Abstract
Background:
Aging is associated with gut dysbiosis, low-grade inflammation, and increased risk of type 2 diabetes (T2D). Prediabetes, which increases T2D and cardiovascular disease risk, is present in 45–50% of mid-life adults. The gut microbiota may link ultra-processed food (UPF) with inflammation and T2D risk.
Methods:
Following a 2-week standardized lead-in diet (59% UPF), adults aged 40–65 years will be randomly assigned to a 6-week diet emphasizing either UPF (81% total energy) or non-UPF (0% total energy). Measurements of insulin sensitivity, 24-h and postprandial glycemic control, gut microbiota composition/function, fecal short chain fatty acids, intestinal inflammation, inflammatory cytokines, and vascular function will be made before and following the 6-week intervention period. Prior to recruitment, menus were developed in order to match UPF and non-UPF conditions based upon relevant dietary factors. Menus were evaluated for palatability and costs, and the commercial additive content of study diets was quantified to explore potential links with outcomes.
Results:
Overall diet palatability ratings were similar (UPF = 7.6 ± 1.0; Non-UPF = 6.8 ± 1.5; Like Moderately = 7, Like Very Much = 8). Cost analysis (food + labor) of the 2000 kcal menu (7-d average) revealed lower costs for UPF compared to non-UPF diets ($20.97/d and $40.23/d, respectively). Additive exposure assessment of the 2000 kcal UPF diet indicated that soy lecithin (16×/week), citric acid (13×/week), sorbic acid (13×/week), and sodium citrate (12×/week) were the most frequently consumed additives.
Conclusions:
Whether UPF consumption impairs glucose homeostasis in mid-life adults is unknown. Findings will address this research gap and contribute information on how UPF consumption may influence T2D development.
Keywords: Ultra-processed food, Gut dysbiosis, Inflammation, Insulin resistance, Type 2 diabetes, Vascular function
1. Background
Advancing age is associated with a low-grade chronic inflammation (i.e., inflammaging) [1] that is associated with increased risk of chronic conditions [2], including type 2 diabetes (T2D) [3–5] and cardiovascular disease (CVD). Inflammaging, characterized by elevated levels of pro-inflammatory cytokines in middle age and beyond, is associated with a progressive reduction in whole-body insulin sensitivity, which is the primary contributor to age-related glucose intolerance [6,7]. In addition, ß-cell function and insulin secretion also decline with aging [8].
The gut microbiota is increasingly recognized as having a pivotal role in health and disease [9]. Microorganisms inhabit the gut and form a complex community that interact with each other and its host [10,11]. The gut microbiota is shaped by host genetics and environment; the latter playing a dominant role. Dysbiosis of the gut microbiota, characterized as adverse changes in the abundance and/or composition of gut microbes, has been implicated in the pathophysiology of a number of chronic diseases including colitis [12], metabolic and cardiovascular diseases [13]. Inflammaging is believed to be a consequence, at least in part, to changes in gut microbiota composition and function.
Ultra-processed foods (UPF) contribute ~60% of total energy in the US diet [14,15]. UPF consumption (based upon the NOVA system [15–17]) is associated with numerous adverse effects [18,19], including weight gain [20], obesity [21,22], increased cardiometabolic disease risk [23–25], and all-cause mortality [26]. NOVA (not an acronym) classifies foods into one of four groups based upon their degree of commercial processing: unprocessed or minimally processed, processed culinary ingredients, processed foods, and ultra-processed foods. A recent observational study in humans linked processed food intake with intestinal inflammation [27]. UPF components linked to alterations in the gut microbiota and increased intestinal inflammation include emulsifiers and non-nutritive sweeteners (NNS), although human in vivo research is sparse [28]. These and other UPF components which may impact gut microbial composition/function have been implicated in accelerating age-related diseases [29].
To date, only one randomized trial has tested a causal relationship between UPF and health. Hall et al [30] reported an increased energy intake (508 ± 106 kcal/d) in adults aged 31.2 ± 1.6 years on a high UPF diet (81% UPF; based on NOVA), resulting in ~1 kg weight gain in 2 weeks. This study provides insight into the impact of UPF on energy intake but no information on the gut microbiota was included. Although there was no consistent negative impact of high UPF intake on cardiometabolic risk factors, the inclusion of 3 daily 20-min cycling bouts may have negated the effect of UPF.
The identification of risk factors that could be targeted in mid-life is an important biomedical research priority, as this age group represents the growing number of Americans older than age 65 in the next two decades. The impact of UPF on glucose homeostasis and vascular function in mid-life adults has not been studied, despite the prevalence of nutrition-related chronic disease increasing with age [31] and association of UPF with adverse outcomes. This article will describe the study design and methods of a controlled feeding trial investigating the influence of UPF consumption on T2D risk and vascular function. Results of activities undertaken prior to recruitment are presented. This research will fill an important void in understanding the links between dietary behaviors and glucose homeostasis in mid-life.
2. Aims and hypotheses
The objective of this trial is to establish proof-of-concept for impairment in glucose homeostasis following increases in UPF consumption in mid-life adults. Our primary aim is to determine the influence of UPF consumption on insulin sensitivity and 24-h glycemic control in mid-life adults. We hypothesize that consumption of a UPF-rich diet will impair insulin sensitivity and 24-h glycemic control in mid-life adults. Our secondary aims are to determine the influence of UPF on gut microbial composition and function, fecal short chain fatty acids (SCFA), intestinal inflammation and permeability, serum endotoxin concentrations, inflammatory cytokines, flow-mediated dilation, and arterial stiffness in mid-life adults. We hypothesize that consumption of a UPF-rich diet will reduce gut microbial diversity and butyrate-producing bacteria, reduce SCFA, increase intestinal inflammation, permeability, endotoxin and inflammatory cytokines concentrations, and adversely affect vascular function. We further hypothesize that changes in gut microbial composition and function, fecal SCFA, intestinal inflammation and permeability, endotoxin, and inflammatory cytokine concentrations with UPF consumption will be correlated with the magnitude of change in glucose homeostasis in these individuals. This trial is registered at ClinicalTrials.gov (NCT05358171).
3. Study design and methods
3.1. Overview
The study will utilize a randomized parallel-group design (Fig. 1). Following a 2-week eucaloric (i.e., weight maintenance) lead-in diet, mid-life adults (40–65 years) will be randomly assigned to a 6-week eucaloric diet emphasizing UPF (81% UPF) or containing no UPF (0% UPF). The 6-week duration was selected based upon previous studies which reported changes in glucose/insulin homeostasis after diet interventions of 1–5 weeks in mid-life adults, preliminary work from a previous study conducted by our group that utilized a 6-week controlled feeding period, and observations of changes to gut microbial composition/function with dietary intervention of similar duration. To enhance rigor, the study diet composition will be verified by chemical analysis. Measurements of insulin sensitivity, 24-h and postprandial glycemic control using continuous glucose monitoring (CGM), gut microbiota composition/function, fecal SCFA, intestinal inflammation, intestinal permeability, serum endotoxin, inflammatory cytokines, flow-mediated dilation, and arterial stiffness will be made before and in the final week of the 6-week intervention period.
Fig. 1.
