Key Points
Question
Is treatment with a coronary paclitaxel-coated balloon superior to an uncoated balloon for 1-year target lesion failure in patients undergoing percutaneous coronary intervention for in-stent restenosis?
Findings
In a multicenter randomized trial of 600 patients designed to support US regulatory approval, target lesion failure was significantly lower in the paclitaxel-coated balloon group (17.9%) compared with the uncoated balloon group (28.6%) (P = .003). Ischemia-driven target lesion revascularization and target vessel myocardial infarction were also lower after treatment with a paclitaxel-coated balloon.
Meaning
Treatment with a paclitaxel-coated balloon offers an effective treatment strategy for the management of coronary in-stent restenosis.
Abstract
Importance
Drug-coated balloons offer a potentially beneficial treatment strategy for the management of coronary in-stent restenosis. However, none have been previously evaluated or approved for use in coronary circulation in the United States.
Objective
To evaluate whether a paclitaxel-coated balloon is superior to an uncoated balloon in patients with in-stent restenosis undergoing percutaneous coronary intervention.
Design, Setting, and Participants
AGENT IDE, a multicenter randomized clinical trial, enrolled 600 patients with in-stent restenosis (lesion length <26 mm and reference vessel diameter >2.0 mm to ≤4.0 mm) at 40 centers across the United States between May 2021 and August 2022. One-year clinical follow-up was completed on October 2, 2023.
Interventions
Participants were randomized in a 2:1 allocation to undergo treatment with a paclitaxel-coated (n = 406) or an uncoated (n = 194) balloon.
Main Outcomes and Measures
The primary end point of 1-year target lesion failure—defined as the composite of ischemia-driven target lesion revascularization, target vessel–related myocardial infarction, or cardiac death—was tested for superiority.
Results
Among 600 randomized patients (mean age, 68 years; 157 females [26.2%]; 42 Black [7%], 35 Hispanic [6%] individuals), 574 (95.7%) completed 1-year follow-up. The primary end point at 1 year occurred in 17.9% in the paclitaxel-coated balloon group vs 28.6% in the uncoated balloon group, meeting the criteria for superiority (hazard ratio [HR], 0.59 [95% CI, 0.42-0.84]; 2-sided P = .003). Target lesion revascularization (13.0% vs 24.7%; HR, 0.50 [95% CI, 0.34-0.74]; P = .001) and target vessel–related myocardial infarction (5.8% vs 11.1%; HR, 0.51 [95% CI, 0.28-0.92]; P = .02) occurred less frequently among patients treated with paclitaxel-coated balloon. The rate of cardiac death was 2.9% vs 1.6% (HR, 1.75 [95% CI, 0.49-6.28]; P = .38) in the coated vs uncoated balloon groups, respectively.
Conclusions and Relevance
Among patients undergoing coronary angioplasty for in-stent restenosis, a paclitaxel-coated balloon was superior to an uncoated balloon with respect to the composite end point of target lesion failure. Paclitaxel-coated balloons are an effective treatment option for patients with coronary in-stent restenosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT04647253
This clinical trial compares a paclitaxel-coated balloon vs an uncoated balloon in patients with in-stent restenosis undergoing percutaneous coronary intervention.
Introduction
Millions of individuals undergo percutaneous coronary intervention worldwide each year, with most receiving treatment with 1 or more drug-eluting stents.1,2 Despite improvements in drug-eluting stent technology that have led to a decreased risk of in-stent restenosis, neointimal hyperplasia and neoatherosclerosis3 still occur within drug-eluting stents, leading to the recurrence of chronic and acute coronary syndromes and necessitating further revascularization procedures when symptoms recur. The incidence of in-stent restenosis is encountered frequently in the United States, occurring in approximately 5% to 10% of patients undergoing percutaneous coronary intervention with drug-eluting stents in the first year.4
Current treatment of coronary in-stent restenosis in the United States commonly involves angioplasty followed by the placement of additional drug-eluting stents, which have been demonstrated to be superior to balloon angioplasty and vascular brachytherapy in reducing subsequent restenosis.5,6,7,8,9 Drug-coated balloons, designed to administer antiproliferative agents to coronary lesions without the use of a metallic stent, were developed as an alternative treatment for coronary artery disease, and specifically for in-stent restenosis, where the introduction of additional layers of stent that further reduce lumen area may be undesirable. While drug-coated balloons currently have a class IA recommendation for the treatment of coronary restenosis in European guidelines,10 prior randomized trials evaluating their use have had small sample sizes,5,11,12,13,14 and none have been conducted in the United States. Consequently, no drug-coated balloons are currently approved in the United States for use in coronary arteries.
The AGENT IDE trial is a multicenter randomized trial conducted to evaluate the treatment of coronary in-stent restenosis by coronary angioplasty using a paclitaxel-coated balloon vs an uncoated balloon.
Methods
Study Design and Oversight
The AGENT IDE (A Clinical Trial to Assess the Agent Paclitaxel Coated PTCA Balloon Catheter for the Treatment of Subjects With In-Stent Restenosis) trial is a multicenter, single-blind, randomized controlled, superiority trial comparing angioplasty with the AGENT paclitaxel-coated balloon (Boston Scientific Corp) vs an uncoated balloon in patients with coronary in-stent restenosis.15 The trial was designed and conducted in consultation with the Food and Drug Administration to support a premarket application for the AGENT drug-coated balloon in the United States and was sponsored and funded by Boston Scientific Corp. The protocol (available in Supplement 1) was approved by the institutional review boards for each site. The trial was performed in accordance with the principles of the Declaration of Helsinki, and the study design adhered to the CONSORT 2010 Statement for randomized clinical trials. An independent steering committee and data and safety monitoring committee oversaw trial progress; an independent clinical events committee adjudicated key clinical outcomes.
