Abstract
Background
We conducted a phase III, non-inferiority trial comparing safety and efficacy of RCP recombinant spike protein Covid-19 vaccine to BBIBP (Sinopharm).
Methods
Adult Iranian population received RCP or BBIBP in a randomized, double blind and an additional non-randomized open labeled trial arms. Eligible participants signed a written informed consent and received two intramuscular injections three weeks apart. In the randomized arm, an intranasal dose of vaccine or adjuvant-only preparation were given to the RCP and BBIBP recipients at day 51 respectively. Participants were actively followed for up to 4 months for safety and efficacy outcomes. Primary outcome was PCR + symptomatic Covid-19 disease two weeks after the second dose. The non-inferiority margin was 10% of reported BBIBP vaccine efficacy (HR = 1.36).
Results
We recruited 23,110 participants (7224 in the randomized and 15,886 in the non-randomized arm). We observed 604 primary outcome events during 4 months of active follow-up including 121 and 133 in the randomized and 157 and 193 cases in the non-randomized arms among recipients of RCP and BBIBP respectively. Adjusted hazard ratios for the primary outcome in those receiving RCP compared with BBIBP interval were 0.91 (0.71–1.16) and 0.62 (0.49–0.77) in the randomized and non-randomized arms respectively. The upper boundary of 99.1% confidence interval of HR = 0.91 (0.67–1.22) remained below the margin of non-inferiority in the randomized arm after observing the early stopping rules using O'Brien Fleming method.
Conclusion
Our study showed that the RCP efficacy is non-inferior and its safety profile is comparable to the BBIBP.
Keywords: Razi-cov-pars, BBIBP, Non-inferiority design, Recombinant Covid-19 vaccine, Vaccine efficacy, Phase III clinical trial
Highlights
-
•
In a phase III, randomized, double blind non-inferiority trial we compared Razi-Cov Pars (RCP) covid-19 vaccine to BBIBP.
-
•
RCP recombinant vaccine efficacy is non-inferior and its safety profile is comparable to the BBIBP inactivated vaccine.
-
•
Estimated vaccine efficacy for prevention of symptomatic Covid-19 infection was 75.5% (95% CI: 51.8–87.7).
1. Introduction
Vaccines played an essential role in controlling the Covid-19 pandemic by dramatically reducing mortality, particularly in vulnerable populations [1,2]. The efficacy and safety of early ones were demonstrated by comparing them with a placebo [[3], [4], [5], [6]]. Comparison with a placebo was no longer ethically feasible when the pioneer vaccines made their way into the vaccine basket of different populations. At the same time, the urgency for vaccinating the whole world's population when the supply was no match necessitated the parallel development of other Covid-19 vaccines [7]. Therefore non-inferiority approaches were needed to evaluate their safety and efficacy.
RCP is a combined intramuscular/intranasal recombinant Covid-19 vaccine based on the monomer of the spike (S) protein subunits S1, S2 of SARS-CoV-2 and S trimer (in its perfusion conformation) of the Wuhan strain formulated in Razi Adjuvant System-01 (RAS-01), an Iranian FDO (Food and Drug Organization) approved oil-in-water emulsion comprised of sesame, olive, and soybean oils and the non-ionic surfactant Tween 80 [8]. It includes two injections on days 0 and 21 and one intranasal dose on day 51 via an intranasal mucosal atomization device. Phase I and II trials of RCP were conducted in 2021 [[9], [10]], and Phase III started in September 2021. In this paper, we are reporting the results of phase III, the non-inferiority trial of RCP compared to BBIBP [11], an inactivated vaccine made by Sinopharm Co, China, that had been listed in WHO's covid-19 EUL (emergency use listing).
2. Methods
2.1. Study design
This is a multicenter, randomized, double-blind, parallel groups, active control, non-inferiority trial comparing the safety and efficacy of RCP, a recombinant spike protein Covid-19 vaccine, to BBIBP in the Iranian adult population. Because of the slow recruitment, two additional non-randomized open-labeled arms were added to the study.
2.2. Participants
Adult Iranian population with no history of Covid-19 vaccination and access to a smartphone were eligible to enter the study. Major exclusion criteria include current COVID-19 infection, pregnancy and breastfeeding, plan to have children in the next six months, any current or new diagnosis of acute or chronic illness requiring continuous ongoing medical care, unstable chronic diseases in the last four weeks, history of allergy to any drug, vaccine or food, long-term use of immunosuppressive drugs or systemic corticosteroids within the past four months, splenectomy for any reason, history of uncontrolled serious psychiatric illnesses, history of chronic neurological diseases (including seizures and epilepsy), current substance or alcohol abuse, and close contact with a confirmed COVID-19 case within two weeks before the first vaccine dose (for more details, please see the study protocol).
2.3. Randomization and masking
We used block randomization stratified by the center to allocate participants into the study groups. Randomization sequences were generated by an independent study epidemiologist using STATA statistical software and integrated into the study online application. A nine-digit code was used to conceal the sequence. Participants and all study team members (including the care providers, outcome assessors, and the entire follow-up team) except the vaccinator nurse were kept blind to the type of intervention. After reaching the injection stage, the assignment group of the participant was displayed on the screen for the vaccinator only and removed once the administration was confirmed.
In the additional non-randomized arms, participants received one of the open-labeled RCP or BBIBP vaccines based on their preference, however, their follow-ups were still carried out while their chosen intervention had been masked within the study application.
2.4. Procedures
Adult Iranian populations with no history of Covid-19 vaccination were recruited through a website. Volunteers who declared their willingness to participate in the study underwent an initial online screening. Those who were successful were invited to attend the clinical units where they signed written informed consent, were assessed for eligibility, and were clinically examined by a physician. All the gathered information was recorded within the study application.