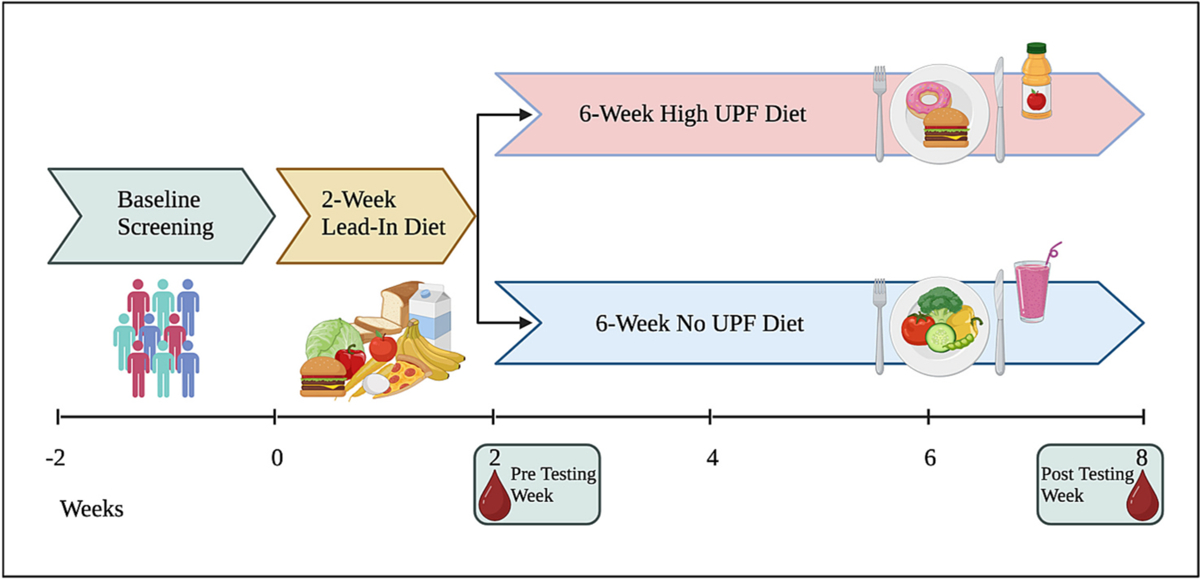
Study Design.
3.2. Participants and randomization
Eligible individuals will be aged 40–65 years, weight stable, body mass index (BMI < 35 kg/m2), sedentary to recreationally active, nondiabetic, and willing to comply with study procedures. To participate, individuals must not have major gastrointestinal disorders or special dietary needs/restrictions, and they must be willing and able to consume all study foods. Randomization will be stratified by age (40 to ≤55 y; 55 to ≤65 y), sex (male/female), and BMI (<30 kg/m2; 30 to ≤35 kg/m2) under the supervision of an individual not involved in data collection or analysis. Separate randomization schemes for sex by age and BMI will be employed to ensure equal numbers are assigned to each group.
3.3. Controlled diet design
Prior to recruitment, study diets were designed and evaluated. Participants will be fed a eucaloric diet (50% carb, 35% fat, 15% protein [10% animal/5% plant]) [32] controlled for potential confounding nutritional factors (i.e., fiber; added sugars; mono- & poly-unsaturated fats, saturated fat; sodium; glycemic index and load, and overall diet quality [Healthy Eating Index [HEI]-2015 [33]]). None of the diets contain non-Nutritive Sweeteners (NNS) due to uncertainty about their effects on the gut microbiota and glucose homeostasis [34]. Soluble (2 g/1000 kcal/d) and total dietary fiber (8 g/1000 kcal/d) will be similar to US averages [35–37]. Intake of added sugars, saturated fat, sodium, and overall diet quality [38] will be comparable to US averages [32,39]. The 2-wk lead-in diet contains 59% energy from UPF, consistent with the US average [14]. The 6-wk UPF diet contains 81% of energy from UPF, and the 6-wk diet containing no UPF (Non-UPF) contains 0% UPF [15,16,30]. NOVA classifications [15,16] will be determined manually using food labels and Nutrition Data Systems for Research (NDSR) 2022 Component/Ingredient output files (NDSR, Nutrition Coordinating Center, University of Minnesota).
First, menus were developed to meet the daily energy, UPF content, and nutrient values expected for each kcal level using NDSR, yielding 84 daily menus and three modules. The optional snack modules are used to provide extra snacks matched to the content of the total diet if needed due to changes in physical activity level or if the participant’s estimated energy needs are between two kcal levels (e.g., 1750 kcal). Accepted variations were ± 5 g of the daily targeted amount for macronutrients and ± 1 g of the daily targeted amount for soluble fiber. Subsequently, a list of available food items from local grocery stores or online vendors was developed to match the NDSR menu items. The nutrient label information for each food item was used to determine NOVA classification, and the product’s nutrient content was used to verify consistency with the menu item in the NDSR database. For the non-UPF diets, recipes were identified to provide homemade alternatives for commercial baked goods, such as bread, muffins, waffles, and buns. Recipes were entered into NDSR to provide detailed nutrient composition information for homemade items. A research dietitian reviewed the menus to verify that the daily targets were achieved. Sample menus are presented in Table 1, and images of the meals and snacks are provided in Supplemental Figs. 2–4. Daily menu targets and the 7-day average of the standardized lead-in, UPF, and Non-UPF diets at the 2000 kcal level are in Tables 2–4.
Table 1.
Sample Daily Menus for the 2000 kcal Standard Lead-in, UPF, and Non-UPF Diets.
| Standardized Lead-In (59% UPF) | UPF (81% UPF) | Non-UPF (0% UPF) | |
|---|---|---|---|
| Breakfast | Eggo waffles with blueberries, served with apple juice and whole milk. | Eggo waffles with syrup, served with orange juice | Homemade waffles with blueberries and pure maple syrup |
| Lunch | Ultra-processed beef patty on commercial white bun with lettuce and ketchup | Ultra-processed beef patty on commercial whole wheat bun with lettuce | 100% ground beef patty with salt on a homemade white bun with lettuce |
| Dinner | Salted chicken breast cooked in canola oil, served over whole grain pasta with broccoli, carrots, and parmesan cheese | Stouffer’s Lasagna with Meat and Sauce (frozen meal) | Salted chicken breast cooked in canola oil, served over whole grain pasta with broccoli, carrots, and parmesan cheese |
| Snack | Chips Ahoy! Cookies, served with Minute Maid Lemonade | Chips Ahoy! Cookies, apple slices with peanut butter, and carrots, served with Minute Maid Lemonade and whole milk | Banana, rice cakes with natural peanut butter, served with homemade lemonade and coconut water |
| Optional Module | Ritz crackers with cheddar cheese, ham, Skittles, and raspberries | Ritz crackers with American cheese, turkey, Skittles, and raspberries | Good Thins rice crackers with cheddar cheese, ham, cantaloupe, and natural fruit licorice candy |
UPF: Ultra-processed Foods.
Table 2.
Daily menu targets and the 7-day average of the Standardized Lead-In menus for the 2000 kcal level.
| Dietary Component | Weekly Average | Target | Difference |
|---|---|---|---|
| Energy (kcal) | 1981 | 2000 | 19 |
| Total Fat (g) | 79 | 78 | −1 |
| Total Carbohydrates (g) | 251 | 250 | −1 |
| Total Protein (g) | 76 | 75 | −1 |
| Animal Protein (g) | 50 | 50 | 0 |
| Vegetable Protein (g) | 26 | 25 | −1 |
| Total Saturated Fatty Acids (SFA) (g) | 27 | 27 | 0 |
| Total Monounsaturated Fatty Acids (MUFA) (g) | 32 | 31 | −1 |
| Total Polyunsaturated Fatty Acids (PUFA) (g) | 13 | 11 | −2 |
| Total Dietary Fiber (g) | 16 | 16 | 0 |
| Soluble Dietary Fiber (g) | 4 | 4 | 0 |
| Added Sugars (by Total Sugars) (g) | 72 | 70 | −2 |
| Sodium (Na, mg) | 3400 | 3400 | 0 |
| Total Vitamin A Activity (Retinol | 761 | 760 | −1 |
| Equivalents) (mcg) | |||
| Vitamin E (Total Alpha-Tocopherol) | 10 | 10 | 0 |
| (mg) | |||
| Vitamin C (ascorbic acid) (mg) | 129 | 130 | 1 |
| Zinc (mg) | 9 | 8 | −1 |
| Selenium (mcg) | 117 | 120 | 3 |
| Calcium (mg) | 960 | 874 | − 86 |
| Magnesium (mg) | 275 | 272 | − 3 |
| Potassium (K, mg) | 2033 | 2400 | 367 |
| % Calories from Fat | 35 | 35 | 0 |
| % Calories from Carbohydrate | 49 | 50 | 1 |
| % Calories from Protein | 15 | 15 | 0 |
| % Calories from SFA | 12 | 12 | 0 |
| % Calories from MUFA | 14 | 14 | 0 |
| % Calories from PUFA | 6 | 5 | −1 |
| Glycemic Index (glucose reference) | 60 | 56 | − 4 |
| Glycemic Load (GL; glucose reference) | 201 | N/Aa | N/Aa |
| Healthy Eating Index (HEI) | 58 | 58 | 0 |
| Ultra-Processed Food, % energy | 59 | 59 | 0 |
| Na/K ratio | 2 | 2 | 0 |
The goal for GL was to be consistent across the days within each diet condition and across diet conditions.