Patient Selection
Patients were eligible for enrollment if they had in-stent restenosis with visually estimated reference vessel diameter greater than 2.0 mm to up to 4.0 mm and length less than 26 mm, and had target lesion stenosis of less than 100% but greater than 50% (in symptomatic patients) or greater than 70% (in asymptomatic patients). Key exclusion criteria included recent ST-elevation or Q-wave myocardial infarction, unprotected left main disease, saphenous vein or arterial graft disease, left ventricular ejection fraction less than 25%, or thrombus in the target vessel. All patients provided signed written informed consent. Additional inclusion and exclusion criteria are provided in eTables 3 and 4 in Supplement 2. Race and ethnicity were evaluated to assess the generalizability of the enrolled study population to real-world practice, and were collected using self-reporting or self-identification based on fixed categories.
Randomization and Treatment
Patients who satisfied the study selection criteria and underwent successful predilation of the target lesion were randomized in a 2:1 ratio to receive either the paclitaxel-coated balloon or an uncoated balloon (Figure 1). Successful predilation was defined as dilation with a balloon catheter of appropriate length and diameter, or pretreatment with directional or rotational coronary atherectomy, laser, or cutting/scoring balloon with no greater than 50% residual stenosis and no dissection greater than National Heart, Lung, and Blood Institute type C. Randomization was stratified by center and whether the target lesion had a single layer vs multiple stent layers of prior stents. Only 1 target lesion was eligible for randomization for each patient. A single nontarget lesion in a nontarget vessel could undergo percutaneous coronary intervention as determined by the operator prior to randomization. Patients, core laboratory personnel, and the clinical events committee remained blinded to the assigned treatment. Operators were not blinded to treatment due to the different packaging of the paclitaxel-coated vs uncoated balloons. The biostatistician remained blinded until analysis of the primary end point.
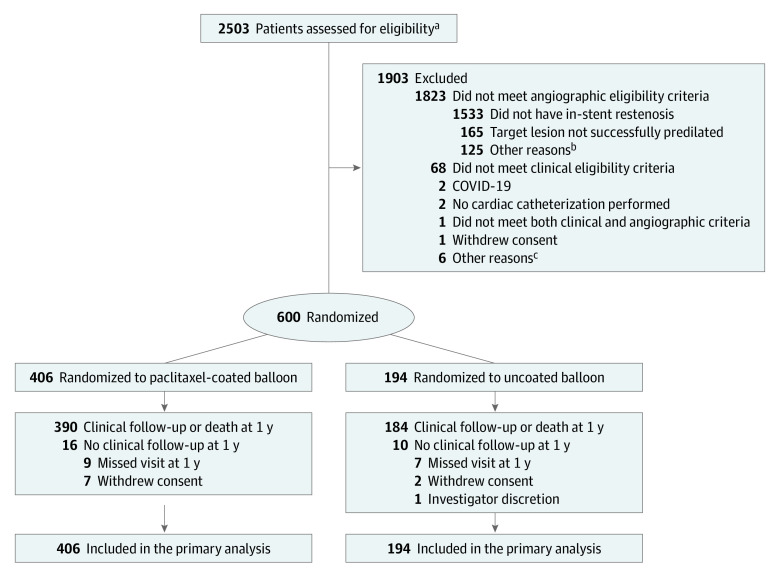
Figure 1. Enrollment, Randomization, and Follow-Up in the AGENT IDE Trial.
aPatients assessed for eligibility were those with suspected in-stent restenosis who provide informed consent to participate in the trial.
bPatients did not meet additional angiographic criteria listed in eTables 3 and 4 in Supplement 2.
cNo additional information available.
Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor was prescribed for at least 1 month post procedure regardless of the randomized assignment. Antiplatelet monotherapy was continued thereafter for the duration of the study.
Clinical Follow-Up and Outcomes
Clinical follow-up was performed in hospital at 30 days, 6 months, and 12 months and will continue through 5 years. The primary end point was the rate of 12-month target lesion failure, defined as the composite occurrence of ischemia-driven target lesion revascularization, target vessel–related myocardial infarction, or cardiac death. Periprocedural myocardial infarction occurring within 48 hours of the index procedure was adjudicated by the Society for Cardiovascular Angiography and Interventions definition,16 and spontaneous myocardial infarction occurring 48 hours after the index procedure was adjudicated according to the fourth universal definition of myocardial infarction.17 Additional prespecified end points were individual components of the primary end point, all-cause death, target vessel revascularization, target vessel failure (defined as the composite of ischemia-driven target vessel revascularization), myocardial infarction related to the target vessel or cardiac death, and stent thrombosis (as defined by the Academic Research Consortium).18 Periprocedural end points included the rates of technical and procedural success. Health status was measured using the EuroQol-5 Dimension Questionnaire scores.19 The definitions of all outcome measures are provided in the study protocol (Supplement 1).
Statistical Analysis
This study used an adaptive group sequential design20 with an initial planned enrollment of 480 patients and 1 formal interim analysis on the 1-year data after randomization of the first 90% of patients, with the expectation that the first 40% of randomized patients would have 1-year follow-up at that time (details provided in Supplement 1). With a sample size of 480 patients, the study had 85% power to demonstrate superiority (1-sided P < .025) of the paclitaxel-coated balloon over the uncoated balloon, assuming respective event rates of 10.6% and 21.2%, with an expected attrition rate of 3%.15 Given uncertainty in the accuracy of these estimated event rates, the interim analysis was prespecified to be performed by the data and safety monitoring committee for potential sample size re-estimation of up to 600 patients.15 Due to rapid enrollment in the trial, no patients had completed 1-year follow-up at the time of the planned interim analysis, and therefore the data and safety monitoring committee recommended to continue enrollment to the maximum of 600 patients. The trial enrolled 600 patients at 40 centers in the United States between May 2021 and August 2022; 1-year clinical follow-up was completed on October 2, 2023. However, to determine final sample size for the primary end point analysis, the Food and Drug Administration proposed that the data and safety monitoring committee perform an interim analysis on the first 40% of patients (n = 192) with 1-year data when those data were available using the prespecified adaptive design strategy. Based on this analysis conducted on April 18, 2023, the data and safety monitoring committee recommended conducting the primary end point analysis on the first 480 patients, consistent with the original adaptive design strategy for the trial. These analyses can be found in eTable 7 in Supplement 2). The results for the full 600-patient cohort are presented within this article.
