Abstract
Peripheral artery disease (PAD) is an atherosclerotic disease associated with limb ischemia that necessitates limb amputation in severe cases. Cell therapies comprised of adult mononuclear or stromal cells have been clinically tested and show moderate benefits. Bioengineering strategies can be applied to modify cell behavior and function in a controllable fashion. Using mechanically tunable or spatially controllable biomaterials, we highlight examples in which biomaterials can increase the survival and function of the transplanted cells to improve their revascularization efficacy in preclinical models. Biomaterials can be used in conjunction with soluble factors or genetic approaches to further modulate the behavior of transplanted cells and the locally implanted tissue environment in vivo. We critically assess the advances in bioengineering strategies such as 3D bioprinting and immunomodulatory biomaterials that can be applied to the treatment of PAD, and then discuss the current challenges and future directions in the implementation of bioengineering strategies.
Graphical Abstract

Introduction
Peripheral artery disease (PAD) is an atherosclerotic occlusive disease that is associated with obstructed blood flow to the limb, leading to limb ischemia. PAD accounts for over 6 million patients in the US and 200 million patients globally.1 Risk factors for PAD include advanced age, smoking, and diabetes2 An advanced form of PAD known as chronic limb-threatening ischemia (CLTI) is associated with gangrene formation, ulceration, and amputation of the limb.3, 4 Surgical and endovascular interventions to restore vascularization to the ischemic limb are effective but not suitable for all PAD patients. A promising approach to induce revascularization is therapeutic angiogenesis, which aims to induce the formation of new blood vessels from preexisting ones.5, 6This therapeutic strategy is well-suited for PAD patients, especially diabetic PAD patients,7 who have impaired regeneration capacity. Numerous strategies to augment therapeutic angiogenesis have been tested in clinical studies, including cell, protein, and gene therapies,8 although the results have only shown minimal to moderate therapeutic benefit. Some of the limitations of the cell-based strategies include poor transplant cell survival, short-lived gene/protein delivery, harsh inflammatory host response, and suboptimal therapeutic dosing or frequency. Although cell-based therapies are not off-the-shelf, compared to protein or gene therapies, autologous cell therapies can be reintroduced into the patient as quickly as on the same day of cell harvest.
Biomaterials and bioengineering strategies have the potential to improve cell-based therapies through several mechanisms including 1) sustaining cell viability during implantation; 2) providing extracellular matrix (ECM) signaling cues that augment therapeutic cell efficacy; 3) modulating the local immune or inflammatory environment for improved therapeutic outcomes; and 4) delivering therapeutic factors locally to the affected tissue. Although non-cellular-based strategies involving gene or protein therapies have been extensively reviewed elsewhere,9, 10 we focus on cell-based therapies and the incorporation of bioengineering strategies to advance cell-based therapies. In this review, we give an overview of the state of cell therapy in clinical and preclinical setting of PAD and describe the emerging bioengineering methods to improve the efficacy of cell-based strategies for treatment of PAD.
What We Do and Do Not Know from Cell Therapy Clinical Trials
Clinical trials for treating PAD using cell therapies are based on the reasoning that the transplanted cells may induce angiogenesis through the paracrine secretion of pro-angiogenic protein growth factors, the release of genetic cargo (ie, extracellular vesicles), the formation of neovasculature, or the incorporation into existing host vasculature. Therapeutic cells that have been tested in clinical trials of PAD include bone marrow-derived mononuclear cells, mesenchymal stromal cells (MSCs), and subpopulations within these cell types based on surface antigen expression.11 Besides some of the notable clinical trials that are described below, we also summarize examples of ongoing or completed trials in Table 1.
Table 1. Clinical Trials of Biological Therapies for Treatment of PAD.
| Study Title | Study Design | Intervention Type |
Intervention | NCT Number |
|---|---|---|---|---|
| Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone-marrow cells: a pilot study and a randomized controlled trial 12 | Randomized No Masking Multi-center |
Biological | Bone marrow mononuclear cell (BM-MNC) | N/A |
| Patients With Intermittent Claudication Injected With ALDH Bright Cells (PACE) 13 | Randomized Quadruple Blind Multi-center |
Biological | Aldehyde dehydrogenase bright (ALDH-br) bone marrow cells (BMC) |
NCT01774097 |
| Safety Study of MultiGeneAngio in Patients With Peripheral Arterial Disease 14 | Single Group Assignment Multi-center |
Biological | MultiGeneAngio (MGA) | NCT00390767 |
| ALD-301 for Critical Limb Ischemia, Randomized Trial 15 | Randomized Triple blind Multi-center |
Procedure | Aldehyde dehydrogenase bright (ALDH-br) bone marrow cells (BMC) and mononuclear bone marrow cells (BM-MNC) |
NCT00392509 |
| Autologous Bone Marrow Mononuclear Cell Implantation for Moderate to Severe Peripheral Arterial Disease 16 | Single Group Assignment Multi-center |
Procedure | Bone marrow mononuclear cell (BM-MNC) | NCT00919516 |
| Granulocyte-Macrophage Stimulating Factor in the Treatment of Peripheral Arterial Disease 17 | Randomized Triple blind Single Center |
Drug | Granulocyte-Macrophage Stimulating Factor (GM-CSF) | NCT01041417 |
| Assessment Of Vascular Health After Niacin Therapy (AVANT) | Randomized Double blind Single Center |
Drug | Niacin | NCT02003638 |
| Treatment of Intermittent Claudication by G-CSF-mobilized PB-MNC | Randomized Single blind Single center |
Procedure | Peripheral blood mononuclease (PB-MNC) | NCT03683628 |
| Treatment of No-option CLI by G-CSF-mobilized PB-MNC | Randomized No Masking Single Center |
Procedure | Peripheral blood mononuclease (PB-MNC) | NCT03686228 |
| BGC101 (EnEPC) Autologous Cell Therapy From Patient's Own Blood for Treatment of Critical Limb Ischemia | Randomized Double blind Multi-center |
Biological | IBGC101 (autologous EnEPC preparation) | NCT02805023 |
| Safety of Intramuscular Injection of Allogeneic PLX-PAD Cells for the Treatment of Critical Limb Ischemia | Single Group Assignment Single Center |
Biological | Placenta-derived adherent stromal cells (PLX-PAD) | NCT00919958 |
| Safety and Preliminary Efficacy of Adipose Derived Stem Cells and Low Frequency Ultrasound in PAD | Randomized No Masking Single Center |
Biological | Low Frequency Ultrasound (LoFU) and Adipose Derived Stem Cells (ADSC) | NCT02756884 |
| A Clinical and Histological Analysis of Mesenchymal Stem Cells in Amputation 18 | Non-Randomized No Masking Single Center |
Biological | Allogeneic bone marrow derived mesenchymal stem cells (MSC) | NCT02685098 |
| Cell Therapy for Peripheral Arterial Disease and Diabetes | Randomized Single Blind Single Center |
Procedure | Cell therapy with a hematopoietic stem cell (HSCs) concentrate | NCT03635970 |
The first cell therapy clinical trial for treatment of PAD was a randomized pilot study of 47 participants called the Therapeutic Angiogenesis using Cell Transplantation (TACT) Study.12 The TACT trial studied the efficacy of autologous bone marrow mononuclear cell and peripheral blood mononuclear blood cell injections (~109 cells per injection) into the ischemic limb of patients with an ankle-brachial index (ABI) below 0.6. Among the 47 participants, 25 were randomized to receive injections of bone marrow mononuclear cells into the gastrocnemius muscle of the relatively more ischemic leg, with the other leg’s gastrocnemius muscle receiving saline. The other 22 participants were injected with bone marrow mononuclear cells into one leg’s gastrocnemius muscle, whereas peripheral blood mononuclear cells were injected into the other one. Characterization of the bone marrow-derived mononuclear cells showed a subpopulation of CD45+ cells that expressed pro-angiogenic factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). Functional measures based on ABI, transcutaneous oxygen pressure (TcO2), and rest pain were quantified at baseline, 4 weeks, and 24 weeks after cell therapy. Participants treated with bone marrow mononuclear cells or peripheral blood mononuclear cells showed significant improvement in ABI and TcO2 between baseline and at 4 weeks, and the results were sustained up to 24 weeks. In contrast, participants treated with saline showed no improvement in ABI and TcO2. In addition, the researchers found that bone marrow mononuclear cells exhibited a greater therapeutic effect compared to peripheral blood mononuclear cells, based on ABI assessment after 4 weeks. This pilot clinical study showed promising results that encouraged larger clinical trials by subsequent investigators.
PACE was a Phase II clinical trial that studied the efficacy of intramuscularly injected autologous bone marrow-derived aldehyde dehydrogenase bright (ALDHbr) cells into participants with PAD and claudication. ALDHbr cells were used as they were shown to be enriched with stem and progenitor cells that may exert therapeutic benefit.19 Patients received 10 intramuscular injections of either autologous ALDHbr cells (n=38) or a cell-free vehicle control (n=40).13 The outcome measured included peak walk time, collateral vessel count, peak hyperemic popliteal flow, and limb perfusion. Importantly, these outcome measures revealed no significant benefit at 6 months after cell implantation.
