Abstract
Major determinants of the biological background or reserve, such as age, biological sex, comorbidities (diabetes, hypertension, obesity, etc.), and medications (e.g., anticoagulants), are known to affect outcome after traumatic brain injury (TBI). With the unparalleled data richness of coronavirus disease 2019 (COVID-19; ∼375,000 and counting!) as well as the chronic form, long-COVID, also called post-acute sequelae SARS-CoV-2 infection (PASC), publications (∼30,000 and counting) covering virtually every aspect of the diseases, pathomechanisms, biomarkers, disease phases, symptomatology, etc., have provided a unique opportunity to better understand and appreciate the holistic nature of diseases, interconnectivity between organ systems, and importance of biological background in modifying disease trajectories and affecting outcomes. Such a holistic approach is badly needed to better understand TBI-induced conditions in their totality. Here, I briefly review what is known about long-COVID/PASC, its underlying—suspected—pathologies, the pathobiological changes induced by TBI, in other words, the TBI endophenotypes, discuss the intersection of long-COVID/PASC and TBI-induced pathobiologies, and how by considering some of the known factors affecting the person's biological background and the inclusion of mechanistic molecular biomarkers can help to improve the clinical management of TBI patients.
Keywords: biological reserve, comorbidities, traumatic brain injury
Background and Introduction
Major determinants of the biological background or reserve, such as age,1–5 biological sex,6–8 pre-existing conditions (diabetes, hypertension, obesity, etc.),9,10 and medications (e.g., anticoagulants),11,12 are known to affect outcome after traumatic brain injury (TBI).
It feels like distant memory, but SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) and its more transmissible variants, Omicron, its subvariants, BA.4 and 5, etc., have infected >100 million persons in the United States and killed >1 million (https://covid.cdc.gov/covid-data-tracker/#datatracker-home) over the past 3 years. Worldwide numbers are ∼700 million infected and ∼7 million killed (https://covid19.who.int). In addition to the tragic loss of lives directly attributable to coronavirus disease 2019 (COVID-19), SARS-CoV-2 infection can cause long-lasting or even permanent pathobiological changes.13 “Long-COVID” or post-acute sequelae of SARS-CoV-2 infection (PASC) is a multi-symptomatic condition that involves virtually all organ systems.14–16 Long-COVID/PASC can adversely affect quality of life and it has become a significant public health challenge.17–19 It has been shown that long-COVID/PASC can increase one's predisposition to other disorders (e.g., stroke) and adversely affect recovery from various disorders.16,20
TBI has been long recognized as the “silent epidemic,”21,22 but given its global nature, it should rather be called a pandemic. However, we still have limited understanding of the biological background, for example, biological sex23,24 or comorbidities.25,26 Reflecting the drastic decline in personal mobility attributable to quarantines and lockdowns, the incidence of TBI caused by traffic accidents also declined during the COVID-19 pandemic, but assaults and severe injuries increased.27 Importantly, outcomes were adversely affected, mortality rates increased, especially in middle-/low-income countries.28
The combination of focusing on developing and testing vaccine(s) for SARS-CoV-2 and public healthcare measures, like social distancing, resulted in major disruptions and/or the complete halt of clinical trials,29–32 including TBI.33 Compensating for the “lost year(s)” in TBI clinical research attributable to COVID-19 pandemic alone has its own challenges—along with new opportunities.34 The increased recognition of long-COVID/PASC as a public health issue has added a new dimension to an already complex health condition arguing for a reassessment of our previous approaches to TBI. The new, post-COVID-19 patient landscape, the additional varying individual vulnerability attributable to long-COVID/PASC to outcomes after TBI will require further stratification of patients. This can be accomplished by using an extended panel of molecular biomarkers, utilizing machine learning (ML)35 that can guide and optimize individualized therapeutic interventions.
Long-COVID/PASC
The real prevalence of long-COVID/PASC is currently not well known because of different criteria used in reporting.16,36 The latest Centers for Disease Control and Prevention data indicate that ∼15% of American adults have had long-COVID/PASC and 5.8% of Americans currently have it (https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm). Studies have shown that only ∼10–40% of COVID-19 patients recovered completely 60 days after being discharged from hospitals,37–39 and the remaining 60–90% of discharged COVID-19 patients have experienced various symptoms that can be attributed only to the infection with SARS-CoV-2.40 Although vaccination protects against severe disease, the level of protection against long-COVID/PASC is currently unknown.41 Importantly, even asymptomatic persons infected with SARS-CoV-2 can develop long-COVID/PASC of varying severity, further complicating one's ability to assess its true prevalence.42,43 The majority (∼90%) of severely ill COVIID-19 patients have reported long-COVID/PASC symptomatology,44 but up to 40% of patients after a mild case of COVID-19 infection have also reported long-COVID/PASC-like symptoms.13,45
Although some studies indicated that long-COVID/PASC is relatively independent of the severity of COVID-19,46 there is a correlation between disease severity and prevalence of long-COVID-PASC. A Swedish study showed that only 1% of mild COVID-19 patients developed long-COVID/PASC, but 32% of severe cases (intensive care unit–treated persons) showed symptoms 1 year post-infection.47 Biological sex also plays an important role; severe COVID-19 with unfavorable outcome mainly affects males, whereas long-COVID/PASC disproportionally affects biological females.47–50
Long-COVID/PASC can affect multiple organ systems, and the most frequently reported symptoms are fatigue, shortness of breath, nausea, palpitations, joint and chest pain, and gastrointestinal and gynecological problems.15,40 Most patients, however, suffer from neurological and -psychiatric symptoms such as “brain fog,” anxiety/depression, memory loss, inattention, disorientation, disturbances of sleep, and headaches.14,51–57 These symptoms are similar to a previously described condition, functional neurological disorders (FNDs),58 and similar symptoms were observed after SARS and MERS infections.59 Patients with chronic fatigue syndrome and fibromyalgia60 as well as post-traumatic stress disorder61 and “chronic” TBI/persistent post-concussive syndromes also have a similar symptomatology.62 It should also be noted that many of these symptoms can be psychogenic, caused by the emotional, mental, and psychological stress attributable to COVID-19-related shutdowns, quarantines, social isolations, etc. Such a lack of specificity and complexity make diagnosing “organic” long-COVID/PASC both important and challenging. A recent study has found evidence that even vaccination without infection can cause FNDs,63 but it is unclear whether the cause is organic or “psychogenic.”
Candidate Pathomechanism(s) of Long-COVID/PASC
The exact pathomechanism of long-COVID/PASC is currently not known. Candidate mechanisms (summarized in Table 1) fell into two categories: direct and indirect.64–66
Table 1.
List of Pathomechanisms Suspected in the Development of Long-COVID/PASC and Their Potential Effect on the Outcome After Various Severities of TBI
| Pathomechanism(s) (selected) | Level of evidence in the pathomechanism of long-COVID/PASC |
Potential influence/effect on outcome after
|
||
|---|---|---|---|---|
| miTBI | MoTBI | sTBI | ||
| Direct | ||||
| Direct viral invasion and/or viral reservoirs in the CNS | Low/uncertain67,68,244,245 | ? | ? | ? |
| Definition: presence of the virus and/or viral particles in cells of the CNS | ||||
| Indirect | ||||
| Endothelial damage/dysfunction/(micro)vascular injury | Moderate to high64,70,75–77,93 | +++ | +++ | +++ |
| Definition: abnormal vascular functions, more reactive endothelial phenotype, injury, blockage of small vessels including capillaries | ||||
| Abnormal coagulation | High (after severe COVID-19)79,246,247 | +++ | +++ | +++ |
| Definition: disruptions in the body's ability to control blood clotting | ||||
| Inflammation | High (after severe COVID-19)81,89,107,248–250 | +/– | ++ | +++ |
| Definition: maladaptive immune response | ||||
| Abnormal immunometabolism | Moderate84–87,251 | +/– | ++ | +++ |
| Definition: changes in intracellular metabolic pathways of immune cells that alter their functionality | ||||
PASC, post-acute sequelae of SARS-CoV-2 infection; TBI, traumatic brain injury; CNS, central nervous system; COVID-19, coronavirus disease 19.