The primary analysis was performed in the intention-to-treat population irrespective of whether the assigned treatment was received. Continuous variables are presented as means (SDs) and differences between groups tested with the t test. Discrete variables are reported as percentages and counts, and differences between groups were assessed by the χ2 or Fisher exact test, as appropriate. A z test with unpooled variance for the difference of 2 proportions was used to test the primary end point hypothesis of superiority of paclitaxel-coated balloon over the uncoated balloon. Differences in the rates of the primary end point and additional prespecified outcomes are reported using survival methodology as cumulative incidence rates and associated hazard ratios (HRs) with 95% CIs. Prespecified subgroup analyses of the primary outcome were analyzed using Cox regression models that included subgroup by treatment group interaction terms. All statistical analyses were performed using SAS version 9.4 or later (SAS Institute Inc).
Results
Patients and Enrollment
A total of 600 patients were randomly assigned to treatment with the paclitaxel-coated balloon (406 patients) vs uncoated balloon (194 patients). After randomization, 16 patients were lost to follow-up, 9 withdrew consent for participation, and 1 was excluded due to investigator discretion (Figure 1). The 1-year primary analysis included data from 600 randomized patients (Figure 1). Baseline characteristics were well balanced between the groups (Table 1). The mean age of patients was 68 years, and 26.2% patients were female. The patients had high rates of coronary risk factors including a 50.7% prevalence of diabetes. Coronary artery disease was extensive, with 78.9% having a history of multivessel coronary artery disease and 30.1% with a history of coronary artery bypass grafting. The index clinical presentation was non–ST-elevation acute coronary syndrome in 37.0% of patients.
Table 1. Baseline Clinical and Lesion Characteristics of the Patientsa.
| Clinical characteristic | Paclitaxel-coated balloon (n = 406) | Uncoated balloon (n = 194) |
|---|---|---|
| Age, mean (SD), y | 68.4 (9.8) | 67.9 (9.7) |
| Sex | ||
| Male | 302 (74.4) | 141 (72.7) |
| Female | 104 (25.6) | 53 (27.3) |
| Race and ethnicityb | ||
| American Indian or Alaska Native | 0 | 1 (0.5) |
| Asian | 9 (2.2) | 6 (3.1) |
| Black, of African heritage | 32 (7.9) | 10 (5.2) |
| Hispanic or Latino | 26 (6.4) | 9 (4.6) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2) | 1 (0.5) |
| White | 304 (74.9) | 148 (76.3) |
| Not disclosed | 23 (5.7) | 18 (9.3) |
| Otherc | 16 (3.9) | 3 (1.5) |
| Body mass index, mean (SD) [No.]d | 30.0 (5.5) [396] | 30.1 (5.9) [191] |
| Currently smokes | 42/406 (10.3) | 19/194 (9.8) |
| Medical history | ||
| Hypertension | 383/405 (94.6) | 186/194 (95.9) |
| Hyperlipidemia | 382/404 (94.6) | 184/194 (94.8) |
| Multivessel coronary artery disease | 317/400 (79.3) | 149/190 (78.4) |
| Current diabetes | 206/404 (51.0) | 97/194 (50.0) |
| Myocardial infarction | 198/398 (49.7) | 95/190 (50.0) |
| Coronary artery bypass graft surgery | 124/403 (30.8) | 55/192 (28.6) |
| Insulin-treated diabetes | 93/404 (23.0) | 49/194 (25.3) |
| Congestive heart failure | 92/401 (22.9) | 41/192 (21.4) |
| Left main coronary artery disease | 89/394 (22.6) | 39/188 (20.7) |
| Peripheral vascular disease | 78/401 (19.5) | 31/192 (16.1) |
| Kidney disease | 74/402 (18.4) | 32/193 (16.6) |
| Transient ischemic attack or cerebrovascular accident | 57/402 (14.2) | 19/194 (9.8) |
| Indication for procedure | ||
| Stable angina | 225 (55.4) | 100 (51.5) |
| Non–ST-elevation acute coronary syndrome | 149 (36.7) | 73 (37.6) |
| Silent ischemia | 7 (1.7) | 5 (2.6) |
| Other indicatione | 25 (6.2) | 16 (8.2) |
| Lesion characteristicsf | ||
| Stent layer in target lesiong | ||
| Single | 230 (56.7) | 112 (57.7) |
| Multiple | 176 (43.3) | 82 (42.3) |
| Target lesion vessel | ||
| Right coronary artery | 156 (38.4) | 69 (35.6) |
| Left anterior descending artery | 141 (34.7) | 69 (35.6) |
| Left circumflex artery | 98 (24.1) | 47 (24.2) |
| Left main coronary artery | 11 (2.7) | 9 (4.6) |
| Lesion length, mean (SD), mm [No.] | 12.8 (6.3) [404] | 11.8 (6.6) [188] |
| Reference vessel diameter, mean (SD), mm [No.] | 2.7 (0.5) [405] | 2.7 (0.5) [192] |
| In-lesion minimum lumen diameter, mean (SD), mm [No.] | 1.0 (0.4) [405] | 0.9 (0.4) [191] |
| In-lesion diameter stenosis, mean (SD), % [No.] | 65.0 (12.1) [405] | 66.4 (12.8) [191] |
| Mehran in-stent restenosis patternh | ||
| 1A (Articulation) | 0/403 | 0/189 |
| 1B (Margin) | 4/403 (1.0) | 2/189 (1.1) |
| 1C (Focal) | 148/403 (36.7) | 84/189 (44.4) |
| 1D (Multifocal) | 3/403 (0.7) | 2/189 (1.1) |
| 2 (Diffuse intrastent) | 230/403 (57.1) | 89/189 (47.1) |
| 3 (Diffuse proliferative) | 16/403 (4.0) | 9/189 (4.8) |
| 4 (Total occlusion) | 2/403 (0.5) | 3/189 (1.6) |
| Thrombolysis In Myocardial Infarction grade flow | ||
| 0 (Complete occlusion) | 8/405 (2.0) | 6/192 (3.1) |
| 1 (Some flow) | 2/405 (0.5) | 3/192 (1.6) |
| 2 (Partial flow) | 21/405 (5.2) | 8/192 (4.2) |
| 3 (Normal flow) | 374/405 (92.3) | 175/192 (91.1) |
All entries are No. (%) or No./total (%) [when N differs from column No. due to missing values], unless otherwise noted.