MSCs show promise as a cell source for treating PAD because in part because they can be sourced from a wide variety of tissues, including bone marrow, adipose tissue, placental tissue, umbilical cord and Wharton’s jelly. In addition, MSCs are known to secrete a range of pro-angiogenic factors that make them attractive for clinical studies.20, 21 Although several dozens of Phase I and II clinical trials have tested the efficacy of MSCs in PAD participants, the patient enrollment size were limited to less than 100 participants, and the cells were usually injected intramuscularly into the ischemic limb.22 In addition, there is significant variance in the number of cells used for treatment as well as what outcomes were measured. Despite this, MSC therapy is generally regarded as safe. With regards to therapeutic efficacy, the outcome measures show promise in these small studies. For example, patients with CLTI originating from Buerger’s disease benefitted from intramuscularly delivered allogeneic bone marrow-derived MSCs, based on pain at rest, ABI, and walking distance, compared to standard of care treatment per month.23
Due to the differences in outcomes of individual clinical studies, systematic reviews and meta-analyses of randomized clinical trials have provided additional insights into the overall benefit of stem cell therapy for treatment of PAD. One systematic review of 28 randomized controlled trials showed that autologous stem cell therapy (ie, bone marrow-derived MSCs, bone marrow- or peripheral blood-derived mononuclear cells) was associated with significant improvement in ABI and TcO2, along with a decline in limb amputation rate, compared to the control group.24 Two separate analyses of 28 and 23 studies found that autologous stem cell therapies show to significantly promote wound healing in patients and is both safe and effective for patients with CLTI.25, 26 Another systematic review of 19 randomized controlled trials drew a similar conclusion, with additional findings that local intramuscular cell injection was more effective as a cell delivery modality than intra-arterial injection.27 However, another meta-analysis of 10 stem cell clinical studies (primarily using bone marrow mononuclear cells of bone marrow-derived MSCs) found no significant benefit in amputation rate, survival, and amputation-free survival, when comparing cell treatment to placebo.28
Based on the various meta-analyses, it is well-accepted that adult stem cell therapies are generally safe and well-tolerated with minimal or transient side effects. However, there is much that we still do not understand. Notably, the efficacy of stem cell therapy remains inconclusive. Contributing to this uncertainty is the fact that the previous clinical studies were relatively small, lacked long-term followup, and were not sufficiently powered for detecting statistical significance. It is possible that the followup was not performed at an optimal time point that would reveal maximal benefit to vascular function. Additionally, the variance in findings reported by the different meta-analyses may be dueto the variability of the transplanted cells, owing to donor-to-donor differences and potential differences in cell harvesting methods. Furthermore, clinical studies do not permit extensive tracking of transplant cell survival, so it is unknown to what extent the transplanted cells survive in the ischemic limb among different donor cells. Such limitations of past clinical studies suggest a need to perform larger clinical studies with more uniform and well-characterized cell populations and longer followup times, as well as to develop more standardized approaches to cell to reduce the donor-to-donor differences during cell harvest and to maximize post-transplant survival. Finally, there is still a lack of knowledge of the phenotypic markers that define the optimal stem cell therapeutic, the ideal frequency and dosage of cells, and the variability of autologous cell quality.
Going beyond existing clinical trials of adult stem cell therapies, some of the emerging opportunities include the use of alternative cell types, including induced pluripotent stem cell (iPSC) derivatives or genetically modified cells to boost cell function, as well as the use of bioengineering strategies to improve the survival and efficacy of therapeutic cells upon transplantation. With the use of non-invasive imaging strategies to detect labeled transplanted cells, we can further track the survival of the transplanted cells in preclinical studies, which would not be allowable in clinical studies. These strategies are overviewed below.
Experimental Endothelial Cell Therapies in Pre-Clinical Testing
A cell type that has shown promise in preclinical setting are endothelial cells (ECs) derived from human pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Pluripotent stem cell-derived ECs can be derived reproducibly using well-established differentiation protocols.29-34 Compared to primary human ECs, pluripotent stem cell-derived ECs have been shown to recapitulate many of the phenotypic, transcriptional, and functional properties of bonafide ECs.35 Although iPSCs and ESCs both have theoretically long-term expansion capability, autologous ECs can only be derived from iPSCs. We and others have demonstrated the therapeutic efficacy of pluripotent stem cell-derived EC injection in murine model of experimental PAD.31, 36-38 We previously showed that injections of human iPSC-ECs in mice with induced hindlimb ischemia could improve blood perfusion and vascular density in ischemic limbs for up to 28 days, whereas delivery of non-therapeutic cells such as fibroblasts did not exert any therapeutic benefit.31 Besides pluripotent stem cell-derived ECs, ECs have been successfully generated directly from somatic cells by the transient activation of the Yamanaka factors (Oct3/4, Sox2, KLF4, C-MYC) in conjunction with endothelial-inducing soluble factors ,39 or by direct endothelial reprogramming of ETS transcription factor ETV240, 41 or miR-208b-3p.42 These experimental cell types serve as alternative sources for therapeutic ECs that may have clinical translational potential.
An important consideration in the preclinical assessment of stem cell therapies is the animal model of PAD. A commonly used model of PAD involves surgical ligation or excision of the murine femoral artery, which induces acute impairment in blood flow to the lower limb.43 Although this model induces limb ischemia that is experienced in clinical subjects, it does not recapitulate other pathological aspects of PAD, including endothelial dysfunction or fewer endogenous stem cells, which are further exacerbated by concurrent diabetes.8, 44 Published studies further demonstrate variability of induced ischemia to varying muscle groups, with the distal anterior hind limb muscles showing the greatest consistency of ischemia induction.45 Alternative animal models involving ameroid constructors to gradually induce ischemia, but it can result in distinctively different molecular signaling processes, compared to an acute ligation model.46 Additionally, considerations of strain, age, and sex differences can further influence the severity of the animal model, as reviewed previously.47 These technical constraints should be considered when interpreting data derived from preclinical models.
Bioengineering Approaches to Enhance Post-Transplant Cell Survival
Since non-cell-based experimental bioengineering approaches i to treat PAD have been reviewed elsewhere,9, 10 here, we focus on bioengineering strategies to enhance cell-based therapies. Despite the therapeutic potential of cell therapies, the viability of cells during and after cell implantation is a bottleneck that limits the efficacy of the cells. In recent decades, a number of biomaterials and bioengineering strategies have sought to mimic the physiological tissue microenvironment, in order to promote cell survival in the ischemic limb and/or augments the therapeutic efficacy of the implanted cells. These strategies utilize controllable hydrogels, spatially nanopatterned biomaterials, and bioengineering strategies to genetically prime the therapeutic cells for transplantation.
i. Cell-Encapsulated Hydrogels
Cell therapies have emerged as promising treatments for PAD by exerting paracrine effects that induce angiogenesis or reduce inflammation, as well as by directly promoting new vasculature formation.48, 49 Nevertheless, direct cell injection faces significant limitations, primarily due to poor cell retention at the site of injury and poor viability in the ischemic environment;50 encapsulation of cells in hydrogels, which are polymer scaffolds that hold a high degree of water content, has proven beneficial to address these challenges. To this end, our research group has been actively developing angiogenic hydrogels designed for encapsulating human induced pluripotent stem cell derived endothelial progenitors (iPSC-EPs) and driving their self-assembly into vascular networks. We have shown that matrix elastic modulus and degradability play crucial roles in plexus formation.34, 51, 52 However, these properties are often interchangeable in most angiogenic hydrogels.53 Therefore, we developed interpenetrating networks of collagen and hyaluronic acid hydrogel that allow independent tunability of elastic modus and degradability to better stimulate angiogenesis.51 These systems underscore the importance of the role of mechano-regulation on vascularization so that angiogenic biomaterials can be effectively deployed in the clinic and ultimately improve vascular health.
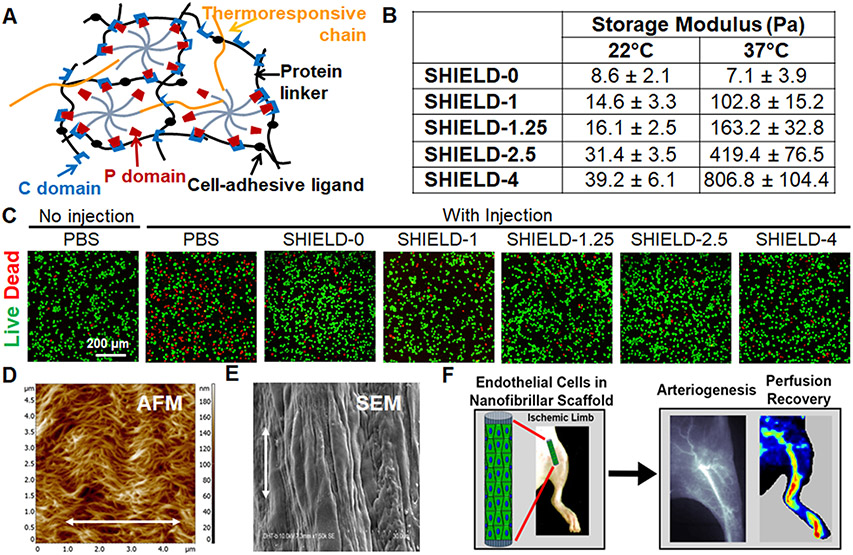
Furthermore, for successful clinical deployment, it is imperative to develop injectable hydrogels, which have shown promise as a minimally invasive method for cell delivery to alleviate PAD. One commonly employed technique for developing injectable hydrogels involves the use of a polymer with temperature-controlled gelation. For example, Foster et al developed shear-thinning injectable hydrogels, termed SHIELD, to improve cell viability of encapsulated iPSC-ECs in vivo.54 SHIELD consists of two polymers: a multi-arm poly(ethylene glycol) (PEG) attached to cell adhesion peptides and poly(N-isopropyl-acrylamide) (PNIPAM), a thermally responsive polymer with a lower critical solution temperature of 32°C (Fig. 1A). The SHIELD system forms an injectable gel below 32°C with a storage modulus under 50 Pa but can stiffen in vivo up to 1000 Pa by adjusting the PNIPAM concentration (Fig. 1B). In vitro, SHIELD hydrogels demonstrated improved cell viability (Fig. 1C) and proliferation of iPSC-ECs. In vivo in hindlimb ischemia models, iPSC-ECs delivered through the hydrogel with ~400 Pa storage modulus exhibited prolonged retention, with 25% of the cells delivered using the SHIELD hydrogel remaining after 3 days compared to only 7% of the cells when delivered through bolus injection. iPSC-ECs encapsulated in SHIELD hydrogel also significantly enhanced arteriole density (100 arterioles/mm2) compared to the PBS control group (26 arterioles/mm2) and iPSC-ECs delivered without the hydrogel (34 arterioles/mm2). These findings suggest that the SHIELD system promotes transplant cell survival as well as the formation of larger-diameter vessels, compared to delivering the cells in saline.