Table 4.
Daily menu targets and the 7-day average of the 0% UPF menus for the 2000 kcal level.
| Dietary Component | 7-day Average | Target | Difference |
|---|---|---|---|
| Energy (kcal) | 1978 | 2000 | 22 |
| Total Fat (g) | 77 | 78 | 1 |
| Total Carbohydrates (g) | 251 | 250 | −1 |
| Total Protein (g) | 77 | 75 | − 2 |
| Animal Protein (g) | 50 | 50 | 0 |
| Vegetable Protein (g) | 26 | 25 | −1 |
| Total Saturated Fatty Acids (SFA) (g) | 28 | 27 | −1 |
| Total Monounsaturated Fatty Acids | 33 | 31 | − 2 |
| (MUFA) (g) | |||
| Total Polyunsaturated Fatty Acids (PUFA) (g) | 10 | 11 | 1 |
| Total Dietary Fiber (g) | 14 | 16 | 2 |
| Soluble Dietary Fiber (g) | 4 | 4 | 0 |
| Added Sugars (by Total Sugars) (g) | 70 | 70 | 0 |
| Sodium (Na, mg) | 3401 | 3400 | −1 |
| Total Vitamin A Activity (Retinol Equivalents) (mcg) | 762 | 760 | − 2 |
| Vitamin E (Total Alpha-Tocopherol) (mg) | 8 | 10 | 2 |
| Vitamin C (ascorbic acid) (mg) | 131 | 130 | −1 |
| Zinc (mg) | 8 | 8 | 0 |
| Selenium (mcg) | 111 | 120 | 9 |
| Calcium (mg) | 738 | 874 | 136 |
| Magnesium (mg) | 241 | 272 | 31 |
| Potassium (K, mg) | 2130 | 2400 | 270 |
| % Calories from Fat | 34 | 35 | 1 |
| % Calories from Carbohydrate | 50 | 50 | 0 |
| % Calories from Protein | 16 | 15 | −1 |
| % Calories from SFA | 12 | 12 | 0 |
| % Calories from MUFA | 15 | 14 | −1 |
| % Calories from PUFA | 5 | 5 | 1 |
| Glycemic Index (glucose reference) | 62 | 56 | − 6 |
| Glycemic Load (GL; glucose reference) | 208 | N/Aa | N/Aa |
| Healthy Eating Index (HEI) | 52 | 58 | 6 |
| Ultra-Processed Food, % energy | 0 | 0 | 0 |
| Na/K ratio | 2 | 2 | 0 |
The goal for GL was to be consistent cross the days within each diet condition, and across diet conditions.
Diets will be prepared in the Metabolic Kitchen and Dining Laboratory at Virginia Tech by ServSafe-certified research assistants, and consist of a 7-d menu cycle with 3 meals and one snack. Participants will eat a supervised breakfast in the laboratory and take the remaining meals with them. Uneaten items will be returned and re-weighed to calculate compliance (g food consumed/g food provided). Trends of >1.5 kg weight loss/gain will be countered by the addition/subtraction of 250 kcal modules with a composition similar to the diet [40,41].
3.3.1. Palatability testing
After menu development, meal and snack palatability were assessed using the adapted United States Department of Agriculture (USDA) Sensory Evaluation Form [42]. Meals and snacks were rated on a 1–9 scale (1 = Dislike Extremely; 9 = Like Extremely). Overall acceptability ratings were similar between diets (Standardized lead-in diet: 7.7 ± 0.7; UPF diet: 7.6 ± 1.0; Non-UPF diet: 6.8 ± 1.5;), corresponding to Like Moderately (7)/Like Very Much (8).
3.3.2. Menu cost analysis
Cost analysis using local food costs (June/July 2023) determined that the daily average foods cost of the 2000 kcal UPF and Non-UPF menus were similar at $9.72 and $10.23, respectively. However, the Non-UPF diet requires significantly more preparation time/staff labor. When factoring in labor at $15/h, the UPF diet was about 50% less costly (Total costs: UPF = $20.97/day; Non-UPF = $40.23/day).
3.3.3. Commercial food additive analysis
The commercial additive content (i.e., frequency of exposure) of the study diets was quantified to enable the investigation of potential causal links with study outcomes [43]. Additives were classified using Codex Alimentarius [44] and determined using labels for foods in the 59% UPF and 81% UPF menus. The 0% diets contain no commercial additives. Table 5 provides the frequency of commercial additive exposure for a week of the 59% UPF and 81% UPF 2000 kcal menus.
Table 5.
Frequency of Commercial Additive Exposure in the 59% and 81% Ultra-Processed Food (UPF) 2000 kcal Weekly Menus.
| 59% UPF Menus | 81% UPF Menus | ||||
|---|---|---|---|---|---|
| Additive | Total # foods containing additive per week | Total # times eaten per week | Additive | Total # foods containing additive per week | Total # times eaten per week |
| Sodium citrate | 2 | 9 | Sodium citrate | 3 | 12 |
| Calcium phosphate | 1 | 6 | Calcium phosphate | 1 | 6 |
| Sodium phosphate | 1 | 6 | Sodium phosphate | 3 | 9 |
| Fumaric acid | 1 | 1 | Calcium carbonate | 2 | 6 |
| Citric acid | 3 | 10 | Citric acid | 4 | 13 |
| Calcium carbonate | 2 | 6 | Fumaric acid | 1 | 3 |
| Monocalcium phosphate | 2 | 6 | Soy lecithin | 4 | 16 |
| Sodium aluminum phosphate | 1 | 2 | Potassium citrate | 1 | 7 |
| Silicon dioxide | 1 | 1 | Tripotassium phosphate | 1 | 1 |
| Powdered cellulose | 1 | 3 | Acetic acid | 1 | 1 |
| Carnauba wax | Sodium aluminum phosphate | 1 | 1 | ||
| Silicon dioxide | 1 | 1 | Glucono delta lactone | 1 | 2 |
| Calcium sulfate | 1 | 4 | Phosphoric acid | 1 | 1 |
| Sunflower lecithin | 1 | 6 | Calcium sulfate | 1 | 6 |
| Soy lecithin | 4 | 11 | Silicon dioxide | 1 | 1 |
| Ascorbic acid | 2 | 2 | Powdered cellulose | 1 | 2 |
| Calcium disodium EDTA | 1 | 7 | Sunflower lecithin | 2 | 8 |
| Tapioca dextrin | Calcium disodium EDTA | 2 | 8 | ||
| Maltodextrin | 1 | 1 | BHA | 1 | 1 |
| Oleoresin paprika | 1 | 6 | Propyl gallate | 1 | 1 |
| Annatto | 1 | 6 | Mixed tocopherols | 1 | 1 |
| Caramel color | 2 | 4 | Ascorbic acid | 1 | 1 |
| Yellow #5 | 1 | 7 | Cellulose gum | 1 | 2 |
| Monoglycerides | 2 | 5 | Carrageenan | 2 | 5 |
| Sodium stearoyl lactylate | 3 | 6 | Maltodextrin | 3 | 6 |
| Glycerol ester of rosin Hydrogenated vegetable | 1 | 7 | Annatto color | 2 | 10 |
| oils | 1 | 1 | Beta carotene | 1 | 1 |
| Datem | 1 | 4 | Oleoresin paprika | 2 | 8 |
| Artificial flavoring | 2 | 4 | Caramel color | 2 | 2 |
| Annatto extract | 1 | 3 | Yellow #5 | 1 | 7 |
| Yeast extract | 1 | 3 | Yellow #6 | 1 | 7 |
| Ammonium sulfate | 3 | 7 | Fruit pectin | 2 | 7 |
| Cellulose gum | 1 | 1 | Xanthan gum | 2 | 4 |
| Calcium iodate | 1 | 4 | DATEM | 2 | 9 |
| Fruit pectin | 2 | 5 | Sodium stearoyl lactylate Sodium | 2 | 7 |
| Celery powder | 2 | 3 | hexametaphosphate | 1 | 1 |
| Hexametaphosphate | Guar gum | 1 | 2 | ||
| Guar gum | 1 | 1 | Glycerol ester of rosin | 1 | 7 |
| High fructose corn syrup | 7 | 19 | Distilled monoglyceride | 2 | 9 |
| Dextrose | 2 | 3 | Hydrogenated vegetable oils | 1 | 4 |
| Turbinado sugar | 1 | 1 | Yeast extract | 1 | 1 |
| Natamycin | 1 | 2 | Artificial flavors | 4 | 11 |
| High fructose corn syrup | 4 | 12 | |||
| Dextrose | 1 | 1 | |||
| Calcium sulfate | 2 | 2 | |||
| Ammonium sulfate | 2 | 7 | |||
| Calcium iodate | 1 | 5 | |||
| Celery powder | 1 | 2 | |||
| Sorbic acid | 5 | 13 | |||
| Monosodium glutamate | 1 | 6 | |||
| Calcium peroxide | 1 | 3 | |||
3.4. Controlled diet delivery
To provide a eucaloric diet for each participant, estimated energy needs are determined using the Mifflin-St. Jeor Equation [45] and self-reported physical activity information collected at the screening visit. Body weight will be monitored daily prior to breakfast to ensure weight stability. Trends of changes in body weight over 2 to 3 days (≥1.5 kg) are countered by the addition/subtraction of 250 kcal food modules or a change in dietary energy level.