Direct mechanisms
Direct mechanism includes, viral invasion, proliferation, and effect of viral particles (e.g., viral proteins) on the various cell types of the brain.67–69 There is in vitro evidence that SARS-CoV-2 can infect cultured human cerebral microvascular endothelial cells.70 Viral particles were found in endothelial cells in SARS-CoV-2-infected non-human primates, and SARS-CoV-2 nucleic acids were detected in supporting cells, choroid plexus, and sustentacular cells, but not in neurons.71 It is possible that some, likely immunocompromised persons may have a hidden viral reservoir, which can cause reinfection. Recurrence of SARS-CoV-2 positivity after antiviral treatment by Paxlovid or other SARS-CoV-2 antivirals demonstrates that there can be an undetectably low level of a viral reservoir that can cause COVID-19 to rebound.72 Based on our current understanding and available evidence, the hidden cerebral viral reservoir likely exists in only a small subset of long-COVID/PASC patients73 and may not be responsible for the majority of long-COVID/PASC cases.74
Indirect mechanisms
The indirect mechanism includes endothelial/vascular damage,70,75–78 coagulopathy,79,80 and abnormal immune functions like altered immunometabolism, chronic inflammation, immune exhaustion, and autoimmunity.54,81–90
Endothelial pathologies
Endothelial damage/dysfunction/(micro)vascular injury has emerged as a leading candidate pathology of long-COVID/PASC.78,91,92 The main target of SARS-CoV-2 is the ACE2 receptor bearing endothelial cells in the central nervous system (CNS),70,71 and the vascular dysfunction, endothelial damage, and (micro)vascular injury resulting in dysfunction of blood vessels has been one of the hallmarks of systemic SARS-CoV-2 infection and one of the leading candidate pathomechanisms of long-COVID/PASC.75–77,93–96 Endothelial cells are key regulators of cell-to-cell adhesion, blood–brain barrier (BBB) formation, and transendothelial transport, including cell migration, coagulation, and inflammation, involving both humoral and cellular pathways.76 Endothelial stress caused by SARS-CoV-2 infection can cause long-lasting changes in the cerebral microvasculature in the forms of microthrombosis and altered BBB functions.97–100 In summary, endothelial and (micro)vascular abnormalities appear to be the leading pathomechanisms classifying long-COVID/PASC as a new vessel disease.78
Coagulopathies
Endothelial/vascular stress and damage can lead to coagulopathies and persistent hyper- and abnormal coagulation, one of the hallmarks of acute COVID-19,101 but studies have also demonstrated the persistence of coagulopathies in long-COVIC/PASC.102,103 The presence of microclots with abnormal protein content, including amyloid, combined with an abnormal fibrinolytic system unable to resolve these clots, can result in a hypercoagulable state, manifested in the circulation of microclots.103,104 Studies have hypothesized that this hypercoagulable state is caused by a chronic inflammatory process104,105 linking vascular abnormalities and the hypercoagulable state to various forms of immune pathologies.46,105,106
Inflammation
The severe form of COVID-19 infection is characterized by a hyperinflammation “cytokine storm,” which can lead to a chronically altered, maladaptive immune response.81,107 The cytokine storm, the hallmark of severe COVID-19, causes—additional—organ damage, the release of damage-associated molecular patterns (DAMPs).108 HMGB1, hsp70, mitochondrial DNA, and other DAMPs further activate the inflammatory process, leading to a vicious cycle that, if not completely broken, can exist for months after the acute phase and lead to long-COVID/PASC.109,110 Indeed, an altered immune system, characterized by chronically elevated levels of proinflammatory molecules, has been identified as one of the leading molecular pathologies of long-COVID/PASC.111,112 Circulating proinflammatory molecules can cause—additional—vascular stress and endothelial activation, leading to fibrinogen accumulation around the vessels, attracting microglia and/or macrophages and inducing a vicious cycle of neuroinflammation and tissue damage in the CNS.113,114 Another form of immune pathologies suspected in the pathology of long-COVID/PASC is altered immunometabolism.86 SARS-CoV-2 infection can modify any of the six major metabolic processes that compromise their functionality, leading/contributing to a chronic inflammatory stage.84,85,87
Autoimmunity
Autoimmunity in long-COVID/PASC
Immunedysregu-lation, especially autoimmunity, has been increasingly recognized as one or the main pathologies responsible for long-COVID/PASC.83,115,116 Several studies have indicated that SAR- CoV-2 infection can result in an unbalance of the affected person's immune homeostasis, resulting in the development of autoantibodies potentially leading to the development autoimmune diseases.116–118 Autoantibodies against brain-tissue–specific epitopes have been found in long-COVID/PASC patients, and their presence correlates with neuropsychiatric abnormalities.119 During the acute phase of SARS-CoV-2 infection, serum levels of neural injury markers of GFAP and NF-L were elevated and, importantly, remained elevated in patients who could be classified as suffering from long-COVID/PASC.120–122 Elevated levels of neural injury markers were associated with elevated levels of inflammatory cytokines and IgM autoantibodies against, for example, myelin-associated glycoprotein and many other brain-specific proteins.119,123 Abnormal protein homeostasis, combined with protease activation and hyperinflammation, can significantly contribute to the autoimmune mechanism,124,125 the generation of autoantibodies against brain-specific proteins.16,115,126
In summary, current evidence points toward three major inter-related pathomechanisms (vascular/endothelial stress/damage, abnormal coagulation, and inflammation) that are the most likely underlying pathologies of long-COVID/PASC. Some of these pathomechanisms may occur individually, but they can also overlap, and can also change over time.
TBI: A Spectrum of Disorders of Different Disease Endophenotypes
TBI is not a single disease, but a spectrum of disorders with the same causation: physical insult to the head and brain.127–132 The only similarity between an unconscious patient with skull fracture, subdural hematoma, and brain contusion and a patient walking into an emergency room with a bump on his or her head feeling dizzy is that there was a physical impact—of different intensities and kinds—to the head.133 Thus, there are two dimensions of TBI that need to be considered; one is severity, traditionally addressed by the Glasgow Coma Scale introduced in 1974 by Teasdale and Jennett.134 However, our biological understanding of the pathophysiological mechanisms underlying the functional abnormalities have become substantially more refined since 1974.135–138 Mild TBI, clinically also called concussion, only causes temporary perturbance of cellular structures and may dislocate membrane-bound ion channels, receptors, and/or intracellular organelles, causing typically transient molecular disturbances reflected in metabolic abnormalities that clinically manifest as a temporary altered state of consciousness.139,140 After moderate TBI, biomechanical forces cause substantial direct tissue and cell damage and cell death, majorly disrupting neuronal signaling and networks manifesting clinically as prolonged loss of consciousness.141 Severe TBI, frequently comorbid with polytrauma, causes major loss of brain parenchyma, severe disruption of neuronal networks, loss of consciousness, and severe neurological dysfunctionality.142–145
The second critical dimension is the temporal aspect of TBI-induced pathobiological changes.146 Though the exact temporal profile of these changes is currently not well understood, available data indicate a dynamic and complex pattern of TBI-induced pathobiological changes that can span weeks or months.147–153
Major Pathobiologies Triggered by TBI: The Disease Endophenotypes
The physical impact to the head—depending on the intensity and type of injury—triggers a variety of pathobiological changes that can occur in partly overlapping fashion, interact in a highly complex fashion, and change over time.154,155 Penetrating TBI causes massive tissue damage, cellular death, and bleeding that release intracellular molecules called damage-associated molecules (DAMPs). DAMPs, like HMGB1, mitochondrial DNA, and S1008/9, rapidly activate the innate immune system by Toll-like receptors (TLRs) with the aim to remove tissue debris and restore homeostasis.156,157 However, the inflammatory process can continue beyond the acute phase and transform into a chronic process.158
Axonal injury is a hallmark of diffuse TBI, but the extent of damage varies from major white matter loss clinically manifested in severe functional deficits to temporary, molecular-level disruption of axonal structures, causing mild and transient neurobehavioral abnormalities.159–162
An important but currently poorly understood TBI (endo)phenotype is characterized by injury to the cerebral vasculature.163–166 The effect of diffuse TBI on the vasculature can range from endothelial stress, damaged BBB function, microbleeding, and hemorrhage.167–169 These pathobiological changes have been detected by neuroimaging163,170 and also by elevated plasma levels of protein biomarkers of endothelial and vascular stress and damage (VEGF, vWF, and cFN)163,166,171–175 and/or endothelial tight junction proteins (Claudin-5 and Occludin).150,176,177 These TBI-induced vascular changes are important inducers of downstream mechanisms such as activation of blood clotting and the innate immune system.178–183 Inflammation is a key adaptive response to any kind of noxious stimuli in all multi-cellular organisms,184,185 and the neuroinflammatory response to TBI—including mild, especially repeated mTBI—is emerging as a key pathobiology responsible for adverse outcomes.183,186,187 The neuroinflammatory response to TBI includes both humoral and cellular players.183 Depending on the type (e.g., closed, diffuse, or penetrating) and severity of the injury, the cellular components can include only intracranial cellular population, microglia, and astroglia or, after penetrating injury, peripheral immune cells (e.g., macrophages also contribute to the inflammatory response).188
Astrocytes play an especially important and complex role in the neuroinflammatory process; they are involved in regulating both innate and adaptive immune responses after TBI.189–191 In the activation of astrocytes, astrogliosis subsequent to penetrating TBI demarcates the injury site and a highly complex bidirectional signaling process between astrocytes and microglia is a key regulator of the acute and chronic neuroinflammatory response.189–191 Moderate and severe TBI disrupt—to varying degrees—the BBB causing the exposure of brain-specific molecules to the adaptive immune system, generating potentially detrimental long-lasting cellular and humoral responses that can cause or contribute to chronic adverse conditions.183,192,193
Intersection of Long-COVID/PASC and TBI-Induced Pathobiologies
Several of the suspected pathobiologies underlying long-COVID/PASC have the potential to affect recovery after various forms of TBI (Table 1). A major challenge for the clinical management of TBI patients has long been the heterogeneity of patients differing, for example, in age, biological sex, comorbidities, comedications, and cofactors—such as alcohol and/or drugs—and pre-existing conditions, such as long-COVID/PASC. The outcome after SARS-CoV-2 infection, especially after the severe form, has shown a sharp age dependence; elderly persons have shown significantly poorer outcome,194,195 but older age as a risk factor for developing long-COVID/PASC has been questioned.196 Conversely, young age has been shown as “protective” against the severe form of COVID-19,194 but not against developing long-COVID/PASC.197,198 Pediatric and adolescent populations represent a significant percentage of TBI cases,199 and it is known that TBI adversely affects later phases of neuronal development.4,200–203 The question is of what unknown is the effect of SARS-CoV-2 infection and/or long-COVID/PASC on the outcome of TBI, specifically the mild/concussive form that is the most frequent among adolescents/young adults. Elderly persons, representing the other predominant age group in TBI, has especially poor recovery after TBI, as reported by the large TRACK-TBI study,8 and long-COVID/PASC can further diminish the recovery process.