Fig. 1. Engineered hydrogels and scaffolds can be engineered to have mechanically and/or biophysically tunable properties.
A. Schematic of engineered SHIELD hydrogel is formed by mixing together two components: C7 engineered protein and 8-arm PEG-P1 with or without thermoresponsive PNIPAM to form a gel. B. By changing the PNIPAM content, the storage (G’) moduli of SHIELD hydrogels can vary at 37 °C among physiologically relevant levels. C. By tuning the mechanical properties of SHIELD, the survival of encapsulated iPSC-ECs within hydrogel formulations after syringe injection are affected. D. Spatially nanopatterned collagen scaffold structure is visualized by AFM imaging. E. ECs cultured on the scaffold forms elongated cells along the direction of the collagen nanofibril orientation. F. Nanopatterned scaffolds seeded with ECs induce revascularization and reperfusion in a mouse model of PAD. A-C. Adapted with permission.54 D-F. Reprinted with permission.61 Copyright 2015 American Chemical Society.
Cell-based therapies for PAD can exert their effects through paracrine signaling rather than directly participate in forming new vasculature.55 Zhao et al developed an injectable hydrogel to enhance retention and viability of MSCs using a chitosan hydrogel.56 Chitosan (CS) was chosen for its thermal responsivity and in vivo degradability. Chitosan was further modified through covalent attachment of the C domain of insulin-like growth factor 1 (IGF-1). The MSCs used in this study were genetically modified to express red fluorescence protein for detection in vivo and luciferase to measure cell viability, since luciferase activity in these cells is directly proportional to MSC viable cell count. For in vivo testing, the researchers induced hindlimb ischemia in mice engineered to express luciferase downstream of the VEGFR2 promoter, thereby allowing for the quantification of the proangiogenic effects of the delivered MSCs. Although for all tested groups, the MSC count significantly decreased by up to 1000-fold 8 days after injection, the IGF-1-CS hydrogel had a significantly higher signal compared to the unmodified CS hydrogel and when MSCs were delivered using PBS. Despite the low retention of MSCs in vivo, the delivered MSCs stimulated angiogenesis, as evidenced by increased luciferase signal from mouse endothelial cells expressing VEGFR2. The highest signal was observed with the IGF-1-CS hydrogel, peaking at day 10. This increase in VEGFR2 signal correlated with a roughly three-fold increase in capillary density in mice receiving MSCs encapsulated in IFG-1-CS compared with MSCs delivered without a hydrogel. Moreover, the IGF-1-CS group showed the highest percentage of limb salvage (70%), with lower rates of necrosis (30%) and no amputations required. In contrast, when MSCs were delivered alone, only 30% achieved limb salvage, with higher rates of necrosis (50%) and limb amputation (20%). Overall, the IGF-1-CS hydrogel improved MSC retention, stimulated angiogenesis, increased capillary density, and enhanced limb salvage rates in the hindlimb ischemia model compared to MSCs delivered without a hydrogel.
Similarly, Young et al developed an injectable hydrogel system for intramuscular delivery of adipose-derived stem cells (ADSCs). ADSCs have well-studied proangiogenic effects through paracrine signaling, much like MSCs.57-59 The researchers utilized a hydrogel composed of methacrylated chitosan with covalently bound RGD for cell attachment, alongside poly(trimethylene carbonate)-b-poly(ethylene glycol)-b-poly(trimethylene carbonate) diacrylate (PEG(PTMC-A)2). The PEG(PTMC-A)2 component enhanced the mechanical properties of chitosan and reduced the hydrogel’s swelling ratio, enabling it to withstand cyclic forces in muscle tissue without causing damage to the surrounding tissue. By modulating the PEG(PTMC-A)2 content, the target compressive modulus (5-15 kPa) and compressive strength at failure (0.5) were achieved. The polymers were crosslinked via a temperature-sensitive free radical polymerization technique employing ammonium persulfate and tetramethylethylenediamine. This polymerization process exhibits a slow crosslinking rate of 9 minutes at room temperature, facilitating comprehensive mixing of the components without premature gelation. However, it enables rapid crosslinking (3 minutes) upon in vivo injection. ADSCs showed high viability under both normoxic (98%) and hypoxic (90%) conditions, with hypoxic conditions promoting increased release of proangiogenic factors, including a 10-fold higher VEGF release and three-fold higher Angiopoietin-1 release after 14 days in culture, supporting their intended use for inducing angiogenesis in ischemic tissue. In a hindlimb ischemia model, the cell-laden hydrogels maintained ADSC density similar to that after 1 week of in vitro culture, equivalent to 30% cell retention, resulting in a significant increase in the number of endothelial CD31+ cells within the muscle tissue when ADSCs were delivered in the hydrogel (170 cells/mm2) compared to bolus injection (140 cells/mm2). Although functional recovery was not reported, these findings hold promise for future applications in PAD treatment. Together, these studies highlight the utility of injectable hydrogels as carriers of the implanted cells that can promote cell survival and improve the angiogenic outcomes.
Some of the advantages of injectable hydrogels for the encapsulation and co-delivery of stem cells include the provision of a biomimetic ECM environment that may improve cell survival and function within the harsh ischemic limb, the tunability of the hydrogel’s mechanical and biochemical properties, and the minimally invasive nature of direct intramuscular injection of the cell-encapsulated hydrogels. These advantages are countered by the limitations, including the scalability and safety of the hydrogels for clinical use, the retention of the hydrogels over time, and potential concerns of inflammatory or immune response to the hydrogels. With the notable exception of alginate, most hydrogels are not currently FDA-approved. Hydrogels that involve complex chemistries or recombinant DNA technology may be subjected to additional concerns of scalability or permissible endotoxin levels, respectively.
ii. Biophysical Patterning Cues in Biomaterials
Besides mechanically tunable hydrogels, another strategy to improve the survival of therapeutic cells is by the presentation of biophysical patterning cues from biomimetic scaffolds. Native ECMs such as collagen confer various topographical patterns within blood vessels. To mimic the woven spiral structure of collagen bundles in relaxed blood vessels60 that have high mechanical strength, aligned collagen fibrillar scaffolds with the woven-like helical and crimped configurations were developed, as shown by atomic force microscopy (AFM) (Fig. 1D).61 These configurations mimic the aligned nano-scale patterning of collagen-based fibrous tissue under reduced load. When these scaffolds were then seeded with primary human ECs, the cells responded to the nano-scale pattern by organizing their cytoskeleton along the axis of the collagen fibrils, as shown by scanning electron microscopy (SEM) (Fig. 1E).61-63 When human iPSC-EC-seeded aligned scaffolds were implanted into ischemic hindlimbs of mice, the transplant cell viability was significantly higher, compared to cell viability on non-patterned scaffolds. Importantly, human iPSC-ECs cultured on aligned scaffolds also persisted for over 28 days, when implanted into ischemic tissue, whereas iPSC-EPs implanted on scaffolds without nanopatterning persisted for only 4 days. Along with improvement in cell viability, ECs seeded on aligned nanofibrillar scaffolds also enhanced vascular perfusion recovery (Fig. 1F).61
Additionally, these EC studies demonstrated that aligned nanofibrillar collagen scaffolds guide EC cellular organization, modulate endothelial inflammatory response, and enhance cell survival after implantation in normal and ischemic tissues.62, 63 The alignment of the ECs on these scaffolds directly influenced their biology, where the aligned ECs were 50% less adhesive for monocytes than the ECs grown on randomly oriented fibrillar scaffolds. The finding of increased cell viability after delivery on aligned scaffolds into ischemic tissue suggested that such nanofibrillar scaffolds may be beneficial as a delivery vehicle for cell therapy. Further studies revealed that the aligned nanofibril pattern promoted greater endothelial outgrowth in vitro than non-patterned scaffolds, in part by integrin α1 activation, and enhanced blood perfusion recovery and arteriogenesis in the murine ischemic hindlimb, compared to cell delivery or scaffold delivery alone.61
With the goal to explore more clinically accessible and abundant therapeutic cell sources, stromal vascular fraction cells (SVF) from adipose tissue were tested for seeding into the cell-seeded aligned nanofibrillar scaffold. In vitro studies showed that SVF cells cultured on the scaffold had a six-fold higher level of VEGF secretion, compared to that of SVF cells cultured in suspension.64 Importantly, when SVF-seeded scaffolds were transplanted into immunodeficient mice with induced hindlimb ischemia, the cell-seeded scaffolds induced a significant higher mean perfusion ratio after 14 days, compared to cells delivered in saline.64 These findings show that both EC and SVF cells delivered on aligned nanofibrillar scaffolds into ischemic tissue stimulated blood perfusion recovery. The recovery mechanisms underlying this therapeutic effect may include both angiogenesis and arteriogenesis, which could be mediated by patterned scaffold-induced activation of integrin α1 and increased VEGF secretion.61, 64, 65 These studies demonstrate an important role of nanopatterning cues in directing cell behavior, which can be applied towards improving the survival and angiogenic function of implanted cells in the setting of limb ischemia.
