoms. Nevertheless, our finding of nearly 90% of patients experiencing PMB is similar to the rate found in a systematic review which included studies using medical record data [31], supporting the validity of our measurement for PMB. Although we recognize that less specific symptoms (such as bloating) may not be accurately captured by diagnosis codes or may not prompt a visit, this should affect both Black and White patients. To the extent that Black patients generally experience greater barriers to care access, the higher rate of diagnosis codes for non-PMB symptoms we found in claims data for Black patients may in fact be a conservative estimate for Black-White differences in such symptoms. Second, our measurement of symptoms was limited to the 18 months prior to uterine cancer diagnosis. Symptoms that occurred earlier than 18 months might have been missed. However, the likelihood that such symptoms did not recur in the 18 months prior to cancer diagnosis (such that they could still be captured) is low. In addition, this measurement window helped reasonably exclude symptoms that might not be related to uterine cancer given that about 90% of uterine cancer patients were diagnosed within one year after symptom presentation [14, 15]. Third, we recognize that because of our large sample size, some of the findings that were statistically significant may only reflect clinically modest effect sizes. However, the reasons for health disparities are complex and are likely the compounding impact of many small differences [32]. Fourth, despite our inclusion of a comprehensive set of covariates, there were potential unmeasured confounders (e.g., unobserved sociocultural and clinician factors). The estimated E value [33] from our fully adjusted model in the overall sample suggested that the unmeasured confounders would need to be associated with both race and advanced-stage diagnosis by a risk ratio of at least 1.37 to explain away the remaining racial disparity in stage at diagnosis. Given this moderate E value, it is plausible that some other factors unobserved in our study may further explain racial disparities in stage at diagnosis. Additional research to identify such factors will be beneficial. Finally, our study was limited to elderly Medicare patients with fee-for-service coverage. The results may not be generalizable to other populations. Future research assessing symptom presentation, as well as potential differences in symptoms across racial/ethnic groups, in younger patients, patients with other types of insurance, and uninsured patients will provide additional insights.
In sum, we found that for patients with uterine cancer, the presence of less specific non-PMB symptoms increased their likelihood of advanced-stage diagnosis. Importantly, compared with White patients, a larger proportion of Black patients experienced non-PMB symptoms. Although this only modestly mediated some of the racial disparity in stage at diagnosis, it offers a new area of attention for promoting early diagnosis and reducing disparities. Nonetheless, residual Black-White disparity in stage at diagnosis remained even after accounting for symptoms and histology, calling for continued research to ascertain other contributing factors.
Supplementary Material
Highlights.
Black patients with uterine cancer were more likely than White patients to be diagnosed at advanced stage.
Having symptoms non-specific to uterine cancer was associated with a higher risk for advanced stage at diagnosis.
Black patients were more likely than White patients to have symptoms non-specific to uterine cancer.
Acknowledgement and Disclaimer:
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Funding:
This research was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD016386. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
Jason D. Wright has received royalties from UpToDate and received research funding from Merck. The other authors have no conflict of interest to declare.
Prior Presentation:
Preliminary results of this study were presented at the Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancer, Tampa, FL, March 25–28, 2023.
CRediT Authorship Contribution Statement:
Xiao Xu: Conceptualization, Methodology, Funding acquisition, Writing – original draft.
Ling Chen: Data curation, Formal analysis, Methodology, Writing – review & editing.
Marcella Nunez-Smith: Funding acquisition, Writing – review & editing.
Mitchell Clark: Writing – review & editing.
Jennifer S. Ferris: Writing – review & editing.
Dawn L. Hershman: Writing – review & editing.
Jason D. Wright: Conceptualization, Methodology, Funding acquisition, Writing – review & editing.