Participants will have daily breakfast supervised, except for on Sundays when all meals are provided in advance on Saturday. The remaining meals and snacks for the day are given to the participant in a portable cooler. Any uneaten items are returned the following morning and reweighed. The total gram weight of food provided vs total grams consumed will be used as the indicator of dietary compliance. To be included in data analysis, participants must achieve >95% adherence on the controlled diet.
3.5. Chemical analysis
The actual nutrient content of the diets will be documented by chemical analysis in the Food Analysis Laboratory Control Center at Virginia Tech. For each diet (UPF, Non-UPF), an extra set of meals and snacks for each daily menu, prepared as they will be consumed by participants, will be assembled and frozen. Homogenized 7-d diet cycle composites will be prepared. Subsamples will be analyzed using established protocols [46]. The following nutrients will be analyzed in the composites, using validated standard methods and quality control measures employed in the generation of data for the USDA national nutrient database [47] and to validate diet composition [48–53]: Proximates (moisture, nitrogen, total fat, ash, yielding calculated energy, protein, total carbohydrates by difference), dietary fiber (McCleary enzymatic-gravimetric method [54] yielding soluble, insoluble, low molecular weight soluble fiber; total fiber by traditional enzymatic-gravimetric analysis [55]), elements (sodium, potassium, calcium, magnesium, phosphorus, iron, copper, manganese, zinc) by Inductively Coupled Plasmas (ICP) [56], selenium by ICP-Mass Spectrometry [57], fatty acids (saturated, mono- and polyunsaturated, trans, major individual fatty acids) by Gas Chromatography-Flame Ionization Detection [58]. Sugars (glucose, fructose, sucrose, lactose, maltose) [59], carotenoids [60], tocopherols and tocotrienols [61], and vitamin C will be quantified by High Performance Liquid Chromatography [62]. Additional subsamples will be stored (−80 °C) to preserve nutrient stability [63–65] for future analysis of other bioactive components or additives.
3.6. Participant recruitment, screening, and testing procedures
Recruitment will take place continuously over a 2-year period. Recruitment strategies will include flyers, ads in university-run List-servs, in-person presentations to campus and community groups, and social media. Interested individuals complete an online prescreening survey to evaluate basic eligibility criteria (age, physical activity, reported height/weight, medication use). Those who meet eligibility criteria (Supplemental Table 6) will be emailed the informed consent document and a study food list to verify the absence of food allergies/aversions.
During screening, eligibility will be determined based on health history, body mass index, blood chemistries (including fasting glucose, HbA1c, plasma lipids, and lipoproteins), and blood pressure (BP). In addition, a 2-h oral glucose tolerance test (OGTT) will be conducted with blood draws at 0 and 120 min to confirm participants do not have T2D. To confirm willingness and ability to consume all study foods, participants will be given a detailed list of all study foods to review.
Following the screening phase, participants will commence the two-week lead-in diet (59% UPF). During the second week of lead-in, baseline data collection will occur. Participants will wear a CGM and a physical activity monitor for 7 days to assess baseline glycemic variability and physical activity (PA). At the end of lead-in, participants will complete 2-day urine and 3-day fecal collections. Participants will also complete a comprehensive 2-h OGTT with blood draws at 0, 30, 60, 90, and 120 min for measures of glucose, HbA1c, insulin, serum lipid and lipoproteins, and inflammatory biomarkers. Lastly, participants will undergo measurements arterial stiffness brachial artery function and body composition.
To evaluate the effects of the controlled diet intervention period, all baseline measures are repeated during the final week of the intervention. Details on these measures are described below, and the schedule of study assessments is presented in Supplemental Fig. 5.
3.7. Description of study assessment procedures
3.7.1. Health history
A health history questionnaire will be completed at screening to confirm eligibility. Premenopausal females will be queried regarding menstrual cycle phase using a menstrual cycle calendar at two time-points (week 2 and week 8 of controlled feeding). An infection/inflammation questionnaire will be completed during screening and during days that fecal samples are collected. Individuals reporting infection/inflammation in the prior 2 weeks will be rescheduled when symptom-free for 2 weeks.
3.7.2. Body mass and composition and energy requirements
Height and body mass (to the nearest 0.1 kg) will be measured using a stadiometer and digital scale (Scale-Tronix 5002, Welch Allyn Inc., Skaneateles Falls, NY). Body mass index will be calculated (kg/m2). Body composition will be measured via DEXA (GE Lunar iDXA) will be completed during the screening period, baseline data collection period, and post-intervention. Premenopausal females provide a urine sample for a pregnancy test prior to the DEXA scan.
3.7.3. Blood chemistries
Fasting plasma glucose, HbA1c, serum lipid and lipoproteins, and inflammatory biomarkers will be measured from blood collected at Time 0 during the baseline and post-intervention OGTT. Serum lipid and lipoproteins are measured in a Clinical Laboratory Improvement Amendments (CLIA)- certified laboratory (Labcorp, Roanoke, VA). Inflammatory cytokines including tumor necrosis factor-alpha, interleuken-6, and monocyte chemoattractant protein–1 will be measured by enzyme-linked immunosorbent assay (American Diagnostica, Inc). Serum endotoxin will be assessed using the PyroGene Recombinant Factor C assay (Lonza, Basel, Switzerland) [66,67].
3.7.4. Blood pressure
Resting blood pressure will be measured via an automated sphygmomanometer (OMRON HEM-907, city state) according to American Heart Association (AHA) guidelines [68]. Participants arrive at the lab after an overnight fast, while withholding caffeine. After a 15-min seated rest period, three to six BP readings will be taken and the average BP will be calculated. Blood pressure will be assessed at 3 timepoints: during each OGT at screening and at baseline and post-intervention testing sessions.
3.7.5. Physical activity
Physical activity (PA) will be recorded by having each individual wear an accelerometer (wGT3X-BT, Actigraph Inc.) on their non-dominant wrist for 7 days during the last week of the lead-in and intervention diets [41] to document unchanging PA. Participants are instructed not to change their habitual PA.
3.7.6. Dietary intake
Habitual dietary intake will be assessed during screening by collecting three unannounced 24-h dietary recalls, for 1 weekend day and 2 weekdays [41,69]. Participants will be instructed on procedures for estimating portion size using 2D food models [70]. Recall forms and dietary analysis results (NDSR 2022, Univ. of Minn. Coord. Center) will be used to determine habitual total energy intake, macronutrient composition, and usual UPF intake [15,16].