Eligible participants randomly received their first dose of either 10-μg/200 μL RCP vaccine, manufactured by Razi Vaccine and Serum Research Institute, Karaj, Iran, or 0.5 ml BBIBP vaccine, manufactured by Sinopharm, Beijing, China, by deep intramuscular injection within the deltoid muscle. The second intramuscular dose was delivered three weeks later on day 21. The third dose was delivered on day 51 via an intranasal mucosal atomization device containing 10-μg/200 μL of RCP vaccine in the RCP group and placebo (an adjuvant-only preparation) in the BBIBP group. In the additional open-labeled non-randomized arms, participants received the vaccine based on their preference, and those receiving the BBIBP did not have a third dose.
Participants were kept under close observation for half an hour after each vaccine dose, and their vital signs and immediate allergic reactions were monitored. Subsequent follow-ups of all participants were managed through an integrated mobile application installed on their smartphones. They were asked to record their daily local and systemic adverse reactions for seven days after vaccination. A 24/7 centralized follow-up center with round-the-clock resident physicians was set up to provide consultation and support for the study participants. They could also report any adverse event, including symptoms suspicious of Covid-19 disease, a medical visit, or medication use at any time via the application triggering an active follow-up at the center until complete resolution. Participants were actively followed for four months for the occurrence of any adverse event (AE) through weekly telephone calls if they failed to report a no-AE status via the mobile application. Upon report of any symptom suggestive of a Covid-19 infection, the patient was referred to the reference clinical laboratories for a rt-PCR test and was followed until complete recovery. All the gathered information during the follow-ups, including the referral visits and test results, was recorded within the application.
3. Outcomes
The primary outcome was the number of symptomatic PCR positive Covid-19 cases two weeks after the second dose. All covid-19 diagnoses were ascertained in the follow-up center.
To record this outcome all clinically Covid-19 suspects (based on their self-reports in the study application) were evaluated in a phone call in the follow-up center and in case of clinical suspicion were referred to the reference lab for nasopharyngeal sampling and PCR.
The secondary outcomes include the numbers of severe illness or death due to covid-19 infection two weeks after the second dose, the number of immediate adverse reactions up to 30 min after vaccination, the number of solicited local and systemic adverse drug reactions up to 7 days after vaccination and the number of all and serious adverse events (SAEs) during the follow-up period. Solicited local and systemic adverse reactions were classified according to FDA Toxicity Grading Scale [12]. Adverse events were coded using International Classification of Diseases (ICD-10). The causality relationship between the intervention and the adverse events was evaluated using the WHO-UMC case causality assessment system [13]. The conduct, data collection, and safety outcomes were overseen by a nine-member data and safety monitoring board (DSMB). Each of the meetings was attended by representatives of the National Ethics Committee (NEC), the Ministry of Health's Food and Drug Organization (FDO), and the Department of Communicable Disease Control (CDC). An internist, a pharmacotherapist, an infectious disease specialist, and an immunology and allergy specialist comprised the four independent members of the DSMB. There were two additional non-voting members representing the sponsor. Specific anti-RBD IgA antibody levels were assessed in a small subgroup of participants in the randomized arm two weeks after the third intranasal dose in saliva and blood sample. The immunogenicity assessment lab staff were blinded to the identity of these samples.
3.1. Statistical analysis
We calculated our sample size in order to test the null hypothesis that the efficacy of the RCP vaccine is at least 10% inferior to the BBIBP vaccine with 72.8% reported efficacy [11] (Non-Inferiority margin of 10%). The risk of contracting SARS-CoV-2 during 6 months for a person who received the BBIBP vaccine was 0.00952 (based on a general population's cumulative incidence rate of 35 per 1000 over a 6-month period), and considering a 10% non-inferiority margin the risk for a person who received the RCP vaccine was calculated at 0.01302. Therefore, the non-inferiority margin for the hazard ratio was calculated as 0.01302/0.00952 = 1.367. Non-inferiority was declared if the upper limit of one-sided 97.5% CI for HR was less than 1.36.
A total of 365 cases of Covid-19 would provide 90% power to detect a 10% non-inferiority in the primary outcome of PCR + symptomatic Covid-19 disease in the RCP group compared to the BBIBP, with two planned interim analyses at approximately 35% and 70% of the target total number of cases, 20% dropout rate, 0.06% PCR positivity during the period reaching the complete vaccination status (from the first dose until two weeks after the second dose), and using the O'Brien-Fleming boundary for adjusting the overall one-sided alpha error rate of 0.025. The non-inferiority of the RCP vaccine could be demonstrated at either the interim or the primary analysis, performed when the target total number of cases had been observed.
We indirectly calculated the RCP vaccine efficacy from the reported BBIBP vaccine efficacy against a placebo using Bayesian network meta-analysis and multiplicative rules (HazardBBIBP/HazardPlacebo × HazardRCP/HazardBBIBP).
The primary efficacy outcome was assessed in the full analysis population (participants who received at least two doses of RCP or BBIBP), the modified intention-to-treat population (participants in the full analysis population without history of Covid-19 before the first dose). Hazard ratios and their one-sided 97.5% confidence intervals derived from Cox proportional hazard models. Mean values for the event of covid-19 disease are reported as incidence per year per 1000 persons. Safety outcomes were analyzed in all participants who received at least one dose of RCP or BBIB. Adverse events were summarized descriptively and their rates and 95% confidence interval were reported. All outcomes were analyzed based on predefined hypothesis without adjustment for multiple comparisons using one-sided alpha of 0.025. All statistical analysis was performed using STATA (version 11) and GraphPad Prism (version 8.0).
4. Result
We recruited 23,110 eligible participants between 5th of September and December 13, 2021.7224 individuals entered the randomized arm of which 3617 received RCP and 3607 received BBIBP. In the non-randomized arm 8054 individuals received RCP and 7832 received BBIBP based on their own preference (Fig. 1). Mean age of study participants was 36.6 years (SD = 16.9), 52.8% were male, and 70% had finished the high school with a diploma or higher degree. Baseline characteristics between the two study groups in the randomized arm were similar. However, in the non-randomized arm those receiving RCP tend to be older males with higher educational achievements and were more likely to have comorbidities (Table 1).
