Abstract
BACKGROUND:
Chronic pain is a common, poorly understood condition. Genetic studies including genome-wide association studies have identified many relevant variants, which have yet to be translated into full understanding of chronic pain. Transcriptome-wide association studies using transcriptomic imputation methods such as S-PrediXcan can help bridge this genotype-phenotype gap.
METHODS:
We carried out transcriptomic imputation using S-PrediXcan to identify genetically regulated gene expression associated with multisite chronic pain in 13 brain tissues and whole blood. Then, we imputed genetically regulated gene expression for over 31,000 Mount Sinai BioMe participants and performed a phenome-wide association study to investigate clinical relationships in chronic pain–associated gene expression changes.
RESULTS:
We identified 95 experiment-wide significant gene-tissue associations (p < 7.97 × 10−7), including 36 unique genes and an additional 134 gene-tissue associations reaching within-tissue significance, including 53 additional unique genes. Of the 89 unique genes in total, 59 were novel for multisite chronic pain and 18 are established drug targets. Chronic pain genetically regulated gene expression for 10 unique genes was significantly associated with cardiac dysrhythmia, metabolic syndrome, disc disorders/dorsopathies, joint/ligament sprain, anemias, and neurologic disorder phecodes. Phenome-wide association study analyses adjusting for mean pain score showed that associations were not driven by mean pain score.
CONCLUSIONS:
We carried out the largest transcriptomic imputation study of any chronic pain trait to date. Results highlight potential causal genes in chronic pain development and tissue and direction of effect. Several gene results were also drug targets. Phenome-wide association study results showed significant associations for phecodes including cardiac dysrhythmia and metabolic syndrome, thereby indicating potential shared mechanisms.
Chronic pain is a common, debilitating condition (1–3). Risk factors for and mechanisms of chronic pain development are not fully understood. Treating chronic pain successfully is a complex process, and many treatments, including pharmacological treatments, are suboptimal [reviewed by (4)].
Genetic studies of chronic pain (5–7) and conditions associated with chronic pain [e.g., rheumatoid arthritis (8), endometriosis (9), and migraine (10)] [see also (11) for a recent review of genetic studies in chronic pain] have revealed hundreds of genetic loci, but these results have not been translated into actionable treatment. In the pathway from genotype to phenotype, transcription and gene expression represent intermediate steps. Understanding expression changes that are associated with chronic pain could aid in increasing understanding of the mechanisms and best pharmaceutical treatments for chronic pain.
Transcriptomic imputation (TI) approaches combine expression quantitative trait loci and genome-wide association study (GWAS) association statistics to identify trait-associated genetically regulated gene expression (GREX), thereby providing directional and tissue-specific context (12–15). This approach is especially useful because changes to the brain and spinal cord, including regional brain activity (functional) changes as measured by functional magnetic resonance imaging, structural plasticity in central nervous system cells and synapses, morphological changes in neurons, changes to cell population sizes, changes in volume, and decreased gray matter, have been widely implicated in the development of chronic pain (16–20), and brain tissue is relatively inaccessible and impossible to assay in living study participants. Furthermore, genes that are involved in axonal guidance and enriched for expression in the brain have also been found to be associated with chronic overlapping pain conditions (21). TI studies have been carried out in a range of conditions (22–24), including complex traits that are commonly associated with chronic pain (10,24–27), but no direct TI analyses of chronic pain have been undertaken. Here, we applied a TI method, S-PrediXcan (13), to impute GREX in 13 brain regions and test for associations with multisite chronic pain (MCP) (5).
There is an unmet need to interrogate consequences of genetic variants in clinical data (28,29). Phenome-wide association studies (PheWASs) test for significant associations between exposures (e.g., genetic variants or other risk factors) and large sets of phenotypes, such as ICD-10 or other electronic health record traits (30). Previous PheWAS analyses have shown a relationship between seronegative rheumatoid arthritis and fibromyalgia (31) and between genetic risk for problematic opioid use and pain-related phenotypes (32). Here, we tested for associations between chronic pain–associated GREX and a phenome of over 1000 phecodes in an ancestrally diverse hospital biobank.
This study involves GWAS summary statistics from one of the largest studies of chronic pain to date, in which chronic pain was examined as a complex disease trait (5). This may represent a more powerful way to uncover genetic variation specific to chronic pain development compared to genetic study of chronic pain–associated conditions. We have highlighted genes of interest through their GREX, in specific tissues, relevant to mechanisms of chronic pain development. We also present the first PheWAS of GREX for chronic pain.
METHODS AND MATERIALS
GWAS Output and Phenotype: MCP
MCP was found to be a complex, polygenic trait genetically correlated with psychiatric and other disorders in a 2019 GWAS (5). Recent changes to ICD-11 coding for chronic pain and International Association for the Study of Pain definitions of chronic pain (33–35) support the study of chronic pain as a disease. Genes involved in central nervous system and immune function were found to be associated with MCP using MAGMA (36), and gene expression of MCP-related genes was enriched in the brain. Summary statistics were used for transcriptome-wide association study analysis through the TI approach S-PrediXcan (13).
Discovery of GREX in Chronic Pain
GREX was imputed using MCP GWAS output and TI models from the GTEx (Genotype-Tissue Expression Project) (37) in 13 brain tissues (Table S1) using S-PrediXcan. Multiple testing correction (Bonferroni) was applied and resulted in 2 thresholds for significance: 1) a per-tissue threshold correcting for all genes tested in each tissue (Table S1), and 2) an experiment-wide threshold correcting for all genes across all tissues (p = 7.9 × 10−7). Then, we sought to replicate our findings using a different TI method, summary–transcriptome-wide association study (38) (see the Supplement).
Replication of Significant TI Gene-Tissue Associations
A recent genetic study of pain intensity was carried out in 598,339 Million Veterans Program participants (39) and included FUSION transcriptome-wide association analysis and prediction models for 6 brain tissues (anterior cingulate cortex, cerebellar hemisphere cortex, frontal cortex, cerebellum, and dorsolateral prefrontal cortex). Pain intensity was significantly genetically correlated with MCP (rg = 0.79) (39). We downloaded the 361 significant gene-tissue results [Supplementary Table 20 in Toikumo et al. (39)] and carried out a Fisher’s exact test to ascertain whether results overlap represented significant replication.
Downstream Analysis: FUMA
Pathway analyses were carried out using FUMA GENE2FUNC (40) including all per-tissue significant gene results (n = 89). We tested for enrichment of all gene sets available in FUMA GENE2FUNC with all genes that had at least one S-PrediXcan prediction model available and were included in FUMA as background (n = 15,588). Significant gene results were also investigated using the FUMA DrugBank (see the Supplement).
Connectivity Map Analysis
We queried Connectivity Map (CMap), a large database of perturbation signatures maintained by the Broad Institute (41,42), using genes up- and downregulated in MCP (Table S2). We filtered results to retain compounds (drugs) passing CMap quality control with significant connectivity scores (−log10(FDR [false discovery rate]–corrected p) > 1.3, FDR–corrected p < .05).
Phenome-wide Association Analysis in Mount Sinai BioMe
To probe relationships between MCP-associated GREX and clinical phenotypes, we performed a series of PheWASs (see the Supplement) in the Mount Sinai BioMe biobank.
BioMe is a large, diverse, hospital-based biobank that includes electronic health record and genotype data for 31,704 participants in the first data freeze. A total of 1236 phecodes for BioMe participants were included in analyses presented in this paper. Phecodes are a high-throughput method that reduce electronic health record dimension and complexity in which ICD-10 codes are manually grouped according to clinical similarity (43). Here, we used previously curated phecodes (44). A full list of phecodes can be searched at https://phewascatalog.org/phecodes_icd10 or through download of the “PheCode Definitions v1.2 ICD-10-CM map” available at https://phewascatalog.org/phecodes_icd10cm.
First, we imputed MCP-GREX (chronic pain–related genetically regulated gene expression) for 31,704 BioMe freeze 1 participants, split across 6 genotype-derived ancestry groups (Table S3).
Specifically, we imputed GREX in all 13 brain regions and in whole blood for all 89 unique genes previously identified as significant MCP-GREX. We tested for associations between these GREX values and BioMe phecodes with at least 10 available cases in at least one ancestry [total phecodes = 1236 (44)]. Results were meta-analyzed using inverse variance-weighted meta-analysis in METAL (45). Multiple testing correction (within-gene FDR) was then applied.
To validate our MCP associations, we tested whether MCP-associated genes were associated with pain. A numeric rating scale (NRS) ranging from 0 to 10, where 10 is the worst pain possible and 0 is no pain, was recorded for BioMe participants and aggregated into a mean pain score across instances in which the pain NRS was recorded. Associations were tested between significant MCP-GREX results and mean pain scores, and results were meta-analyzed across ancestry groups using inverse variance-weighted meta-analysis in METAL. FDR correction was performed as previously described.
RESULTS
Novel Brain-Specific Genes and Pathways Associated With Chronic Pain Identified With TI
We applied S-PrediXcan to the largest available summary statistics for MCP (n = 387,649). We identified 95 experiment-wide significant gene-tissue associations (p < 7.97 × 10−7), including 36 unique genes (Table 1). An experiment-wide threshold is likely overly conservative because many expression quantitative trait loci are shared between tissues; therefore, we also applied a within-tissue Bonferroni threshold (Table S1; Figure 1A, B). We identified an additional 134 gene-tissue associations that reached within-tissue significance, including 53 additional unique genes.
Table 1.