The advantages of biophysically patterning include the activation of topography-mediated molecular signaling in adherent therapeutic cells that can induce cell survival or therapeutic function, as well as the induction of cellular reorganization and alignment that can lead to further effects on cell migration and immunomodulation to cell types such as ECs.65 Compared to injectable hydrogels, however, biophysically patterned scaffolds require more invasive delivery techniques, such as a trocar for intramuscular delivery, and should be mechanically strong enough to withstand surgical manipulation. Another disadvantage of spatially patterned scaffolds is the lack of knowledge of how cells will respond to topographical patterning upon partial degradation when the topographical cues may not be as evident.
iii. Biomaterials-Based Genetic Cell Modification
The process of angiogenesis in PAD treatment necessitates the sustained presentation of numerous growth factors at different time points to facilitate revascularization treatment.66, 67 However, achieving consistent and spatiotemporal release kinetics for multiple growth factors over an extended period presents a significant challenge in biomaterial engineering. Gene delivery emerges as a promising solution by combining the advantages of cell therapy and growth factor delivery systems. Through viral or plasmid transfection, cells can be genetically modified to overexpress specific growth factors, which are then introduced to the ischemic tissue. This innovative approach allows for tunable and long-term release of growth factors, surpassing the limitations of conventional drug delivery systems. Moreover, these genetically modified cells leverage their inherent capacity to stimulate vascularization, thereby presenting an additional advantage for enhancing therapeutic outcomes.68 Some representative examples of using genetic engineering to treat PAD can be found in Table 2, containing a variety of target genes and delivery methods. Vascular endothelial growth factor A (VEGFA) is a widely studied growth factor known for its potent proangiogenic effects69. Previous studies have demonstrated the beneficial outcomes of VEGFA delivery in murine hindlimb ischemia models, leading to functional recovery.70-72 Similarly, the overexpression of VEGFA in transplanted cells has shown promising results, as demonstrated by Park et al.73 In their work, they successfully transfected MSCs with VEGFA and enhanced green fluorescent protein (EGFP). Upon injection of the transduced MSCs into the ischemic hindlimbs of mice, the authors observed notable improvements in blood perfusion in the affected limbs, with perfusion reaching 79% of normal levels in mice receiving transduced MSCs, compared to 53% and 26% for mice receiving non-transduced MSCs and no treatment, respectively. In addition, they observed a 4.5-fold reduction in fibrosis in mice treated with transduced MSCs compared to non-transduced MSCs. This work exemplifies the utility of genetic modification techniques to enhance the pro-angiogenic characteristics of implanted cells.
Table 2. Overview of preclinical testing of genetic engineering for PAD treatment.
Column two differentiates between treatments in which cells were modified prior to implantation in the host (in vitro) and treatments in which factors for upregulating the gene of interest were delivered directly to the host (in vivo).
| Gene(s) of Interest |
In vitro or in vivo |
Cell Type |
Delivery Method | Observations |
|---|---|---|---|---|
| VEGFA | in vitro | MSCs | Nonviral plasmid |
|
| VEGFA and CXCR4 | in vitro | ADSCs | PBAE nanoparticles |
|
| HIF-1a | in vitro | ADSCs | Nonviral plasmid |
|
| Nestin-1 | in vitro | ADSCs | Adenovirus |
|
| ADAM-12 and miR-29aINH | in vivo | N/A | Lipid nanoparticles with F-triggered release |
|
| MiR-140-3p | in vivo | N/A | Lentivirus |
|
| HIF-1a | in vivo | N/A | Baculovirus |
|
| FGF2 | in vivo | N/A | Gene gun (pyro-driven jet injector) |
|
| PTGIS | in vivo | N/A | Glutaraldehyde-polyethyleneimine nanoparticles |
|
| HGF | in vivo | N/A | Modified mRNA |
|
Besides the use of viral transduction for genetic modification of cells, polymer nanoparticles are also effective for cellular transfection. Nanoparticles composed of poly(β-amino ester) (PBAE) can modify the DNA into the nanoparticle structure and thereby be transfected into cells.74 The PBAE nanoparticles had a 1-2 fold higher transfection efficiency compared to lipofectamine with reduced cytotoxicity. Using this nanoparticle technology, Deveza et al modified ADSCs with plasmids encoding either the G protein-coupled receptor C-X-C chemokine receptor 4 (CXCR4) or VEGF, as demonstrated by gene and protein expression analysis.75 When the genetically modified ADSCs were injected into the murine ischemic limb, the cells that were genetically modified with CXCR4 alone or together with VEGF showed the highest degree of cell survival at 10 days post-transplantation. Furthermore, CXCR4-modified ADSCs had 100% limb salvage, compared to the negative control group that only had ~40% limb salvage. Along with the improvement in limb salvage, there was a significant improvement in blood perfusion recovery in the group treated with CXCR4-modified ADSCs, compared to cells modified with an irrelevant gene. This study highlights the potential benefit of a nanoparticle-based genetic modification strategy that could encounter less safety concerns towards clinical translation. An additional example of using nanoparticles to deliver genetic material is research by Lamin et al.76 The researchers targeted ADAM12 and miR29aINH, which are normally upregulated and downregulated in ischemic tissues, respectively. Given the disruption of these regulatory mechanisms in PAD, they employed lipid nanoparticles with ultrasound-triggered release to deliver plasmids containing the ADAM12 gene or a miR29aINH inhibitor to the ischemic limb. They observed that while both ADAM12 upregulation or miR29aINH inhibition improved limb perfusion and muscle twitch force, indicating functional recovery, inhibiting the miRNA had a more significant positive effect on ischemic recovery. Together, these examples highlight the utility of various bioengineering and biomaterials strategies to improve the survival or therapeutic efficacy of transplanted cells for treatment of limb ischemia.
The advantages of biomaterials-based genetic cell modification include the tunability of cell transfection kinetics, thereby potentially enabling more sustained duration of transfection, compared to traditional cell transfection methods in solution. Furthermore, biomaterials are well-suited for localized cell transfection, thereby limiting transfection only to the transplanted cells or other cells in the immediate vicinity of the biomaterial. In contrast, one of the limitations of biomaterials-based genetic cell modification include the possibility of genomic integration, thereby raising concerns of the safety of this strategy for clinical use. However, recent developments in modified mRNA-based transfection technologies may circumvent this obstacle.77
Emerging Cell-Based Bioengineering Technologies for PAD treatment:
i. 3D Bioprinting:
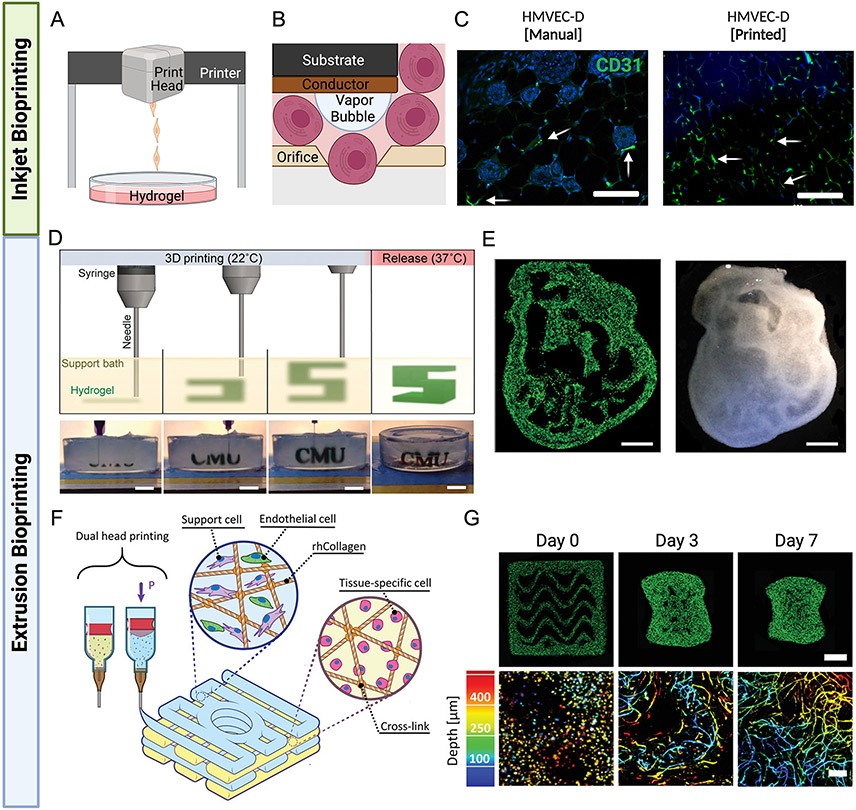
3D bioprinting is an emerging technology that enables the precise positioning of cells and biomaterials in defined spatial geometries. Among the different types of 3D bioprinting, thermal inkjet and extrusion-based bioprinting are most common (Fig. 2). Thermal inkjet bioprinting utilizes heat, laser, or piezoelectric energy sources to deposit cells and bio-inks in defined geometries (Fig. 2A). The orifice of the inkjet print head allows single cells to pass through after being subjected to high energy sources (Fig. 2B).87 Thermal inkjet printing was the first technology that was used for bioengineering applications with protein arrays and DNA chips as the first bioprinting products.88-90 Following these successes, printing bacteria and mammalian cells with high viability rates paved the way for a wider range of applications.91 For example, Oropeza et al demonstrated the ability to print human microvascular endothelial cells (HMVEC) using a modified inkjet bioprinter and successfully implanted the constructs into a murine model, resulting in a significant increase in the vascular formations in the implant area (Fig. 2C).87, 92
Fig. 2. Advancements in 3D bioprinting for vascular graft replacements.
