Abstract
Environmental pollution stands as one of the most critical challenges affecting human health, with an estimated mortality rate linked to pollution-induced non-communicable diseases projected to range from 20% to 25%. These pollutants not only disrupt immune responses but can also trigger immunotoxicity. Phosphoinositide signaling, a pivotal regulator of immune responses, plays a central role in the development of autoimmune diseases and exhibits high sensitivity to environmental stressors. Among these stressors, environmental pollutants have become increasingly prevalent in our society, contributing to the initiation and exacerbation of autoimmune conditions. In this review, we summarize the intricate interplay between phosphoinositide signaling and autoimmune diseases within the context of environmental pollutants and contaminants. We provide an up-to-date overview of stress-induced phosphoinositide signaling, discuss 14 selected examples categorized into three groups of environmental pollutants and their connections to immune diseases, and shed light on the associated phosphoinositide signaling pathways. Through these discussions, this review advances our understanding of how phosphoinositide signaling influences the coordinated immune response to environmental stressors at a biological level. Furthermore, it offers valuable insights into potential research directions and therapeutic targets aimed at mitigating the impact of environmental pollutants on the pathogenesis of autoimmune diseases.
Keywords: Environmental pollutants, Phosphoinositide signaling, Autoimmunity
Graphical Abstract

1. INTRODUCTION
Environmental pollution stands as one of the most critical challenges affecting human health (Collaborators, 2016; Landrigan et al., 2018). It involves the introduction of harmful substances or contaminants into the natural environment, with potentially devastating effects on living organisms, ecosystems, and overall environmental quality. Alarmingly, the estimated mortality rate linked to pollution-induced non-communicable diseases is projected to range from 20% to 25%, making it a formidable threat to public health in the modern world (Collaborators, 2016).
Pollutants have the capacity to disrupt immune responses and trigger immunotoxicity, with autoimmunity being a significant category of immune disorders impacting humans (Suzuki et al., 2020). Autoimmunity is characterized by the immune system’s misguided attack on the body’s own cells and tissues. While the exact origins of these disorders remain elusive, a complex interplay of genetic, hormonal, immunological, and environmental factors, including environmental pollutants (Suzuki et al., 2020), plays a substantial role in their development.
Phosphoinositide (PI) signaling, a vital pathway governing immune cell activation and function, has emerged as a key contributor to autoimmune disease pathogenesis (Stark et al., 2015). Recent research has unveiled the impact of cellular stressors, including environmental pollutants, on PI signaling (Chen et al., 2020; Cong et al., 2020). These pollutants, found ubiquitously in our daily surroundings and increasingly prevalent in society, have the potential to disrupt PI signaling and impair immune responses, thereby exacerbating patient symptoms and disease outcomes.
This paper delves into the intricate interplay between environmental pollutants, PI signaling, and autoimmune diseases. We provide an updated overview of stress-induced PI signaling, highlighting prominent pollutants associated with immune diseases and shedding light on the underlying signaling pathways involved. By advancing our comprehension of how PI signaling shapes immune responses to environmental stressors, this review unveils opportunities for innovative research and potential therapeutic targets aimed at mitigating the impact of these increasingly relevant pollutants on the development of autoimmune diseases.
1.1. Autoimmune Diseases
Autoimmune diseases encompass a broad range of conditions, with approximately 80 known disorders affecting millions of individuals worldwide (Rad et al., 2019). These diseases occur when the immune system, including most tissues and organs, which is designed to protect the body from harmful xenobiotics, mistakenly targets healthy cells and tissues (Fig. 1). Such malfunctioning immune responses lead to a diverse array of symptoms and complications. It is estimated that around 3-5 percent of the global population experiences symptoms associated with immune response disorders (Eaton et al., 2007). Common autoimmune diseases include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), chronic tonsillitis, multiple sclerosis (MS), type 1 diabetes (T1D), inflammatory bowel disease (IBD), psoriasis, and Hashimoto’s thyroiditis (HT), among others (Wang et al., 2015). Despite variations in their specific manifestations and target organs, these conditions share a common underlying feature of self-directed immune system dysregulation. Understanding the mechanisms that initiate and facilitate autoimmune diseases and subsequently developing effective therapeutic interventions are critical areas of research in order to alleviate the burden of these chronic conditions on individuals and society.
Fig. 1.

Immune system and autoimmune diseases. The schematic diagram provides an overview of the key components of the immune system and their associated autoimmune diseases. It is important to recognize that autoimmune diseases can manifest in diverse ways and affect multiple organ systems. Furthermore, a single autoimmune disease can arise from various immune system disorders, and the malfunction of specific immune system components can contribute to the development of multiple autoimmune diseases. While certain autoimmune disorders are not exclusively linked to the immune system components listed, the fundamental elements here play a crucial role in their development. The diagram is generated using BioRender.
1.2. Phosphoinositide Signaling
PIs are a family of signaling lipids that play crucial roles in various cellular processes, including intracellular signaling, cytoskeletal dynamics, membrane trafficking, and cell growth and survival (Chen et al., 2020). They are synthesized by the differential phosphorylation of the inositol ring of phosphatidylinositol (PtdIns) lipids at the 3, 4, and 5 positions, leading to the generation of seven distinct phosphorylated forms of PtdIns, including PtdIns3P, PtdIns4P, PtdIns5P, PtdIns(3,4)P2, PtdIns(3,5)P2, PtdIns(4,5)P2, and PtdIns(3,4,5)P3 (Fig. 2A, B) (Chen et al., 2020). The phosphorylated forms of PtdIns are known as polyphosphoinositides (PIPns). PIPns are transported, converted, and interchanged through various PI-metabolizing enzymes, such as PI-transfer proteins, kinases, phosphatases, and phospholipases. Once generated, these lipids exert their function through recruiting and regulating the activity of effector proteins through their interaction with specialized lipid binding domains such as PH (pleckstrin homology) (Li, D. et al., 2022; Wang, X. et al., 2014), FYVE (Fab1, YOTB, Vac1, and EEA1) (Eitzen et al., 2019), and PX (Phox homology) (Sato et al., 2001) domains. These phospholipids are canonically considered to be triggered by agonist stimulation and enriched in plasma membranes. However, accumulating evidence continues to expand our conventional understanding of these signaling lipids from the cell surface to endomembrane and from membrane systems into non-membranous compartments like the nucleoplasm where PIPns complex with proteins ( Carrillo et al., 2023; Chen, M. et al., 2022; Chen et al., 2020; Tribble et al., 2016). Regardless of subcellular localization, PIPns are widely recognized as essential second messengers that hold central positions in various cellular functions. Among these, the key second messenger PtdIns(3,4,5)P3, generated by phosphoinositide 3-kinase (PI3K), activates the PDK1/mTORC2/Akt/FOXO (pyruvate dehydrogenase kinase isozyme 1/mechanistic target of rapamycin complex 2/Akt/forkhead box O) signaling cascade, which plays important role in proliferation, growth, and survival (Hoxhaj and Manning, 2020) (Fig. 2C). Dysregulation of PI signaling, particularly the PI3K-Akt pathway, has been implicated in many human afflictions, including cancer, neurodegeneration, autoimmunity, and chronic inflammatory diseases, making PI signaling an important area of research for combating these diseases (Bankaitis and Carman, 2019; Kandoth et al., 2013).
Fig. 2.

Phosphoinositides metabolism. (A) Phosphatidylinositol (PtdIns) structure; (B) phosphoinositide metabolism cycle; and (C) PI3K-Akt signaling pathway. The three hydroxyl groups of the inositol ring can be phosphorylated, forming seven distinct phosphoinositide isomers. These phosphoinositide species differ in the number and position of phosphate groups and can be interconverted by phosphoinositide kinases, phosphoinositide phosphatases, and phospholipases.
1.3. Environmental Pollutants
Environmental pollutants can arise from industrial emissions, air, and water contamination, agricultural practices, and other human activities. They exert detrimental effects on human health and have been increasingly recognized as contributors to the development and progression of autoimmune diseases (Alb et al., 2003). Environmental pollutants can be broadly categorized into three main classifications: physical, chemical, and biological (Fig. 3). Physical pollutants include sun exposure, noise, radiation, microplastics, and temperature extremes, which can exert stress on the human body and disrupt typical biological processes. Chemical pollutants encompass many of the commonly recognized and more often discussed compounds such as heavy metals, pesticides, industrial chemicals such as trihalomethanes, and air pollutants which can contaminate air, water, and soil. Lastly, biological pollutants are largely comprised of microorganisms, such as virus, bacteria, and fungi, as well as allergens and toxins produced by living organisms. Given the growing impact of pollution across the globe, the intricate reciprocity between environmental pollutants and PI signaling is an increasingly pertinent research direction due to its significant roles in the development and progression of autoimmune diseases.
Fig. 3.
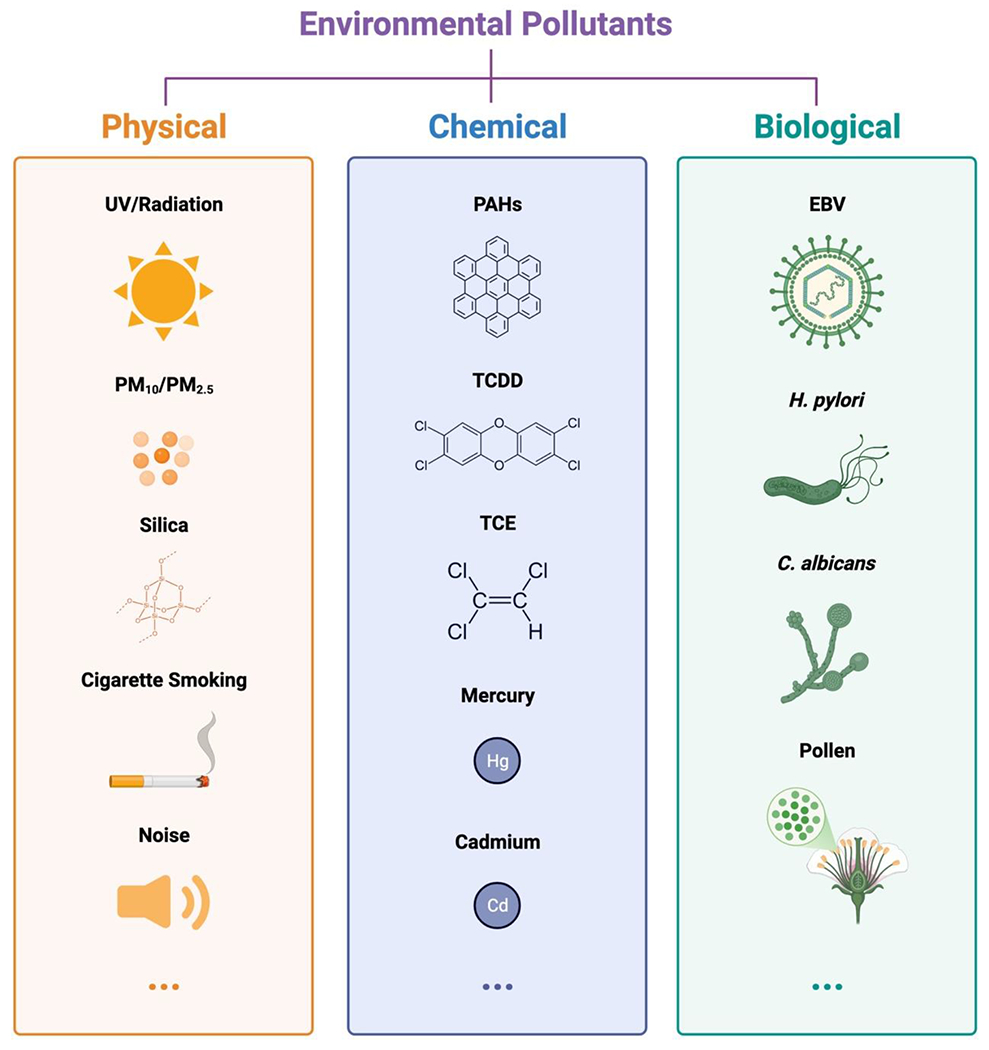
Environmental Pollutants. This schematic diagram provides an overview of environmental pollutants, broadly categorized into three main groups: physical, chemical, and biological. The symbols represent typical examples discussed in the text with dedicated sections. It’s important to note that each category encompasses additional pollutants not depicted here, and the list of pollutants related to PI signaling and autoimmunity is continuously expanding. This diagram is generated using BioRender.
Recent emphasis has been shown on the connection between environmental stresses and autoimmune diseases (Alb et al., 2003) (Table 1) with substantial intrinsic involvement of PI signaling pathways (Table 2). Understanding these pathways in autoimmune conditions enhances our knowledge of the underlying pathological processes and reveals avenues for effective interventions. This review delves into a comprehensive discussion of various environmental stresses, categorized based on their physical, chemical, or biological designations. We will explore the autoimmune diseases associated with each stressor and examine the precise involvement of PI signaling in the pathological processes of these diseases. By shedding light on the interconnection between environmental stresses, PI signaling, and autoimmune diseases, we aim to provide valuable insights that can expose targets for developing novel therapeutic strategies intended to alleviate the burden of autoimmune conditions on affected individuals.
Table 1.
Environmental Pollutants’ Relative Autoimmune Diseases and Impact on PI Signaling.
| Pollution Type | Environmental Pollutants | Relative Autoimmune Diseases | Impact on PI signaling |
|---|---|---|---|
| Physical Pollution | UV | SLE (Cooper et al., 2010; Fava and Petri, 2019; Garcia-Romo et al., 2011; Lood et al., 2016; Wolf et al., 2018), RA (Alex et al., 2020; Arkema et al., 2013), Vitiligo (Baldini et al., 2017; Le Poole, 2021; Wan et al., 2017) | UV exposure induces the assemble of PtsIns4P, PtdIns(4,5)P2, PtdIns(3,4,5)P3, IPMK, PIPn effector p53, and nuclear Akt activation at the DNA damage sites (Chen, M. et al., 2022; Choi et al., 2019; Wang, Y.H. et al., 2017; Wang, Y.H. et al., 2022). |
| PM10/PM2.5 | RA (Adami et al., 2022), SS (Zhang, T.P. et al.,2022), IBD (Opstelten et al., 2016), SLE (Bernatsky et al., 2011; Stojan et al., 2020; Yariwake et al., 2021), MN (Zhou et al., 2022) | PM10 activates PI3K/Akt/FOXO3a pathway (Liu, X. et al., 2022); PM2.5 induces PI3K/Akt/mTOR pathway (Zhang, F. et al., 2020a). | |
| Silica | SLE (Cooper et al., 2010; Sanchez-Roman et al., 1993), RA (McDermott and Sparks, 2023; Prisco et al., 2020; Vega Miranda et al., 2014), SS (Sanchez-Roman et al.,1993; Vega Miranda et al., 2014; Yi et al., 2016), sarcoidosis (Vega Miranda et al., 2014), SSc (Vega Miranda et al., 2014; Yi et al., 2016) | Silica particles activate PI3K/Akt pathway, leading to c-Src phosphorylation and subsequent expression of TGF-β1 (Hao et al., 2023). | |
| Cigarette Smoking | SLE (Barbhaiya et al., 2018; Chen, J. et al., 2022b; Fava and Petri, 2019; Majka and Holers, 2006), RA (McDermott and Sparks, 2023; Pisetsky, 2023; Prisco et al., 2020), MS (Olsson et al., 2017), GD (Aranyosi et al., 2022; Bartalena et al., 2020; Ferrari et al., 2017; Kau et al., 2016), PBC (Gershwin et al., 2005; Rutledge and Asgharpour, 2020), FMS (Bokarewa et al., 2014; Orhurhu et al., 2015) | Nicotine and tobacco carcinogen in cigarette smoke rapidly activate Akt (West et al., 2003). | |
| Noise | NIHL (Fan et al., 2023; Wang et al., 2020; Zhang, A. et al., 2020), IBD (Zhang, A. et al., 2020) | Noise exposure inhibits the PI signaling pathway and decreases the levels of phosphorylated PI3K and Akt (Fan et al., 2023). | |
| Chemical Pollution | PAHs | RA (Tamaki et al., 2004; Ye et al., 2022), SLE (Gilcrease et al., 2020; Yu et al., 2022; Zhu et al., 2023, T1D (Yu et al., 2022), MS (Yu et al., 2022) | PAH metabolites mutate p53, which amplifies nuclear PI signaling and Akt activation (Chen, M. et al., 2022; Choi et al., 2019; Pan et al., 2020; Shen et al., 2006); PAH metabolites target AhR and leads to induced PI3K/Akt signaling (Therachiyil et al., 2022) and sequential TNF-α and IL-6 expression (Guo et al.,2021). |
| TCDD | AIH (Cannon et al., 2022), RA (Tamaki et al., 2004), SS (Ishimaru et al., 2009) | TCDD targets AhR and leads to induced PI3K-Akt pathway (Davis et al., 2000; Therachiyil et al., 2022). | |
| TCE | AIH (Wang, et al.,2014; Wang, et al.,2019), SLE (Wang, G. et al., 2014; Wang, H. et al., 2019), SSc (Wang, G. et al.,2014) | TCE exposure increases Akt phosphorylation, and the inhibition of Akt blocks the TCE-induced cell proliferation (Xia et al., 2022). | |
| Mercury | SLE (Barbhaiya and Costenbader, 2016; Crowe et al., 2015; Fava and Petri, 2019), HT (Benvenga et al., 2022; Pamphlett et al., 2021), MS (Anglen et al., 2015) | Mercury exposure leads to an increase in PI3K/Akt signaling (Chen et al., 2006). | |
| Cadmium | RA (Joo et al., 2019) | Cadmium exposure activates PI3K/Akt/mTOR signaling axis (Kulkarni et al., 2020). | |
| Biological Pollution | EBV | SLE (Barbhaiya et al., 2018; Chen, J. et al., 2022b; Fava and Petri, 2019; Jog and James, 2020; Majka and Holers, 2006), MS (Bjornevik et al., 2022; Lanz et al., 2022; Olsson et al., 2017; Soldan and Lieberman, 2023), SSc (Farina et al., 2017), RA (Fadlallah et al., 2021; Kroos et al., 2022; Svendsen et al., 2021), APS (Kwok et al., 2022; Ogawa et al., 2002), SS (Drozdzik et al., 2014; Inoue et al., 2012) | EBV infection activates PI4K (Suzuki et al., 1992); EBNA1 induces PI4K and PIPK activity, leading to increased PtdIns4P and PtdIns(4,5)P2 (Suzuki et al., 1992); EBV latent proteins LMP1 and LMP2A activate the PI3K/Akt pathway and enhance the activity of IRF4 (Luo et al., 2021; Wang, L. et al., 2017); LMP1 downregulates the expression of PTEN by increasing the expression of miR-21 to amplify PI3K/Akt signaling (Yang et al., 2016); EBV-miRNA-BART7-3P contributes to the PI3K/Akt signaling through PTEN inhibition (Luo et al., 2021); EBV envelope protein gp350 binds CR2/CD21 receptor to activate PI3K (D’Addario et al., 1999; Pan et al., 1996). |
| H. pylori | IBD (Lord et al., 2018), ITP (Vanegas and Vishnu, 2019), PBC (Goo et al., 2008), RA (Zentilin et al., 2002), SLE (Wu et al., 2020) | H. pylori infection inhibits platelet autophagy through the phosphorylation and inactivation of PTEN (Suzuki et al., 2009; Zhu et al., 2008). | |
| C. albicans | Psoriasis (Whitley et al., 2022), T1D (Soyucen et al., 2014), IBD (Gerard et al., 2015) | C. albicans infection activates PI3K/Akt/mTORC1 signaling pathway (Shi et al., 2011); mTORC1 increases the phosphorylation level of STAT3 and promotes the differentiation (Delgoffe et al., 2011). | |
| Pollen | IBD (Johnson et al., 2019), SLE (Jimenez-Alonso et al., 2004), ITP (Ring et al., 2009), sarcoidosis (Van Gundy and Sharma, 1987) | Pollen exposure activates PI3K/Akt signaling pathway (Kampe et al., 2012); p-Akt enhances the phosphorylation and degradation of IκB-α , and leads to the activation of NF-κB and upregulation of TNF-α (Shimamura et al., 2003). |
Table 2.