3.7.7. Glucose control
Oral glucose tolerance will be assessed in response to a 75 g glucose load. During the screening OGTT, blood glucose will be measured at baseline (0 min) and 120 min post-glucose beverage consumption. During lead-in baseline and post-intervention testing sessions, OGTT blood samples will be collected every 30 min (0, 30, 60, 90, 120 min). Glucose tolerance, insulin sensitivity [71], and ß-cell function will be calculated from concentrations of glucose (Hemocue Glucose 201, Brea, CA), insulin, and c-peptide (high-sensitivity ELISA, R&D Systems) [72]. Glucose and insulin area under the curve (AUC) will be calculated using the trapezoidal method. Homeostasis Model of Assessment of Insulin Resistance (HOMA-IR) will be calculated as a surrogate for hepatic insulin sensitivity [73]. Insulin clearance will be estimated using the ratio of fasting C-peptide/insulin concentrations. 24-h and postprandial glycemic control will be assessed using a continuous glucose monitor (CGM) (Lifestyle Libre Pro, Alameda, CA) inserted into the back of the upper arm, and free-living glycemia will be assessed for 6 days during the last week of the lead-in and intervention diets. The 24-h incremental area under the curve (AUC) will be the primary CGM outcome. Mean 24-h glucose, glycemic variability, time in/out of range, and 2-h postprandial glucose responses will be calculated according to recent recommendations [74]. Only recordings with the CGM active for at least 70% of the total time will be used [74].
3.7.8. Urinalysis
Urine will be collected for 48 h at baseline and post-intervention sessions for assessment of urinary sodium, potassium, phosphorus, albumin, creatinine, and nitrogen. Urine containers and instructions will be provided to participants during the second week of the lead-in diet and during the final week of the controlled feeding period. Participants will return urine containers in biohazard coolers with freezer packs to keep the urine cold/refrigerated.
3.7.9. Gut microbiome DNA and SCFA
Fecal samples are collected daily during the final 3 days during the lead-in and intervention periods. Samples are saved in sterile plastic containers and frozen (−80 °C) within 24–48 h of collection until final processing and analysis. Fecal samples are homogenized and total bacterial DNA will be extracted using Qiagen PowerSoil kit (Qiagen, Germantown, MD). Bacterial 16S ribosomal RNA will be amplified using field-standard barcoded primers [75] and amplicons will be sequenced using an Illumina MiSeq. The resulting sequence data will be preprocessed using the QIIME2 platform [75] and bacteria identified using the DADA2 software package [76]. Statistics associated with diet treatment will be performed using linear models implemented with the lme4 package in R and the lmerTest package to generate p values. A mixed-effects model will be used by including a random intercept term (1 | ID) in the model formula within the lmer() function to account for repeated sampling. Short-chain fatty acid concentrations will be measured via gas chromatography with a flame ionizing detector using reported protocols [77]. Intestinal inflammation will be assessed using fecal calprotectin, lactoferrin, and lipocalin-2, measured using ELISA [78–81]. Intestinal permeability will be assessed using serum zonulin (Immunodiagnostik AG, Bensheim, Germany) concentrations via ELISA [82,83].
3.7.10. Arterial stiffness and brachial artery function
Participants will report to the laboratory after an overnight fast and without having performed any vigorous physical activity for the past 48 h. Participants will lie on a hospital bed for 10 min before having their blood pressure and heart rate measured three times using an automated oscillometric blood pressure Omron HEM 907XL (OmronHealth care Inc., West Field Court Lake Forest, IL, USA). Endothelial function will be assessed using the Flow-Mediated Dilation (FMD) technique. Brachial artery FMD will be assessed with duplex ultrasonography (GE Logiq E, GE Healthcare, WI) using a high-resolution linear array transducer (with stereotaxic holder) according to published guidelines and recommendations [84,85]. Reactive hyperemia will be produced by inflation of a pediatric blood pressure cuff placed around the participant’s forearm for 5 min and endothelium vasodilation will be assessed by measuring the diameter of the artery for 2 min following deflation of the pediatric cuff. Endothelium independent vasodilation (EID) will be assessed by measuring the diameter of the brachial artery for 5 min following administration of 0.4 mg of sublingual nitroglycerine. Edge detection software (Vascular Analysis Tools, Medical Imaging Applications, Inc) will be used to analyze baseline and post-reactive hyperemic diameters of the brachial artery of the participant. FMD and EID are expressed as mm and % change from the baseline diameter of the brachial artery. Arterial stiffness will be measured by calculating the carotid-femoral pulse wave velocity (C–F PWV) from tonometry waveforms (NIHem, Cardiovascular Engineering, Inc., Norwood, MA) and body distances (distances of the carotid and femoral pulse recording site to the suprasternal notch).
3.8. Statistical analysis and power calculation
Intention-to-treat will be the primary analytic approach. Effects on primary/secondary outcomes will be assessed using mixed-effect linear models to estimate treatment differences between diet conditions. Exploratory path analysis with multiple mediators will be utilized to understand mechanisms of change and estimate effect sizes. Covariates will be included to control for baseline group differences, if any. With a 2 × 2 design and α = 0.05, we will have >80% power to detect significant diet intervention condition differences for effect size (Cohen’s f) as small as 0.25 and change in insulin sensitivity of 20 ± 32% with a minimum sample of n = 17/group [86]. Links between additive exposures and study outcomes will be explored [43].
4. Discussion
Middle-aged adults are at increased risk for glucose intolerance, T2D, and cardiovascular diseases. Ultra-processed food consumption is associated with an increased risk of T2D and CVD even after controlling for overall diet quality [25]. A 10% increase in UPF intake is associated with a 15% and 13% increase in risk of T2D and coronary heart disease, respectively. To date, no experimental trials have evaluated the impact of UPF on glucose homeostasis or vascular function in mid-life adults.
UPF consumption may result in a gut microbiota with a low abundance of SCFA-producing bacteria, increased intestinal inflammation and permeability [87]. Compared to the Bacteriodetes-rich microbiota associated with foraging and agriculture, the UPF-microbiota has a lower microbial diversity, which could contribute to immune system changes that lead to chronic inflammation and associated disease risk. Shifts in gut microbiota with industrialization are associated with an increased abundance of Bacteroidaceae and Verrucomicrobia, reduced SCFA production, reduced barrier function, and increased colonization resistance [87].
Numerous components of UPF could mechanistically link UPF consumption with inflammation and T2D risk. Emulsifiers (e.g., polysorbate 80 [P80], carboxymethylcellulose [CMC]) are widely present in our food supply, including UPF [88]. Evidence from ex vivo, animal, and preclinical studies suggest that emulsifier exposure alters microbiota composition and initiates a cascade of events leading to intestinal inflammation and impaired glycemic control. Chassaing et al [89] reported that P80 and CMC altered microbial gene expression related to flagella expression in a simulator of the human intestinal microbial ecosystem. Transferring these emulsifier-treated microbiotas to germ-free mice increased Proteobacteria and Enterobacteriacae and decreased Bacteroidaceae, increased lipopolysaccharide and flagellin, and induced chronic low-grade inflammation. Treatment of mice with these emulsifiers reduced mucous thickness, increased microbial encroachment and intestinal inflammation, and impaired glycemic control [90]. Fecal samples fermented with P80 reduced microbial diversity, bifidobacterial abundance, fermentation capacity, and SCFA production [91]. Recent evidence extends these findings to a wider variety of commonly-used commercial additives/emulsifiers [43]. Increased intestinal permeability was reported in rodents exposed to a thermally-processed diet, which generated advanced glycation end products (common in UPF) [92]. Human clinical studies are needed to understand the implications of these findings.
UPF are becoming increasingly recognized as a contributing factor to the development of major chronic diseases and are now addressed in the American Heart Association guidelines [93], the American Cancer Society guidelines [94], and the American Diabetes Association Consensus Report [95]. The USDA Dietary Guidelines for Americans do not address the potential adverse health effects of a high UPF diet [96]. Our study may contribute to an evidence base needed to inform guidelines which do not currently address total UPF consumption due to a lack of rigorously-designed trials.