A-B. Thermal inkjet printers use heat to force droplets through the small nozzle. The small orifice of the inkjet print head allows single cells to pass thorough after being subjected to high temperatures and pressures, which in turn cause activation of angiogenic factors. C. In vitro studies demonstrate the increase in vascular formation for inkjet printed constructs, compared to those with manually seeded cells (100 μm scale bar). D. A gelatin slurry is used as a support bath throughout the 3D bioprinting process, allowing for precise printing of alginate prior to crosslinking. E. The cross section of a 3D printed embryonic chick heart (scaled 10X) made using fluorescent (green) alginate, the internal structures can be seen through the translucent wall (1 mm scale bar) F. Collagen, endothelial cells, and support materials are extruded using a dual print head to form vascularized constructs. G. Vessel network images at days 0, 3, and 7. The top row shows the full construct (1 mm scale bar) and the bottom row shows the vascular depth using color-coded projection (200μm scale bar). B-C. Adapted with permission.87 D-E. Adapted with permission.96 F-G. Adapted with permission.97
Advances in 3D printing have led to the development of new approaches to treat PAD, specifically in generating large tissue engineered vascular graft replacements.93 For example, Mirabella et al used 3D printing to create parallel polysaccharide filaments, each surrounded by fibrin gels seed with endothelial cells.94 Subsequently, the polysaccharide filaments were dissolved to create small diameter channels. Upon implantation in a hindlimb ischemia model, they found that different channel geometries resulted in varying degrees of perfusion recovery. Specifically, endothelialized channels oriented in parallel exhibited nearly complete recovery five days post-surgery, whereas gels with endothelial cells but lacking channels showed significantly less recovery. Freeman et al developed a method for 3D bioprinting vascular grafts directly onto a rotating surface.95 Unlike previous methods where scaffolds were printed on a flat surface requiring support material, this approach eliminates that need by printing on a cylindrical surface, enabling the creation of arbitrarily large vessels. The bioink utilized contains human dermal fibroblasts, gelatin, and fibrinogen. Cooling the bio-ink to room temperature allows partial solidification of gelatin, facilitating printing on the rotating cylinder. Following printing, the vascular graft is immersed in thrombin to crosslink the dissolved fibrinogen. By subsequently raising the temperature to 37°C, the gelatin melts and clears from the construct. Control over the viscosity and stiffness of the printed graft was achieved by altering the gelatin concentration in the bioink and adjusting the gelatin heat treatment time. The researchers successfully cultured fibroblasts within the construct for up to two months. Notably, this approach for generating large diameter vessels is highly scalable, and further incorporation of endothelial or smooth muscle cells could serve as a valuable treatment for PAD patients.
Another significant advancement involved the use of Freeform Reversible Embedding of Suspended Hydrogels (FRESH) bioprinting, in which a support bath consisting of gelatin slurry enables precise printing of bio-inks in defined geometries such as a 3D printed embryonic chick heart cross section (Fig. 2D-E).96 Szklanny et al applied FRESH printing technology to create a large diameter vessel surrounded by a dense capillary bed (Fig. 2F-G).97 In this work, a biocompatible poly(lactide-co-glycolide) (PLGA)-poly(l-lactide) (PLLA) polymer with fenestrations was employed to construct the large diameter vessel, enabling capillary sprouting. Endothelialization of the vessel involved coating it with fibrinogen and flowing endothelial cells through the tube. Surrounding the large diameter vessel, a methacrylated collagen bioink was printed, consisting of a combination of human adipose-derived ECs and dental pulp stem cells as support cells. These cells were chosen because they are both easy to isolate and can form vascular networks.98-100 In vivo experiments were performed in rats by resecting and clamping the femoral artery and using the large diameter vessel as a replacement. The authors first implanted a defenestrated large diameter vessel without the surrounding collagen gel to demonstrate that the large diameter vessel could anastomose with other vessels. While using a non-endothelialized vessel showed some improvement in blood flow (65% compared to a ligated femoral artery), significantly greater improvements were observed when an endothelialized vessel was employed (85% of normal blood flow). The authors then repeated this experiment using both the large diameter vessel as well as the surrounding collagen to verify integration of the collagen hydrogel’s capillary bed with the host vasculature as well as with the large diameter vessel. Both approaches demonstrate promising possibilities for the future of vascular grafting using 3D printing technology.
The primary advantage of 3D bioprinting lies in its capacity to create intricate macro-scale structures, particularly beneficial for treating PAD, where large diameter vessels are essential. Additionally, 3D bioprinting facilitates the production of personalized vascular grafts and tissues tailored to meet the specific needs of individual patients, enhancing compatibility and integration upon implantation. The incorporation of different bioinks allows for the introduction of multiple cell types, contributing to a more accurate recreation of native tissue organization. However, a significant drawback of this approach is the challenge of identifying suitable bioinks for printing—ones that are both biocompatible and possess the necessary mechanical properties. Ensuring that these bioinks support cell survival and function while maintaining structural integrity poses a key hurdle. Maintaining cell viability during the printing process and ensuring that the printed cells continue to function as intended after implantation are crucial considerations, especially given the specificity required for each cell and polymer type. In addition, while bioprinting enables the creation of large diameter vessels, current technologies lack the resolution to print smaller scale structures, such as capillaries. Further research and clinical trials are imperative to validate the effectiveness, safety, and long-term outcomes of 3D bioprinting in PAD treatment. Additionally, ongoing exploration and development are needed to address regulatory considerations and enhance the scalability of the technology for widespread clinical use.
ii. Immunomodulatory Biomaterials
In the context of PAD, the inflammatory microenvironment in ischemic tissue poses a significant challenge to angiogenesis. Genetic engineering approaches have been employed to tackle this issue. For example, Jiang et al genetically engineered ADSCs to overexpress Nestin-181, a protein normally associated with neuronal development101 that has also been shown to improve endothelial cell viability102 and promote an anti-inflammatory macrophage M2 phenotype.103 The objective of their research was to reduce restenosis following angioplasty, and the retention of M2 macrophages is crucial in achieving this goal, as they aid in the clearance of senescent cells, known to contribute to restenosis.104 Additionally, the Nestin-1 overexpressing ADSCs were encapsulated in a hydrogel composed of graphene oxide, polydopamine, and polyacrylamide. This composite hydrogel facilitated the wrapping of the affected arteries and provided support to the cells. Implanting the hydrogel in mice resulted in a greater than 50% reduction in expression of the pro-inflammatory markers TNFα and IL-6, associated with M1 macrophages, and a four-fold increase in expression of the autoinflammatory markers Arg-1 and IL-10, associated with M2 macrophages. This study highlights the role of hydrogels, in conjunction with genetically modified stem cells, to regulate inflammation.
Even in the absence of transplanted cells, biomaterials can exert immunomodulation of the ischemic muscle tissue through the release of anti-inflammatory factors. This strategy can potentially obviate the genetic modification of cells. One potential benefit of delivering anti-inflammatory factors using biomaterials is the higher degree of control in the release kinetics, compared to conventional bolus delivery of soluble factors in saline. For example, when IL-4-releasing nanoparticles were injected to the ischemic limbs of mice for 15 days, the IL4-releasing nanoparticles were able to significantly improve muscle contraction force by 40%, compared to the group receiving only phosphate buffered saline treatment.105 The contractile velocity also showed a similar degree of improvement, although vascular perfusion was not significantly different among the treatment groups. Mechanistically, flow cytometry analysis of the hindlimb tissue demonstrated twice as many M2 macrophages and half as many M1 macrophages in the group receiving IL-4-releasing nanoparticles, compared the saline control group. . An alternative approach was developed by Kwee et al106 who implanted an antigen-releasing scaffold in animals previously vaccinated with the same antigen. Mice with hindlimb ischemia that were previously vaccinated with ovalbumin (OVA) and aluminum then received OVA-releasing scaffolds. This implantation led to the accumulation of antigen-specific TH2 T cells at the site of limb ischemia, thereby promoting blood perfusion recovery of ischemic tissue. These examples illustrate examples in which biomaterials-based delivery of immunomodulatory factors can influence functional recovery of the ischemic limb muscle.
The primary advantage of immunomodulation for treating PAD is that it leverages a patient’s innate ability for repair and regeneration. This approach eliminates the need for cell encapsulation, streamlining both the development of the treatment and the regulatory approval process. One notable disadvantage is the lack of clarity regarding the optimal timing for introducing these materials, and their long-term effects. Additionally, individual patient responses to immunomodulatory biomaterials may vary, posing challenges in predicting and ensuring consistent treatment outcomes. Factors such as the patient's overall health, immune system status, and genetic variability could influence the effectiveness of the treatment. It is important to note that while immunomodulatory biomaterials show promise, further research and clinical trials are needed to fully understand their efficacy and safety in the context of PAD treatment. Table 3 summarizes the pros and cons of the various biomaterials-based strategies to treat PAD.