Fig. 1.
Participant flow diagram.
Table 1.
Comparison of baseline characteristics in the study groups in the randomized and non-randomized arms.
| Random arm (n = 7224) |
Nonrandom arm (n = 15,886) |
Total |
|||
|---|---|---|---|---|---|
| RCP n = 3617 | BBIBP n = 3607 | RCP n = 8054 | BBIBP n = 7832 | n = 23,110 | |
| Sex, n (%) | |||||
| Male | 2079 (57.5) | 2089 (57.9) | 4466 (55.5) | 3572 (45.6) | 12,205 (52.8) |
| Female | 1538 (42.5) | 1518 (42.1) | 3583 (44.5) | 4260 (54.4) | 10,893 (47.2) |
| Age (year) | |||||
| Mean (SD) | 36.10 (9.85) | 35.68 (9.81) | 37.84 (11.17) | 35.73 (11.23) | 36.61 (16.88) |
| Age group, n (%) | |||||
| 18-28 | 804 (22.2) | 861 (23.9) | 1484 (20.9) | 2006 (28.2) | 5155 (24.0%) |
| 28-38 | 1459 (40.4) | 1417 (39.3) | 2424 (34.1) | 2558 (35.9) | 7858 (36.6%) |
| 38-48 | 1005 (27.8) | 985 (27.3) | 2051 (28.8) | 1619 (22.7) | 5660 (26.4%) |
| 48-58 | 255 (7.1) | 260 (7.2) | 842 (11.8) | 676 (9.5) | 2033 (9.5%) |
| 58-68 | 75 (2.1) | 63 (1.8) | 237 (3.3) | 193 (2.7) | 568 (2.7%) |
| >68 | 16 (0.4) | 14 (0.4) | 69 (1.0) | 68 (1.0) | 167 (0.8%) |
| Body-mass index | |||||
| Mean (SD) | 26.03 (4.6) | 25.93 (4.5) | 26.19 (4.6) | 25.84 (4.6) | 26.00 (4.6) |
| Education, n (%) | |||||
| Elementary | 91 (2.5) | 89 (2.5) | 182 (2.3) | 428 (5.5) | 790 (3.4) |
| Under Diploma | 890 (24.6) | 915 (25.4) | 1608 (20.0) | 2752 (35.1) | 6165 (26.7) |
| Diploma | 1498 (41.4) | 1443 (40.0) | 3158 (39.3) | 3230 (41.2) | 9329 (40.4) |
| Diploma plus | 262 (7.2) | 255 (7.1) | 615 (7.6) | 424 (5.4) | 1556 (6.7) |
| Bachelor | 655 (18.1) | 672 (18.6) | 1809 (22.5) | 857 (10.9) | 3993 (17.3) |
| Master | 190 (5.3) | 205 (5.7) | 565 (7.0) | 126 (1.6) | 1086 (4.7) |
| Doctoral and above | 31 (0.9) | 28 (0.8) | 105 (1.3) | 15 (0.2) | 179 (0.8) |
| History of covid-19 | 163 (4.5) | 138 (3.8) | 1742 (21.6) | 971 (12.4) | 3014 (13.0) |
| Type of Comorbidities | |||||
| Hypertension | 126 (3.5) | 93 (2.6) | 356 (5.0) | 272 (3.8) | 847 (4.0) |
| Chronic heart diseases | 26 (0.7) | 33 (0.9) | 79 (1.1) | 65 (0.9) | 203 (1.0) |
| Chronic non-asthma lung dis. | 3 (0.1) | 5 (0.1) | 21 (0.3) | 14 (0.2) | 43 (0.2) |
| Asthma | 28 (0.8) | 21 (0.6) | 49 (0.7) | 61 (0.9) | 159 (0.7) |
| Chronic kidney diseases | 11 (0.3) | 7 (0.2) | 19 (0.3) | 23 (0.3) | 60 (0.3) |
| Moderate or severe liver dis. | 6 (0.2) | 7 (0.2) | 12 (0.2) | 18 (0.3) | 43 (0.2) |
| Mild liver diseases (fatty liver) | 126 (3.5) | 125 (3.5) | 263 (3.7) | 183 (2.6) | 697 (3.3) |
| Chronic neurological diseases | 41 (1.1) | 43 (1.2) | 94 (1.3) | 111 (1.6) | 289 (1.4) |
| Diabetes | 61 (1.7) | 68 (1.9) | 193 (2.7) | 182 (2.6) | 504 (2.4) |
| Diabetes with complications | 2 (0.1) | 3 (0.1) | 11 (0.2) | 10 (0.1) | 26 (0.1) |
| Chronic blood diseases | 18 (0.5) | 17 (0.5) | 38 (0.5) | 29 (0.4) | 102 (0.5) |
| Rheumatic diseases | 9 (0.3) | 12 (0.3) | 32 (0.5) | 34 (0.5) | 87 (0.4) |
| Dementia | 1 (0.0) | 2 (0.1) | 2 (0.0) | 6 (0.1) | 11 (0.1) |
