Abstract
Scopoletin is a coumarin synthesized by diverse medicinal and edible plants, which plays a vital role as a therapeutic and chemopreventive agent in the treatment of a variety of diseases. In this review, an overview of the pharmacology, pharmacokinetics, and toxicity of scopoletin is provided. In addition, the prospects and outlook for future studies are appraised. Scopoletin is indicated to have antimicrobial, anticancer, anti-inflammation, anti-angiogenesis, anti-oxidation, antidiabetic, antihypertensive, hepatoprotective, and neuroprotective properties and immunomodulatory effects in both in vitro and in vivo experimental trials. In addition, it is an inhibitor of various enzymes, including choline acetyltransferase, acetylcholinesterase, and monoamine oxidase. Pharmacokinetic studies have demonstrated the low bioavailability, rapid absorption, and extensive metabolism of scopoletin. These properties may be associated with its poor solubility in aqueous media. In addition, toxicity research indicates the non-toxicity of scopoletin to most cell types tested to date, suggesting that scopoletin will neither induce treatment-associated mortality nor abnormal performance with the test dose. Considering its favorable pharmacological activities, scopoletin has the potential to act as a drug candidate in the treatment of cancer, liver disease, diabetes, neurodegenerative disease, and mental disorders. In view of its merits and limitations, scopoletin is a suitable lead compound for the development of new, efficient, and low-toxicity derivatives. Additional studies are needed to explore its molecular mechanisms and targets, verify its toxicity, and promote its oral bioavailability.
Keywords: scopoletin, plant sources, pharmacological activities, pharmacokinetics, toxicology
1 Introduction
In recent years, functional components of food sources have aroused considerable interest because of their benefits in preventing illnesses and promoting health (Iahtisham Ul et al., 2019). Scopoletin (6-methoxy-7-hydroxycoumarin, Figure 1) is a phenolic coumarin that is extracted from numerous medicinal and edible plants and has various pharmacological activities. Scopoletin is synthesized by diverse medicinal plants, such as Erycibe obtusifolia (Pan et al., 2011a), Aster tataricus (Ng et al., 2003), Foeniculum vulgare (Kwon et al., 2002), Artemisia annua (Tzeng et al., 2007), Sinomenium acutum (Shaw et al., 2003), Melia azedarach (Carpinella et al., 2005), and Artemisia iwayomogi, as well as certain edible plants, such as Lycium barbarum and Morinda citrifolia (Dou et al., 2013; Lee et al., 2014; Forino et al., 2016; Mohamad Shalan et al., 2016). In addition, it is a component of numerous fruit and vegetable crop plants, including Avena sativa, Allium ampeloprasum, Apium graveolens, Capsicum annuum, Capsicum frutescens, Daucus carota, Cichorium intybus, Citrus limon, and Citrus aurantium, demonstrating its low toxicity as well as its safe application as a synergistic compound together with synthetic or other natural substances, such as vanillin (Carpinella et al., 2005).
FIGURE 1.
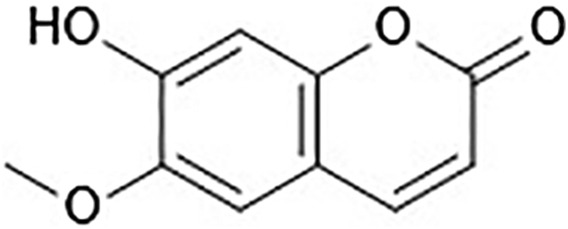
Structure of scopoletin.
Scopoletin has attracted the attention of medicinal chemists and health professionals because of its broad range of beneficial properties, such as antibacterial, antifungal, antiparasitic, anticancer, anti-inflammation, hepatoprotective, antihyperlipidemic, antidiabetic, neuroprotective, antioxidant, anti-angiogenesis, anti-hypertensive, analgesic, anxiolytic, immunomodulatory, anti-osteoporosis, anti-allergic, anti-aging, and anti-gout activities. In addition, scopoletin is an inhibitor of various enzymes, including choline acetyltransferase, acetylcholinesterase inhibitor (Rollinger et al., 2004; Hornick et al., 2011), aldose reductase (Lee et al., 2010), γ-aminotransferase (half-maximal inhibitory concentration [IC50] = 10.57 μM) (Mishra et al., 2010), monoamine oxidase (Yun et al., 2001), quinone oxidoreductase (Khunluck et al., 2019), and inducible nitric oxide synthase (iNOS) (Kim et al., 1999).
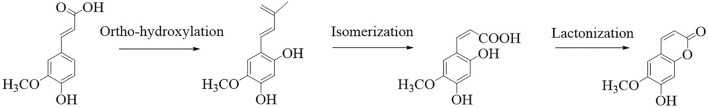
Scopoletin is a hydroxycoumarin with a molecular weight of 192.7 g/mol and a melting point of 204°C–206°C. The empirical formula of the compound is C10H8O4. It is slightly soluble in water or cold ethanol, soluble in hot ethanol or hot acetic acid, easily soluble in chloroform, and almost insoluble in benzene. Scopoletin is biosynthesized by ortho-hydroxylation of feruloyl-CoA in Arabidopsis thaliana (Figure 2) (Kai et al., 2008). Scopoletin is widely distributed in medicinal plants of various families and genera, including Malva (Lagunas-Herrera et al., 2019; Medrano-Jiménez et al., 2019), Cynodon (Patel et al., 2013), Convolvulus (Kaur et al., 2016; Garg et al., 2018), Artemisia (Kang et al., 1999; Choi et al., 2012; Kim et al., 2018), Erycibe (Pan et al., 2010), Canarium (Mogana et al., 2013), and Brunfelsia (Oliveira et al., 2001). It is also present in the whole plant of Viola mandshurica W. Becker (Park et al., 2017), Polygala sabulosa A.W. Bennett (Meotti et al., 2006; Ribas et al., 2008; Capra et al., 2010; Pereira Dos Santos Nascimento et al., 2016), Hedyotis diffusa Willd. (Chen et al., 2018), and Artemisia annua L. (Seo et al., 2016); in the aerial parts of Artemisia capillaris Thunb. (Navarro-García et al., 2011), Mitracarpus frigidus Willd. (Lemos et al., 2020), Solanum lyratum Thunb. (Kang et al., 1998), and Cirsium setidens (Dunn) Nakai (Ahn et al., 2014); in the roots of Hibiscus syriacus L. (Yun et al., 2001), Urtica dioica L. (Nahata and Dixit, 2012), Biebersteinia multifida DC. (Monsef-Esfahani et al., 2013), Gelsemium sempervirens L. (Khuda-Bukhsh et al., 2010), Saposhnikovia divaricata Turcz. Schischk (Kamino et al., 2016), Hypochaeris radicata L. (Jamuna et al., 2015), Argyreia speciosa L.f. (Kashyap et al., 2020), and Angelica pubescens Maxim. (Yang et al., 2009); the fruit of Tetrapleura tetraptera (Schumach. and Thonn.) Taub (Ojewole and Adesina, 1983); the flowers of Tilia cordata Mill. (Barreiro Arcos et al., 2006), the heartwood of Acer nikoense Miq. (Iizuka et al., 2007); and the inner shell of the nut of Castanea crenata (Noh et al., 2010). In addition, scopoletin has been analyzed in Morinda citrifolia L. (Kishore Kumar et al., 2017; Wigati et al., 2017; Ahmadi et al., 2019), Fraxinus rhynchophylla Hance (Kim et al., 1999), Torilis radiata (Ezzat et al., 2012), Brunfelsia hopeana Benth. (Oliveira et al., 2001), and Canarium patentinervium Miq. (Mogana et al., 2013). Table 1 summarizes the main plant sources of scopoletin and the associated bioactivities.
FIGURE 2.
Synthesis of scopoletin (Kai et al., 2008).
TABLE 1.
Main plant sources of scopoletin and their bioactivities.
| Family | Species | Parts of plant | Content W/W (%) | Bioactivity | Reference |
|---|---|---|---|---|---|
| Malvaceae | Malva parviflora L. | Whole plant | Not measured | Anti-hypertension and anti-AD | Lagunas-Herrera et al. (2019), Medrano-Jiménez et al. (2019) |
| Poaceae | Cynodon dactylon Linn. | Whole plant | Not measured | Anti-asthma | Patel et al. (2013) |
| Convolvulaceae | Convolvulus pluricaulis Chois. | Whole plant | Not measured | Anti-oxidant and anti-hyperlipidemia | Kaur et al. (2016), Garg et al. (2018) |
| Violaceae | Viola mandshurica W.Becker | Whole plant | Not measured | Anti-atherosclerosis | Park et al. (2017) |
| Rubiaceae | Hedyotis diffusa Willd. | Whole plant | 0.0528 | Chen et al. (2018) | |
| Polygalaceae | Polygala sabulosa A.W. Bennett | Whole plant | 0.0287, 0.112 | Anti-pleurisy, anti-depression, and antinociceptive | Meotti et al. (2006); Ribas et al. (2008); Capra et al. (2010); Pereira Dos Santos Nascimento et al. (2016) |
| Compositae | Artemisia annua Linn. | Whole plant | Not measured | Anti-cancer | Seo et al. (2016) |
| Apiaceae | Torilis radiata Moench. | Aerial parts | 0.0045 | Hepatoprotective | Ezzat et al. (2012) |
| Compositae | Artemisia capillaris Thunb. | Aerial parts | Not measured | Downregulated MMP-1 and suppressed primary splenocyte proliferation | Kim et al. (2018); Lee et al. (2017) |
| Rubiaceae | Mitracarpus frigidus Willd. | Aerial parts | 23.07 (scopoletin/extract) | Antifungal | Lemos et al. (2020) |
| Polemoniaceae | Loeselia mexicana Lamb. | Aerial parts | 0.005 | Antifungal | Navarro-García et al. (2011) |
| Solanaceae | Solanum lyratum Thunb. | Aerial parts | 0.00023 | Hepatoprotective activity | (Kang et al., 1998) |
| Compositae | Cirsium setidens Nakai. | Aerial parts | Not measured | Increased melanin synthesis | Ahn et al. (2014) |
| Compositae | Artemisia feddei Lev. | Aerial parts | 0.024 | Inhibited NO | Kang et al. (1999) |
| Asteraceae | Artemisia iwayomogi Kitam. | Leaves and stems | Not measured | Anti-inflammatory | Choi et al. (2012) |
| Convolvulaceae | Erycibe obtusifolia Benth. | Stems | 0.022 | Anti-angiogenesis and anti-arthritic | Pan et al. (2010) |
| Solanaceae | Nicotiana glauca Graham. | Leaves | Not measured | Anti-tumor | Tabana et al. (2016) |
| Rutaceae | Melicope lunuankenda | Leaves | Not measured | Inhibited α-glucosidase activity | Al-Zuaidy et al. (2016) |
| Rutaceae | Aegle marmelos Linn. | Leaves | Not measured | Decreased serum glucose | Panda and Kar (2006) |
| Solanaceae | Nicotiana glauca Graham. | Leaves | Not measured | Anti-tumor | Tabana et al. (2016) |
| Burseraceae Kunth | Canarium patentinervium Miq. | Leaves and barks | 0.98 | Anti-inflammatory, anti-cholinesterase, and anti-oxidant | Mogana et al. (2013) |
| Solanaceae | Nicotiana glauca Graham. | Leaves | Not measured | Anti-tumor | Tabana et al. (2016) |
| Convolvulaceae | Erycibe schmidtii Craib. | Twigs and leaves | 0.066 | Anti-rheumatoid arthritis | Ren et al. (2020) |
| Rubiaceae | Morinda citrifolia L. | Fruits and leaves | 0.46 ± 0.05 (leaf) | Antioxidant, anti-inflammatory, anti-leukemia, anti-hypertension, anti-PD, antibacterial, anti-angiogenic, anti-gastric ulcer, gastrokinetic activity, and anti-dyslipidemic | Mandukhail et al. (2010); Mahattanadul et al. (2011); Beh et al. (2012); Nima et al. (2012); Mohamad Shalan et al. (2016); Kishore Kumar et al. (2017); Wigati et al. (2017); Ahmadi et al. (2019); De La Cruz-Sánchez et al. (2019); Narasimhan et al. (2019) |
| Rubiaceae | Morinda citrifolia L. | Fruits | 0.06 | Parkinson’s disease | Kishore Kumar et al. (2017) |
| Mimosaceae | Tetrapleura tetraptera (Taub) | Fruits | Not measured | Spasmolytic activity | Ojewole and Adesina (1983) |
| Malvaceae | Hibiscus syriacus Linn. | Root bark | 0.0021 | Anti-oxidative | Yun et al. (2001) |
| Urticaceae | Stinging nettle (Urtica dioica L.) | Roots | Not measured | Benign prostatic hyperplasia | Nahata and Dixit (2012) |
| Solanaceae | Brunfelsia hopeana Benth. | Roots | 0.01125 | Anti-hypertension | Oliveira et al. (2001) |
| Geraniaceae | Biebersteinia multifida DC. | Roots | Not measured | Anxiolytic effects | Monsef-Esfahani et al. (2013) |
| Loganiaceae | Gelsemium sempervirens L. | Roots and rhizomes | Not measured | Anticancer | Khuda-Bukhsh et al. (2010) |
| Umbelliferae | Saposhnikovia divaricata Turcz. | Roots and rhizomes | 0.0039 | Inhibited NO | Kamino et al. (2016) |
| Asteraceae | Hypochaeris radicata Var. | Roots | Not measured | Anti-inflammatory | Jamuna et al. (2015) |
| Convolvulaceae | Argyreia speciosa Linn. | Roots | 0.56% (scopoletin/extract) | Anti-amyloidogenic | Kashyap et al. (2020) |
| Umbelliferae | Angelicae Pubescentis Maxim. | Root | 0.0001 | Yang et al. (2009) | |
| Fagaceae | Castanea crenata | Inner shells | Not measured | Anti-oxidant | Noh et al. (2010) |
| Aceraceae | Acer nikoense Miq. | Heartwood | Not measured | Vasorelaxant | Iizuka et al. (2007) |
| Tiliaceae | Tilia cordata Mill. | Flowers | Not measured | Anti-tumor | Barreiro Arcos et al. (2006) |
| Oleaceae | Fraxinus rhynchophylla Hance. | Barks | 0.0025 | Inhibited NO | Kim et al. (1999) |
The present review concentrates on recent research progress associated with the role of scopoletin in the prevention and/or treatment of illnesses and disorders, stressing the mechanism of its action and discussion of its toxicity and pharmacokinetic characteristics. Accordingly, recent literature concerning the bioactivity and uses of scopoletin as a chemotherapeutic agent was collated from multiple databases.
2 Pharmacological activities of scopoletin
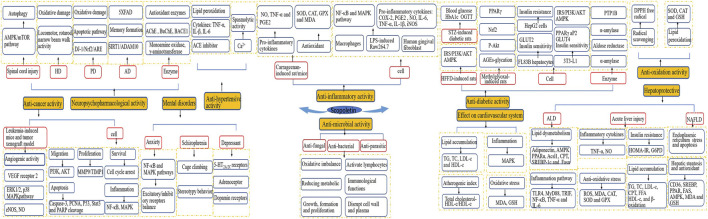
A considerable body of evidence has proven the benefits of scopoletin in human health (Figure 3). Table 2 summarizes the health-promoting activities of scopoletin and the underlying mechanism of action for various illnesses and disorders. Details concerning the activity of scopoletin against various illnesses and disorders are discussed in the following sections.
FIGURE 3.
Main biological activities and possible molecular mechanisms of scopoletin.
TABLE 2.
Treatment perspectives of scopoletin.