Data on race and ethnicity were collected in a combined format using self-reporting or self-identification. A patient may be identified in multiple ethnicity and race categories. The category terms were those used during data collection.
No additional information provided as determined by the site.
Body mass index is the weight in kilograms divided by height in meters squared.
Indicated for procedure due to cardiac conditions other than stable angina, non–ST-elevation acute coronary syndrome, or silent ischemia.
Baseline lesion characteristics were quantified by the angiographic core laboratory.
Site-reported values.
Mehran in-stent restenosis pattern proposed by Mehran et al.21
Baseline lesion characteristics were relatively similar between the 2 groups (Table 1). Multiple-layer in-stent restenosis was present in 43.0% of patients. Angiographic core laboratory–reported mean reference vessel diameter was similar in both groups (2.7 mm), and mean lesion length was 12.8 mm and 11.8 mm in the paclitaxel-coated balloon and uncoated balloon groups, respectively. Prior to randomization, lesions were treated with a combination of conventional angioplasty techniques (eTable 5 in Supplement 2); intravascular imaging use was performed in 73.8% of patients and well-balanced between groups. Clinical procedural success (based on site assessment including estimated diameter stenosis <30%) was 92.1% in the paclitaxel-coated balloon group and 88.7% in the uncoated balloon group (P = .17). The rates of technical success were also similar (paclitaxel-coated balloon group: 93.4%, uncoated balloon group: 89.7%; P = .12). Three patients in the paclitaxel-coated balloon group and 1 patient in the uncoated balloon group received bailout stents. Most patients continued dual antiplatelet therapy through 12 months (eTable 6 in Supplement 2). Specifically, the respective treatment rates with dual antiplatelet therapy to 6 and 12 months were 85.7% and 79.6% for the paclitaxel-coated balloon group and 83.2% and 77.8% for the uncoated balloon group, with no significant differences between groups.
Primary End Point and Components
Kaplan-Meier estimates of the primary composite end point and individual components in the full trial population are shown in Figure 2. At 1 year, the incidence of the primary end point of target lesion failure occurred in 17.9% of patients in the paclitaxel-coated balloon group and 28.6% patients in the uncoated balloon group (HR, 0.59 [95% CI, 0.42-0.84]; 2-sided P = .003 for superiority). Results were similar after adjustment for stratification variables using Cox regression (eTable 8 in Supplement 2) as well as after considering the competing risk of noncardiac death (eTable 9 in Supplement 2). Target lesion revascularization occurred less frequently in the paclitaxel-coated balloon group (13.0% vs 24.7%; HR, 0.50 [95% CI, 0.34-0.74]; P = .001), as did myocardial infarction related to the target vessel (5.8% vs 11.1%; HR, 0.51 [95% CI, 0.28-0.92]; P = .02). There was no difference in the incidence of cardiac death between the groups (paclitaxel-coated balloon, 2.9% vs uncoated balloon, 1.6%; HR, 1.75 [95% CI, 0.49-6.28]; P = .38).
Figure 2. One-Year Time-to-Event Curves for the Primary End Point and Individual Components.
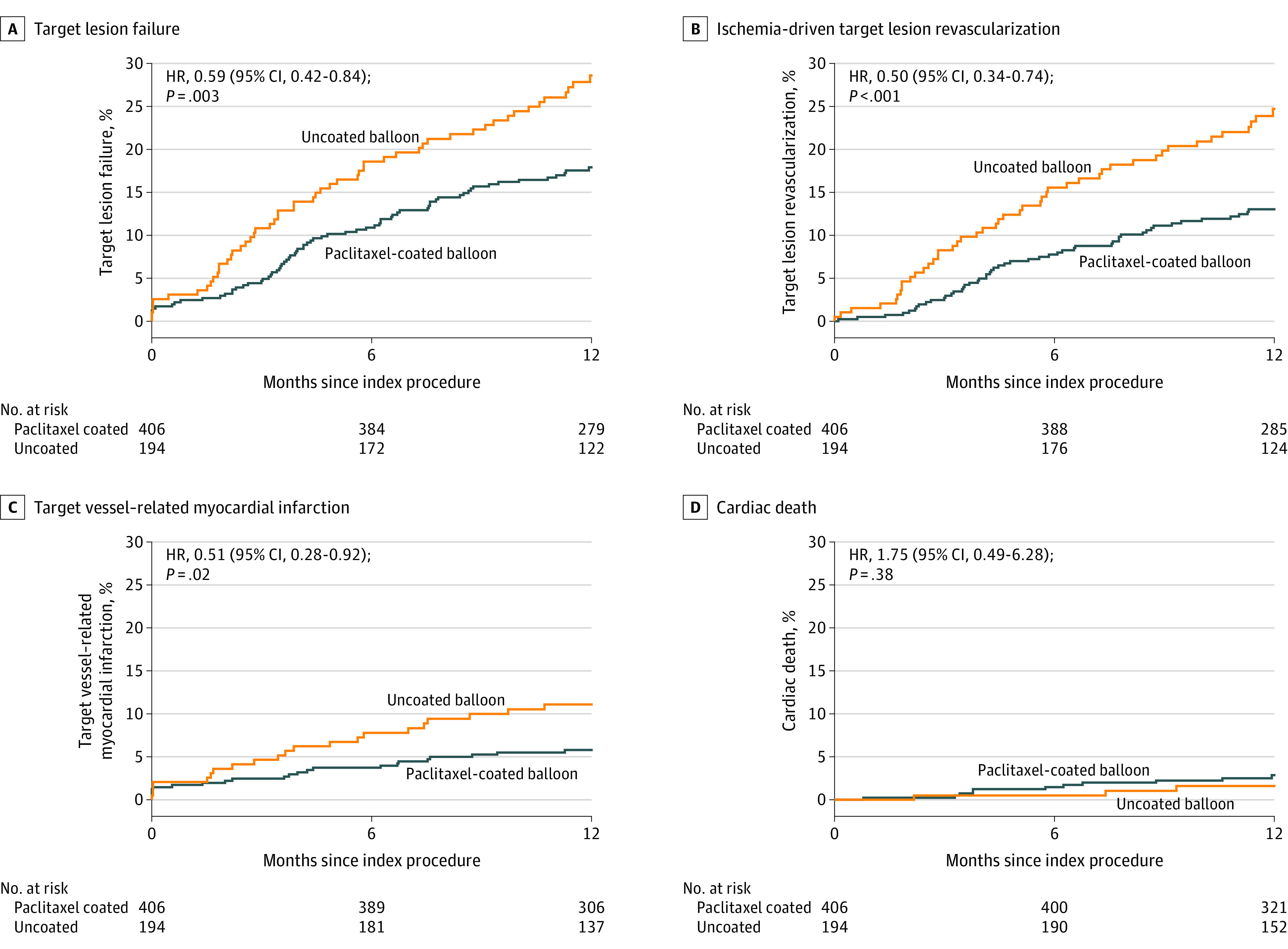
The primary end point of 1-year target lesion failure is defined as the composite occurrence of ischemia-driven target lesion revascularization, target vessel–related myocardial infarction, or cardiac death. The reported P values were obtained from Cox proportional hazard models. HR indicates hazard ratio; and MI, myocardial infarction.