Eighty-nine Unique Genes Associated With Multisite Chronic Pain
| Gene Symbol | Tissue | z Score | Effect Size | p (Unadjusted) |
|---|---|---|---|---|
| ECM1 | Hippocampus | 6.43 | 0.182 | 1.24 × 10−10a |
| TARS2 | Cerebellum | 6.43 | 0.157 | 1.29 × 10−10a |
| GPX1 | Frontal cortex, BA 9 | 6.25 | 0.083 | 4.03 × 10−10a |
| GPX1 | Cerebellar hemisphere | 6.20 | 0.113 | 5.54 × 10−10a |
| GMPPB | Anterior cingulate cortex, BA 24 | −6.17 | 0.051 | 6.77 × 10−10a |
| SNRPC | Anterior cingulate cortex, BA 24 | −6.17 | −0.082 | 6.95 × 10−10a |
| GMPPB | Hypothalamus | 6.17 | 0.046 | 6.96 × 10−10a |
| GMPPB | Caudate, basal ganglia | 6.15 | 0.041 | 7.77 × 10−10a |
| GMPPB | Cerebellum | 6.14 | 0.032 | 8.38 × 10−10a |
| GMPPB | Nucleus accumbens, basal ganglia | 6.12 | 0.037 | 9.08 × 10−10a |
| GMPPB | Cerebellar hemisphere | 6.07 | 0.039 | 1.30 × 10−9a |
| CELSR3 | Amygdala | 6.02 | 0.218 | 1.77 × 10−9a |
| SEMA3B | Nucleus accumbens, basal ganglia | −5.97 | −0.267 | 2.32 × 10−9a |
| GMPPB | Spinal cord cervical C1 | −5.97 | 0.038 | 2.40 × 10−9a |
| GMPPB | Whole blood | 5.95 | 0.120 | 2.70 × 10−9a |
| AMT | Hypothalamus | −5.91 | −0.110 | 3.44 × 10−9a |
| GMPPB | Cortex | 5.90 | 0.032 | 3.53 × 10−9a |
| NMT1 | Putamen, basal ganglia | 5.90 | 0.075 | 3.63 × 10−9a |
| NMT1 | Anterior cingulate cortex, BA 24 | 5.89 | 0.120 | 3.83 × 10−9a |
| VPS33B | Whole blood | −5.84 | −0.069 | 5.35 × 10−9a |
| RP11–24H2.3 | Amygdala | −5.84 | −0.059 | 5.36 × 10−9a |
| FUBP1 | Cerebellum | −5.81 | −0.227 | 6.06 × 10−9a |
| RPRD2 | Nucleus accumbens, basal ganglia | 5.81 | 0.290 | 6.10 × 10−9a |
| GMPPB | Hippocampus | 5.78 | 0.032 | 7.51 × 10−9a |
| C6orf106 (ILRUN) | Hypothalamus | 5.73 | 0.163 | 1.01 × 10−8a |
| GMPPB | Substantia nigra | 5.69 | 0.031 | 1.24 × 10−8a |
| UHRF1BP1 | Spinal cord cervical C1 | 5.66 | 0.070 | 1.49 × 10−8a |
| CSK | Caudate, basal ganglia | −5.58 | −0.138 | 2.35 × 10−8a |
| SNRPC | Frontal cortex, BA 9 | −5.54 | −0.065 | 3.04 × 10−8a |
| AMT | Whole blood | −5.51 | −0.054 | 3.50 × 10−8a |
| GPX1 | Cortex | 5.47 | 0.063 | 4.49 × 10−8a |
| SDCCAG8 | Whole blood | 5.44 | 0.043 | 5.31 × 10−8a |
| C6orf106 (ILRUN) | Putamen, basal ganglia | 5.43 | 0.068 | 5.60 × 10−8a |
| ECM1 | Nucleus accumbens, basal ganglia | 5.43 | 0.101 | 5.70 × 10−8a |
| ZNF501 | Frontal cortex, BA 9 | −5.41 | −0.066 | 6.26 × 10−8a |
| RBM6 | Nucleus accumbens, basal ganglia | −5.40 | −0.049 | 6.68 × 10−8a |
| SNRPC | Nucleus accumbens, basal ganglia | −5.37 | −0.038 | 7.95 × 10−8a |
| AMT | Putamen, basal ganglia | −5.36 | −0.050 | 8.10 × 10−8a |
| C6orf106 (ILRUN) | Cortex | 5.36 | 0.078 | 8.50 × 10−8a |
| SUOX | Whole blood | 5.34 | 0.073 | 9.14 × 10−8a |
| C6orf106 (ILRUN) | Frontal cortex, BA 9 | 5.33 | 0.120 | 9.78 × 10−8a |
| UHRF1BP1 | Hypothalamus | 5.30 | 0.072 | 1.14 × 10−7a |
| RP11–24H2.3 | Anterior cingulate cortex, BA 24 | −5.30 | −0.043 | 1.18 × 10−7a |
| MST1 | Whole blood | −5.30 | −0.125 | 1.18 × 10−7a |
| GMPPB | Frontal cortex, BA 9 | 5.29 | 0.034 | 1.19 × 10−7a |
| RPS26 | Frontal cortex, BA 9 | −5.28 | −0.017 | 1.30 × 10−7a |
| RNF123 | Nucleus accumbens, basal ganglia | 5.27 | 0.119 | 1.36 × 10−7a |
| RPS26 | Putamen, basal ganglia | −5.24 | −0.014 | 1.64 × 10−7a |
| AMT | Substantia nigra | −5.23 | −0.077 | 1.68 × 10−7a |
| GPX1 | Cerebellum | 5.23 | 0.060 | 1.69 × 10−7a |
| GPR27 | Cortex | 5.19 | 0.183 | 2.06 × 10−7a |
| C6orf106 (ILRUN) | Nucleus accumbens, basal ganglia | 5.19 | 0.079 | 2.10 × 10−7a |
| SNRPC | Hippocampus | −5.19 | −0.086 | 2.13 × 10−7a |
| AMT | Anterior cingulate cortex, BA 24 | −5.19 | −0.075 | 2.13 × 10−7a |
| SUOX | Nucleus accumbens, basal ganglia | 5.18 | 0.054 | 2.18 × 10−7a |
| UHRF1BP1 | Cerebellar hemisphere | 5.18 | 0.068 | 2.18 × 10−7a |
| MRPS21 | Frontal cortex, BA 9 | −5.18 | −0.182 | 2.27 × 10−7a |
| SNRPC | Putamen, basal ganglia | −5.16 | −0.054 | 2.50 × 10−7a |
| RPS26 | Cerebellum | −5.15 | −0.015 | 2.58 × 10−7a |
| RPRD2 | Whole blood | 5.13 | 0.250 | 2.87 × 10−7a |
| SNRPC | Whole blood | −5.13 | −0.486 | 2.91 × 10−7a |
| GPX1 | Caudate, basal ganglia | 5.12 | 0.078 | 2.98 × 10−7a |
| UHRF1BP1 | Cortex | 5.12 | 0.044 | 3.07 × 10−7a |
| CEP170 | Whole blood | 5.10 | 0.159 | 3.34 × 10−7a |
| SUOX | Putamen, basal ganglia | 5.10 | 0.079 | 3.40 × 10−7a |
| GMPPB | Amygdala | 5.09 | 0.027 | 3.55 × 10−7a |
| AMT | Nucleus accumbens, basal ganglia | −5.09 | −0.049 | 3.65 × 10−7a |
| SDCCAG8 | Caudate, basal ganglia | 5.08 | 0.114 | 3.70 × 10−7a |
| P4HTM | Cerebellum | −5.08 | −0.072 | 3.83 × 10−7a |
| RBM6 | Caudate, basal ganglia | −5.06 | −0.050 | 4.14 × 10−7a |
| INTS1 | Spinal cord cervical C1 | −5.06 | −0.025 | 4.19 × 10−7a |
| RBM6 | Cortex | −5.06 | −0.031 | 4.27 × 10−7a |
| RP11–160H22.5 | Whole blood | −5.06 | −0.059 | 4.28 × 10−7a |
| UHRF1BP1 | Caudate, basal ganglia | 5.04 | 0.062 | 4.59 × 10−7a |
| UHRF1BP1 | Whole blood | 5.04 | 0.029 | 4.71 × 10−7a |
| SUOX | Cerebellum | 5.03 | 0.027 | 4.88 × 10−7a |
| SUOX | Cerebellar hemisphere | 5.03 | 0.044 | 4.90 × 10−7a |
| UHRF1BP1 | Frontal cortex, BA 9 | 5.02 | 0.157 | 5.17 × 10−7a |
| SP4 | Nucleus accumbens, basal ganglia | 5.00 | 0.243 | 5.61 × 10−7a |
| MON1B | Whole blood | 5.00 | 0.105 | 5.63 × 10−7a |
| SUOX | Caudate, basal ganglia | 5.00 | 0.075 | 5.82 × 10−7a |
| SDCCAG8 | Putamen, basal ganglia | 4.99 | 0.087 | 5.89 × 10−7a |
| RPRD2 | Substantia nigra | 4.99 | 0.102 | 5.91 × 10−7a |
| ZNF197 | Whole blood | −4.99 | −0.037 | 5.98 × 10−7a |
| GPR27 | Frontal cortex, BA 9 | 4.98 | 0.137 | 6.21 × 10−7a |
| UHRF1BP1 | Cerebellum | 4.98 | 0.080 | 6.28 × 10−7a |
| CTBP2 | Cerebellum | −4.98 | −0.080 | 6.40 × 10−7a |
| GPX1 | Nucleus accumbens, basal ganglia | 4.96 | 0.055 | 7.03 × 10−7a |
| PTK2 | Nucleus accumbens, basal ganglia | 4.95 | 0.079 | 7.56 × 10−7a |
| SLC25A13 | Whole blood | 4.94 | 0.070 | 7.66 × 10−7a |
| RPS26 | Caudate, basal ganglia | −4.94 | −0.016 | 7.80 × 10−7a |
| SEMA3F | Anterior cingulate cortex, BA 24 | −4.94 | −0.205 | 7.80 × 10−7a |
| RPS26 | Nucleus accumbens, basal ganglia | −4.94 | −0.014 | 7.89 × 10−7a |
| RNF123 | Cerebellum | 4.94 | 0.042 | 7.94 × 10−7a |
| SUOX | Cortex | 4.94 | 0.071 | 7.94 × 10−7a |
| SUOX | Amygdala | 4.94 | 0.081 | 7.99 × 10−7 |
| SUOX | Hypothalamus | 4.93 | 0.073 | 8.10 × 10−7 |
| RBM6 | Cerebellar hemisphere | −4.92 | −0.044 | 8.50 × 10−7 |
| RPS26 | Whole blood | −4.92 | −0.013 | 8.54 × 10−7 |
| SP4 | Cerebellum | 4.92 | 0.265 | 8.85 × 10−7 |
| MRPS21 | Cerebellum | −4.92 | −0.048 | 8.86 × 10−7 |
| NUDT18 | Putamen, basal ganglia | −4.91 | −0.062 | 8.95 × 10−7 |
| MRPS21 | Hypothalamus | −4.91 | −0.181 | 8.99 × 10−7 |
| GMPPB | Putamen, basal ganglia | 4.91 | 0.032 | 9.01 × 10−7 |
| SUOX | Spinal cord cervical C1 | 4.91 | 0.062 | 9.02 × 10−7 |
| RPS26 | Cortex | −4.90 | −0.016 | 9.78 × 10−7 |
| TARS2 | Anterior cingulate cortex, BA 24 | 4.89 | 0.141 | 9.99 × 10−7 |
| RNF123 | Cortex | 4.88 | 0.093 | 1.05 × 10−6 |
| UHRF1BP1 | Putamen, basal ganglia | 4.88 | 0.032 | 1.07 × 10−6 |
| RBM6 | Frontal cortex, BA 9 | −4.88 | −0.050 | 1.08 × 10−6 |
| AMT | Cortex | −4.87 | −0.033 | 1.09 × 10−6 |
| RPS26 | Hypothalamus | −4.87 | −0.016 | 1.11 × 10−6 |
| GPX1 | Putamen, basal ganglia | 4.87 | 0.118 | 1.14 × 10−6 |
| NUDT18 | Whole blood | −4.86 | −0.036 | 1.14 × 10−6 |