| Disorder | Mechanism | Reference |
|---|---|---|
| Antifungal | Cell wall and membrane damage ↑, apoptosis ↑, oxidative imbalance ↑, and metabolic activities ↓ | Das et al. (2020a); Lemos et al. (2020) |
| Antibacterial | Bacterial cell division ↓ | Duggirala et al. (2014) |
| Cancer | Cell proliferative ↓ | Barreiro Arcos et al. (2006); Manuele et al. (2006) |
| Cell cycle arrest ↑, apoptosis ↑, and caspase-3 ↑ | Liu et al. (2001) | |
| Caspase-3 ↑ and NF-κB ↑ | Kim et al. (2005) | |
| Caspase-3 ↑, caspase-8 ↑, bone marrow myeloblast levels ↓, CSF3 ↓, SOCS1 ↓, PTEN ↓, TRP53 ↓, VEGF↓, IL-10 ↑, and IL-4 ↑ | Ahmadi et al. (2019) | |
| Cyclin-D1↓, PCNA ↓, STAT-3 ↓, P53 ↑, and caspase-3 ↑ | Khuda-Bukhsh et al. (2010); Bhattacharyya et al. (2011) | |
| Cell cycle arrest ↑ and apoptosis ↑ | Asgar et al. (2015) | |
| NQO1↓ and cell migration ↓ | Khunluck et al. (2019) | |
| Cell cycle arrest ↑, cleaved-3/8/9 ↑, PARP ↑, cell invasion ↓, p-PI3K ↓, and p-AKT ↓ | Tian et al. (2019) | |
| Tumor growth ↓, vascularization ↓, and anti-vascular effect ↑ | Tabana et al. (2016) | |
| NF-κB ↓ | Seo et al. (2016) | |
| Arthritis | Synovial angiogenesis ↓ and apoptosis ↑ | Ying et al. (2009); Pan et al. (2010); Pan et al. (2011a) |
| IL-6 ↓, cell proliferation ↓, MAPKs ↓, PKC ↓, and CREB ↓ | Dou et al. (2013) | |
| COX-2 ↓ | Chen et al. (2021) | |
| Cell proliferation ↓ | Ren et al. (2020) | |
| IL-β ↓, TNF-α ↓, H2S ↓, chemoattractant protein 1↓, IL-33 ↓, preprotachykinin A ↓, and NF-κB ↓ | Leema and Tamizhselvi (2018) | |
| Pleurisy | NF-κB ↓ and MAPKs ↓ | Pereira Dos Santos Nascimento et al. (2016) |
| Asthma | K+ ↓ and Ca2+ ↓ | Patel et al. (2013) |
| Autoimmune encephalomyelitis | NF-κB ↓, demyelination ↓, MHC class II ↓, CD80 ↓, and CD86↓ | Zhang et al. (2019) |
| Acute liver injury | ALT ↑, AST ↑, LDH ↑, TNF-α ↓, NO ↓, NAG ↓, MPO ↓, MDA ↓, CAT ↑, and GSH ↑ | Ezzat et al. (2012) |
| GSH ↑, SOD ↑, and MDA ↓ | Kang et al. (1998) | |
| ROS ↓, CAT ↑, SOD ↑, GPx ↑, and MDA ↓ | Noh et al. (2010) | |
| Alcoholic liver | AMPK ↑, ACC ↓, SREBP-1c ↓, FAS ↓, PAP ↓, G6PD ↓, CYP2E1 ↓, SOD ↑, CAT ↑, GSH-Px ↑, GST ↑, and GSH ↑ | Lee et al. (2014) |
| TG ↓, adiponectin↑, AMPK ↑, SREBP-1c ↓, FAS ↓, PPARα ↑, ACSL1 ↑, CPT ↑, ACOX ↑, ACAA1A ↑, TLR4 ↓, MyD88 ↓, TRIF ↓, NF-κB ↓, TNF-α ↓, and IL-6 ↓ | Lee and Lee (2015a) | |
| Glucose intolerance ↓, HOMA-IR ↓, serum insulin level ↓, IR ↓, PI3K ↑, GK ↑, G6Pase ↓, SOD ↑, CAT ↑, GPx ↑, H2O2 ↓, and MDA ↓ | Lee and Lee (2015b) | |
| ALT ↓, AST ↓, TG ↓, TBARS ↓, CAT ↑, SOD ↑, GPx ↑, GR ↑, and CYP2E1 ↓ | Noh et al. (2011) | |
| Non-alcoholic fatty liver | Body weight ↓, visceral fat ↓, leptin ↓, TG ↓, TC ↓, TNF-α ↓, IL-6 ↓, IFNγ ↓, MCP-1 ↓, serum glucose ↓, insulin ↓, HOMA-IR ↓, IPGTT ↓, ERRFI1 ↑, APOA4 ↓, CYP7A1 ↓, COL1A1 ↓, MMP-13 ↓, CDKN1A ↓, and GDF-15 ↓ | Ham et al. (2016) |
| Body/liver weight ↓, TG ↓, TC ↓, LDL ↓, glucose ↓, ALT ↓, AST ↓, ACC ↓, SREBP-1c ↓, and p-AMPK ↑ | Park et al. (2017) | |
| Hyperlipidemia | TG ↓ and TC ↓ | Kim et al. (2017a) |
| TG ↓, TC ↓, and glucose ↓ | Mandukhail et al. (2010) | |
| TG ↓, TC ↓, LDL ↓, and MDA ↓ | Garg et al. (2018) | |
| Diabetic | α-glucosidase ↓, α-amylase ↓, and postprandial hyperglycemia ↓ | Abdullah et al. (2016); Jang et al. (2018) |
| α-glucosidase ↓, DPPH radical activity↓, and fasting blood glucose ↓ | Al-Zuaidy et al. (2016) | |
| Glucose uptake ↑, GLUT4 ↑, p-Akt ↑, PI3K ↑, and AMPK ↑ | Jang et al. (2020) | |
| PPARγ 2 ↑, IR ↓, p-Akt ↑, and p-PKB ↑ | Zhang et al. (2010) | |
| Serum glucose ↓ and G6PD ↓ | Panda and Kar (2006) | |
| Insulin secretion ↑, blood glucose ↓, TG ↓, and TC ↓ | Verma et al. (2013) | |
| HbA1 ↓, blood glucose ↓, FFA ↓, TG ↓, TC ↓, ALT ↓, TLR4 ↓, MyD88 ↓, TNF-α ↓, IL-6 ↓, PPARγ ↓, DGAT2 ↓, and MCP-1 ↓ | Choi et al. (2017) | |
| AGEs ↓, TG ↓, TC ↓, LDL ↓, HDL ↑, D-lactic acid ↑, IR ↓, blood glucose ↓, Nrf2 ↑, PPARγ ↑, p-AKT ↑, PTP1B ↓, and GLUT2 ↑ | Chang et al. (2015) | |
| AMPK ↑, IRS1 ↑, PI3K ↑, Akt ↑, TBARS ↓, LHP ↓, PC ↓, SOD ↑, GPX ↑, CAT ↑, HOMA-IR ↓, TC ↓, TG ↓, HDL-C ↑, LDL ↓, and VLDL ↓ | Kalpana et al. (2019) | |
| AGE formation ↓ and RLAR ↓ | Lee et al. (2010) | |
| AR ↓, oxidative stress ↓, galactitol ↑, and GSH ↑ | Kim et al. (2013) | |
| Alzheimer’s | Aβ42 fibril ↓, AChE ↓, BuChE ↓, oxidative stress ↓, and neurotoxicity ↓ | Kashyap et al. (2020) |
| AChE ↑, LTP ↑, LTD ↓, and memory retention ↑ | Hornick et al. (2011); Das et al. (2020a) | |
| Microglia phagocytic ↑, PPARγ ↑, CD36 ↑, TNF-α ↓, IL-6 ↓, and amyloid plaque ↓ | Medrano-Jiménez et al. (2019) | |
| Apoptotic ↓, ROS ↓, SIRT1 ↑, FOXO3A ↑, ADAM10 ↑, Bcl2 ↑, CAT ↑, and SOD ↑ | Gay et al. (2020) | |
| Parkinsonism | SOD ↑, CAT ↑, GST ↑, Nrf2 ↑, GR ↑, ARE ↑, DJ-1 ↑, Keap1 ↓, and GUL3 ↑ | Narasimhan et al. (2019) |
| Cell death ↓, striatal neuronal loss ↑, Bax ↓, Bcl2 ↑, and cytochrome C ↓ | Kishore Kumar et al. (2017) | |
| GSH ↑, redox balance ↑, mitochondrial function ↑, and oxidative damage ↓ | Pradhan et al. (2020) | |
| Huntington’s disease | Body weight ↑, locomotor activity ↑, grip strength ↑, gait abnormalities ↑, MDA ↓, nitrite ↓, SOD ↑, and GSH ↑ | Kaur et al. (2016) |
| Spinal cord injury | AMPK ↑, mTOR ↑, motor neuronal ↑, apoptosis ↓, autophagy ↑, beclin-1 ↑, and LC3B-positive neuronal cells ↑ | Zhou et al. (2020) |
| Anxiety | MAOs ↓ | Monsef-Esfahani et al. (2013) |
| Microglia activation ↓, IL-1β ↓, IL-6 ↓, TNF-α ↓, MAPK ↓, NF-κB ↓, and GABA-T ↓ | Luo et al. (2020) | |
| Schizophrenia | Climbing behavior ↓ and stereotyped behavior ↓ | Pandy and Vijeepallam (2017) |
| Depressant | Depression-like behavior ↓ and immobility time ↓ | Capra et al. (2010) |
| Antioxidant | Superoxide anion ↓, SOD ↑, CAT ↑, and GSH ↑ | Shaw et al. (2003) |
| LDL oxidation ↓ and TBARS ↓ | Thuong et al. (2005) | |
| Anti-angiogenesis | Branching pattern of blood vessels ↓ | Beh et al. (2012) |
| Blood vessels ↓, tube formation ↓, proliferation ↓, and migration ↓ | Pan et al. (2011b) | |
| VEGFR2↓, proliferation ↓, migration ↓, tube formation ↓, and ERK1/2 ↓ | Pan et al. (2009) | |
| Hypertension | Relaxed smooth muscles ↑, spasmogenic activities ↓, ACE ↓, and portal vein contractions ↓ | Ojewole and Adesina (1983) |
| Contractile responses ↓ | Iizuka et al. (2007) | |
| SBP ↓, DBP ↓, TNF-α ↓, MDA ↓, IL-6 ↓, and IL-1β ↓ | Lagunas-Herrera et al. (2019) | |
| SBP ↓ and DBP ↓ | Wigati et al. (2017) | |
| Anti-gout | Uric acid ↓ | Ding et al. (2005) |
| Allergic | PMA ↓, IL-4 ↓, IL-5 ↓, IL-10 ↓, IFN-γ ↑, NFAT↓, GATA-3 ↓ | Cheng et al. (2012) |
| Vitiligo | MITF ↑, tyrosinase ↑, p-CREB↑, cAMP/PKA ↑, and P38MAPK ↑ | Ahn et al. (2014); Kim et al. (2017b); Heriniaina et al. (2018) |
| Antiaging | MMP-1↓, IL-1α ↓, TNF-α ↓, and p-P38 ↓ | Kim et al. (2018) |
| SA-β-Gal staining ↓, HDAC1↑, SIRT1 ↑, SIRT6↑, Nrf-2 ↑, p-FoxO1↑, and P53 ↑ | Nam and Kim (2015) | |
| Anticonvulsant | GABA transaminase ↓ | Mishra et al. (2010) |
| Immunomodulatory | Macrophage phagocytic activity ↑ | Mankar et al. (2013) |
| Macrophage phagocytic activity ↑ and phagocytosis-linked genes (CDC42 ↓, FCGR1A ↓, FCGR1C ↓, ITGA9 ↓, ITGB3 ↓, PLCE1 ↓, RHOD ↓, RND3 ↓, DIRAS3 ↑, ITGA1 ↑, PIK3CA ↑, PIK3R3↑, and PLCD1↑) | Alkorashy et al. (2020) | |
| Splenocyte proliferation ↓ and adaptive immune cell activation ↓ | Lee et al. (2017) | |
| Osteoporosis | Osteoclastic differentiation ↓, ROS ↓, superoxide anion ↓, NF-κB ↓, and peroxyl radical-scavenging ↑ | Lee et al. (2013) |
| Analgesic | Glutamatergic transmission ↓, TNF-α ↓, and IL-1β ↓ | Ribas et al. (2008) |
↓, downregulation, inactivation, and inhibition; ↑, upregulation and activation.
2.1 Anti-microbial activities
2.1.1 Antifungal activity
The microbial population, inside and outside the human body, plays a vital role in human health because many microbes may induce illnesses. Scopoletin shows maximum antifungal activity against Trichophyton mentagrophytes, Aspergillus niger, and Candida albicans (Navarro-García et al., 2011). The minimum inhibitory concentration (MIC) of scopoletin against Candida glabrata and Candida tropicalis is 67.22 and 119 μg/mL, respectively, which initiates an oxidative imbalance and reduces metabolism to achieve its antibacterial effect on these two Candida species (Das et al., 2020). Scopoletin has antifungal properties effective against a multidrug-resistant strain of C. tropicalis. Its mechanism of action is interference in the synthesis of essential fungal cell components, disruption of cell walls and plasma membranes, and impairment of C. tropicalis biofilm growth, formation, and proliferation (Lemos et al., 2020). Recent studies have reported that scopoletin has strong antitubercular activity; the compound isolated from Morinda citrifolia roots exhibits high activity against Mycobacterium tuberculosis with an MIC of 50 μg/mL (Sam-Ang et al., 2023). The MIC of the crude ethanol extract from the stem bark of Hymenodictyon floribundum BL Rob. against Mycobacterium indicum and Mycobacterium madagascariense is 195 and 781.25 μg/mL, respectively (Marealle et al., 2023).
2.1.2 Antibacterial activity
Several studies have reported that scopoletin is active against Staphylococcus aureus (Buathong et al., 2019; De La Cruz-Sánchez et al., 2019; Ramírez-Reyes et al., 2019; Chandrasekhar et al., 2023). Scopoletin exerts antimycobacterial activity against Streptococcus pyogenes, Pseudomonas aeruginosa, P. aeruginosa DMSC 37166, Mycobacterium tuberculosis H37Rv, Actinomyces israelii, Actinomyces naeslundii, and Salmonella typhi (Chiang et al., 2010; More et al., 2012; Acharya et al., 2013; Meerungrueang and Panichayupakaranant, 2014; Napiroon et al., 2018). In addition, Duggirala et al. reported that scopoletin inhibited both the polymerization and GTPase activity of filamentous temperature-sensitive protein Z, a target of anti-bacterial drugs, so it may be used as a lead structure for anti-filamentous temperature-sensitive protein Z drug design (Duggirala et al., 2014). Molokoane et al. reported that the compound from Artemisia afra (62.5 μg/mL) showed good activity against Escherichia coli (Molokoane et al., 2023).
2.1.3 Antiparasitic activity
Scopoletin significantly inhibits the growth of Plasmodium yoelii and Trypanosoma brucei brucei (Mamoon Ur et al., 2014; Li et al., 2018).
2.1.4 Antiviral activity
Individual fractions of scopoletin isolated from Artemisia annua exert strong virucidal and antiviral effects at a minimum concentration of 50 μg/mL in vitro and have been shown to inhibit SARS-CoV-2 infection (Baggieri et al., 2023).
2.2 Anticancer activity
The antitumor activity of scopoletin may result from its anti-proliferation, anti-migration, pro-apoptotic, anti-invasion, and anti-angiogenic inhibition of multiple drug resistance, regulation of the mitogen-activated protein kinase (MAPK) and PI3K/AKT/mTOR pathways, and its effect on cell cycle arrest (Antika et al., 2023).
Scopoletin shows anti-proliferative action on BW5147 murine lymphoma cells and MCF-7 human adenocarcinoma cells (Barreiro Arcos et al., 2006; Manuele et al., 2006; Nasseri et al., 2022). It exerts anticancer effects on human cervical cancer cell lines by inducing apoptosis and cell cycle arrest and inhibiting cell invasion and the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway (Tian et al., 2019). It is indicated to play a role in triggering cell cycle arrest and increasing apoptosis in PC3 cells via activation of caspase-3 (Liu et al., 2001). Scopoletin has been reported to activate nuclear factor-kappa B (NF-κB), caspase-3, and PARP cleavage, leading to apoptosis of promyeloleukemic HL-60 cells (Kim et al., 2005). Scopoletin causes human melanoma cell A375 apoptosis through downregulation of cyclin-D1; proliferation of cell nuclear antigen, survivin, and Stat-3; and upregulation of p53 and caspase-3 (Khuda-Bukhsh et al., 2010). Similar outcomes have been observed for cholangiocarcinoma cells and cervical cancer cells with respect to cell cycle arrest (G0/G1) and apoptosis induction, and an increase in cytotoxicity by co-administration of cisplatin and scopoletin is indicated (Asgar et al., 2015). Scopoletin has a significant inhibitory effect on A549 cells, with an IC50 of approximately 16 μg/mL (Yuan et al., 2021). In human Jurkat leukemia cells and leukemia-induced BALB/c mice, scopoletin shows anti-leukemia activity associated with cancer cell apoptosis and inhibition of inflammation and angiogenesis and mitigation of bone marrow myeloblast imbalance (Ahmadi et al., 2019). Angiogenesis plays an important role in tumor growth and metastasis (Yamakawa et al., 2018). Beh et al. observed that scopoletin (10, 30, and 100 nmol/egg) decreases the number of vascular branch points in a dose-dependent manner in chick embryo chorioallantoic membranes (Beh et al., 2012). Tabana et al. concluded that scopoletin (100 and 200 mg/kg, p.o.) shows anti-tumorigenic and anti-angiogenic activity in a nude mouse xenograft model by inhibiting vascular endothelial growth factor A (VEGFA), fibroblast growth factor 2 (FGF2), and extracellular signal-regulated kinase-1 (ERK-1) (Tabana et al., 2016). Scopoletin inhibits in vitro tube formation, proliferation, and migration in human umbilical vein endothelial cells and functions by obstructing VEGFR2 autophosphorylation and inhibiting ERK1/2, p38 MAPK, and Akt activation (Pan et al., 2009; Pan et al., 2011b; Cai et al., 2013). Scopoletin exposure upregulates cell cycle arrest in cancer cells, including cervical (Tian et al., 2019), cholangiocarcinoma (Prompipak et al., 2021), breast cancer (Yu et al., 2021), hepatoma, and lung cancer cells (Shi et al., 2020). A recent study has reported that matrine and scopoletin are effective ingredients of the Qinghao–Kushen combination combating liver cancer, which reduce the expression of GSK-3β in HepG2 cells and upregulate GSK-3β in HepG2.2.15 cells (Ji et al., 2022).
2.3 Anti-inflammatory activity
Administration of scopoletin inhibits mouse ear edema induced by ethyl phenylpropiolate, 12-O-tetradecanoylphorbol-13-acetate, croton oil, carrageenan, and 2,4-dinitrochlorobenzene, as well as paw and skin inflammation (Farah and Samuelsson, 1992; Muschietti et al., 2001; Ding et al., 2008; Selim and Ouf, 2012; Jamuna et al., 2015; Bak et al., 2022), which may be associated with modulation of the generation of pro-inflammatory mediators, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), prostaglandin E2, and IL-6, which suppresses cyclooxygenase-2 (COX-2) and iNOS expression. In lipopolysaccharide (LPS)-stimulated human gingival fibroblast and RAW 264.7 cells, scopoletin significantly inhibits the expression levels of the pro-inflammatory mediators IL-6 and TNF-α, thus prohibiting COX-2, iNOS, and nitric oxide (NO) expression (Kim et al., 1999; Kim et al., 2004; Choi et al., 2012; Kamino et al., 2016; Chaingam et al., 2021). However, the mechanism by which scopoletin influences the generation of inflammatory cytokines remains unclear. Moon et al. revealed that scopoletin (0.01–0.2 mM) suppresses the generation of inflammatory cytokines induced by phorbol 12-myristate 13-acetate plus A23187 by suppressing IκBα phosphorylation and degradation to obstruct NF-κB activation (Moon et al., 2007). In vitro assays indicate that scopoletin (100 and 200 mg/kg, i.p.) suppresses monosodium urate crystal-induced leukocyte infiltration and activation by inhibiting the synthesis and release of inflammatory mediators of activated macrophages. Scopoletin may exert anti-inflammatory effects through prevention of NF-κB signaling and the MAPK pathway (Yao et al., 2012). Pereira et al. reported that the anti-pleurisy effect of scopoletin is mainly mediated by inhibition of pro-inflammatory cytokines (TNF-α and IL-1β), NF-κB, and p38 MAPKs (Pereira Dos Santos Nascimento et al., 2016) and reduction in central nervous system inflammation via suppression of NF-κB signaling (Zhang et al., 2019). In addition, the regulation of the NF-κB signaling pathway reduces airway inflammation in platelet-derived growth factor BB-induced airway smooth muscle cells (Fan et al., 2022). In addition, a scopoletin-rich Morinda citrifolia leaf extract reduces TNF-α, IL-1β, and NO levels in serum, which relieves osteoarthritis symptoms (Wan Osman et al., 2019).
2.3.1 Anti-dendritic cell activity
Rheumatoid arthritis is an autoimmune disorder characterized by synovial hyperplasia and inflammation as well as resulting in joint destruction and deformity (Sajti et al., 2004). The synovium relies on blood supply for proliferation and formation of a pannus that invades the cartilage and bone, causing osteoclast activation and cartilage and bone destruction (Kimura et al., 2007). Dendritic cells are bone marrow-derived cells that arise from lymphoid–bone marrow hematopoiesis and coordinate innate and adaptive immune responses (Collin and Ginhoux, 2019). Immature dendritic cells are preferentially localized at the lining or sub-lining layer of the rheumatoid arthritis synovium (Page et al., 2002). Scopoletin (1 and 5 μM) functionally decreases the proliferation of bone marrow immature dendritic cells (Ren et al., 2020). Scopoletin (50 and 100 mg/kg), in part, improves the clinical state of rat adjuvant-induced arthritis through ameliorating synovial inflammation and destruction of cartilage and bone, thus blocking synovial angiogenesis, triggering apoptosis of fibroblast-like synoviocytes, and inhibiting COX-2 (Ying et al., 2009; Pan et al., 2010; Chen et al., 2021; Gao et al., 2011). The anti-rheumatoid arthritis activity of scopoletin (15, 30, and 60 μM) is likely exerted by suppression of IL-6 generation by fibroblast-like synoviocytes of adjuvant arthritis and potential activation of the MAPK/protein kinase C/cAMP response element-binding protein (CREB) (Dou et al., 2013). Furthermore, scopoletin (30, 40, and 50 μM) inhibits fibroblast-like synovial cells and blocks NF-κB signal transduction, thus combating rheumatoid arthritis (Chen et al., 2022). In addition, scopoletin isolated from Bouvardia ternifolia (Cav.) Schltdl. inhibits NF-κB expression, thereby exerting its anti-rheumatoid arthritis action in Freund’s complete adjuvant-induced ICR mice (Zapata Lopera et al., 2022).