Association between Autoimmune Diseases and PI Signaling.
| Autoimmune Diseases | Involved Phosphoinositide Signaling |
|---|---|
| Systemic Lupus Erythematosus (SLE) | Inhibition of PI3Kδ and PI3Kγ reduces macrophage kidney infiltration and ameliorates systemic lupus (Barber et al., 2005; Suarez-Fueyo et al., 2014). |
| Inhibition of the PI3K/Akt/mTOR signaling pathway mitigates the inflammatory response and alleviates SLE (Bacalao and Satterthwaite, 2020; Dong et al., 2023). | |
| Rheumatoid Arthritis (RA) | Suppression of PDK1-induced Akt activation and RSK2 phosphorylation inhibits migration and invasion of RA-FLS (Ma et al., 2019). |
| Activation of the PI3K/Akt signaling pathway regulates anti-apoptotic properties and inflammatory cytokines production in RA-FLS (Aihaiti et al., 2021). | |
| Inhibition of PI3Kγ reduces late-stage joint-specific inflammation in collagen-induced arthritis (CIA) (Camps et al., 2005). | |
| Activation of the PI3K/Akt/mTOR/SREBP1/SCD-1 axis reduces ferroptosis of synovial fibroblasts in RA (Cheng et al., 2022). | |
| Inhibition of the PI3K/Akt/mTOR/HIF-1α pathway interrupts angiogenesis in RA (Ba et al., 2021). | |
| Sjögren’s Syndrome (SS) | Inhibition of the PI3K/Akt/mTOR pathway improves the inflammatory microenvironment and relieves SS symptoms (Zeng et al., 2022a; Zeng et al., 2022b). |
| Systemic Sclerosis (SSc) | Inhibition of the PI3K/Akt pathway may mediate the role of calpain in macrophage polarization toward the M1 phenotype in bleomycin model of SSc-ILD (Zhang, L. et al., 2022). |
| Regulating FAK/PI3K/Akt signaling pathway via integrins effectively attenuates skin fibrosis (Huang, Y. et al., 2023). | |
| Antiphospholipid Syndrome (APS) | The PI3K/Akt signaling pathway negatively regulates autophagy by mediating the mTOR expression in endothelial cells (Zhang et al., 2021). |
| Fibromyalgia Syndrome (FMS) | Phosphorylation of the PI3K/Akt/mTOR/NF-κB signaling pathway is increased in the mouse thalamus and SSC after inducing FM (Chen et al., 2019). |
| Inflammatory Bowel Disease (IBD) | Inhibition of the PI3K/Akt/mTOR signaling pathway promotes autophagy in IECs and limits colonic inflammation (Chen, S.L. et al., 2023; Larabi et al., 2020; Zhao et al., 2015). |
| Multiple Sclerosis (MS) | Increased phosphorylation of various proteins in the PI3K/Akt/mTOR pathway potentially enhances Oligodendrocyte progenitor (OLP) cell proliferation, reduces apoptosis, and enhances myelination (Kumar et al., 2013). |
| PI3K serves as the critical signaling mediator of CD28, regulating cell metabolism and amplifying specific inflammatory T cell phenotypes in MS (Kunkl et al., 2019). | |
| Graves’ Disease (GD) | OPN activates OFs and induces VEGF and collagen I mRNA expression in OFs through the PI3K/Akt signaling pathway (Lou et al., 2021). |
| TSHR signaling in TED OFs stimulates cell proliferation directly through PI3K/Akt signaling pathway (Woeller et al., 2019). | |
| Activation of the PI3K/Akt/mTOR pathway promotes the expression of GT1b-induced HAS2 (Yoo et al., 2021). | |
| Inhibition of phosphorylation and activation of the PI3K/Akt signaling pathways promotes cell apoptosis and GD remission (Xin et al., 2021). | |
| Hashimoto’s Thyroiditis (HT) | High expression of the PIK3CG gene may play a role in PI3K mediated signaling in HT (Gan et al., 2021; Li, H. et al., 2018; Wojciechowska-Durczynska et al., 2012). |
| Autoimmune Hepatitis (AIH) | CCN1 stimulates IL-6 production via α6β1/PI3K/AKT/NF-κB signaling pathway in the pathogenesis of AIH (Jiang et al., 2022). |
| Suppressing the activation of PI3K/Akt pathway relieves inflammation and fibrosis in AIH (Huang, Z. et al., 2023; Wang, S. et al., 2022). | |
| inhibiting GRK2 translocation mediated PI3K/Akt/mTOR signaling modulates lipid metabolism in T lymphocytes of AIH (Jin et al., 2022). | |
| Primary Biliary Cirrhosis (PBC) | Up-regulating the PI3K/Akt signaling activates HSCs in liver fibrosis (Ma et al., 2017). |
| Type 1 Diabetes (T1D) | Dysregulation of the insulin/PI3K/Akt pathway is implicated in diabetes (Guastafierro et al., 2017). |
| Diabetic BMDMs exhibit impaired PI3K expression with lower p-PI3K p55 levels and higher levels of PI3K p110α (Galvao Tessaro et al., 2020). | |
| Over-expression of the PI3Kδ isoform mediates increased Ca2+ current through CaV1.2 channels, driving increased contractility in diabetic vessels (Pinho et al., 2010). | |
| Membranous Nephropathy (MN) | Inhibiting PI3K/Akt signaling activates renal autophagy, reducing cell proliferation and inflammation in MN (Chen, J. et al., 2022a). |
| Sarcoidosis | Both downregulation and upregulation of the PI3K/Akt signaling pathway have been associated with sarcoidosis (Konigsberg et al., 2023). |
| Activation of PI3K relates to the upregulation of apoptotic, immune response and development pathways in sarcoidosis patients (Vukmirovic et al., 2021). | |
| Inhibition of programmed death impairs CD4+ T cell proliferation in Sarcoidosis by inhibiting the PI3K/Akt/mTOR signaling pathway (Celada et al., 2017). | |
| Psoriasis | mTORC1 increases the expression of HIF-1α and positively regulates Th17 differentiation by enhancing Th17-related gene expression (Shi et al., 2011). |
| Activating PI3K/Akt/mTORC1 signaling pathway increases the phosphorylation level of STAT3, resulting in the differentiation of Th17 cells and the expression of related cytokine IL-17, and leading to the release of IL-6, IL-1β and TNF-α (Chen et al.,2021). | |
| Vitiligo | High expression of PTEN in the skin junction between normal and lesional areas of vitiligo leads to the inhibition of Akt phosphorylation at S473 (Zhu et al., 2020). |
| Impaired activation of Akt is observed in vitiligo lesions (Kim et al., 2007; Kim and Lee, 2010). | |
| Immune Thrombocytopenic Purpura (ITP) | PTEN inhibits PI3K/Akt/mTOR signaling pathway, thereby enhancing the expression of autophagy-related proteins, inhibiting cell apoptosis and improving platelet viability (Guo et al., 2015; Wang, C.Y. et al., 2019). |
| Noise-Induced Hearing Loss (NIHL) | Activation of the PI3K/Akt signaling pathway provides protection against NIHL (Chen et al., 2015; Fan et al., 2023). |
2. IMPACT OF PHYSICAL POLLUTANT-INDUCED PI SIGNALING ON AUTOIMMUNE DISEASES
Physical pollution refers to the introduction of materials or objects into the environment that can lead to toxicity or harm. This category encompasses various factors, including but not limited to ultraviolet (UV) radiation, particulate matter (PM), silica, cigarette smoking, and noise. In the subsequent section, we delve into their effects on PI signaling within the context of autoimmunity, exploring the role of physical environmental stress-induced lipid signaling in immune disorders.
2.1. UV
Radiation exists all around us, from both natural and manmade sources, and is in two forms: ionizing and non-ionizing radiation. UV radiation, a form of non-ionizing radiation, in the context of pervasive environmental exposure to electromagnetic radiation from the sun, has the potential to cause severe skin damage especially for sensitive populations (Wolf et al., 2018). Notably, sensitivity to UV light is a characteristic shared among various autoimmune diseases such as SLE (Cooper et al., 2010; Fava and Petri, 2019; Garcia-Romo et al., 2011; Lood et al., 2016; Wolf et al., 2018), RA (Alex et al., 2020; Arkema et al., 2013), and vitiligo (Baldini et al., 2017; Le Poole, 2021; Wan et al., 2017). While the biological mechanisms of autoimmune photosensitivity are not fully understood, they may involve UV-driven DNA damage, apoptosis, autoantigen exposure, cytokine production, inflammatory cell recruitment, and systemic flare induction (Wolf et al., 2018). Significant for many of these processes (Patel and Mohan, 2005), phosphoinositide signaling is activated as a stress response to UV radiation (Chen et al., 2020; Strozyk and Kulms, 2013) and has reported links to SLE (Barbhaiya et al., 2022; Bijl and Kallenberg, 2006), RA (Ding, Q. et al., 2023), and vitiligo (Teng et al., 2021). This overlapping relevance suggests PI signaling may operate at the intersection of UV-driven repercussions and the associated autoimmune responses. For instance, UV exposure can potentially trigger the onset of SLE in susceptible females leading to the appearance of a malar rash (Barbhaiya et al., 2022). In individuals with active SLE, blood lymphocytes express relatively higher levels of the p53 protein (Miret et al., 2003), which has been shown to be a novel effector of PIPns (Chen, M. et al., 2022; Choi et al., 2019). Upon UV radiation, p53 and nuclear PI signaling components PtdIns4P, PtdIns(4,5)P2, PtdIns(3,4,5)P3, and a nuclear PI3K, inositol polyphosphate multikinase (IPMK), are rapidly assembled at the DNA damage sites (Wang, Y.H. et al., 2017; Wang, Y.H. et al., 2022). Our recent studies demonstrated that stress-induced p53 binds with PtdIns(4,5)P2 which recruits small heat shock proteins for stabilization (Choi et al., 2019). The p53-PtdIns(4,5)P2 complex is further processed by IPMK into p53-PtdIns(3,4,5)P3 resulting in nuclear Akt activation and stress resistance (Chen, M. et al., 2022). Meanwhile, increased PI3K activity has been reported in SLE, leading to the activation of the PtdIns(3,4,5)P3/Akt/mechanistic target of rapamycin (mTOR) cascade (Pan et al., 2020). This cascade, in turn, stimulates the synthesis of proteins involved in cellular division, proliferation, and lymphocyte survival (Pan et al., 2020). The relationship between UV radiation, PI signaling, and autoimmune responses appears to be intricately intertwined (Fig. 4). As a result, targeting the phosphoinositide signaling pathway shows promise in managing autoimmune photosensitivity.
Fig. 4.

Proposed model of UV radiation-induced PI signaling in SLE. Upon UV radiation, p53 and nuclear PI signaling components PtsIns4P, PtdIns(4,5)P2, PtdIns(3,4,5)P3, and a nuclear PI3K IPMK are rapidly assembled in the DNA damage sites. Additionally, p53 facilitates the activation of nuclear Akt through the presentation of PtdIns(3,4,5)P3. Activation of the PI3K/Akt signaling pathway can stimulate lymphocyte activation and subsequent inflammation, potentially contributing to the development of SLE. The diagram is generated using BioRender.
2.2. PM
PM most commonly refers to fine solid or liquid particles suspended in the air, primarily from the daily use of fuels in appliances without emissions abatement systems, industrial emissions, and vehicular traffic (Izzotti et al., 2022). PM pollution, especially PM10 and PM2.5, is one of the major global public health concerns in large part because of its association with an increased risk for autoimmune diseases, such as RA (Adami et al., 2022), Sjögren’s syndrome (SS) (Zhang, T.P. et al., 2022), IBD (Opstelten et al., 2016), SLE (Bernatsky et al., 2011; Stojan et al., 2020; Yariwake et al., 2021), and membranous nephropathy (MN) (Zhou et al., 2022). Previous reviews have outlined the primary pathogenic mechanisms of PM, highlighting oxidative stress (Feng et al., 2016; Weichenthal et al., 2013; You et al., 2022), inflammation (Feng et al., 2016), apoptosis (Adami et al., 2022; Chen, Y. et al., 2023; Wang et al., 2021), autophagy (Chen, Y. et al., 2023; Wang et al., 2021), and DNA damage (Liu, X. et al., 2022) as the main mechanisms leading to its adverse effects (Li, T. et al., 2022). Since PM pollutants could activate PI3K/Akt pathway (Garcia-Cuellar et al., 2020; Zhang, F. et al., 2020a), PI signaling may participate in these pathogenic mechanisms and play a significant role in the occurrence and development of RA (Ba et al., 2021), SS (Zeng et al., 2022a; Zeng et al., 2022b), IBD (Chen, S.L. et al., 2023; Larabi et al., 2020), SLE (Suarez-Fueyo et al., 2014), and MN (Chiou et al., 2021). For example, ingestion of PM, one common route of exposure, can directly impact epithelial cells, inducing proinflammatory responses and increasing gut permeability (Beamish et al., 2011). In IBD, upregulated colonic glial-derived neurotrophic factor (GDNF) has been shown to have anti-apoptotic properties in colonic epithelial cells through the activation of the PI3K/Akt signaling pathway, contributing to chronic inflammation and affecting intestinal epithelial barrier/integrity (Steinkamp et al., 2003). Additionally, the PI3K/Akt/mTOR signaling pathway is involved in the pathogenesis of IBD through the regulation of autophagy (Jiang et al., 2019; Zhao et al., 2015). Inhibition of the PI3K/Akt/mTOR pathway has been shown to promote autophagy in intestinal epithelial cells and exhibit a protective role in ulcerative colitis (UC) (Zhang, F. et al.,2020b). Considering the increasing prevalence of exposure to PM pollution, strengthening personal protection is a practical approach to safeguard the autoimmune system. Further research on the PI signaling pathway in the context of autoimmunity will provide new insights for preventing and treating PM-induced autoimmune diseases.
2.3. Silica
Silica (or silicon dioxide, SiO2) is a highly prevalent environmental component which mainly exists as polymorph quartz, cristobalite and tridymite in rocks, sand, and soil (Cavalin et al., 2022). While silica is considered an important trace element and has predominantly natural origins compared to other pollutants discussed here, it has been found to be remarkably deleterious to human health (Bai et al., 2023). Numerous studies have established links between silica and serious lung diseases, as well as systemic autoimmune diseases such as systemic sclerosis (SSc) (Vega Miranda et al., 2014; Yi et al., 2016), RA (McDermott and Sparks, 2023; Prisco et al., 2020; Vega Miranda et al., 2014), SLE (Cooper et al., 2010; Sanchez-Roman et al., 1993), SS (Sanchez-Roman et al., 1993; Vega Miranda et al., 2014; Yi et al., 2016), and sarcoidosis (Vega Miranda et al., 2014). Silica may facilitate the onset of autoimmune diseases by causing cell death, such as apoptosis (Gambelli et al., 2004), stimulating the polarization and pyroptosis of macrophages (Bierschenk et al., 2023; Lescoat et al., 2020), as well as cytokine secretion (Lafyatis and York, 2009). Previous studies have demonstrated the involvement of the PI3K/Akt signaling axis in these cellular processes, playing a critical role in developing the aforementioned diseases (Aihaiti et al., 2021; Zhang, L. et al., 2022). Indeed, silica particles activate the PI3K/Akt pathway, leading to c-Src phosphorylation and subsequent expression of transforming growth factor-beta 1 (TGF-β1) (Hao et al., 2023). SSc is a systemic autoimmune disease, characterized by immune system activation, fibrosis of skin and vasculopathy, and accompanied with various complications in internal organs (Gabrielli et al., 2009), particularly lung fibrosis (Yi et al., 2016; Zhang, L. et al., 2022). It has been demonstrated that calpains have an inhibitory role in the regulation of the PI3K/Akt pathway activity (Beltran et al., 2011). Indeed, pharmacological inhibition of calpain increases the protein levels of PI3K and subsequent Akt activation in lung tissues of the bleomycin model of SSc-ILD mice (Zhang, L. et al., 2022). As the PI3K/Akt signaling axis has been associated with macrophage polarization, it is plausible that inhibiting the PI3K/Akt pathway may mediate the role of calpains in promoting macrophage M1 polarization and regulating bleomycin-induced pulmonary fibrosis in mice (Tan et al., 2017). The complex connections between silica, PI signaling, and autoimmune diseases highlight the potential significance of targeting the PI pathways for treating silica-induced autoimmune disorders.
2.4. Cigarette Smoking
Cigarette smoking is an addictive behavior and is recognized as the primary risk factor for many chronic diseases (Ding, R. et al., 2023; Jiang and Alfredsson, 2020). It is firmly established as a significant public health concern, representing a complex environmental pollutant that encompasses both physical and chemical dimensions which cooperatively contribute to its environmental impact (Qiu et al., 2017; Wu, W. et al., 2022). The combustion of tobacco releases visible particulate matter, toxic chemicals, and harmful substances that give rise to various health issues including well-documented conditions such as lung cancer (Cheng et al., 2023) and multiple organ diseases (Kamimura et al., 2018; Larsson and Burgess, 2022). Moreover, smoking has been linked to negative impacts on mental health (Pham et al., 2020) and has been shown to trigger adaptive immunity, leading to the development of autoimmune diseases such as RA (McDermott and Sparks, 2023; Pisetsky, 2023; Prisco et al., 2020), SLE (Barbhaiya et al., 2018; Chen, J. et al., 2022b; Ekblom-Kullberg et al., 2014; Fava and Petri, 2019; Majka and Holers, 2006), MS (Olsson et al., 2017), Graves’ disease (GD) (Aranyosi et al., 2022; Bartalena et al., 2020; Ferrari et al., 2017; Kau et al., 2016), primary biliary cirrhosis (PBC) (Gershwin et al., 2005; Rutledge and Asgharpour, 2020), and fibromyalgia syndrome (FMS) (Bokarewa et al., 2014; Orhurhu et al., 2015). Cigarette smoking contributes to the development of autoimmune diseases through several mechanisms, including cell apoptosis (Dang et al., 2020), autophagy (Yoshida et al., 2019), oxidative stress (Dang et al., 2020; Lugg et al., 2022; Perricone et al., 2016), macrophage polarization (Lugg et al., 2022; Mu et al., 2018), cytokines expression (Rodriguez-Rabassa et al., 2018; van der Vaart et al., 2005; Yang and Chen, 2018), and chronic inflammation (Polverino et al., 2021; Sparks and Karlson, 2016), among others. PI signaling, which is rapidly activated by nicotine and tobacco carcinogen in cigarette smoke (West et al., 2003), plays an important role in these smoking-induced pathological processes. A paradigmatic example is found in RA, where cigarette smoking represents the most well-recognized risk factor (Klareskog et al., 2010). RA is a systemic autoimmune disease involving chronic synovial inflammation as the main pathological feature (Cheng et al., 2022) and where angiogenesis in the synovial tissue is considered an important early event in its development (Elshabrawy et al., 2015). PI3K/Akt signaling activates mTOR to upregulate the expression of hypoxia-inducible factor-1α (HIF-1α) which is stabilized under inflammatory or hypoxic environments and quickly translocated to the nucleus. Once there, HIF-1α induces the expression of vascular endothelial growth factor (VEGF), promoting angiogenesis in the synovial tissue (Ferrara, 2004; Latacz et al., 2020). The PI3K/Akt signaling pathway is involved in the expression of various inflammatory factors during the pathogenesis of RA (Wu, N. et al., 2022). These inflammatory factors form a complex network, interact, influence each other, and ultimately directly or indirectly regulate the expression of VEGF thereby promoting inflammation and synovial pannus formation (Wu, N. et al., 2022). Furthermore, lysosomal-associated protein transmembrane 4B (LAPTM4B), identified as a pathogenic protein associated with smoking-induced lung diseases, acts as a PtdIns(4,5)P2 effector, regulating epidermal growth factor receptor (EGFR) signaling (Tan et al., 2015a). EGFR activates the PI3K/Akt/mTOR pathway, which inhibits autophagy and may be linked to the aggravation of synovial inflammatory responses and the exacerbation of RA (Han et al., 2020; Tan et al., 2015b). Consequently, interrupting the PI3K/Akt/mTOR/HIF-1α pathway to reduce angiogenesis in synovial tissue may offer an effective strategy for impeding the progression of RA (Ba et al., 2021). Cigarette smoking affects the progression of autoimmune diseases through various pathways, however, PI signaling has emerged as a significant player. Therefore, further research on PI signaling holds promise in identifying potential therapeutic targets for the treatment of autoimmune diseases like RA.