REFERENCES
- [1].National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. Bethesda, MD. https://seer.cancer.gov/statfacts/html/corp.html. Accessed March 24, 2023. [Google Scholar]
- [2].Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- [3].Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine Cancer Incidence and Mortality - United States, 1999–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Doll KM, Winn AN, Goff BA. Untangling the Black-White mortality gap in endometrial cancer: a cohort simulation. Am J Obstet Gynecol. 2017;216:324–5. [DOI] [PubMed] [Google Scholar]
- [5].ACOG Committee Opinion No. 734: The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124–e9. [DOI] [PubMed] [Google Scholar]
- [6].Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 2015;125:1006–26. [DOI] [PubMed] [Google Scholar]
- [7].Marchetti M, Vasile C, Chiarelli S. Endometrial cancer: asymptomatic endometrial findings. Characteristics of postmenopausal endometrial cancer. Eur J Gynaecol Oncol. 2005;26:479–84. [PubMed] [Google Scholar]
- [8].Passarello K, Kurian S, Villanueva V. Endometrial cancer: An overview of pathophysiology, management, and care. Semin Oncol Nurs. 2019;35:157–65. [DOI] [PubMed] [Google Scholar]
- [9].Walker S, Hyde C, Hamilton W. Risk of uterine cancer in symptomatic women in primary care: case-control study using electronic records. Br J Gen Pract. 2013;63:e643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ryerson AB, Eheman C, Burton J, McCall N, Blackman D, Subramanian S, et al. Symptoms, diagnoses, and time to key diagnostic procedures among older U.S. women with ovarian cancer. Obstet Gynecol. 2007;109:1053–61. [DOI] [PubMed] [Google Scholar]
- [11].Doll KM, Hempstead B, Alson J, Sage L, Lavallee D. Assessment of prediagnostic experiences of black women with endometrial cancer in the United States. JAMA Netw Open. 2020;3:e204954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Freeman HP, Chu KC. Determinants of cancer disparities: Barriers to cancer screening, diagnosis, and treatment. Surg Oncol Clin N Am. 2005;14(4):655–669. [DOI] [PubMed] [Google Scholar]
- [13].National Cancer Institute. SEER-Medicare: Brief Description of the SEER-Medicare Database. Bethesda, MD. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed March 27, 2023. [Google Scholar]
- [14].Liu JR, Conaway M, Rodriguez GC, Soper JT, Clarke-Pearson DL, Berchuck A. Relationship between race and interval to treatment in endometrial cancer. Obstet Gynecol. 1995;86:486–90. [DOI] [PubMed] [Google Scholar]
- [15].Vandborg MP, Christensen RD, Kragstrup J, Edwards K, Vedsted P, Hansen DG, et al. Reasons for diagnostic delay in gynecological malignancies. Int J Gynecol Cancer. 2011;21:967–74. [DOI] [PubMed] [Google Scholar]
- [16].Huepenbecker SP, Sun CC, Fu S, Zhao H, Primm K, Giordano SH, et al. Factors impacting the time to ovarian cancer diagnosis based on classic symptom presentation in the United States. Cancer. 2021;127:4151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].National Cancer Institute. Race and Hispanic Ethnicity Changes: For the 1975–2019 SEER Research Data (November 2021 Submission). Bethesda, MD. https://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/. Accessed March 29, 2023. [Google Scholar]
- [18].Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Chapter 3. Defining Categorization Needs for Race and Ethnicity Data. Content last reviewed April 2018. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/research/findings/final-reports/iomracereport/reldata3.html [Google Scholar]
- [19].Johnson CH, Adamo M. The SEER program coding and staging manual 2007. Bethesda, MD: National Cancer Institute; 2008. https://seer.cancer.gov/archive/manuals/2007/SPCSM_2007_maindoc.pdf. [Google Scholar]
- [20].National Cancer Institute Surveillance, Epidemiology, and End Results Program. Census Tract-level SES and Rurality Database (2006–2018). Bethesda, MD. https://seer.cancer.gov/seerstat/databases/census-tract/index.html. Accessed March 29, 2023. [Google Scholar]
- [21].National Cancer Institute. NCI Comorbidity Index Overview. Bethesda, MD. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html. Accessed July 25, 2023. [Google Scholar]
- [22].Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- [23].Johnson AL, Medina HN, Schlumbrecht MP, Reis I, Kobetz EN, Pinheiro PS. The role of histology on endometrial cancer survival disparities in diverse Florida. PLoS One. 2020;15:e0236402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Whetstone S, Burke W, Sheth SS, Brooks R, Cavens A, Huber-Keener K, et al. Health disparities in uterine cancer: Report from the Uterine Cancer Evidence Review Conference. Obstet Gynecol. 2022;139:645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J Clin Oncol. 2019;37:1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Balasubramaniam K, Ravn P, Larsen PV, Sondergaard J, Jarbol DE. Specific and unspecific gynecological alarm symptoms--prevalence estimates in different age groups: A population-based study. Acta Obstet Gynecol Scand. 2015;94:191–7. [DOI] [PubMed] [Google Scholar]
- [27].Din NU, Ukoumunne OC, Rubin G, Hamilton W, Carter B, Stapley S, et al. Age and gender variations in cancer diagnostic intervals in 15 cancers: Analysis of data from the UK Clinical Practice Research Datalink. PLoS One. 2015;10:e0127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Doll KM, Nguyen A, Alson JG. A conceptual model of vulnerability to care delay among women at risk for endometrial cancer. Gynecol Oncol. 2022;164:318–24. [DOI] [PubMed] [Google Scholar]
- [29].Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: A public health issue. Am J Obstet Gynecol. 2014;210(3):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Doll KM, Khor S, Odem-Davis K, He H, Wolff EM, Flum DR, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol. 2018;219:593 e1–e14. [DOI] [PubMed] [Google Scholar]
- [31].Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Phillips AR, Reitz KM, Myers S, Thoma F, Andraska EA, Jano A, Sridharan N, Smith RE, Mulukutla SR, Chaer R. Association between Black race, clinical severity, and management of acute pulmonary embolism: A retrospective cohort study. J Am Heart Assoc. 2021;10(17):e021818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