2.4 Effects of scopoletin on liver diseases
The common clinical liver diseases are mainly viral diseases caused by hepatitis B and C viral infection, drug-induced liver injury, alcoholic fatty liver disease, non-alcoholic fatty liver disease (NAFLD), cirrhosis, and liver cancer.
2.4.1 Acute liver injury
Acute liver injury is the beginning of the progression of many liver diseases leading to liver failure; hence, it is a crucial research focus. Models to simulate acute liver injury mainly include a chemical liver injury model induced by carbon tetrachloride (CCl4), drug-induced liver injury model, drug liver injury model induced by lipopolysaccharide, and alcohol-induced alcoholic liver injury model.
Scopoletin (1, 5, and 10 mg/kg) reduces the activity of an antioxidant enzyme (superoxide dismutase, SOD) and reduced glutathione (GSH) content, inhibits the production of malondialdehyde (MDA), and resists oxidative stress during acute liver injury induced by CCl4 in rats so as to protect the liver (Sharma et al., 2022). Scopoletin (1, 5, and 10 mg/kg) significantly improves alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) activities in rat livers under toxicity induced by 100 mg/kg isoniazid, 300 mg/kg rifampicin, and 700 mg/kg pyrazinamide (Sharma et al., 2023).
2.4.2 Chronic liver injury
2.4.2.1 Alcoholic fatty liver disease
Lee et al. reported that orally administered scopoletin (0.05%, w/w) decreased lipid contents in the liver and plasma and the activities of hepatic lipogenic enzymes in alcohol plus 35% kcal high-fat diet (HFD)-induced mice. The potential mechanism for these effects was modulation of AMP-activated protein kinase (AMPK)-sterol regulatory element-binding protein 1C (SREBP-1c) pathway-mediated lipogenesis in HFD-induced mice. The hepatoprotective effect of scopoletin is associated with its stimulation of the antioxidant defense system (Lee et al., 2014). In alcohol-fed rats, scopoletin regulates AMPK and the toll-like receptor 4 (TLR4)/myeloid differentiation major response gene 88 (MyD88)/NF-κB pathway and alleviates alcoholic hepatic steatosis and inflammation (Lee and Lee, 2015). In addition, Lee et al. reported that scopoletin (0.01 and 0.05 g/L) weakened chronic alcohol-induced insulin resistance and activated the antioxidant defense system through regulation of genes involved in liver glucose and antioxidant metabolism (Lee and Lee, 2015). Scopoletin is among the predominant compounds in the inner shell of chestnut (Castanea crenata) and has protective effects on ethanol-induced oxidative damage in vivo. Its hepatoprotective effects are associated with inhibition of lipid accumulation, peroxidation, and reinforcement of the antioxidant defense system in ethanol-induced mice. Scopoletin (50 μg/mL) increases antioxidant enzyme activities (SOD, catalase, glutathione peroxidase, and glutathione reductase) in alcohol-induced HepG2 cells (Noh et al., 2011).
2.4.3 Non-alcoholic fatty liver disease
In the HFD-induced obesity mice model, scopoletin (0.01% and 0.05% in diet) may mitigate NAFLD and prevent the development of liver fibrosis by regulating lipid metabolism and inflammation. The specific mechanism involves reduction of liver lipid accumulation, improvement in insulin resistance, and reduction in inflammatory factors (TNF-α, IL-6, and IFNγ), chemokine monocyte chemoattractant protein-1 (MCP-1), and leptin levels (Ham et al., 2016). Administration of scopoletin to HFD-fed mice decreases the body weight, liver weight, and serum levels for lipids and liver damage markers (ALT and AST) and regulates the AMPK/SREBP signaling pathway (Park et al., 2017). Scopoletin promotes palmitic acid-induced intracellular accumulation of triglycerides (TGs) and total cholesterol in HepG2 cells (Kim et al., 2017a). Scopoletin (6.25–50 μmol/L) inhibits endoplasmic reticulum stress and reactive oxygen species (ROS) production in primary liver cells of rats and reduces c-Jun N-terminal kinase (JNK) phosphorylation to prevent palmitic acid- and bile acid-induced liver cell death (Wu et al., 2022).
2.5 Effects on the cardiovascular system
2.5.1 Hypotensive activity
Ojewole and Adesina, (1983) observed that scopoletin isolated from Tetrapleura tetraptera fruit had non-specific spasmolytic activity on smooth muscles(Ojewole and Adesina, 1983). Subsequently, Oliveira et al. proposed that, with regard to scopoletin derived from the roots of Brunfelsia hopeana, the non-specific spasmolitic action is exerted through interference with the mobilization of intracellular calcium from norepinephrine (NE)-sensitive stores (Oliveira et al., 2001), and the release of sarcoplasmic reticulum Ca2+ induced by NE is inhibited, resulting in vasodilation of aortic rings (Iizuka et al., 2007). Wigati et al. reported that scopoletin decreases systolic pressure (SBP), diastolic pressure (DBP), and mean arterial blood pressure (MABP) in dexamethasone-induced mice. The mechanism is associated with the activity of an angiotensin-converting enzyme (ACE) inhibitor and the antioxidant activity of scopoletin (Wigati et al., 2017). Recently, scopoletin (0.01, 0.1, 1, 2, and 5 mg/kg, p.o.) has been shown to have antihypertensive effects on chronic and acute hypertensive mice induced by administration of angiotensin II. Scopoletin decreases the pharmacodynamic parameters for SBP and DBP by 75% and 92.8%, respectively (Lagunas-Herrera et al., 2019). Among the complications associated with hypertension, the onset of intracerebral hemorrhage is a devastating stage and is the most disabling type of stroke with the highest mortality rate. Zhang et al. observed that scopoletin improves rat ischemia induced by collagenase injection by reducing the expression of brain edema and other inflammatory mediators, such as TNF-α and IL-1β (Zhang et al., 2021).
2.5.2 Anti-atherosclerotic activity
The relevant literature clearly indicates that lipid accumulation (Cartolano et al., 2018), inflammation (Geovanini and Libby, 2018), and oxidative stress (Förstermann et al., 2017) are the most important risk factors for atherosclerosis. Scopoletin (10 μg/mL) attenuates lipid accumulation and inflammation in the aorta in HFD-induced apolipoprotein E-deficient (ApoE−/−) mice, which reduces vascular inflammation by AMPK activation to suppress the expression of cell-cycle regulators (cyclin and cyclin-dependent kinase adhesion molecule) in human aortic smooth muscle cells (Park et al., 2017). Subsequently, Garg et al. observed that scopoletin (the main component isolated from Convolvulus pluricaulis extract, 0.4 mg/kg) significantly decreases the levels of atherogenic lipid biomarkers, atherogenic index, and MDA and increases the levels of HDL-C and GSH in tyloxapol-induced hyperlipidemia rats (Garg et al., 2018). In addition, Batra et al. reported that orally administered scopoletin (1, 5, and 10 mg/kg) reduces total cholesterol, low-density lipoprotein (LDL), and TG contents and improves the plasma atherosclerosis index and Castelli risk index in the high-fructose high-fat diet (HFHFD)-induced dyslipidemia model of Wistar rats (Batra et al., 2023a).
2.5.3 Anti-myocardial infarction activity
Recently, in an isoproterenol-induced myocardial infarction rat model, pretreatment with scopoletin (25 and 50 mg/kg) was observed to significantly reduce the heart-to-body weight ratio, cardiac diagnostic markers, MDA content, inflammatory markers, and apoptotic markers (Rong et al., 2023). In addition, Li et al. reported that scopolamine induces endothelial-dependent relaxation mediated through the NO and prostacyclin pathways, thereby alleviating acute myocardial ischemia (Li et al., 2023).
2.6 Antidiabetic activity
Diabetes mellitus may be the fastest-growing metabolic disease in the world. Approximately 2.5%–7% of the global population suffers from diabetes, which is a leading cause of illness and death. In diabetes, chronic hyperglycemia results from an interruption of carbohydrate and fat metabolism owing to insufficient insulin secretion, insufficient insulin function, or both (Rauf et al., 2017).
Scopoletin regulates hyperglycemia and diabetes. In the streptozotocin (STZ)-induced diabetic rat model, scopoletin has hypoglycemic and lipid-lowering effects (Verma et al., 2013; Al-Zuaidy et al., 2016). Choi et al. reported that scopoletin (0.01%) ameliorates hyperglycemia and hepatic steatosis in HFD- and STZ-induced diabetic mice through suppression of lipid biosynthesis and the TLR4–MyD88 pathways (Choi et al., 2017). In addition, scopoletin enhances the postprandial blood glucose levels by inhibiting the activity of carbohydrate digestive enzymes (α-glucosidase and α-amylase) in STZ-induced diabetes mice (Jang et al., 2018).
In 3T3-L1 adipocytes and high-glucose-induced HepG2 cells, scopoletin (10, 20, and 50 µM) improves insulin resistance and enhances glucose uptake by activating the PI3K/Akt signaling pathway (Zhang et al., 2010; Jang et al., 2020). Scopoletin (10 and 25 μM) improves insulin sensitivity in methylglyoxal-induced FL83B hepatocytes by activating the PPARγ/Akt pathway and restoring the plasma translocation of GLUT2 (Chang et al., 2015). In addition, improvement in insulin sensitivity in response to scopoletin (1 mg/kg/day, p.o.) can activate the AMPK and the IRS1–PI3K–Akt pathways in pancreatic β cells of high-fructose diet (HFHFD) rats and improves glucose homeostasis in HFHFD-induced diabetes rats (Kalpana et al., 2019; Batra et al., 2023b). Scopoletin (1–5 µM) stimulates insulin secretion by the KATP channel-dependent pathway in INS-1 pancreas β cells (Park et al., 2022). Furthermore, scopoletin (5, 10, 25, and 50 μM) protects INS-1 pancreatic β cells from glycotoxicity induced by high glucose and thus has potential as a drug to protect pancreatic β cells (Park and Han, 2023). Lee and Kim showed that scopoletin has potent inhibitory activity on both advanced glycation end-product (AGE) formation and rat lens aldose reductase (RLAR) in an in vitro bioassay, with an IC50 of 2.93 ± 0.06 μM and 22.51 ± 2.01 μM, respectively (Lee et al., 2010). The accumulation of AGEs is associated with an increase in the risk of fracture in patients with type 2 diabetes and has a direct adverse effect on bone quality (Yamamoto and Sugimoto, 2016). In vitro studies have revealed that scopoletin (1–20 µM) improves osteoclast formation in diabetes through RANKL and enhances osteoclast formation in diabetes by inducing BMP-2 and Runx2. Oral administration of 10 mg/kg scopoletin promotes the formation of bone trabeculae and collagen fibers in the femoral epiphysis and metaphysis of type 2 diabetes mice (Lee et al., 2021). Aldose reductase (AR) is a crucial rate-limiting enzyme that contributes to cataract induction among patients with diabetes. Scopoletin (10 and 50 mg/kg) mitigates diabetes cataract formation through inhibiting AR activity, polyol accumulation, and GSH generation in galactose-fed rats (Kim et al., 2013). To further explore the specific mechanism by which scopoletin alleviates diabetes retinopathy, Pan et al reported that scopoletin protected retinal ganglion cells from high glucose-induced damage through ROS-dependent p38 and JNK signaling cascades in a high glucose-induced retinal ganglia cell model (Pan et al., 2022). Diabetes nephropathy is among the most common microvascular complications of type 1 and type 2 diabetes; it is observed in approximately 40% of diabetes patients and is the main cause of chronic kidney disease worldwide (Vučić Lovrenčić et al., 2023). Scopoletin inhibits the proliferation of rat glomerular mesangial cells, reduces extracellular matrix proliferation and cell hypertrophy, reduces extracellular matrix protein accumulation, reduces the expression of the crucial fibrotic factor TGF-β and connective tissue growth factor, inhibits renal fibrosis, and thus improves diabetes glomerulosclerosis (Liang et al., 2021).
2.7 Effect of scopoletin on neurodegenerative disorders
A neurodegenerative disorder indicates the progressive loss of functions and structures and neuronal cell death arising from different conditions, such as genetic and environmental factors (Gay et al., 2020). Motor neuron degeneration is an important pathological process in many types of nervous system diseases. Motor neuron disease is characterized by chronic progressive degeneration of motor neurons. Many studies have shown that scopoletin has a neuroprotective effect, which is mainly affected via 1) inhibition of monoamine oxidase (MAO) and acetylcholinesterase (AChE), 2) reduction of oxidative damage and chronic inflammation, and 3) protection of the activity of neurotrophic factors.
2.7.1 Anti-Alzheimer’s disease activity
Monoamine oxidase can be used to treat neurological disorders as a validated drug target (Carradori and Petzer, 2015). Its main function is to catalyze the oxidative deamination of neurotransmitters and biogenic amines (Edmondson et al., 2004). Yun et al. observed that scopoletin suppresses MAO in a dose-dependent manner with an IC50 value of 19.4 μg/mL (Yun et al., 2001). Furthermore, scopoletin (80 mg/kg, i.p.) is a reversible and selective MAO inhibitor causing an increase in the levels of dopamine and its metabolite (DOPAC) in the mouse brain (Mogana et al., 2013; Basu et al., 2016).
Acetylcholine (ACh) is widely distributed in the brain. The cholinergic system plays a role in crucial physiological processes, such as attention, learning, memory, stress response, wakefulness, and sleep, or sensory information (Ferreira-Vieira et al., 2016). Scopoletin can serve as an inhibitor of AChE, as indicated by the pharmacophore-based virtual screening method. The IC50 for AChE inhibition is 168.6 µM and 0.27 ± 0.02 mM (Rollinger et al., 2004; Mogana et al., 2013). Scopoletin shows AChE inhibitory activity in the range of 13.92%–34.18% at a concentration of 100 μg/mL (Suchaichit et al., 2018).
Neuronal cell death is an important feature of neurodegenerative disorders. In SH-SY5Y cells subject to hydrogen peroxide (H2O2) injury, scopoletin (5 μΜ) attenuates neurodegeneration via restoration of antioxidant enzyme activity, reduction in cell apoptosis, and activation of the SIRT1–ADAM10 signaling pathway, which is implicated in reduction in amyloid β (Aβ) production (Gay et al., 2020). In addition, Aβ is the main component of neuritic plaques in Alzheimer’s disease (AD) (Greenberg et al., 2020). Administration of 23 mg/kg scopoletin ameliorates the detrimental impacts of Aβ deposition on memory and learning among 5XFAD transgenic mice under a HFD diet, which is associated with microglia-enhanced phagocytic capacity and weakened microglia M1 phenotype activation (Medrano-Jiménez et al., 2019). Subsequently, Kashyap et al. proposed that scopoletin improves Aβ42-induced neurotoxicity and H2O2-induced cytotoxicity in PC12 cells. This effect may mediate inhibition of Aβ42 aggregation, AChE, butyrylcholinesterase, Aβ-site precursor protein-cleaving enzyme 1, MAO-B, and oxidative stress (Kashyap et al., 2020).
In conclusion, the imbalance of AChE and MAO, nerve cell death, and Aβ deposition may lead to cognitive and memory impairment. In cholinergically impaired and age-impaired mice models, scopoletin induces a significant increase in presynaptic activity-dependent acetylcholine release, enhances long-term potentiation (LTP) in the hippocampus, and exerts memory-improving properties (Hornick et al., 2011). The stimulatory role of Convolvulus pluricaulis extracts (500 mg/kg, scopoletin as the active ingredient), which modulate synaptic plasticity in the hippocampal cornu ammonis, enhances LTP and reduces long-term depression, which are the two major synaptic plasticity forms of memory formation (Das R. et al., 2020).
2.7.2 Anti-Parkinsonism disease activity
Although the pathogenesis of Parkinson’s disease (PD) is not entirely resolved, it has been reported that excessive production of ROS, mitochondrial dysfunction, neuroinflammation, and environmental toxins can promote the loss of dopaminergic neurons in PD (Ryan et al., 2015). Rotenone-induced Sprague‒Dawley (SD) rats and SH-SY5Y cell models have shown that scopoletin inhibits cell apoptosis and oxidative stress by activating DJ-1–Nrf2–antioxidant response element (ARE) signaling (Narasimhan et al., 2019). In the same model, scopoletin attenuates rotenone-induced apoptosis of dopaminergic neurons in SD rats. The mechanism involves inhibition of the mitochondrial pathway of internal apoptosis, regulated by the Bcl2 family (Kishore Kumar et al., 2017). In addition, scopoletin (2.5 mM) is an antioxidant, reducing mitochondrial dysfunction and oxidative stress caused by an increase in ROS concentrations so as to restore motor ability and enhance the mitochondrial and cellular health of dopaminergic neurons in a Drosophila fly model of PD (Pradhan et al., 2020).
2.7.3 Anti-Huntington’s activity
In a 3-nitropropionic acid-induced model of Huntington’s disease, administration of 20 mg/kg scopoletin attenuates motor deficits and oxidative damage in rats where it improves behavioral parameters (locomotor, rotarod, and narrow beam walking activity) and biochemical parameters (MDA, SOD, GSH, and nitrite) (Kaur et al., 2016).
2.8 Anti-mental disorder
Worldwide, the prevalence of mental illness is approximately 25%. The mental illness mentioned in this paper refers to the medical concept of mental pain defined in the DSM-5 diagnostic criteria traditionally used for research. However, due to its long-term effects, mental illness is also considered a disability (Littlewood, 2001; Sayce and Boardman, 2008).
2.8.1 Anti-anxiety activity
Biebersteinia multifida root extract (45 mg/kg, i.p., including scopoletin) exhibits an anxiolytic effect that shows the same anti-anxiety effect as that of diazepam but lasts longer for 90 min (Monsef-Esfahani et al., 2013). In the Freund’s adjuvant-induced chronic inflammation anxiety mouse model, scopoletin (50 mg/kg, i.p.) exerts an anxiolytic effect through ameliorating anxiety-like behaviors, for which the mechanism is associated with suppression of the NF-κB/MAPK signaling pathways involving anti-inflammatory activities and regulation of the excitatory/inhibitory receptor balance (Luo et al., 2020).
2.8.2 Anti-schizophrenia activity
Scopoletin at a specific dose of 0.1 mg/kg can alleviate the positive symptoms of schizophrenia. Scopoletin exerts anti-climbing and anti-stereotypy effects on apomorphine-induced cage climbing and methamphetamine-induced stereotypy behaviors, respectively, in mice (Pandy and Vijeepallam, 2017).
2.8.3 Anti-depressant activity
Scopoletin (10–100 mg/kg, p.o.) shows particular antidepressant-like effects, as observed in the tail suspension test. Antidepressant effects are associated with the interaction of serotonergic (5-HT2A/2C receptors), noradrenergic (α1-and α2-adrenoceptor), and dopaminergic (D1 and D2 receptors) systems (Capra et al., 2010).