Table 3. Benefits and limitations of bioengineering strategies for cell delivery.
| Bioengineering Strategy for Cell Delivery |
Pros | Cons |
|---|---|---|
| Cell-Encapsulated Hydrogels |
|
|
| Cells on Spatially Patterned Scaffolds |
|
|
| Biomaterials-Based Genetic Cell Modification |
|
|
| 3D Bioprinting of Cells |
|
|
| Immunomodulatory Biomaterials |
|
|
Current Challenges & Future Directions
Despite the progress made in the advancements in bioengineering technology, the application of bioengineering-assisted cell therapy has been largely limited to preclinical PAD studies. Some of the challenges in adapting these technologies for clinical translation include the scalability of the biomaterials to clinical sizes, along with the safety of the biomaterials, especially when prepared with recombinant DNA technology or xenoproteins. From the commercialization perspective, the incorporation of bioengineered elements can lead to a more complicated regulatory pathway, compared to cell therapy alone. Engineered tissues and other biomaterials-based biologics can fall into multiple categories of therapeutic agents (ie, tissues, biological products, drugs, medical devices) which can prolong or complicate the regulatory process for FDA approval.107 Some biomaterials already have FDA approval, such as PLGA and collagen. Therefore, as more biomaterials become FDA-cleared, biomaterials will be more widely adopted for clinical use.
In addition, the mouse hindlimb ischemia model for preclinical testing fails to recapitulate conditions seen in clinical settings. A recent review by Krishna et al concluded that the acute ischemia induced from ligating the femoral artery results in significantly different cell responses compared to the chronic ischemia that occurs in PAD. In addition, success in hindlimb ischemia models is often measured based on presence or absence of limb necrosis rather than metrics more commonly used in clinical settings such as blood perfusion rate, treadmill walking, and pain at rest.108
Besides the regulatory hurdles associated with bioengineering-related devices, further advancements in bioengineering technology are necessary. For example, the geometric complexity of 3D bioprinted constructs has improved in the past decade, but the resolution of bioprinted structures using conventional extrusion-based bioprinting is limited to 200-500 μm.88, 109, 110 The recent development of lithography-based bioprinting can produce larger bioprinted constructs within minutes at a resolution approaching 25 μm.111, 112 It is therefore anticipated that these technologies will continue to break the boundaries to create more physiologically relevant tissue geometries.
With the increasing popularization of personalized medicine, we envision that cell therapy and biomaterials will become increasingly tailored to address patient-specific disease conditions. For example, emerging research in preclinical models suggests that lifestyle choices such as tobacco exposure can negatively affect the efficacy of stem cell therapy for treatment of PAD.113, 114 Therefore, autologous stem cells from some patients may need pre-conditioning to reverse or reprogram the cells into a functional state prior to transplantation. In this regard, biomaterials can become a tool to modulate cellular function.
In summary, cell therapies have been tested in a limited number of clinical trials with results that suggest minor to moderate improvement. Bioengineering and biomaterials approaches have the potential to improve the effectiveness of cell-based therapies for treatment of PAD through the modulation of cell survival and function of the transplanted cells as well as the cells in the recipient tissue. As technical and regulatory hurdles become overcome, we anticipate that bioengineering strategies will become more widely used in conjunction with therapeutic cells for treatment of PAD.
HIGHLIGHTS.
Bioengineering and biomaterials can improve the survival or efficacy of cell therapy for treatment of PAD in the preclinical setting
Hydrogels and spatially patterned biomaterials allow for tunable mechanical and biophysical properties, respectively, to exert functional effects on encapsulated cells
Biomaterials can be used to genetically modify transplanted cell behavior in the setting of the ischemic limb
Emerging technologies include 3D bioprinting and immunomodulatory materials that can further advance cell therapies in the setting of PAD
ACKNOWLEDGEMENTS:
Biorender was used for figure preparation.
FUNDING SOURCES:
This work was supported in part by grants to NFH from the US National Institutes of Health (R01 HL127113, R01 HL142718, R41HL170875, R21 HL172096-01), the US Department of Veterans Affairs (1I01BX004259 and RX001222), the National Science Foundation (1829534 and 2227614), the Tobacco Related Disease Research Program (T33IP6580), and the American Heart Association (20IPA35360085 and 20IPA35310731). NFH is a recipient of a Research Career Scientist award (IK6BX006309) from the Department of Veterans Affairs. JZ gratefully acknowledges the financial support of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (R21EB027812-01A1 and R01HL15829, respectively). BPO was supported in part by a diversity supplement from the US National Institutes of Health (3R01HL151997-03S1).
Nonstandard Abbreviations and Acronyms
- ABI
ankle-brachial index
- ADSC
adipose-derived stem cells
- AFM
atomic force microscopy
- ALDHbr
aldehyde dehydrogenase bright
- bFGF
basic fibroblast growth factor
- CLTI
chronic limb-threatening ischemia
- CS
chitosan
- CXCR4
C-X-C chemokine receptor 4
- EC
endothelial cell
- ECM
extracellular matrix
- ESC
embryonic stem cell
- FRESH
freeform reversible embedding of suspended hydrogel
- GFP
green fluorescent protein
- IGF-1
insulin-like growth factor 1
- iPSC
induced pluripotent stem cell
- iPSC-EP
induced pluripotent stem cell derived endothelial progenitor
- MSC
mesenchymal stromal cell
- OVA
ovalbumin
- PAD
peripheral artery disease
- PBAE
poly(β-amino ester)
- PEG
poly(ethylene glycol)
- PEG(PTMC-A)2
poly(trimethylene carbonate)-b-poly(ethylene glycol)-b-poly(trimethylene carbonate) diacrylate
- PLGA
poly(lactide-co-glycolide)
- PLLA
poly(l-lactide)
- PNIPAM
poly(N-isopropyl-acrylamide)
- SEM
scanning electron microscopy
- SVF
stromal vascular fraction cells
- TACT
Therapeutic Angiogenesis using Cell Transplantation
- TcO2
transcutaneous oxygen pressure
- VEGF
vascular endothelial growth factor
- VEGFA
vascular endothelial growth factor A
Footnotes
DISCLOSURES: TSZ and MVP are employees of Fibralign Corporation that manufactures a nanopatterned collagen medical device. The authors declare no conflicts of interest.
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: A report from the american heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Eraso LH, Fukaya E, Mohler ER 3rd, Xie D, Sha D, Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704–711. doi: 10.1177/2047487312452968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: Current challenges and future prospects. Vasc Health Risk Manag. 2018;14:63–74. doi: 10.2147/VHRM.S125065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073 [DOI] [PubMed] [Google Scholar]
- 5.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183 [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadini GP, Spinetti G, Santopaolo M, Madeddu P. Impaired regeneration contributes to poor outcomes in diabetic peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:34–44. doi: 10.1161/ATVBAHA.119.312863 [DOI] [PubMed] [Google Scholar]
- 8.Cooke JP, Meng S. Vascular regeneration in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1627–1634. doi: 10.1161/ATVBAHA.120.312862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Kitzerow O, Nie F, Dai J, Liu X, Carlson MA, Casale GP, Pipinos II, Li X. Bioengineering strategies for the treatment of peripheral arterial disease. Bioact Mater. 2021;6:684–696. doi: 10.1016/j.bioactmat.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qamar SUR, Spahic L, Benolic L, Zivanovic M, Filipovic N. Treatment of peripheral artery disease using injectable biomaterials and drug-coated balloons: Safety and efficacy perspective. Pharmaceutics. 2023;15:1813. doi: 10.3390/pharmaceutics15071813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells. 2018;36:161–171. doi: 10.1002/stem.2751 [DOI] [PubMed] [Google Scholar]
- 12.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8 [DOI] [PubMed] [Google Scholar]
- 13.Perin EC, Murphy MP, March KL, et al. Evaluation of cell therapy on exercise performance and limb perfusion in peripheral artery disease: The cctrn pace trial (patients with intermittent claudication injected with aldh bright cells). Circulation. 2017;135:1417–1428. doi: 10.1161/CIRCULATIONAHA.116.025707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman PM, Mohler ER 3rd, Roessler BJ, et al. Phase i study of multi-gene cell therapy in patients with peripheral artery disease. Vasc Med. 2016;21:21–32. doi: 10.1177/1358863X15612148 [DOI] [PubMed] [Google Scholar]
- 15.Perin EC, Silva G, Gahremanpour A, et al. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2011;78:1060–1067. doi: 10.1002/ccd.23066 [DOI] [PubMed] [Google Scholar]
- 16.Franz RW, Shah KJ, Pin RH, Hankins T, Hartman JF, Wright ML. Autologous bone marrow mononuclear cell implantation therapy is an effective limb salvage strategy for patients with severe peripheral arterial disease. J Vasc Surg. 2015;62:673–680. doi: 10.1016/j.jvs.2015.02.059 [DOI] [PubMed] [Google Scholar]
- 17.Poole J, Mavromatis K, Binongo JN, et al. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: A randomized clinical trial. JAMA. 2013;310:2631–2639. doi: 10.1001/jama.2013.282540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SK, Green LA, Drucker NA, Motaganahalli RL, Fajardo A, Murphy MP. Rationale and design of the clinical and histologic analysis of mesenchymal stromal cells in amputations (champ) trial investigating the therapeutic mechanism of mesenchymal stromal cells in the treatment of critical limb ischemia. J Vasc Surg. 2018;68:176–181 e171. doi: 10.1016/j.jvs.2017.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnaird T, Stabile E, Burnett M, Lee C, Barr S, Fuchs S, Epstein S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. [DOI] [PubMed] [Google Scholar]