2.5. Noise
Noise is a widely existing stressor in daily life that can cause surprisingly significant alterations to the immune system and has been connected to several physical and mental disorders (Hahad et al., 2019). Prolonged exposure to high levels of noise can lead to hearing loss, stress, sleep disturbances, and other problems (Zaman et al., 2022). Many studies have shown that noise may also play a vital role in the occurrence and progression of certain autoimmune diseases, such as noise-induced hearing loss (NIHL) (Fan et al., 2023; Wang et al., 2020; Zhang, A. et al., 2020) and IBD (Zhang, A. et al., 2020). There is substantial evidence suggesting that noise not only participates in hearing defects via affecting the function of hair cells in the cochlea but can also influences multiple systems and suffices to induce changes in the immune system (Carlson and Neitzel, 2018; Zhang, A. et al., 2020). NIHL is a prominent example of the negative impact of excessive noise exposure (Gupta and Sataloff, 2003). Research in the past decade has focused on the role of the PI3K/Akt signaling pathway in maintaining the integrity of cochlear hair cells and their protection mechanisms under stress conditions like noise (Jadali et al., 2017; Kucharava et al., 2019). Studies have shown that noise exposure inhibited the PI signaling pathway and decreased the levels of phosphorylated PI3K and Akt. Consistently, when phosphatase and tensin homolog (PTEN) is knocked down, the activity of the PI3K/Akt pathway increases, providing protection against cochlear hair cell loss (Fan et al., 2023). Furthermore, noise-induced sleep disorders have been found to increase the levels of cytokines such as interleukin (IL)-1, IL-17 and tumor necrosis factor α (TNF-α) (Irwin, 2019). Elevated levels of these cytokines are associated with a variety of autoimmune diseases, including IBD, as they promote the production of specific autoantibodies (Hsiao et al., 2015). The underlying pathological mechanisms of noise-induced autoimmune diseases still requires additional attention. Targeting the activation of the PI signaling pathway may offer a new approach for the development of effective pharmacological interventions in the context of these conditions.
3. IMPACT OF CHEMICAL POLLUTANT-INDUCED PI SIGNALING ON AUTOIMMUNE DISEASES
A chemical environmental pollutant is a harmful substance or compound released into the environment that can have adverse effects on ecosystems, human health, and other living organisms due to its toxic or damaging chemical properties. In the following section, we discuss five representative types of chemical environmental pollutants and examine their impacts on PI signaling within the context of autoimmunity, highlighting the significance of chemical environmental stress-induced lipid signaling in immune disorders.
3.1. Polycyclic Aromatic Hydrocarbons (PAHs)
PAHs are a group of organic compounds commonly found in combustion processes. In modern societies, PAHs are derived mainly from vehicle traffic, industrial activities, cooking with wood or petroleum, and tobacco smoke (Cetin et al., 2018; Liu, Y. et al., 2015). As persistent pollutants, PAHs have been identified as toxicants capable of carcinogenic and immunotoxic effects (Senturk, 2013; Shukla et al., 2022). These environmental pollutants are associated with various autoimmune diseases, including RA (Tamaki et al., 2004; Ye et al., 2022), SLE (Gilcrease et al., 2020; Yu et al., 2022; Zhu et al., 2023), T1D (Yu et al., 2022), and MS (Yu et al., 2022). The mechanisms through which PAHs contribute to these diseases commonly involve DNA mutation, oxidative damage, T-cell activation, and cytokine secretion (Yu et al., 2022). PAHs must be metabolically transformed by a series of xenobiotic-metabolizing enzymes (XMEs) into more reactive metabolites. These metabolites can directly react with DNA and proteins to form adducts, leading to DNA mutations and altered protein functions (Moorthy et al., 2015). A more recent study has reported that PAH metabolites mutate p53 (Shen et al., 2006), a known regulator that amplifies nuclear PI signaling, activates Akt, and dysregulates lymphocytes as previous discussed (Chen, M. et al., 2022; Choi et al., 2019; Pan et al., 2020). This supports the connection between PAH exposure, PI signaling, and autoimmune responses. Additionally, PAH metabolites target a protein receptor named aryl hydrocarbon receptor (AhR) (Vondracek et al., 2020). The activation of AhR leads to induced PI3K-Akt signaling (Therachiyil et al., 2022). Consistently, inhibition of PI3K has been shown to alleviate the expression of inflammatory cytokines TNF-α and IL-6 induced by PAHs (Guo et al., 2021). Taking MS, the most common demyelinating and neurodegenerative diseases affecting the central nervous system (CNS) in young adults (Filippi et al., 2018), as one example, TNF and IL are major cytokines playing pivotal roles in pathogenesis and progression (Fiedler et al., 2017; Fresegna et al., 2020). PI3K/PDK1/Akt/mTOR signaling participates in pro-inflammatory cytokine levels, regulating cell metabolism, and amplifying specific inflammatory T cell phenotypes in MS (Kunkl et al., 2019; Park et al., 2009). Taken together, these connections indicate the mediating role of PI signaling in PAH-induced immune disorders (Fig. 5).
Fig. 5.

Proposed model of PAHs-induced PI signaling in MS. PAHs undergo metabolic transformation upon cellular entry, forming active metabolites. These metabolites can react with DNA and proteins, resulting in the formation of adducts that lead to DNA mutations and altered protein functions. PAH metabolites have the potential to induce mutations in the p53 gene, leading to enhanced nuclear PI signaling and activation of Akt. Additionally, PAH metabolites can target AhR, triggering the PI3K/Akt pathway and subsequent expression of inflammatory cytokines such as TNF-α and IL-6. The resulting inflammation may contribute to the development of MS. The diagram is generated using BioRender.
3.2. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)
TCDD, perhaps the most notorious pollutant, is an extremely toxic environmental contaminant belonging to the dioxin family (Gogal and Holladay, 2008). It is produced as a byproduct of chemical synthesis, including the manufacturing of certain chlorinated herbicides and fungicides. TCDD is also released during the combustion of chlorinated organic compounds and chlorine-based wood pulp bleaching (Sund et al., 1993). The adverse health effects associated with TCDD exposure range from cancer to reproductive and developmental abnormalities to dysfunction of the immune system (Bell et al., 2010; Canga et al., 1993; Mead, 2008; Peterson et al., 1993). TCDD has been shown to modulate the human immune system and mediate certain autoimmune diseases like autoimmune hepatitis (AIH) (Cannon et al., 2022), RA (Tamaki et al., 2004), and SS (Ishimaru et al., 2009) through triggering immune cell apoptosis, altering B/T cells subsets and functions, and regulating the expression of cytokines and co-stimulatory molecules (Camacho et al., 2005; Ito et al., 2002; Singh et al., 2008). Similar to PAH, TCDD also targets AhR and leads to induced PI3K-Akt signaling (Davis et al., 2000; Therachiyil et al., 2022), which may be implicated in the pathogenesis of these diseases. Sjögren’s syndrome is primarily considered a B/T cell-mediated autoimmune disorder characterized by lymphocyte infiltration, destruction of exocrine glands, especially salivary glands, and systemic production of autoantibodies (Fox, 2005; Fox et al., 2000; Kruize et al., 1995). Inhibition of phosphorylation events in the PI3K/Akt/mTOR pathway have been reported to decrease the secretion levels of inflammatory cytokines such as IL-17, alleviate the focal infiltration of lymphocytes, modulate the inflammatory microenvironment, and thus attenuate clinical symptoms and improve life quality for patients with SS (Zeng et al., 2022a; Zeng et al., 2022b). Studies have also demonstrated significant expression of phosphorylated ribosomal protein S6 (pS6), a downstream mediator of the PI3Kδ pathway, within the salivary glands. The prophylactic or therapeutic blocking of PI3K activity by seletalisib, a PI3Kδ selective inhibitor, has shown promise in significantly reducing the aggregation of lymphocytes and plasma cells in the salivary gland, thus improving clinical symptoms (Nayar et al., 2019). The immune system changes caused by TCDD exposure are interrelated to autoimmune diseases. Besides simple avoidance of environmental pollutants, exploring the connection between PI signaling pathways and TCDD-induced autoimmune diseases not only enhances our understanding of disease mechanisms but also holds the potential for developing effective treatments by targeting this pathway.
3.3. Trichloroethylene (TCE)
Chlorinated ethenes, including vinyl chloride (VC), dichloroethylene (DCE), perchloroethylene (PCE), and especially TCE, are commonly used as industrial solvents and degreasers in the chemical industry (Liu, S. et al., 2022). However, they are also pervasive environmental contaminants with adverse effects on ecosystems and human health through similar mechanisms (Anders et al., 2016; Chen et al., 2002; Liu, C. et al., 2015). TCE is perhaps the most relevant chlorinated ethene as it is classified as a primary carcinogen due to its asymmetric molecular structure and active chemical properties (Henschler et al., 1976; Kotik et al., 2013). In this section, we focus on TCE as a representative chlorinated ethane to explore its connection with PI signaling pathways and autoimmune diseases. Prolonged exposure to TCE through inhalation, ingestion, or skin contact can lead to a range of health issues, including certain cancers, liver and kidney damage, neurological effects, and autoimmune diseases (Cooper et al., 2009; Purdue, 2013; Wang et al., 2023). In recent years, many investigations of immune-related effects of TCE have been conducted, and part of this work focused on autoimmune diseases such as AIH, SLE, and SSc (Wang, G. et al., 2014; Wang, H. et al., 2019). Several biological mechanisms and pathways have been delineated in the pathogenesis of TCE-mediated autoimmune diseases, including systemic oxidative stress, apoptosis, autophagy, and inflammasome activation (Cooper et al., 2009; Wang et al., 2008; Wang, G. et al., 2019; Wang, H. et al., 2019). Like other environmental pollutants, the PI3K/Akt pathway plays a vital role in the cellular processes triggered by TCE. For instance, TCE exposure increases Akt phosphorylation, and the inhibition of Akt blocks TCE-induced cell proliferation (Xia et al., 2022). Activating α6β1/PI3K/Akt/nuclear factor-κB (NF-κB) signaling pathway may upregulate the production of specific cytokines, such as IL-6, and promote inflammation in AIH (Jiang et al., 2022). Conversely, suppressing the activation of the PI3K/Akt pathway and reducing the phosphorylation of Akt and PI3K have been suggested to significantly ameliorate hepatitis and liver fibrosis (Huang, Z. et al., 2023; Wang, S. et al., 2022). Similarly, activation of PI3K signaling contributes to exacerbating inflammation in SLE (Dong et al., 2023). In addition to TCE, other chlorinated ethenes, including VC (Anders et al., 2016), DCE (Chen et al., 2002), and PCE (Liu, C. et al., 2015), have been reported to be associated with PI signaling. Although the mechanisms underlying the connection to autoimmune diseases are not fully elucidated, future research directions may explore the potential therapeutic approaches involving the interference with PI signaling in chlorinated ethene-related autoimmune diseases.
3.4. Mercury
Mercury is a well-known heavy metal that is ubiquitous in the environment (Yang et al., 2020). It has the potential to cause toxic effects on the nervous, digestive, and immune systems, as well as various organs (Cappelletti et al., 2019; Paduraru et al., 2022). In severe cases of mercury intoxication, it can even lead to death (Pereira et al., 2019). Mercury exerts its impact on human health through several biological mechanisms, including cell apoptosis, autophagy, and oxidative stress (El Asar et al., 2019; Han et al., 2022; Zhao et al., 2021), leading to certain autoimmune diseases including SLE (Barbhaiya and Costenbader, 2016; Crowe et al., 2015; Fava and Petri, 2019), HT (Benvenga et al., 2022; Pamphlett et al., 2021), and MS (Anglen et al., 2015). Mercury is a widely recognized toxic metal that is known to induce oxidative stress (Chen et al., 2006). There is an emerging association between mercury exposure and stress responsive PI signaling. It has been observed that mercury exposure leads to an increase in PI3K activity, as well as the phosphorylation of its downstream effector, Akt (Chen et al., 2006). Studies have also demonstrated that exposure to mercury can induce autophagic cell death, which may be linked to the regulation of PI3K signaling cascades. The PI signaling pathway plays an important role in controlling the activation of inflammatory cells and the release of inflammatory transmitters during the chronic inflammatory response in HT (Gan et al., 2021; Li, H. et al., 2018). Luteolin and Kaempferol, reported PI3K/Akt signaling modulators, offer critical regulation against inflammation and show exciting potential in the treatment of HT (Gan et al., 2021). The relationship between mercury, PI signaling, and autoimmune responses seems to be convoluted. Overall, mercury’s association with autoimmune diseases and its influence on cellular processes, including the PI signaling pathway, requires further research for a comprehensive understanding. By deepening our knowledge of these connections, we can potentially develop targeted interventions to mitigate the adverse effects of mercury and improve treatment outcomes for autoimmune diseases.
3.5. Cadmium
Cadmium is a hazardous heavy metal found in industrial emissions, batteries, and certain fertilizers (He et al., 2022). Long-term exposure to cadmium can cause organ damage, increase the risk of cancer, and induce the occurrence of autoimmune diseases (Frangos and Maret, 2020). Cadmium exposure can exacerbate inflammatory responses in diseases through various pathways, including oxidative stress, DNA damage, and apoptosis (Ma et al., 2022; Yan and Allen, 2021). Studies have shown that cadmium plays a role in certain arthritic diseases, such as RA (Frangos and Maret, 2020; Joo et al., 2019), as an effector of the immune system, inflammation, and cellular metabolism. Specifically, inhalation of cadmium has been suggested as a trigger of a specific form of RA known as nodular RA (Murphy et al., 2019). The PI3K/Akt/mTOR signaling axis is reported to be the primary molecular pathway activated by cadmium exposure (Kulkarni et al., 2020) and may contribute to the cellular processes associated with RA and nodular RA. RA is characterized by systemic synovitis, resulting in joint destruction (Liu et al., 2021). Interestingly, evidence supports the role of pro-inflammatory cytokines in triggering the PI3K/Akt signaling pathway, which is responsible for immune-mediated inflammation in RA. Activation of this signaling pathway leads to the production of pro-inflammatory cytokines, further exacerbating the disease (Cheng et al., 2022; Liu et al., 2021). The research exploring the relationship between cadmium and autoimmune diseases is still limited, and more in-depth investigation is needed. A better understanding of the PI signaling pathway and its inhibitors in relation to these pathogenic mechanisms may help identify therapeutic targets and develop new drugs for cadmium-induced autoimmune diseases.
4. IMPACT OF BIOLOGICAL POLLUTANT-INDUCED PI SIGNALING ON AUTOIMMUNE DISEASES
Biological environmental pollutants encompass a diverse array of living organisms, such as bacteria, viruses, fungi, and other microorganisms, as well as larger species like insects and plants, that can have detrimental effects on ecosystems, human health, and other living organisms. In the upcoming section, we delve into the influence of selected biological environmental pollutants on PI signaling within the context of autoimmunity, covering one virus, one bacterium, one fungus, as well as plant-based pollen as examples. This exploration underscores the relevance of considering the role of biological environmental stress-induced lipid signaling in immune disorders and its potential implications for future studies and therapeutic strategies.
4.1. Epstein-Barr Virus (EBV)
EBV, the first human tumor virus to be identified (Soldan and Lieberman, 2023), is a widespread biological pollutant and establishes lifelong infection in more than 90% of adults worldwide (Shannon-Lowe and Rickinson, 2019) serving as a conditional pathogen once the equilibrium between the host and the virus is disrupted. EBV is also closely associated with multiple human cancers. Besides carcinomas derived from epithelial cells (nasopharyngeal carcinoma and gastric carcinoma), EBV is mainly linked to lymphomas derived from B cells and T cells (Luo et al., 2021), such as Burkitt lymphoma, Hodgkin lymphoma, NK/T-cell lymphoma, and posttransplant lymphoproliferative disorder (PTLD) indicating a deep connection with the immune system. Indeed, EBV profoundly affects the immune system with researchers revealing the presence of EBV antibodies in the plasma of patients with various autoimmune diseases, such as SLE (Barbhaiya et al., 2018; Chen,J. et al., 2022b; Fava and Petri, 2019; Jog and James, 2020; Majka and Holers, 2006), MS (Bjornevik et al., 2022; Lanz et al., 2022; Olsson et al., 2017; Soldan and Lieberman, 2023), SSc (Farina et al., 2017), RA (Fadlallah et al., 2021; Kroos et al., 2022; Svendsen et al., 2021), antiphospholipid syndrome (APS) (Kwok et al., 2022; Ogawa et al., 2002), and SS (Drozdzik et al., 2014; Inoue et al., 2012), suggesting that EBV may be an important factor in the progression of these diseases (Damania et al., 2022; Sausen et al., 2021). When the human immune surveillance fails, latent EBV can undergo reactivation, resulting in abnormal cell proliferation and pathogenesis. Efficient virus replication relies on the host PI metabolism, where phosphatidylinositol-4-kinase (PI4K) and PtdIns4P play important roles (Delang et al., 2012). During these processes, certain EBV-encoded latent proteins and miRNAs play notable roles as well. Following EBV infection, PI4K activity is significantly induced in B cells (Suzuki et al., 1992). Expression of a latent EBV protein called EBV nuclear antigen 1 (EBNA1) can induce PI4K and PI4P-kinase (PIPK) activity, leading to increased levels of PtdIns4P and PtdIns(4,5)P2 (Suzuki et al., 1992). Additionally, two other EBV latent proteins, LMP1 and LMP2A, activate the PI3K/Akt signaling pathway and enhance the activity of interferon regulatory factor 4 (IRF4) (Luo et al., 2021; Wang, L. et al., 2017). IRF4 is known to be involved in the differentiation of B cells, T cells, and macrophages (Shaffer et al., 2009). Additionally, LMP1 downregulates the expression of the PI3-phosphatase, PTEN, by increasing the expression of miR-21 (Yang et al., 2016). This, in turn, amplifies the PI3K/Akt signaling pathway. Besides, EBV-miRNA-BART7-3P could also contribute to the PI3K/Akt signaling through PTEN inhibition (Luo et al., 2021). Additionally, EBV utilizes its envelope protein gp350 to bind CR2/CD21 receptor, modulating proinflammatory cytokines including IL-6, IL-1β, and TNF-α (D’Addario et al., 1999). NF-κB pathway activation in gp350-treated cells depends on PKC and PI3K, potentially regulating IL-1β gene expression (D’Addario et al., 1999; Pan et al., 1996). The involvement of PI3K in B lymphocyte activation is still under exploration (Bouillie et al., 1999). While limited, research suggests a link between EBV-induced inflammatory changes and the PI signaling pathway (D’Addario et al., 2000; Soldan and Lieberman, 2023). Further investigation is needed to understand EBV’s role in autoimmune disorders and the PI signaling pathway (Fig. 6).
Fig. 6.

Proposed model of EBV-induced PI signaling in autoimmune diseases. Upon EBV infection, PI signaling is activated through multiple mechanisms. Firstly, the EBV protein EBNA1 induces the activity of PI4K and PIPK, resulting in elevated levels of PtdIns4P and PtdIns(4,5)P2. Secondly, the EBV latent proteins LMP1 and LMP2A activate the PI3K/Akt pathway. LMP1 downregulates PTEN expression by upregulating miR-21, thereby amplifying PI3K/Akt signaling. Thirdly, EBV-miRNA-BART7-3P contributes to PI3K/Akt signaling by inhibiting PTEN. Furthermore, the EBV envelope protein gp350 binds to the CR2/CD21 receptor, activating PI3K. Induction of the PI3K/Akt pathway leads to the activation of Src and NF-κB, resulting in the release of various cytokines including IRF4, IL-6, IL-1β, and TNF-α. This inflammatory response may contribute to the development of autoimmune diseases. The diagram is generated using BioRender.