| Having one comorbidity | 336 (9.3) | 292 (8.1) | 857 (10.6) | 964 (8.9) | 2179 (9.4) |
| Having two or more comorbidities | 55 (1.5) | 56 (1.6) | 205 (2.5) | 184 (2.3) | 500 (2.2) |
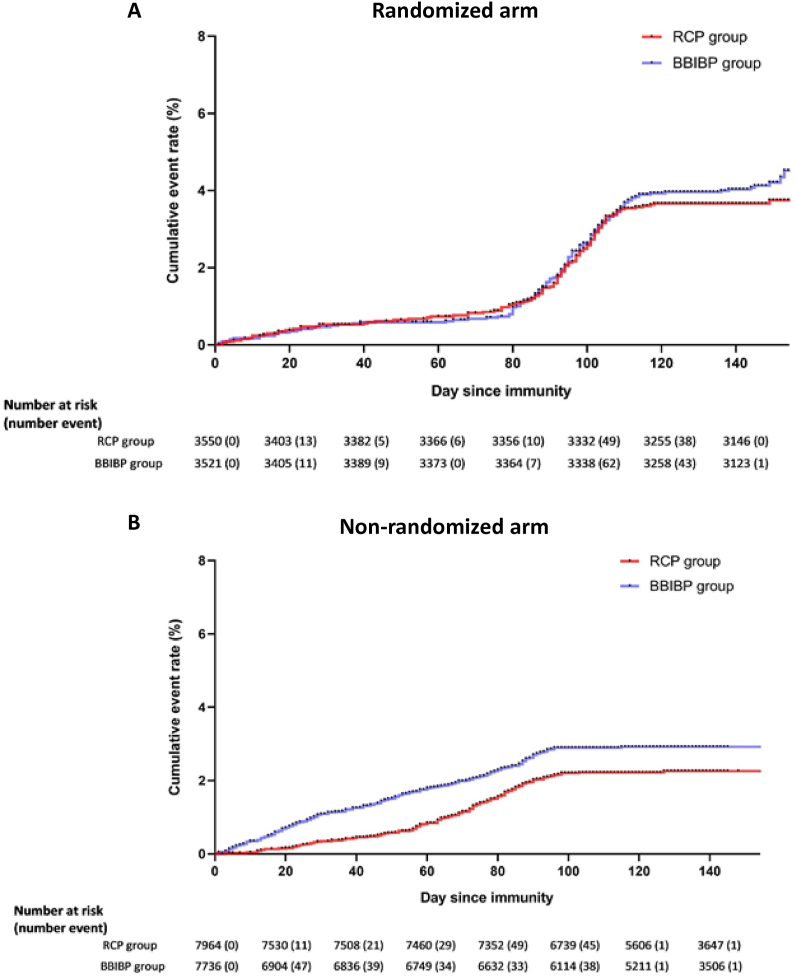
During the study follow-up period, we performed about 2100 PC R tests on symptomatic patients suspicious of Covid-19 disease. We identified 133 cases of symptomatic PCR positive Covid-19 disease over 479,843 person-days of active follow-up in the recipients of BBIBP in the randomized arm (incidence = 27.7, 95% CI: 23.4–32.8). The number of cases and person-days of active follow-up were 121 and 480,298 in the RCP group (incidence = 25.2, 95% CI: 21.1–30.1) (Fig. 4). People who had been vaccinated with RCP in the randomized arm were 9% less likely to acquire Covid-19 (HR = 0.91, 97.5% one-sided CI: 0.71–1.16). The higher bound of confidence interval was lower than the non-inferiority margin of 1.36 (Fig. 2). Adjusting for possible confounding factors of age, sex, education, week of the first injection, and history of covid-19 did not changed the result. Taking into account the information fraction (number of events achieved in the randomized arm versus the originally planned in the target sample size) using O'Brien-Fleming method increased the upper bound of the confidence interval to 1.22, however it did not cross the 1.36 non-inferiority margin (Table 2). We repeated the analyses after excluding subjects with a history of Covid-19 and the results remained unchanged. Based on the results from randomized arm, estimated vaccine efficacy for prevention of symptomatic Covid-19 infection was 75.5%.
Fig. 4.
Cumulative hazard curve for PCR positive Covid-19 compared between two study interventions during four months of active follow-up A) Cumulative hazard curve for PCR positive Covid-19 in the randomized arm, B) Cumulative hazard curve for PCR positive Covid-19 in the non-randomized arm.
Fig. 2.
Estimated hazard ratios for PCR positive Covid-19 disease in RCP compared with BBIBP-CorV recipients using Cox proportional hazard regression model. In the randomized arm confidence intervals have been modified according to the information fraction using O'Brien-Fleming method.
The non-inferiority margin for the hazard ratio was assumed Δ = 1.36. It was calculated based on a 10% margin of non-inferiority to the reported efficacy of 72.8% for BBIBP in preventing PCR-positive Covd-19 disease. All four presented point estimates of the hazard ratios indicates a better performance of RCP compared with BBIBP. The upper limit of the 97.5% and 99.1% (taking into account the information fraction using O'Brien-Fleming method) confidence intervals in the randomized arm still does not cross the non-inferiority margin rejecting the null hypothesis of inferiority.
Table 2.
Efficacy outcomes in the RCP and BBIBP study groups in the randomized and non-randomized arms.