Biological sex seems to play a significant role in outcome after SARS-CoV-2 infections. Severe COVID-19 with an unfavorable outcome was observed mainly in males, whereas long-COVID/PASC disproportionally affected biological females.47–50 The large TRACK-TBI study has found that women are more vulnerable to develop persistent neurobehavioral symptoms than men after mTBI and suffer from chronic post-concussion syndromes.8 Accordingly, women suffering from long-COVID/PASC will likely have poorer long-term functional outcomes after TBI.
There are currently very limited data on how long the long-COVID/PASC really lasts and whether it has distinct disease phases.45 The current view is that long-COVID/PASC is a chronic condition with relatively steady pathobiologies,15,103 but there are reports indicating time-dependent changes in the underlying pathobiologies.44 TBI induces severity- and injury-type–dependent pathobiological responses that change dynamically over time, although the exact temporal pattern of these changes is currently poorly understood.153,182,193,204 Accordingly, not all the pathologies are present at every post-injury period,148,150–152 and their co-occurrence with pathologies of long-COVID/PASC—especially an abnormal inflammatory profile—has the potential to negatively affect the recovery process. SARS-CoV-2 infection itself can cause brain injury—defined as elevated serum levels of brain-injury markers NF-L, Tau, and GFAP—during the acute phase of COVID-19.122
Elevated levels of these brain injury biomarkers were associated with elevated serum levels of inflammatory cytokines (TNFa, IL-1b, and IL-6) and autoantibodies—both IgM and IgG—against brain-specific proteins (e.g., myelin-associated glycoprotein). Importantly, 4 months after the infection, the convalescent phase that can qualify for long-COVID/PASC, serum levels of brain injury markers, especially Tau, were still significantly elevated over normal controls along with inflammatory cytokines and autoantibodies. These findings implicate an ongoing inflammatory and autoimmune process, given that manifestations of immune dysregulation, one of the main pathomechanisms of SARS-CoV-2 infection, are also suspected in causing long-COVID/PASC.83,118,205
The altered biological background in long-COVID/PASC characterized by vascular abnormalities and, importantly, by a chronic inflammatory landscape can majorly affect the disease process after TBI (Fig. 1). An ongoing neuroinflammation has been proposed as the key pathomechanism of chronic TBI and TBI-induced pathological processes (e.g., chronic traumatic encephalopathy and Alzheimer's disease).191,206 Additional parenchymal damage caused by TBI will take place on an already dysregulated immune system causing additional and/or maintaining the long-term parenchymal damage, releasing DAMPs, further activating the adaptive immune response through TLR signaling and resulting in a vicious cycle that delays and or adversely affects the recovery process after TBI.120,122
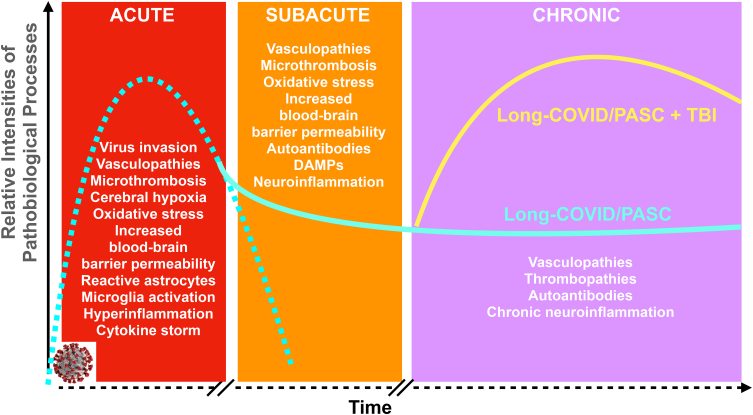
FIG. 1.
Onset and extent of major pathobiological changes during the various phases of severe COVID-19 and the hypothesized role of long-COVID/PASC affecting the outcome of TBI. The pathobiological changes identified or suspected during the acute, subacute, and chronic (long-COVID/PASC) phases of COVID-19 are listed. The dashed turquoise line indicates the relative intensity and relative timeline of pathobiologies of COVID-19. The solid turquoise line indicates the relative intensities of pathobiological changes associated with long-COVID/PASC. The solid yellow curve indicates the relative intensity and temporal changes of pathobiologies of TBI on a long-COVID/PASC background. Relevant references are in the text and listed in the references. DAMPs, damage-associated molecular patterns; PASC, post-acute sequelae SARS-CoV-2 infection; TBI, traumatic brain injury.
The Role of Biomarkers
Identification of TBI endophenotypes is a critical step toward developing evidence-based individualized clinical management of TBI patients.166,207–209 Because of their rich information content, blood-based (and other biofluid) protein biomarkers have currently the most potential to perform molecular phenotyping of TBI patients.210–214 In order to identify increased vulnerability of TBI patients attributable to pathologies underlying/suspected in long-COVID/PASC, an expanded biomarker panel should be used. Such a panel should include markers of any of the pathomechanisms suspected in the development of long-COVID/PASC (Table 2). These markers should be in addition and used in the context of the current, most commonly used, well-established markers of neural injury (GFAP, UCH-L1, Tau, and NF-L).211 There is an increasing recognition to use “mechanistic” biomarkers in TBI in conjunction with “classical” injury markers.215 However, markers of coagulopathies (e.g., vWF, D-dimer), endothelial stress (e.g., VEGF-A, Ang-1/2, and ET-1), vascular damage (CLDN5, Occludin, VCAM-1, and cFN),166,174,216—220 and inflammation (e.g., CRF, IL-1b, IL-6, TNFa, IL-8, and CXCL12)84,221—223 that have already been used in clinical settings should be coanalyzed with the panel of injury markers. The caveat is that elevated serum levels of the neural injury marker NF-L have been found in patients who could qualify as suffering from long-COVID/PASC (4 months after infection). This finding indicates an ongoing neuronal damage likely caused by an ongoing inflammation.120–122
Table 2.
Biomarkers of Selected Pathomechanisms Suspected in PASC for Blood-Borne Phenotyping of TBI Patients
| Biomarker of | Full name, abbreviation, references, and notes |
|---|---|
| Autoimmunity | Cyclic citrullinated peptide (CCP),252 protein microarray-based assays253,254 |
| Abnormal immune response; altered immunometabolism, immune dysregulation, hyperinflammation | Chemerin (ChM)84,221,222 |
| C-reactive peptide (CRP), tumor necrosis factor alpha (TNF-a), interleukin-1 beta (IL-1b), interleukin-6 (IL-6)223 | |
| Endothelial (vascular) damage, abnormal coagulation | von Willebrand factor (vWF), IL-18, vascular endothelial growth factor (VEGF), cellular fibronectin (cFN), D-dimer (Dd), fibrinogen (Fb)166,174,216–220 |
PASC, post-acute sequelae of SARS-CoV-2 infection; TBI, traumatic brain injury.
The major challenge will be how to analyze, harmonize, and correlate the large volume of—various—biomarker data in order to help clinical decision making. Potential solutions should include massive use of Big Data approaches like ML.35,207,224 Critically, any successful ML approach critically relies on a high quality and high quantity of primary-protein, physiology, imaging, etc., biomarker data and well-structured/machine-readable clinical reports.35,225–227
The critical unmet need is the availability of normal, reference ranges of biofluid blood—plasma and serum—and cerebrospinal fluid–based protein biomarkers. Astonishingly, no such publicly available database exists. Combined with issues of pre-analytical and analytical variables (e.g., different assay platforms),228 the current use of biomarker data has limited value.
The Role of TBI Models
Pioneering works have developed high-fidelity animal models of various forms of TBI,229–234 enabling one to identify the anatomical, cellular, and molecular substrates of the primary and secondary injury processes.185,235–237 These experiments have—mostly if not exclusively—been performed using healthy young male rats and only recently females have also been included.238,239 Though studies have started to address how the young, developing brain responds to and recovers from TBI,240 much less is known about the aging brain's response241 despite the huge and increasing number of aging persons affected by TBI.242 These elderly patients frequently suffer from comorbidities (e.g., long-COVID/PASC) and under (multiple) medications that combined can substantially alter patients' biological background in addition to age.26,243 In order to generate clinically relevant, translatable information, there is a need to develop and use animal models of human conditions/diseases (e.g., chronic inflammation) that are prevalent in the aged population.
Summary
Long-COVID/PASC has been identified as a significant public health issue. It reduces the quality of life, increases the vulnerability of affected persons to other diseases, and negatively affects disease processes. The underlying—suspected—pathologies, vascular abnormalities, coagulopathies, and inflammation can adversely affect the recovery process after TBI. Expanded diagnostics aimed to better inform about the biological background and comorbidities, such as long-COVID/PASC, of TBI patients will help to develop individualized clinical management.
Acknowledgments
The opinions or assertions contained herein are those of the author and are not to be construed as official or reflecting the views of the Department of Defense, Department of the Navy, or Uniformed Services University.
Abbreviations Used
- BBB
blood–brain barrier
- CNS
central nervous system
- COVID-19
coronavirus disease 2019
- DAMPs
damage-associated molecular patterns
- FNDs
functional neurological disorders
- ML
machine learning
- PASC
post-acute sequelae SARS-CoV-2 infection
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TBI
traumatic brain injury
- TLRs
Toll-like receptors
Funding Information
No funding was received for this work.
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Agoston DV. Traumatic brain injury in the long-COVID era. Neurotrauma Reports 2024:5(1):81–94. doi: 10.1089/neur.2023.0067.