- 21.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC [DOI] [PubMed] [Google Scholar]
- 22.Shirbaghaee Z, Hassani M, Heidari Keshel S, Soleimani M. Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia. Stem Cell Res Ther. 2022;13:462. doi: 10.1186/s13287-022-03148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta PK, Krishna M, Chullikana A, et al. Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to buerger's disease: Phase ii study report suggests clinical efficacy. Stem Cells Transl Med. 2017;6:689–699. doi: 10.5966/sctm.2016-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao W, Chen D, Liu G, Ran X. Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2019;10:140. doi: 10.1186/s13287-019-1254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang KJ, Chiu LC, Kang YN, Chen C. Autologous stem cell therapy for chronic lower extremity wounds: A meta-analysis of randomized controlled trials. Cells. 2021;10:3307. doi: 10.3390/cells10123307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie B, Luo H, Zhang Y, Wang Q, Zhou C, Xu D. Autologous stem cell therapy in critical limb ischemia: A meta-analysis of randomized controlled trials. Stem Cells Int. 2018;2018:7528464. doi: 10.1155/2018/7528464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326–1340. doi: 10.1161/CIRCRESAHA.116.309045 [DOI] [PubMed] [Google Scholar]
- 28.Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone marrow derived cell therapy in critical limb ischemia: A meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg. 2015;50:775–783. doi: 10.1016/j.ejvs.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 29.Adams WJ, Zhang Y, Cloutier J, et al. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports. 2013;1:105–113. doi: 10.1016/j.stemcr.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belair DG, Whisler JA, Valdez J, et al. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev Rep. 2015;11:511–525. doi: 10.1007/s12015-014-9549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rufaihah AJ, Huang NF, Jame S, et al. Endothelial cells derived from human ipscs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KW, Shin YJ, Kim BM, Cui S, Ko EJ, Lim SW, Yang CW, Chung BH. Modeling of endothelial cell dysfunction using human induced pluripotent stem cells derived from patients with end-stage renal disease. Kidney Res Clin Pract. 2021;40:698–711. doi: 10.23876/j.krcp.20.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, Dunn KK, Shusta EV, Palecek SP. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of wnt signaling. Stem Cell Reports. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosby CO, Valliappan D, Shu D, Kumar S, Tu C, Deng W, Parekh SH, Zoldan J. Quantifying the vasculogenic potential of induced pluripotent stem cell-derived endothelial progenitors in collagen hydrogels. Tissue Eng Part A. 2019;25:746–758. doi: 10.1089/ten.TEA.2018.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White MP, Rufaihah AJ, Liu L, Ghebremariam YT, Ivey KN, Cooke JP, Srivastava D. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31:92–103. doi: 10.1002/stem.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayton ZE, Yuen GS, Sadeghipour S, Hywood JD, Wong JW, Huang NF, Ng MK, Cooke JP, Patel S. A comparison of the pro-angiogenic potential of human induced pluripotent stem cell derived endothelial cells and induced endothelial cells in a murine model of peripheral arterial disease. Int J Cardiol. 2017;234:81–89. doi: 10.1016/j.ijcard.2017.01.125 [DOI] [PubMed] [Google Scholar]
- 37.Park JK, Lee TW, Do EK, Moon HJ, Kim JH. Role of notch1 in the arterial specification and angiogenic potential of mouse embryonic stem cell-derived endothelial cells. Stem Cell Res Ther. 2018;9:197. doi: 10.1186/s13287-018-0945-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacAskill MG, Saif J, Condie A, et al. Robust revascularization in models of limb ischemia using a clinically translatable human stem cell-derived endothelial cell product. Mol Ther. 2018;26:1669–1684. doi: 10.1016/j.ymthe.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ets factors and tgfbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veldman MB, Zhao C, Gomez GA, et al. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor etv2. PLoS Biol. 2013;11:e1001590. doi: 10.1371/journal.pbio.1001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho J, Kim S, Lee H, et al. Regeneration of infarcted mouse hearts by cardiovascular tissue formed via the direct reprogramming of mouse fibroblasts. Nat Biomed Eng. 2021;5:880–896. doi: 10.1038/s41551-021-00783-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niiyama H, Huang NF, Rollins MD, Cooke JP. Murine model of hindlimb ischemia. J Vis Exp. 2009;23:1035. doi: 10.3791/1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88 [DOI] [PubMed] [Google Scholar]
- 45.Lee JJ, Arpino JM, Yin H, Nong Z, Szpakowski A, Hashi AA, Chevalier J, O'Neil C, Pickering JG. Systematic interrogation of angiogenesis in the ischemic mouse hind limb: Vulnerabilities and quality assurance. Arterioscler Thromb Vasc Biol. 2020;40:2454–2467. doi: 10.1161/ATVBAHA.120.315028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Tang G, Yan J, Park B, Hoffman A, Tie G, Wang R, Messina LM. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J Vasc Surg. 2008;48:1546–1558. doi: 10.1016/j.jvs.2008.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons M, Alitalo K, Annex BH, et al. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: A scientific statement from the american heart association. Circ Res. 2015;116:e99–132. doi: 10.1161/RES.0000000000000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botham CM, Bennett WL, Cooke JP. Clinical trials of adult stem cell therapy for peripheral artery disease. Methodist Debakey Cardiovasc J. 2013;9:201–205. doi: 10.14797/mdcj-9-4-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060 [DOI] [PubMed] [Google Scholar]
- 50.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/circresaha.109.208991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crosby CO, Hillsley A, Kumar S, Stern B, Parekh SH, Rosales A, Zoldan J. Phototunable interpenetrating polymer network hydrogels to stimulate the vasculogenesis of stem cell-derived endothelial progenitors. Acta Biomater. 2021;122:133–144. doi: 10.1016/j.actbio.2020.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crosby CO, Zoldan J. An in vitro 3d model and computational pipeline to quantify the vasculogenic potential of ipsc-derived endothelial progenitors. J Vis Exp. 2019: 10.3791/59342. doi: 10.3791/59342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crosby CO, Stern B, Kalkunte N, Pedahzur S, Ramesh S, Zoldan J. Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering. Rev Chem Eng. 2022;38:347–361. doi: 10.1515/revce-2020-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster AA, Dewi RE, Cai L, Hou L, Strassberg Z, Alcazar CA, Heilshorn SC, Huang NF. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater Sci. 2018;6:614–622. doi: 10.1039/c7bm00883j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016;2016:1314709. doi: 10.1155/2016/1314709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao N, Yue Z, Cui J, et al. Igf-1c domain-modified hydrogel enhances therapeutic potential of mesenchymal stem cells for hindlimb ischemia. Stem Cell Res Ther. 2019;10:129. doi: 10.1186/s13287-019-1230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496 [DOI] [PubMed] [Google Scholar]
- 58.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140 [DOI] [PubMed] [Google Scholar]
- 59.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- 60.Arkill KP, Moger J, Winlove CP. The structure and mechanical properties of collecting lymphatic vessels: An investigation using multimodal nonlinear microscopy. J Anat. 2010;216:547–555. doi: 10.1111/j.1469-7580.2010.01215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakayama KH, Hong G, Lee JC, et al. Aligned-braided nanofibrillar scaffold with endothelial cells enhances arteriogenesis. ACS Nano. 2015;9:6900–6908. doi: 10.1021/acsnano.5b00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang NF, Okogbaa J, Lee JC, et al. The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds. Biomaterials. 2013;34:4038–4047. doi: 10.1016/j.biomaterials.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang NF, Lai ES, Ribeiro AJ, Pan S, Pruitt BL, Fuller GG, Cooke JP. Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature. Biomaterials. 2013;34:2928–2937. doi: 10.1016/j.biomaterials.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu C, Zaitseva TS, Alcazar C, et al. Delivery of human stromal vascular fraction cells on nanofibrillar scaffolds for treatment of peripheral arterial disease. Front Bioeng Biotechnol. 2020;8:689. doi: 10.3389/fbioe.2020.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama KH, Surya VN, Gole M, et al. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Lett. 2016;16:410–419. doi: 10.1021/acs.nanolett.5b04028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei Z, Volkova E, Blatchley MR, Gerecht S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv Drug Deliv Rev. 2019;149-150:95–106. doi: 10.1016/j.addr.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tu C, Das S, Baker AB, Zoldan J, Suggs LJ. Nanoscale strategies: Treatment for peripheral vascular disease and critical limb ischemia. ACS Nano. 2015;9:3436–3452. doi: 10.1021/nn507269g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han J, Luo L, Marcelina O, Kasim V, Wu S. Therapeutic angiogenesis-based strategy for peripheral artery disease. Theranostics. 2022;12:5015–5033. doi: 10.7150/thno.74785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouma GO, Jonas RA, Usman MHU, Mohler ER III. Targets and delivery methods for therapeutic angiogenesis in peripheral artery disease. Vasc Med. 2012;17:174–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, Fuster V, Badimon JJ, Sauter BV. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004;110:2430–2435. doi: 10.1161/01.Cir.0000145120.37891.8a [DOI] [PubMed] [Google Scholar]