| Randomized arm |
Nonrandom arm |
|||
|---|---|---|---|---|
| BBIBP (n = 3607) | RCP (n = 3617) | BBIBP (n = 7832) | RCP (n = 8054) | |
| Symptomatic PCR + Covid-19 two weeks after the 2nd injection | ||||
| Follow-up (person-day) | 479,843 | 480,298 | 778,521 | 850,438 |
| Event | 133 | 121 | 193 | 157 |
| Incidence rate (%95 CI)a | 27.7 (23.4–32.8) | 25.2 (21.1–30.1) | 24.8 (21.5–28.5) | 18.5 (15.8–21.6) |
| Unadjusted hazard ratio (97.5% CI)b | 1.00 | 0.91 (0.71–1.16) | 1.00 | 0.73 (0.59–0.91)b |
| Adjusted hazard ratio (97.5% CI)c | 1.00 | 0.91 (0.71–1.16) | 1.00 | 0.60 (0.48–0.75)c |
| Adjusted hazard ratio (97.5% CI)d | 1.00 | 0.91 (0.71–1.16) | 1.00 | 0.61 (0.48–0.76)d |
| Adjusted hazard ratio (97.5% CI)e | 1.00 | 0.91 (0.71–1.16) | 1.00 | 0.62 (0.49–0.77)e |
| Adjusted hazard ratio (99.1% CI)f | 1.00 | 0.91 (0.67–1.22) | – | – |
| Symptomatic PCR + Covid-19 two weeks after the 2nd injection excluding those with history of covid-19g | ||||
| Follow-up (person-day) | 461,910 | 458,658 | 690,903 | 671,979 |
| Event | 127 | 113 | 176 | 126 |
| Incidence rate (95 % CI)a | 27.5 (23.1–32.7) | 24.6 (20.5–29.6) | 25.5 (21.9–29.5) | 18.7 (15.7–22.3) |
| Unadjusted hazard ratio (97.5% CI)b | 1.00 | 0.89 (0.69–1.15) | 1.00 | 0.73 (0.58–0.91)b |
| Adjusted hazard ratio (97.5% CI)c | 1.00 | 0.87 (0.61–1.12) | 1.00 | 0.61 (0.48–0.78)c |
| Adjusted hazard ratio (97.5% CI)d | 1.00 | 0.90 (0.69–1.16) | 1.00 | 0.62 (0.48–0.78)d |
| Estimated vaccine efficacy | 1.00 | 75.5% (51.8–87.7) | 1.00 | 83.5% (68.6–91.5) |
| Hospitalizations due to Covid-19 two weeks after the 2nd injection (n)h | 1 | 2 | 7 | 1 |
Incidence per 100,000.
Hazard ratios (HR) and their confidence intervals (α error = 0.025) derived from cox proportional hazard model.
HRs from cox proportional hazard model adjusted for age, sex, education.
HRs from cox proportional hazard model adjusted for age, sex, education, and week of the first injection.
HRs from cox proportional hazard model adjusted for age, sex, education, week of the first injection, and history of covid-19.
HRs from cox proportional hazard model adjusted for age, sex, education, week of the first injection, and history of covid-19 taking into account the information fraction using O'Brien-Fleming method (α error = 0.009).
Previous Covid-19 infection was defined as a history of positive PCR test or lung CT scan confirmed by a health professional.
All patients were recovered and discharged with no reports of ICU admission and mortality.
In the non-randomized arm the number of diagnosed symptomatic PCR positive Covid-19 disease was 193 over 778,521 person-days of active follow-up (incidence = 24.8, 95% CI: 21.5–28.5) in the BBIBP group that was higher than the 157 identified cases over 850,438 person-days in the RCP group (incidence = 18.5, 95% CI: 15.8–21.6).
12 fully vaccinated participants (4 in the RCP and 8 in the BBIBP group) were admitted to hospital due to moderate to severe Covid-19 disease over the follow-up period within the whole study population. One of the admissions in the RCP group was due to hyperglycemia following corticosteroid prescription in a known diabetic patient.
In a randomly selected subsample of participants in the randomized arm, mean serum IgA-specific antibody levels in the RCP group two weeks after the intranasal dose was more than 6 times higher than the BBIBP group, which had received the adjuvant-only preparation (GMR = 6.2, 95% CI: 3.57–10.93) while similar differences were not observed in saliva (GMR = 1.29, 95% CI: 0.74–2.27). (Figs. S1 and S2 in the supplement file.).
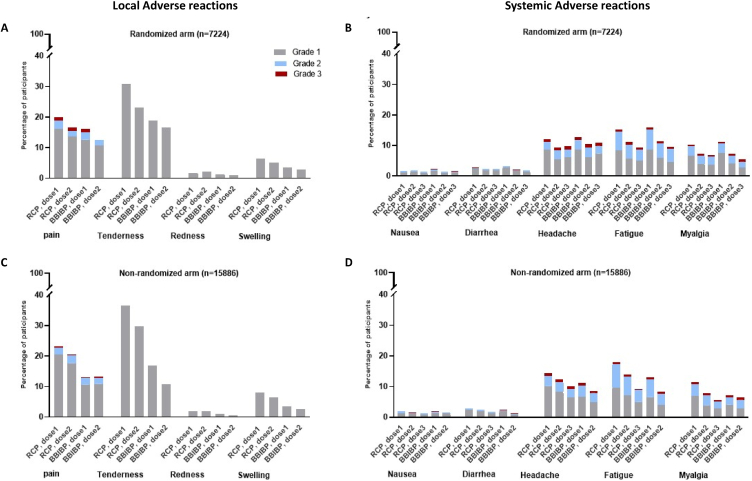
Frequency of solicited local adverse reaction were generally low in the recipients of both vaccines. No grade three or higher local redness, tenderness or swelling was seen. Grade III local pain was seen in a very small proportion of cases (33 in the RCP group and 30 in the BBIBP group) which resolved completely within few days. The most common solicited systemic adverse reaction was fatigue (17 in the RCP group and 12 in the BBIBP group) which was seen in both vaccine recipients followed by headache and myalgia. We observed no grade IV solicited systemic adverse reaction in the study participants and the very few grade III were followed until complete resolution (Fig. 3).
Fig. 3.
Solicited local and systemic adverse reactions up to 7 days after injection A) Local adverse reactions in the randomized arm, B) Systemic adverse reactions in the randomized arm, C) Local adverse reactions in the non-randomized arm D) Systemic adverse reactions in the non-randomized arm.
We observed a total of 15,682 adverse events over the 2,589,100 person-days of active follow-up. 1226 adverse events were classified as possibly vaccine related with slightly higher rates in RCP vaccine recipients (rates per 100,000 b y intervention groups have been summarized in Table 3, for more details please see Tables S6 and S7). Overall 72 serious adverse events were observed in the randomized arm of which 3 were classified as possibly vaccine related (a case of myocarditis in the RCP group and two cases of multiple sclerosis (MS) flare-up and newly diagnosed psoriasis in the BBIBP group) (Tables S2–5). Of the 143 serious adverse events in the non-randomized arm, 9 were possibly vaccine related. They included a missed abortion, an asthma attack, a radiologically isolated syndrome (MS), a post vaccination generalized myoclonus syndrome, and a portal vein thrombosis in the RCP vaccine recipients, and a missed abortion, a Crohn's disease activation, a clinically isolated syndrome (MS) and a case of blurred vision in the BBIBP vaccine recipients. All cases were followed-up and medically managed during the follow-up period.