| RBM6 | Whole blood | −4.86 | −0.025 | 1.15 × 10−6 |
| RBM6 | Putamen, basal ganglia | −4.86 | −0.039 | 1.15 × 10−6 |
| RPS26 | Cerebellar hemisphere | −4.86 | −0.017 | 1.15 × 10−6 |
| PRKAR2A | Substantia nigra | 4.86 | 0.099 | 1.16 × 10−6 |
| ECM1 | Cerebellar hemisphere | 4.85 | 0.033 | 1.25 × 10−6 |
| RPS26 | Anterior cingulate cortex, BA 24 | −4.84 | −0.014 | 1.27 × 10−6 |
| MRPS21 | Caudate, basal ganglia | −4.84 | −0.090 | 1.29 × 10−6 |
| UFL1 | Cerebellum | 4.84 | 0.068 | 1.31 × 10−6 |
| ZNF501 | Caudate, basal ganglia | −4.82 | −0.059 | 1.41 × 10−6 |
| SCAMP2 | Cerebellum | 4.81 | 0.093 | 1.48 × 10−6 |
| MRPS21 | Nucleus accumbens, basal ganglia | −4.81 | −2.206 | 1.50 × 10−6 |
| NMT1 | Caudate, basal ganglia | 4.81 | 0.080 | 1.54 × 10−6 |
| TSKU | Cerebellar hemisphere | 4.80 | 0.038 | 1.55 × 10−6 |
| UBA7 | Caudate, basal ganglia | −4.80 | −0.240 | 1.56 × 10−6 |
| LANCL1 | Cortex | 4.80 | 0.102 | 1.59 × 10−6 |
| GRK4 | Anterior cingulate cortex, BA 24 | 4.80 | 0.060 | 1.62 × 10−6 |
| ZNF501 | Cerebellum | −4.79 | −0.046 | 1.71 × 10−6 |
| UHRF1BP1 | Nucleus accumbens, basal ganglia | 4.78 | 0.149 | 1.73 × 10−6 |
| SNRPC | Caudate, basal ganglia | −4.78 | −0.059 | 1.77 × 10−6 |
| C15orf57 | Cortex | −4.78 | −0.035 | 1.79 × 10−6 |
| UHRF1BP1 | Anterior cingulate cortex, BA 24 | 4.78 | 0.058 | 1.79 × 10−6 |
| RBM6 | Anterior cingulate cortex, BA 24 | −4.77 | −0.035 | 1.80 × 10−6 |
| MST1R | Caudate, basal ganglia | 4.77 | 0.068 | 1.82 × 10−6 |
| KLHDC8B | Cerebellum | 4.77 | 0.113 | 1.86 × 10−6 |
| TSPYL4 | Cerebellum | 4.76 | 0.099 | 1.93 × 10−6 |
| C15orf57 | Nucleus accumbens, basal ganglia | −4.76 | −0.040 | 1.93 × 10−6 |
| ZNF35 | Whole blood | −4.76 | −0.127 | 1.93 × 10−6 |
| RBM6 | Spinal cord cervical C1 | −4.75 | −0.043 | 2.07 × 10−6 |
| LIN28B-AS1 | Putamen, basal ganglia | 4.73 | 0.120 | 2.24 × 10−6 |
| AMT | Caudate, basal ganglia | −4.73 | −0.031 | 2.29 × 10−6 |
| MAU2 | Cerebellum | −4.72 | −0.124 | 2.31 × 10−6 |
| TSKU | Cerebellum | 4.71 | 0.044 | 2.48 × 10−6 |
| RPS26 | Amygdala | −4.71 | −0.015 | 2.53 × 10−6 |
| SNRPC | Amygdala | −4.69 | −0.045 | 2.68 × 10−6 |
| ACADL | Frontal cortex, BA 9 | −4.69 | −0.208 | 2.71 × 10−6 |
| PACSIN3 | Cortex | −4.69 | −0.095 | 2.72 × 10−6 |
| C6orf106 (ILRUN) | Amygdala | 4.68 | 0.089 | 2.80 × 10−6 |
| MPI | Putamen, basal ganglia | 4.68 | 0.058 | 2.81 × 10−6 |
| PTK2 | Caudate, basal ganglia | 4.68 | 0.097 | 2.85 × 10−6 |
| NUP43 | Cerebellum | −4.68 | −0.033 | 2.93 × 10−6 |
| KNDC1 | Cerebellum | 4.68 | 0.035 | 2.94 × 10−6 |
| NUP43 | Cerebellar hemisphere | −4.67 | −0.037 | 3.04 × 10−6 |
| RBM6 | Hippocampus | −4.67 | −0.071 | 3.08 × 10−6 |
| SNRPC | Cortex | −4.66 | −0.036 | 3.15 × 10−6 |
| GINM1 | Whole blood | 4.66 | 0.054 | 3.17 × 10−6 |
| FASTKD5 | Cortex | 4.66 | 0.108 | 3.20 × 10−6 |
| UBOX5 | Nucleus accumbens, basal ganglia | −4.65 | −0.080 | 3.27 × 10−6 |
| AMT | Hippocampus | −4.65 | −0.049 | 3.36 × 10−6 |
| HEXIM1 | Frontal cortex, BA 9 | −4.65 | −0.129 | 3.37 × 10−6 |
| KCNH2 | Cerebellar hemisphere | −4.64 | −0.057 | 3.46 × 10−6 |
| NELFA | Cerebellum | 4.64 | 0.097 | 3.47 × 10−6 |
| P4HTM | Cerebellar hemisphere | −4.64 | −0.065 | 3.50 × 10−6 |
| ERICH2 | Amygdala | −4.63 | −0.072 | 3.74 × 10−6 |
| RNF123 | Cerebellar hemisphere | 4.60 | 0.055 | 4.30 × 10−6 |
| LATS1 | Cerebellum | −4.59 | −0.067 | 4.51 × 10−6 |
| RNF123 | Amygdala | 4.58 | 0.106 | 4.65 × 10−6 |
| DCAKD | Frontal cortex, BA 9 | −4.58 | −0.052 | 4.68 × 10−6 |
| NUDT18 | Amygdala | −4.58 | −0.186 | 4.69 × 10−6 |
| DCAKD | Whole blood | −4.58 | −0.044 | 4.75 × 10−6 |
| RBM6 | Cerebellum | −4.57 | −0.022 | 4.80 × 10−6 |
| C6orf106 (ILRUN) | Cerebellar hemisphere | 4.57 | 0.115 | 4.85 × 10−6 |
| RNF123 | Anterior cingulate cortex, BA 24 | 4.57 | 0.102 | 4.87 × 10−6 |
| AC007405.6 | Caudate, basal ganglia | −4.57 | −0.080 | 4.93 × 10−6 |
| NUDT18 | Nucleus accumbens, basal ganglia | −4.57 | −0.033 | 4.98 × 10−6 |
| PPP6C | Anterior cingulate cortex, BA 24 | 4.56 | 0.109 | 5.04 × 10−6 |
| LLGL1 | Anterior cingulate cortex, BA 24 | −4.56 | −0.222 | 5.07 × 10−6 |
| NUP43 | Whole blood | 4.56 | 0.043 | 5.19 × 10−6 |
| C15orf57 | Cerebellum | −4.55 | −0.028 | 5.42 × 10−6 |
| ZNF23 | Hippocampus | 4.54 | 0.049 | 5.51 × 10−6 |
| RPS26 | Substantia nigra | −4.54 | −0.017 | 5.54 × 10−6 |
| PPP6C | Cortex | 4.54 | 0.289 | 5.58 × 10−6 |
| SLC38A3 | Frontal cortex, BA 9 | −4.54 | −0.065 | 5.62 × 10−6 |
| ZNF502 | Hippocampus | −4.54 | −0.081 | 5.63 × 10−6 |
| DNAH11 | Frontal cortex, BA 9 | 4.54 | 0.093 | 5.68 × 10−6 |
| ZNF502 | Nucleus accumbens, basal ganglia | −4.54 | −0.140 | 5.71 × 10−6 |
| SCAMP2 | Whole blood | 4.54 | 0.088 | 5.75 × 10−6 |
| RAD51 | Caudate, basal ganglia | −4.53 | −0.070 | 5.81 × 10−6 |
| ZNF502 | Cortex | −4.52 | −0.029 | 6.05 × 10−6 |
| DCAKD | Cerebellum | −4.52 | −0.027 | 6.25 × 10−6 |
| URM1 | Whole blood | −4.52 | −0.148 | 6.32 × 10−6 |
| LATS1 | Caudate, basal ganglia | −4.51 | −0.151 | 6.36 × 10−6 |
| BAK1 | Cerebellum | 4.51 | 0.071 | 6.40 × 10−6 |
| NUDT18 | Caudate, basal ganglia | −4.50 | −0.059 | 6.71 × 10−6 |
| MPI | Anterior cingulate cortex, BA 24 | 4.50 | 0.037 | 6.76 × 10−6 |
| FAM180B | Hypothalamus | −4.50 | −0.052 | 6.77 × 10−6 |
| IL23A | Hypothalamus | 4.50 | 0.059 | 6.85 × 10−6 |
| ZNF502 | Whole blood | −4.50 | −0.034 | 6.86 × 10−6 |
| DNMT3B | Cerebellum | 4.50 | 0.047 | 6.93 × 10−6 |
| LANCL1 | Cerebellar hemisphere | 4.49 | 0.113 | 6.97 × 10−6 |
| MPI | Cerebellum | 4.49 | 0.079 | 6.97 × 10−6 |
| SCAI | Cortex | 4.49 | 0.133 | 7.06 × 10−6 |
| SLC25A13 | Cerebellar hemisphere | 4.48 | 0.058 | 7.29 × 10−6 |
| CDK14 | Cortex | 4.48 | 0.164 | 7.36 × 10−6 |
| ACSF3 | Cortex | −4.47 | −0.023 | 7.73 × 10−6 |
| KIF3B | Amygdala | 4.47 | 0.064 | 7.81 × 10−6 |
| RP11–147L13.8 | Frontal cortex, BA 9 | 4.47 | 0.058 | 7.96 × 10−6 |
| RP11–147L13.11 | Spinal cord cervical C1 | −4.46 | −0.146 | 8.16 × 10−6 |
| RNF123 | Hypothalamus | 4.46 | 0.106 | 8.30 × 10−6 |
| MST1R | Nucleus accumbens, basal ganglia | 4.46 | 0.041 | 8.34 × 10−6 |
| LINC01671 | Nucleus accumbens, basal ganglia | −4.45 | −0.090 | 8.42 × 10−6 |
| CYB561D2 | Cortex | −4.45 | −0.172 | 8.44 × 10−6 |
| S100A1 | Cortex | 4.45 | 0.163 | 8.58 × 10−6 |
| RBM6 | Hypothalamus | −4.44 | −0.043 | 8.96 × 10−6 |
| RBM6 | Substantia nigra | −4.41 | −0.035 | 1.01 × 10−5 |
| DNAH11 | Putamen, basal ganglia | 4.41 | 0.045 | 1.03 × 10−5 |
| C15orf57 | Hypothalamus | −4.40 | −0.032 | 1.06 × 10−5 |
| ZNF502 | Frontal cortex, BA 9 | −4.40 | −0.041 | 1.10 × 10−5 |
| COX11 | Anterior cingulate cortex, BA 24 | −4.39 | −0.049 | 1.11 × 10−5 |
| NMT1 | Hippocampus | 4.39 | 0.085 | 1.13 × 10−5 |
| BAK1 | Hippocampus | 4.39 | 0.070 | 1.15 × 10−5 |
| SHMT1 | Hypothalamus | 4.38 | 0.044 | 1.18 × 10−5 |
| COX11 | Amygdala | −4.37 | −0.059 | 1.22 × 10−5 |
| COX11 | Hippocampus | −4.37 | −0.069 | 1.27 × 10−5 |
| RP11–147L13.11 | Anterior cingulate cortex, BA 24 | −4.36 | −0.102 | 1.30 × 10−5 |
| GINM1 | Substantia nigra | 4.36 | 0.076 | 1.31 × 10−5 |
All entries are tissue-wide significant. A positive sign indicates increased genetically regulated gene expression in these genes is associated with increased trait value (number of chronic pain sites).
BA, Brodmann area.
p Values reaching experiment-wide significance.
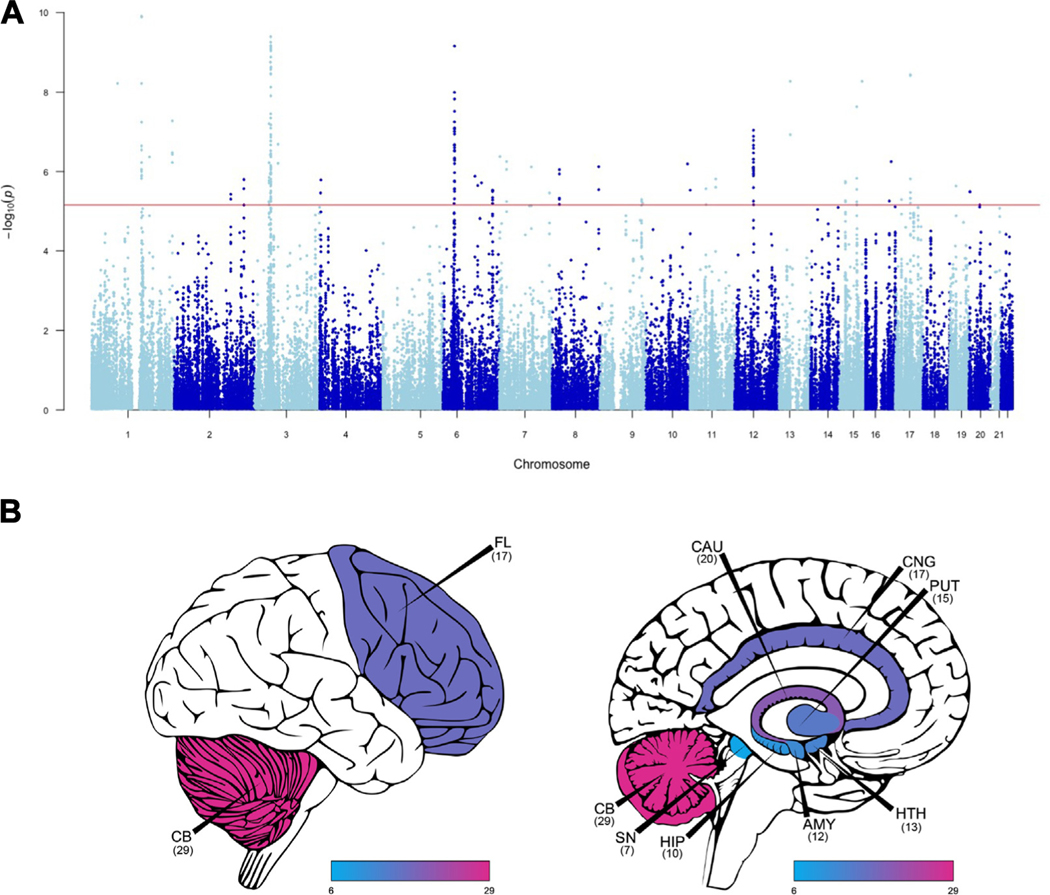
Figure 1.