2.9 Anti-oxidant activity
Scopoletin hinders oxidation in the ABTS, diphenyl-2-picrylhydrazyl (DPPH), FRAP, and β-carotene bleaching assays with half-maximal effective concentration (EC50) values of 5.62 ± 0.03 μM, 0.19 ± 0.01 mM, 0.25 ± 0.03 mM, and 0.65 ± 0.07 mM, respectively (Mogana et al., 2013). Scopoletin scavenges xanthine/xanthine oxidase-generated superoxide anions in a dose-dependent manner while xanthine oxidase activity is maintained and enhances the activity of endogenous antioxidant enzymes, such as SOD, catalase, and GSH (Shaw et al., 2003; Panda and Kar, 2006). Furthermore, scopoletin inhibits xanthine oxidase, maintains mitochondrial functioning to reduce ROS amounts, and suppresses LDL oxidation mediated by either Cu2+ or free radicals generated with an azo compound (Thuong et al., 2005). In addition, scopoletin shows antioxidant potential against DPPH (IC50 = 0.82 mg/mL) and NO (IC50 = 0.64 mg/mL) radicals (Sam-Ang et al., 2023).
2.10 Miscellaneous properties
2.10.1 Anti-gout-lowering uric acid activity
Ding et al. showed that scopoletin (100 and 200 mg/kg, i.p.) causes a significant reduction in uric acid activity associated with potassium oxonate by decreasing the serum uric acid level and enhancing urine urate (Ding et al., 2005), although the mechanism is not clear. Scopoletin (200 mg/kg, p.o.) remarkably lowers the serum uric acid level of a yeast extract in potassium oxonate-induced mice; the therapeutic mechanisms are associated with inhibition of the activity of hepatic xanthine oxidase and promotion of uric acid excretion (Zeng et al., 2017).
2.10.2 Anti-allergic activity
Scopoletin (50 μΜ) exerts anti-allergic activity mainly by inhibiting the production of cytokines (IL-4, IL-5 IL-10, and IFN-γ) and suppressing nuclear factor and GATA3 expression in activated T cells and PMA-/ionomycin-induced EL-4 T cells (Cheng et al., 2012).
2.10.3 Anti-vitiligo activity
Vitiligo is a skin disease. The death or loss-of-function of skin melanocytes leads to partial discoloration of the skin (Pichler et al., 2006). Ahn et al. reported that scopoletin increases melanin synthesis in B16F10 cells by activating cAMP-responsive CREB phosphorylation and microphthalmia-associated transcription factor (MITF), resulting in an increase in the expression of tyrosinase (Ahn et al., 2014). In addition, scopoletin (40 μg/mL) stimulates melanin synthesis through activation of the cAMP/PKA/p38 MAPK pathway in B16 melanoma cells (Kim et al., 2017b). Furthermore, scopoletin (10, 20, and 25 μΜ) enhances melanogenesis responses in zebrafish and B16F10 cells, which is associated with increases in melanin content and expression of tyrosinase-related protein 1 and MITF (Heriniaina et al., 2018).
2.10.4 Anti-aging activity
For human lung fibroblasts, scopoletin has anti-aging effects, which promotes autophagy induction via inactivation of p53 and enhance FoxO transportation, thereby inducing anti-aging-related autophagy and longevity (Nam and Kim, 2015). In HaCaT human keratinocytes with UVB, scopoletin (30, 100, and 300 µM) inhibits the expression of pro-inflammatory cytokines and matrix metallopeptidase (MMP)-1 by inhibiting the phosphorylation of p38 MAPK (Kim et al., 2018).
2.10.5 Immunomodulatory activity
Alkorashy et al. demonstrated that scopoletin (50 μg/mL) stimulates U937-derived macrophages and significantly affects the expression of certain phagocytosis-linked genes (Alkorashy et al., 2020). Scopoletin (10 and 100 μΜ) suppresses ConA- and LPS-induced adaptive immune cell activation (Lee et al., 2017).
2.10.6 Anti-nociceptive activity
Scopoletin, found in a Polygala sabulosa hydroalcoholic extract (0.01–10 mg/kg, i.p.), inhibits the acetic acid-induced visceral nociceptive response (Meotti et al., 2006). In addition, scopoletin (10 mg/kg, i.p.) counteracts nociception induced by glutamate in mice (Ribas et al., 2008).
2.10.7 Anti-spinal cord injury activity
In a rat model of spinal cord injury, scopoletin (100 mg/kg, i.p.) improves locomotion 325 recovery and motor neuronal loss through stimulation of autophagy by triggering the AMPK/mammalian 326 target of the rapamycin (mTOR) signaling pathway (Zhou et al., 2020).
2.10.8 Facilitating the digestion activity
Sun et al. preliminarily confirmed that scopoletin isolated from Cynachum auriculatum has an anti-functional dyspepsia effect (Sun et al., 2024). Scopoletin remarkably prevents acid reflux esophagitis production, with a similar efficiency to that of standard anti-secretory agents (ranitidine and lansoprazole) through its anti-inflammatory and anti-secretory attributes, such as its pro-kinetic activity, which can accelerate gastric emptying and intestinal transit (Mahattanadul et al., 2011). The potential mechanism is partially ascribed to the active component stimulating the 5-HT4 receptor (Nima et al., 2012).
2.10.9 Inducing the expression of latent HIV
Reversing the incubation period of HIV-1 can promote the killing of infected cells, which is crucial for treatment strategies. In HIV-1 latently infected Jurkat T cell lines, scopoletin (2.0 mM) can significantly influence the incubation period of HIV-1 without cytotoxicity in a dose-dependent manner (Zhu et al., 2023).
2.10.10 Inducing metabolomic profile disturbances
Yao et al. evaluated the metabolic effects of scopoletin in zebrafish embryos using non-targeted metabolomics methods. Exposure to scopoletin (2.1, 6.2, and 18.5 μg/mL) resulted in significant metabolic disorders, mainly involving phosphonate and phosphinate metabolism, vitamin B6 metabolism, histidine metabolism, sphingolipid metabolism, and folate biosynthesis (Yao et al., 2022).
2.10.11 Ameliorating nephrotoxicity
Scopoletin (50 mg/kg/once daily, i.p.), via the Keap1–Nrf2/HO-1 and IκBα–P65–NF-κB–P38/MAPK signaling pathways, effectively improves renal function, oxidative stress biomarkers, and inflammatory mediators in vancomycin-treated rats (Khalaf et al., 2022).
3 Pharmacokinetics of scopoletin
Pharmacokinetics is the study of the time course of the absorption, metabolism, distribution, and excretion of a drug, compound, or novel chemical entity upon its administration to the body (Fan and de Lannoy, 2014). Pharmacokinetics research provides compound-/drug-specific data to determine doses and dosing routes for individual patients, minimize toxicity, and offer a cornerstone for illnesses (Visser, 2018).
3.1 Absorption, metabolism, and elimination
Absorption, metabolism, and elimination transformations of scopoletin are widely used for monitoring its possible effects on different lifestyle-related disorders.
Following intragastric administration of 50 mg/kg scopoletin in rats, Xia et al. used high-performance liquid chromatography (HPLC) to tentatively detect the parameters (T max (min) = 10, C max (g/m) = 8.2 ± 0.8, T 1/2 (min) = 14.1 ± 0.6, AUCt (g min/mL) = 145.9 ± 11.8, and Ke (min−1) = 0.051 ± 0.005) associated with the absorption process by rat plasma (Xia et al., 2007). Given the low sensitivity of the HPLC method, it is unsuitable to study the in vivo absorption characteristics of scopoletin in detail. Therefore, Liu et al. studied its pharmacokinetics by HPLC/tandem mass spectrometry (Liu et al., 2011; Zeng et al., 2015; Li et al., 2019). The average percentage of scopoletin excreted from urine, above the dose administered, was 14.93%, and the cumulative biliary excretion of scopoletin above the dose administered was 0.16% after oral administration of Glehniae Radix extract to male SD rats (Liu et al., 2011). The results revealed that less than 15% of the analytes unchanged from the extract were excreted in the urine and less than 1% of the analytes unchanged from the extract were excreted in the bile, indicating that scopoletin undergoes major metabolism in the body. A pharmacokinetic study on rats after oral administration of scopoletin (5, 10, and 20 mg/kg) revealed that the oral bioavailability following a dose of 5 mg/kg was 6.62% ± 1.72%, for a dose of 10 mg/kg, it was 5.59% ± 1.16%, and for 20 mg/kg 5.65% ± 0.75% in the rat plasma (Zeng et al., 2015). Pharmacokinetic studies of dog plasma after oral administration of scopoletin (10, 25, and 50 mg/kg) showed that the bioavailability was 7.08%, 5.87%, and 5.69%, respectively (Zhao et al., 2019), similar to the bioavailability of coumarin (3.40% ± 2.60%) (Ritschel et al., 1977). Thus, these results indicated the statistical similarity of the oral bioavailability among the three p.o. groups and thus was found to be independent of the delivery of the administered dose. Zhang et al. applied the UHPLC-LTQ-Orbitrap-MS method to study the pharmacokinetics of scopoletin in dog plasma after intravenous (1 mg/kg) and oral administration (10, 25, and 50 mg/kg). The main relevant measurement parameters were as follows: AUC0-t (ng/h/mL) = 186.54 ± 36.45, 131.83 ± 19.23, 277.78 ± 35.12, and 528.19 ± 45.78; AUC0-∞ (ng/h/mL) = 197.97 ± 35.21, 140.43 ± 21.10, 284.69 ± 39.87, and 546.61 ± 51.28; T 1/2 (h) = 2.05 ± 0.27, 2.36 ± 0.45, 1.87 ± 0.21, and 1.65 ± 0.45; C max (ng/mL) = 423.23 ± 39.45, 85.47 ± 15.78, 253.78 ± 45.27, and 410.79 ± 57.19; and CL/F (L/kg/h) = 5.05 ± 0.89, 71.22 ± 15.23, 87.87 ± 15.56, and 91.47 ± 17.28 (Zhao et al., 2019). Li et al. reported that in rats administered 100 mg/kg scopoletin by gavage, the relevant pharmacokinetic parameters are as follows: AUC0-t (μg/L/h) = 203 ± 29.5; AUC0-∞ (μg/L/h) = 206 ± 29.1; T 1/2 (min): 69.6 ± 5.4; C max (μg/mL) = 72.7 ± 8.7; and CL/F (L/kg/min) = 418.9 ± 36.8 (Tian et al., 2023). Li et al. reported that, after the oral administration of 30 mg/kg scopoline, which is a metabolite of scopoletin, significant differences in certain parameters were observed between male and female rats (p < 0.05), i.e., AUC (9783.33 ± 157.61 ng/mL/min vs. 12,966.66 ± 1771.97 ng/mL/min), T max (14.00 ± 5.48 min vs. 6.67 ± 2.58 min), and CL/F (3.07 ± 0.05 L/min/kg vs. 2.36 ± 0.36 L/min/kg). Further investigation is needed to elucidate the potential mechanism of gender differences; however, the maximal excretion rates of scopoletin were 31.68 μg/h and 25.58 μg/h in male and female rats, respectively (Li et al., 2019).
Coumarin is quickly absorbed from the human gastrointestinal tract and is thoroughly metabolized by the liver, and only 2%–6% of the coumarin enters the systemic circulation intact (Ritschel et al., 1977; Ritschel et al., 1979). Scopoletin is a coumarin analog, and its rapid absorption, metabolism, and excretion from the human body may explain the poor bioavailability (Zeng et al., 2015). One study has shown that scopoletin is eliminated by first-order kinetics after intraperitoneal injection of Ding Gong Teng in mice. It showed the characteristics of a two-compartment open model: rapid absorption, rapid distribution, rapid action, and slow elimination. After intramuscular injection of Ding Gong Teng (scopoletin content: 2030 mg/L) in rabbits, the absorption showed double peaks: the first peak appeared at 8.08 min, and the concentration of scopoletin was 145.45 μg/L; the second peak appeared at 2.45 h, and the scopoletin concentration was 48.66 μg/L (Min et al., 2000). The pharmacokinetic study of Ding Gong Teng injection in rabbits showed that scopoletin was eliminated quickly, the elimination rate constant was 0.56 h, the half-life was 1.81 h, and the concentration at 4 h after administration was 5.32 μg/L. An additional study reported that scopoletin was well-absorbed in a human colon adenocarcinoma cell line model, indicating that it is well-absorbed in the gut lumen (Galkin et al., 2009). The aforementioned results suggest that scopoletin undergoes extensive metabolism in the body. Wang et al. determined that hepatic injury does not significantly influence the pharmacokinetics of scopoletin (Wang et al., 2018). The reason for this may be that cytochrome P450 enzymes had underwent partial change in the process of liver injury. It is also possible that scopoletin is not a P-glycoprotein substrate (Nabekura et al., 2015; Yang et al., 2015), which would explain the decrease in the bioavailability of scopoletin. The bioavailability of scopoletin is low (approximately 6.0%) (Zeng et al., 2017), which may be associated with its low water solubility and instability in physiological media. It may also reflect limited solubility, poor absorption and metabolism, or decomposition in the gastrointestinal tract (Issell et al., 2008).
Nevertheless, after oral administration of a Hedyotis diffusa extract (4.837 g/kg equivalent to 30.45 mg/kg of scopoletin), scopoletin was rapidly absorbed into the circulatory system in rats, and the half-life and average retention time were more than 10 h (Chen et al., 2018), indicating that the clearance rate of scopoletin in the plasma was slow.
3.2 Distribution
Following oral administration of 6 g/kg of Angelicae Pubescentis Radix extract to rats at the lower limit of quantification levels (2.16 ng/mL), scopoletin could not be determined in the rat plasma. Analysis of its tissue distribution showed that scopoletin was extensively distributed in multiple tissues, particularly the heart, liver, and kidneys, reflecting its pharmacological roles (Chang et al., 2013).
The pharmacokinetic deficiencies and outlook for scopoletin can be briefly summarized as follows: 1) the optimal method to investigate the pharmacokinetics of scopoletin requires clarification; 2) there are distinct differences in pharmacokinetic parameters between mice of different genders, and additional studies should be conducted to explore the underlying mechanism of gender differences; 3) the pharmacokinetic parameters of scopoletin have only been studied in mice/rat and rabbit models.
4 Toxicology of scopoletin
No strict boundary is proposed to portray or differentiate favorable or detrimental chemicals. The degree of harmfulness and safety seems to depend on the chemical dose. Therefore, this concept has become the hub of modern toxicology, meaning that dose determines toxicity (Hayes and Dixon, 2017).
4.1 Toxicity
As indicated by the acute toxicity test, scopoletin failed to generate treatment-associated mortality and abnormal performance at the limit test dose (2000 mg/kg, p.o.) for 14 days in SD rats (Tabana et al., 2016). This research shows the safety of scopoletin at the dose level, and, therefore, the LD50 value of scopoletin for oral toxicity is > 2000 mg/kg. Jamuna et al. observed rats for 14 days after administration of oral doses of 10, 50, and 100 mg/kg scopoletin and detected no obvious acute toxicity signs, no net gain or loss of body weight, or gross behavioral variation (Jamuna et al., 2015). In vivo experiments have administered a dose of 50–200 mg/kg (i.p.) scopoletin to SD rats and ICR mice (Ding et al., 2005; Pan et al., 2010; Yao et al., 2012). Table 3 summarizes the reported dosages of scopoletin for different animals. Thus, previous research has defined scopoletin as a relatively safe natural product, but there is a lack of long-term toxicity studies on animals. Strict experiments in vivo should be conducted for improved estimation of the side effects of scopoletin to ensure its safe use.
TABLE 3.
Dose range of scopoletin examined in animal models.
| Strain | Animal model | Administration approach | Experimental duration | Tested dosage | Result | Reference |
|---|---|---|---|---|---|---|
| SD rats | Ether-induced arthritis model | i.p. | 23 days | 50 and 100 mg/kg | Suppressed new vessel formation | Pan et al. (2010) |
| ICR mice | Murine air pouch model | i.p. | 6 h | 50, 100, and 200 mg/kg | Inhibited monosodium urate crystal-induced inflammation | Yao et al. (2012) |
| SD rats | Drug-induced liver injury model | p.o. | 21 days | 1.5 and 10 mg/kg | Decreased ALT, AST, and ALP levels | Sharma et al. (2023) |
| Wistar male rats | Type IV collagenase I was injected into the left striatum | 6 weeks | 100 mg/kg | Reduced the expression of brain edema and inhibited inflammation | Zhang et al. (2021) | |
| Wistar rats | HFHFD-induced dyslipidemia model | p.o. | 60 days | 1.5 and 10 mg/kg | Reduced TG, TC, and LDL-H and improved plasma atherosclerosis index and Castelli risk index | Batra et al. (2023a) |
| Albino male rats | Isoproterenol-induced myocardial infarction model | p.o. | 28 days | 50 mg/kg | Reduced heart to body weight ratio, cardiac diagnostic markers, inflammatory markers, and apoptotic markers | Rong et al. (2023) |
| db/db mice | p.o. | 10 weeks | 10 mg/kg | Decreased weight and increased the serum RANKL/OPG ratio | Lee et al. (2021) | |
| ICR mice | Potassium oxonate-induced hyperuricemia | i.p. | 1 h | 50, 100, and 200 mg/kg | Decreased hyperuricemia | Ding et al. (2005) |
| SD rats | Acute oral toxicity | p.o. | 14 days | 2000 mg/kg | Did not produce any considerable toxic behavioral effects | Tabana et al. (2016) |
| Athymic nude mice | HCT116 cell xenograft mouse model | p.o. | 21 days | 50, 100, and 200 mg/kg/day | Suppressed tumor growth by 94.7% relative to the vehicle-treated group | |
| Female Swiss albino mice | Acute oral toxicity | p.o. | 14 days | 10, 50, and 100 mg/kg | Possessed high safety profile | Jamuna et al. (2015) |
| Swiss albino mice | Cerulein-induced acute pancreatitis | i.p. | 6 h | 10 mg/kg | Improved acute pancreatitis | Leema and Tamizhselvi (2018) |
| Female Swiss mice | Carrageenan-induced pleurisy | i.p. | 4.5 h | 0.1, 1, and 5 mg/kg | Decreased neutrophil migration | Pereira Dos Santos Nascimento et al. (2016) |
| Female Swiss mice | Forced swimming test, tail suspension test, or open-field test | p.o. | 1 h | 0.1, 1, 10, and 100 mg/kg | Antidepressant-like action | Capra et al. (2010) |
| Swiss mice | Acetic acid-induced visceral nociceptive | i.p. | 0.5 h | 0.01, 0.1, 1, and 10 mg/kg | Improved visceral and inflammatory pain | Meotti et al. (2006) |
| Swiss albino mice | i.p. | 4 weeks | 80 mg/kg | Increased brain DA and decreased DOPAC | Basu et al. (2016) | |
| Female Wistar rats | T4-induced | p.o. | 1 week | 0.5 and 1.0 mg/kg/day | Anti-hyperthyroid | Panda and Kar (2006) |
| Wistar albino rats | STZ-induced diabetic rats | p.o. | 6 weeks | 1 mg/kg | Decreased blood glucose level and lipid level | Verma et al. (2013) |
| C57BL/6N mice | STZ-induced diabetic rats | 11 weeks | 0.01%, w/w | Ameliorates steatosis and inflammation | Choi et al. (2017) | |
| SD rats | HFFD-induced type 2 diabetes | p.o. | 45 days | 1 mg/kg/day | Improves insulin sensitivity | Kalpana et al. (2019) |
| SD rats | HFHFD-induced diabetes model | p.o. | 74 days | 1.5 and 10 mg/kg/day | Reversed insulin resistance | Batra et al. (2023b) |
| SD rats | Galactose-fed rats | p.o. | 2 weeks | 10, 50 mg/kg/day | Kim et al. (2013) | |
| C57BL/6 mice | CFA-induced anxiety | p.o. | 2 weeks | 2, 10, and 50 mg/kg | Ameliorates anxiety-like behaviors | Luo et al. (2020) |
4.2 Cytotoxicity
Scopoletin cytotoxicity has been assessed in numerous cell types in previous in vitro research, such as cancer cells, normal cells, immune cells, and nerve cells, illustrating that scopoletin is a relatively safe natural product. Table 4 summarizes the reported dosages of scopoletin for different cell lines.