- 71.Koga J, Matoba T, Egashira K, Kubo M, Miyagawa M, Iwata E, Sueishi K, Shibuya M, Sunagawa K. Soluble flt-1 gene transfer ameliorates neointima formation after wire injury in flt-1 tyrosine kinase-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:458–464. doi: 10.1161/atvbaha.109.183772 [DOI] [PubMed] [Google Scholar]
- 72.Kondoh K, Koyama H, Miyata T, Takato T, Hamada H, Shigematsu H. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc Res. 2004;61:132–142. doi: 10.1016/j.cardiores.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 73.Park JS, Bae SH, Jung S, Lee M, Choi D. Enrichment of vascular endothelial growth factor secreting mesenchymal stromal cells enhances therapeutic angiogenesis in a mouse model of hind limb ischemia. Cytotherapy. 2019;21:433–443. doi: 10.1016/j.jcyt.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 74.Anderson DG, Akinc A, Hossain N, Langer R. Structure/property studies of polymeric gene delivery using a library of poly(beta-amino esters). Mol Ther. 2005;11:426–434. doi: 10.1016/j.ymthe.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 75.Deveza L, Choi J, Lee J, Huang N, Cooke J, Yang F. Polymer-DNA nanoparticle-induced cxcr4 overexpression improves stem cell engraftment and tissue regeneration in a mouse hindlimb ischemia model. Theranostics. 2016;6:1176–1189. doi: 10.7150/thno.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamin V, Verry J, Eigner-Bybee I, Fuqua JD, Wong T, Lira VA, Dokun AO. Modulation of mir-29a and adam12 reduces post-ischemic skeletal muscle injury and improves perfusion recovery and skeletal muscle function in a mouse model of type 2 diabetes and peripheral artery disease. International Journal of Molecular Sciences. 2022;23:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaur K, Hadas Y, Kurian AA, et al. Direct reprogramming induces vascular regeneration post muscle ischemic injury. Mol Ther. 2021;29:3042–3058. doi: 10.1016/j.ymthe.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park JS, Bae S-H, Jung S, Lee M, Choi D. Enrichment of vascular endothelial growth factor secreting mesenchymal stromal cells enhances therapeutic angiogenesis in a mouse model of hind limb ischemia. Cytotherapy. 2019;21:433–443. doi: 10.1016/j.jcyt.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 79.Deveza L, Choi J, Lee J, Huang N, Cooke J, Yang F. Polymer-DNA nanoparticle-induced cxcr4 overexpression improves stem cell engraftment and tissue regeneration in a mouse hindlimb ischemia model. Theranostics. 2016;6:1176–1189. doi: 10.7150/thno.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Est-Witte SE, Farris AL, Tzeng SY, Hutton DL, Gong DH, Calabresi KG, Grayson WL, Green JJ. Non-viral gene delivery of hif-1α promotes angiogenesis in human adipose-derived stem cells. Acta Biomater. 2020;113:279–288. doi: 10.1016/j.actbio.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang Y, Cai Y, Hu J, et al. Adhesive hydrogel wrap loaded with netrin-1-modified adipose-derived stem cells: An effective approach against periarterial inflammation after endovascular intervention. Front Bioeng Biotechnol. 2022;10:944435. doi: 10.3389/fbioe.2022.944435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu ZR, He Q, Wu WB, Chang GQ, Yao C, Zhao Y, Wang M, Wang SM. Mir-140-3p is involved in in-stent restenosis by targeting c-myb and bcl-2 in peripheral artery disease. J Atheroscler Thromb. 2018;25:1168–1181. doi: 10.5551/jat.44024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giménez CS, Castillo MG, Simonin JA, et al. Effect of intramuscular baculovirus encoding mutant hypoxia-inducible factor 1-alpha on neovasculogenesis and ischemic muscle protection in rabbits with peripheral arterial disease. Cytotherapy. 2020;22:563–572. doi: 10.1016/j.jcyt.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 84.Nakae T, Obana M, Maeda T, et al. Title: Gene transfer by pyro-drive jet injector is a novel therapeutic approach for muscle diseases. Gene. 2021;788:145664. doi: 10.1016/j.gene.2021.145664 [DOI] [PubMed] [Google Scholar]
- 85.Guo X, Liu J, Zhang R, et al. A polymer nanoplatform with high transfection rate for the efficient gene therapy of peripheral arterial diseases. J Drug Deliv Sci Technol. 2023;86:104645. doi: 10.1016/j.jddst.2023.104645 [DOI] [Google Scholar]
- 86.Zaitseva TS, Yang G, Dionyssiou D, et al. Delivery of hepatocyte growth factor mrna from nanofibrillar scaffolds in a pig model of peripheral arterial disease. Regen Med. 2020;15:1761–1773. doi: 10.2217/rme-2020-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oropeza BP, Serna C 3rd, Furth ME, et al. Assessment of angiogenesis and cell survivability of an inkjet bioprinted biological implant in an animal model. Materials (Basel). 2022;15:4468. doi: 10.3390/ma15134468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandrycky C, Wang Z, Kim K, Kim DH. 3d bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okamoto T, Suzuki T, Yamamoto N. Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nat Biotechnol. 2000;18:438–441. doi: 10.1038/74507 [DOI] [PubMed] [Google Scholar]
- 90.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3 [DOI] [PubMed] [Google Scholar]
- 91.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–99. doi: 10.1016/j.biomaterials.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 92.Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1:910–917. doi: 10.1002/biot.200600081 [DOI] [PubMed] [Google Scholar]
- 93.Murphy SV, Atala A. 3d bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 94.Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS. 3d-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat Biomed Eng. 2017;1:0083. doi: 10.1038/s41551-017-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freeman S, Ramos R, Alexis Chando P, Zhou L, Reeser K, Jin S, Soman P, Ye K. A bioink blend for rotary 3d bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019;95:152–164. doi: 10.1016/j.actbio.2019.06.052 [DOI] [PubMed] [Google Scholar]
- 96.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szklanny AA, Machour M, Redenski I, et al. 3d bioprinting of engineered tissue flaps with hierarchical vessel networks (vesselnet) for direct host-to-implant perfusion. Adv Mater. 2021;33:e2102661. doi: 10.1002/adma.202102661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luzuriaga J, Irurzun J, Irastorza I, Unda F, Ibarretxe G, Pineda JR. Vasculogenesis from human dental pulp stem cells grown in matrigel with fully defined serum-free culture media. Biomedicines. 2020;8:483. doi: 10.3390/biomedicines8110483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo S, Redenski I, Landau S, Szklanny A, Merdler U, Levenberg S. Prevascularized scaffolds bearing human dental pulp stem cells for treating complete spinal cord injury. Adv Healthc Mater. 2020;9:e2000974. doi: 10.1002/adhm.202000974 [DOI] [PubMed] [Google Scholar]
- 100.Redenski I, Guo S, Machour M, et al. Engineered vascularized flaps, composed of polymeric soft tissue and live bone, repair complex tibial defects. Adv Funct Mater. 2021;31:2008687. doi: 10.1002/adfm.202008687 [DOI] [Google Scholar]
- 101.Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/dcc signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J Neurosci. 2007;27:4342–4350. doi: 10.1523/jneurosci.0700-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding Q, Liao S-J, Yu J. Axon guidance factor netrin-1 and its receptors regulate angiogenesis after cerebral ischemia. Neurosci Bull. 2014;30:683–691. doi: 10.1007/s12264-013-1441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing m2 polarization. Am J Physiol Renal Physiol. 2013;304:F948–F957. doi: 10.1152/ajprenal.00580.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elder SS, Emmerson E. Senescent cells and macrophages: Key players for regeneration? Open Biol. 2020;10:200309. doi: 10.1098/rsob.200309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raimondo TM, Mooney DJ. Functional muscle recovery with nanoparticle-directed m2 macrophage polarization in mice. Proc Natl Acad Sci U S A. 2018;115:10648–10653. doi: 10.1073/pnas.1806908115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih TY, White D, Mooney DJ. Treating ischemia via recruitment of antigen-specific t cells. Sci Adv. 2019;5:eaav6313. doi: 10.1126/sciadv.aav6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webber MJ, Khan OF, Sydlik SA, Tang BC, Langer R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng. 2015;43:641–656. doi: 10.1007/s10439-014-1104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishna SM, Omer SM, Golledge J. Evaluation of the clinical relevance and limitations of current pre-clinical models of peripheral artery disease. Clin Sci (Lond). 2016;130:127–150. doi: 10.1042/cs20150435 [DOI] [PubMed] [Google Scholar]
- 109.Agarwal T, Fortunato GM, Hann SY, et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater Sci Eng C Mater Biol Appl. 2021;124:112057. doi: 10.1016/j.msec.2021.112057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of notch signalling by the coup-tfii transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- 111.Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daly AC, Lim KS. High resolution lithography 3d bioprinting. Trends Biotechnol. 2023;41:262–263. doi: 10.1016/j.tibtech.2022.11.007 [DOI] [PubMed] [Google Scholar]
- 113.Chan AHP, Hu C, Chiang G,CF, Ekweume C, Huang NF. Chronic nicotine impairs the angiogenic capacity of human induced pluripotent stem cell-derived endothelial cells in a murine model of peripheral arterial disease. JVS Vasc Sci. 2023;4:100115. doi: 10.1016/j.jvssci.2023.100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan AH, Huang NF. Effects of nicotine on the translation of stem cell therapy. Regen Med. 2020;15:1679–1688. doi: 10.2217/rme-2020-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]