S-PrediXcan analysis identifies 89 unique genes associated with chronic pain. (A) S-PrediXcan analyses identified 89 unique significant gene associations across 14 tissues. Red line indicates most conservative per-tissue significance threshold. (B) Number of significant multisite chronic pain–genetically regulated gene expression genes per brain region. Created using cerebroViz (157). AMY, amygdala; CAU, caudate; CB, cerebellum; CNG, anterior cingulate cortex; FL, frontal lobe; HIP, hippocampus; HTH, hypothalamus; PUT, putamen; SN, substantia nigra.
Of these 89 genes, 59 were not previously associated with MCP (5) (Table S4; Figure S2). We also found significant levels of replication of our gene-tissue findings in summary–transcriptome-wide association study (Supplement; Tables S5, S6). We also found significant replication of S-PrediXcan findings within significant TI findings for pain intensity. Six significant gene-tissue associations for MCP (Tables S7, S8) were also significant in analyses of pain intensity, representing significant replication (p = 4 × 10−9). To test whether significant associations were enriched in specific brain regions, we compared the proportion of experiment-wide significant associations per region with the proportion of genes tested in that region (binomial enrichment tests). We found significantly more experiment-wide significant associations in the nucleus accumbens basal ganglia than would be expected by chance (14.7% vs. 7.6%, pBinomial = .0075) and significantly fewer in the cerebellar hemisphere (4.2% vs. 9.0%, pBinomial = .038). Repeating this test for nominally associated genes, 3 brain regions showed fewer associations than would be expected by chance: the hippocampus (5.3% vs. 5.8%, pBinomial = .033), spinal cord cervical C1 (4.4% vs. 5.1%, pBinomial = .0014), and substantia nigra (3.4% vs. 4.0%, pBinomial = .0035).
Downstream Analyses Indicate Potential Chronic Pain Drug Targets
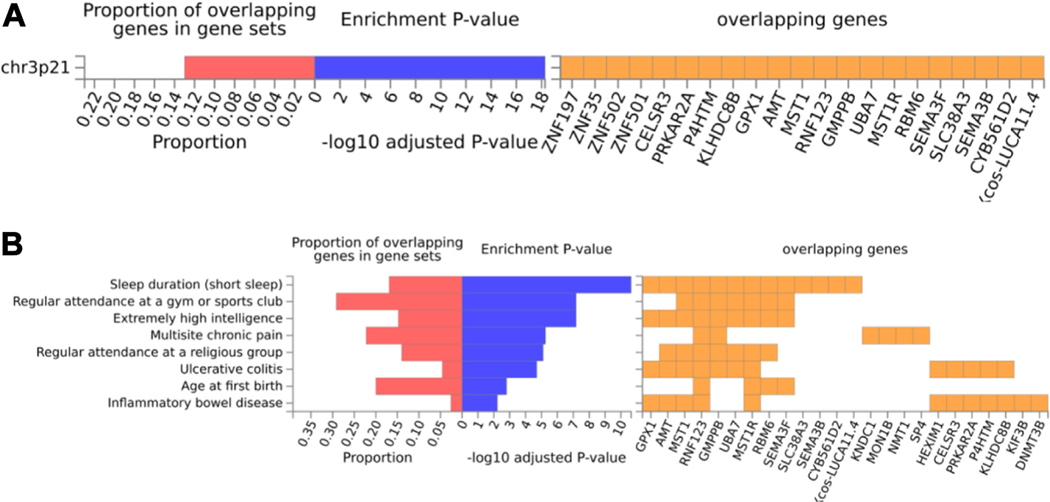
To identify functional patterns of MCP-GREX associations, we conducted a gene set enrichment analysis using FUMA (see the Supplement). Genes associated with MCP-GREX were significantly enriched in the positional gene set chr3p21 (p = 5.27 × 10−19) (Figure 2A), which was also implicated in anorexia nervosa (46). MCP-GREX genes were significantly enriched for genes associated with 8 GWASs (Figure 2B). This included a previous GWAS of MCP (p = 5.54 × 10−6) (5), sleep duration (short sleep) (p = 2.27 × 10−11), extremely high intelligence (p = 6.66 × 10−8), regular attendance at gyms and sports clubs (p = 6.66 × 10−8), and religious group attendance (p = 7.66 × 10−6), as well as inflammatory conditions (ulcerative colitis, p = 1.95 × 10−5, inflammatory bowel disease, p = 5.9 × 10−3) and age at first birth (p = 1.57 × 10−3). FUMA DrugBank lookups (Table S9) identified 19 genes as drug targets. CMap analyses identified 23 compounds with significant connectivity scores (Table 2).
Figure 2.
Gene set enrichment analysis identifies positional and genome-wide association study enrichments. (A) FUMA gene set enrichment identified one positional gene set (chr3p21) enriched for multisite chronic pain–genetically regulated gene expression genes. (B) Enrichment analyses showed 9 genome-wide association study catalog traits significantly enriched for multisite chronic pain–genetically regulated gene expression genes.
Table 2.
CMap Compounds With Significant Connectivity Scores With MCP-GREX
| Compound Name | Mechanism of Action | CS (Normalized) |
|---|---|---|
| PX-12 | Thioredoxin inhibitor | −1.62 |
| Physostigmine | Cholinesterase inhibitor, acetylcholinesterase inhibitor | −1.62 |
| Ibrutinib | BTK inhibitor | −1.62 |
| SR-2640 | Leucotriene receptor antagonist | −1.62 |
| Aspirin | Cyclooxygenase inhibitor | −1.63 |
| Fenoterol | Adrenergic receptor agonist | −1.64 |
| Nimesulide | Cyclooxygenase inhibitor | −1.64 |
| Arcyriaflavin-a | CDK inhibitor | −1.65 |
| BRD-A04553218 | Histamine receptor antagonist | −1.67 |
| Ponatinib | Bcr-abl inhibitor, FLT3 inhibitor, PDGFR inhibitor | −1.67 |
| SB-525334 | TGF-β receptor inhibitor | −1.67 |
| Sorbinil | Aldose reductase inhibitor | −1.68 |
| L-689560 | Glutamate receptor antagonist | −1.68 |
| Entecavir | DNA inhibitor, reverse transcriptase inhibitor | −1.68 |
| Ursolic acid | 11-beta-HSD1 inhibitor, acetylcholinesterase inhibitor, caspase inhibitor, HIV protease inhibitor, lipid peroxidase inhibitor, quorum sensing signaling modulator, stearyl sulfatase inhibitor, tyrosine phosphatase inhibitor, ATPase inhibitor, NF-κB inhibitor, STAT inhibitor | −1.68 |
| Palmitoylethanolamide | Cannabinoid receptor agonist | −1.68 |
| Luteolin | Glucosidase inhibitor | −1.69 |
| Resiquimod | TLR agonist | −1.69 |
| Tiabendazole | Angiogenesis inhibitor | −1.72 |
| BRD-K18059238 | Cyclooxygenase inhibitor, prostanoid receptor agonist | −1.74 |
| KO-143 | Breast cancer resistance protein inhibitor | −1.75 |
| PD-153035 | EGFR inhibitor | −1.76 |
| Dutasteride | 5-alpha reductase inhibitor | −1.85 |
CMap, Connectivity Map; CS, connectivity score; GREX, genetically regulated gene expression; MCP, multisite chronic pain.
Clinical Associations With Chronic Pain GREX Revealed Through PheWAS
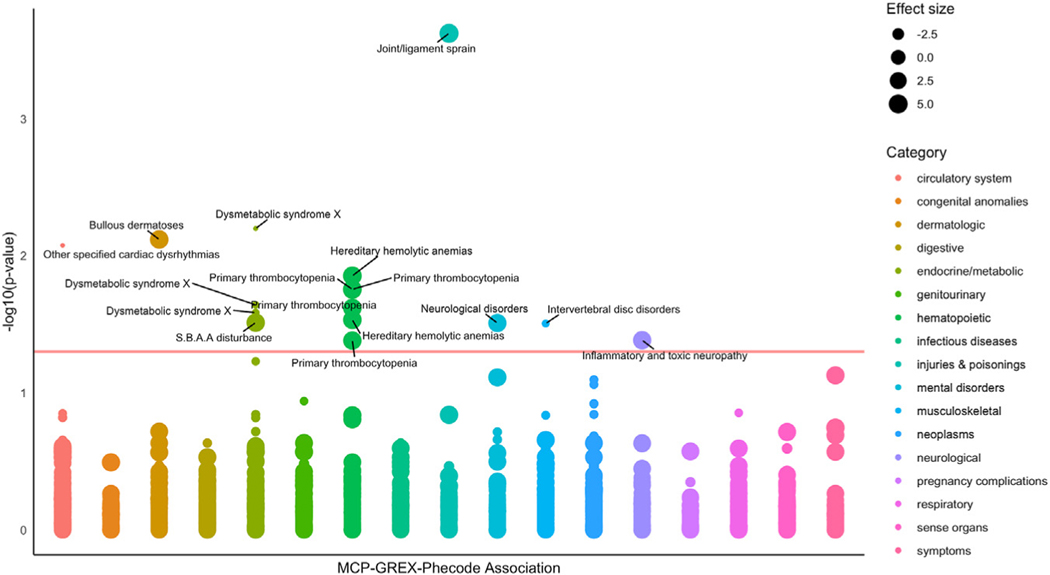
To probe clinical consequences of our MCP-associated genes, we performed a PheWAS in the Mount Sinai BioMe biobank. First, we imputed MCP-GREX for 89 significant MCP-GREX gene-tissue associations for 18,806 biobank participants who had available mean pain score data and tested for association between GREX and mean pain score. We identified 37 associations including 10 unique genes between MCP-GREX and mean pain score (Table 3). Next, we tested for phenome-wide associations, imputing MCP-GREX for 89 significant MCP-GREX gene-tissue associations for 31,704 BioMe participants across 6 ancestry groups. Then, we meta-analyzed across ancestry using METAL and applied multiple testing correction (FDR). We identified 16 significant GREX-phecode associations across 9 brain regions, including 10 unique gene-phecode associations (Table 3; Figure 3). Associated phecodes included cardiac dysrhythmia, metabolic syndrome, disc disorders/dorsopathies, joint/ligament sprain, anemias, and neurological disorders.
Table 3.