TABLE 4.
Cytotoxic effects of scopoletin in different cell lines.
| Cell line | Species | Category | Detection method | Tested concentration or IC50 | Result | Reference |
|---|---|---|---|---|---|---|
| PC3 | Human | Androgen adenocarcinoma cell | MTT assay | 33, 66, 133, 266, and 533 mg/mL (72 h), IC50 = 157 ± 25 mg/mL | Inhibited cell proliferation | Liu et al. (2001) |
| HL-60 | Human | Leukemia cell line | MTT assay | 0.025, 0.05, 0.1, 0.2, and 0.5 mg/mL (24 h) IC50 = 0.5 mg/mL | Induced apoptosis via activation of NF-κB and caspase-3 | Kim et al. (2005) |
| A375 | Human | Melanoma cell | MTT assay | 6, 12, 24, 30, 48, and 60 μΜ (24 h) | Inhibited cell proliferation and induced apoptosis | Khuda-Bukhsh et al. (2010) |
| KKU-100 | Human | Cholangiocarcinoma cell line | MTT assay | 250, 300, 350, 400, and 500 μΜ (72 h) IC50 = 486.2 ± 1.5 µM | Induced cell apoptosis | Asgar et al. (2015) |
| KKU-M214 | Human | Cholangiocarcinoma cell line | MTT assay | 50, 300, 350, 400, and 500 (72 h) IC50 = 493.5 ± 4.7 µM | Induced cell apoptosis | |
| H69 cells | Human | Bile duct epithelial cell line | MTT assay | (20–72 h) IC50 > 500 µM | Lower cytotoxic effects | |
| BW 5147 | Human | T-cell lymphoma cell line | Trypan blue exclusion method | 10, 50, 100, and 500 μg/mL (72 h) | Inhibited cell proliferation | Barreiro Arcos et al. (2006); Manuele et al. (2006) |
| EC50 = 251 ± 15 μg/mL | ||||||
| MCF-7 | Human | Human breast adenocarcinoma | MTT assay | 297.17 ± 7.99 μg/mL | Inhibited cell proliferation | Nasseri et al. (2022) |
| KKU-100 | Human | Cholangiocarcinoma cell line | Sulforhodamine B assay | 0.08, 0.16, 0.33, 0.65, 1.3, and 2.6 mM (24–48 h), IC50 24 h = 1.92 mM IC50 48 h = 0.89 mM | Anti-migration effect | Khunluck et al. (2019) |
| DoTc2 | Human | Cervical cancer cell lines | Cell counting assay | 3.56, 6.12, 12.5, 25, 50, and 100 μM (24 h) IC50 = 25 μM | Triggered apoptosis and cell cycle arrest and inhibited cell invasion | Tian et al. (2019) |
| SiHa | Human | Cervical cancer cell lines | Cell counting assay | 3.56, 6.12, 12.5, 25, 50, and 100 μM (24 h) IC50 = 15 μM | ||
| HeLa | Human | Cervical cancer cell lines | Cell counting assay | 3.56, 6.12.12.5, 25, 50, and 100 μM (24 h) IC50 = 7.5 μM | ||
| C33A | Human | Cervical cancer cell lines | Cell counting assay | 3.56, 6.12, 12.5, 25, 50, and 100 μM (24 h) IC50 = 25 μM | ||
| HCvEpC | Human | Normal cell | Cell counting assay | 3.56, 6.12,12.5, 25, 50, and 100 μM (24 h) IC50 = 90 μM | ||
| KKU-100/KKU-213B | Human | Cholangiocarcinoma cell | MTT assay | 1, 2, 3, and 4 mg/mL (24 h, 48 h, and 72 h) | Antiproliferative | Prompipak et al. (2021) |
| IC50 = 1.06 ± 0.03 mg/mL, 2.14 ± 0.08 mg/mL | ||||||
| RAW 264.7 | Mouse | Murine macrophage cell line | MTT assay | 1, 5, 10, 25, and 50 μg/mL, did not affect the cell viability | Kim et al. (2004) | |
| RAW 264.7 | Mouse | Murine macrophage cell line | MTT assay | 1, 10, 25, and 50 μg/mL, did not affect the cell viability | Inhibited iNOS | Kim et al. (1999) |
| RAW 264.7 | Mouse | Murine macrophage cell line | MTT assay | 30, 100, and 300 μΜ (20 h) | Anti-inflammatory | Yao et al. (2012) |
| RA-FLS | Human | Fibroblast-like synoviocytes | MTT assay | 10, 20, 30, 40, and 50 μΜ (24 h, 48 h) | Inhibited proliferation, migration, and invasion | Chen et al. (2022) |
| HASMCs | Human | Airway smooth muscle cells | CCK-8 assay | Inhibited proliferation | Fan et al. (2022) | |
| Fibroblast-like synoviocytes | Mouse | Adjuvant arthritis rat cells | MTT assay | 125, 250, 500, and 1,000 μΜ (24 h) | Ying et al. (2009) | |
| Fibroblast-like synoviocytes | Mouse | Adjuvant arthritis rat cells | MTT assay | 15, 30, and 60 mΜ (24 h) did not display remarkable cytotoxicity | Anti-rheumatoid arthritis | Dou et al. (2013) |
| INS-1 pancreatic β cells | Mouse | Pancreatic β cells | MTT assay | 5, 10, 20, 25, 50, and 100 µM | Stimulated the secretion of insulin | Park and Han (2023); Park et al. (2022) |
| RGC-5 cell | Mouse | Retinal ganglion cell | MTT assay | 62.5, 125, 250, and 500 mM (6 h) | Protected RGC-5 cells exposed to high-glucose environments | Pan et al. (2022) |
| RGMCs HBZY-1 cells | Mouse | Mesangial cell | MTT assay | 0.01, 5, 0.1, and 1 μM (48 h) | Inhibited rat glomerular mesangial cells proliferation and ECM proliferation and cell hypertrophy | Liang et al. (2021) |
| SH-SY5Y cells | Human | Dopaminergic cell | MTT assay | 1, 5, 10, and 50 μM (24 h) | Attenuated neurodegeneration | Gay et al. (2020) |
| PC12 cells | Mouse | Nerve cell | MTT assay | 10, 20, and 40 μM | Anti-amyloidogenic and anti-cholinesterase | Kashyap et al. (2020) |
| EL-4 T cells | Mouse | T-lymphoma cell | Trypan blue dye exclusion | 1, 10, 25, and 50 μM | Attenuates allergy | Cheng et al. (2012) |
| B16F10 cells | Mouse | Melanoma cells | Crystal violet assay | 0.1, 1, 10, 20, and 50 μΜ (24 h) | Increases melanin synthesis | Ahn et al. (2014) |
| B16F10 cells | Mouse | Melanoma cells | MTT assay | 0–40 μg/mL (24 h) | Stimulates melanogenesis | Kim et al. (2017a) |
| B16F10 cells | Mouse | Melanoma cells | MTT assay | 0.5, 10, 25, 50, 100, 150, 200, and 250 µM (48 h) | Stimulates melanogenesis | Heriniaina et al. (2018) |
| IMR 90 | Human | Lung fibroblast cells | MTT assay | 1, 2, 4.8, and 16 μΜ (24 h) | Anti-aging | Nam and Kim (2015) |
| HaCaT keratinocytes | Human | Fibroblasts | MTT assay | 0.5, 5, 15, 50, 150, 250, and 500 µM (24 h) | Downregulated MMP-1 expression | Kim et al. (2018) |
| U937 | Human | Macrophage cell line | CytoTox96 LDH-release kit | 10, 50, and 100 μg/mL | No detectable cytotoxic effects | Acharya et al. (2013) |
| U937 | Human | Monocytic cell line | MTT assay | 12.5, 25, 50, 100, 200, and 400 μg/mL | No detectable cytotoxic effects | Alkorashy et al. (2020) |
| Caco-2 | Human | Colonic adenocarcinoma | WST-1 cytotoxicity assay | 250 mM | No detectable cytotoxic effects | Galkin et al. (2009) |
| HUVECs | Human | Human endothelial cell | MTS assay | IC50 = 0.73 μM | Antitumor | Tabana et al. (2016) |
| 3T3-L1 cells | Mouse | Embryo fibroblasts | MTT assay | 5, 10, 25, 50, and 100 mM (24 h) | Did not affect the viability | Jang et al. (2018) |
5 Conclusion and future research prospects
In summary, this review summarizes the multiple physiological effects of scopoletin, confirming its significant positive effects on different illnesses, as stated previously. Consequently, many therapeutic intervention measures should be proposed in accordance with the potential mechanisms of the active agent and its derivatives. Moreover, as a natural compound, scopoletin provides a safer alternative for pharmaceutical applications targeting hepatic, neural, and cancer illnesses. Considering the therapeutic activities and the weak oral bioavailability of scopoletin, a large number of its derivatives and pharmaceutical dosages can be designed. Shi et al. considered isoxazole-based hybrids of scopoletin as an efficient chemical modification that improved the anticancer activity of scopoletin(Shi et al., 2017). The 2-fluorobenzylpyridinium derivative is the most potent tested compound, with an IC50 value of 0.215 ± 0.015 μM, which is significantly ameliorated compared with that of scopoletin (Khunnawutmanotham et al., 2016). Multiple substituted 8,8-dimethyl-8H-pyrano [2,3-f] chromen-2-ones (chromeno-coumarin hybrids) have been synthesized based on scopoletin as vasorelaxing agents. Compared with the parent molecule scopoletin, the sensitivity of these derivatives to experimental tissues was increased by 29.40–70.89 times (Singh et al., 2020). In addition, Soluplus-based scopoletin micelles (Sco-Ms) have been produced using a simple thin-film hydration technique, and the oral bioavailability of Sco-Ms was enhanced by 438% compared with that of free scopoletin. Oral delivery of Sco-Ms showed distinctly higher hypouricemic efficiency in hyperuricemic mice compared with that of scopoletin (Zeng et al., 2020). Polymeric nanoparticle encapsulation of scopoletin induced massive apoptosis in the human melanoma cell line A375 (Bhattacharyya et al., 2011).
The poor solubility of scopoletin limits the oral absorption and bioavailability of the compound. Therefore, methods to improve the bioavailability of scopoletin, reduce its toxicity, develop a suitable method for administration, and improve its clinical efficacy should be the focus of future research. In addition, scopoletin is a constituent in many edible plants and food products and thus could be developed as a health food and functional food. Further research on human subjects should be conducted to guarantee its safety, and decomposition products in the human body should be assessed to ensure its safe application in treatments. Similarly, further efforts should be made to verify the effects of food supplements, explore their diverse effects on humans and elucidate the mechanisms of action.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author contributions
X-YG: writing–original draft, writing–review and editing, and investigation. X-YL: Writing–original draft. C-YZ: Writing–original draft. C-YB: Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| AGEs | Advanced glycation end products |
| AUC | Area under the curve of the glucose tolerance test |
| APOA4 | Apolipoprotein A-IV |
| AMPK | AMP-activated protein kinase |
| Aβ | Amyloid β |
| ACC | Acetyl-CoA carboxylase |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| ADAM | A disintegrin and metalloproteinases |
| ACE | Angiotensin-converting enzyme |
| AGII | Angiotensin II |
| AR | Aldose reductase |
| AChE | Acetylcholinesterase |
| CYP | Cytochrome P450 isoforms |
| BuChE | Butyrylcholinesterase |
| BCL-2 | B-cell lymphoma 2 |
| BACE1 | Aβ-site precursor protein-cleaving enzyme 1 |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP response element binding protein |
| CSF3 | Colony-stimulating factor 3 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A |
| CIDEA | Cell death-inducing DFFA-like effector A |
| COL1A1 | Collagen type 1 |
| CPT | Carnitine palmitoyltransferase |
| CYP7A1 | Cholesterol 7 alpha-hydroxylase |
| ERRFI1 | ERBB receptor feedback inhibitor 1 |
| DGAT2 | Diacylglycerol O-acyltransferase 2 |
| DPPH | Diphenyl-2-picrylhydrazyl |
| DA | Dopamine |
| DBP | Diastolic pressure |
| ERK-1 | Extracellular signal-regulated kinases-1 |
| EC 50 | Concentration for 50% of maximal effect |
| FFA | Free fatty acid |
| FOXO | Forkhead box O transcription factor |
| FAS | Fatty acid synthase |
| FGF2 | Fibroblast growth factor 2 |
| FoxO | Family of mammalian forkhead box O |
| FtsZ | Filamentous temperature sensitive protein Z |
| GK | Glucokinase |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GLUT4 | Glucose transporter 4 |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GABA | Gamma-aminobutyric acid |
| GPX | Glutathione peroxidase |
| GPT | Glutamic pyruvic transaminase |
| GDF-15 | Growth differentiation factor 15 |
| HbA1c | Glycosylated hemoglobin |
| H 2 O 2 | Hydrogen peroxide |
| HUVECs | Human umbilical vein endothelial cells |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HFFD | High-fat high-fructose diet |
| IFN-γ | Interferon-y |
| IC 50 | Half maximal inhibitory concentration |
| IPGTT | Intraperitoneal glucose tolerance test |
| IL | Interleukin |
| iNOS | Nitric oxide synthase |
| IRS | Insulin receptor substrate |
| LDH | Lactic dehydrogenase |
| LDL | Low-density lipoprotein |
| VLDL | Very-low density lipoprotein |
| LPS | Lipopolysaccharide |
| LHP | Lipid hydroperoxide |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| MAPK | Mitogen-activated protein kinase |
| MPO | Myeloperoxidase |
| MITF | Microphthalmia-associated transcription factor |
| MDA | Malondialdehyde |
| MABP | Mean arterial blood pressure |
| MMP | Matrix metallopeptidase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MAO | Monoamine oxidase |
| mTOR | Mammalian target of rapamycin |
| MAOs | Monoamine oxidases |
| MyD88 | Myeloid differentiation primary response gene 88 |
| MMP-13 | Matrix metallopeptidase 13 |
| MIC | Minimum inhibitory concentration |
| NE | Norepinephrine |
| NF-κB | Nuclear factor-kappa B |
| NAG | N-acetyl-β-D-glucosaminidase |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| NFAT | Nuclear factor of activated T cells |
| NQO1 | Quinone oxidoreductase |
| nAChR | Nicotinic ACh receptor |
| OGTT | Oral glucose tolerance test |
| PC | Protein carbonyl |
| PI3K | Phosphoinositide 3-kinase |
| PGE2 | Prostaglandin E2 |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PTP1B | Protein tyrosine phosphatase 1B |
| PAP | Phosphatidate phosphohydrolase |
| PMA | Phorbol myristate acetate |
| PCNA | Proliferating cell nuclear antigen |
| PKC | Protein kinase C |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| RLAR | Rat lens aldose reductase; SD, Sprague‒Dawley |
| SREBP | Sterol regulatory element-binding protein 1C |
| STZ | Streptozotocin |
| SOD | Superoxide dismutase |
| SDH | Sorbitol dehydrogenase |
| SIRT1 | Sirtuin-1 |
| SBP | Systolic pressure |
| SOCS1 | Suppressor of cytokine signaling 1 |
| TC | Total cholesterol |
| TG | Triglycerides |
| TBARS | Thiobarbituric acid reactive substance |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor α |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| TRP53 | Protein 53 tumor suppressor |
| VEGF-A | Vascular endothelial growth factor A |
| WAT | White adipose tissue |
References
- Abdullah N. H., Salim F., Ahmad R. (2016). Chemical constituents of Malaysian U. Cordata var. ferruginea and their in vitro α-glucosidase inhibitory activities. Molecules 21 (5), 525. 10.3390/molecules21050525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya D., Bogati B., Risal P. (2013). Scopoletin reduces intracellular survival of Salmonella typhi within U937 human macrophage cell line in vitro . Afr. J. Microbiol. Res. 1 (6), 47–51. [Google Scholar]
- Ahmadi N., Mohamed S., Sulaiman Rahman H., Rosli R. (2019). Epicatechin and scopoletin-rich Morinda citrifolia leaf ameliorated leukemia via anti-inflammatory, anti-angiogenesis, and apoptosis pathways in vitro and in vivo . J. Food Biochem. 43 (7), e12868. 10.1111/jfbc.12868 [DOI] [PubMed] [Google Scholar]
- Ahn M. J., Hur S. J., Kim E. H., Lee S. H., Shin J. S., Kim M. K., et al. (2014). Scopoletin from Cirsium setidens increases melanin synthesis via CREB phosphorylation in B16F10 cells. Korean J. Physiol. Pharmacol. 18 (4), 307–311. 10.4196/kjpp.2014.18.4.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkorashy A. I., Doghish A. S., Abulsoud A. I., Ewees M. G., Abdelghany T. M., Elshafey M. M., et al. (2020). Effect of scopoletin on phagocytic activity of U937-derived human macrophages: insights from transcriptomic analysis. Genomics 112 (5), 3518–3524. 10.1016/j.ygeno.2020.03.022 [DOI] [PubMed] [Google Scholar]
- Al-Zuaidy M. H., Hamid A. A., Ismail A., Mohamed S., Abdul Razis A. F., Mumtaz M. W., et al. (2016). Potent antidiabetic activity and metabolite profiling of melicope lunu-ankenda leaves. J. Food Sci. 81 (5), C1080–C1090. 10.1111/1750-3841.13293 [DOI] [PubMed] [Google Scholar]
- Antika L., Meilawati L., Dewi R., Tasfiyati A., Septama A. (2023). Scopoletin: anticancer potential and mechanism of action. Asian Pac. J. Trop. Biomed. 13 (1), 1. 10.4103/2221-1691.367685 [DOI] [Google Scholar]
- Asgar M. A., Senawong G., Sripa B., Senawong T. (2015). Scopoletin potentiates the anti-cancer effects of cisplatin against cholangiocarcinoma cell lines. Bangladesh J. Pharmacol. 10 (1), 69–77. 10.3329/bjp.v10i1.21202 [DOI] [Google Scholar]
- Baggieri M., Gioacchini S., Borgonovo G., Catinella G., Marchi A., Picone P., et al. (2023). Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2. Biomed. Pharmacother. 168, 115682. 10.1016/j.biopha.2023.115682 [DOI] [PubMed] [Google Scholar]
- Bak S. G., Lim H. J., Won Y. S., Lee S., Cheong S. H., Lee S. J., et al. (2022). Regulatory effects of Lycium barbarum extract and isolated scopoletin on atopic dermatitis-like skin inflammation. Biomed. Res. Int. 2022, 2475699. 10.1155/2022/2475699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro Arcos M. L., Cremaschi G., Werner S., Coussio J., Ferraro G., Anesini C. (2006). Tilia cordata Mill. Extracts and scopoletin (isolated compound): differential cell growth effects on lymphocytes. Phytother. Res. 20 (1), 34–40. 10.1002/ptr.1798 [DOI] [PubMed] [Google Scholar]
- Basu M., Mayana K., Xavier S., Balachandran S., Mishra N. (2016). Effect of scopoletin on monoamine oxidases and brain amines. Neurochem. Int. 93, 113–117. 10.1016/j.neuint.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Batra G. K., Anand A., Sharma S., Sharma S., Bhansali S., Patil A. N. (2023a). Scopoletin improves glucose homeostasis in the high-fructose high-fat diet-induced diabetes model in wistar rats. J. Med. Food 26 (4), 270–274. 10.1089/jmf.2022.K.0153 [DOI] [PubMed] [Google Scholar]