5. Strengths and limitations
This trial uses a controlled feeding design with diets matched for potential confounding dietary factors, which is a major strength. A 2-week standardized diet lead-in period is included to minimize baseline differences in habitual diet and the gut microbiota [70,97,98]. Although a crossover design was considered, the lack of human clinical evidence [70] and uncertainty of how long-lasting possible effects of UPF and/or commercial additives on the gut microbiota [43] or glucose tolerance may be, it is difficult to determine the duration of an adequate washout period (a limitation in existing studies [97]). Participant burden, retention, dietary compliance, and carryover effects may also be problematic with more lengthy controlled feeding periods [98,99]. Previous studies indicate changes in glucose/insulin homeostasis after diet interventions of 1–5 weeks in mid-life adults [100,101] and observations of changes to gut microbial composition/function with dietary intervention of similar duration [97]. For these reasons, a parallel design and a 6-week diet duration were selected.
This is an outpatient trial so dietary compliance cannot be definitively determined, however dietary biomarker data (urinary excretion) will be used to evaluate compliance in addition to self-reported information. The gut microbiome analysis will be limited to 16S rRNA sequencing, targeted metabolic analyses of SCFA, and targeted qRT-PCR. Untargeted metabolomics and shot gun metagenomic approaches would extend future work to better understand the meta-organismal pathway contributing to impaired glucose homeostasis.
6. Conclusions and future directions
Mid-life is a vulnerable stage with 45–50% of adults in this age group having prediabetes [102]. This highlights the significance of T2D prevention strategies for these individuals. Our research could contribute to the evidence base informing dietary guidelines and T2D prevention strategies for this age group. Based upon these findings, future studies could examine the time course of changes, match diets in UPF but vary additive content to isolate the effects of individual UPF components/additives,43,and investigate behavioral strategies to reduce UPF intake.
Supplementary Material
Table 3.
Daily menu targets and the 7-day average of the 81% UPF menus for the 2000 kcal level.
| Dietary Component | Weekly Average | Target | Difference |
|---|---|---|---|
| Energy (kcal) | 1986 | 2000 | 14 |
| Total Fat (g) | 79 | 78 | −1 |
| Total Carbohydrates (g) | 252 | 250 | − 2 |
| Total Protein (g) | 76 | 75 | −1 |
| Animal Protein (g) | 49 | 50 | 1 |
| Vegetable Protein (g) | 27 | 25 | − 2 |
| Total Saturated Fatty Acids (SFA) (g) | 29 | 27 | − 2 |
| Total Monounsaturated Fatty Acids | 29 | 31 | 2 |
| (MUFA) (g) | |||
| Total Polyunsaturated Fatty Acids | 14 | 11 | − 3 |
| (PUFA) (g) | |||
| Total Dietary Fiber (g) | 15 | 16 | 1 |
| Soluble Dietary Fiber (g) | 5 | 4 | −1 |
| Added Sugars (by Total Sugars) (g) | 71 | 70 | −1 |
| Sodium (Na, mg) | 3405 | 3400 | − 5 |
| Total Vitamin A Activity (Retinol Equivalents) (mcg) | 760 | 760 | 0 |
| Vitamin E (Total Alpha-Tocopherol) (mg) | 8 | 10 | 2 |
| Vitamin C (ascorbic acid) (mg) | 131 | 130 | −1 |
| Zinc (mg) | 9 | 8 | −1 |
| Selenium (mcg) | 117 | 120 | 3 |
| Calcium (mg) | 1004 | 874 | −130 |
| Magnesium (mg) | 267 | 272 | 5 |
| Potassium (K, mg) | 2268 | 2400 | 132 |
| % Calories from Fat | 35 | 35 | 0 |
| % Calories from Carbohydrate | 50 | 50 | 0 |
| % Calories from Protein | 15 | 15 | 0 |
| % Calories from SFA | 13 | 12 | −1 |
| % Calories from MUFA | 13 | 14 | 1 |
| % Calories from PUFA | 6 | 5 | −1 |
| Glycemic Index (glucose reference) | 61 | 56 | − 5 |
| Glycemic Load (GL; glucose reference) | 205 | N/Aa | N/Aa |
| Healthy Eating Index (HEI) | 50 | 58 | 8 |
| Ultra-Processed Food, % energy | 81 | 81 | 0 |
| Na/K ratio | 2 | 2 | 0 |
The goal for GL was to be consistent cross the days within each diet condition, and across diet conditions.
Acknowledgments
This work was supported by the National Institutes of Health (grant number: R21AG077143 and R21AG075930).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2024.107427.
Data availability
Data will be made available on request.
References
- [1].Ferrucci L, Fabbri E, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nat. Rev. Cardiol 15 (9) (2018) 505–522, 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fulop T, Witkowski JM, Olivieri F, Larbi A, The integration of inflammaging in age-related diseases, Semin. Immunol 40 (2018) 17–35, 10.1016/j.smim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- [3].Pickup JC, Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes, Diabetes Care 27 (3) (2004) 813–823, 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- [4].Pickup JC, Crook MA, Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41 (10) (1998) 1241–1248, 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- [5].Shoelson SE, Lee J, Goldfine AB, Inflammation and insulin resistance, J. Clin. Invest 116 (7) (2006) 1793–1801, 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee PG, Halter JB, The pathophysiology of hyperglycemia in older adults: clinical considerations, Diabetes Care 40 (4) (2017) 444–452, 10.2337/dc16-1732. [DOI] [PubMed] [Google Scholar]
- [7].Defronzo RA, Glucose intolerance and aging: evidence for tissue insensitivity to insulin, Diabetes 28 (12) (1979) 1095–1101, 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- [8].Chang AM, Halter JB, Aging and insulin secretion, Am. J. Physiol. Endocrinol. Metab 284 (1) (2003) E7–12, 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- [9].Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI, The human microbiome project, Nature 449 (7164) (2007) 804–810, 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gill SR, Pop M, Deboy RT, et al. , Metagenomic analysis of the human distal gut microbiome, Science (1979) 312 (5778) (2006) 1355–1359, 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Savage DC, Microbial ecology of the gastrointestinal tract, Annu. Rev. Microbiol 31 (1977) 107–133, 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- [12].Garrett WS, Lord GM, Punit S, et al. , Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system, Cell 131 (1) (2007) 33–45, 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tang WHW, Backhed F, Landmesser U, Hazen SL, Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review, J. Am. Coll. Cardiol 73 (16) (2019) 2089–2105, 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA, Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study, BMJ Open 8 (3) (2018) e020574, 10.1136/bmjopen-2017-020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Monteiro CA, Cannon G, Levy RB, et al. , Ultra-processed foods: what they are and how to identify them, Public Health Nutr 22 (5) (2019) 936–941, 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC, The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing, Public Health Nutr 21 (1) (2018) 5–17, 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC, Freshly prepared meals and not ultra-processed foods, Cell Metab 30 (1) (2019) 5–6, 10.1016/j.cmet.2019.06.006. [DOI] [PubMed] [Google Scholar]
- [18].Chen X, Zhang Z, Yang H, et al. , Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies, Nutr. J 19 (1) (2020) 86, 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elizabeth L, Machado P, Zinocker M, Baker P, Lawrence M, Ultra-processed foods and health outcomes: a narrative review, Nutrients 12 (7) (2020), 10.3390/nu12071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB, Changes in diet and lifestyle and long-term weight gain in women and men, N. Engl. J. Med 364 (25) (2011) 2392–2404, 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mendonca RD, Pimenta AM, Gea A, et al. , Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-up (SUN) cohort study, Am. J. Clin. Nutr 104 (5) (2016) 1433–1440, 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- [22].Rauber F, Chang K, Vamos EP, et al. , Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK biobank, Eur. J. Nutr (2020), 10.1007/s00394-020-02367-1. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martinez Steele E, Juul F, Neri D, Rauber F, Monteiro CA, Dietary share of ultra-processed foods and metabolic syndrome in the US adult population, Prev. Med 125 (2019) 40–48, 10.1016/j.ypmed.2019.05.004. [DOI] [PubMed] [Google Scholar]
- [24].Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N, Ultra-processed foods and incident cardiovascular disease in the Framingham offspring study, J. Am. Coll. Cardiol 77 (12) (2021) 1520–1531, 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- [25].Srour B, Fezeu LK, Kesse-Guyot E, et al. , Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante), BMJ 365 (2019) l1451, 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schnabel L, Kesse-Guyot E, Alles B, et al. , Association between Ultraprocessed food consumption and risk of mortality among middle-aged adults in France, JAMA Intern. Med 179 (4) (2019) 490–498, 10.1001/jamainternmed.2018.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bolte LA, Vich Vila A, Imhann F, et al. , Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome, Gut (2021), 10.1136/gutjnl-2020-322670. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khoshbin K, Camilleri M, Effects of dietary components on intestinal permeability in health and disease, Am. J. Physiol. Gastrointest. Liver Physiol (2020), 10.1152/ajpgi.00245.2020. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zinocker MK, Lindseth IA, The Western diet-microbiome-host interaction and its role in metabolic disease, Nutrients 10 (3) (2018), 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hall KD, Ayuketah A, Brychta R, et al. , Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake, Cell Metab 30 (1) (2019) 226, 10.1016/j.cmet.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Prince MJ, Wu F, Guo Y, et al. , The burden of disease in older people and implications for health policy and practice, Lancet 385 (9967) (2015) 549–562, 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- [32].Shan Z, Rehm CD, Rogers G, et al. , Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016, JAMA 322 (12) (2019) 1178–1187, 10.1001/jama.2019.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Healthy Eating Index: Comparing the HEI-2015, HEI-2010 & HEI-2005.