Associations Between Mean Pain Score and MCP-GREX
| Gene | Tissue | z Score | pFDR | pRaw |
|---|---|---|---|---|
| SDCCAG8 | Brain: cerebellar hemisphere | −2.25 | .049 | .025 |
| Brain: putamen, basal ganglia | −2.65 | .043 | .008 | |
| Whole blood | −2.45 | .043 | .014 | |
| UHRF1BP1 | Brain: amygdala | −3.58 | .002 | .000 |
| Brain: anterior cingulate cortex, BA 24 | −2.78 | .011 | .005 | |
| Brain: caudate, basal ganglia | −2.72 | .011 | .006 | |
| Brain: cerebellar hemisphere | −2.68 | .011 | .007 | |
| Brain: cerebellum | −2.63 | .011 | .009 | |
| Brain: cortex | −3.56 | .002 | .000 | |
| Brain: frontal cortex, BA 9 | −2.67 | .011 | .008 | |
| Brain: hypothalamus | −2.71 | .011 | .007 | |
| Brain: nucleus accumbens, basal ganglia | −2.90 | .011 | .004 | |
| Brain: putamen, basal ganglia | −2.35 | .020 | .019 | |
| Brain, spinal cord cervical C1 | −3.03 | .011 | .002 | |
| Whole blood | −2.52 | .014 | .012 | |
| DNMT3B | Brain: anterior cingulate cortex, BA 24 | −2.91 | .011 | .004 |
| ACADL | Brain: frontal cortex, BA 9 | 2.42 | .031 | .015 |
| SNRPC | Brain: amygdala | 2.95 | .017 | .003 |
| Brain: caudate, basal ganglia | 2.13 | .046 | .033 | |
| Brain: cerebellum | 2.69 | .017 | .007 | |
| Brain: cortex | 2.67 | .017 | .008 | |
| Brain: hippocampus | 2.69 | .017 | .007 | |
| Brain: nucleus accumbens, basal ganglia | 2.50 | .021 | .012 | |
| Brain: putamen, basal ganglia | 2.48 | .021 | .013 | |
| Whole blood | 3.02 | .017 | .003 | |
| TARS2 | Brain: anterior cingulate cortex, BA 24 | 3.26 | .006 | .001 |
| Brain: cerebellar hemisphere | 2.39 | .028 | .017 | |
| Brain: cerebellum | 2.98 | .007 | .003 | |
| CEP170 | Whole blood | −2.52 | .023 | .012 |
| HEXIM1 | Brain: cortex | 2.60 | .028 | .009 |
| ILRUN | Brain: hippocampus | −3.07 | .024 | .002 |
| MRPS21 | Brain: caudate, basal ganglia | 2.48 | .025 | .013 |
| Brain: cerebellum | −3.15 | .015 | .002 | |
| Brain: cortex | −2.45 | .025 | .014 | |
| Brain: frontal cortex, BA 9 | −2.53 | .025 | .011 | |
| Brain: hypothalamus | −2.37 | .027 | .018 | |
| Brain: nucleus accumbens, basal ganglia | 2.71 | .025 | .007 |
BA, Brodmann area; FDR, false discovery rate; GREX, genetically regulated gene expression; MCP, multisite chronic pain.
Figure 3.
Phenome-wide associations with chronic pain–associated genes. Effect size = z score value for the association between MCP-GREX and phecode. Red horizontal line indicates p value significance threshold (−log10(0.05) = 1.3); phecodes are color-coded according to wider phecode category [using mapping tables made available at https://phewascatalog.org/phecodes_icd10 and associated with Wu et al. (44)]. GREX, genetically regulated gene expression; MCP, multisite chronic pain; S.B.A.A, sulfur-bearing amino acid.
Because pain and chronic pain are core symptoms of many of these diagnoses, and some genes with significant MCP-GREX were significantly associated with pain NRS, it is difficult to discern whether our MCP genes are associated with pain experience or directly with the trait itself. Therefore, we repeated our PheWAS on a subset of BioMe participants and included mean pain scores derived from pain NRS information as covariates. We also carried out a PheWAS with adjustment identical to our main analyses (no adjustment for mean pain score) on the same subset of participants. We found the results to be significantly different from the main PheWAS results, but after comparison with the unadjusted-subset PheWAS, this appears to have been driven by a reduction in sample size rather than by mean pain score (Tables S10, S11). Sample size is significantly reduced when adjusting for pain score because many BioMe participants do not have pain NRS information available.
DISCUSSION
These results reveal novel genes, theoretically enriched for causal effect, that are relevant to chronic pain development, thus providing new insight into mechanisms of chronic pain. By applying TI using S-PrediXcan, we were able to perform a well-powered study of gene expression in brain tissue and whole blood, which is currently not feasible with existing cohorts in which chronic pain phenotyping, genotype, and expression data are available together due to limited sample sizes. In the following section, we contextualize our findings with a focus on MCP-GREX genes found to be significantly associated with clinical traits (phecodes) in our BioMe PheWAS analysis.
Gene Findings Give Insight Into Shared Pathways Between Chronic Pain and Other Medical Conditions
GREX of ILRUN, involved in innate immune response and highly expressed in B cells (47), was significantly associated with MCP in the basal ganglia of the nucleus accumbens, hypothalamus, amygdala, and cortex in the original S-PrediXcan analysis and with primary thrombocytopenia across all 4 tissues in our PheWAS (Table 4 ). Primary thrombocytopenia is an autoimmune platelet disorder that causes low peripheral plate counts and symptoms including joint and abdominal pain, bleeding, and bruising. ILRUN has also been linked to the renin-angiotensin-aldosterone system (involved in blood volume, sodium reabsorption, and vascular tone among other processes) in a study of SARS-CoV-2 infection (48). Peripheral small Ad and C fibers that transmit pain signals contain cells expressing renin-angiotensin-aldosterone system components, and renin-angiotensin-aldosterone system modulators have been shown to affect pain relief (49). Our results suggest a role for ILRUN in the brain in chronic pain development, in addition to in pain perception in the periphery.
Table 4.
Significant GREX-Phecode Associations
| Gene | Phecode Description | Tissue | Full Analysis | Correcting for Pain Scores | ||||
|---|---|---|---|---|---|---|---|---|
| z Score | p FDR | p Raw | z Score | p FDR | p Raw | |||
| DCAKD | Cardiac dysrhythmias | Caudate, basal ganglia | −4.98 | .0084 | 6.18 × 10−7 | −5.52 | .0046 | 3.47 × 10−8 |
| ECM1 | Dysmetabolic syndrome X | Cerebellar hemisphere | 4.58 | .0063 | 6.39 × 10−7 | – | – | – |
| Cerebellum | −4.99 | .0228 | 4.61 ×10−6 | – | – | – | ||
| Nucleus accumbens, basal ganglia | 5.33 | .026 | 7.89 ×10−6 | – | – | – | ||
| ERICH2 | Disc disorders/dorsopathies | Amygdala | 4.7 | .0312 | 8.4 × 10−6 | −4.42 | .033 | 9.83 × 10−6 |
| ILRUN (C6orf106) | Primary thrombocytopenia | Amygdala | −4.47 | .0176 | 1.98 ×10−6 | – | – | – |
| Hypothalamus | 4.81 | .0176 | 2.59 × 10−6 | – | – | – | ||
| Nucleus accumbens, basal ganglia | 4.46 | .024 | 5.3 × 10−6 | – | – | – | ||
| Cortex | −4.45 | .0415 | 1.22 × 10−5 | – | – | – | ||
| MON1B | Anemias | Spinal cord cervical C1 | −4.58 | .014 | 1.14 × 10−6 | – | – | – |
| Amygdala | 4.21 | .0293 | 4.74 × 10−6 | – | – | – | ||
| PACSIN3 | Bullous dermatoses | Nucleus accumbens, basal ganglia | 4.87 | .0076 | 1.54 × 10−6 | – | – | – |
| RAD51 | Disturbances of sulfur-bearing amino acid metabolism | Substantia nigra | 4.76 | .0307 | 1.24 ×10−5 | – | – | – |
| SCAI | Inflammatory and toxic neuropathy | Cortex | 4.55 | .0412 | 8.34 × 10−6 | – | – | – |
| SLC38A3 | Joint/ligament sprain | Caudate, basal ganglia | 4.37 | .0002 | 9.62 × 10−8 | 6.00 | 4.34 × 10−06 | 1.92 × 10−9 |
| Neurological disorders | Caudate, basal ganglia | 4.37 | .0309 | 2.5 × 10−5 | – | – | – | |
| ZNF197 | Hand/finger injuries and lacerations | Substantia nigra | – | – | – | 4.45 | .048 | 8.45 × 10−6 |
| ENSG00000278730 (Novel Transcript, lncRNA, a.k.a. RP11–147L13.11) | Spondylosis with myelopathy | Anterior cingulate cortex, BA 24 | – | – | – | 4.93 | .0046 | 8.21 × 10−7 |
| Cerebellum | – | – | – | 4.59 | .017 | 4.41 × 10−6 | ||
| Cortex | – | – | – | 5.05 | .0046 | 4.46 × 10−7 | ||
| Hypothalamus | – | – | – | 4.37 | .035 | 1.25 × 10−5 | ||
FDR correction carried out within gene; z score presents PheWAS z score value; tissue represents GREX tissue; pFDR presents GREX-phecode phenome-wide association study p value (FDR corrected); and pRaw presents uncorrected p value.
a.k.a., also known as; FDR, false discovery rate; GREX, genetically regulated gene expression; lncRNA, long noncoding RNA.
MCP-GREX of MON1B in both the amygdala and cervical spinal cord C1 was found to be significantly associated with anemias (Table 4); this phecode includes sickle cell anemia, thalassemia, and hemolytic anemias, all of which have often been associated with significant pain (50). Iron deficiency and iron-deficiency anemia are also generally associated with chronic inflammatory disease and chronic pain (51). Dysregulation of iron metabolism can play a key role in immune cell homeostasis and inflammation (52,53). MON1B also encodes a protein for which defects are associated with autoimmune pathology (54), a process that plays a significant role in chronic pain (55). This protein is also a key regulator of endocytic sorting by Numb, and so is linked to cell migration, asymmetrical cell division, and differentiation (56).
DCAKD encodes a protein linked to neurodevelopment (57) that is expressed widely in the brain (58), and MCP-GREX of this gene in the caudate basal ganglia was negatively associated with cardiac dysrhythmia (Table 4). Previous studies indicate a relationship between magnetic resonance imaging markers of cerebral small vessel disease and DCAKD (59) and Friedrich’s ataxia (60), a disease of progressive neurodegeneration, heart, and spinal problems (61,62). Heart rate variability is thought to represent hyperarousal and has been linked to emotion regulation and chronic pain (63,64). In addition, certain nerve blocks can treat both cardiac and chronic pain conditions (65).
ECM1 encodes a protein involved in type 2 helper T cell migration (66) and skin development (67). In PheWAS analyses, ECM1 MCP-GREX was associated with dysmetabolic syndrome X (aka metabolic syndrome) in 3 different brain tissues (68,69) (Table 4). This syndrome has been associated with increased risk of cardiovascular disease and type 2 diabetes (68,70). T cells have been associated with insulin resistance development in obesity (71); having metabolic syndrome can affect T cell development [reviewed by (72)]; and the amount of memory T cells has been associated with a proinflammatory state (73). These cell types could be therapeutic targets in chronic pain treatment (74–77) and could represent a sex-dimorphic mediator of pain hypersensitivity [reviewed by (78,79)].
PACSIN3 encodes a protein involved in the actin cytoskeleton and formation of vesicles (80). This protein also binds TRPV4; channelopathy mutations in the TRPV4 gene lead to skeletal dysplasias, Charcot-Marie-Tooth disease subtype 2C, premature osteoarthritis, and neurological disorders (81). TRPV4 channels are also important in skin function (82) and are involved in the itch-scratch cycle (83,84). TRP channels have also been implicated in chronic low back pain (85) and investigated as a therapeutic target in fibromyalgia (86,87). PACSIN3 MCP-GREX in the basal ganglia of the nucleus accumbens was significantly associated with bullous dermatoses in PheWAS analyses (Table 4). Bullous dermatoses are autoimmune skin conditions of painful blistering (88–91). Although itch and pain are considered to be distinct (84), they share many similarities (92). Results here suggest that TRPV4 ion channels and their interaction with PACSIN3 could be a point of overlap between chronic pain and itch.
RAD51 is involved in DNA repair (93,94). RAD51 mutations have been linked to congenital mirror movement disorder (95) and cancers (96). MCP-GREX at this gene in the substantia nigra was significantly associated with disturbances of sulfur-bearing amino acid metabolism (Table 4). This phecode includes homocystinuria (the body is unable to process methionine) and methylenetetrahydrofolate reductase (MTHFR) deficiency (homocysteine levels are elevated) (97). Both processes are part of DNA metabolism (98), and elevated homocysteine levels are associated with a range of illnesses and neurotoxicity (99). RAD51 foci (indicators of cellular replication stress) (100) were increased in experiments examining folate deficiency (101). Previous studies in rodents showed that elevated homocysteine caused mechanical allodynia (102), and PheWAS results indicate a role for this mechanism of sensitization in human chronic pain.
SCAI encodes a transcriptional cofactor that regulates invasive cell migration (103), including in gliomas (104). MCP-GREX of this gene in the cortex was associated with toxic/inflammatory neuropathy in PheWAS analyses, and this gene was differentially expressed in rat models of diabetic neuropathy in the spinal cord (105). Our findings suggest a similar role for human SCAI in neuropathy.