Additional Outcomes and Subgroup Analyses
There were no occurrences of definite or probable stent thrombosis at 1 year in the paclitaxel-coated balloon group, while 6 patients in the uncoated balloon group experienced definite or probable stent thrombosis (Table 2). Quality-of-life outcomes were similar between the 2 groups through 1 year of follow-up (eTable 10 in Supplement 2).
Table 2. Prespecified Clinical Outcomes Through 1 Year.
| Clinical outcomesa | No. % | Difference (95% CI), % | HR (95% CI) | Log-rank P value | |
|---|---|---|---|---|---|
| Paclitaxel-coated balloon (n = 406) | Uncoated balloon (n = 194) | ||||
| Primary outcome | |||||
| Target lesion failureb | 71 (17.9) | 54 (28.6) | −10.7 (−18.2 to −3.2) | 0.59 (0.42 to 0.84) | .003 |
| Additional prespecified outcomes | |||||
| Target lesion revascularizationc | |||||
| Overall | 51 (13.0) | 46 (24.7) | −11.6 (−18.7 to −4.5) | 0.50 (0.34 to 0.74) | .001 |
| PCI | 39 (10.0) | 43 (22.8) | −12.9 (−19.6 to −6.1) | 0.41 (0.26 to 0.63) | <.001 |
| CABG | 13 (3.3) | 8 (4.5) | −1.2 (−4.8 to 2.4) | 0.77 (0.32 to 1.86) | .56 |
| Target vessel revascularizationc | |||||
| Overall | 56 (14.3) | 49 (26.2) | −11.8 (−19.1 to −4.6) | 0.51 (0.35 to 0.75) | .001 |
| PCI | 44 (11.3) | 46 (24.4) | −13.1 (−20.0 to −6.2) | 0.43 (0.28 to 0.64) | <.001 |
| CABG | 14 (3.6) | 8 (4.5) | −0.9 (−4.5 to 2.7) | 0.83 (0.35 to 1.98) | .68 |
| Target vessel revascularization not in target lesionc,d | |||||
| Overall | 20 (5.1) | 13 (6.9) | −1.8 (−6.0 to 2.4) | 0.72 (0.36 to 1.46) | .36 |
| PCI | 17 (4.3) | 11 (5.8) | −1.5 (−5.3 to 2.4) | 0.73 (0.34 to 1.55) | .41 |
| CABG | 4 (1.0) | 3 (1.6) | −0.6 (−2.6 to 1.5) | 0.64 (0.14 to 2.85) | .55 |
| Target vessel failuree | 73 (18.4) | 57 (30.1) | −11.7 (−19.3 to −4.1) | 0.57 (0.40 to 0.81) | .001 |
| Myocardial infarction | 29 (7.5) | 23 (12.1) | −4.7 (−10.0 to 0.7) | 0.58 (0.34 to 1.01) | .05 |
| Related to target vessel | 23 (5.8) | 21 (11.1) | −5.3 (−10.3 to −0.2) | 0.51 (0.28 to 0.92) | .02 |
| Q-wave myocardial infarction | 1 (0.3) | 1 (0.5) | −0.3 (−1.4 to 0.9) | 0.48 (0.03 to 7.64) | .59 |
| Related to target vessel | 0 | 1 (0.5) | −0.5 (−1.5 to 0.5) | NA | .15 |
| Non–Q-wave myocardial infarction | 28 (7.2) | 22 (11.6) | −4.4 (−9.7 to 0.9) | 0.59 (0.34 to 1.03) | .06 |
| Related to target vessel | 23 (5.8) | 20 (10.6) | −4.8 (−9.7 to 0.2) | 0.54 (0.30 to 0.98) | .04 |
| Related to target vessel-unknownf | 11 (2.8) | 4 (2.2) | 0.7 (−2.0 to 3.3) | 1.32 (0.42 to 4.16) | .63 |
| Not related to target vessel | 5 (1.4) | 2 (1.1) | 0.4 (−1.5 to 2.3) | 1.19 (0.23 to 6.12) | .84 |
| All deathg | 16 (4.1) | 7 (3.7) | 0.4 (−3.0 to 3.7) | 1.09 (0.45 to 2.65) | .85 |
| Cardiac | 11 (2.9) | 3 (1.6) | 1.3 (−1.2 to 3.7) | 1.75 (0.49 to 6.28) | .38 |
| Noncardiac | 5 (1.3) | 4 (2.2) | −0.9 (−3.3 to 1.5) | 0.60 (0.16 to 2.22) | .43 |
| Stent thrombosisg | 1 (0.4) | 7 (3.7) | −3.3 (−6.1 to −0.5) | 0.07 (0.01 to 0.55) | .001 |
| Definite or probable | 0 | 6 (3.2) | −3.2 (−5.7 to −0.7) | NA | <.001 |
| Definite | 0 | 6 (3.2) | −3.2 (−5.7 to −0.7) | NA | <.001 |
| Probable | 0 | 0 | 0.0 (0.0 to 0.0) | NA | Undefined |
| Possible | 1 (0.4) | 1 (0.5) | −0.2 (−1.4 to 1.1) | 0.48 (0.03 to 7.63) | .59 |
Abbreviations: CABG, coronary artery bypass graft surgery; HR, hazard ratio; NA, not available; PCI, percutaneous coronary intervention.