References
- 1. Karr JE, Iverson GL, Berghem K, et al. Complicated mild traumatic brain injury in older adults: post-concussion symptoms and functional outcome at one week post injury. Brain Inj 2020;34(1):26–33; doi: 10.1080/02699052.2019.1669825 [DOI] [PubMed] [Google Scholar]
- 2. Levin SG, Pershina EV, Bugaev-Makarovskiy NA, et al. Why do levels of anti-inflammatory cytokines increase during memory acquisition? Neuroscience 2021;473:159–169; doi: 10.1016/j.neuroscience.2021.08.007 [DOI] [PubMed] [Google Scholar]
- 3. dell'Aquila M, Maiese A, De Matteis A, et al. Traumatic brain injury: estimate of the age of the injury based on neuroinflammation, endothelial activation markers and adhesion molecules. Histol Histopathol 2021;36(8):795–806; doi: 10.14670/hh-18-319 [DOI] [PubMed] [Google Scholar]
- 4. Delage C, Taib T, Mamma C, et al. Traumatic brain injury: an age-dependent view of post-traumatic neuroinflammation and its treatment. Pharmaceutics 2021;13(10):1624; doi: 10.3390/pharmaceutics13101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim T, Chelluboina B, Chokkalla AK, et al. Age and sex differences in the pathophysiology of acute CNS injury. Neurochem Int 2019;127:22–28; doi: 10.1016/j.neuint.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biegon A. Considering biological sex in traumatic brain injury. Front Neurol 2021;12:576366; doi: 10.3389/fneur.2021.576366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krukowski K. Short review: The impact of sex on neuroimmune and cognitive outcomes after traumatic brain injury. Brain Behav Immun Health 2021;16:100327; doi: 10.1016/j.bbih.2021.100327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin HS, Temkin NR, Barber J, et al. Association of sex and age with mild traumatic brain injury-related symptoms: a TRACK-TBI study. JAMA Netw Open 2021;4(4):e213046; doi: 10.1001/jamanetworkopen.2021.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saunders LL, Selassie AW, Hill EG, et al. Pre-existing health conditions and repeat traumatic brain injury. Arch Phys Med Rehabil 2009;90(11):1853–1859; doi: 10.1016/j.apmr.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 10. Dell KC, Grossner EC, Staph J, et al. A population-based study of pre-existing health conditions in traumatic brain injury. Neurotrauma Rep 2021;2(1):255–269; doi: 10.1089/neur.2020.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spano PJ, 2nd, Shaikh S, Boneva D, et al. Anticoagulant chemoprophylaxis in patients with traumatic brain injuries: a systematic review. J Trauma Acute Care Surg 2020;88(3):454–460; doi: 10.1097/ta.0000000000002580 [DOI] [PubMed] [Google Scholar]
- 12. Wiegele M, Schöchl H, Haushofer A, et al. Diagnostic and therapeutic approach in adult patients with traumatic brain injury receiving oral anticoagulant therapy: an Austrian interdisciplinary consensus statement. Crit Care 2019;23(1):62; doi: 10.1186/s13054-019-2352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garg M, Maralakunte M, Garg S, et al. The conundrum of ‘long-COVID-19’: a narrative review. Int J Gen Med 2021;14:2491–2506; doi: 10.2147/ijgm.S316708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, et al. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry 2023;28(2):564–578; doi: 10.1038/s41380-022-01836-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021;12:698169; doi: 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21(3):133–146; doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pye A, Roberts SR, Blennerhassett A, et al. A public health approach to estimating the need for long COVID services. J Public Health (Oxf) 2023;45(1):169–175; doi: 10.1093/pubmed/fdab365 [DOI] [PubMed] [Google Scholar]
- 18. Nittas V, Gao M, West EA, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev 2022;43:1604501; doi: 10.3389/phrs.2022.1604501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facing up to long COVID. Lancet 2020;396(10266):1861; doi: 10.1016/s0140-6736(20)32662-3 [DOI] [PMC free article] [PubMed]
- 20. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 2020;77(11):1–7; doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstein M. Traumatic brain injury: a silent epidemic. Ann Neurol 1990;27(3):327; doi: 10.1002/ana.410270315 [DOI] [PubMed] [Google Scholar]
- 22. Pascrell B Jr. Traumatic brain injury: the silent epidemic. N J Med 2001;98(11):47–48. [PubMed] [Google Scholar]
- 23. Hanafy S, Amodio V, Haag HL, et al. Is it prime time for sex and gender considerations in traumatic brain injury? Perspectives of rehabilitation care professionals. Disabil Rehabil 2022;44(5):684–692; doi: 10.1080/09638288.2020.1774670 [DOI] [PubMed] [Google Scholar]
- 24. Mollayeva T, Bordignon C, Ishtiaq M, et al. Knowledge of sex and gender and related information needs in patients with traumatic brain injury: in-depth interview study. Disabil Rehabil 2021;43(13):1872–1882; doi: 10.1080/09638288.2019.1683235 [DOI] [PubMed] [Google Scholar]
- 25. Hanafy S, Xiong C, Chan V, et al. Comorbidity in traumatic brain injury and functional outcomes: a systematic review. Eur J Phys Rehabil Med 2021;57(4):535–550; doi: 10.23736/s1973-9087.21.06491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maas AIR, Menon DK, Manley GT, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol 2022;21(11):1004–1060; doi: 10.1016/s1474-4422(22)00309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajalu BM, Indira Devi B, Shukla DP, et al. Traumatic brain injury during COVID-19 pandemic-time-series analysis of a natural experiment. BMJ Open 2022;12(4):e052639; doi: 10.1136/bmjopen-2021-052639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Damara FA, Muchamad GR, Anton A, et al. Epidemiological pattern of traumatic brain injury in the COVID-19 pandemic: a systematic review and meta-analysis. World Neurosurg 2022;161:e698–e709; doi: 10.1016/j.wneu.2022.02.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sathian B, Asim M, Banerjee I, et al. Impact of COVID-19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol 2020;10(3):878–887; doi: 10.3126/nje.v10i3.31622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller P, Williams A.. COVID-19's Impact on Clinical Research. In: RTI Press Research Brief. RTI Press: Research Triangle Park, NC; 2014. [PubMed] [Google Scholar]
- 31. Teng H, Wang Z, Yang X, et al. The impact of COVID-19 on clinical outcomes in people undergoing neurosurgery: a systematic review and meta-analysis. Syst Rev 2023;12(1):137; doi: 10.1186/s13643-023-02291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Dorn A. COVID-19 and readjusting clinical trials. Lancet 2020;396(10250):523–524; doi: 10.1016/s0140-6736(20)31787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lester A, Leach P, Zaben M. The impact of the COVID-19 pandemic on traumatic brain injury management: lessons learned over the first year. World Neurosurg 2021;156:28–32; doi: 10.1016/j.wneu.2021.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agoston DV. COVID-19 and traumatic brain injury (TBI); what we can learn from the viral pandemic to better understand the biology of TBI, improve diagnostics and develop evidence-based treatments. Front Neurol 2021;12:752937; doi: 10.3389/fneur.2021.752937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agoston DV. Big Data, Artificial Intelligence and Machine Learning in Neurotrauma. In: Leveraging Biomedical and Healthcare Data: Semantics, Analytics and Knowledge. (Kobeissy F, Alawieh A, Zaraket FA, Wang K. ed.) Academic Press: London; 2019; pp. 53–75. [Google Scholar]
- 36. Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open 2022;5(10):e2238804; doi: 10.1001/jamanetworkopen.2022.38804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022;226(9):1593–1607; doi: 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep 2022;12(1):9950; doi: 10.1038/s41598-022-13495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugiyama A, Miwata K, Kitahara Y, et al. Long COVID occurrence in COVID-19 survivors. Sci Rep 2022;12(1):6039; doi: 10.1038/s41598-022-10051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27(4):601–615; doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wisnivesky JP, Govindarajulu U, Bagiella E, et al. Association of vaccination with the persistence of post-COVID symptoms. J Gen Intern Med 2022;37(7):1748–1753; doi: 10.1007/s11606-022-07465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scherlinger M, Pijnenburg L, Chatelus E, et al. Effect of SARS-CoV-2 vaccination on symptoms from post-acute sequelae of COVID-19: results from the nationwide VAXILONG Study. Vaccines (Basel) 2021;10(1):46; doi: 10.3390/vaccines10010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wynberg E, Han AX, Boyd A, et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): a prospective cohort study. Vaccine 2022;40(32):4424–4431; doi: 10.1016/j.vaccine.2022.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022;185(5):881–895.e20; doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18(9):e1003773; doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramakrishnan RK, Kashour T, Hamid Q, et al. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol 2021;12:686029; doi: 10.3389/fimmu.2021.686029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hedberg P, Granath F, Bruchfeld J, et al. Post COVID-19 condition diagnosis: a population-based cohort study of occurrence, associated factors, and healthcare use by severity of acute infection. J Intern Med 2023. Feb;293(2):246–258; doi: 10.1111/joim.13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sylvester SV, Rusu R, Chan B, et al. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin 2022;38(8):1391–1399; doi: 10.1080/03007995.2022.2081454 [DOI] [PubMed] [Google Scholar]
- 49. Pelà G, Goldoni M, Solinas E, et al. Sex-related differences in long-COVID-19 syndrome. J Womens Health (Larchmt) 2022;31(5):620–630; doi: 10.1089/jwh.2021.0411 [DOI] [PubMed] [Google Scholar]