SLC38A3 encodes a glutamine transporter (106) involved in cell energy metabolism. Glutamine is the preferred energy source for rapidly proliferating cell populations in the nervous system, immune system, and cancer cells (107–111). SLC38A3 is also expressed in muscles, and significant MCP-GREX in the caudate basal ganglia was found to be associated with joint and muscle sprain (Table 4), suggesting that the glutamine transporter encoded by SLC38A3 has a central as well as a peripheral role. SLC38A3 MCP-GREX in the same brain area was also significantly associated with neurological disorders (Table 4), consistent with research showing relationships between glutamine metabolism in the brain and neurological conditions (112–115). GABAergic (gamma-aminobutyric acidergic) gene regulatory elements have also been implicated in neurological and psychiatric diseases (116–120), glutamate receptors in neurological dysfunction (121), and treating neurodegeneration through targeting glutamate transporters (122). Activity-dependent synaptic plasticity also involves glutamate and glutamine metabolism (123). Glutamine has also been investigated as a chronic pain biomarker because concentrations vary in individuals with chronic pain compared with control participants (124,125), and glutamine supplementation may be helpful in vaso-occlusive crisis in sickle cell disease (126). Glutamine levels have also been associated with individual pain sensitivity differences (127) and migraine (128). Finally, glutamine supplementation was associated with reduced opioid use in sickle cell disease in a small study, highlighting potential as a harm- and pain-reducing compound in chronic pain treatment (129). Finally, ERICH2 MCP-GREX in the amygdala was significantly associated with dorsopathies (Table 4).
Comparison With Genetic Correlation Results
Psychiatric disorder–related phecodes and phecodes assigned to chronic pain conditions, e.g., rheumatoid arthritis or endometriosis, were not significantly associated with MCP-GREX. In contrast, significant genetic correlations between MCP and, e.g., major depressive disorder and MCP and rheumatoid arthritis were found in a previous study (5). Genetic correlations are calculated using all single nucleotide polymorphism associations genome wide rather than at a gene level, which may explain these differences. In addition, in theory, S-PrediXcan results represent gene expression changes that occur before chronic pain development (whereas GWAS summary statistics used in linkage disequilibrium score regression represent genetic associations more generally). This suggests that the gene expression changes that contribute to chronic pain development do not directly contribute to psychiatric conditions (e.g., major depressive disorder), which is consistent with previous studies that have suggested that chronic pain can have a causal effect on major depression development but not vice versa (5). Another possibility is that tissues that were not examined in this study are associated with MCP-GREX and would show associations with psychiatric disorder or other expected phecodes in a PheWAS. However, it is difficult to explain why these nonbrain tissues, and not brain tissue, would show this result. We chose to examine brain and whole blood because chronic pain involves significant changes in the brain and spinal cord (16–19), and whole blood represents a tissue of interest due to immune components and ease of testing for, e.g., potential chronic pain biomarkers. Finally, phecodes generally represent a broad category of diagnoses; for example, the phecode for mood disorder (296) encompasses depression associated with major depressive disorder, bipolar disorder, and schizophrenia, and this heterogeneity could affect PheWAS results.
Changes to PheWAS Findings When Adjusting for Mean Pain Score
After adjusting our PheWAS association testing for mean pain score, results were significantly different compared with the main PheWAS analyses. However, these changes appear to be driven by reduction in sample size because unadjusted and adjusted analyses in the same subset of individuals showed similar results. Although NRS is a widely used pain reporting measure in clinical and research settings (130), it can change in unpredictable ways over time in chronic pain (131,132), may not accurately reflect treatment outcome when used alone (133), and may not be the most useful measure for identifying clinically important pain (134) or changes in pain (135). Pain NRS may not represent an ideal assessment tool in nonacute pain at the population or group level despite some studies demonstrating stability when an NRS was used to assess improvement in individuals over time (136) because perception of pain, which influences NRS ratings, is likely to be significantly different between individuals with and without chronic pain (137). People with chronic pain may rate moderate to high levels of pain as tolerable (138); conversely, depression or depressive symptoms that are commonly comorbid with chronic pain could lead to the reporting of higher NRS scores (139–141).
Drug Targets in Chronic Pain
Chronic pain is complex and difficult to treat successfully. The results shown here could inform treatment development; genes where MCP-GREX is associated with upregulation may present better targets in genomic medicine (downregulation of a gene can be easier to induce than upregulation), and genes where significant MCP-GREX is shown in a singular tissue may present a better target for potential animal modeling of chronic pain compared with genes where MCP-GREX is widespread. DrugBank lookups provide suggestions for drug repurposing, and several drugs highlighted are already used experimentally in chronic pain treatment, e.g., monoclonal antibodies in migraine (142–144) and drugs that increase inhibitory glycinergic neurotransmission in the spinal cord (145,146). Several compounds identified in CMap analysis also show potential in chronic pain treatment; PX-12 showed anti-allodynia effects in a rodent model of chronic pain (147); physostigmine showed an antihyperalgesic effect in clinical trials (148); and SR-2640 activates TREK-1 channels that are associated with nociceptive hypersensitivity in rodent models (149). Arcyriaflavin-a is a potential therapeutic compound in endometriosis (150), as sorbinil (151) and fenoterol (152) are in diabetic neuropathy. Ursolic acid has demonstrated antinociceptive properties in animal models (153), and analgesic properties of palmitoylethanolamide (154) and luteolin (155) have been shown in multiple studies. Other findings are established pain treatments, e.g., aspirin and nimesulide. Other compounds, e.g., epidermal growth factor receptor (EGFR) inhibitor PD-153035, affect cancer-related pathways, which are also implicated in chronic pain (156), thus presenting novel treatment targets.
Conclusions
We carried out the largest TI study of a chronic pain trait to date, making important progress in translating GWAS findings into insights into chronic pain development and beginning to bridge the gap between genotype (GWAS output) and phenotype (MCP). Specific brain tissues and the direction of effect of MCP-GREX are also given; pathways of interest and potential mechanistic overlap with other medical conditions are indicated; and several genes showing significant MCP-GREX are also potential drug targets. We also identified several compounds with opposite expression perturbation signatures to MCP (i.e., potentially therapeutic compounds in chronic pain). Results of our PheWAS in which we adjusted for mean pain score indicate that associations tend not to be driven solely by pain perception. PheWAS results indicate potential shared causal pathways between chronic pain and conditions such as metabolic syndrome, anemias, and cardiac dysrhythmia.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | GTEx | https://doi.org/10.1038/ng.2653 | https://gtexportal.org/home/ | |
| Deposited Data; Public Database | CMap | https://doi.org/10.1016/j.cell.2017.10.049 | https://www.broadinstitute.org/connectivity-map-cmap | |
| Deposited Data; Public Database | Phewas Catalog | https://doi.org/10.2196/14325 | https://phewascatalog.org/ | |
| Deposited Data; Public Database | Multisite Chronic Pain GWAS summary statistics | https://doi.org/10.1371/journal.pgen.1008164 | https://researchdata.gla.ac.uk/822/ | |
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | S-PrediXcan | https://doi.org/10.1038/s41467–018-03621–1 | ||
| Software; Algorithm | FUSION | https://doi.org/10.1038/ng.3506 | http://gusevlab.org/projects/fusion/ | |
| Software; Algorithm | PheWAS | https://doi.org/10.1093/bioinformatics/btu197 | https://github.com/PheWAS/PheWAS | |
| Software; Algorithm | FUMA | https://doi.org/10.1038/s41467–017-01261–5 | https://fuma.ctglab.nl/ | |
| Transfected Construct | ||||
| Other |
ACKNOWLEDGMENTS
KJAJ is supported by National Institute of Mental Health (Grant Nos. R01MH118278 and R01MH124839). JJ and LMH are supported by the Klarman Family Foundation. LMH is supported by National Institute of Mental Health (Grant Nos. R01MH118278 and R01MH124839) and National Institute of Environmental Health Sciences (Grant No. R01ES033630).
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2023.08.023.
Contributor Information
Keira J.A. Johnston, Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
Alanna C. Cote, Pamela Sklar Division of Psychiatric Genetics, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, New York
Emily Hicks, Pamela Sklar Division of Psychiatric Genetics, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, New York.
Jessica Johnson, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Laura M. Huckins, Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
REFERENCES
- 1.Mills SEE, Nicolson KP, Smith BH (2019): Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J Anaesth 123:e273–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018): Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macfarlane GJ (2016): The epidemiology of chronic pain. Pain 157:2158–2159. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Singh V, Kaye AD, Hirsch JA (2020): Lessons for better pain management in the future: Learning from the past. Pain Ther 9:373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, et al. (2019): Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet 15:e1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsepilov YA, Freidin MB, Shadrina AS, Sharapov SZ, Elgaeva EE, van Zundert JV, et al. (2020): Analysis of genetically independent phenotypes identifies shared genetic factors associated with chronic musculoskeletal pain conditions. Commun Biol 3:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman MS, Winsvold BS, Chavez Chavez SO, Børte S, Tsepilov YA, Sharapov SZ, et al. (2021): Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann Rheum Dis 80:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha E, Bae SC, Kim K (2021): Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann Rheum Dis 80:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, De Vivo I, et al. (2017): Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun 8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AVE, Kogelman LJA, et al. (2022): Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 54:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diatchenko L, Parisien M, Jahangiri Esfahani S, Mogil JS (2022): Omics approaches to discover pathophysiological pathways contributing to human pain. Pain 163:S69–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. (2015): A gene-based association method for mapping traits using reference transcriptome data. Nat Genet 47:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbeira AN, Dickinson SP, Bonazzola R, Zheng J, Wheeler HE, Torres JM, et al. (2018): Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics [no. 1]. Nat Commun 9:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Ritchie MD (2021): From GWAS to gene: Transcriptome-wide association studies and other methods to functionally understand GWAS discoveries. Front Genet 12:713230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainberg M, Sinnott-Armstrong N, Mancuso N, Barbeira AN, Knowles DA, Golan D, et al. (2019): Opportunities and challenges for transcriptome-wide association studies. Nat Genet 51:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baliki MN, Mansour AR, Baria AT, Apkarian AV (2014): Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9:e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apkarian VA, Hashmi JA, Baliki MN (2011): Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 152(suppl):S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. (2013): Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136:2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuner R, Flor H (2017): Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18:20–30. [DOI] [PubMed] [Google Scholar]
- 20.Farrell SF, Campos AI, Kho PF, de Zoete RMJ, Sterling M, Rentería ME, et al. (2021): Genetic basis to structural grey matter associations with chronic pain. Brain 144:3611–3622. [DOI] [PubMed] [Google Scholar]
- 21.Khoury S, Parisien M, Thompson SJ, Vachon-Presseau E, Roy M, Martinsen AE, et al. (2022): Genome-wide analysis identifies impaired axonogenesis in chronic overlapping pain conditions. Brain 145:1111–1123. [DOI] [PubMed] [Google Scholar]
- 22.Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, et al. (2018): Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet 50:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall LS, Medway CW, Pain O, Pardiñas AF, Rees EG, Escott-Price V, et al. (2020): A transcriptome-wide association study implicates specific pre- and post-synaptic abnormalities in schizophrenia. Hum Mol Genet 29:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doke T, Huang S, Qiu C, Liu H, Guan Y, Hu H, et al. (2021): Transcriptome-wide association analysis identifies DACH1 as a kidney disease risk gene that contributes to fibrosis. J Clin Invest 131: e141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Díez-Obrero V, Moratalla-Navarro F, Ibáñez-Sanz G, Guardiola J, Rodríguez-Moranta F, Obón-Santacana M, et al. (2022): Transcriptome-wide association study for inflammatory bowel disease reveals novel candidate susceptibility genes in specific colon subsites and tissue categories. J Crohns Colitis 16:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Y, Pei G, Zhao Z, Jia P (2019): A convergent study of genetic variants associated with Crohn’s disease: Evidence from GWAS, gene expression, methylation, eQTL and TWAS. Front Genet 10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X, Kim K, Suetsugu H, Bang SY, Wen L, Koido M, et al. (2022): Biological insights into systemic lupus erythematosus through an immune cell-specific transcriptome-wide association study. Ann Rheum Dis 81:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheutlin AB, Dennis J, Karlsson Linnér RK, Moscati A, Restrepo N, Straub P, et al. (2019): Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry 176:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huckins LM (2023): Thoughtful phenotype definitions empower participants and power studies. Complex Psychiatry 8:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Zhang X, Meng X, Koskeridis F, Georgiou A, Yu L, et al. (2021): Methodology in phenome-wide association studies: A systematic review. J Med Genet 58:720–728. [DOI] [PubMed] [Google Scholar]
- 31.Doss J, Mo H, Carroll RJ, Crofford LJ, Denny JC (2017): Phenome-wide association study of rheumatoid arthritis subgroups identifies association between seronegative disease and fibromyalgia. Arthritis Rheumatol 69:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Roige S, Fontanillas P, Jennings MV, Bianchi SB, Huang Y, Hatoum AS, et al. (2021): Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Mol Psychiatry 26:6209–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, et al. (2019): The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 160:28–37. [DOI] [PubMed] [Google Scholar]
- 34.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. (2019): Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160:19–27. [DOI] [PubMed] [Google Scholar]
- 35.IASP (2020): IASP announces revised definition of pain. Available at: https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/. Accessed September 20, 2022.