- [34].Suez J, Korem T, Zeevi D, et al. , Artificial sweeteners induce glucose intolerance by altering the gut microbiota, Nature 514 (7521) (2014) 181–186, 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- [35].Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK, Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and nutrition examination survey I epidemiologic follow-up study, Arch. Intern. Med 163 (16) (2003) 1897–1904, 10.1001/archinte.163.16.1897. [DOI] [PubMed] [Google Scholar]
- [36].King DE, Mainous AG 3rd, Lambourne CA, Trends in dietary fiber intake in the United States, 1999–2008, J. Acad. Nutr. Diet 112 (5) (2012) 642–648, 10.1016/j.jand.2012.01.019. [DOI] [PubMed] [Google Scholar]
- [37].Ma Y, Hebert JR, Li W, et al. , Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative observational study, Nutrition 24 (10) (2008) 941–949, 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Ajami NJ, El-Serag HB, et al. , Dietary quality and the colonic mucosa-associated gut microbiome in humans, Am. J. Clin. Nutr 110 (3) (2019) 701–712, 10.1093/ajcn/nqz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cogswell ME, Zhang Z, Carriquiry AL, et al. , Sodium and potassium intakes among US adults: NHANES 2003–2008, Am. J. Clin. Nutr 96 (3) (2012) 647–657, 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mitchell CM, Davy B, Ponder MA, et al. , The effect of prebiotic supplementation with inulin on insulin sensitivity in adults at elevated risk for type 2 diabetes: A pilot randomized controlled trial, In review. Published online, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mitchell CM, Davy BM, Halliday TM, et al. , The effect of prebiotic supplementation with inulin on cardiometabolic health: rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes, Contemp. Clin. Trials 45 (Pt B) (2015) 328–337, 10.1016/j.cct.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].USDA, Sensory Evaluation Form - Fill and Sign Printable Template Online https://www.uslegalforms.com/form-library/54246-usda-sensory-evaluation-form. (Accessed 17 September 2023).
- [43].Naimi S, Viennois E, Gewirtz AT, Chassaing B, Direct impact of commonly used dietary emulsifiers on human gut microbiota, Microbiome 9 (1) (2021) 66, 10.1186/s40168-020-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].FAO/WHO, International Numbering System for Food Additives. Codex Committee on Food Additives and Contaminants. Codex Alimentarius http://www.fao.org/fao-who-codexalimentarius/about-codex/en/.
- [45].Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO, A new predictive equation for resting energy expenditure in healthy individuals, Am. J. Clin. Nutr 51 (2) (1990) 241–247, 10.1093/AJCN/51.2.241. [DOI] [PubMed] [Google Scholar]
- [46].Phillips KM, Stewart KK, Valdiating diet composition by chemical analysis, in: Dennis BH, Ershow A, Obarzanek E, Clevidence B (Eds.), Well-Controlled Diet Studies in Humans: A Practical Guide to Design and Management, American Dietetic Association, 1999, pp. 336–367. [Google Scholar]
- [47].Haytowitz DB, Pehrsson PR, USDA’s National Food and nutrient analysis program (NFNAP) produces high-quality data for USDA food composition databases: two decades of collaboration, Food Chem 238 (2018) 134–138, 10.1016/j.foodchem.2016.11.082. [DOI] [PubMed] [Google Scholar]
- [48].Carey VJ, Bishop L, Charleston J, et al. , Rationale and design of the optimal macro-nutrient intake heart trial to prevent heart disease (OMNI-heart), Clin. Trials 2 (6) (2005) 529–537, 10.1191/1740774505cn123oa. [DOI] [PubMed] [Google Scholar]
- [49].Dennis BH, Stewart P, Wang CH, et al. , Diet design for a multicenter controlled feeding trial: the DELTA program. Delta research group, J. Am. Diet. Assoc 98 (7) (1998) 766–776, 10.1016/s0002-8223(98)00173-4. [DOI] [PubMed] [Google Scholar]
- [50].Kris-Etherton PM, Etherton TD, Pearson TA, et al. , Stanol supplemented margarine lowers LDL-C in moderately hypercholesterolemic subjects fed an average American diet, FASEB J 12 (1998). [Google Scholar]
- [51].Phillips KM, Patterson KY, Rasor AS, et al. , Quality-control materials in the USDA National Food and nutrient analysis program (NFNAP), Anal. Bioanal. Chem 384 (6) (2006) 1341–1355, 10.1007/s00216-005-0294-0. [DOI] [PubMed] [Google Scholar]
- [52].Phillips KM, Stewart KK, Karanja NM, et al. , Validation of diet composition for the dietary approaches to stop hypertension trial. DASH collaborative research group, J. Am. Diet. Assoc 99 (8 Suppl) (1999) S60–S68, 10.1016/s0002-8223(99)00418-6. [DOI] [PubMed] [Google Scholar]
- [53].Racette SB, Spearie CA, Phillips KM, Lin X, Ma L, Ostlund RE Jr., Phytosterol-deficient and high-phytosterol diets developed for controlled feeding studies, J. Am. Diet. Assoc 109 (12) (2009) 2043–2051, 10.1016/j.jada.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Insoluble, soluble, and Total dietary Fiber in foods-enzymatic-gravimetric-liquid chromatography, in: Official Method 2011.25 (32.1.43). AOAC Official Methods of Analysis, Association of Official Analytical Chemists International, 2011. [Google Scholar]
- [55].Total Dietary Fiber in Foods-Enzymatic-Graviemtric Method. Official Method 985.29 (45.4.07). AOAC Official Methods of Analysis, Association of Official Analytical Chemists International, 2005. [Google Scholar]
- [56].Metals in Foods by ICP. Methods 985.01 (3.2.06) and 984.27 (50.1.15). Official Methods of Analysis of the Association of Official Analytical Chemists, 2011. [Google Scholar]
- [57].Chromium, Selenium, and Molybdenum Infant Formula and Adult Nutritional Products-Inductively Coupled Plasma-Mass Spectrometry. Method 2011.19. Official Methods of Analysis of the Association of Official Analytical Chemists, Assocation of Official Analytical Chemists, 2011. [Google Scholar]
- [58].Fat (Total, Saturated, and Unsaturated) in Foods, Hydrolytic Extraction Gas Chromatographic Method. Official Method 996.06. AOAC Official Methods of Analysis, Association of Official Analytical Chemists International, 2005. [Google Scholar]
- [59].Official Method 982.14.AOAC Official Method of Analysis, Association of Official Analutical Chemists International, 2005. [Google Scholar]
- [60].Y1004–2 Carotenoid Profile. Quantitatively Measure Individual Carotenoids by LC-VIS/DAD. Technical Sheet, Eurofins SF Analytical Laboratories Inc., 2020. [Google Scholar]
- [61].Kamal-Eldi A, Gorgen S, Pettersson J, Lampi AM, Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols. Comparison of different chromatographic columns, J. Chromatogr. A 881 (1–2) (2000) 217–227, 10.1016/s0021-9673(99)01346-1. [DOI] [PubMed] [Google Scholar]
- [62].Tarrago-Trani M, Phillips K, Cotty M, Matrix-specific method validation for quantitative analysis of vitamin C in diverse foods, J. Food Compos. Anal 26 (1–2) (2012) 12–25. [Google Scholar]
- [63].Phillips K, Tarrago-Trani M, Gebhardt S, et al. , Stability of vitamin C in frozen raw fruit and vegetable homogenates, J. Food Compos. Anal 23 (2010) 253–259. [Google Scholar]
- [64].Phillips K, Wunderlich KM, Exler J, et al. , Stability of 5-methyltatrahydrofolate in fresh frozen fruits and vegetables, Food Chem 94 (2) (2005) 587–595. [Google Scholar]
- [65].Phillips KM, Simpkins AH, Amanna KR, et al. , Long-term stability of nutrients in a frozen mixed food control material, Fresenius J. Anal. Chem 370 (2–3) (2001) 297–302, 10.1007/s002160100785. [DOI] [PubMed] [Google Scholar]