- 50. Fernández-de-Las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 Symptoms: the LONG-COVID-EXP-CM Multicenter Study. J Clin Med 2022;11(2):413; doi: 10.3390/jcm11020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stefanou MI, Palaiodimou L, Bakola E, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis 2022;13:20406223221076890; doi: 10.1177/20406223221076890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol 2022;23(2):194–202; doi: 10.1038/s41590-021-01104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med 2021;114(9):428–442; doi: 10.1177/01410768211032850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Al-Hakeim HK, Al-Rubaye HT, Almulla AF, et al. Chronic fatigue, depression and anxiety symptoms in long COVID are strongly predicted by neuroimmune and neuro-oxidative pathways which are caused by the inflammation during acute infection. J Clin Med 2023;12(2):511; doi: 10.3390/jcm12020511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cha C, Baek G. Symptoms and management of long COVID: a scoping review. J Clin Nurs 2021; doi: 10.1111/jocn.16150 [DOI] [PubMed] [Google Scholar]
- 56. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (Long COVID): a scoping review. Front Med (Lausanne) 2021;8:750378; doi: 10.3389/fmed.2021.750378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Healey Q, Sheikh A, Daines L, et al. Symptoms and signs of long COVID: a rapid review and meta-analysis. J Glob Health 2022;12:05014; doi: 10.7189/jogh.12.05014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75(9):1132–1141; doi: 10.1001/jamaneurol.2018.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clin Med (Lond) 2021;21(1):e68–e70; doi: 10.7861/clinmed.2020-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Malkova AM, Shoenfeld Y. Autoimmune autonomic nervous system imbalance and conditions: chronic fatigue syndrome, fibromyalgia, silicone breast implants, COVID and post-COVID syndrome, sick building syndrome, post-orthostatic tachycardia syndrome, autoimmune diseases and autoimmune/inflammatory syndrome induced by adjuvants. Autoimmun Rev 2023;22(1):103230; doi: 10.1016/j.autrev.2022.103231 [DOI] [PubMed] [Google Scholar]
- 61. Nguyen J, Whiteside LK, Bulger EM, et al. Post-traumatic stress disorder (PTSD) symptoms and alcohol and drug use comorbidity at 25 US level I trauma centers. Trauma Surg Acute Care Open 2022;7(1):e000913; doi: 10.1136/tsaco-2022-000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013;9(4):211–221; doi: 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alonso-Canovas A, Kurtis MM, Gomez-Mayordomo V, et al. Functional neurological disorders after COVID-19 and SARS-CoV-2 vaccines: a national multicentre observational study. J Neurol Neurosurg Psychiatry 2023;94(9):776–777; doi: 10.1136/jnnp-2022-330885 [DOI] [PubMed] [Google Scholar]
- 64. Jarrott B, Head R, Pringle KG, et al. “LONG COVID”—a hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol Res Perspect 2022;10(1):e00911; doi: 10.1002/prp2.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mantovani A, Morrone MC, Patrono C, et al. Long Covid: where we stand and challenges ahead. Cell Death Differ 2022;29(10):1891–1900; doi: 10.1038/s41418-022-01052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53(10):737–754; doi: 10.1080/23744235.2021.1924397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bauer L, Laksono BM, de Vrij FMS, et al. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci 2022;45(5):358–368; doi: 10.1016/j.tins.2022.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McQuaid C, Brady M, Deane R. SARS-CoV-2: is there neuroinvasion? Fluids Barriers CNS 2021;18(1):32; doi: 10.1186/s12987-021-00267-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen B, Julg B, Mohandas S, et al. ; RECOVER Mechanistic Pathways Task Force. Viral persistence, reactivation, and mechanisms of long COVID. Elife 2023;12:e86015; doi: 10.7554/eLife.86015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Torices S, Motta C, da Rosa B, et al. SARS-CoV-2 infection of human brain microvascular endothelial cells leads to inflammatory activation through NF-κB non-canonical pathway and mitochondrial remodeling. Res Sq 2022; doi: 10.21203/rs.3.rs-1762855/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rutkai I, Mayer MG, Hellmers LM, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun 2022;13(1):1745; doi: 10.1038/s41467-022-29440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang L, Berger NA, Davis PB, et al. COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. medRxiv 2022; doi: 10.1101/2022.06.21.22276724 [DOI] [Google Scholar]
- 73. Viszlayová D, Sojka M, Dobrodenková S, et al. SARS-CoV-2 RNA in the cerebrospinal fluid of a patient with long COVID. Ther Adv Infect Dis 2021;8:20499361211048572; doi: 10.1177/20499361211048572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schweitzer F, Goereci Y, Franke C, et al. Cerebrospinal fluid analysis post-COVID-19 is not suggestive of persistent central nervous system infection. Ann Neurol 2022;91(1):150–157; doi: 10.1002/ana.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu SW, Ilyas I, Weng JP. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin 2023;44(4):695–709; doi: 10.1038/s41401-022-00998-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ambrosino P, Calcaterra IL, Mosella M, et al. Endothelial dysfunction in COVID-19: a unifying mechanism and a potential therapeutic target. Biomedicines 2022;10(4):812; doi: 10.3390/biomedicines10040812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep 2021;9(3):e14726; doi: 10.14814/phy2.14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zanini G, Selleri V, Roncati L, et al. Vascular “long COVID”: a new vessel disease? Angiology 2024;75(1):8–14; doi: 10.1177/00033197231153204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Acanfora D, Acanfora C, Ciccone MM, et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses 2021;13(10):1904; doi: 10.3390/v13101904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Turner S, Khan MA, Putrino D, et al. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab 2023;34(6):321–344; doi: 10.1016/j.tem.2023.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tan LY, Komarasamy TV, Rmt Balasubramaniam V. Hyperinflammatory immune response and COVID-19: a double edged sword. Front Immunol 2021;12:742941; doi: 10.3389/fimmu.2021.742941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med (Lausanne) 2023;10:1011936; doi: 10.3389/fmed.2023.1011936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Opsteen S, Files JK, Fram T, et al. The role of immune activation and antigen persistence in acute and long COVID. J Investig Med 2023;71(5):545–562; doi: 10.1177/10815589231158041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koelman L, Reichmann R, Börnhorst C, et al. Determinants of elevated chemerin as a novel biomarker of immunometabolism: data from a large population-based cohort. Endocr Connect 2021;10(9):1200–1211; doi: 10.1530/ec-21-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Voss K, Hong HS, Bader JE, et al. A guide to interrogating immunometabolism. Nat Rev Immunol 2021;21(10):637–652; doi: 10.1038/s41577-021-00529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rudiansyah M, Jasim SA, Mohammad Pour ZG, et al. Coronavirus disease 2019 (COVID-19) update: from metabolic reprogramming to immunometabolism. J Med Virol 2022;94(10):4611–4627; doi: 10.1002/jmv.27929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang S, Zeng X, Wang Y, et al. Immunometabolism and potential targets in severe COVID-19 peripheral immune responses. Asian J Pharm Sci 2021;16(6):665–667; doi: 10.1016/j.ajps.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maes M, Al-Rubaye HT, Almulla AF, et al. Lowered quality of life in long COVID is predicted by affective symptoms, chronic fatigue syndrome, inflammation and neuroimmunotoxic pathways. Int J Environ Res Public Health 2022;19(16):10362; doi: 10.3390/ijerph191610362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vollbracht C, Kraft K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: implications for the benefit of high-dose intravenous vitamin C. Front Pharmacol 2022;13:899198; doi: 10.3389/fphar.2022.899198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yin K, Peluso MJ, Thomas R, et al. Long COVID manifests with T cell dysregulation, inflammation, and an uncoordinated adaptive immune response to SARS-CoV-2. bioRxiv 2023; doi: 10.1101/2023.02.09.527892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ahamed J, Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest 2022;132(15):e161167; doi: 10.1172/jci161167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sherif ZA, Gomez CR, Connors TJ, et al. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023;12:e86002; doi: 10.7554/eLife.86002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ahmad SJ, Feigen CM, Vazquez JP, et al. Neurological sequelae of COVID-19. J Integr Neurosci 2022;21(3):77; doi: 10.31083/j.jin2103077 [DOI] [PubMed] [Google Scholar]
- 94. Acharya Y, Alameer A, Calpin G, et al. A comprehensive review of vascular complications in COVID-19. J Thromb Thrombolysis 2022;53(3):586–593, doi: 10.1007/s11239-021-02593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Siddiqi HK, Libby P, Ridker PM. COVID-19—a vascular disease. Trends Cardiovasc Med 2021;31(1):1–5; doi: 10.1016/j.tcm.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res 2020;116(14):2177–2184; doi: 10.1093/cvr/cvaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Logroscino G, Beghi E. Stroke epidemiology and COVID-19 pandemic. Curr Opin Neurol 2021;34(1):3–10; doi: 10.1097/wco.0000000000000879 [DOI] [PubMed] [Google Scholar]
- 98. Siepmann T, Sedghi A, Simon E, et al. Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur J Neurol 2021;28(1):238–247; doi: 10.1111/ene.14535 [DOI] [PubMed] [Google Scholar]
- 99. Shahjouei S, Naderi S, Li J, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine 2020;59:102939; doi: 10.1016/j.ebiom.2020.102939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fridman S, Bullrich MB, Jimenez-Ruiz A, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology 2020;95(24):e3373–e3385; doi: 10.1212/wnl.0000000000010851 [DOI] [PubMed] [Google Scholar]