Table 3.
Frequency and rate of adverse events (AE) per 100,000 person-day of follow up according to their relationship to the intervention in the study groups.
| Randomized arm |
Non- randomized arm |
|||
|---|---|---|---|---|
| RCP (n = 3617) | BBIBP (n = 3607) | RCP (n = 8054) | BBIBP (n = 7832) | |
| Person day of follow-up | 480,298 | 479,843 | 850,438 | 778,521 |
| All adverse events (n, ratea) | 2335, 486.2 (467.1–505.8) |
2158, 449.7 (431.0–468.1) |
5791, 680.9 (635.6–698.6) |
5398, 693.4 (675.0–712.0) |
| All adverse events (possibly vaccine related) | 209, 43.5 (37.8–49.8) | 196, 40.8 (35.3–46.9) | 443, 56.9 (51.7–62.4) | 378, 48.5 (43.8–53.7) |
| Serious adverse event except deaths | 44, 9.2 (6.7–12.3) | 28, 5.8 (3.9–8.4) | 65, 7.6 (5.9–9.7) | 78, 10.0 (7.9–12.5) |
| Non-elective surgery | 16, 3.3 (1.9–5.4) | 3, 0.6 (0.1–1.8) | 20, 2.3 (1.4–3.6) | 26, 3.3 (2.2–4.9) |
| Elective surgery | 10, 2.1 (1.0–3.8) | 14, 2.9 (1.6–4.9) | 14, 1.6 (1.0–2.7) | 17, 2.2 (1.3–3.5) |
| Medical admissions (Non-cardiovascular) | 8, 1.7 (0.7–3.3) | 4, 0.8 (0.2–2.1) | 14, 1.6 (1.0–2.7) | 13, 1.7 (0.9–2.8) |
| Medical admissions (Cardiovascular) | 4, 0.8 (0.2–2.1) | 2, 0.4 (0.1–1.5) | 9, 1.0 (0.5–2.0) | 11, 1.4 (0.7–2.5) |
| Admission due to Covid-19 | 6, 1.2 (0.4–2.7) | 5, 1.0 (0.3–2.4) | 8, 0.9 (0.4–1.8) | 11, 1.4 (0.7–2.5) |
| Death | 2, 0.4 (0.1–1.5) | 1, 0.2 (0.0–1.2) | 2, 0.2 (0.0–0.8) | 5, 0.6 (0.2–1.5) |
Event rates per 100,000 and their 95% confidence interval.
We identified 10 deaths, none vaccine-related, during the study follow-up (4 cases in the RCP group and 6 in the BBIBP). They included 4 cases of trauma-related, one sudden cardiac arrest in a patient with Sjogren's syndrome, one self-poisoning, one multiple myeloma, one gastrointestinal bleeding, one iatrogenic intracranial hemorrhage, and one case of Covid-19 disease that occurred six days after the first vaccine dose (Table S1). DSMB meeting was convened within a week of each death, all the circumstances around the death were reviewed, and its relationship to the vaccine was discussed.
5. Discussion
We found that the RCP vaccine is non-inferior to BBIBP regarding the primary outcome of the rate of symptomatic PCR positive Coivd-19 disease. The hazard ratio of the primary outcome in the randomized arm was 0.91, and the upper bound of the 95% confidence interval was 1.16, which was within the non-inferiority margin of 1.36. Findings from the non-randomized arm were also in conformity with the results of the randomized arm. We observed 70% of the required events of the primary outcome based on the target study sample size in the randomized arm only. Taking into account the information fraction at the time of analysis using the O'Brien Fleming method increased the upper bound of 0.95% confidence interval to 1.22, which was again within the non-inferiority margin of 1.36. The overall safety profile of RCP was comparable to the BBIBP, although we detected a slightly higher rate of adverse reactions in the former. All common adverse reactions were self-limited and resolved over the study period. No vaccine-related death was observed.
Direct calculation of vaccine efficacy was impossible in our study because we did not have a control group that had not received a vaccine. WHO-approved Covid-19 vaccines had become available for the general Iranian population when the current study was started, and it was unethical to deprive the control group of an effective vaccine. Therefore, we used a non-inferiority design comparing the RCP with the BBIBP vaccine as the active control and estimated the RCP efficacy using the Bayesian network meta-analysis method [14].
The results in the non-randomized arm pointed towards the superiority of the RCP vaccine versus the BBIBP in the primary outcome, even after adjusting for age, sex, education, time of first vaccination dose, and history of Covid-19 infection. We interpreted these findings with caution because of potential unknown confounders. Therefore, in a conservative approach, we made our conclusions based on the results from the randomized and double-blinded arm. As the number of observed events of the primary outcome in the randomized arm was 254 and had not reached the target number of events of 365, we adjusted for the 70% information fraction using O’ Brien- Fleming method, and the results indicated that the RCP is non-inferior to the BBIBP.
Serious vaccine adverse reactions detected in our study were generally expected and reasonably balanced in the RCP and BBIBP groups. From 143 serious adverse events (SAE) observed during the follow-up period, only 12 could not be attributed to another disease or drug intake (WHO case causality assessment criteria) and were categorized as possibly vaccine-related (a complete list of all SAEs have been presented in Tables S2–S5). The observed SAEs had also been reported by other vaccine studies, such as Myocarditis [14,15], Multiple Sclerosis [16,17], and Psoriasis [18,19]. Following a case of asthma attack seen 9 days after an intranasal dose of RCP classified as vaccine-related, people with a history of asthma were exempted from receiving the intranasal doses by the principal investigator's decision. Cardiovascular SAEs mainly occurred in participants with previously known risk factors such as diabetes, hypertension, and heavy smoking resulting in difficulty in establishing a causal relationship between these events and vaccination. Other non-serious possibly-related adverse events had a slightly higher rate in the RCP compared to the BBIBP recipients. There were mostly self-limiting and resolved completely and did not have high clinical significance.