- Batra G. K., Mothsara C., Sharma S., Anand A., Bhatia A., Bhansali S., et al. (2023b). Dose-response evaluation of scopoletin, a phytochemical, in a high-fructose high-fat diet-induced dyslipidemia model in wistar rats. J. Med. Food 26 (5), 319–327. 10.1089/jmf.2022.K.0120 [DOI] [PubMed] [Google Scholar]
- Beh H. K., Seow L. J., Asmawi M. Z., Abdul Majid A. M., Murugaiyah V., Ismail N., et al. (2012). Anti-angiogenic activity of Morinda citrifolia extracts and its chemical constituents. Nat. Prod. Res. 26 (16), 1492–1497. 10.1080/14786419.2011.562208 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. S., Paul S., De A., Das D., Samadder A., Boujedaini N., et al. (2011). Poly (lactide-co-glycolide) acid nanoencapsulation of a synthetic coumarin: cytotoxicity and bio-distribution in mice, in cancer cell line and interaction with calf thymus DNA as target. Toxicol. Appl. Pharmacol. 253 (3), 270–281. 10.1016/j.taap.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Buathong R., Chamchumroon V., Schinnerl J., Bacher M., Santimaleeworagun W., Kraichak E., et al. (2019). Chemovariation and antibacterial activity of extracts and isolated compounds from species of Ixora and Greenea (Ixoroideae, Rubiaceae). PeerJ 7, e6893. 10.7717/peerj.6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Yang J., Zhou J., Lu W., Hu C., Gu Z., et al. (2013). Synthesis and biological evaluation of scopoletin derivatives. Bioorg Med. Chem. 21 (1), 84–92. 10.1016/j.bmc.2012.10.059 [DOI] [PubMed] [Google Scholar]
- Capra J. C., Cunha M. P., Machado D. G., Zomkowski A. D., Mendes B. G., Santos A. R., et al. (2010). Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 643 (2-3), 232–238. 10.1016/j.ejphar.2010.06.043 [DOI] [PubMed] [Google Scholar]
- Carpinella M. C., Ferrayoli C. G., Palacios S. M. (2005). Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 53 (8), 2922–2927. 10.1021/jf0482461 [DOI] [PubMed] [Google Scholar]
- Carradori S., Petzer J. P. (2015). Novel monoamine oxidase inhibitors: a patent review (2012 - 2014). Expert Opin. Ther. Pat. 25 (1), 91–110. 10.1517/13543776.2014.982535 [DOI] [PubMed] [Google Scholar]
- Cartolano F. C., Pappiani C., Freitas M. C. P., Figueiredo Neto A. M., Carioca A. A. F., Damasceno N. R. T. (2018). Is lipid accumulation product associated with an atherogenic lipoprotein profile in Brazilian subjects? Arq. Bras. Cardiol. 110 (4), 339–347. 10.5935/abc.20180054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaingam J., Juengwatanatrakul T., Yusakul G., Kanchanapoom T., Putalun W. (2021). HPLC-UV-Based simultaneous determination of canthin-6-one alkaloids, quassinoids, and scopoletin: the active ingredients in eurycoma longifolia jack and eurycoma harmandiana pierre, and their anti-inflammatory activities. J. AOAC Int. 104 (3), 802–810. 10.1093/jaoacint/qsaa141 [DOI] [PubMed] [Google Scholar]
- Chandrasekhar G., Shukla M., Kaul G., K R., Chopra S., Pandey R. (2023). Characterization and antimicrobial evaluation of anthraquinones and triterpenes from Rubia cordifolia. J. Asian Nat. Prod. Res. 25, 1110–1116. 10.1080/10286020.2023.2193698 [DOI] [PubMed] [Google Scholar]
- Chang W. C., Wu S. C., Xu K. D., Liao B. C., Wu J. F., Cheng A. S. (2015). Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation endproducts (AGEs) generation and anti-glycation. Molecules 20 (2), 2786–2801. 10.3390/molecules20022786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. X., Zhang Q. H., Li J., Zhang L., Guo X. R., He J., et al. (2013). Simultaneous determination of scopoletin, psoralen, bergapten, xanthotoxin, columbianetin acetate, imperatorin, osthole and isoimperatorin in rat plasma by LC-MS/MS for pharmacokinetic studies following oral administration of Radix Angelicae Pubescentis extract. J. Pharm. Biomed. Anal. 77, 71–75. 10.1016/j.jpba.2012.12.031 [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhou W., Huang Y., Tian Y., Wong S. Y., Lam W. K., et al. (2022). Umbelliferone and scopoletin target tyrosine kinases on fibroblast-like synoviocytes to block NF-κB signaling to combat rheumatoid arthritis. Front. Pharmacol. 13, 946210. 10.3389/fphar.2022.946210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zhu L., Yip K. M., Tang Y., Liu Y., Jiang T., et al. (2021). A hybrid platform featuring nanomagnetic ligand fishing for discovering COX-2 selective inhibitors from aerial part of Saussurea laniceps Hand.-Mazz. J. Ethnopharmacol. 271, 113849. 10.1016/j.jep.2021.113849 [DOI] [PubMed] [Google Scholar]
- Chen X., Zhu P., Liu B., Wei L., Xu Y. (2018). Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 159, 490–512. 10.1016/j.jpba.2018.07.023 [DOI] [PubMed] [Google Scholar]
- Cheng A. S., Cheng Y. H., Chang T. L. (2012). Scopoletin attenuates allergy by inhibiting Th2 cytokines production in EL-4 T cells. Food Funct. 3 (8), 886–890. 10.1039/c2fo30054k [DOI] [PubMed] [Google Scholar]
- Chiang C. C., Cheng M. J., Peng C. F., Huang H. Y., Chen I. S. (2010). A novel dimeric coumarin analog and antimycobacterial constituents from Fatoua pilosa. Chem. Biodivers. 7 (7), 1728–1736. 10.1002/cbdv.200900326 [DOI] [PubMed] [Google Scholar]
- Choi R. Y., Ham J. R., Lee H. I., Cho H. W., Choi M. S., Park S. K., et al. (2017). Scopoletin supplementation ameliorates steatosis and inflammation in diabetic mice. Phytother. Res. 31 (11), 1795–1804. 10.1002/ptr.5925 [DOI] [PubMed] [Google Scholar]
- Choi Y. G., Yeo S., Kim S. H., Lim S. (2012). Anti-inflammatory changes of gene expression by Artemisia iwayomogi in the LPS-stimulated human gingival fibroblast: microarray analysis. Arch. Pharm. Res. 35 (3), 549–563. 10.1007/s12272-012-0319-0 [DOI] [PubMed] [Google Scholar]
- Collin M., Ginhoux F. (2019). Human dendritic cells. Semin. Cell Dev. Biol. 86, 1–2. 10.1016/j.semcdb.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Das R., Sengupta T., Roy S., Chattarji S., Ray J. (2020a). Convolvulus pluricaulis extract can modulate synaptic plasticity in rat brain hippocampus. Neuroreport 31 (8), 597–604. 10.1097/wnr.0000000000001446 [DOI] [PubMed] [Google Scholar]
- Das S., Czuni L., Báló V., Papp G., Gazdag Z., Papp N., et al. (2020b). Cytotoxic action of artemisinin and scopoletin on planktonic forms and on biofilms of Candida species. Molecules 25 (3), 476. 10.3390/molecules25030476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De LaCruz-Sánchez N. G., Gómez-Rivera A., Alvarez-Fitz P., Ventura-Zapata E., Pérez-García M. D., Avilés-Flores M., et al. (2019). Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog. 128, 347–353. 10.1016/j.micpath.2019.01.030 [DOI] [PubMed] [Google Scholar]
- Ding Z., Dai Y., Hao H., Pan R., Yao X., Wang Z. (2008). Anti-inflammatory effects of scopoletin and underlying mechanisms. Pharm. Biol. 46 (12), 854–860. 10.1080/13880200802367155 [DOI] [Google Scholar]
- Ding Z., Dai Y., Wang Z. (2005). Hypouricemic action of scopoletin arising from xanthine oxidase inhibition and uricosuric activity. Planta Med. 71 (2), 183–185. 10.1055/s-2005-837789 [DOI] [PubMed] [Google Scholar]
- Dou Y., Tong B., Wei Z., Li Y., Xia Y., Dai Y. (2013). Scopoletin suppresses IL-6 production from fibroblast-like synoviocytes of adjuvant arthritis rats induced by IL-1β stimulation. Int. Immunopharmacol. 17 (4), 1037–1043. 10.1016/j.intimp.2013.10.011 [DOI] [PubMed] [Google Scholar]
- Duggirala S., Nankar R. P., Rajendran S., Doble M. (2014). Phytochemicals as inhibitors of bacterial cell division protein FtsZ: coumarins are promising candidates. Appl. Biochem. Biotechnol. 174 (1), 283–296. 10.1007/s12010-014-1056-2 [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Mattevi A., Binda C., Li M., Hubálek F. (2004). Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 11 (15), 1983–1993. 10.2174/0929867043364784 [DOI] [PubMed] [Google Scholar]
- Ezzat S. M., Abdallah H. M., Fawzy G. A., El-Maraghy S. A. (2012). Hepatoprotective constituents of Torilis radiata moench (apiaceae). Nat. Prod. Res. 26 (3), 282–285. 10.1080/14786419.2011.587422 [DOI] [PubMed] [Google Scholar]
- Fan J., de Lannoy I. A. (2014). Pharmacokinetics. Biochem. Pharmacol. 87 (1), 93–120. 10.1016/j.bcp.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Fan Z., Tang D., Wu Q., Huang Q., Song J., Long Q. (2022). Scopoletin inhibits PDGF-BB-induced proliferation and migration of airway smooth muscle cells by regulating NF-κB signaling pathway. Allergol. Immunopathol. Madr. 50 (1), 92–98. 10.15586/aei.v50i1.517 [DOI] [PubMed] [Google Scholar]
- Farah M. H., Samuelsson G. (1992). Pharmacologically active phenylpropanoids from Senra incana. Planta Med. 58 (1), 14–18. 10.1055/s-2006-961380 [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira T. H., Guimaraes I. M., Silva F. R., Ribeiro F. M. (2016). Alzheimer's disease: targeting the cholinergic system. Curr. Neuropharmacol. 14 (1), 101–115. 10.2174/1570159x13666150716165726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forino M., Tartaglione L., Dell'Aversano C., Ciminiello P. (2016). NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 194, 1254–1259. 10.1016/j.foodchem.2015.08.129 [DOI] [PubMed] [Google Scholar]
- Förstermann U., Xia N., Li H. (2017). Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120 (4), 713–735. 10.1161/circresaha.116.309326 [DOI] [PubMed] [Google Scholar]
- Galkin A., Fallarero A., Vuorela P. M. (2009). Coumarins permeability in Caco-2 cell model. J. Pharm. Pharmacol. 61 (2), 177–184. 10.1211/jpp/61.02.0006 [DOI] [PubMed] [Google Scholar]
- Garg G., Patil A., Singh J., Kaushik N., Praksah A., Pal A., et al. (2018). Pharmacological evaluation of Convolvulus pluricaulis as hypolipidaemic agent in Triton WR-1339-induced hyperlipidaemia in rats. J. Pharm. Pharmacol. 70 (11), 1572–1580. 10.1111/jphp.13004 [DOI] [PubMed] [Google Scholar]
- Gay N. H., Suwanjang W., Ruankham W., Songtawee N., Wongchitrat P., Prachayasittikul V., et al. (2020). Butein, isoliquiritigenin, and scopoletin attenuate neurodegeneration via antioxidant enzymes and SIRT1/ADAM10 signaling pathway. RSC Adv. 10 (28), 16593–16606. 10.1039/c9ra06056a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geovanini G. R., Libby P. (2018). Atherosclerosis and inflammation: overview and updates. Clin. Sci. (Lond) 132 (12), 1243–1252. 10.1042/cs20180306 [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Bacskai B. J., Hernandez-Guillamon M., Pruzin J., Sperling R., van Veluw S. J. (2020). Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol. 16 (1), 30–42. 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J. R., Lee H.-I., Choi R.-Y., Sim M.-O., Choi M.-S., Kwon E.-Y., et al. (2016). Anti-obesity and anti-hepatosteatosis effects of dietary scopoletin in high-fat diet fed mice. J. Funct. Foods 25, 433–446. 10.1016/j.jff.2016.06.026 [DOI] [Google Scholar]
- Hayes A. W., Dixon D. (2017). Cornerstones of toxicology. Toxicol. Pathol. 45 (1), 57–63. 10.1177/0192623316675768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heriniaina R. M., Dong J., Kalavagunta P. K., Wu H. L., Yan D. S., Shang J. (2018). Effects of six compounds with different chemical structures on melanogenesis. Chin. J. Nat. Med. 16 (10), 766–773. 10.1016/s1875-5364(18)30116-x [DOI] [PubMed] [Google Scholar]
- Hornick A., Lieb A., Vo N. P., Rollinger J. M., Stuppner H., Prast H. (2011). The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic- and age-impaired memory. Neuroscience 197 (1), 280–292. 10.1016/j.neuroscience.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iahtisham Ul H., Butt M. S., Randhawa M. A., Shahid M. (2019). Hepatoprotective effects of red beetroot-based beverages against CCl(4) -induced hepatic stress in Sprague Dawley rats. J. Food Biochem. 43 (12), e13057. 10.1111/jfbc.13057 [DOI] [PubMed] [Google Scholar]
- Iizuka T., Nagumo S., Yotsumoto H., Moriyama H., Nagai M. (2007). Vasorelaxant effects of Acer nikoense extract and isolated coumarinolignans on rat aortic rings. Biol. Pharm. Bull. 30 (6), 1164–1166. 10.1248/bpb.30.1164 [DOI] [PubMed] [Google Scholar]
- Issell B. F., Franke A., Fielding R. M. (2008). Pharmacokinetic study of Noni fruit extract. J. Diet. Suppl. 5 (4), 373–382. 10.1080/19390210802519671 [DOI] [PubMed] [Google Scholar]
- Jamuna S., Karthika K., Paulsamy S., Thenmozhi K., Kathiravan S., Venkatesh R. (2015). Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Industrial Crops Prod. 70, 221–230. 10.1016/j.indcrop.2015.03.039 [DOI] [Google Scholar]
- Jang J. H., Park J. E., Han J. S. (2018). Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 834, 152–156. 10.1016/j.ejphar.2018.07.032 [DOI] [PubMed] [Google Scholar]
- Jang J. H., Park J. E., Han J. S. (2020). Scopoletin increases glucose uptake through activation of PI3K and AMPK signaling pathway and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. 74, 52–61. 10.1016/j.nutres.2019.12.003 [DOI] [PubMed] [Google Scholar]
- Ji J., Zhang Z., Peng Q., Hao L., Guo Y., Xue Y., et al. (2022). The effects of qinghao-kushen and its active compounds on the biological characteristics of liver cancer cells. Evid. Based Complement. Altern. Med. 2022, 8763510. 10.1155/2022/8763510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai K., Mizutani M., Kawamura N., Yamamoto R., Tamai M., Yamaguchi H., et al. (2008). Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana . Plant J. 55 (6), 989–999. 10.1111/j.1365-313X.2008.03568.x [DOI] [PubMed] [Google Scholar]
- Kalpana K., Sathiya Priya C., Dipti N., Vidhya R., Anuradha C. V. (2019). Supplementation of scopoletin improves insulin sensitivity by attenuating the derangements of insulin signaling through AMPK. Mol. Cell Biochem. 453 (1-2), 65–78. 10.1007/s11010-018-3432-7 [DOI] [PubMed] [Google Scholar]
- Kamino T., Shimokura T., Morita Y., Tezuka Y., Nishizawa M., Tanaka K. (2016). Comparative analysis of the constituents in Saposhnikoviae Radix and Glehniae Radix cum Rhizoma by monitoring inhibitory activity of nitric oxide production. J. Nat. Med. 70 (2), 253–259. 10.1007/s11418-016-0969-1 [DOI] [PubMed] [Google Scholar]
- Kang S. Y., Sung S. H., Park J. H., Kim Y. C. (1998). Hepatoprotective activity of scopoletin, a constituent of Solanum lyratum. Arch. Pharm. Res. 21 (6), 718–722. 10.1007/bf02976764 [DOI] [PubMed] [Google Scholar]
- Kang T. H., Pae H. O., Jeong S. J., Yoo J. C., Choi B. M., Jun C. D., et al. (1999). Scopoletin: an inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med. 65 (5), 400–403. 10.1055/s-1999-14014 [DOI] [PubMed] [Google Scholar]
- Kashyap P., Ram H., Shukla S. D., Kumar S. (2020). Scopoletin: antiamyloidogenic, anticholinesterase, and neuroprotective potential of a natural compound present in Argyreia speciosa roots by in vitro and in silico study. Neurosci. Insights 15, 2633105520937693. 10.1177/2633105520937693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M., Prakash A., Kalia A. N. (2016). Neuroprotective potential of antioxidant potent fractions from Convolvulus pluricaulis Chois. in 3-nitropropionic acid challenged rats. Nutr. Neurosci. 19 (2), 70–78. 10.1179/1476830515y.0000000022 [DOI] [PubMed] [Google Scholar]
- Khalaf M. M., Hassan S. M., Sayed A. M., Abo-Youssef A. M. (2022). Ameliorate impacts of scopoletin against vancomycin-induced intoxication in rat model through modulation of Keap1-Nrf2/HO-1 and IκBα-P65 NF-κB/P38 MAPK signaling pathways: molecular study, molecular docking evidence and network pharmacology analysis. Int. Immunopharmacol. 102, 108382. 10.1016/j.intimp.2021.108382 [DOI] [PubMed] [Google Scholar]
- Khuda-Bukhsh A. R., Bhattacharyya S. S., Paul S., Boujedaini N. (2010). Polymeric nanoparticle encapsulation of a naturally occurring plant scopoletin and its effects on human melanoma cell A375. Zhong Xi Yi Jie He Xue Bao 8 (9), 853–862. 10.3736/jcim20100909 [DOI] [PubMed] [Google Scholar]
- Khunluck T., Kukongviriyapan V., Senggunprai L., Duangarsong W., Prawan A. (2019). The inhibition kinetics and potential anti-migration activity of NQO1 inhibitory coumarins on cholangiocarcinoma cells. Integr. Cancer Ther. 18, 1534735418820444. 10.1177/1534735418820444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunnawutmanotham N., Chimnoi N., Saparpakorn P., Techasakul S. (2016). Synthesis and anti-acetylcholinesterase activity of scopoletin derivatives. Bioorg Chem. 65, 137–145. 10.1016/j.bioorg.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Kim D. S., Cha S. B., Park M. C., Park S. A., Kim H. S., Woo W. H., et al. (2017a). Scopoletin stimulates melanogenesis via cAMP/PKA pathway and partially p38 activation. Biol. Pharm. Bull. 40 (12), 2068–2074. 10.1248/bpb.b16-00690 [DOI] [PubMed] [Google Scholar]
- Kim E. K., Kwon K. B., Shin B. C., Seo E. A., Lee Y. R., Kim J. S., et al. (2005). Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor kappaB and caspase-3. Life Sci. 77 (7), 824–836. 10.1016/j.lfs.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Kim H. G., Lee S. B., Lee J. S., Kim W. Y., Choi S. H., Son C. G. (2017b). Artemisia iwayomogi plus curcuma longa synergistically ameliorates nonalcoholic steatohepatitis in HepG2 cells. Evid. Based Complement. Altern. Med., 2017, 4390636. 10.1155/2017/4390636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Jang S. I., Kim Y. J., Chung H. T., Yun Y. G., Kang T. H., et al. (2004). Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia 75 (3-4), 261–266. 10.1016/j.fitote.2003.12.021 [DOI] [PubMed] [Google Scholar]