- 101. Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev 2021;47:100761; doi: 10.1016/j.blre.2020.100761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Astin R, Banerjee A, Baker MR, et al. Long COVID: mechanisms, risk factors and recovery. Exp Physiol 2023;108(1):12–27; doi: 10.1113/ep090802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021;20(1):172; doi: 10.1186/s12933-021-01359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J 2022;479(4):537–559; doi: 10.1042/bcj20220016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022;54(1):1473–1487; doi: 10.1080/07853890.2022.2076901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 2021;36(6):109518; doi: 10.1016/j.celrep.2021.109518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shimohata T. Neuro-COVID-19. Clin Exp Neuroimmunol 2021; doi: 10.1111/cen3.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jentho E, Weis S. DAMPs and Innate Immune Training. Front Immunol 2021;12:699563; doi: 10.3389/fimmu.2021.699563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Klimova EM, Bozhkov AI, Lavinska OV, et al. Low molecular weight cytotoxic components (DAMPs) form the post-COVID-19 syndrome. Immunobiology 2023;228(1):152316; doi: 10.1016/j.imbio.2022.152316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Islam MS, Wang Z, Abdel-Mohsen M, et al. Tissue injury and leukocyte changes in post-acute sequelae of SARS-CoV-2: review of 2833 post-acute patient outcomes per immune dysregulation and microbial translocation in long COVID. J Leukoc Biol 2023;113(3):236–254; doi: 10.1093/jleuko/qiac001 [DOI] [PubMed] [Google Scholar]
- 111. Liang CS, Gałecki P, Su KP. Unresolved Systemic Inflammation, Long COVID, and the Common Pathomechanisms of Somatic and Psychiatric Comorbidity. J Clin Med 2022;11(17):5114; doi: 10.3390/jcm11175114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stefano GB, Büttiker P, Weissenberger S, et al. Editorial: the pathogenesis of long-term neuropsychiatric COVID-19 and the role of microglia, mitochondria, and persistent neuroinflammation: a hypothesis. Med Sci Monit 2021;27:e933015; doi: 10.12659/msm.933015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Petersen M. Brain imaging and neuropsychological assessment of individuals recovered from a mild to moderate SARS- CoV-2 infection. medRxiv 2022; doi: <h/> 10.1101/2022.07.08.22277420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Petersen MS, Kristiansen MF, Hanusson KD, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months' follow-up. Int J Infect Dis 2022;122:437–441; doi: 10.1016/j.ijid.2022.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. El-Rhermoul FZ, Fedorowski A, Eardley P, et al. Autoimmunity in Long Covid and POTS. Oxf Open Immunol 2023;4(1):iqad002; doi: 10.1093/oxfimm/iqad002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sin DD. Is long COVID an autoimmune disease? Eur Respir J 2023;61(1):2202272; doi: 10.1183/13993003.02272-2022 [DOI] [PubMed] [Google Scholar]
- 117. Anaya JM, Herrán M, Beltrán S, et al. Is post-COVID syndrome an autoimmune disease? Expert Rev Clin Immunol 2022;18(7):653–666; doi: 10.1080/1744666x.2022.2085561 [DOI] [PubMed] [Google Scholar]
- 118. Votto M, Castagnoli R, Marseglia GL, et al. COVID-19 and autoimmune diseases: is there a connection? Curr Opin Allergy Clin Immunol 2023;23(2):185–192; doi: 10.1097/aci.0000000000000888 [DOI] [PubMed] [Google Scholar]
- 119. Franke C, Boesl F, Goereci Y, et al. Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav Immun 2023;109:139–143; doi: 10.1016/j.bbi.2023.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Needham E. Brain Injury in COVID-19 is Associated with Autoinflammation and Autoimmunity. medRxiv 2021; doi: 10.1101/2021.12.03.21266112 [DOI] [Google Scholar]
- 121. Needham EJ, Chou SH, Coles AJ, et al. Neurological implications of COVID-19 infections. Neurocrit Care 2020;32(3):667–671; doi: 10.1007/s12028-020-00978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Needham EJ, Ren AL, Digby RJ, et al. Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain 2022;145(11):4097–4107; doi: 10.1093/brain/awac321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lindqvist I, Cunningham JL, Mulder J, et al. Myoclonus in patients with COVID-19: Findings of autoantibodies against brain structures in cerebrospinal fluid. Eur J Neurol 2023; doi: 10.1111/ene.15958 [DOI] [PubMed] [Google Scholar]
- 124. Kwak N, Lee KH, Woo J, et al. Synergistic cycles of protease activity and inflammation via PPARγ degradation in chronic obstructive pulmonary disease. Exp Mol Med 2021;53(5):947–955; doi: 10.1038/s12276-021-00626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhao L, Zhao J, Zhong K, et al. Targeted protein degradation: mechanisms, strategies and application. Signal Transduct Target Ther 2022;7(1):113; doi: 10.1038/s41392-022-00966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Venkataramani V, Winkler F. Cognitive deficits in Long Covid-19. N Engl J Med 2022;387(19):1813–1815; doi: 10.1056/NEJMcibr2210069 [DOI] [PubMed] [Google Scholar]
- 127. Agoston DV, Elsayed M. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front Neurol 2012;3:107; doi: 10.3389/fneur.2012.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bremner JD, Wittbrodt MT. Stress, the brain, and trauma spectrum disorders. Int Rev Neurobiol 2020;152:1–22; doi: 10.1016/bs.irn.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Decuypere M, Klimo P Jr. Spectrum of traumatic brain injury from mild to severe. Surg Clin North Am 2012;92(4):939–957, ix; doi: 10.1016/j.suc.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 130. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 2013;9(4):222–230; doi: 10.1038/nrneurol.2013.33 [DOI] [PubMed] [Google Scholar]
- 131. Mayer AR, Quinn DK, Master CL. The spectrum of mild traumatic brain injury: A review. Neurology 2017;89(6):623–632; doi: 10.1212/wnl.0000000000004214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pugh MJ, Kennedy E, Prager EM, et al. Phenotyping the spectrum of traumatic brain injury: a review and pathway to standardization. J Neurotrauma 2021;38(23):3222–3234; doi: 10.1089/neu.2021.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Si B, Dumkrieger G, Wu T, et al. Sub-classifying patients with mild traumatic brain injury: a clustering approach based on baseline clinical characteristics and 90-day and 180-day outcomes. PLoS One 2018;13(7):e0198741; doi: 10.1371/journal.pone.0198741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81–84; doi: 10.1016/s0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 135. Dikmen S, Machamer J, Manley GT, et al. ; TRACK-TBI Investigators. Functional Status Examination versus Glasgow Outcome Scale Extended as outcome measures in traumatic brain injuries: how do they compare? J Neurotrauma 2019;36(16):2423–2429; doi: 10.1089/neu.2018.6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Reith FC, Synnot A, van den Brande R, et al. Factors influencing the reliability of the Glasgow Coma Scale: a systematic review. Neurosurgery 2017;80(6):829–839; doi: 10.1093/neuros/nyw178 [DOI] [PubMed] [Google Scholar]
- 137. Reith FC, Van den Brande R, Synnot A, et al. The reliability of the Glasgow Coma Scale: a systematic review. Intensive Care Med 2016;42(1):3–15; doi: 10.1007/s00134-015-4124-3 [DOI] [PubMed] [Google Scholar]
- 138. Segatore M, Way C. The Glasgow Coma Scale: time for change. Heart Lung 1992;21(6):548–557. [PubMed] [Google Scholar]
- 139. Giza C, Greco T, Prins ML. Concussion: pathophysiology and clinical translation. Handb Clin Neurol 2018;158:51–61; doi: 10.1016/b978-0-444-63954-7.00006-9 [DOI] [PubMed] [Google Scholar]
- 140. Echemendia RJ, Meeuwisse W, McCrory P, et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): background and rationale. Br J Sports Med 2017;51(11):848–850; doi: 10.1136/bjsports-2017-097506 [DOI] [PubMed] [Google Scholar]
- 141. Nasrallah F, Bellapart J, Walsham J, et al. PREdiction and Diagnosis using Imaging and Clinical biomarkers Trial in Traumatic Brain Injury (PREDICT-TBI) study protocol: an observational, prospective, multicentre cohort study for the prediction of outcome in moderate-to-severe TBI. BMJ Open 2023;13(4):e067740; doi: 10.1136/bmjopen-2022-067740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Takahashi CE, Virmani D, Chung DY, et al. Blunt and penetrating severe traumatic brain injury. Neurol Clin 2021;39(2):443–469; doi: 10.1016/j.ncl.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 143. Dijkland SA, Foks KA, Polinder S, et al. Prognosis in moderate and severe traumatic brain injury: a systematic review of contemporary models and validation studies. J Neurotrauma 2020;37(1):1–13; doi: 10.1089/neu.2019.6401 [DOI] [PubMed] [Google Scholar]
- 144. Abdelmalik PA, Draghic N, Ling GSF. Management of moderate and severe traumatic brain injury. Transfusion 2019;59(S2):1529–1538; doi: 10.1111/trf.15171 [DOI] [PubMed] [Google Scholar]
- 145. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol 2017;16(6):452–464; doi: 10.1016/s1474-4422(17)30118-7 [DOI] [PubMed] [Google Scholar]
- 146. Golding EM. Sequelae following traumatic brain injury. The cerebrovascular perspective. Brain Res Brain Res Rev 2002;38(3):377–388. [DOI] [PubMed] [Google Scholar]
- 147. Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol 2016;73(5):551–560; doi: 10.1001/jamaneurol.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rostami E, Gyorgy A, Davidsson J, et al. Time-dependent changes in serum level of protein biomarkers after focal traumatic brain injury. Int J Neurorehabilitation 2015;2(3):2–6. [Google Scholar]
- 149. Agoston DV, Vink B, Helmy A, et al. How to translate time; the temporal aspects of rodent and human pathobiological processes in traumatic brain injury. J Neurotrauma 2019;36(11):1724–1737; doi: 10.1089/neu.2018.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ahmed F, Gyorgy A, Kamnaksh A, et al. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 2012;33(24):3705–3711; doi: 10.1002/elps.201200299 [DOI] [PubMed] [Google Scholar]