Rates of outcomes with their associated HRs and 95% CIs were calculated using survival methodology.
Defined as any ischemia-driven revascularization of the target lesion, myocardial infarction (Q-wave and non–Q-wave) related to the target vessel, or cardiac death. If it could not be determined with certainty whether the myocardial infarction was related to the target vessel, it was considered a target lesion failure.
The numbers may not equal the overall totals because patients could have multiple events.
Refers to lesions more than 5 mm from the stent borders, but within the same major coronary vessel.
Defined as any ischemia-driven revascularization of the target vessel, myocardial infarction (Q-wave and non–Q-wave) related to the target vessel, or cardiac death. If it could not be determined with certainty whether the myocardial infarction was related to the target vessel by the clinical events committee, it is considered a target vessel failure.
All unknown non–Q-wave myocardial infarction counted as related to target vessel.
As defined by the Academic Research Consortium.18
The results of the primary end point analyses in the prespecified subgroups are shown in Figure 3. The results were largely consistent across prespecified subgroups: the relative risk of target lesion failure was lower in patients treated with a paclitaxel-coated balloon than an uncoated balloon. Among patients with multiple stent layers, the rate of target lesion failure was 23.8% in the paclitaxel-coated balloon group and 40.0% in the uncoated balloon group (HR, 0.55 [95% CI, 0.34-0.87]; P = .009). Among patients with diabetes, the rates of target lesion failure were 21.6% in the paclitaxel-coated balloon group vs 29.2% in the uncoated balloon group (HR, 0.71 [95% CI, 0.43-1.17]; P = .18).
Figure 3. Prespecified Subgroup Analyses of the Primary Outcome at 1 Year.
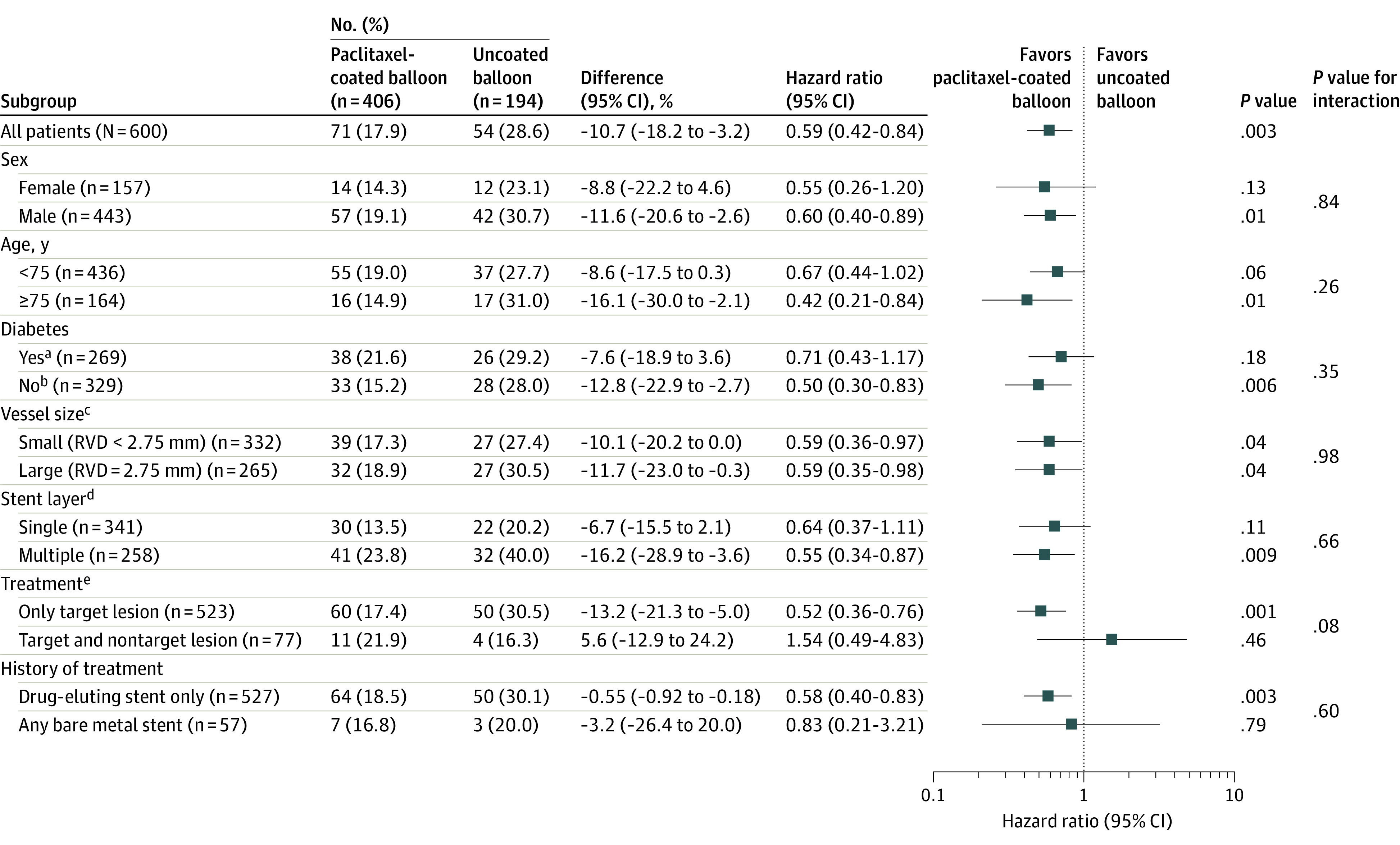
Shown is the forest plot of the hazard ratio for the primary outcome of 1-year target lesion failure (composite of ischemia-driven revascularization of the target lesion, myocardial infarction related to the target vessel, or cardiac death). RVD indicates reference vessel diameter.