- Kim H. L., Woo S. M., Choi W. R., Kim H. S., Yi C., Kim K. H., et al. (2018). Scopoletin downregulates MMP-1 expression in human fibroblasts via inhibition of p38 phosphorylation. Int. J. Mol. Med. 42 (4), 2285–2293. 10.3892/ijmm.2018.3757 [DOI] [PubMed] [Google Scholar]
- Kim J., Kim C. S., Lee Y. M., Sohn E., Jo K., Shin S. D., et al. (2013). Scopoletin inhibits rat aldose reductase activity and cataractogenesis in galactose-fed rats. Evid. Based Complement. Altern. Med., 2013, 787138. 10.1155/2013/787138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. Y., Pae H. O., Ko Y. S., Yoo J. C., Choi B. M., Jun C. D., et al. (1999). In vitro inducible nitric oxide synthesis inhibitory active constituents from Fraxinus rhynchophylla. Planta Med. 65 (7), 656–658. 10.1055/s-2006-960840 [DOI] [PubMed] [Google Scholar]
- Kimura M., Kawahito Y., Obayashi H., Ohta M., Hara H., Adachi T., et al. (2007). A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J. Immunol. 178 (5), 3316–3322. 10.4049/jimmunol.178.5.3316 [DOI] [PubMed] [Google Scholar]
- Kishore Kumar S. N., Deepthy J., Saraswathi U., Thangarajeswari M., Yogesh Kanna S., Ezhil P., et al. (2017). Morinda citrifolia mitigates rotenone-induced striatal neuronal loss in male Sprague-Dawley rats by preventing mitochondrial pathway of intrinsic apoptosis. Redox Rep. 22 (6), 418–429. 10.1080/13510002.2016.1253449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. S., Choi W. G., Kim W. J., Kim W. K., Kim M. J., Kang W. H., et al. (2002). Antimicrobial constituents of Foeniculum vulgare. Arch. Pharm. Res. 25 (2), 154–157. 10.1007/bf02976556 [DOI] [PubMed] [Google Scholar]
- Lagunas-Herrera H., Tortoriello J., Herrera-Ruiz M., Martínez-Henández G. B., Zamilpa A., Santamaría L. A., et al. (2019). Acute and chronic antihypertensive effect of fractions, tiliroside and scopoletin from Malva parviflora. Biol. Pharm. Bull. 42 (1), 18–25. 10.1248/bpb.b18-00355 [DOI] [PubMed] [Google Scholar]
- Lee E. J., Na W., Kang M. K., Kim Y. H., Kim D. Y., Oh H., et al. (2021). Hydroxycoumarin scopoletin inhibits bone loss through enhancing induction of bone turnover markers in a mouse model of type 2 diabetes. Biomedicines 9 (6), 648. 10.3390/biomedicines9060648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. E., Yang G., Choi J. S., Lee J. Y. (2017). Suppression of primary splenocyte proliferation by Artemisia capillaris and its components. Toxicol. Res. 33 (4), 283–290. 10.5487/tr.2017.33.4.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-I., Lee M.-K. (2015a). Effects of scopoletin supplementation on insulin resistance and antioxidant defense system in chronic alcohol-fed rats. J. Korean Soc. Food Sci. Nutr. 44 (2), 173–181. 10.3746/jkfn.2015.44.2.173 [DOI] [Google Scholar]
- Lee H. I., Lee M. K. (2015b). Coordinated regulation of scopoletin at adipose tissue-liver axis improved alcohol-induced lipid dysmetabolism and inflammation in rats. Toxicol. Lett. 237 (3), 210–218. 10.1016/j.toxlet.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Lee H. I., Yun K. W., Seo K. I., Kim M. J., Lee M. K. (2014). Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK-SREBP pathway in diet-induced obese mice. Metabolism 63 (4), 593–601. 10.1016/j.metabol.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Lee J., Kim N. H., Nam J. W., Lee Y. M., Jang D. S., Kim Y. S., et al. (2010). Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo . Arch. Pharm. Res. 33 (9), 1317–1323. 10.1007/s12272-010-0904-z [DOI] [PubMed] [Google Scholar]
- Lee S. H., Ding Y., Yan X. T., Kim Y. H., Jang H. D. (2013). Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J. Nat. Prod. 76 (4), 615–620. 10.1021/np300824h [DOI] [PubMed] [Google Scholar]
- Leema G., Tamizhselvi R. (2018). Protective effect of scopoletin against cerulein-induced acute pancreatitis and associated lung injury in mice. Pancreas 47 (5), 577–585. 10.1097/mpa.0000000000001034 [DOI] [PubMed] [Google Scholar]
- Lemos A. S. O., Florêncio J. R., Pinto N. C. C., Campos L. M., Silva T. P., Grazul R. M., et al. (2020). Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front. Microbiol. 11, 1525. 10.3389/fmicb.2020.01525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lu M., Chu Z., Lei S., Sun P., Xiong S., et al. (2019). Evaluation of pharmacokinetics, bioavailability and urinary excretion of scopolin and its metabolite scopoletin in Sprague Dawley rats by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 33 (12), e4678. 10.1002/bmc.4678 [DOI] [PubMed] [Google Scholar]
- Li J., Zhang C., Gong M., Wang M. (2018). Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria: pharmacodynamic and pharmacokinetic studies. Phytother. Res. 32 (7), 1415–1420. 10.1002/ptr.6077 [DOI] [PubMed] [Google Scholar]
- Li S., Zhan J., Wang Y., Oduro P. K., Owusu F. B., Zhang J., et al. (2023). Suxiao Jiuxin Pill attenuates acute myocardial ischemia via regulation of coronary artery tone. Front. Pharmacol. 14, 1104243. 10.3389/fphar.2023.1104243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Zeng X., Guo J., Liu H., He B., Lai R., et al. (2021). Scopoletin and umbelliferone from Cortex Mori as protective agents in high glucose-induced mesangial cell as in vitro model of diabetic glomerulosclerosis. Chin. J. Physiol. 64 (3), 150–158. 10.4103/cjp.cjp_9_21 [DOI] [PubMed] [Google Scholar]
- Littlewood R. (2001). From psychiatric patient to citizen: overcoming discrimination and social exclusion by liz Sayce. Basingstoke: macmillan. 2000. 280 pp. £13.99 (pb). ISBN 0 333 69890 8. Psychiatric Patient Citiz. Overcoming Discrimination Soc. Exclusion By Liz Sayce. Basingstoke Macmillan Br. J. Psychiatry 179 (2), 182. 10.1192/bjp.179.2.182 [DOI] [Google Scholar]
- Liu M., Shi X., Yang W., Liu S., Wang N., Shi R., et al. (2011). Quantitative analysis of nine coumarins in rat urine and bile after oral administration of Radix Glehniae extract by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 25 (7), 783–793. 10.1002/bmc.1517 [DOI] [PubMed] [Google Scholar]
- Liu X. L., Zhang L., Fu X. L., Chen K., Qian B. C. (2001). Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 22 (10), 929–933. [PubMed] [Google Scholar]
- Luo L., Sun T., Yang L., Liu A., Liu Q. Q., Tian Q. Q., et al. (2020). Scopoletin ameliorates anxiety-like behaviors in complete Freund's adjuvant-induced mouse model. Mol. Brain 13 (1), 15. 10.1186/s13041-020-0560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahattanadul S., Ridtitid W., Nima S., Phdoongsombut N., Ratanasuwon P., Kasiwong S. (2011). Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J. Ethnopharmacol. 134 (2), 243–250. 10.1016/j.jep.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Mamoon Ur R., Ali S., Alamzeb M., Igoli J., Clements C., Shah S. Q., et al. (2014). Phytochemical and antitrypanosomal investigation of the fractions and compounds isolated from Artemisia elegantissima. Pharm. Biol. 52 (8), 983–987. 10.3109/13880209.2013.874534 [DOI] [PubMed] [Google Scholar]
- Mandukhail S. U., Aziz N., Gilani A. H. (2010). Studies on antidyslipidemic effects of Morinda citrifolia (Noni) fruit, leaves and root extracts. Lipids Health Dis. 9, 88. 10.1186/1476-511x-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankar M., Akhtar S., Kumar C. S., Ravi S., Kumar S., Gothlwal R., et al. (2013). Evaluation of noni extract on mouse macrophages for its immune-enhancer property. Int. J. Phytomedicine 5 (4), 499. [Google Scholar]
- Manuele M. G., Ferraro G., Barreiro Arcos M. L., López P., Cremaschi G., Anesini C. (2006). Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci. 79 (21), 2043–2048. 10.1016/j.lfs.2006.06.045 [DOI] [PubMed] [Google Scholar]
- Marealle A. I., Moyo A. A., Machumi F., Qwarse M., Chenyambuga Y. M., Heydenreich M., et al. (2023). Antimycobacterial activity of scopoletin from ethanolic extract of Hymenodictyon floribundum (Hochst. and Steud.) B.L.Rob. Stem bark. Sci. Afr. 21, e01778. 10.1016/j.sciaf.2023.e01778 [DOI] [Google Scholar]
- Medrano-Jiménez E., Jiménez-Ferrer Carrillo I., Pedraza-Escalona M., Ramírez-Serrano C. E., Álvarez-Arellano L., Cortés-Mendoza J., et al. (2019). Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer's disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflammation 16 (1), 143. 10.1186/s12974-019-1515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerungrueang W., Panichayupakaranant P. (2014). Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharm. Biol. 52 (9), 1104–1109. 10.3109/13880209.2013.877493 [DOI] [PubMed] [Google Scholar]
- Meotti F. C., Ardenghi J. V., Pretto J. B., Souza M. M., d' Avila Moura J., Junior A. C., et al. (2006). Antinociceptive properties of coumarins, steroid and dihydrostyryl-2-pyrones from Polygala sabulosa (Polygalaceae) in mice. J. Pharm. Pharmacol. 58 (1), 107–112. 10.1211/jpp.58.1.0013 [DOI] [PubMed] [Google Scholar]
- Min W., Li_ling Z., Rui L. (2000). Determination of plasma concentration of total scopoletin by RP-HPLC. TRADITIONAL Chin. DRUG Res. Clin. Pharmacol. 1. [Google Scholar]
- Mishra N., Oraon A., Dev A., Jayaprakash V., Basu A., Pattnaik A. K., et al. (2010). Anticonvulsant activity of Benkara malabarica (Linn.) root extract: in vitro and in vivo investigation. J. Ethnopharmacol. 128 (2), 533–536. 10.1016/j.jep.2010.01.042 [DOI] [PubMed] [Google Scholar]
- Mogana R., Teng-Jin K., Wiart C. (2013). Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from canarium patentinervium Miq. (Burseraceae kunth). Evid. Based Complement. Altern. Med., 2013, 734824. 10.1155/2013/734824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad Shalan N. A., Mustapha N. M., Mohamed S. (2016). Morinda citrifolia leaf enhanced performance by improving angiogenesis, mitochondrial biogenesis, antioxidant, anti-inflammatory and stress responses. Food Chem. 212, 443–452. 10.1016/j.foodchem.2016.05.179 [DOI] [PubMed] [Google Scholar]
- Molokoane T. L., Kemboi D., Siwe-Noundou X., Famuyide I. M., McGaw L. J., Tembu V. J. (2023). Extractives from Artemisia afra with anti-bacterial and anti-fungal properties. Plants (Basel) 12 (19), 3369. 10.3390/plants12193369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsef-Esfahani H. R., Amini M., Goodarzi N., Saiedmohammadi F., Hajiaghaee R., Faramarzi M. A., et al. (2013). Coumarin compounds of Biebersteinia multifida roots show potential anxiolytic effects in mice. Daru 21 (1), 51. 10.1186/2008-2231-21-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon P. D., Lee B. H., Jeong H. J., An H. J., Park S. J., Kim H. R., et al. (2007). Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IkappaB/NF-kappaB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 555 (2-3), 218–225. 10.1016/j.ejphar.2006.10.021 [DOI] [PubMed] [Google Scholar]
- More G., Lall N., Hussein A., Tshikalange T. E. (2012). Antimicrobial constituents of Artemisia afra jacq. Ex Willd. Against periodontal pathogens. Evid. Based Complement. Altern. Med. 2012, 252758. 10.1155/2012/252758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschietti L., Gorzalczany S., Ferraro G., Acevedo C., Martino V. (2001). Phenolic compounds with anti-inflammatory activity from Eupatorium buniifolium. Planta Med. 67 (8), 743–744. 10.1055/s-2001-18355 [DOI] [PubMed] [Google Scholar]
- Nabekura T., Hiroi T., Kawasaki T., Uwai Y. (2015). Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 70, 140–145. 10.1016/j.biopha.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Nahata A., Dixit V. K. (2012). Ameliorative effects of stinging nettle (Urtica dioica) on testosterone-induced prostatic hyperplasia in rats. Andrologia 44 (Suppl. 1), 396–409. 10.1111/j.1439-0272.2011.01197.x [DOI] [PubMed] [Google Scholar]
- Nam H., Kim M. M. (2015). Scopoletin has a potential activity for anti-aging via autophagy in human lung fibroblasts. Phytomedicine 22 (3), 362–368. 10.1016/j.phymed.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Napiroon T., Bacher M., Balslev H., Tawaitakham K., Santimaleeworagun W., Vajrodaya S. (2018). Scopoletin from Lasianthus lucidus Blume (Rubiaceae): a potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa . J. Appl. Pharm. Sci. 8 (9), 1–6. 10.7324/JAPS.2018.8901 [DOI] [Google Scholar]
- Narasimhan K. K. S., Jayakumar D., Velusamy P., Srinivasan A., Mohan T., Ravi D. B., et al. (2019). Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/nrf2/ARE signaling pathway. Oxid. Med. Cell Longev., 2019, 2761041. 10.1155/2019/2761041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri S., Delnavazi M. R., Shirazi F. H., Mojab F. (2022). Cytotoxic activity and phytochemical analysis of Artemisia haussknechtii boiss. Iran. J. Pharm. Res. 21 (1), e126917. 10.5812/ijpr-126917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-García V. M., Rojas G., Avilés M., Fuentes M., Zepeda G. (2011). In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 54 (5), e569–e571. 10.1111/j.1439-0507.2010.01993.x [DOI] [PubMed] [Google Scholar]
- Ng T. B., Liu F., Lu Y., Cheng C. H., Wang Z. (2003). Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 136 (2), 109–115. 10.1016/s1532-0456(03)00170-4 [DOI] [PubMed] [Google Scholar]
- Nima S., Kasiwong S., Ridtitid W., Thaenmanee N., Mahattanadul S. (2012). Gastrokinetic activity of Morinda citrifolia aqueous fruit extract and its possible mechanism of action in human and rat models. J. Ethnopharmacol. 142 (2), 354–361. 10.1016/j.jep.2012.04.044 [DOI] [PubMed] [Google Scholar]
- Noh J. R., Gang G. T., Kim Y. H., Yang K. J., Hwang J. H., Lee H. S., et al. (2010). Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4-and high-fat diet-treated mice. Food Chem. Toxicol. 48 (11), 3177–3183. 10.1016/j.fct.2010.08.018 [DOI] [PubMed] [Google Scholar]
- Noh J. R., Kim Y. H., Gang G. T., Hwang J. H., Lee H. S., Ly S. Y., et al. (2011). Hepatoprotective effects of chestnut (Castanea crenata) inner shell extract against chronic ethanol-induced oxidative stress in C57BL/6 mice. Food Chem. Toxicol. 49 (7), 1537–1543. 10.1016/j.fct.2011.03.045 [DOI] [PubMed] [Google Scholar]
- Ojewole J. A., Adesina S. K. (1983). Mechanism of the hypotensive effect of scopoletin isolated from the fruit of Tetrapleura tetraptera. Planta Med. 49 (1), 46–50. 10.1055/s-2007-969809 [DOI] [PubMed] [Google Scholar]
- Oliveira E. J., Romero M. A., Silva M. S., Silva B. A., Medeiros I. A. (2001). Intracellular calcium mobilization as a target for the spasmolytic action of scopoletin. Planta Med. 67 (7), 605–608. 10.1055/s-2001-17355 [DOI] [PubMed] [Google Scholar]
- Page G., Lebecque S., Miossec P. (2002). Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J. Immunol. 168 (10), 5333–5341. 10.4049/jimmunol.168.10.5333 [DOI] [PubMed] [Google Scholar]
- Pan J., Liu H., Wu Q., Zhou M. (2022). Scopoletin protects retinal ganglion cells 5 from high glucose-induced injury in a cellular model of diabetic retinopathy via ROS-dependent p38 and JNK signaling cascade. Cent. Eur. J. Immunol. 47 (1), 20–29. 10.5114/ceji.2022.115710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R., Dai Y., Gao X. H., Lu D., Xia Y. F. (2011a). Inhibition of vascular endothelial growth factor-induced angiogenesis by scopoletin through interrupting the autophosphorylation of VEGF receptor 2 and its downstream signaling pathways. Vasc. Pharmacol. 54 (1-2), 18–28. 10.1016/j.vph.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Pan R., Dai Y., Yang J., Li Y., Yao X., Xia Y. (2009). Anti-angiogenic potential of scopoletin is associated with the inhibition of ERK1/2 activation. Drug Dev. Res. 70 (3), 214–219. 10.1002/ddr.20297 [DOI] [Google Scholar]
- Pan R., Gao X., Lu D., Xu X., Xia Y., Dai Y. (2011b). Prevention of FGF-2-induced angiogenesis by scopoletin, a coumarin compound isolated from Erycibe obtusifolia Benth, and its mechanism of action. Int. Immunopharmacol. 11 (12), 2007–2016. 10.1016/j.intimp.2011.08.012 [DOI] [PubMed] [Google Scholar]
- Pan R., Gao X. H., Li Y., Xia Y. F., Dai Y. (2010). Anti-arthritic effect of scopoletin, a coumarin compound occurring in Erycibe obtusifolia Benth stems, is associated with decreased angiogenesis in synovium. Fundam. Clin. Pharmacol. 24 (4), 477–490. 10.1111/j.1472-8206.2009.00784.x [DOI] [PubMed] [Google Scholar]
- Panda S., Kar A. (2006). Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother. Res. 20 (12), 1103–1105. 10.1002/ptr.2014 [DOI] [PubMed] [Google Scholar]
- Pandy V., Vijeepallam K. (2017). Antipsychotic-like activity of scopoletin and rutin against the positive symptoms of schizophrenia in mouse models. Exp. Anim. 66 (4), 417–423. 10.1538/expanim.17-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Han J. S. (2023). Scopoletin protects INS-1 pancreatic β cells from glucotoxicity by reducing oxidative stress and apoptosis. Toxicol Vitro 93, 105665. 10.1016/j.tiv.2023.105665 [DOI] [PubMed] [Google Scholar]