- 36.de Leeuw CA, Mooij JM, Heskes T, Posthuma D (2015): MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput Biol 11: e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GTEx Consortium (2020): The GTEx Consortium atlas of genetic regulatory effects across human tissues 2020;. 369:1318–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BWJH, et al. (2016): Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet 48:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toikumo S, Vickers-Smith R, Jinwala Z, Xu H, Saini D, Hartwell E, et al. (2023): The genetic architecture of pain intensity in a sample of 598,339 U.S. veterans. medRxiv. 10.1101/2023.03.09.23286958. [DOI] [Google Scholar]
- 40.Watanabe K, Taskesen E, van Bochoven A, Posthuma D (2017): Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. (2006): The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 313:1929–1935. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. (2017): A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 171:1437–1452.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastarache L (2021): Using phecodes for research with the electronic health record: From PheWAS to PheRS. Annu Rev Biomed Data Sci 4:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu P, Gifford A, Meng X, Li X, Campbell H, Varley T, et al. (2019): Mapping ICD-10 and ICD-10-CM codes to phecodes: Workflow development and initial evaluation. JMIR Med Inform 7:e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willer CJ, Li Y, Abecasis GR (2010): METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JS, Cote AC, Dobbyn A, Sloofman LG, Xu J, Cotter L, et al. (2022): Mapping anorexia nervosa genes to clinical phenotypes. Psychol Med 53:2619–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambrose RL, Brice AM, Caputo AT, Alexander MR, Tribolet L, Liu YC, et al. (2020): Molecular characterisation of ILRUN, a novel inhibitor of proinflammatory and antimicrobial cytokines. Heliyon 6:e04115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tribolet L, Alexander MR, Brice AM, van Vuren PJ, Rootes CL, Mara K, et al. (2021): ILRUN downregulates ACE2 expression and blocks infection of human cells by SARS-CoV-2. J Virol 95: e0032721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bessaguet F, Magy L, Desmoulière A, Demiot C (2016): The therapeutic potential of renin angiotensin aldosterone system (RAAS) in chronic pain: From preclinical studies to clinical trials. Expert Rev Neurother 16:331–339. [DOI] [PubMed] [Google Scholar]
- 50.Lal A (2016): Assessment and treatment of pain in thalassemia. Ann N Y Acad Sci 1368:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cappellini MD, Comin-Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, et al. (2017): Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol 92:1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cronin SJF, Woolf CJ, Weiss G, Penninger JM (2019): The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukhbaatar N, Weichhart T (2018): Iron regulation: Macrophages in control. Pharmaceuticals (Basel) 11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinchen JM, Ravichandran KS (2010): Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 464:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacagnina MJ, Heijnen CJ, Watkins LR, Grace PM (2021): Autoimmune regulation of chronic pain. PAIN Rep 6:e905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao X, Liu Y, Yu Q, Ding Z, Qian W, Zhang L, et al. (2016): Numb regulates vesicular docking for homotypic fusion of early endosomes via membrane recruitment of Mon1b. Cell Res 26:593–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Lozano MA, Klemmer P, Gebuis T, Hassan C, van Nierop P, van Kesteren RE, et al. (2016): Dynamics of the mouse brain cortical synaptic proteome during postnatal brain development. Sci Rep 6:35456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latourelle JC, Dumitriu A, Hadzi TC, Beach TG, Myers RH (2012): Evaluation of Parkinson disease risk variants as expression-QTLs. PLoS One 7:e46199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persyn E, Hanscombe KB, Howson JMM, Lewis CM, Traylor M, Markus HS (2020): Genome-wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat Commun 11:2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMackin MZ, Durbin-Johnson B, Napierala M, Napierala JS, Ruiz L, Napoli E, et al. (2019): Potential biomarker identification for Friedreich’s ataxia using overlapping gene expression patterns in patient cells and mouse dorsal root ganglion. PLoS One 14:e0223209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanson E, Sheldon M, Pacheco B, Alkubeysi M, Raizada V (2019): Heart disease in Friedreich’s ataxia. World J Cardiol 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institute of Neurological Disorders and Stroke (2018), Friedreich ataxia fact sheet. Available at: https://www.ninds.nih.gov/friedreich-ataxia-fact-sheet. Accessed September 2, 2022.
- 63.Beauchaine T (2001): Vagal tone, development, and gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol 13:183–214. [DOI] [PubMed] [Google Scholar]
- 64.Koenig J, Loerbroks A, Jarczok MN, Fischer JE, Thayer JF (2016): Chronic pain and heart rate variability in a cross-sectional occupational sample: Evidence for impaired vagal control. Clin J Pain 32:218–225. [DOI] [PubMed] [Google Scholar]
- 65.Deshpande K, Gama W, Emerick T (2021): An alternative role for the pain physician: Utilization of stellate block for treatment resistant cardiac arrhythmias. Pain Med 22:1447–1451. [DOI] [PubMed] [Google Scholar]
- 66.He L, Gu W, Wang M, Chang X, Sun X, Zhang Y, et al. (2018): Extracellular matrix protein 1 promotes follicular helper T cell differentiation and antibody production. Proc Natl Acad Sci USA 115:8621–8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oyama N, Merregaert J (2017): The extracellular matrix Protein 1 (ECM1) in molecular-based skin biology. In: Farage MA, Miller KW, Maibach HI, editors. Textbook of Aging Skin. Berlin, Heidelberg: Springer, 91–110. [Google Scholar]
- 68.Nilsson PM, Tuomilehto J, Rydén L (2019): The metabolic syndrome – What is it and how should it be managed? Eur J Prev Cardiol 26:33–46. [DOI] [PubMed] [Google Scholar]
- 69.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. (2008): The metabolic syndrome. Endocr Rev 29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ-H (2020): Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr 23:189–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghazarian M, Revelo XS, Nøhr MK, Luck H, Zeng K, Lei H, et al. (2017): Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci Immunol 2:eaai7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu HC, Mountz JD (2010): Metabolic syndrome, hormones, and maintenance of T cells during aging. Curr Opin Immunol 22:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, et al. (2012): Effector Memory T cells Are Associated with Atherosclerosis in Humans and Animal Models. J Am Heart Assoc 1:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A (2019): T cells as an emerging target for chronic pain therapy. Front Mol Neurosci 12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bethea JR, Fischer R (2021): Role of peripheral immune cells for development and recovery of chronic pain. Front Immunol 12: 641588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding YQ, Luo H, Qi JG (2020): MHCII-restricted T helper cells: An emerging trigger for chronic tactile allodynia after nerve injuries. J Neuroinflammation 17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galvin DA, McCrory C (2021): The role of T-lymphocytes in neuropathic pain initiation, development of chronicity and treatment. Brain Behav Immun Health 18:100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorge RE, Totsch SK (2017): Sex differences in pain. J Neurosci Res 95:1271–1281. [DOI] [PubMed] [Google Scholar]
- 79.Mapplebeck JCS, Beggs S, Salter MW (2016): Sex differences in pain: A tale of two immune cells. Pain 157(suppl 1):S2–S6. [DOI] [PubMed] [Google Scholar]
- 80.Dumont V, Lehtonen S (2022): PACSIN proteins in vivo: Roles in development and physiology. Acta Physiol (Oxf) 234:e13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ürel-Demir G, Şimşek-Kiper PÖ, Öncel İ, Utine GE, Haliloğlu G, Boduroğlu K (2021): Natural history of TRPV4-Related disorders: From skeletal dysplasia to neuromuscular phenotype. Eur J Paediatr Neurol 32:46–55. [DOI] [PubMed] [Google Scholar]
- 82.Maglie R, Souza Monteiro de Araujo D, Antiga E, Geppetti P, Nassini R, De Logu F (2021): The role of TRPA1 in skin physiology and pathology. Int J Mol Sci 22:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Q, Henry G, Chen Y (2021): Emerging role of transient receptor potential vanilloid 4 (TRPV4) ion channel in acute and chronic itch. Int J Mol Sci 22:7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mack MR, Kim BS (2018): The itch–scratch cycle: A neuroimmune perspective. Trends Immunol 39:980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fozzato S, Baranzini N, Bossi E, Cinquetti R, Grimaldi A, Campomenosi P, Surace MF (2021): TRPV4 and TRPM8 as putative targets for chronic low back pain alleviation. Pflugers Arch 473:151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin JG, Hsieh CL, Lin YW (2015): Analgesic effect of electro-acupuncture in a mouse fibromyalgia model: Roles of TRPV1, TRPV4, and pERK. PLoS One 10:e0128037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McNulty AL, Leddy HA, Liedtke W, Guilak F (2015): TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch Pharmacol 388:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Egami S, Yamagami J, Amagai M (2020): Autoimmune bullous skin diseases, pemphigus and pemphigoid. J Allergy Clin Immunol 145:1031–1047. [DOI] [PubMed] [Google Scholar]
- 89.Patrício P, Ferreira C, Gomes MM, Filipe P (2009): Autoimmune bullous dermatoses: A review. Ann N Y Acad Sci 1173:203–210. [DOI] [PubMed] [Google Scholar]
- 90.Edwards G, Diercks GFH, Seelen MAJ, Horvath B, van Doorn MBA, Damman J (2019): Complement activation in autoimmune bullous dermatoses: A comprehensive review. Front Immunol 10:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saschenbrecker S, Karl I, Komorowski L, Probst C, Dähnrich C, Fechner K, et al. (2019): Serological diagnosis of autoimmune bullous skin diseases. Front Immunol 10:1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anzelc M, Burkhart CG (2020): Pain and pruritus: A study of their similarities and differences. Int J Dermatol 59:159–164. [DOI] [PubMed] [Google Scholar]
- 93.Orhan E, Velazquez C, Tabet I, Sardet C, Theillet C (2021): Regulation of RAD51 at the transcriptional and functional levels: What prospects for cancer therapy? Cancers 13:2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramanyam S, Ismail M, Bhattacharya I, Spies M (2016): Tyrosine phosphorylation stimulates activity of human RAD51 recombinase through altered nucleoprotein filament dynamics. Proc Natl Acad Sci USA 113:E6045–E6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Depienne C, Bouteiller D, Méneret A, Billot S, Groppa S, Klebe S, et al. (2012): RAD51 haploinsufficiency causes congenital mirror movements in humans. Am J Hum Genet 90:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grundy MK, Buckanovich RJ, Bernstein KA (2020): Regulation and pharmacological targeting of RAD51 in cancer. NAR Cancer 2:zcaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dean L (2012): Methylenetetrahydrofolate reductase deficiency. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, a MS, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries. Bethesda, (MD): National Center for Biotechnology Information (US). [PubMed] [Google Scholar]
- 98.Bolufer P, Barragan E, Collado M, Cervera J, López JA, Sanz MA (2006): Influence of genetic polymorphisms on the risk of developing leukemia and on disease progression. Leuk Res 30:1471–1491. [DOI] [PubMed] [Google Scholar]