aDiabetic subgroup includes patients with diabetes requiring medical treatment (oral or injection) for control of blood glucose levels.
bNondiabetic subgroup includes patients with diabetes treated with diet only or patients without diabetes.
cReference vessel diameter (RVD) is based on angiographic core laboratory data.
dRefers to patients with single stent layer treatment or patients with multiple stent layer treatment.
eRefers to patients with target lesion treatment only or patients with both target and nontarget lesion treatment.
Discussion
This trial is the largest randomized clinical trial to date examining the efficacy and safety of a drug-coated balloon in patients with coronary in-stent restenosis, and the first trial of coronary drug-coated balloons to be conducted in the United States. Patients treated with a paclitaxel-coated balloon had a lower rate of target lesion failure, largely driven by a lower rate of ischemia-driven revascularization and target vessel myocardial infarction, compared with patients treated with an uncoated balloon.
Drug-coated balloons have emerged internationally as an alternative treatment option that may provide the benefits of localized drug delivery to reduce neointimal growth, while avoiding the placement of additional stent layers within existing stents. These devices have been approved internationally for use, principally on the basis of smaller randomized trials.11,12,13,14 Paclitaxel-coated balloons were previously shown to reduce restenosis compared with balloon angioplasty among 52 patients randomized at 5 centers in Germany.11 Additionally, the ISAR-DESIRE 3 trial that was conducted at 3 German sites demonstrated significantly reduced occurrence of repeat revascularization through 10 years of follow-up in patients treated with a paclitaxel-coated balloon (n = 137 patients) compared with an uncoated balloon (n = 134 patients).22
Despite these promising international data, drug-coated balloons have not been previously evaluated or approved for use in the United States for various reasons, including the challenge and cost of conducting adequately powered randomized trials needed to support regulatory approval as well as significant improvements in drug-eluting stent technology. The introduction of drug-eluting stents represented an important breakthrough in device technology to limit the occurrence of in-stent restenosis, which previously occurred in 20% to 40% of patients treated with bare metal stents.23 However, there has been growing recognition that despite these advances in stent technology, patients with coronary in-stent restenosis continue to comprise approximately 10% of individuals undergoing percutaneous coronary intervention each year and have poor long-term outcomes.4,24 Due to the growing sentiment that drug-coated balloons could address an unmet clinical need among patients with coronary artery disease in the United States, the AGENT drug-coated balloon was designated as a Breakthrough Device by the Food and Drug Administration.
The AGENT drug-coated balloon catheter consists of a semicompliant balloon coated with a low-dose formation of paclitaxel and the excipient acetyl tri-n-butyl citrate that allows paclitaxel to be transferred to the vessel wall and dwell for an average of 90 days. The current trial randomized patients to treatment with either the paclitaxel-coated balloon or an uncoated balloon after successful angioplasty, with less than 50% residual stenosis and no significant coronary artery dissection. However, angioplasty without local administration of antiproliferative therapy can lead to localized neointimal hyperplasia that can result in coronary restenosis. In this trial, treatment with the paclitaxel-coated balloon lowered the rate of target lesion failure compared with an uncoated balloon, providing further evidence supporting the beneficial effects of localized drug delivery after angioplasty. In addition to a reduction in ischemia-driven target lesion revascularization, the rate of target vessel myocardial infarction at 1 year was 5.8% in the paclitaxel-coated balloon group compared with 11.1% in the uncoated balloon group, and no cases of definite or probable stent thrombosis occurred among the paclitaxel-coated balloon group compared with 6 cases (3.2%) within the uncoated balloon group. These data—in conjunction with the presentation with non–ST-elevation acute coronary syndrome in one-third of the enrolled cohort—support prior observations demonstrating that in-stent restenosis is not necessarily a benign or slowly progressive process.4,25
More than 40% of the patients in this trial had multiple prior stents at the site of the target lesion, representing a patient group at very high risk for recurrent restenosis.26 Rates of 1-year target lesion failure were nearly 2-fold greater in these patients compared with those who had previously had only a single layer of stent in the target lesion. Nevertheless, treatment with the paclitaxel-coated balloon in this high-risk group had a consistent relative risk reduction and numerically greater absolute risk reduction in events. A drug-coated balloon may be particularly useful in this setting, where additional stenting would result in 3 or more layers of stent that may further reduce lumen area and limit future therapeutic options by further constraining the vessel wall.
Limitations
This trial had several limitations. First, although patients and event adjudicators were not aware of the assigned treatment, operators were not blinded due to the different appearance of the paclitaxel-coated vs uncoated balloons. In addition, the study was conducted at high-volume sites and rates of intravascular ultrasound were higher than in conventional practice in the United States. Whether findings might differ at less-experienced centers and those with less-frequent intravascular imaging use is unknown. Second, a strategy of treatment with a drug-coated balloon is predicated on achievement of a successful conventional angioplasty without dissection or flow limitation (ie, a result that can be durable without the scaffolding provided by a stent). Third, an alternative design might have assessed noninferiority of the paclitaxel-coated balloon against a drug-eluting stent. A superiority assessment was conducted against an uncoated balloon because many patients, particularly those with multiple layers of stent as were enrolled in this trial, are treated with conventional angioplasty. There was a strong preference to include such patients in this trial because they represent a population with potentially the greatest need for new treatment options. This study does not answer the question of whether treatment with a drug-eluting stent or a drug-coated balloon might be the optimal approach for patients with in-stent restenosis. Fourth, the study was not powered to examine differences in treatment effect in potentially important subgroups and low-frequency events (ie, stent thrombosis).