4.2. Helicobacter pylori (H. pylori)
H. pylori is a widely prevalent gram-negative microaerophilic bacterium, serving as a biological pollutant that colonizes the human stomach lining in more than half of the world’s population (Suerbaum and Michetti, 2002). Clinically, it is known to cause active chronic gastritis and is associated with various gastrointestinal disorders (Sharma et al., 2010). Infection with H. pylori is commonly linked to peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphomas, resulting in this bacterium being classified as a class I carcinogen by the World Health Organization (WHO) (Coats et al., 2003; Stasi and Provan, 2008). Chronic infection with H. pylori is considered as a source of persistent antigenic stimulation and underlies the causative agents’ ability to induce systemic inflammatory responses (Jackson et al., 2009). Studies have suggested a potential role for H. pylori in the development of various autoimmune diseases, including IBD (Lord et al., 2018), immune thrombocytopenic purpura (ITP) (Vanegas and Vishnu, 2019), PBC (Goo et al., 2008), RA (Zentilin et al., 2002), and SLE (Wu et al., 2020). H. pylori appears to participate in these diseases through multiple mechanisms, such as T/B cells activation and autophagy (Amedei et al., 2003; Ruan et al., 2023; Yamanishi et al., 2006). As early as 1998, researchers discovered a relevant connection between H. pylori infection and the onset of ITP (Gasbarrini et al., 1998). ITP is a complex autoimmune-mediated bleeding disease characterized by autoreactive antibodies produced due to immune dysregulation of T and B cells, resulting in decreased platelet production and/or increased platelet destruction (Shan et al., 2016). Studies have shown that PTEN protein expression is lower in all B cell subsets of ITP patients, excluding memory B cells, contributing to B cell hyper-responsiveness (Xie et al., 2018). Lower programmed death 1 (PD1) and PTEN protein expressions have also been observed in CD4+ T cells from ITP patients compared to healthy controls, leading to disturbances of CD4+ T - cell homeostasis (Zhao et al., 2019). Furthermore, platelet autophagy is believed to be regulated by PTEN in patients with ITP through the PI3K/Akt/mTOR signaling pathway. PTEN serves as a key positive regulator for autophagy, blocking the inhibitory effect of PI3K on autophagy and enhancing autophagy-related proteins in the PI3K/Akt/mTOR signaling pathway, thus inhibiting cell apoptosis, and improving platelet viability (Guo et al., 2015; Wang, C.Y. et al., 2019). However, genome sequencing results suggest that several genes, including PTEN, harbor an excess number of rare and damaging mutations in ITP patients, leading to the activation of the PI3K/Akt/mTOR pathway and inhibition of autophagy which plays an important role in the initiation and progression of ITP (Ruan et al., 2023). ITP triggered by the infection of H. pylori has been confirmed through various proposed mechanisms. A number of studies have shown a significant increase in platelet count of ITP patients upon eradication of H. pylori, which may also inhibit the production of antiplatelet autoantibodies and lead to alleviation or complete regression of the disease in many patients (Aljarad et al., 2018; Sheema et al., 2017). Interestingly, H. pylori infection has been suggested to phosphorylate and inactivate PTEN in vivo and in vitro (Suzuki et al., 2009; Zhu et al., 2008). Therefore, after long-term infection, in addition to generating cross-reactivity with platelet surface antigens in the presence of H. pylori cytotoxin-associated gene A (CagA), H. pylori may also inhibit platelet autophagy through phosphorylation and inactivation of PTEN thereby suppressing platelet destruction and shortening the lifespan of platelets in ITP patients. Considering the close association between H. pylori and ITP, every patient with unexplained thrombocytopenia should undergo an H. pylori test. Although there is no established mechanism to explain how H. pylori could be implicated in the pathogenesis of autoimmune diseases through influencing the PI3K/Akt signaling pathway, chronic infections and the persistent activation of the immune system might contribute to immune dysregulation, potentially affecting individuals with underlying autoimmune conditions. Thus, further study of the connection between H. pylori, PI signaling pathway and autoimmune diseases, as well as their role in the pathogenesis of autoimmune diseases, is warranted.
4.3. Candida albicans (C. albicans)
C. albicans is an opportunistic pathogen, acting as a biological pollutant that overgrows and leads to fungal infections of the mucosa and skin when the balance of microorganisms in the body is disrupted (Garber, 2001). In such cases, the immune system responds to the infection by mounting an inflammatory response. Some research suggests that chronic C. albicans infections may contribute to immune dysregulation, possibly influencing the development or exacerbation of autoimmune diseases in susceptible individuals, including psoriasis (Whitley et al., 2022), T1D (Soyucen et al., 2014), and IBD (Gerard et al., 2015). T helper 17 cells (Th17) play a crucial role in protecting against mucocutaneous C. albicans infection (Li, J. et al., 2018). Under the stimulation of C. albicans, CD4+ effector T cells can differentiate into Th17 cells that secrete cytokines such as IL-17A, IL-17F, and IL-22, with IL-23 promoting their own development and proliferation. These cytokines mediate protective immunity against fungi by promoting the recruitment of neutrophils and enhancing barrier functions (Li, J. et al., 2018; Whitley et al., 2022). The IL-23/IL-17/IL-22 inflammatory axis protects the human body through the antifungal immune response. However, if dysregulated, the IL-17 response can promote immune pathology of infection or autoimmune diseases, which may also contribute to C. albicans infection aggravating autoimmune diseases such as psoriasis (Sparber and LeibundGut-Landmann, 2019). Psoriasis is an immune-mediated chronic, recurrent, inflammatory, and systemic disease induced by the combined effects of an individual’s genetics and their environment (Rademaker et al., 2019). One of its typical characteristics is the excessive proliferation and abnormal apoptosis of keratinocytes (Nograles et al., 2010). Although the pathogenesis of psoriasis is not completely clear, it is currently believed that Th17 cells are abnormally activated and release a number of cytokines that act on keratinocytes and other subtypes of Th cells causing them to proliferate and secrete inflammatory factors while expressing some chemokines. These cytokines cooperate to sustain persistent inflammation, ultimately evolving into chronic inflammatory damage to the skin (Hawkes et al., 2018; Whitley et al., 2022). Numerous studies have shown that the PI signaling pathway is closely related to the function of T cells, and the PI3K/Akt/mTOR complex 1 (mTORC1) signaling pathway is mainly involved in the regulation of Th17 cells (Kurebayashi et al., 2021). mTORC1 is indirectly activated by Thr308-phosphorylated Akt. Thus far, several distinct mechanisms are believed to participate in the regulation of Th17 differentiation via mTORC1. One is the increased expression of HIF-1α, downstream of mTORC1, which positively regulates Th17 differentiation by enhancing Th17-related gene expression (Shi et al., 2011). In addition, activating the PI3K/Akt/mTORC1 signaling pathway can increase the phosphorylation level of signal transducers and activators of transcription 3 (STAT3), a transcription factor expressed in the nucleus of Th17 cells which promotes the differentiation of Th17 cells and the expression of related cytokines, including IL-17 (Delgoffe et al., 2011). IL-17A directly activates keratinocytes to express a variety of cytokines such as IL-6. It may also synergize with TNF-α to induce the release of IL-6, IL-1β, and TNF-α, thereby enhancing inflammatory actions and contributing to the deterioration of autoimmune diseases such as psoriasis (Chen et al., 2021). In conclusion, the activation of Th17 cells caused by C. albicans infection plays a predominantly antifungal role. However, under the regulation of PI3K signaling pathway, Th17 cells are also considered as the main participants in the pathogenesis of various autoimmune diseases due to the recruitment of neutrophils and the release of inflammatory factors. Therefore, prevention and treatment of C. albicans infection are effective measures to improve the symptoms of autoimmune diseases in this capacity. Moreover, an in-depth study of the relationship between C. albicans, the PI signaling pathway, and autoimmunity will help to identify new therapeutic targets for disease treatment.
4.4. Pollen
Pollen, the reproductive particle produced by plants, is a common biological environmental pollutant (Lara et al., 2023). Research has shown that the vast majority of pollen grains in humans do not reach the thoracic respiratory tract but are swallowed and come into direct contact with the mucosa of the digestive tract (Wilson et al., 1973). When inhaled or in contact with mucous membranes, pollen particles can activate the immune system, leading to the release of histamines and many other inflammatory mediators, thus triggering allergies in susceptible individuals (Buhner et al., 2023; Kampe et al., 2012). In some cases, continuous exposure to allergens such as pollen may contribute to immune dysregulation and aggravate autoimmune conditions in susceptible populations associated with autoimmune diseases including IBD (Johnson et al., 2019), SLE (Jimenez-Alonso et al., 2004), ITP (Ring et al., 2009), and sarcoidosis (Van Gundy and Sharma, 1987). Pollen grains themselves trigger local inflammation in the gastrointestinal mucosa, similar to their role in the nasal mucosa (Magnusson et al., 2003). Regarding IBD, pollen sensitization is often discussed as a potential factor in the pathogenesis process (Johnson et al., 2019). When exposed to pollen, the number of eosinophils in the peripheral blood increases significantly under the stimulation of allergens which can cause inflammatory actions in target tissues (Jedrzejczak-Czechowicz et al., 2011; Kampe et al., 2012). The intestinal mucosal barrier (IMB) is the first barrier against harsh environments, but it is disrupted in IBD patients (Wu et al., 2019). Studies have demonstrated that the number of eosinophils in the colonic mucosa of patients with IBD is significantly increased (Neubauer et al., 2018). Peripheral blood eosinophilia (PBE) is regarded as a biomarker of the aggressive multiyear natural history in adults with IBD (Prathapan et al., 2020). Eosinophils are multifunctional leukocytes participated in host defense and inflammatory response, as well as immune modulation. Once activated and recruited to the intestinal tissue, eosinophils secrete an array of inflammatory mediators and histotoxic granule proteins through degranulation, leading to tissue damage (Weller and Spencer, 2017). Pharmacological studies have found that the PI3K/Akt signaling pathway is involved in the regulation of eosinophils. Wortmannin, a PI3K inhibitor, specifically blocks PI3K and enhances cell apoptosis and autophagy. By inhibiting the PI3K/Akt signaling pathway, Wortmannin suppresses the proliferation, migration, and degranulation of eosinophils, decreases the release of inflammatory factors, and blocks the epithelial cell damage caused by inflammatory cytokines which may result in improving intestinal inflammation and protecting the IMB of patients with IBD (Huang et al., 2011; Lee, 2004). In addition to the influence of eosinophils, studies have confirmed that PI3K activation exists in the gastrointestinal mucosa of IBD patients. p-Akt enhances the phosphorylation and degradation of NF-κB inhibitory protein (mainly IκB-α), which may cause the activation of NF-κB and upregulation of TNF-α, thus sustaining and amplifying the inflammatory response (Shimamura et al., 2003). Ongoing exposure to allergens like pollen may lead to long-term hypersensitivity in susceptible individuals resulting in an imbalance in immune function which is likely to cause allergic diseases or autoimmune diseases. Although the involvement of pollen in the pathogenesis of autoimmune diseases is not yet well understood, inhibiting the PI3K/Akt pathway may be beneficial to alleviate the symptoms of the disease. Therefore, studying the role of PI signaling pathway in the pathogenic mechanisms of pollen is crucial for the treatment of the relevant autoimmune diseases.
5. CONCLUSION
In this study, we delve into the pivotal role of PI signaling in cellular responses to environmental contaminants within the context of autoimmune diseases. Understanding how PI signaling contributes to the initiation and progression of autoimmune diseases is essential for advancing our comprehension of the underlying pathological processes and for formulating effective prevention and treatment strategies. Environmental pollutants are categorized based on their physical, chemical, and biological nature, and their connections to specific autoimmune diseases are continuously being explored. Here, we have focused on discussing a few representative types of pollutants within each pollution category (Fig. 3). Each category encompasses additional pollutants not explicitly presented, and the list of pollutants associated with PI signaling and autoimmunity is expanding with growing scientific attention.
There are over 80 different types of autoimmune diseases, and each may have distinct factors contributing to their development (Rad et al., 2019). We acknowledge that autoimmune diseases can have diverse triggers and mechanisms, and not all of them may be directly related to PI signaling. However, it is crucial to note that the pollutants and autoimmune diseases we have examined bear notable implications for PI signaling (Table 1, 2), indicating a profound and fundamental relationship between PI signaling in the development of environmentally induced autoimmunity. Adding complexity to this relationship, pollutants from different sources may share common or even synergistic impacts on PI signaling. As shown in Table 1, both UV radiation and EBV influence PtdIns4P. Additionally, both UV radiation and PAHs affect the PI effector p53. Furthermore, it is noteworthy that all the listed environmental pollutants have an impact on the PI3K/Akt pathway. However, each pollutant may also exhibit specificity, necessitating further investigation.
Given the significance of PI signaling as a promising drug target, with a range of inhibitors and therapeutics available, future research endeavors could investigate the role of PI signaling in immune disorders related to environmental stress or assess the effectiveness of implementing PI signaling interventions using strategies that selectively target various PI kinases or lipid effectors, including PI4K (Li et al., 2021), PIPK (Semenas et al., 2014), PI3K (Sun and Meng, 2020), and Akt (Hua et al., 2021). Collectively, our review offers valuable insights for the development of interventions aimed at addressing immune disorders associated with environmental pollutants.
ENVIRONMENTAL IMPLICATION.
The review unveils significant environmental implications concerning PI signaling in autoimmune diseases. Through an examination of varied physical, chemical, and biological environmental pollutants, the study highlights the crucial role of stress-induced PI signaling in immune regulation and its intricate connection to the initiation of autoimmune disorders. The paper strongly emphasizes the pressing requirement to comprehend the intricate interplay between PI signaling pathways and environmental pollutants. These insights hold substantial importance for understanding autoimmune disease pathogenesis, paving the route for strategic approaches that can address their impact on both public health and the environment.
SYNOPSIS:
Phosphoinositide signaling at the intersection of environmental pollutants and autoimmunity provides novel insights for managing autoimmune diseases aggravated by pollutants.
HIGHLIGHTS:
First reviewed environmental pollutants’ impact on PI signaling in autoimmunity.
Responsible pollutants are grouped by physical, chemical, and biological origins.
Exploring pollutant-responsive PI signaling in autoimmunity informs public health.
ACKNOWLEDGMENTS
This work was funded by the research startup grant by the Shenzhen municipal government (Y011146105, M.C.) and Southern University of Science and Technology (Y011146205, M.C.). Additional sponsorship was provided by the Shenzhen Medical Academy of Research and Translation (D2301007, M.C.). This work was supported in part by a National Institutes of Health grant R35GM134955 (R.A.A.), Department of Defense Breast Cancer Research Program grants W81XWH-17-1-0258 (R.A.A.), W81XWH-17-1-0259 (V.L.C.) and W81XWH-21-1-0129 (V.L.C.), and a grant from the Breast Cancer Research Foundation (V.L.C.).
ABBREVIATIONS
- AhR
aryl hydrocarbon receptor
- AIH
autoimmune hepatitis
- APS
antiphospholipid syndrome
- C. albicans
Candida albicans
- CagA
cytotoxin-associated gene A
- CNS
central nervous system
- DCE
dichloroethylene
- EBNA1
EBV nuclear antigen 1
- EBV
Epstein-Barr virus
- EGFR
epidermal growth factor receptor
- FMS
fibromyalgia syndrome
- FOXO
forkhead box O
- FYVE
Fab1, YOTB, Vac1, and EEA1
- GD
Graves’ disease
- GDNF
glial-derived neurotrophic factor
- H. pylori
Helicobacter pylori
- HIF-1α
hypoxia-inducible factor-1α
- HT
Hashimoto’s thyroiditis
- IBD
inflammatory bowel disease
- IL-1
interleukin-1
- IMB
intestinal mucosal barrier
- IPMK
inositol polyphosphate multikinase
- IRF4
interferon regulatory factor 4
- ITP
immune thrombocytopenic purpura
- LAPTM4B
lysosomal-associated protein transmembrane 4B
- MALT
mucosa-associated lymphoid tissue
- MN
membranous nephropathy
- MS
multiple sclerosis
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR complex 1; mTORC2, mTOR complex 2
- NF-κB
nuclear factor-κB
- NIHL
noise-induced hearing loss
- PAH
polycyclic aromatic hydrocarbon
- PBC
primary biliary cirrhosis
- PBE
peripheral blood eosinophilia
- PCE
perchloroethylene
- PD1
programmed death 1
- PDK1
pyruvate dehydrogenase kinase isozyme 1
- PH
pleckstrin homology
- PI
phosphoinositide
- PI3K
phosphoinositide 3-kinase
- PIPK
PI4P-kinase
- PIPns
polyphosphoinositides
- PM
particulate matter
- PtdIns
phosphatidylinositol
- PTEN
phosphatase and tensin homolog
- PTLD
posttransplant lymphoproliferative disorder
- PX
phox homology
- RA
rheumatoid arthritis
- SiO2
silicon dioxide
- SLE
systemic lupus erythematosus
- SS
Sjogren’s syndrome
- SSc
systemic sclerosis
- STAT3
signal transducers and activators of transcription 3
- T1D
type 1 diabetes
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TCE
trichloroethylene
- TGF-β1
transforming growth factor-beta 1
- Th17
T helper cells 17
- TNF-α
tumor necrosis factor-alpha
- UC
ulcerative colitis
- UV
ultraviolet
- VC
vinyl chloride
- VEGF
vascular endothelial growth factor
- WHO
world health organization
- XME
xenobiotic-metabolizing enzyme
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DATA AVAILABILITY
No data was used for the research described in the article.