- Park J. E., Kim S. Y., Han J. S. (2022). Scopoletin stimulates the secretion of insulin via a KATP channel-dependent pathway in INS-1 pancreatic β cells. J. Pharm. Pharmacol. 74 (9), 1274–1281. 10.1093/jpp/rgab143 [DOI] [PubMed] [Google Scholar]
- Park S. H., Sung Y. Y., Nho K. J., Kim D. S., Kim H. K. (2017). Effects of Viola mandshurica on atherosclerosis and hepatic steatosis in ApoE[formula: see text] via the AMPK pathway. Am. J. Chin. Med. 45 (4), 757–772. 10.1142/s0192415x17500409 [DOI] [PubMed] [Google Scholar]
- Patel M. R., Bhalodia Y. S., Pathak N. L., Patel M. S., Suthar K., Patel N., et al. (2013). Study on the mechanism of the bronchodilatory effects of Cynodon dactylon (Linn.) and identification of the active ingredient. J. Ethnopharmacol. 150 (3), 946–952. 10.1016/j.jep.2013.09.053 [DOI] [PubMed] [Google Scholar]
- Pereira Dos Santos Nascimento M. V., Arruda-Silva F., Gobbo Luz A. B., Baratto B., Venzke D., Mendes B. G., et al. (2016). Inhibition of the NF-κB and p38 MAPK pathways by scopoletin reduce the inflammation caused by carrageenan in the mouse model of pleurisy. Immunopharmacol. Immunotoxicol. 38 (5), 344–352. 10.1080/08923973.2016.1203929 [DOI] [PubMed] [Google Scholar]
- Pichler R., Sfetsos K., Badics B., Gutenbrunner S., Auböck J. (2006). Vitiligo patients present lower plasma levels of alpha-melanotropin immunoreactivities. Neuropeptides 40 (3), 177–183. 10.1016/j.npep.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Pradhan P., Majhi O., Biswas A., Joshi V. K., Sinha D. (2020). Enhanced accumulation of reduced glutathione by Scopoletin improves survivability of dopaminergic neurons in Parkinson's model. Cell Death Dis. 11 (9), 739. 10.1038/s41419-020-02942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompipak J., Senawong T., Sripa B., Ketterman A. J., Utaiwat S., Woranam K., et al. (2021). Anticancer effects of the combined Thai noni juice ethanolic extracts and 5-fluorouracil against cholangiocarcinoma cells in vitro and in vivo . Sci. Rep. 11 (1), 14866. 10.1038/s41598-021-94049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Reyes T. I., Aguilar-Colorado Á S., Murrieta-León D. L., Licona-Velázquez L. S., Bonilla-Landa I., Durán-Espinosa C., et al. (2019). Identification of antibacterial phenolics in selected plant species from Mexican cloud forest by mass spectrometry dereplication. Chem. Biodivers. 16 (4), e1800603. 10.1002/cbdv.201800603 [DOI] [PubMed] [Google Scholar]
- Rauf A., Imran M., Suleria H. A. R., Ahmad B., Peters D. G., Mubarak M. S. (2017). A comprehensive review of the health perspectives of resveratrol. Food Funct. 8 (12), 4284–4305. 10.1039/c7fo01300k [DOI] [PubMed] [Google Scholar]
- Ren W., Wang Y., He Q., Zhou Y., Li C., Wang W., et al. (2020). Chemical composition of Erycibe schmidtii and antiproliferative activity of scopoletin on immature dendritic cells. Nat. Prod. Res. 34 (18), 2581–2588. 10.1080/14786419.2018.1547292 [DOI] [PubMed] [Google Scholar]
- Ribas C. M., Meotti F. C., Nascimento F. P., Jacques A. V., Dafre A. L., Rodrigues A. L. S., et al. (2008). Antinociceptive effect of the Polygala sabulosa hydroalcoholic extract in mice: evidence for the involvement of glutamatergic receptors and cytokine pathways. Basic and Clin. Pharmacol. Toxicol. 103 (1), 43–47. 10.1111/j.1742-7843.2008.00245.x [DOI] [PubMed] [Google Scholar]
- Ritschel W. A., Brady M. E., Tan H. S. (1979). First-pass effect of coumarin in man. Int. J. Clin. Pharmacol. Biopharm. 17 (3), 99–103. [PubMed] [Google Scholar]
- Ritschel W. A., Brady M. E., Tan H. S., Hoffmann K. A., Yiu I. M., Grummich K. W. (1977). Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur. J. Clin. Pharmacol. 12 (6), 457–461. 10.1007/bf00561066 [DOI] [PubMed] [Google Scholar]
- Rollinger J. M., Hornick A., Langer T., Stuppner H., Prast H. (2004). Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 47 (25), 6248–6254. 10.1021/jm049655r [DOI] [PubMed] [Google Scholar]
- Rong N., Yang R., Ibrahim I. A. A., Zhang W. (2023). Cardioprotective role of scopoletin on isoproterenol-induced myocardial infarction in rats. Appl. Biochem. Biotechnol. 195 (2), 919–932. 10.1007/s12010-022-04123-z [DOI] [PubMed] [Google Scholar]
- Ryan B. J., Hoek S., Fon E. A., Wade-Martins R. (2015). Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem. Sci. 40 (4), 200–210. 10.1016/j.tibs.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Sajti E., van Meeteren N., Kavelaars A., van der Net J., Gispen W. H., Heijnen C. (2004). Individual differences in behavior of inbred Lewis rats are associated with severity of joint destruction in adjuvant-induced arthritis. Brain Behav. Immun. 18 (6), 505–514. 10.1016/j.bbi.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Sam-Ang P., Phanumartwiwath A., Liana D., Sureram S., Hongmanee P., Kittakoop P. (2023). UHPLC-QQQ-MS and RP-HPLC detection of bioactive alizarin and scopoletin metabolites from Morinda citrifolia root extracts and their antitubercular, antibacterial, and antioxidant activities. ACS Omega 8 (32), 29615–29624. 10.1021/acsomega.3c03656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayce L., Boardman J. (2008). Disability rights and mental health in the UK: recent developments of the Disability Discrimination Act. Adv. Psychiatric Treat. 14 (4), 265–275. 10.1192/apt.bp.106.003103 [DOI] [Google Scholar]
- Selim Y. A., Ouf N. H. (2012). Anti-inflammatory new coumarin from the Ammi majus L. Org. Med. Chem. Lett. 2 (1), 1. 10.1186/2191-2858-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E. J., Saeed M., Law B. Y., Wu A. G., Kadioglu O., Greten H. J., et al. (2016). Pharmacogenomics of scopoletin in tumor cells. Molecules 21 (4), 496. 10.3390/molecules21040496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Anand A., Bhatia A., Sharma V., Singh A. K., Banerjee D., et al. (2022). Pharmacological evaluation of scopoletin in the carbon tetrachloride-induced hepatotoxicity model in wistar rats. J. Pharm. Bioallied Sci. 14 (4), 201–206. 10.4103/jpbs.jpbs_333_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Sharma V., Taneja S., Alka B., Anand A., Patil A. N., et al. (2023). Scopoletin a potential phytochemical therapy for antitubercular treatment drug induced liver injury (ATT-DILI) model in Wistar rats. J. Complement. Integr. Med. 20, 797–803. 10.1515/jcim-2023-0168 [DOI] [PubMed] [Google Scholar]
- Shaw C. Y., Chen C. H., Hsu C. C., Chen C. C., Tsai Y. C. (2003). Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother. Res. 17 (7), 823–825. 10.1002/ptr.1170 [DOI] [PubMed] [Google Scholar]
- Shi W., Hu J., Bao N., Li D., Chen L., Sun J. (2017). Design, synthesis and cytotoxic activities of scopoletin-isoxazole and scopoletin-pyrazole hybrids. Bioorg Med. Chem. Lett. 27 (2), 147–151. 10.1016/j.bmcl.2016.11.089 [DOI] [PubMed] [Google Scholar]
- Shi Z., Li N., Chen C., Wang Y., Lei Z., Chen L., et al. (2020). Novel NO-releasing scopoletin derivatives induce cell death via mitochondrial apoptosis pathway and cell cycle arrest. Eur. J. Med. Chem. 200, 112386. 10.1016/j.ejmech.2020.112386 [DOI] [PubMed] [Google Scholar]
- Singh S., Agarwal K., Iqbal H., Yadav P., Yadav D., Chanda D., et al. (2020). Synthesis and evaluation of substituted 8,8-dimethyl-8H-pyrano[2,3-f]chromen-2-one derivatives as vasorelaxing agents. Bioorg Med. Chem. Lett. 30 (1), 126759. 10.1016/j.bmcl.2019.126759 [DOI] [PubMed] [Google Scholar]
- Suchaichit N., Kanokmedhakul S., Kanokmedhakul K., Moosophon P., Boonyarat C., Plekratoke K., et al. (2018). Phytochemical investigation and acetylcholinesterase inhibitory activity of bark of Hymenodictyon orixense. Nat. Prod. Res. 32 (24), 2936–2939. 10.1080/14786419.2017.1389930 [DOI] [PubMed] [Google Scholar]
- Sun J., Gou J., Qin L., Liu T., Huang Y., Lu Y., et al. (2024). Screening of anti-functional dyspepsia compounds in Cynanchum auriculatum: a spectrum-effect relationship analysis, and ATP-binding cassette transporters inhibitor evaluation. J. Ethnopharmacol. 318 (Pt A), 116867. 10.1016/j.jep.2023.116867 [DOI] [PubMed] [Google Scholar]
- Tabana Y. M., Hassan L. E., Ahamed M. B., Dahham S. S., Iqbal M. A., Saeed M. A., et al. (2016). Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 107, 17–33. 10.1016/j.mvr.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Thuong P. T., Na M., Su N. D., Seong R. S., Lee Y. M., Sok D. E., et al. (2005). Inhibitory effect of coumarins from Weigela subsessilis on low density lipoprotein oxidation. Biol. Pharm. Bull. 28 (6), 1095–1097. 10.1248/bpb.28.1095 [DOI] [PubMed] [Google Scholar]
- Tian Q., Jin H., Huo X., Zhao Y., Wu W., Xu L., et al. (2023). Comparative pharmacokinetics of scoparone and its metabolite scopoletin in normal and ANIT-induced intrahepatic cholestatic rats. Curr. Drug Metab. 24 (4), 303–311. 10.2174/1389200224666230510125610 [DOI] [PubMed] [Google Scholar]
- Tian Q., Wang L., Sun X., Zeng F., Pan Q., Xue M. (2019). Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. J. buon 24 (3), 997–1002. [PubMed] [Google Scholar]
- Tzeng T. C., Lin Y. L., Jong T. T., Chang C. M. J. (2007). Ethanol modified supercritical fluids extraction of scopoletin and artemisinin from Artemisia annua L. Sep. Purif. Technol. 56 (1), 18–24. 10.1016/j.seppur.2007.01.010 [DOI] [Google Scholar]
- Verma A., Dewangan P., Kesharwani D., Kela S. P. (2013). Hypoglycemic and hypolipidemic activity of scopoletin (coumarin derivative) in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 22 (1), 79–83. [Google Scholar]
- Visser M. (2018). Translating pharmacokinetic and pharmacodynamic data into practice. Vet. Clin. North Am. Exot. Anim. Pract. 21 (2), 169–182. 10.1016/j.cvex.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Vučić Lovrenčić M., Božičević S., Smirčić Duvnjak L. (2023). Diagnostic challenges of diabetic kidney disease. Biochem. Med. Zagreb. 33 (3), 030501. 10.11613/bm.2023.030501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xing X., Cao Y., Zhao L., Sun S., Chen Y., et al. (2018). Development and application of an UHPLC-MS/MS method for comparative pharmacokinetic study of eight major bioactive components from yin chen hao tang in normal and acute liver injured rats. Evid. Based Complement. Altern. Med., 2018, 3239785. 10.1155/2018/3239785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Osman W. N., Che Ahmad Tantowi N. A., Lau S. F., Mohamed S. (2019). Epicatechin and scopoletin rich Morinda citrifolia (Noni) leaf extract supplementation, mitigated Osteoarthritis via anti-inflammatory, anti-oxidative, and anti-protease pathways. J. Food Biochem. 43 (3), e12755. 10.1111/jfbc.12755 [DOI] [PubMed] [Google Scholar]
- Wigati D., Anwar K., Sudarsono S., Nugroho A. E. (2017). Hypotensive activity of ethanolic extracts of Morinda citrifolia L. Leaves and fruit in dexamethasone-induced hypertensive rat. J. Evid. Based Complement. Altern. Med. 22 (1), 107–113. 10.1177/2156587216653660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Geng Y., Buist-Homan M., Moshage H. (2022). Scopoletin and umbelliferone protect hepatocytes against palmitate- and bile acid-induced cell death by reducing endoplasmic reticulum stress and oxidative stress. Toxicol. Appl. Pharmacol. 436, 115858. 10.1016/j.taap.2021.115858 [DOI] [PubMed] [Google Scholar]
- Xia Y., Dai Y., Wang Q., Liang H. (2007). Determination of scopoletin in rat plasma by high performance liquid chromatographic method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 857 (2), 332–336. 10.1016/j.jchromb.2007.07.023 [DOI] [PubMed] [Google Scholar]
- Yamakawa M., Doh S. J., Santosa S. M., Montana M., Qin E. C., Kong H., et al. (2018). Potential lymphangiogenesis therapies: learning from current antiangiogenesis therapies-A review. Med. Res. Rev. 38 (6), 1769–1798. 10.1002/med.21496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Sugimoto T. (2016). Advanced glycation end products, diabetes, and bone strength. Curr. Osteoporos. Rep. 14 (6), 320–326. 10.1007/s11914-016-0332-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. W., Zhang C. Y., Zhang B. G., Lu Y., Luan J. W., Zheng Q. T. (2009). Novel coumarin and furan from the roots of Angelica pubescens f. biserrata. J. Asian Nat. Prod. Res. 11 (8), 698–703. 10.1080/10286020802619140 [DOI] [PubMed] [Google Scholar]
- Yang Y. F., Xu W., Song W., Ye M., Yang X. W. (2015). Transport of twelve coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR cell monolayer-an in vitro model for blood-brain barrier permeability. Molecules 20 (7), 11719–11732. 10.3390/molecules200711719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Chen J., Lin Z., Wang N., Wang A., Wang B., et al. (2022). Scopoletin induced metabolomic profile disturbances in zebrafish embryos. Metabolites 12 (10), 934. 10.3390/metabo12100934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ding Z., Xia Y., Wei Z., Luo Y., Feleder C., et al. (2012). Inhibition of monosodium urate crystal-induced inflammation by scopoletin and underlying mechanisms. Int. Immunopharmacol. 14 (4), 454–462. 10.1016/j.intimp.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Ying L., Yue D., Mei L., Pan R., Luo Y., Xia Y., et al. (2009). Scopoletin induces apoptosis of fibroblast-like synoviocytes from adjuvant arthritis rats by a mitochondrial-dependent pathway. Drug Dev. Res. 70, 378–385. 10.1002/ddr.20314 [DOI] [Google Scholar]
- Yu N., Li N., Wang K., Deng Q., Lei Z., Sun J., et al. (2021). Design, synthesis and biological activity evaluation of novel scopoletin-NO donor derivatives against MCF-7 human breast cancer in vitro and in vivo . Eur. J. Med. Chem. 224, 113701. 10.1016/j.ejmech.2021.113701 [DOI] [PubMed] [Google Scholar]
- Yuan C., Wang M. H., Wang F., Chen P. Y., Ke X. G., Yu B., et al. (2021). Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 270, 119105. 10.1016/j.lfs.2021.119105 [DOI] [PubMed] [Google Scholar]
- Yun B. S., Lee I. K., Ryoo I. J., Yoo I. D. (2001). Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from Hibiscus syriacus. J. Nat. Prod. 64 (9), 1238–1240. 10.1021/np0100946 [DOI] [PubMed] [Google Scholar]
- Zapata Lopera Y. M., Jiménez-Ferrer E., Herrera-Ruiz M., Zamilpa A., González-Cortazar M., Rosas-Salgado G., et al. (2022). New chromones from Bouvardia ternifolia (cav.) Schltdl with anti-inflammatory and immunomodulatory activity. Plants (Basel) 12 (1), 1. 10.3390/plants12010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Li S., Wang X., Gong T., Sun X., Zhang Z. (2015). Validated LC-MS/MS method for the determination of scopoletin in rat plasma and its application to pharmacokinetic studies. Molecules 20 (10), 18988–19001. 10.3390/molecules201018988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Ma Y., Yang Z., Mao J., Zheng Y. (2020). Antihyperuricemic efficacy of Scopoletin-loaded Soluplus micelles in yeast extract/potassium oxonate-induced hyperuricemic mice. Drug Dev. Ind. Pharm. 46 (9), 1550–1557. 10.1080/03639045.2020.1811302 [DOI] [PubMed] [Google Scholar]
- Zeng Y. C., Li S., Liu C., Gong T., Sun X., Fu Y., et al. (2017). Soluplus micelles for improving the oral bioavailability of scopoletin and their hypouricemic effect in vivo . Acta Pharmacol. Sin. 38 (3), 424–433. 10.1038/aps.2016.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zhang Y., Yang T., Ye Z. Q., Tian J., Fang H. R., et al. (2019). Scopoletin suppresses activation of dendritic cells and pathogenesis of experimental autoimmune encephalomyelitis by inhibiting NF-κB signaling. Front. Pharmacol. 10, 863. 10.3389/fphar.2019.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao W., Ge C., Li X., Sun Z. (2021). Scopoletin attenuates intracerebral hemorrhage-induced brain injury and improves neurological performance in rats. Neuroimmunomodulation 28 (2), 74–81. 10.1159/000505731 [DOI] [PubMed] [Google Scholar]
- Zhang W. Y., Lee J. J., Kim Y., Kim I. S., Park J. S., Myung C. S. (2010). Amelioration of insulin resistance by scopoletin in high-glucose-induced, insulin-resistant HepG2 cells. Horm. Metab. Res. 42 (13), 930–935. 10.1055/s-0030-1265219 [DOI] [PubMed] [Google Scholar]
- Zhao Y. X., Wang M., Dong W. T., Li Y. (2019). Pharmacokinetics, bioavailability and metabolism of scopoletin in dog by ultra-high-performance liquid chromatography combined with linear ion trap-Orbitrap tandem mass spectrometry. Biomed. Chromatogr. 33 (3), e4436. 10.1002/bmc.4436 [DOI] [PubMed] [Google Scholar]
- Zhou R., Kan S., Cai S., Sun R., Yuan H., Yu B. (2020). Scopoletin activates adenosine monophosphate-activated protein kinase/mammalian target of rapamycin signaling pathway and improves functional recovery after spinal cord injury in rats. Pharmacology 105 (5-6), 349–359. 10.1159/000503866 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Jiang Z., Liu L., Yang X., Li M., Cheng Y., et al. (2023). Scopoletin reactivates latent HIV-1 by inducing NF-κB expression without global T cell activation. Int. J. Mol. Sci. 24 (16), 12649. 10.3390/ijms241612649 [DOI] [PMC free article] [PubMed] [Google Scholar]