- 151. Gyorgy A, Ling G, Wingo D, et al. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J Neurotrauma 2011;28(6):1121–1126; doi: 10.1089/neu.2010.1561 [DOI] [PubMed] [Google Scholar]
- 152. Lin IH, Kamnaksh A, Aniceto R, et al. Time-dependent changes in the biofluid levels of neural injury markers in severe traumatic brain injury patients—cerebrospinal fluid and cerebral microdialysates: a longitudinal prospective pilot study. Neurotrauma Rep 2023;4(1):107–117; doi: 10.1089/neur.2022.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Thelin EP, Zeiler FA, Ercole A, et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front Neurol 2017;8:300; doi: 10.3389/fneur.2017.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mohamadpour M, Whitney K, Bergold PJ. The importance of therapeutic time window in the treatment of traumatic brain injury. Front Neurosci 2019;13:07; doi: 10.3389/fnins.2019.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med 2017;51(12):935–940; doi: 10.1136/bjsports-2016-097464 [DOI] [PubMed] [Google Scholar]
- 156. Balança B, Desmurs L, Grelier J, et al. DAMPs and RAGE pathophysiology at the acute phase of brain injury: an overview. Int J Mol Sci 2021;22(5):2439; doi: 10.3390/ijms22052439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci 2015;35(2):583–598; doi: 10.1523/jneurosci.2439-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Thundyil J, Lim KL. DAMPs and neurodegeneration. Ageing Res Rev 2015;24(Pt A):17–28; doi: 10.1016/j.arr.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 159. Tsitsopoulos PP, Abu Hamdeh S, Marklund N. Current opportunities for clinical monitoring of axonal pathology in traumatic brain injury. Front Neurol 2017;8:599; doi: 10.3389/fneur.2017.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Smith DH, Nonaka M, Miller R, et al. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J Neurosurg 2000;93(2):315–322; doi: 10.3171/jns.2000.93.2.0315 [DOI] [PubMed] [Google Scholar]
- 161. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013;246:35–43; doi: 10.1016/j.expneurol.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Povlishock JT, Erb DE, Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J Neurotrauma 1992;9(Suppl 1):S189–S200. [PubMed] [Google Scholar]
- 163. Haber M, Amyot F, Lynch CE, et al. Imaging biomarkers of vascular and axonal injury are spatially distinct in chronic traumatic brain injury. J Cereb Blood Flow Metab 2021; doi: 10.1177/0271678x20985156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kenney K, Amyot F, Haber M, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 2016;275 Pt 3:353–366; doi: 10.1016/j.expneurol.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 165. Lynch CE, Eisenbaum M, Algamal M, et al. Impairment of cerebrovascular reactivity in response to hypercapnic challenge in a mouse model of repetitive mild traumatic brain injury. J Cereb Blood Flow Metab 2020; doi: 10.1177/0271678x20954015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sandsmark DK, Bashir A, Wellington CL, et al. Cerebral microvascular injury: a potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration. Neuron 2019;103(3):367–379; doi: 10.1016/j.neuron.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Schneider ALC, Barber J, Temkin N, et al. Associations of preexisting vascular risk factors with outcomes after traumatic brain injury: a TRACK-TBI study. J Head Trauma Rehabil 2023;38(2):e88–e98; doi: 10.1097/htr.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Baker TL, Agoston DV, Brady RD, et al. Targeting the cerebrovascular system: next-generation biomarkers and treatment for mild traumatic brain injury. Neuroscientist 2021; doi: 10.1177/10738584211012264 [DOI] [PubMed] [Google Scholar]
- 169. Werhane ML, Evangelista ND, Clark AL, et al. Pathological vascular and inflammatory biomarkers of acute- and chronic-phase traumatic brain injury. Concussion 2017;2(1):Cnc30; doi: 10.2217/cnc-2016-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Amyot F, Kenney K, Moore C, et al. Imaging of cerebrovascular function in chronic traumatic brain injury. J Neurotrauma 2018;35(10):1116–1123; doi: 10.1089/neu.2017.5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sun M, Symons GF, O'Brien WT, et al. Serum protein biomarkers of inflammation, oxidative stress, and cerebrovascular and glial injury in concussed Australian football players. J Neurotrauma 2022;39(11–12):800–808; doi: 10.1089/neu.2021.0493 [DOI] [PubMed] [Google Scholar]
- 172. Major BP, McDonald SJ, O'Brien WT, et al. Serum protein biomarker findings reflective of oxidative stress and vascular abnormalities in male, but not female, collision sport athletes. Front Neurol 2020;11:549624; doi: 10.3389/fneur.2020.549624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wright DK, Brady RD, Kamnaksh A, et al. Repeated mild traumatic brain injuries induce persistent changes in plasma protein and magnetic resonance imaging biomarkers in the rat. Sci Rep 2019;9(1):14626; doi: 10.1038/s41598-019-51267-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Thomas R, Gatson J, Silverman E, et al. von Willebrand factor as a biomarker of traumatic brain injury. Neurology 2021;96(15 Supplement):4717. [Google Scholar]
- 175. Sandsmark DK, Bogoslovsky T, Qu BX, et al. Changes in plasma von Willebrand factor and cellular fibronectin in MRI-defined traumatic microvascular injury. Front Neurol 2019;10:246; doi: 10.3389/fneur.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kempuraj D, Ahmed ME, Selvakumar GP, et al. Mast cell activation, neuroinflammation, and tight junction protein derangement in acute traumatic brain injury. Mediators Inflamm 2020;2020:4243953; doi: 10.1155/2020/4243953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bhowmick S, D'Mello V, Caruso D, et al. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp Neurol 2019;317:260–270; doi: 10.1016/j.expneurol.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 178. Hubbard WB, Dong JF, Cruz MA, et al. Links between thrombosis and inflammation in traumatic brain injury. Thromb Res 2021;198:62–71; doi: 10.1016/j.thromres.2020.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kempuraj D, Ahmed ME, Selvakumar GP, et al. Acute traumatic brain injury-induced neuroinflammatory response and neurovascular disorders in the brain. Neurotox Res 2021;39(2):359–368; doi: 10.1007/s12640-020-00288-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sulhan S, Lyon KA, Shapiro LA, et al. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res 2020;98(1):19–28; doi: 10.1002/jnr.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Frederick N, Louveau A. Meningeal lymphatics, immunity and neuroinflammation. Curr Opin Neurobiol 2020;62:41–47; doi: 10.1016/j.conb.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 182. Thelin EP, Tajsic T, Zeiler FA, et al. Monitoring the neuroinflammatory response following acute brain injury. Front Neurol 2017;8:351; doi: 10.3389/fneur.2017.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Needham EJ, Helmy A, Zanier ER, et al. The immunological response to traumatic brain injury. J Neuroimmunol 2019;332:112–125; doi: 10.1016/j.jneuroim.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 184. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454(7203):428–435; doi: 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 185. McGinn MJ, Povlishock JT. Pathophysiology of traumatic brain injury. Neurosurg Clin N Am 2016;27(4):397–407; doi: 10.1016/j.nec.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 186. Marklund N, Vedung F, Lubberink M, et al. Tau aggregation and increased neuroinflammation in athletes after sports-related concussions and in traumatic brain injury patients—a PET/MR study. Neuroimage Clin 2021;30:102665; doi: 10.1016/j.nicl.2021.102665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nitta ME, Savitz J, Nelson LD, et al. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology 2019;93(5):e497–e507; doi: 10.1212/wnl.0000000000007864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 2013;35(5):601–612; doi: 10.1007/s00281-013-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron 2020;108(4):608–622; doi: 10.1016/j.neuron.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bhusal A, Afridi R, Lee WH, et al. Bidirectional communication between microglia and astrocytes in neuroinflammation. Curr Neuropharmacol 2023;21(10):2020–2029; doi: 10.2174/1570159x21666221129121715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Patani R, Hardingham GE, Liddelow SA. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol 2023;19(7):395–409; doi: 10.1038/s41582-023-00822-1 [DOI] [PubMed] [Google Scholar]
- 192. Wang KK, Yang Z, Yue JK, et al. Plasma anti-glial fibrillary acidic protein autoantibody levels during the acute and chronic phases of traumatic brain injury: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot Study. J Neurotrauma 2016;33(13):1270–1277; doi: 10.1089/neu.2015.3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Needham EJ, Stoevesandt O, Thelin EP, et al. Complex autoantibody responses occur following moderate to severe traumatic brain injury. J Immunol 2021;207(1):90–100; doi: 10.4049/jimmunol.2001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tisminetzky M, Delude C, Hebert T, et al. Age, multiple chronic conditions, and COVID-19: a literature review. J Gerontol A Biol Sci Med Sci 2022;77(4):872–878; doi: 10.1093/gerona/glaa320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Polidori MC, Sies H, Ferrucci L, et al. COVID-19 mortality as a fingerprint of biological age. Ageing Res Rev 2021;67:101308; doi: 10.1016/j.arr.2021.101308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med 2022;11(24):7314; doi: 10.3390/jcm11247314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Stephenson T, Shafran R, Ladhani SN. Long COVID in children and adolescents. Curr Opin Infect Dis 2022;35(5):461–467; doi: 10.1097/qco.0000000000000854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Brugler Yonts A. Pediatric Long-COVID: a review of the definition, epidemiology, presentation, and pathophysiology. Pediatr Ann 2022;51(11):e416–e420; doi: 10.3928/19382359-20220913-06 [DOI] [PubMed] [Google Scholar]