- [66].Anderson AS, Haynie KR, McMillan RP, et al. , Early skeletal muscle adaptations to short-term high-fat diet in humans before changes in insulin sensitivity, Obesity (Silver Spring) 23 (4) (2015) 720–724, 10.1002/oby.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bowser SM, McMillan RP, Boutagy NE, et al. , Serum endotoxin, gut permeability and skeletal muscle metabolic adaptations following a short term high fat diet in humans, Metabolism 103 (2019) 154041, 10.1016/j.metabol.2019.154041. [DOI] [PubMed] [Google Scholar]
- [68].Muntner P, Shimbo D, Carey RM, et al. , Measurement of blood pressure in humans: a scientific statement from the American Heart Association, Hypertension 73 (5) (2019) E35–E66, 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moshfegh AJ, Rhodes DG, Baer DJ, et al. , The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes, Am. J. Clin. Nutr 88 (2) (2008) 324–332, 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- [70].Leeming ER, Louca P, Gibson R, Menni C, Spector TD, Le Roy CI, The complexities of the diet-microbiome relationship: advances and perspectives, Genome Med 13 (1) (2021) 10, 10.1186/s13073-020-00813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Matsuda M, DeFronzo RA, Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp, Diabetes Care 22 (9) (1999) 1462–1470, 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- [72].Kashyap SR, Bhatt DL, Wolski K, et al. , Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment, Diabetes Care 36 (8) (2013) 2175–2182, 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia 28 (7) (1985) 412–419, 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- [74].Battelino T, Danne T, Bergenstal RM, et al. , Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range, Diabetes Care 42 (8) (2019) 1593–1603, 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Caporaso JG, Lauber CL, Walters WA, et al. , Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample, Proc. Natl. Acad. Sci. U. S. A 108 (Suppl. 1) (2011) 4516–4522, 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Callahan B, DADA2 and the State of the Art, Accessed at: https://benjjneb.github.io/dada2/SotA.html. [Google Scholar]
- [77].David LA, Maurice CF, Carmody RN, et al. , Diet rapidly and reproducibly alters the human gut microbiome, Nature 505 (7484) (2014) 559–563, 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M, Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation, PLoS One 7 (9) (2012) e44328, 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kane SV, Sandborn WJ, Rufo PA, et al. , Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation, Am. J. Gastroenterol 98 (6) (2003) 1309–1314, 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- [80].Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H, Lipocalin-2: a master mediator of intestinal and metabolic inflammation, Trends Endocrinol. Metab 28 (5) (2017) 388–397, 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [81].Schwiertz A, Spiegel J, Dillmann U, et al. , Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease, Parkinsonism Relat. Disord 50 (2018) 104–107, 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- [82].Morkl S, Lackner S, Meinitzer A, et al. , Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women, Eur. J. Nutr 57 (8) (2018) 2985–2997, 10.1007/s00394-018-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Qi Y, Goel R, Kim S, et al. , Intestinal permeability biomarker Zonulin is elevated in healthy aging, J. Am. Med. Dir. Assoc 18(9):810 (2017) e1–810 e4, 10.1016/j.jamda.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Corretti MC, Plotnick GD, Vogel RA, Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound, Am. J. Phys 268 (4 Pt 2) (1995), 10.1152/AJPHEART.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- [85].Thijssen DHJ, Black MA, Pyke KE, et al. , Assessment of flow-mediated dilation in humans: a methodological and physiological guideline, Am. J. Physiol. Heart Circ. Physiol 300 (1) (2011), 10.1152/AJPHEART.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Perreault L, Bergman BC, Hunerdosse DM, Playdon MC, Eckel RH, Inflexibility in intramuscular triglyceride fractional synthesis distinguishes prediabetes from obesity in humans, Obesity (Silver Spring) 18 (8) (2010) 1524–1531, 10.1038/oby.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sonnenburg JL, Sonnenburg ED, Vulnerability of the industrialized microbiota, Science (1979) 366 (6464) (2019), 10.1126/science.aaw9255. [DOI] [PubMed] [Google Scholar]
- [88].Shah R, Kolanos R, DiNovi MJ, Mattia A, Kaneko KJ, Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety, Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 34 (6) (2017) 905–917, 10.1080/19440049.2017.1311420. [DOI] [PubMed] [Google Scholar]
- [89].Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT, Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation, Gut 66 (8) (2017) 1414–1427, 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chassaing B, Koren O, Goodrich JK, et al. , Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome, Nature 519 (7541) (2015) 92–96, 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gerasimidis K, Bryden K, Chen X, et al. , The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity, Eur. J. Nutr (2019), 10.1007/s00394-019-02161-8. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Snelson M, Tan SM, Clarke RE, et al. , Processed foods drive intestinal barrier permeability and microvascular diseases, Sci. Adv 7 (14) (2021), 10.1126/sciadv.abe4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lichtenstein AH, Appel LJ, Vadiveloo M, et al. , 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association, Circulation 144 (23) (2021) e472–e487, 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- [94].Rock CL, Thomson C, Gansler T, et al. , American Cancer Society guideline for diet and physical activity for cancer prevention, CA Cancer J. Clin 70 (4) (2020) 245–271, 10.3322/CAAC.21591. [DOI] [PubMed] [Google Scholar]
- [95].Evert AB, Dennison M, Gardner CD, et al. , Nutrition therapy for adults with diabetes or prediabetes: a consensus report, Diabetes Care 42 (5) (2019) 731–754, 10.2337/DCI19-0014/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans-2020–2025, 9th edition, 2020. Accessed February 13, 2022, https://www.dietaryguidelines.gov/.
- [97].So D, Whelan K, Rossi M, et al. , Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis, Am. J. Clin. Nutr 107 (6) (2018) 965–983, 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- [98].Davy KP, Davy BM, Advances in nutrition science and integrative physiology: insights from controlled feeding studies, Front. Physiol 10 (2019) 1341, 10.3389/fphys.2019.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hall DM, Most MM, Dietary adherence in well-controlled feeding studies, J. Am. Diet. Assoc 105 (8) (2005) 1285–1288, 10.1016/j.jada.2005.05.009. [DOI] [PubMed] [Google Scholar]
- [100].Sacks FM, Carey VJ, Anderson CA, et al. , Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial, JAMA 312 (23) (2014) 2531–2541, 10.1001/jama.2014.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Solverson PM, Henderson TR, Debelo H, Ferruzzi MG, Baer DJ, Novotny JA, An anthocyanin-rich mixed-berry intervention may improve insulin sensitivity in a randomized trial of overweight and obese adults, Nutrients 11 (12) (2019), 10.3390/nu11122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Menke A, Casagrande S, Geiss L, Cowie CC, Prevalence of and trends in diabetes among adults in the United States, 1988–2012, JAMA 314 (10) (2015) 1021–1029, 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