- 99.Ansari R, Mahta A, Mallack E, Luo JJ (2014): Hyperhomocysteinemia and neurologic disorders: A review. J Clin Neurol 10:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zellweger R, Dalcher D, Mutreja K, Berti M, Schmid JA, Herrador R, et al. (2015): Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J Cell Biol 208:563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamm N, Maoz K, Bester AC, Im MM, Shewach DS, Karni R, Kerem B (2015): Folate levels modulate oncogene-induced replication stress and tumorigenicity. EMBO Mol Med 7:1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaifullina AS, Lazniewska J, Gerasimova EV, Burkhanova GF, Rzhepetskyy Y, Tomin A, et al. (2019): A potential role for T-type calcium channels in homocysteinemia-induced peripheral neuropathy. Pain 160:2798–2810. [DOI] [PubMed] [Google Scholar]
- 103.Brandt DT, Baarlink C, Kitzing TM, Kremmer E, Ivaska J, Nollau P, Grosse R (2009): SCAI acts as a suppressor of cancer cell invasion through the transcriptional control of β1-integrin. Nat Cell Biol 11:557–568. [DOI] [PubMed] [Google Scholar]
- 104.Chen X, Hu W, Xie B, Gao H, Xu C, Chen J (2014): Downregulation of SCAI enhances glioma cell invasion and stem cell like phenotype by activating Wnt/β-catenin signaling. Biochem Biophys Res Commun 448:206–211. [DOI] [PubMed] [Google Scholar]
- 105.Hong Q-X, Xu S-Y, Dai S-H, Zhao W-X (2016): Expression profiling of spinal genes in peripheral neuropathy model rats with type 2 diabetes mellitus. Int J Clin Exp Med 9:6376–6384. [Google Scholar]
- 106.Rubio-Aliaga I, Wagner CA (2016): Regulation and function of the SLC38A3/SNAT3 glutamine transporter. Channels (Austin) 10:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Albrecht J, Zielińska M (2019): Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology 161: 107560. [DOI] [PubMed] [Google Scholar]
- 108.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P (2018): Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 10:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith RJ (1990): Glutamine metabolism and its physiologic importance. JPEN J Parenter Enteral Nutr 14(suppl):40S–44S. [DOI] [PubMed] [Google Scholar]
- 110.Yoo HC, Yu YC, Sung Y, Han JM (2020): Glutamine reliance in cell metabolism. Exp Mol Med 52:1496–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shyer JA, Flavell RA, Bailis W (2020): Metabolic signaling in T cells. Cell Res 30:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jayakumar AR, Norenberg MD (2016): Glutamine synthetase: Role in neurological disorders. In: Schousboe A, Sonnewald U, editors. The Glutamate/GABA-Glutamine Cycle: Amino Acid Neurotransmitter Homeostasis. Cham, Germany: Springer International Publishing, 327–350. [Google Scholar]
- 113.Sandhu MRS, Gruenbaum BF, Gruenbaum SE, Dhaher R, Deshpande K, Funaro MC, et al. (2021): Astroglial glutamine synthetase and the pathogenesis of mesial temporal lobe epilepsy. Front Neurol 12:665334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y, Wang Q, Wang Y, Li J, Lu G, Liu Z (2019): Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ Health Prev Med 24:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aldana BI, Zhang Y, Jensen P, Chandrasekaran A, Christensen SK, Nielsen TT, et al. (2020): Glutamate-glutamine homeostasis is perturbed in neurons and astrocytes derived from patient iPSC models of frontotemporal dementia. Mol Brain 13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duba-Kiss R, Niibori Y, Hampson DR (2021): GABAergic gene regulatory elements used in adeno-associated viral vectors. Front Neurol 12:745159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maragakis NJ, Rothstein JD (2001): Glutamate transporters in neurologic disease. Arch Neurol 58:365–370. [DOI] [PubMed] [Google Scholar]
- 118.Reddy-Thootkur M, Kraguljac NV, Lahti AC (2022): The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders – A systematic review of magnetic resonance spectroscopy studies. Schizophr Res 249:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koshiyama D, Kirihara K, Tada M, Nagai T, Fujioka M, Ichikawa E, et al. (2018): Electrophysiological evidence for abnormal glutamate-GABA association following psychosis onset. Transl Psychiatry 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benarroch EE (2018): Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: Emerging mechanisms. Neurology 91:125–132. [DOI] [PubMed] [Google Scholar]
- 121.Lau A, Tymianski M (2010): Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542. [DOI] [PubMed] [Google Scholar]
- 122.Lin CL, Kong Q, Cuny GD, Glicksman MA (2012): Glutamate transporter EAAT2: A new target for the treatment of neurodegenerative diseases. Future Med Chem 4:1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnes JR, Mukherjee B, Rogers BC, Nafar F, Gosse M, Parsons MP (2020): The relationship between glutamate dynamics and activity-dependent synaptic plasticity. J Neurosci 40:2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peek AL, Rebbeck T, Puts NAJ, Watson J, Aguila M-ER, Leaver AM (2020): Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 210:116532. [DOI] [PubMed] [Google Scholar]
- 125.Cruz-Almeida Y, Forbes M, Cohen RC, Woods AJ, Fillingim RB, Riley JL, Porges ES (2021): Brain gamma-aminobutyric acid, but not glutamine and glutamate levels are lower in older adults with chronic musculoskeletal pain: Considerations by sex and brain location. PAIN Rep 6:e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cieri-Hutcherson NE, Hutcherson TC, Conway-Habes EE, Burns BN, White NA (2019): Systematic Review of l-glutamine for Prevention of vaso-occlusive Pain Crisis in Patients with sickle cell disease. Pharmacotherapy 39:1095–1104. [DOI] [PubMed] [Google Scholar]
- 127.Zunhammer M, Schweizer LM, Witte V, Harris RE, Bingel U, Schmidt-Wilcke T (2016): Combined glutamate and glutamine levels in pain-processing brain regions are associated with individual pain sensitivity. Pain 157:2248–2256. [DOI] [PubMed] [Google Scholar]
- 128.Bathel A, Schweizer L, Stude P, Glaubitz B, Wulms N, Delice S, Schmidt-Wilcke T (2018): Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain 19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson S, Wright F, Carden MA (2019): L-glutamine decreases opioid use in individuals with sickle cell disease and chronic pain: A case series. Blood 134(suppl 1):4849–4849. [Google Scholar]
- 130.Safikhani S, Gries KS, Trudeau JJ, Reasner D, Rüdell K, Coons SJ, et al. (2017): Response scale selection in adult pain measures: Results from a literature review. J Patient Rep Outcomes 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haefeli M, Elfering A (2006): Pain assessment. Eur Spine J 15(suppl 1):S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nugent SM, Lovejoy TI, Shull S, Dobscha SK, Morasco BJ (2021): Associations of pain numeric rating scale scores collected during usual care with research administered patient reported pain outcomes. Pain Med 22:2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pilitsis JG Fahey M, Custozzo A, Chakravarthy K, Capobianco R (2021): Composite score is a better reflection of patient response to chronic pain therapy compared with pain intensity alone. Neuromodulation 24:68–75. [DOI] [PubMed] [Google Scholar]
- 134.Krebs EE, Carey TS, Weinberger M (2007): Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med 22:1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, Hróbjartsson A (2017): Pain relief that matters to patients: Systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Euasobhon P, Atisook R, Bumrungchatudom K, Zinboonyahgoon N, Saisavoey N, Jensen MP (2022): Reliability and responsivity of pain intensity scales in individuals with chronic pain. Pain 163:e1184–e1191. [DOI] [PubMed] [Google Scholar]
- 137.Majedi H, Mohammadi M, Tafakhori A, Khazaeipour Z (2020): The Influence of Chronic Pain on Number Sense and Numeric Rating Scale: A prospective Cohort Study. Anesth Pain Med 10:e103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Markman JD, Gewandter JS, Frazer ME (2020): Comparison of a pain tolerability question with the numeric rating scale for assessment of self-reported chronic pain. JAMA Netw Open 3:e203155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stompór M, Grodzicki T, Stompór T, Wordliczek J, Dubiel M, Kurowska I (2019): Prevalence of chronic pain, particularly with neuropathic component, and its effect on overall functioning of elderly patients. Med Sci Monit 25:2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rapti E, Damigos D, Apostolara P, Roka V, Tzavara C, Lionis C (2019): Patients with chronic pain: Evaluating depression and their quality of life in a single center study in Greece. BMC Psychol 7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nitzan U, Hecht M, Braw Y, Maoz H, Levkovitz Y, Yarnitsky D, et al. (2019): Initial evaluation of pain intensity among depressed patients as a possible mediator between depression and pain complaints. Front Psychiatry 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diener HC, Förderreuther S, Gaul C, Giese F, Hamann T, Holle-Lee D, et al. (2020): Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: Addition to the S1 guideline: Therapy of migraine attacks and prevention of migraine. Recommendations of the Germany society of neurology and the German Migraine and headache society. Neurol Res Pract 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ibekwe A (2016): Monoclonal antibodies to prevent migraine headaches. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. 167. [PubMed] [Google Scholar]
- 144.Vandervorst F, Van Deun L, Van Dycke A, Paemeleire K, Reuter U, Schoenen J, Versijpt J (2021): CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain 22:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vandenberg RJ, Ryan RM, Carland JE, Imlach WL, Christie MJ (2014): Glycine transport inhibitors for the treatment of pain. Trends Pharmacol Sci 35:423–430. [DOI] [PubMed] [Google Scholar]
- 146.Imlach WL (2017): New approaches to target glycinergic neurotransmission for the treatment of chronic pain. Pharmacol Res 116:93–99. [DOI] [PubMed] [Google Scholar]
- 147.Hsiao HT, Lin YC, Wang JC-F, Tsai YC, Liu YC (2016): Hypoxia inducible factor-1α inhibition produced anti-allodynia effect and suppressed inflammatory cytokine production in early stage of mouse complex regional pain syndrome model. Clin Exp Pharmacol Physiol 43:355–359. [DOI] [PubMed] [Google Scholar]
- 148.Klivinyi C, Rumpold-Seitlinger G, Dorn C, Sampl L, Sivro N, Lang-Illievich K, et al. (2021): Perioperative use of physostigmine to reduce opioid consumption and peri-incisional hyperalgesia: A randomised controlled trial. Br J Anaesth 126:700–705. [DOI] [PubMed] [Google Scholar]
- 149.García G, Martínez-Rojas VA, Murbartián J (2021): TREK-1 potassium channels participate in acute and long-lasting nociceptive hypersensitivity induced by formalin in rats. Behav Brain Res 413:113446. [DOI] [PubMed] [Google Scholar]
- 150.Hirakawa T, Nasu K, Aoyagi Y, Takebayashi K, Narahara H (2017): Arcyriaflavin a, a cyclin D1–cyclin-dependent kinase4 inhibitor, induces apoptosis and inhibits proliferation of human endometriotic stromal cells: A potential therapeutic agent in endometriosis. Reprod Biol Endocrinol 15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang Q, Liu Q, Ouyang D (2019): Sorbinil, an aldose reductase inhibitor, in Fighting Against Diabetic Complications. Med Chem 15:3–7. [DOI] [PubMed] [Google Scholar]
- 152.Paul A, Kumar M, Das P, Guha N, Rudrapal M, Zaman MdK (2022): Drug repurposing – A search for novel therapy for the treatment of diabetic neuropathy. Biomed Pharmacother 156:113846. [DOI] [PubMed] [Google Scholar]
- 153.Bhat RA, Lingaraju MC, Pathak NN, Kalra J, Kumar D, Kumar D, Tandan SK (2016): Effect of ursolic acid in attenuating chronic constriction injury-induced neuropathic pain in rats. Fundam Clin Pharmacol 30:517–528. [DOI] [PubMed] [Google Scholar]
- 154.Gabrielsson L, Mattsson S, Fowler CJ (2016): Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br J Clin Pharmacol 82:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ntalouka F, Tsirivakou A (2023): Luteolin: A promising natural agent in management of pain in chronic conditions. Front Pain Res (Lausanne) 4:1114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borges JP, Mekhail K, Fairn GD, Antonescu CN, Steinberg BE (2021): Modulation of pathological pain by epidermal growth factor receptor. Front Pharmacol 12:642820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bahl E, Koomar T, Michaelson JJ (2017): cerebroViz: An R package for anatomical visualization of spatiotemporal brain data. Bioinformatics 33:762–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.