REFERENCES
- Adami G, Pontalti M, Cattani G, Rossini M, Viapiana O, Orsolini G, Benini C, Bertoldo E, Fracassi E, Gatti D, Fassio A, 2022. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open 8(1). 10.1136/rmdopen-2021-002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihaiti Y, Song Cai Y, Tuerhong X, Ni Yang Y, Ma Y, Shi Zheng H, Xu K, Xu P, 2021. Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation. Front Pharmacol 12, 672054. 10.3389/fphar.2021.672054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alb JG Jr, Cortese JD, Phillips SE, Albin RL, Nagy TR, Hamilton BA, Bankaitis VA, 2003. Mice lacking phosphatidylinositol transfer protein-alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia. J Biol Chem 278(35), 33501–33518. 10.1074/jbc.M303591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex AM, Kunkel G, Sayles H, Flautero Arcos JD, Mikuls TR, Kerr GS, 2020. Exposure to ambient air pollution and autoantibody status in rheumatoid arthritis. Clin Rheumatol 39(3), 761–768. 10.1007/s10067-019-04813-w. [DOI] [PubMed] [Google Scholar]
- Aljarad S, Alhamid A, Sankari Tarabishi A, Tarabishi AS, Suliman A, Aljarad Z, 2018. The impact of helicobacter pylori eradication on platelet counts of adult patients with idiopathic thrombocytopenic purpura. BMC Hematol 18, 28. 10.1186/s12878-018-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D’Elios MM, Del Prete G, 2003. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med 198(8), 1147–1156. 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders LC, Lang AL, Anwar-Mohamed A, Douglas AN, Bushau AM, Falkner KC, Hill BG, Warner NL, Arteel GE, Cave M, McClain CJ, Beier JI, 2016. Vinyl Chloride Metabolites Potentiate Inflammatory Liver Injury Caused by LPS in Mice. Toxicol Sci 151(2), 312–323. 10.1093/toxsci/kfw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglen J, Gruninger SE, Chou HN, Weuve J, Turyk ME, Freels S, Stayner LT, 2015. Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists. J Am Dent Assoc 146(9), 659–668 e651. 10.1016/j.adaj.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Aranyosi JK, Galgoczi E, Erdei A, Katko M, Fodor M, Ujhelyi Z, Bacskay I, Nagy EV, Ujhelyi B, 2022. Different Effects of Cigarette Smoke, Heated Tobacco Product and E-Cigarette Vapour on Orbital Fibroblasts in Graves’ Orbitopathy; a Study by Real Time Cell Electronic Sensing. Molecules 27(9). 10.3390/molecules27093001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkema EV, Hart JE, Bertrand KA, Laden F, Grodstein F, Rosner BA, Karlson EW, Costenbader KH, 2013. Exposure to ultraviolet-B and risk of developing rheumatoid arthritis among women in the Nurses’ Health Study. Ann Rheum Dis 72(4), 506–511. 10.1136/annrheumdis-2012-202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Huang Y, Shen P, Huang Y, Wang H, Han L, Lin WJ, Yan HJ, Xu LJ, Qin K, Chen Z, Tu SH, 2021. WTD Attenuating Rheumatoid Arthritis via Suppressing Angiogenesis and Modulating the PI3K/AKT/mTOR/HIF-1alpha Pathway. Front Pharmacol 12, 696802. 10.3389/fphar.2021.696802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacalao MA, Satterthwaite AB, 2020. Recent Advances in Lupus B Cell Biology: PI3K, IFNgamma, and Chromatin. Front Immunol 11, 615673. 10.3389/fimmu.2020.615673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Li FF, Zhang Y, Ding YB, 2023. Silicon dioxide nanoparticles compromise decidualization via autophagy impairment to possibly cause embryo resorption. Toxicol Lett 381, 72–82. 10.1016/j.toxlet.2023.05.003. [DOI] [PubMed] [Google Scholar]
- Baldini E, Odorisio T, Sorrenti S, Catania A, Tartaglia F, Carbotta G, Pironi D, Rendina R, D’Armiento E, Persechino S, Ulisse S, 2017. Vitiligo and Autoimmune Thyroid Disorders. Front Endocrinol (Lausanne) 8, 290. 10.3389/fendo.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Carman GM, 2019. The Role of Phosphoinositides in Signaling and Disease: Introduction to the Thematic Review Series. J Lipid Res 60(2), 227–228. 10.1194/jlr.E091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz MK, Rodriguez S, Martinez AC, Balomenos D, Rommel C, Carrera AC, 2005. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11(9), 933–935. 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- Barbhaiya M, Costenbader KH, 2016. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol 28(5), 497–505. 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya M, Hart JE, Malspeis S, Tedeschi SK, VoPham T, Sparks JA, Karlson EW, Laden F, Costenbader KH, 2022. Association of Ultraviolet B Radiation and Risk of Systemic Lupus Erythematosus Among Women in the Nurses’ Health Studies. Arthritis Care Res (Hoboken). 10.1002/acr.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, Karlson EW, Costenbader KH, 2018. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann Rheum Dis 77(2), 196–202. 10.1136/annrheumdis-2017-211675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML, 2020. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front Endocrinol (Lausanne) 11, 615993. 10.3389/fendo.2020.615993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E, 2011. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis 5(4), 279–286. 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Bell DR, Clode S, Fan MQ, Fernandes A, Foster PM, Jiang T, Loizou G, MacNicoll A, Miller BG, Rose M, Tran L, White S, 2010. Interpretation of studies on the developmental reproductive toxicology of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring. Food Chem Toxicol 48(6), 1439–1447. 10.1016/j.fct.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran L, Chaussade C, Vanhaesebroeck B, Cutillas PR, 2011. Calpain interacts with class IA phosphoinositide 3-kinases regulating their stability and signaling activity. Proc Natl Acad Sci U S A 108(39), 16217–16222. 10.1073/pnas.1107692108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenga S, Fama F, Perdichizzi LG, Antonelli A, Brenta G, Vermiglio F, Moleti M, 2022. Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids. Front Endocrinol (Lausanne) 13, 891233. 10.3389/fendo.2022.891233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, Smargiassi A, 2011. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE). Environ Health Perspect 119(1), 45–49. 10.1289/ehp.1002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierschenk D, Papac-Milicevic N, Bresch IP, Kovacic V, Bettoni S, Dziedzic M, Wetsel RA, Eschenburg S, Binder CJ, Blom AM, King BC, 2023. C4b-binding protein inhibits particulate- and crystalline-induced NLRP3 inflammasome activation. Front Immunol 14, 1149822. 10.3389/fimmu.2023.n49822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl M, Kallenberg CG, 2006. Ultraviolet light and cutaneous lupus. Lupus 15(11), 724–727. 10.1177/0961203306071705. [DOI] [PubMed] [Google Scholar]
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A, 2022. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375(6578), 296–301. 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- Bokarewa MI, Erlandsson MC, Bjersing J, Dehlin M, Mannerkorpi K, 2014. Smoking is associated with reduced leptin and neuropeptide Y levels and higher pain experience in patients with fibromyalgia. Mediators Inflamm 2014, 627041. 10.1155/2014/627041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillie S, Barel M, Frade R, 1999. Signaling through the EBV/C3d receptor (CR2, CD21) in human B lymphocytes: activation of phosphatidylinositol 3-kinase via a CD19-independent pathway. J Immunol 162(1), 136–143. [PubMed] [Google Scholar]
- Buhner S, Schauffele S, Giesbertz P, Demir IE, Zeller F, Traidl-Hoffmann C, Schemann M, Gilles S, 2023. Allergen-free extracts from birch, ragweed, and hazel pollen activate human and guinea-pig submucous and spinal sensory neurons. Neurogastroenterol Motil 35(7), e14559. 10.1111/nmo.14559. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS, 2005. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol 175(1), 90–103. 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C, 2005. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11(9), 936–943. 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Canga L, Paroli L, Blanck TJ, Silver RB, Rifkind AB, 1993. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases cardiac myocyte intracellular calcium and progressively impairs ventricular contractile responses to isoproterenol and to calcium in chick embryo hearts. Mol Pharmacol 44(6), 1142–1151. [PubMed] [Google Scholar]
- Cannon AS, Holloman BL, Wilson K, Miranda K, Dopkins N, Nagarkatti P, Nagarkatti M, 2022. AhR Activation Leads to Attenuation of Murine Autoimmune Hepatitis: Single-Cell RNA-Seq Analysis Reveals Unique Immune Cell Phenotypes and Gene Expression Changes in the Liver. Front Immunol 13, 899609. 10.3389/fimmu.2022.899609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti S, Piacentino D, Fineschi V, Frati P, D’Errico S, Aromatario M, 2019. Mercuric chloride poisoning: symptoms, analysis, therapies, and autoptic findings. A review of the literature. Crit Rev Toxicol 49(4), 329–341. 10.1080/10408444.2019.1621262. [DOI] [PubMed] [Google Scholar]
- Carlson K, Neitzel RL, 2018. Hearing loss, lead (Pb) exposure, and noise: a sound approach to ototoxicity exploration. J Toxicol Environ Health B Crit Rev 21(5), 335–355. 10.1080/10937404.2018.1562391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo ND, Chen M, Cryns VL, Anderson RA, 2023. Lipid transfer proteins initiate nuclear phosphoinositide signaling. bioRxiv. 10.n01/2023.05.08.539894. [DOI] [Google Scholar]
- Cavalin C, Lescoat A, Sigaux J, Macchi O, Ballerie A, Catinon M, Vincent M, Semerano L, Boissier MC, Rosental PA, 2022. Crystalline silica exposure in patients with rheumatoid arthritis and systemic sclerosis: a nationwide cross-sectional survey. Rheumatology (Oxford). 10.1093/rheumatology/keac675. [DOI] [PubMed] [Google Scholar]
- Celada LJ, Rotsinger JE, Young A, Shaginurova G, Shelton D, Hawkins C, Drake WP, 2017. Programmed Death-1 Inhibition of Phosphatidylinositol 3-Kinase/AKT/Mechanistic Target of Rapamycin Signaling Impairs Sarcoidosis CD4(+) T Cell Proliferation. Am J Respir Cell Mol Biol 56(1), 74–82. 10.1165/rcmb.2016-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin B, Yurdakul S, Gungormus E, Ozturk F, Sofuoglu SC, 2018. Source apportionment and carcinogenic risk assessment of passive air sampler-derived PAHs and PCBs in a heavily industrialized region. Sci Total Environ 633, 30–41. 10.1016/j.scitotenv.2018.03.145. [DOI] [PubMed] [Google Scholar]
- Chen CT, Lin JG, Huang CP, Lin YW, 2019. Electroacupuncture attenuates chronic fibromyalgia pain through the phosphorylated phosphoinositide 3-kinase signaling pathway in the mouse brain. Iran J Basic Med Sci 22(9), 1085–1090. 10.22038/ijbms.2019.35887.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, Lo CH, Huang CC, Lu MP, Hu PY, Chen CS, Chueh DY, Chen P, Lin TN, Lo YH, Hsiao YP, Hsu DK, Liu FT, 2021. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J Clin Invest 131(1). 10.1172/JCI130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hu Q, Luo Y, Luo L, Lin H, Chen D, Xu Y, Liu B, He Y, Liang C, Liu Y, Zhou J, Wu J, 2022a. Salvianolic acid B attenuates membranous nephropathy by activating renal autophagy via microRNA-145-5p/phosphatidylinositol 3-kinase/AKT pathway. Bioengineered 13(5), 13956–13969. 10.1080/21655979.2022.2083822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liao S, Pang W, Guo F, Yang L, Liu HF, Pan Q, 2022b. Life factors acting on systemic lupus erythematosus. Front Immunol 13, 986239. 10.3389/fimmu.2022.986239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yuan H, Talaska AE, Hill K, Sha SH, 2015. Increased Sensitivity to Noise-Induced Hearing Loss by Blockade of Endogenous PI3K/Akt Signaling. J Assoc Res Otolaryngol 16(3), 347–356. 10.1007/s10162-015-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Choi S, Wen T, Chen C, Thapa N, Lee JH, Cryns VL, Anderson RA, 2022. A p53-phosphoinositide signalosome regulates nuclear AKT activation. Nat Cell Biol 24(7), 1099–1113. 10.1038/s41556-022-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wen T, Horn HT, Chandrahas VK, Thapa N, Choi S, Cryns VL, Anderson RA, 2020. The nuclear phosphoinositide response to stress. Cell Cycle 19(3), 268–289. 10.1080/15384101.2019.1711316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Wang JL, Chen JH, Huang RN, 2002. Possible involvement of glutathione and p53 in trichloroethylene- and perchloroethylene-induced lipid peroxidation and apoptosis in human lung cancer cells. Free Radic Biol Med 33(4), 464–472. 10.1016/s0891-5849(02)00817-1. [DOI] [PubMed] [Google Scholar]
- Chen SL, Li CM, Li W, Liu QS, Hu SY, Zhao MY, Hu DS, Hao YW, Zeng JH, Zhang Y, 2023. How autophagy, a potential therapeutic target, regulates intestinal inflammation. Front Immunol 14, 1087677. 10.3389/fimmu.2023.1087677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu Y, Qi Y, Liu S, 2023. Cell Death Pathways: The Variable Mechanisms Underlying Fine Particulate Matter-Induced Cytotoxicity. ACS Nanosci Au 3(2), 130–139. 10.1021/acsnanoscienceau.2c00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH, 2006. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes 55(6), 1614–1624. 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- Cheng C, Wang P, Yang Y, Du X, Xia H, Liu J, Lu L, Wu H, Liu Q, 2023. Smoking-Induced M2-TAMs, via circEML4 in EVs, Promote the Progression of NSCLC through ALKBH5-Regulated m6A Modification of SOCS2 in NSCLC Cells. Adv Sci (Weinh), e2300953. 10.1002/advs.202300953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Chen M, Liu M, Chen X, Zhu L, Xu J, Xue J, Wu H, Du Y, 2022. Semaphorin 5A suppresses ferroptosis through activation of PI3K-AKT-mTOR signaling in rheumatoid arthritis. Cell Death Dis 13(7), 608. 10.1038/s41419-022-05065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TT, Chau YY, Chen JB, Hsu HH, Hung SP, Lee WC, 2021. Rapamycin attenuates PLA2R activation-mediated podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed Pharmacother 144, 112349. 10.1016/j.biopha.2021.112349. [DOI] [PubMed] [Google Scholar]
- Choi S, Chen M, Cryns VL, Anderson RA, 2019. A nuclear phosphoinositide kinase complex regulates p53. Nat Cell Biol 21(4), 462–475. 10.1038/s41556-019-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats AJ, Henein M, Flather M, Sigwart U, Seggewiss H, Wang D, Yousufuddin M, Shamim W, 2003. Retraction: Shamim et Al. Nonsurgical reduction of the interventricular septum in patients with hypertrophic cardiomyopathy. N Engl J Med 2002;347:1326–33. N Engl J Med 348(10), 951. 10.1056/NEJMc035061. [DOI] [PubMed] [Google Scholar]
- Collaborators GBDRF, 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053), 1659–1724. 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong LH, Li T, Wang H, Wu YN, Wang SP, Zhao YY, Zhang GQ, Duan J, 2020. IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J Cell Mol Med 24(15), 8532–8544. 10.1111/jcmm.15475. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cooper GS, Makris SL, Nietert PJ, Jinot J, 2009. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect 117(5), 696–702. 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Wither J, Bernatsky S, Claudio JO, Clarke A, Rioux JD, Ca NGI, Fortin PR, 2010. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology (Oxford) 49(11), 2172–2180. 10.1093/rheumatology/keq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe W, Doherty L, Watson G, Armstrong D, Ball E, Magee P, Allsopp P, Bell A, Strain JJ, McSorley E, 2015. Mercury in Hair Is Inversely Related to Disease Associated Damage in Systemic Lupus Erythematosus. Int J Environ Res Public Health 13(1), ijerph13010075. 10.3390/ijerph13010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario M, Ahmad A, Morgan A, Menezes J, 2000. Binding of the Epstein-Barr virus major envelope glycoprotein gp350 results in the upregulation of the TNF-alpha gene expression in monocytic cells via NF-kappaB involving PKC, PI3-K and tyrosine kinases. J Mol Biol 298(5), 765–778. 10.1006/jmbi.2000.3717. [DOI] [PubMed] [Google Scholar]
- D’Addario M, Ahmad A, Xu JW, Menezes J, 1999. Epstein-Barr virus envelope glycoprotein gp350 induces NF-kappaB activation and IL-1beta synthesis in human monocytes-macrophages involving PKC and PI3-K. FASEB J 13(15), 2203–2213. 10.1096/fasebj.13.15.2203. [DOI] [PubMed] [Google Scholar]
- Damania B, Kenney SC, Raab-Traub N, 2022. Epstein-Barr virus: Biology and clinical disease. Cell 185(20), 3652–3670. 10.1016/j.cell.2022.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, He B, Ning Q, Liu Y, Guo J, Niu G, Chen M, 2020. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-kappaB pathways. Respir Res 21(1), 95. 10.1186/s12931-020-01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JW 2nd, Melendez K, Salas VM, Lauer FT, Burchiel SW, 2000. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits growth factor withdrawal-induced apoptosis in the human mammary epithelial cell line, MCF-10A. Carcinogenesis 21(5), 881–886. 10.1093/carcin/21.5.881. [DOI] [PubMed] [Google Scholar]
- Delang L, Paeshuyse J, Neyts J, 2012. The role of phosphatidylinositol 4-kinases and phosphatidylinositol 4-phosphate during viral replication. Biochem Pharmacol 84(11), 1400–1408. 10.1016/j.bcp.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD, 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12(4), 295–303. 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Hu W, Wang R, Yang Q, Zhu M, Li M, Cai J, Rose P, Mao J, Zhu YZ, 2023. Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct Target Ther 8(1), 68. 10.1038/s41392-023-01331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, Ren X, Sun Q, Sun Z, Duan J, 2023. An integral perspective of canonical cigarette and e-cigarette-related cardiovascular toxicity based on the adverse outcome pathway framework. J Adv Res 48, 227–257. 10.1016/jjare.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Gao L, Sun Q, Jia L, Liu D, 2023. Increased levels of IL-17 and autoantibodies following Bisphenol A exposure were associated with activation of PI3K/AKT/mTOR pathway and abnormal autophagy in MRL/lpr mice. Ecotoxicol Environ Saf 255, 114788. 10.1016/j.ecoenv.2023.114788. [DOI] [PubMed] [Google Scholar]
- Drozdzik A, Dziedziejko V, Kurzawski M, 2014. IL-1 and TNF-alpha regulation of aryl hydrocarbon receptor (AhR) expression in HSY human salivary cells. Arch Oral Biol 59(4), 434–439. 10.1016/j.archoralbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB, 2007. Epidemiology of autoimmune diseases in Denmark. J Autoimmun 29(1), 1–9. 10.1016/jjaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G, Smithers CC, Murray AG, Overduin M, 2019. Structure and function of the Fgd family of divergent FYVE domain proteins (1). Biochem Cell Biol 97(3), 257–264. 10.1139/bcb-2018-0185. [DOI] [PubMed] [Google Scholar]
- Ekblom-Kullberg S, Kautiainen H, Alha P, Leirisalo-Repo M, Miettinen A, Julkunen H, 2014. Smoking, disease activity, permanent damage and dsDNA autoantibody production in patients with systemic lupus erythematosus. Rheumatol Int 34(3), 341–345. 10.1007/s00296-013-2889-7. [DOI] [PubMed] [Google Scholar]
- El Asar HM, Mohammed EA, Aboulhoda BE, Emam HY, Imam AA, 2019. Selenium protection against mercury neurotoxicity: Modulation of apoptosis and autophagy in the anterior pituitary. Life Sci 231, 116578. 10.1016/j.lfs.2019.116578. [DOI] [PubMed] [Google Scholar]
- Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S, 2015. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 18(4), 433–448. 10.1007/s10456-015-9477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadlallah S, Hussein H, Jallad MA, Shehab M, Jurjus AR, Matar GM, Rahal EA, 2021. Effect of Epstein-Barr Virus DNA on the Incidence and Severity of Arthritis in a Rheumatoid Arthritis Mouse Model. Front Immunol 12, 672752. 10.3389/fimmu.2021.672752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Lu F, Du WJ, Chen J, An XG, Wang RF, Li W, Song YL, Zha DJ, Chen FQ, 2023. PTEN inhibitor bisperoxovanadium protects against noise-induced hearing loss. Neural Regen Res 18(7), 1601–1606. 10.4103/1673-5374.358606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina A, Peruzzi G, Lacconi V, Lenna S, Quarta S, Rosato E, Vestri AR, York M, Dreyfus DH, Faggioni A, Morrone S, Trojanowska M, Farina GA, 2017. Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes. Arthritis Res Ther 19(1), 39. 10.1186/s13075-017-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava A, Petri M, 2019. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun 96, 1–13. 10.1016/jjaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Gao D, Liao F, Zhou F, Wang X, 2016. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf 128, 67–74. 10.1016/j.ecoenv.2016.01.030. [DOI] [PubMed] [Google Scholar]
- Ferrara N, 2004. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4), 581–611. 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Ferrari SM, Fallahi P, Antonelli A, Benvenga S, 2017. Environmental Issues in Thyroid Diseases. Front Endocrinol (Lausanne) 8, 50. 10.3389/fendo.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler SE, George JD, Love HN, Kim E, Spain R, Bourdette D, Salinthone S, 2017. Analysis of IL-6, IL-1beta and TNF-alpha production in monocytes isolated from multiple sclerosis patients treated with disease modifying drugs. J Syst Integr Neurosci 3(3). 10.15761/JSIN.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA, 2017. Multiple sclerosis. Nat Rev Dis Primers 4(1), 43. 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- Fox RI, 2005. Sjogren’s syndrome. Lancet 366(9482), 321–331. 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- Fox RI, Stern M, Michelson P, 2000. Update in Sjogren syndrome. Curr Opin Rheumatol 12(5), 391–398. 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Frangos T, Maret W, 2020. Zinc and Cadmium in the Aetiology and Pathogenesis of Osteoarthritis and Rheumatoid Arthritis. Nutrients 13(1). 10.3390/nu13010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresegna D, Bullitta S, Musella A, Rizzo FR, De Vito F, Guadalupi L, Caioli S, Balletta S, Sanna K, Dolcetti E, Vanni V, Bruno A, Buttari F, Stampanoni Bassi M, Mandolesi G, Centonze D, Gentile A, 2020. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 9(10). 10.3390/cells9102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli A, Avvedimento EV, Krieg T, 2009. Scleroderma. N Engl J Med 360(19), 1989–2003. 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- Galvao Tessaro FH, Ayala TS, Bella LM, Martins JO, 2020. Macrophages from a type 1 diabetes mouse model present dysregulated Pl3K/AKT, ERK 1/2 and SAPK/JNK levels. Immunobiology 225(2), 151879. 10.1016/j.imbio.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Gambelli F, Di P, Niu X, Friedman M, Hammond T, Riches DW, Ortiz LA, 2004. Phosphorylation of tumor necrosis factor receptor 1 (p55) protects macrophages from silica-induced apoptosis. J Biol Chem 279(3), 2020–2029. 10.1074/jbc.M309763200. [DOI] [PubMed] [Google Scholar]
- Gan XX, Zhong LK, Shen F, Feng JH, Li YY, Li SJ, Cai WS, Xu B, 2021. Network Pharmacology to Explore the Molecular Mechanisms of Prunella vulgaris for Treating Hashimoto’s Thyroiditis. Front Pharmacol 12, 700896. 10.3389/fphar.2021.700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber G, 2001. An overview of fungal infections. Drugs 61 Suppl 1, 1–12. 10.2165/00003495-200161001-00001. [DOI] [PubMed] [Google Scholar]
- Garcia-Cuellar CM, Chirino YI, Morales-Barcenas R, Soto-Reyes E, Quintana-Belmares R, Santibanez-Andrade M, Sanchez-Perez Y, 2020. Airborne Particulate Matter (PM(10)) Inhibits Apoptosis through PI3K/AKT/FoxO3a Pathway in Lung Epithelial Cells: The Role of a Second Oxidant Stimulus. Int J Mol Sci 21(2). 10.3390/ijms21020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V, 2011. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3(73), 73ra20. 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G, 1998. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 352(9131), 878. 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- Gerard R, Sendid B, Colombel JF, Poulain D, Jouault T, 2015. An immunological link between Candida albicans colonization and Crohn’s disease. Crit Rev Microbiol 41(2), 135–139. 10.3109/1040841X.2013.810587. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM, Group UPE, 2005. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology 42(5), 1194–1202. 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilcrease GW, Padovan D, Heffler E, Peano C, Massaglia S, Roccatello D, Radin M, Cuadrado MJ, Sciascia S, 2020. Is air pollution affecting the disease activity in patients with systemic lupus erythematosus? State of the art and a systematic literature review. Eur J Rheumatol 7(1), 31–34. 10.5152/eurjrheum.2019.19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogal RM Jr., Holladay SD, 2008. Perinatal TCDD exposure and the adult onset of autoimmune disease. J Immunotoxicol 5(4), 413–418. 10.1080/10408360802483201. [DOI] [PubMed] [Google Scholar]
- Goo MJ, Ki MR, Lee HR, Hong IH, Park JK, Yang HJ, Yuan DW, Hwang OK, Do SH, Yoo SE, Jeong KS, 2008. Primary biliary cirrhosis, similar to that in human beings, in a male C57BL/6 mouse infected with Helicobacter pylori. Eur J Gastroenterol Hepatol 20(10), 1045–1048. 10.1097/MEG.0b013e3282f5e9db. [DOI] [PubMed] [Google Scholar]
- Guastafierro T, Bacalini MG, Marcoccia A, Gentilini D, Pisoni S, Di Blasio AM, Corsi A, Franceschi C, Raimondo D, Spano A, Garagnani P, Bondanini F, 2017. Genome-wide DNA methylation analysis in blood cells from patients with Werner syndrome. Clin Epigenetics 9, 92. 10.1186/s13148-017-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Huang Y, Wang H, Zhang Z, Li C, Hu F, Zhang W, Liu Y, Zeng Y, Wang J, 2021. Low molecular weight-PAHs induced inflammation in A549 cells by activating PI3K/AKT and NF-kappaB signaling pathways. Toxicol Res (Camb) 10(1), 150–157. 10.1093/toxres/tfaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB, Liu WC, 2015. Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/Akt/mTOR signaling pathway. Apoptosis 20(8), 1109–1121. 10.1007/s10495-015-1138-9. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sataloff RT, 2003. Noise-induced autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol 112(7), 569–573. 10.1177/000348940311200701. [DOI] [PubMed] [Google Scholar]
- Hahad O, Prochaska JH, Daiber A, Muenzel T, 2019. Environmental Noise-Induced Effects on Stress Hormones, Oxidative Stress, and Vascular Dysfunction: Key Factors in the Relationship between Cerebrocardiovascular and Psychological Disorders. Oxid Med Cell Longev 2019, 4623109. 10.1155/2019/4623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Lv Z, Han X, Li S, Han B, Yang Q, Wang X, Wu P, Li J, Deng N, Zhang Z, 2022. Harmful Effects of Inorganic Mercury Exposure on Kidney Cells: Mitochondrial Dynamics Disorder and Excessive Oxidative Stress. Biol Trace Elem Res 200(4), 1591–1597. 10.1007/s12011-021-02766-3. [DOI] [PubMed] [Google Scholar]
- Han J, Wan M, Ma Z, Hu C, Yi H, 2020. Prediction of Targets of Curculigoside A in Osteoporosis and Rheumatoid Arthritis Using Network Pharmacology and Experimental Verification. Drug Des Devel Ther 14, 5235–5250. 10.2147/DDDT.S282112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Jin Y, Zhang Y, Li S, Cui J, He H, Guo L, Yang F, Liu H, 2023. Inhibition of Oncogenic Src Ameliorates Silica-Induced Pulmonary Fibrosis via PI3K/AKT Pathway. Int J Mol Sci 24(1). 10.3390/ijms24010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Yan BY, Chan TC, Krueger JG, 2018. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol 201(6), 1605–1613. 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Shen P, Feng L, Hao H, He Y, Fan G, Liu Z, Zhu K, Wang Y, Zhang N, Hu X, Fu Y, Wu J, 2022. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicol Environ Saf 245, 114123. 10.1016/j.ecoenv.2022.114123. [DOI] [PubMed] [Google Scholar]
- Henschler D, Bonse G, Greim H, 1976. Carcinogenic potential of chlorinated ethylenes tentative molecular rules. IARC Sci Publ (1971)(13), 171–175. [PubMed] [Google Scholar]
- Hoxhaj G, Manning BD, 2020. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 20(2), 74–88. 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YH, Chen YT, Tseng CM, Wu LA, Lin WC, Su VY, Perng DW, Chang SC, Chen YM, Chen TJ, Lee YC, Chou KT, 2015. Sleep disorders and increased risk of autoimmune diseases in individuals without sleep apnea. Sleep 38(4), 581–586. 10.5665/sleep.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Zhang H, Chen J, Wang J, Liu J, Jiang Y, 2021. Targeting Akt in cancer for precision therapy. J Hematol Oncol 14(1), 128. 10.1186/s13045-021-01137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XL, Xu J, Zhang XH, Qiu BY, Peng L, Zhang M, Gan HT, 2011. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm Res 60(8), 727–734. 10.1007/s00011-011-0325-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao H, zhang Y, Tang Y, Shi X, Jiang S, Pu W, Liu J, Ma Y, Lin J, Lin J, Wu W, Gong Y, Wang J, Liu Q, 2023. Enhancement of Zyxin Promotes Skin Fibrosis by Regulating FAK/PI3K/AKT and TGF-beta Signaling Pathways via Integrins. Int J Biol Sci 19(8), 2394–2408. 10.7150/ijbs.77649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Nie S, Wang S, Wang H, Gong J, Yan W, Tian D, Liu M, 2023. Therapeutic Effect of Costunolide in Autoimmune Hepatitis: Network Pharmacology and Experimental Validation. Pharmaceuticals (Basel) 16(2). 10.3390/ph16020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Mishima K, Yamamoto-Yoshida S, Ushikoshi-Nakayama R, Nakagawa Y, Yamamoto K, Ryo K, Ide F, Saito I, 2012. Aryl hydrocarbon receptor-mediated induction of EBV reactivation as a risk factor for Sjogren’s syndrome. J Immunol 188(9), 4654–4662. 10.4049/jimmunol.1101575. [DOI] [PubMed] [Google Scholar]
- Irwin MR, 2019. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 19(11), 702–715. 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Takagi A, Kohashi M, Yamada A, Arakaki R, Kanno J, Hayashi Y, 2009. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol 182(10), 6576–6586. 10.4049/jimmunol.0802289. [DOI] [PubMed] [Google Scholar]
- Ito T, Inouye K, Fujimaki H, Tohyama C, Nohara K, 2002. Mechanism of TCDD-induced suppression of antibody production: effect on T cell-derived cytokine production in the primary immune reaction of mice. Toxicol Sci 70(1), 46–54. 10.1093/toxsci/70.E46. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Spatera P, Khalid Z, Pulliero A, 2022. Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter. Int J Environ Res Public Health 19(17). 10.3390/ijerph191710587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L, Britton J, Lewis SA, McKeever TM, Atherton J, Fullerton D, Fogarty AW, 2009. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter 14(5), 108–113. 10.1111/j.1523-5378.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- Jadali A, Ying YM, Kwan KY, 2017. Activation of CHK1 in Supporting Cells Indirectly Promotes Hair Cell Survival. Front Cell Neurosci 11, 137. 10.3389/fncel.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejczak-Czechowicz M, Lewandowska-Polak A, Jarzebska M, Kowalski ML, 2011. Mast cell and eosinophil activation during early phase of grass pollen-induced ocular allergic reaction. Allergy Asthma Proc 32(1), 43–48. 10.2500/aap.2011.32.3402. [DOI] [PubMed] [Google Scholar]
- Jiang R, Tang J, Zhang X, He Y, Yu Z, Chen S, Xia J, Lin J, Ou Q, 2022. CCN1 Promotes Inflammation by Inducing IL-6 Production via alpha6beta1/PI3K/Akt/NF-kappaB Pathway in Autoimmune Hepatitis. Front Immunol 13, 810671. 10.3389/fimmu.2022.810671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Han YP, Hu M, Bao XQ, Yan Y, Chen G, 2019. A study on regulatory mechanism of miR-223 in ulcerative colitis through PI3K/Akt-mTOR signaling pathway. Eur Rev Med Pharmacol Sci 23(11), 4865–4872. 10.26355/eurrev_201906_18074. [DOI] [PubMed] [Google Scholar]
- Jiang X, Alfredsson L, 2020. Modifiable environmental exposure and risk of rheumatoid arthritis-current evidence from genetic studies. Arthritis Res Ther 22(1), 154. 10.1186/s13075-020-02253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Alonso J, Jimenez-Jaimez J, Almazan MV, 2004. Pollen allergies in patients with systemic lupus erythematosus. J Rheumatol 31(9), 1873; author reply 1873-1874. [PubMed] [Google Scholar]
- Jin C, Gao BB, Zhou WJ, Zhao BJ, Fang X, Yang CL, Wang XH, Xia Q, Liu TT, 2022. Hydroxychloroquine attenuates autoimmune hepatitis by suppressing the interaction of GRK2 with PI3K in T lymphocytes. Front Pharmacol 13, 972397. 10.3389/fphar.2022.972397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog NR, James JA, 2020. Epstein Barr Virus and Autoimmune Responses in Systemic Lupus Erythematosus. Front Immunol 11, 623944. 10.3389/fimmu.2020.623944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Therkelsen SP, Nentwich I, Nissen-Meyer LSH, Hetland G, 2019. IgE-sensitization to food and inhalant allergens in IBD patients compared with normal blood donors at Oslo University Hospital, Norway. Scand J Gastroenterol 54(9), 1107–1110. 10.1080/00365521.2019.1663445. [DOI] [PubMed] [Google Scholar]
- Joo SH, Lee J, Hutchinson D, Song YW, 2019. Prevalence of rheumatoid arthritis in relation to serum cadmium concentrations: cross-sectional study using Korean National Health and Nutrition Examination Survey (KNHANES) data. BMJ Open 9(1), e023233. 10.1136/bmjopen-2018-023233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, DeFilippis AP, Fox ER, Rodriguez CJ, Keith RJ, Benjamin EJ, Butler J, Bhatnagar A, Robertson RM, Winniford MD, Correa A, Hall ME, 2018. Cigarette Smoking and Incident Heart Failure: Insights From the Jackson Heart Study. Circulation 137(24), 2572–2582. 10.1161/CIRCULATIONAHA.117.031912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe M, Lampinen M, Stolt I, Janson C, Stalenheim G, Carlson M, 2012. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflammation 35(1), 230–239. 10.1007/s10753-011-9309-5. [DOI] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MDM, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L, 2013. Mutational landscape and significance across 12 major cancer types. Nature 502(7471), 333–339. 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau HC, Wu SB, Tsai CC, Liu CJ, Wei YH, 2016. Cigarette Smoke Extract-Induced Oxidative Stress and Fibrosis-Related Genes Expression in Orbital Fibroblasts from Patients with Graves’ Ophthalmopathy. Oxid Med Cell Longev 2016, 4676289. 10.1155/2016/4676289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Jeon S, Lee HJ, Lee AY, 2007. Impaired PI3K/Akt activation-mediated NF-kappaB inactivation under elevated TNF-alpha is more vulnerable to apoptosis in vitiliginous keratinocytes. J Invest Dermatol 127(11), 2612–2617. 10.1038/sj.jid.5700900. [DOI] [PubMed] [Google Scholar]
- Kim NH, Lee AY, 2010. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol 130(9), 2231–2239. 10.1038/jid.2010.99. [DOI] [PubMed] [Google Scholar]
- Klareskog L, Gregersen PK, Huizinga TW, 2010. Prevention of autoimmune rheumatic disease: state of the art and future perspectives. Ann Rheum Dis 69(12), 2062–2066. 10.1136/ard.2010.142109. [DOI] [PubMed] [Google Scholar]
- Konigsberg IR, Lin NW, Liao SY, Liu C, MacPhail K, Mroz MM, Davidson E, Restrepo CI, Sharma S, Li L, Maier LA, Yang IV, 2023. Multi-Omic Signatures of Sarcoidosis and Progression in Bronchoalveolar Lavage Cells. bioRxiv. 10.1101/2023.01.26.525601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotik M, Davidova A, Voriskova J, Baldrian P, 2013. Bacterial communities in tetrachloroethene-polluted groundwaters: a case study. Sci Total Environ 454-455, 517–527. 10.1016/j.scitotenv.2013.02.082. [DOI] [PubMed] [Google Scholar]
- Kroos S, Kampstra ASB, Toes REM, Slot LM, Scherer HU, 2022. Absence of Epstein-Barr virus DNA in anti-citrullinated protein antibody-expressing B cells of patients with rheumatoid arthritis. Arthritis Res Ther 24(1), 230. 10.1186/s13075-022-02919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruize AA, Smeenk RJ, Kater L, 1995. Diagnostic criteria and immunopathogenesis of Sjogren’s syndrome: implications for therapy. Immunol Today 16(12), 557–559. 10.1016/0167-5699(95)80075-1. [DOI] [PubMed] [Google Scholar]
- Kucharava K, Sekulic-Jablanovic M, Horvath L, Bodmer D, Petkovic V, 2019. Pasireotide protects mammalian cochlear hair cells from gentamicin ototoxicity by activating the PI3K-Akt pathway. Cell Death Dis 10(2), 110. 10.1038/s41419-019-1386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P, Dasgupta P, Bhat NS, Hashimoto Y, Saini S, Shahryari V, Yamamura S, Shiina M, Tanaka Y, Dahiya R, Majid S, 2020. Role of the PI3K/Akt pathway in cadmium induced malignant transformation of normal prostate epithelial cells. Toxicol Appl Pharmacol 409, 115308. 10.1016/j.taap.2020.115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Patel R, Moore S, Crawford DK, Suwanna N, Mangiardi M, Tiwari-Woodruff SK, 2013. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis 56, 131–144. 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkl M, Sambucci M, Ruggieri S, Amormino C, Tortorella C, Gasperini C, Battistini L, Tuosto L, 2019. CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis. Cells 8(6). 10.3390/cells8060575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, Koyasu S, 2021. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep 34(12), 108886. 10.1016/j.celrep.2021.108886. [DOI] [PubMed] [Google Scholar]
- Kwok YFR, Asti D, Mudduluru BM, Skaradinskiy Y, 2022. Catastrophic antiphospholipid syndrome post-Epstein-Barr virus infection: a case report. Hematol Transfus Cell Ther 44(3), 419–421. 10.1016/j.htct.2020.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R, York M, 2009. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol 21(6), 617–622. 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K, Zhong M, 2018. The Lancet Commission on pollution and health. Lancet 391(10119), 462–512. 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, Fernandes RA, Gomez AM, Nadj GS, Bartley CM, Schubert RD, Hawes IA, Vazquez SE, Iyer M, Zuchero JB, Teegen B, Dunn JE, Lock CB, Kipp LB, Cotham VC, Ueberheide BM, Aftab BT, Anderson MS, DeRisi JL, Wilson MR, Bashford-Rogers RJM, Platten M, Garcia KC, Steinman L, Robinson WH, 2022. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603(7900), 321–327. 10.1038/s41586-022-04432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara B, Rojo J, Costa AR, Burgos-Montero AM, Antunes CM, Perez-Badia R, 2023. Atmospheric pollen allergen load and environmental patterns in central and southwestern Iberian Peninsula. Sci Total Environ 858(Pt 2), 159630. 10.1016/j.scitotenv.2022.159630. [DOI] [PubMed] [Google Scholar]
- Larabi A, Barnich N, Nguyen HTT, 2020. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 16(1), 38–51. 10.1080/15548627.2019.1635384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Burgess S, 2022. Appraising the causal role of smoking in multiple diseases: A systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine 82, 104154. 10.1016/j.ebiom.2022.104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latacz E, Caspani E, Barnhill R, Lugassy C, Verhoef C, Grunhagen D, Van Laere S, Fernandez Moro C, Gerling M, Dirix M, Dirix LY, Vermeulen PB, 2020. Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis 23(1), 43–54. 10.1007/s10456-019-09690-0. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, 2021. Myron Gordon Award paper: Microbes, T-cell diversity and pigmentation. Pigment Cell Melanoma Res 34(2), 244–255. 10.1111/pcmr.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, 2004. The role of PTEN in allergic inflammation. Arch Immunol Ther Exp (Warsz) 52(4), 250–254. [PubMed] [Google Scholar]