In the current study, we did not see an increase in the specific anti-RBD IgA antibody levels in the RCP group in the saliva samples following the intranasal vaccine dose. Besides technical difficulties in taking, preparing, and detecting the specific IgA antibodies in saliva samples [20], the observed antibody levels are comparable to those in the phase I study [8], suggesting that saliva IgA antibody levels were already high and could not rise further [20]. With hindsight, a nasal swap sample could be a more appropriate alternative to saliva [21,22]. However, serum-specific anti-RBD IgA antibody levels, which is associated with stronger long-term protection [23], did show an increase particularly in the RCP group. Although the amount of increase and the baseline levels in the RCP group were higher than the BBIBP group, the overall rise in both groups could be interpreted as the antibody response to the vaccination as a whole rather than the intranasal component [24].
We were unable to recruit enough participants in the randomized arm to achieve the target number of 365 cases of symptomatic PCR-positive covid-19 as initially planned. Therefore, we were compelled to allow participants willing to choose the vaccine they receive to do so in a non-randomized open-labeled arm. However, as the number of primary outcome events in the randomized arm reached 70% of the original target, we concluded based on the interim analysis after observing early trial-stopping rules using the O'Brien-Fleming method as planned in the protocol. Furthermore, we were obliged to use a non-inferiority design because of ethical considerations. Therefore, we could not directly calculate the vaccine efficacy and had to estimate it using Bayesian network meta-analysis methodology, accepting the limitation of changing the Covid-19 virus variant and different positions of the target population in the epidemic curve. Our rigorous active follow-up of all participants in the first four months of the planned study duration was one of our strong points. We are confident of recording all the adverse events, as the total number of observed adverse events shows, during this period using weekly follow-up calls from a centralized round-the-clock center. All detected adverse events thoroughly went through the causality assessment process by resident physicians and, were needed, referred to the relevant subspecialties for additional assessments. Our study also provided valuable post-BBIBP vaccination data on adverse events in a sizable cohort of the general Iranian population.
6. Conclusion
In summary, our study showed that the RCP efficacy is non-inferior to the BBIBP and estimated vaccine efficacy for protection against symptomatic Covid-19 infection is 75.5% (95% CI: 51.8–87.7). It is a safe and effective recombinant S-protein Covid-19 vaccine and has a comparable safety profile to the inactive BBIBP vaccine, which has the emergency approval of the WHO.
Ethical approval
The study was conducted according to the Helsinki Declaration and approved by the national committee of Ethics in medical research (Reference number: IR. NREC.1400.007). The study was registered with the Iranian Registry of clinical trials (IRCT) (Reference number: IRCT20201214049709N3).
Role of funding source
The study is funded by the Razi Vaccine and Serum Research Institute. The study design, data collection, data management, analysis, interpretation, and writing of the report were done by IUMS-CTC. The study's sponsor helped with the design and performed the immunogenicity tests, but it was blinded to the identity of the participants in all blood specimens. IUMS-CTC and Razi participated in the decision to submit the paper for publication.
Data availability statement
Data will be available upon request by sending an email to the corresponding authors.
CRediT authorship contribution statement
Masoud Solaymani-Dodaran: Writing – original draft, Supervision, Software, Investigation, Formal analysis, Conceptualization. Saeed Kalantari: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Data curation, Conceptualization. Seyed Reza Banihashemi: Writing – review & editing, Project administration, Methodology, Conceptualization. Ali Es-haghi: Writing – review & editing, Supervision, Resources, Project administration, Conceptualization. Mojtaba Nofeli: Writing – review & editing, Validation, Supervision, Resources, Project administration. Arash Mohazzab: Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation. Ladan Mokhberalsafa: Writing – review & editing, Supervision, Project administration, Funding acquisition. Fariba Sadeghi: Writing – review & editing, Validation, Supervision, Resources, Project administration. Ali Rezae Mokaram: Writing – review & editing, Supervision, Resources, Project administration, Conceptualization. Monireh Haji Moradi: Validation, Data curation. Seyad Hossein Razaz: Validation, Investigation, Data curation. Maryam Taghdiri: Validation, Data curation. Mohsen Lotfi: Visualization, Supervision, Data curation. Seyed Amin Setarehdan: Validation, Supervision, Software, Investigation, Conceptualization. Safdar Masoumi: Visualization, Software, Formal analysis, Conceptualization. Akram Ansarifar: Software, Methodology, Conceptualization. Saeedeh Ebrahimi: Supervision, Investigation, Data curation. Neda Esmailzadehha: Writing – review & editing, Validation, Supervision. Zahra Boluki: Supervision, Investigation, Data curation. Malihe Khoramdad: Validation, Supervision. Leila Molaipour: Validation, Supervision. Mohamad Hassan Rabiei: Validation, Supervision, Data curation. Fahimeh Bagheri Amiri: Validation, Supervision, Data curation. Sara Filsoof: Validation, Investigation, Data curation. Behrooz Bani-vaheb: Validation, Supervision, Investigation, Data curation. Maryam Raghami Derakhshani: Supervision, Data curation. Sheno Bayazidi: Data curation. Rezvan Golmoradizadeh: Data curation. Masoumeh Shahsavan: Data curation. Shiva Safari: Data curation. Neda Ghahremanzadeh: Data curation. Vahideh Mohseni: Data curation. Saeed Erfanpoor: Validation, Supervision, Data curation. Mohammad Hossein Fallah Mehrabadi: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The Iran University of Medical Sciences Clinical Trial Center (IUMS-CTC), were primarily responsible for the conduct of the trial. SRB, AE, MN, ARM, LMS, FS, MHM, SHR, MT, MB, ML, AK, AG, and MHFM were Razi Vaccine and Serum Research Institute, employees. SRB is the inventor of the RCP vaccine. MSD, SK,AM,SAS,SM, AA,SE,NE,ZB, MK, MLP,SE,MHR,FBA, BBV,SF,MRD,ShB,RG,MS,SS,NG, and VM are employees or postgraduate students in IUMS.