- 199. Peters ME, Hsu M, Rao V, et al. Influence of study population definition on the effect of age on outcomes after blunt head trauma. Brain Inj 2018;32(13–14):1725–1730; doi: 10.1080/02699052.2018.1520301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mayer AR, Meier TB, Dodd AB, et al. Prospective study of gray matter atrophy following pediatric mild traumatic brain injury. Neurology 2023;100(5):e516–e527; doi: 10.1212/wnl.0000000000201470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Keenan HT, Hooper SR, Wetherington CE, et al. Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics 2007;119(3):e616–e623; doi: 10.1542/peds.2006-2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Koerte IK, Wiegand TLT, Bonke EM, et al. Diffusion imaging of sport-related repetitive head impacts—a systematic review. Neuropsychol Rev 2023;33(1):122–143; doi: 10.1007/s11065-022-09566-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Badea A, Kamnaksh A, Anderson RJ, et al. Repeated mild blast exposure in young adult rats results in dynamic and persistent microstructural changes in the brain. Neuroimage Clin 2018;18:60–73; doi: 10.1016/j.nicl.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Fletcher-Sandersjoo A, Lindblad C, Thelin EP, et al. Serial S100B sampling detects intracranial lesion development in patients on extracorporeal membrane oxygenation. Front Neurol 2019;10:512; doi: 10.3389/fneur.2019.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tsilingiris D, Vallianou NG, Karampela I, et al. Laboratory findings and biomarkers in Long COVID: what do we know so far? Insights into epidemiology, pathogenesis, therapeutic perspectives and challenges. Int J Mol Sci 2023;24(13):10458; doi: 10.3390/ijms241310458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sun L, Shan W, Yang H, et al. The role of neuroinflammation in post-traumatic epilepsy. Front Neurol 2021;12:646152; doi: 10.3389/fneur.2021.646152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Folweiler KA, Sandsmark DK, Diaz-Arrastia R, et al. Unsupervised machine learning reveals novel traumatic brain injury patient phenotypes with distinct acute injury profiles and long-term outcomes. J Neurotrauma 2020;37(12):1431–1444; doi: 10.1089/neu.2019.6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Sayed N, Culver C, Dams-O'Connor K, et al. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma 2013;30(13):1117–1122; doi: 10.1089/neu.2012.2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Diaz-Arrastia R, Agostini MA, Madden CJ, et al. Posttraumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia 2009;50(Suppl 2):14–20; doi: 10.1111/j.1528-1167.2008.02006.x [DOI] [PubMed] [Google Scholar]
- 210. Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj 2017;31(9):1195–1203; doi: 10.1080/02699052.2017.1357836 [DOI] [PubMed] [Google Scholar]
- 211. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn 2018;18(2):165–180; doi: 10.1080/14737159.2018.1428089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Mondello S, Sorinola A, Czeiter E, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. J Neurotrauma 2021;38(8):1086–1106; doi: 10.1089/neu.2017.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Yue JK, Kobeissy FH, Jain S, et al. Neuroinflammatory biomarkers for traumatic brain injury diagnosis and prognosis: a TRACK-TBI Pilot Study. Neurotrauma Rep 2023;4(1):171–183; doi: 10.1089/neur.2022.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gardner RC, Puccio AM, Korley FK, et al. Effects of age and time since injury on traumatic brain injury blood biomarkers: a TRACK-TBI study. Brain Commun 2023;5(1):fcac316; doi: 10.1093/braincomms/fcac316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Tenovuo O, Diaz-Arrastia R, Goldstein LE, et al. Assessing the severity of traumatic brain injury—time for a change? J Clin Med 2021;10(1):148; doi: 10.3390/jcm10010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Joly BS, Darmon M, Dekimpe C, et al. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. J Thromb Haemost 2021;19(9):2193–2198; doi: 10.1111/jth.15445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yatsiv I, Morganti-Kossmann MC, Perez D, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J Cereb Blood Flow Metab 2002;22(8):971–978. [DOI] [PubMed] [Google Scholar]
- 218. Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 2005;9(4):777–794; doi: 10.1111/j.1582-4934.2005.tb00379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB Life 2001;52(1–2):61–66; doi: 10.1080/15216540252774784 [DOI] [PubMed] [Google Scholar]
- 220. Xu LB, Yue JK, Korley F, et al. High-sensitivity C-reactive protein is a prognostic biomarker of six-month disability after traumatic brain injury: results from the TRACK-TBI Study. J Neurotrauma 2021;38(7):918–927; doi: 10.1089/neu.2020.7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Fischer TF, Beck-Sickinger AG. Chemerin—exploring a versatile adipokine. Biol Chem 2022;403(7):625–642; doi: 10.1515/hsz-2021-0409 [DOI] [PubMed] [Google Scholar]
- 222. Wong LR, Perlman S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses—are we our own worst enemy? Nat Rev Immunol 2022;22(1):47–56; doi: 10.1038/s41577-021-00656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med 2022;3(6):100663; doi: 10.1016/j.xcrm.2022.100663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Liu NT, Salinas J. Machine learning for predicting outcomes in trauma. Shock 2017;48(5):504–510; doi: 10.1097/shk.0000000000000898 [DOI] [PubMed] [Google Scholar]
- 225. Khalili H, Rismani M, Nematollahi MA, et al. Prognosis prediction in traumatic brain injury patients using machine learning algorithms. Sci Rep 2023;13(1):960; doi: 10.1038/s41598-023-28188-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Courville E, Kazim SF, Vellek J, et al. Machine learning algorithms for predicting outcomes of traumatic brain injury: a systematic review and meta-analysis. Surg Neurol Int 2023;14:262; doi: 10.25259/sni_312_2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Dietz N, Vaitheesh J, Alkin V, et al. Machine learning in clinical diagnosis, prognostication, and management of acute traumatic spinal cord injury (SCI): a systematic review. J Clin Orthop Trauma 2022;35:102046; doi: 10.1016/j.jcot.2022.102046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. McDonald SJ, Shultz SR, Agoston DV. The known unknowns: an overview of the state of blood-based protein biomarkers of mild traumatic brain injury. J Neurotrauma 2021;38(19):2652–2666; doi: 10.1089/neu.2021.0011 [DOI] [PubMed] [Google Scholar]
- 229. Finnie J. Animal models of traumatic brain injury: a review. Aust Vet J 2001;79(9):628–633. [DOI] [PubMed] [Google Scholar]
- 230. Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol 2011;164(4):1207–1229; doi: 10.1111/j.1476-5381.2010.01163.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Agoston DV, Kamnaksh A.. Modeling the Neurobehavioral Consequences of Blast-Induced Traumatic Brain Injury Spectrum Disorder and Identifying Related Biomarkers. In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. (Kobeissy FH. ed.) CRC Press/Taylor & Francis: Boca Raton, FL; 2015. [PubMed] [Google Scholar]
- 232. Kochanek PM, Bramlett HM, Dixon CE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury: operation brain trauma therapy. J Neurotrauma 2016;33(6):513–522; doi: 10.1089/neu.2015.4113 [DOI] [PubMed] [Google Scholar]
- 233. Shultz SR, McDonald SJ, Vonder Haar C, et al. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci Biobehav Rev 2017;76(Pt B):396–414; doi: 10.1016/j.neubiorev.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 234. Brady RD, Casillas-Espinosa PM, Agoston DV, et al. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol Dis 2019;123:8–19; doi: 10.1016/j.nbd.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Povlishock JT. Pathophysiology of neural injury: therapeutic opportunities and challenges. Clin Neurosurg 2000;46:113–126. [PubMed] [Google Scholar]
- 236. Kochanek PM, Dixon CE, Mondello S, et al. Multi-center pre-clinical consortia to enhance translation of therapies and biomarkers for traumatic brain injury: operation brain trauma therapy and beyond. Front Neurol 2018;9:640; doi: 10.3389/fneur.2018.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kochanek PM, Bramlett HM, Shear DA, et al. Synthesis of findings, current investigations, and future directions: operation brain trauma therapy. J Neurotrauma 2016;33(6):606–614; doi: 10.1089/neu.2015.4133 [DOI] [PubMed] [Google Scholar]
- 238. Jullienne A, Salehi A, Affeldt B, et al. Male and female mice exhibit divergent responses of the cortical vasculature to traumatic brain injury. J Neurotrauma 2018;35(14):1646–1658; doi: 10.1089/neu.2017.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Valera EM, Joseph AC, Snedaker K, et al. Understanding traumatic brain injury in females: a state-of-the-art summary and future directions. J Head Trauma Rehabil 2021;36(1):e1–e17; doi: 10.1097/htr.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma 2003;20(2):123–137; doi: 10.1089/08977150360547053 [DOI] [PubMed] [Google Scholar]
- 241. Hamm RJ, Jenkins LW, Lyeth BG, et al. The effect of age on outcome following traumatic brain injury in rats. J Neurosurg 1991;75(6):916–921; doi: 10.3171/jns.1991.75.6.0916 [DOI] [PubMed] [Google Scholar]
- 242. Stocchetti N, Paterno R, Citerio G, et al. Traumatic brain injury in an aging population. J Neurotrauma 2012;29(6):1119–1125; doi: 10.1089/neu.2011.1995 [DOI] [PubMed] [Google Scholar]
- 243. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 2013;9(4):231–236; doi: 10.1038/nrneurol.2013.22 [DOI] [PubMed] [Google Scholar]