- Lescoat A, Ballerie A, Lelong M, Augagneur Y, Morzadec C, Jouneau S, Jego P, Fardel O, Vernhet L, Lecureur V, 2020. Crystalline Silica Impairs Efferocytosis Abilities of Human and Mouse Macrophages: Implication for Silica-Associated Systemic Sclerosis. Front Immunol 11, 219. 10.3389/fimmu.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sun F, Yang Y, Tu H, Cai H, 2022. Gradients of PI(4,5)P(2) and PI(3,5)P(2) Jointly Participate in Shaping the Back State of Dictyostelium Cells. Front Cell Dev Biol 10, 835185. 10.3389/fcell.2022.835185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Min J, Mao X, Wang X, Yang Y, Chen Y, 2018. Edaravone ameliorates experimental autoimmune thyroiditis in rats through HO-1-dependent STAT3/PI3K/Akt pathway. Am J Transl Res 10(7), 2037–2046. [PMC free article] [PubMed] [Google Scholar]
- Li J, Casanova JL, Puel A, 2018. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol 11(3), 581–589. 10.1038/mi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yu Y, Sun Z, Duan J, 2022. A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence. Part Fibre Toxicol 19(1), 67. 10.1186/s12989-022-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Mikrani R, Hu YF, Faran Ashraf Baig MM, Abbas M, Akhtar F, Xu M, 2021. Research progress of phosphatidylinositol 4-kinase and its inhibitors in inflammatory diseases. Eur J Pharmacol 907, 174300. 10.1016/j.ejphar.2021.174300. [DOI] [PubMed] [Google Scholar]
- Liu C, Li L, Ha M, Qi S, Duan P, Yang K, 2015. The PI3K/Akt and ERK pathways elevate thyroid hormone receptor beta1 and TRH receptor to decrease thyroid hormones after exposure to PCB153 and p,p’-DDE. Chemosphere 118, 229–238. 10.1016/j.chemosphere.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Liu S, Ma H, Zhang H, Deng C, Xin P, 2021. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin Immunol 230, 108793. 10.1016/j.clim.2021.108793. [DOI] [PubMed] [Google Scholar]
- Liu S, Yan EZ, Turyk ME, Katta SS, Rasti AF, Lee JH, Alajlouni M, Wallace TE, Catt W, Aikins EA, 2022. A pilot study characterizing tetrachloroethylene exposure with exhaled breath in an impacted community. Environ Pollut 297, 118756. 10.1016/j.envpol.2021.118756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ge P, Lu Z, Yang R, Liu Z, Zhao F, Chen M, 2022. Reproductive toxicity and underlying mechanisms of fine particulate matter (PM(2.5)) on Caenorhabditis elegans in different seasons. Ecotoxicol Environ Saf 248, 114281. 10.1016/j.ecoenv.2022.114281. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao Y, Yu N, Zhang C, Wang S, Ma L, Zhao J, Lohmann R, 2015. Particulate matter, gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) in an urban traffic tunnel of China: Emission from on-road vehicles and gas-particle partitioning. Chemosphere 134, 52–59. 10.1016/j.chemosphere.2015.03.065. [DOI] [PubMed] [Google Scholar]
- Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ, 2016. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 22(2), 146–153. 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord AR, Simms LA, Hanigan K, Sullivan R, Hobson P, Radford-Smith GL, 2018. Protective effects of Helicobacter pylori for IBD are related to the cagA-positive strain. Gut 67(2), 393–394. 10.1136/gutjnl-2017-313805. [DOI] [PubMed] [Google Scholar]
- Lou H, Wu LQ, Wang H, Wei RL, Cheng JW, 2021. The Potential Role of Osteopontin in the Pathogenesis of Graves’ Ophthalmopathy. Invest Ophthalmol Vis Sci 62(12), 18. 10.1167/iovs.62.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugg ST, Scott A, Parekh D, Naidu B, Thickett DR, 2022. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax 77(1), 94–101. 10.1136/thoraxjnl-2020-216296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Liu Y, Wang C, Gan R, 2021. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int 21(1), 93. 10.1186/s12935-021-01793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JD, Jing J, Wang JW, Yan T, Li QH, Mo YQ, Zheng DH, Gao JL, Nguyen KA, Dai L, 2019. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther 21(1), 153. 10.1186/s13075-019-1935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Cai X, Wang Z, Huang L, Wang C, Jiang S, Hua Y, Liu Q, 2017. miR-200c Accelerates Hepatic Stellate Cell-Induced Liver Fibrosis via Targeting the FOG2/PI3K Pathway. Biomed Res Int 2017, 2670658. 10.1155/2017/2670658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Su Q, Yue C, Zou H, Zhu J, Zhao H, Song R, Liu Z, 2022. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int J Mol Sci 23(21). 10.3390/ijms232113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson J, Lin XP, Dahlman-Hoglund A, Hanson LL, Telemo E, Magnusson O, Bengtsson U, Ahlstedt S, 2003. Seasonal intestinal inflammation in patients with birch pollen allergy. J Allergy Clin Immunol 112(1), 45–50. 10.1067/mai.2003.1604. [DOI] [PubMed] [Google Scholar]
- Majka DS, Holers VM, 2006. Cigarette smoking and the risk of systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis 65(5), 561–563. 10.1136/ard.2005.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott GC, Sparks JA, 2023. Invited Perspective: Air Pollutants, Genetics, and the Mucosal Paradigm for Rheumatoid Arthritis Risk. Environ Health Perspect 131(3), 31303. 10.1289/EHP12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead MN, 2008. Cancer and TCDD: the mitochondrial connection. Environ Health Perspect 116(3), A112. 10.1289/ehp.116-a112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret C, Molina R, Filella X, Garcia-Carrasco M, Claver G, Ingelmo M, Ballesta A, Font J, 2003. Relationship of p53 with other oncogenes, cytokines and systemic lupus erythematosus activity. Tumour Biol 24(4), 185–188. 10.1159/000074428. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Chu C, Carlin DJ, 2015. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145(1), 5–15. 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Patters BJ, Midde NM, He H, Kumar S, Cory TJ, 2018. Tobacco and Antiretrovirals Modulate Transporter, Metabolic Enzyme, and Antioxidant Enzyme Expression and Function in Polarized Macrophages. Curr HIV Res 16(5), 354–363. 10.2174/1570162X17666190130114531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D, Sinha-Royle E, Bellis K, Harrington C, Hutchinson D, 2019. Nodular rheumatoid arthritis (RA): A distinct disease subtype, initiated by cadmium inhalation inducing pulmonary nodule formation and subsequent RA-associated autoantibody generation. Med Hypotheses 122, 48–55. 10.1016/j.mehy.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Colafrancesco S, Pipi E, Kollert F, Hunter KJ, Brewer C, Buckley CD, Bowman SJ, Priori R, Valesini G, Juarez M, Fahy WA, Fisher BA, Payne A, Allen RA, Barone F, 2018. Phosphatidylinositol 3-kinase delta pathway: a novel therapeutic target for Sjogren’s syndrome. Ann Rheum Dis 78(2), 249–260. 10.1136/annrheumdis-2017-212619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer K, Matusiewicz M, Bednarz-Misa I, Gorska S, Gamian A, Krzystek-Korpacka M, 2018. Diagnostic Potential of Systemic Eosinophil-Associated Cytokines and Growth Factors in IBD. Gastroenterol Res Pract 2018, 7265812. 10.1155/2018/7265812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Davidovici B, Krueger JG, 2010. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg 29(1), 3–9. 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J, Koike R, Sugihara T, Hagiyama H, Nishio J, Kohsaka H, Kubota T, Kawachi H, Kasahara I, Miyasaka N, 2002. [An autopsied case of chronic active Epstein-Barr virus infection complicated in systemic lupus erythematosus and antiphospholipid antibody syndrome]. Nihon Rinsho Meneki Gakkai Kaishi 25(6), 458–465. 10.2177/jsci.25.458. [DOI] [PubMed] [Google Scholar]
- Olsson T, Barcellos LF, Alfredsson L, 2017. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13(1), 25–36. 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- Opstelten JL, Beelen RMJ, Leenders M, Hoek G, Brunekreef B, van Schaik FDM, Siersema PD, Eriksen KT, Raaschou-Nielsen O, Tjonneland A, Overvad K, Boutron-Ruault MC, Carbonnel F, de Hoogh K, Key TJ, Luben R, Chan SSM, Hart AR, Bueno-de-Mesquita HB, Oldenburg B, 2016. Exposure to Ambient Air Pollution and the Risk of Inflammatory Bowel Disease: A European Nested Case-Control Study. Dig Dis Sci 61(10), 2963–2971. 10.1007/s10620-016-4249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhurhu VJ, Pittelkow TP, Hooten WM, 2015. Prevalence of smoking in adults with chronic pain. Tob Induc Dis 13(1), 17. 10.1186/s12971-015-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paduraru E, Iacob D, Rarinca V, Rusu A, Jijie R, Ilie OD, Ciobica A, Nicoara M, Doroftei B, 2022. Comprehensive Review Regarding Mercury Poisoning and Its Complex Involvement in Alzheimer’s Disease. Int J Mol Sci 23(4). 10.3390/ijms23041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Doble PA, Bishop DP, 2021. Mercury in the human thyroid gland: Potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PLoS One 16(2), e0246748. 10.1371/journal.pone.0246748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Lu MP, Wang JH, Xu M, Yang SR, 2020. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr 16(1), 19–30. 10.1007/s12519-019-00229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZK, Zuraw BL, Lung CC, Prossnitz ER, Browning DD, Ye RD, 1996. Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts. J Clin Invest 98(9), 2042–2049. 10.1172/JCn19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S, 2009. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol 10(2), 158–166. 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RK, Mohan C, 2005. PI3K/AKT signaling and systemic autoimmunity. Immunol Res 31(1), 47–55. 10.1385/IR:31:1:47. [DOI] [PubMed] [Google Scholar]
- Pereira P, Korbas M, Pereira V, Cappello T, Maisano M, Canario J, Almeida A, Pacheco M, 2019. A multidimensional concept for mercury neuronal and sensory toxicity in fish - From toxicokinetics and biochemistry to morphometry and behavior. Biochim Biophys Acta Gen Subj 1863(12), 129298. 10.1016/j.bbagen.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Perricone C, Versini M, Ben-Ami D, Gertel S, Watad A, Segel MJ, Ceccarelli F, Conti F, Cantarini L, Bogdanos DP, Antonelli A, Amital H, Valesini G, Shoenfeld Y, 2016. Smoke and autoimmunity: The fire behind the disease. Autoimmun Rev 15(4), 354–374. 10.1016/j.autrev.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL, 1993. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol 23(3), 283–335. 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Pham T, Williams JVA, Bhattarai A, Dores AK, Isherwood LJ, Patten SB, 2018. Electronic cigarette use and mental health: A Canadian population-based study. J Affect Disord 260, 646–652. 10.1016/jjad.2019.09.026. [DOI] [PubMed] [Google Scholar]
- Pinho JF, Medeiros MA, Capettini LS, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS, 2010. Phosphatidylinositol 3-kinase-delta up-regulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br J Pharmacol 161(7), 1458–1471. 10.1111/j.1476-5381.2010.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky DS, 2023. Pathogenesis of autoimmune disease. Nat Rev Nephrol, 1–16. 10.1038/s41581-023-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverino F, Wu TD, Rojas-Quintero J, Wang X, Mayo J, Tomchaney M, Tram J, Packard S, Zhang D, Cleveland KH, Cordoba-Lanus E, Owen CA, Fawzy A, Kinney GL, Hersh CP, Hansel NN, Doubleday K, Sauler M, Tesfaigzi Y, Ledford JG, Casanova C, Zmijewski J, Konhilas J, Langlais PR, Schnellmann R, Rahman I, McCormack M, Celli B, 2021. Metformin: Experimental and Clinical Evidence for a Potential Role in Emphysema Treatment. Am J Respir Crit Care Med 204(6), 651–666. 10.1164/rccm.202012-4510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathapan KM, Ramos Rivers C, Anderson A, Koutroumpakis F, Koutroubakis IE, Babichenko D, Tan X, Tang G, Schwartz M, Proksell S, Johnston E, Hashash JG, Dunn M, Wilson A, Barrie A, Harrison J, Hartman D, Kim SC, Binion DG, 2020. Peripheral Blood Eosinophilia and Long-term Severity in Pediatric-Onset Inflammatory Bowel Disease. Inflamm Bowel Dis 26(12), 1890–1900. 10.1093/ibd/izz323. [DOI] [PubMed] [Google Scholar]
- Prisco LC, Martin LW, Sparks JA, 2020. Inhalants other than personal cigarette smoking and risk for developing rheumatoid arthritis. Curr Opin Rheumatol 32(3), 279–288. 10.1097/BOR.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue MP, 2013. Trichloroethylene and cancer. J Natl Cancer Inst 105(12), 844–846. 10.1093/jnci/djt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z, 2017. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 8(1), 268–284. 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad F, Ghorbani M, Mohammadi Roushandeh A, Habibi Roudkenar M, 2019. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep 46(1), 1533–1549. 10.1007/s11033-019-04588-y. [DOI] [PubMed] [Google Scholar]
- Rademaker M, Agnew K, Anagnostou N, Andrews M, Armour K, Baker C, Foley P, Gebauer K, Gupta M, Marshman G, Rubel D, Sullivan J, Wong LC, 2019. Psoriasis and infection. A clinical practice narrative. Australas J Dermatol 60(2), 91–98. 10.1111/ajd.12895. [DOI] [PubMed] [Google Scholar]
- Ring J, Belloni B, Behrendt H, 2009. Looking ahead in dermatology: skin and allergy. Actas Dermosifiliogr 100 Suppl 2, 32–39. 10.1016/s0001-7310(09)73376-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rabassa M, Lopez P, Rodriguez-Santiago RE, Cases A, Felici M, Sanchez R, Yamamura Y, Rivera-Amill V, 2018. Cigarette Smoking Modulation of Saliva Microbial Composition and Cytokine Levels. Int J Environ Res Public Health 15(11). 10.3390/ijerph15112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan JS, Sun RJ, Wang JP, Sui XH, Qu HT, Yuan D, Shan NN, 2023. Gene mutations in the PI3K/Akt signaling pathway were related to immune thrombocytopenia pathogenesis. Medicine (Baltimore) 102(7), e32947. 10.1097/MD.0000000000032947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge SM, Asgharpour A, 2020. Smoking and Liver Disease. Gastroenterol Hepatol (N Y) 16(12), 617–625. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roman J, Wichmann I, Salaberri J, Varela JM, Nunez-Roldan A, 1993. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis 52(7), 534–538. 10.1136/ard.52.7.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Overduin M, Emr SD, 2001. Location, location, location: membrane targeting directed by PX domains. Science 294(5548), 1881–1885. 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- Sausen DG, Bhutta MS, Gallo ES, Dahari H, Borenstein R, 2021. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 11(9). 10.3390/biom11091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenas J, Hedblom A, Miftakhova RR, Sarwar M, Larsson R, Shcherbina L, Johansson ME, Harkonen P, Sterner O, Persson JL, 2014. The role of PI3K/AKT-related PIP5K1alpha and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc Natl Acad Sci U S A 111(35), E3689–3698. 10.1073/pnas.1405801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk Z, 2013. Analysis of carcinogenic Polycyclic Aromatic Hydrocarbons (PAHS): an overview of modern electroanalytical techniques and their applications. Curr Drug Deliv 10(1), 76–91. 10.2174/1567201811310010014. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Emre NC, Romesser PB, Staudt LM, 2009. IRF4: Immunity. Malignancy! Therapy? Clin Cancer Res 15(9), 2954–2961. 10.1158/1078-0432.CCR-08-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan NN, Dong LL, Zhang XM, Liu X, Li Y, 2016. Targeting autophagy as a potential therapeutic approach for immune thrombocytopenia therapy. Crit Rev Oncol Hematol 100, 11–15. 10.1016/j.critrevonc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Shannon-Lowe C, Rickinson A, 2019. The Global Landscape of EBV-Associated Tumors. Front Oncol 9, 713. 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermuller J, Reinhardt R, Stadler PF, Vogel J, 2010. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464(7286), 250–255. 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- Sheema K, Ikramdin U, Arshi N, Farah N, Imran S, 2017. Role of Helicobacter pylori Eradication Therapy on Platelet Recovery in Chronic Immune Thrombocytopenic Purpura. Gastroenterol Res Pract 2017, 9529752. 10.1155/2017/9529752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YM, Troxel AB, Vedantam S, Penning TM, Field J, 2006. Comparison of p53 mutations induced by PAH o-quinones with those caused by anti-benzo[a]pyrene diol epoxide in vitro: role of reactive oxygen and biological selection. Chem Res Toxicol 19(11), 1441–1450. 10.1021/tx0601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H, 2011. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208(7), 1367–1376. 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura H, Terada Y, Okado T, Tanaka H, Inoshita S, Sasaki S, 2003. The PI3-kinase-Akt pathway promotes mesangial cell survival and inhibits apoptosis in vitro via NF-kappa B and Bad. J Am Soc Nephrol 14(6), 1427–1434. 10.1097/01.asn.0000066140.99610.32. [DOI] [PubMed] [Google Scholar]
- Shukla S, Khan R, Bhattacharya P, Devanesan S, AlSalhi MS, 2022. Concentration, source apportionment and potential carcinogenic risks of polycyclic aromatic hydrocarbons (PAHs) in roadside soils. Chemosphere 292, 133413. 10.1016/j.chemosphere.2021.133413. [DOI] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti P, 2008. Primary peripheral T cells become susceptible to 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated apoptosis in vitro upon activation and in the presence of dendritic cells. Mol Pharmacol 73(6), 1722–1735. 10.1124/mol.107.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan SS, Lieberman PM, 2023. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol 21(1), 51–64. 10.1038/s41579-022-00770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyucen E, Gulcan A, Aktuglu-Zeybek AC, Onal H, Kiykim E, Aydin A, 2014. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int 56(3), 336–343. 10.1111/ped.12243. [DOI] [PubMed] [Google Scholar]
- Sparber F, LeibundGut-Landmann S, 2019. Interleukin-17 in Antifungal Immunity. Pathogens 8(2). 10.3390/pathogens8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JA, Karlson EW, 2016. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep 18(3), 15. 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AK, Sriskantharajah S, Hessel EM, Okkenhaug K, 2015. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol 23, 82–91. 10.1016/j.coph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi R, Provan D, 2008. Helicobacter pylori and Chronic ITP. Hematology Am Soc Hematol Educ Program, 206–211. 10.1182/asheducation-2008.1.206. [DOI] [PubMed] [Google Scholar]
- Steinkamp M, Geerling I, Seufferlein T, von Boyen G, Egger B, Grossmann J, Ludwig L, Adler G, Reinshagen M, 2003. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 124(7), 1748–1757. 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Stojan G, Kvit A, Curriero FC, Petri M, 2020. A Spatiotemporal Analysis of Organ-Specific Lupus Flares in Relation to Atmospheric Variables and Fine Particulate Matter Pollution. Arthritis Rheumatol 72(7), 1134–1142. 10.1002/art.41217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk E, Kulms D, 2013. The role of AKT/mTOR pathway in stress response to UV-irradiation: implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence. Int J Mol Sci 14(8), 15260–15285. 10.3390/ijms140815260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Fueyo A, Rojas JM, Cariaga AE, Garcia E, Steiner BH, Barber DF, Puri KD, Carrera AC, 2014. Inhibition of PI3Kdelta reduces kidney infiltration by macrophages and ameliorates systemic lupus in the mouse. J Immunol 193(2), 544–554. 10.4049/jimmunol.1400350. [DOI] [PubMed] [Google Scholar]