Acknowledgment
The investigators express their gratitude for the contribution of all trial participants. The investigators would like to thank the members of the National Ethics Committee (Dr. Akbar Fotouhi), the Ministry of Health's Food and Drug Organization (Dr. Reza Mosaed), and Communicable Disease Control (Dr. Mohsen Zahraei), as well as the staff of Razi Vaccine and Serum Research Institute, other IUMS-CTC members, Hazrat Rasol hospital staff for their cooperation in the conduction of trial. Also, we thank Dr. Zeynab Yasin, Dr. Alireza Hejrati, Dr. Somaye Nasiri, and Dr. Sima Shokri as independent members of the data monitoring and safety committee. We express our gratitude to Dr. Parmida Shahbazi and Dr. Nima Hemmaty, for their diligent contributions in coordination of the follow-up team.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27370.
Contributor Information
Seyed Reza Banihashemi, Email: reza7471@gmail.com.
Mohammad Hossein Fallah Mehrabadi, Email: mhf2480@yahoo.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang Y.Z., Kuan C.C. Vaccination to reduce severe COVID-19 and mortality in COVID-19 patients: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022;26(5):1770–1776. doi: 10.26355/eurrev_202203_28248. [DOI] [PubMed] [Google Scholar]
- 2.Rahmani K., Shavaleh R., Forouhi M., Disfani H.F., Kamandi M., Oskooi R.K., et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: a systematic review and meta-analysis. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N. Engl. J. Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 covid-19 vaccine. N. Engl. J. Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nohynek H., Wilder-Smith A. Does the world still need new covid-19 vaccines? N. Engl. J. Med. 2022;386(22):2140–2142. doi: 10.1056/NEJMe2204695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banihashemi S.R., Es-haghi A., Fallah Mehrabadi M.H., Nofeli M., Mokarram A.R., Ranjbar A., et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: a preclinical study in several animal models. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.836745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohazzab A., Fallah Mehrabadi M.H., Es-haghi A., Kalantari S., Mokhberalsafa L., Setarehdan S.A., et al. Phase II, safety and immunogenicity of razi cov Pars (RCP) sars cov-2 vaccine in adults aged 18–70 Years; A randomized, double-blind clinical trial. J. Pharmaceut. Sci. 2023;112(12):3012–3021. doi: 10.1016/j.xphs.2023.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Solaymani Dodaran M., Banihashemi S.R., Es-haghi A., Kalantari S., Nofeli M., Rezaei Mokarram A., et al. Vaccines; Basel: 2023. Immunogenicity and Safety of a Combined Intramuscu-Lar/intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: a Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidance F.D.A. Food and Drug Administration. US Department of Health and Human Services; 2007. For industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. [Google Scholar]
- 13.Who . Uppsala Monitoring Centre; 2013. The Use of the WHO-UMC System for Standardised Case Causality Assessment. [Google Scholar]
- 14.Fleming T.R., Krause P.R., Nason M., Longini I.M., Henao-Restrepo A.M. COVID-19 vaccine trials: the use of active controls and non-inferiority studies. Clin. Trials. 2021;18(3):335–342. doi: 10.1177/1740774520988244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nabizadeh F., Ramezannezhad E., Kazemzadeh K., Khalili E., Ghaffary E.M., Mirmosayyeb O. Multiple sclerosis relapse after COVID-19 vaccination: a case report-based systematic review. J. Clin. Neurosci. : official journal of the Neurosurgical Society of Australasia. 2022;104:118–125. doi: 10.1016/j.jocn.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alluqmani M. New onset multiple sclerosis post-COVID-19 vaccination and correlation with possible predictors in a case-control study. Cureus. 2023;15(3) doi: 10.7759/cureus.36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P.C., Huang I.H., Wang C.W., Tsai C.C., Chung W.H., Chen C.B. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am. J. Clin. Dermatol. 2022;23(6):775–799. doi: 10.1007/s40257-022-00721-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran T.N.A., Nguyen T.T.P., Pham N.N., Pham N.T.U., Vu T.T.P., Nguyen H.T. New onset of psoriasis following COVID-19 vaccination. Dermatol. Ther. 2022;35(8) doi: 10.1111/dth.15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D., Calderone R., Nsouli T.M., Reznikov E., Bellanti J.A. Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines. Allergy Asthma Proc. 2022;43(5):419–430. doi: 10.2500/aap.2022.43.220045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aita A., Basso D., Cattelan A.M., Fioretto P., Navaglia F., Barbaro F., et al. SARS-CoV-2 identification and IgA antibodies in saliva: one sample two tests approach for diagnosis. Clinica chimica acta. international journal of clinical chemistry. 2020;510:717–722. doi: 10.1016/j.cca.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roubidoux E.K., Brigleb P.H., Vegesana K., Souquette A., Whitt K., Freiden P., et al. Utility of nasal swabs for assessing mucosal immune responses towards SARS-CoV-2. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-44989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh-Mohamed S., Isho B., Chao G.Y.C., Zuo M., Cohen C., Lustig Y., et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15(5):799–808. doi: 10.1038/s41385-022-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montague B.T., Wipperman M.F., Chio E., Crow R., Hooper A.T., O'Brien M.P., et al. Elevated serum IgA following vaccination against SARS-CoV-2 in a cohort of high-risk first responders. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-19095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon request by sending an email to the corresponding authors.