- Suerbaum S, Michetti P, 2002. Helicobacter pylori infection. N Engl J Med 347(15), 1175–1186. 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- Sun P, Meng LH, 2020. Emerging roles of class I PI3K inhibitors in modulating tumor microenvironment and immunity. Acta Pharmacol Sin 41(11), 1395–1402. 10.1038/s41401-020-00500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund KG, Carlo GL, Crouch RL, Senefelder BC, 1993. Background soil concentrations of phenolic compounds, chlorinated herbicides, PCDDs and PCDFs in the Melbourne metropolitan area. Aust J Public Health 17(2), 157–161. 10.1111/j.1753-6405.1993.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, Berg DE, Sasakawa C, 2009. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe 5(1), 23–34. 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hidaka T, Kumagai Y, Yamamoto M, 2020. Environmental pollutants and the immune response. Nat Immunol 21(12), 1486–1495. 10.1038/s41590-020-0802-6. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ohsugi K, Ono Y, 1992. EBV increases phosphoinositide kinase activities in human B cells. J Immunol 149(1), 207–213. [PubMed] [Google Scholar]
- Svendsen AJ, Westergaard MCW, Draborg AH, Holst R, Kyvik KO, Jakobsen MA, Junker P, Houen G, 2021. Altered Antibody Response to Epstein-Barr Virus in Patients With Rheumatoid Arthritis and Healthy Subjects Predisposed to the Disease. A Twin Study. Front Immunol 12, 650713. 10.3389/fimmu.2021.650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki A, Hayashi H, Nakajima H, Takii T, Katagiri D, Miyazawa K, Hirose K, Onozaki K, 2004. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol Pharm Bull 27(3), 407–410. 10.1248/bpb.27.407. [DOI] [PubMed] [Google Scholar]
- Tan WJ, Tan QY, Wang T, Lian M, Zhang L, Cheng ZS, 2017. Calpain 1 regulates TGF-beta1-induced epithelial-mesenchymal transition in human lung epithelial cells via PI3K/Akt signaling pathway. Am J Transl Res 9(3), 1402–1409. [PMC free article] [PubMed] [Google Scholar]
- Tan X, Sun Y, Thapa N, Liao Y, Hedman AC, Anderson RA, 2015a. LAPTM4B is a PtdIns(4,5)P2 effector that regulates EGFR signaling, lysosomal sorting, and degradation. EMBO J 34(4), 475–490. 10.15252/embj.201489425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Thapa N, Sun Y, Anderson RA, 2015b. A kinase-independent role for EGF receptor in autophagy initiation. Cell 160(1-2), 145–160. 10.1016/j.cell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Fan Y, Ma J, Lu W, Liu N, Chen Y, Pan W, Tao X, 2021. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 10(5). 10.3390/cells10051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therachiyil L, Krishnankutty R, Ahmad F, Mateo JM, Uddin S, Korashy HM, 2022. Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, beta-Catenin, and Epithelial to Mesenchymal Transition Pathways. Int J Mol Sci 23(12). 10.3390/ijms23126395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble EK, Ivanova PT, Grabon A, Alb JG Jr., Faenza I, Cocco L, Brown HA, Bankaitis VA, 2016. Quantitative profiling of the endonuclear glycerophospholipidome of murine embryonic fibroblasts. J Lipid Res 57(8), 1492–1506. 10.1194/jlr.M068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart H, Postma DS, Timens W, Hylkema MN, Willemse BW, Boezen HM, Vonk JM, de Reus DM, Kauffman HF, ten Hacken NH, 2005. Acute effects of cigarette smoking on inflammation in healthy intermittent smokers. Respir Res 6(1), 22. 10.1186/1465-9921-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gundy K, Sharma OP, 1987. Pathogenesis of sarcoidosis. West J Med 147(2), 168–174. [PMC free article] [PubMed] [Google Scholar]
- Vanegas YAM, Vishnu P, 2019. Management of Helicobacter pylori in Patients with Immune Thrombocytopenia. Hamostaseologie 39(3), 279–283. 10.1055/s-0039-1683974. [DOI] [PubMed] [Google Scholar]
- Vega Miranda J, Pinto Penaranda LF, Marquez Hernandez JD, Velasquez Franco CJ, 2014. Microscopic polyangiitis secondary to silica exposure. Reumatol Clin 10(3), 180–182. 10.1016/j.reuma.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Vondracek J, Pencikova K, Ciganek M, Pivnicka J, Karasova M, Hyzdalova M, Strapacova S, Palkova L, Neca J, Matthews J, Lom MV, Topinka J, Milcova A, Machala M, 2020. Environmental six-ring polycyclic aromatic hydrocarbons are potent inducers of the AhR-dependent signaling in human cells. Environ Pollut 266(Pt 2), 115125. 10.1016/j.envpol.2020.115125. [DOI] [PubMed] [Google Scholar]
- Vukmirovic M, Yan X, Gibson KF, Gulati M, Schupp JC, DeIuliis G, Adams TS, Hu B, Mihaljinec A, Woolard TN, Lynn H, Emeagwali N, Herzog EL, Chen ES, Morris A, Leader JK, Zhang Y, Garcia JGN, Maier LA, Collman RG, Drake WP, Becich MJ, Hochheiser H, Wisniewski SR, Benos PV, Moller DR, Prasse A, Koth LL, Kaminski N, Investigators G, 2021. Transcriptomics of bronchoalveolar lavage cells identifies new molecular endotypes of sarcoidosis. Eur Respir J 58(6). 10.1183/13993003.02950-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Lin F, Zhang W, Xu A, DeGiorgis J, Lu H, Wan Y, 2017. Novel approaches to vitiligo treatment via modulation of mTOR and NF-kappaB pathways in human skin melanocytes. Int J Biol Sci 13(3), 391–400. 10.7150/ijbs.17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Ma S, Bi SJ, Su L, Huang SY, Miao JY, Ma CH, Gao CJ, Hou M, Peng J, 2019. Enhancing autophagy protects platelets in immune thrombocytopenia patients. Ann Transl Med 7(7), 134. 10.21037/atm.2019.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Konig R, Ansari GA, Khan MF, 2008. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic Biol Med 44(7), 1475–1482. 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ma H, Wang J, Khan MF, 2019. Contribution of poly(ADP-ribose)polymerase-1 activation and apoptosis in trichloroethene-mediated autoimmunity. Toxicol Appl Pharmacol 362, 28–34. 10.1016/j.taap.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Luo X, Ansari GA, Khan MF, 2014. Nitrosative stress and nitrated proteins in trichloroethene-mediated autoimmunity. PLoS One 9(6), e98660. 10.1371/journal.pone.0098660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Banerjee N, Wang G, Firoze Khan M, 2023. Autophagy dysregulation in trichloroethene-mediated inflammation and autoimmune response. Toxicology 487, 153468. 10.1016/j.tox.2023.153468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang G, Liang Y, Du X, Boor PJ, Sun J, Khan MF, 2019. Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity. Free Radic Biol Med 143, 223–231. 10.1016/j.freeradbiomed.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ren J, Li G, Moorman JP, Yao ZQ, Ning S, 2017. LMP1 signaling pathway activates IRF4 in latent EBV infection and a positive circuit between PI3K and Src is required. Oncogene 36(16), 2265–2274. 10.1038/onc.2016.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang FS, Gershwin ME, 2015. Human autoimmune diseases: a comprehensive update. J Intern Med 278(4), 369–395. 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen Y, Hu H, Fan C, Zhang A, Ding R, Ye B, Xiang M, 2020. Systematic Transcriptome Analysis of Noise-Induced Hearing Loss Pathogenesis Suggests Inflammatory Activities and Multiple Susceptible Molecules and Pathways. Front Genet 11, 968. 10.3389/fgene.2020.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Huang Z, Lei Y, Han X, Tian D, Gong J, Liu M, 2022. Celastrol Alleviates Autoimmune Hepatitis Through the PI3K/AKT Signaling Pathway Based on Network Pharmacology and Experiments. Front Pharmacol 13, 816350. 10.3389/fphar.2022.816350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Boyken SE, Hu J, Xu X, Rimer RP, Shea MA, Shaw AS, Andreotti AH, Huang YH, 2014. Calmodulin and PI(3,4,5)P(3) cooperatively bind to the Itk pleckstrin homology domain to promote efficient calcium signaling and IL-17A production. Sci Signal 7(337), ra74. 10.1126/scisignal.2005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhong Y, Liao J, Wang G, 2021. PM2.5-related cell death patterns. Int J Med Sci 18(4), 1024–1029. 10.7150/ijms.46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Hariharan A, Bastianello G, Toyama Y, Shivashankar GV, Foiani M, Sheetz MP, 2017. DNA damage causes rapid accumulation of phosphoinositides for ATR signaling. Nat Commun 8(1), 2118. 10.1038/s41467-017-01805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Ho TLF, Hariharan A, Goh HC, Wong YL, Verkaik NS, Lee MY, Tam WL, van Gent DC, Venkitaraman AR, Sheetz MP, Lane DP, 2022. Rapid recruitment of p53 to DNA damage sites directs DNA repair choice and integrity. Proc Natl Acad Sci U S A 119(10), e2113233119. 10.1073/pnas.2113233119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal SA, Godri-Pollitt K, Villeneuve PJ, 2013. PM2.5, oxidant defence and cardiorespiratory health: a review. Environ Health 12, 40. 10.1186/1476-069X-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller PF, Spencer LA, 2017. Functions of tissue-resident eosinophils. Nat Rev Immunol 17(12), 746–760. 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA, 2003. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111(1), 81–90. 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley SK, Li M, Kashem SW, Hirai T, Igyarto BZ, Knizner K, Ho J, Ferris LK, Weaver CT, Cua DJ, McGeachy MJ, Kaplan DH, 2022. Local IL-23 is required for proliferation and retention of skin-resident memory T(H)17 cells. Sci Immunol 7(77), eabq3254. 10.1126/sciimmunol.abq3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AF, Novey HS, Berke RA, Surprenant EL, 1973. Deposition of inhaled pollen and pollen extract in human airways. N Engl J Med 288(20), 1056–1058. 10.1056/NEJM197305172882006. [DOI] [PubMed] [Google Scholar]
- Woeller CF, Roztocil E, Hammond C, Feldon SE, 2019. TSHR Signaling Stimulates Proliferation Through PI3K/Akt and Induction of miR-146a and miR-155 in Thyroid Eye Disease Orbital Fibroblasts. Invest Ophthalmol Vis Sci 60(13), 4336–4345. 10.1167/iovs.19-27865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska-Durczynska K, Krawczyk-Rusiecka K, Cyniak-Magierska A, Zygmunt A, Sporny S, Lewinski A, 2012. The role of phosphoinositide 3-kinase subunits in chronic thyroiditis. Thyroid Res 5(1), 22. 10.1186/1756-6614-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SJ, Estadt SN, Gudjonsson JE, Kahlenberg JM, 2018. Human and Murine Evidence for Mechanisms Driving Autoimmune Photosensitivity. Front Immunol 9, 2430. 10.3389/fimmu.2018.02430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MC, Huang JY, Chen HH, Wei JC, 2020. Effect of early eradication therapy on systemic lupus erythematosus risk in patients with Helicobacter pylori infection: a nationwide population-based cohort study. Lupus 29(7), 751–760. 10.1177/0961203320923393. [DOI] [PubMed] [Google Scholar]
- Wu N, Yuan T, Yin Z, Yuan X, Sun J, Wu Z, Zhang Q, Redshaw C, Yang S, Dai X, 2022. Network Pharmacology and Molecular Docking Study of the Chinese Miao Medicine Sidaxue in the Treatment of Rheumatoid Arthritis. Drug Des Devel Ther 16, 435–466. 10.2147/DDDT.S330947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Alexander JS, Metcalf JP, 2022. In Vivo and In Vitro Studies of Cigarette Smoke Effects on Innate Responses to Influenza Virus: A Matter of Models? Viruses 14(8). 10.3390/v14081824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XX, Huang XL, Chen RR, Li T, Ye HJ, Xie W, Huang ZM, Cao GZ, 2019. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 42(6), 2215–2225. 10.1007/s10753-019-01085-z. [DOI] [PubMed] [Google Scholar]
- Xia Y, Jiang B, Teng Z, Liu T, Wang J, Aniagu S, Zhang G, Chen T, Jiang Y, 2022. Cx43 overexpression is involved in the hyper-proliferation effect of trichloroethylene on human embryonic stem cells. Toxicology 465, 153065. 10.1016/j.tox.2021.153065. [DOI] [PubMed] [Google Scholar]
- Xie C, Yi J, Lu J, Nie M, Huang M, Rong J, Zhu Z, Chen J, Zhou X, Li B, Chen H, Lu N, Shu X, 2018. N-Acetylcysteine Reduces ROS-Mediated Oxidative DNA Damage and PI3K/Akt Pathway Activation Induced by Helicobacter pylori Infection. Oxid Med Cell Longev 2018, 1874985. 10.1155/2018/1874985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Cheng W, Yu Y, Chen J, Zhang X, Shao S, 2021. Diosgenin From Dioscorea Nipponica Rhizoma Against Graves’ Disease-On Network Pharmacology and Experimental Evaluation. Front Pharmacol 12, 806829. 10.3389/fphar.2021.806829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi S, Iizumi T, Watanabe E, Shimizu M, Kamiya S, Nagata K, Kumagai Y, Fukunaga Y, Takahashi H, 2006. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect Immun 74(1), 248–256. 10.1128/IAI.74.1.248-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Allen DC, 2021. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 11(11). 10.3390/biom11111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Yang GD, Huang TJ, Li R, Chu QQ, Xu L, Wang MS, Cai MD, Zhong L, Wei HJ, Huang HB, Huang JL, Qian CN, Huang BJ, 2016. EB-virus latent membrane protein 1 potentiates the stemness of nasopharyngeal carcinoma via preferential activation of PI3K/AKT pathway by a positive feedback loop. Oncogene 35(26), 3419–3431. 10.1038/onc.2015.402. [DOI] [PubMed] [Google Scholar]
- Yang DC, Chen CH, 2018. Cigarette Smoking-Mediated Macrophage Reprogramming: Mechanistic Insights and Therapeutic Implications. J Nat Sci 4(11). [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Wang F, Luo Z, Guo S, Strahle U, 2020. Toxicity of mercury: Molecular evidence. Chemosphere 245, 125586. 10.1016/j.chemosphere.2019.125586. [DOI] [PubMed] [Google Scholar]
- Yariwake VY, Torres JI, Dos Santos ARP, Freitas SCF, De Angelis K, Farhat SCL, Camara NOS, Veras MM, 2021. Chronic exposure to PM2.5 aggravates SLE manifestations in lupus-prone mice. Part Fibre Toxicol 18(1), 15. 10.1186/s12989-021-00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Xi X, Fan D, Cao X, Wang Q, Wang X, Zhang M, Wang B, Tao Q, Xiao C, 2022. Polycyclic aromatic hydrocarbons in bone homeostasis. Biomed Pharmacother 146, 112547. 10.1016/j.biopha.2021.112547. [DOI] [PubMed] [Google Scholar]
- Yi MK, Choi WJ, Han SW, Song SH, Lee DH, Kyung SY, Han SH, 2016. Overlap syndrome with Sjogren’s syndrome and systemic sclerosis in a steel rolling mill worker: a case report. Ann Occup Environ Med 28, 24. 10.1186/s40557-016-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HK, Park H, Hwang HS, Kim HJ, Choi YH, Kook KH, 2021. Ganglioside GT1b increases hyaluronic acid synthase 2 via PI3K activation with TLR2 dependence in orbital fibroblasts from thyroid eye disease patients. BMB Rep 54(2), 136–141. 10.5483/BMBRep.2021.54.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Minagawa S, Araya J, Sakamoto T, Hara H, Tsubouchi K, Hosaka Y, Ichikawa A, Saito N, Kadota T, Sato N, Kurita Y, Kobayashi K, Ito S, Utsumi H, Wakui H, Numata T, Kaneko Y, Mori S, Asano H, Yamashita M, Odaka M, Morikawa T, Nakayama K, Iwamoto T, Imai H, Kuwano K, 2019. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun 10(1), 3145. 10.1038/s41467-019-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You R, Ho YS, Chang RC, 2022. The pathogenic effects of particulate matter on neurodegeneration: a review. J Biomed Sci 29(1), 15. 10.1186/s12929-022-00799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YY, Jin H, Lu Q, 2022. Effect of polycyclic aromatic hydrocarbons on immunity. J Transl Autoimmun 5, 100177. 10.1016/jjtauto.2022.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M, Muslim M, Jehangir A, 2022. Environmental noise-induced cardiovascular, metabolic and mental health disorders: a brief review. Environ Sci Pollut Res Int 29(51), 76485–76500. 10.1007/s11356-022-22351-y. [DOI] [PubMed] [Google Scholar]
- Zeng P, Jiang Z, Huang Z, Huang Y, Xu H, Chen C, Ma W, 2022a. PI3K/AKT/mTOR Signaling Pathway Is Downregulated by Runzaoling (RZL) in Sjogren’s Syndrome. Mediators Inflamm 2022, 7236118. 10.1155/2022/7236118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P, Liu W, Yang X, Zhang S, Du S, Fan Y, Zhao L, Wang A, 2022b. Qing Zao Fang (QZF) Alleviates the Inflammatory Microenvironment of the Submandibular Gland in Sjogren’s Syndrome Based on the PI3K/Akt/HIF-1alpha/VEGF Signaling Pathway. Dis Markers 2022, 6153459. 10.1155/2022/6153459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zentilin P, Seriolo B, Dulbecco P, Caratto E, Iiritano E, Fasciolo D, Bilardi C, Mansi C, Testa E, Savarino V, 2002. Eradication of Helicobacter pylori may reduce disease severity in rheumatoid arthritis. Aliment Pharmacol Ther 16(7), 1291–1299. 10.1046/j.1365-2036.2002.01284.x. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zou T, Guo D, Wang Q, Shen Y, Hu H, Ye B, Xiang M, 2020. The Immune System Can Hear Noise. Front Immunol 11, 619189. 10.3389/fimmu.2020.619189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Ma H, Wang ZL, Li WH, Liu H, Zhao YX, 2020a. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J Int Med Res 48(7), 300060520927919. 10.1177/0300060520927919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang W, Niu J, Yang G, Luo J, Lan D, Wu J, Li M, Sun Y, Wang K, Miao Y, 2020b. Heat-shock transcription factor 2 promotes sodium butyrate-induced autophagy by inhibiting mTOR in ulcerative colitis. Exp Cell Res 388(1), 111820. 10.1016/j.yexcr.2020.111820. [DOI] [PubMed] [Google Scholar]
- Zhang G, He C, Wu Q, Xu G, Kuang M, Wang T, Xu L, Zhou H, Yuan W, 2021. Impaired Autophagy Induced by oxLDL/beta2GPI/anti-beta2GPI Complex through PI3K/AKT/mTOR and eNOS Signaling Pathways Contributes to Endothelial Cell Dysfunction. Oxid Med Cell Longev 2021, 6662225. 10.1155/2021/6662225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zheng D, Yan Y, Yu Y, Chen R, Li Z, Greer PA, Peng T, Wang Q, 2022. Myeloid cell-specific deletion of Capns1 prevents macrophage polarization toward the M1 phenotype and reduces interstitial lung disease in the bleomycin model of systemic sclerosis. Arthritis Res Ther 24(l), 148. 10.1186/sl3075-022-02833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TP, Dou J, Wang L, Wang S, Wang P, Zhou XH, Yang CM, Li XM, 2022. Exposure to particulate pollutant increases the risk of hospitalizations for Sjogren’s syndrome. Front Immunol 13, 1059981. 10.3389/fimmu.2022.1059981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Qi L, Wang Y, Li X, Li Q, Tang X, Wang X, Wu C, 2021. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol Environ Saf 222, 112529. 10.1016/j.ecoenv.2021.112529. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sun Y, Shi P, Dong JN, Zuo LG, Wang HG, Gong JF, Li Y, Gu L, Li N, Li JS, Zhu WM, 2015. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int Immunopharmacol 26(1), 221–228. 10.1016/j.intimp.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Han P, Liu L, Wang X, Xu P, Wang H, Yu T, Sun Y, Li L, Sun T, Liu X, Zhou H, Qiu J, Wang L, Peng J, Xu S, Hou M, 2019. Indirubin modulates CD4(+) T-cell homeostasis via PD1/PTEN/AKT signalling pathway in immune thrombocytopenia. J Cell Mol Med 23(3), 1885–1898. 10.1111/jcmm.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Dai H, Jiang H, Rui H, Liu W, Dong Z, Zhang N, Zhao Q, Feng Z, Hu Y, Hou F, Zheng Y, Liu B, 2022. MicroRNAs: Potential mediators between particulate matter 2.5 and Th17/Treg immune disorder in primary membranous nephropathy. Front Pharmacol 13, 968256. 10.3389/fphar.2022.968256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lin X, Zhi L, Fang Y, Lin K, Li K, Wu L, 2020. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res Ther 11(1), 26. 10.1186/sl3287-019-1543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Kennicott K, Liang Y, 2023. Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice. Int J Mol Sci 24(7). 10.3390/ijms24076163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Shu X, Chen J, Xie Y, Xu P, Huang DQ, Lu NH, 2008. Effect of Helicobacter pylori eradication on oncogenes and cell proliferation. Eur J Clin Invest 38(9), 628–633. 10.1111/j.1365-2362.2008.01987.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