Conclusions
Among patients undergoing coronary angioplasty for in-stent restenosis, a paclitaxel-coated balloon was superior to an uncoated balloon with respect to the composite end point of target lesion failure. Paclitaxel-coated balloons are an effective treatment option for patients with coronary in-stent restenosis.
Trial Protocol
eTable 1. AGENT IDE Investigators, Study Support, and Enrollment by Site Name
eTable 2. AGENT IDE Committees
eTable 3. Agent IDE Study Inclusion Criteria
eTable 4. Agent IDE Study Exclusion Criteria
eTable 5. Procedural and Postprocedural Outcomes
eTable 6. Antiplatelet Medication Usage Through 1-Year
eTable 7. One-Year Primary End Point Results in the Analysis Cohort
eTable 8. Stratified Primary End Point Results
eTable 9. Competing Risk Analysis for the Primary Endpoint
eTable 10. EQ-5D Scores Through 1-Year
eReferences
Nonauthor Collaborators. AGENT IDE Investigators
Data Sharing Statement
References
- 1.Inohara T, Kohsaka S, Spertus JA, et al. Comparative trends in percutaneous coronary intervention in Japan and the United States, 2013 to 2017. J Am Coll Cardiol. 2020;76(11):1328-1340. doi: 10.1016/j.jacc.2020.07.037 [DOI] [PubMed] [Google Scholar]
- 2.Timmis A, Townsend N, Gale CP, et al. ; European Society of Cardiology . European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12-85. doi: 10.1093/eurheartj/ehz859 [DOI] [PubMed] [Google Scholar]
- 3.Jinnouchi H, Kuramitsu S, Shinozaki T, et al. Difference of tissue characteristics between early and late restenosis after second-generation drug-eluting stents implantation: an optical coherence tomography study. Circ J. 2017;81(4):450-457. doi: 10.1253/circj.CJ-16-1069 [DOI] [PubMed] [Google Scholar]
- 4.Moussa ID, Mohananey D, Saucedo J, et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521-1531. doi: 10.1016/j.jacc.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Byrne RA, Neumann FJ, Mehilli J, et al. ; ISAR-DESIRE 3 investigators . Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381(9865):461-467. doi: 10.1016/S0140-6736(12)61964-3 [DOI] [PubMed] [Google Scholar]
- 6.Kufner S, Cassese S, Valeskini M, et al. ; ISAR-DESIRE 3 Investigators . Long-term efficacy and safety of paclitaxel-eluting balloon for the treatment of drug-eluting stent restenosis: 3-year results of a randomized controlled trial. JACC Cardiovasc Interv. 2015;8(7):877-884. doi: 10.1016/j.jcin.2015.01.031 [DOI] [PubMed] [Google Scholar]
- 7.Kastrati A, Mehilli J, von Beckerath N, et al. ; ISAR-DESIRE Study Investigators . Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293(2):165-171. doi: 10.1001/jama.293.2.165 [DOI] [PubMed] [Google Scholar]
- 8.Holmes DR Jr, Teirstein P, Satler L, et al. ; SISR Investigators . Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295(11):1264-1273. doi: 10.1001/jama.295.11.1264 [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Ellis SG, O’Shaughnessy CD, et al. ; TAXUS V ISR Investigators . Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253-1263. doi: 10.1001/jama.295.11.1253 [DOI] [PubMed] [Google Scholar]
- 10.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 11.Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113-2124. doi: 10.1056/NEJMoa061254 [DOI] [PubMed] [Google Scholar]
- 12.Hamm CW, Dörr O, Woehrle J, et al. A multicentre, randomised controlled clinical study of drug-coated balloons for the treatment of coronary in-stent restenosis. EuroIntervention. 2020;16(4):e328-e334. doi: 10.4244/EIJ-D-19-00051 [DOI] [PubMed] [Google Scholar]
- 13.Scheller B, Clever YP, Kelsch B, et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012;5(3):323-330. doi: 10.1016/j.jcin.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 14.Rittger H, Waliszewski M, Brachmann J, et al. Long-term outcomes after treatment with a paclitaxel-coated balloon versus balloon angioplasty: insights from the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] In-Stent Restenosis With SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc Interv. 2015;8(13):1695-1700. doi: 10.1016/j.jcin.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 15.Yeh RW, Bachinsky W, Stoler R, et al. Rationale and design of a randomized study comparing the agent drug coated balloon to plain old balloon angioplasty in patients with in-stent restenosis. Am Heart J. 2021;241:101-107. doi: 10.1016/j.ahj.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 16.Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62(17):1563-1570. doi: 10.1016/j.jacc.2013.08.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, et al. ; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231-2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med. 2011;30(28):3267-3284. doi: 10.1002/sim.4102 [DOI] [PubMed] [Google Scholar]
- 21.Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872-1878. doi: 10.1161/01.CIR.100.18.1872 [DOI] [PubMed] [Google Scholar]
- 22.Giacoppo D, Alvarez-Covarrubias HA, Koch T, et al. Coronary artery restenosis treatment with plain balloon, drug-coated balloon, or drug-eluting stent: 10-year outcomes of the ISAR-DESIRE 3 trial. Eur Heart J. 2023;44(15):1343-1357. doi: 10.1093/eurheartj/ehad026 [DOI] [PubMed] [Google Scholar]
- 23.Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030-1039. doi: 10.1056/NEJMoa067484 [DOI] [PubMed] [Google Scholar]
- 24.Tamez H, Secemsky EA, Valsdottir LR, et al. Long-term outcomes of percutaneous coronary intervention for in-stent restenosis among Medicare beneficiaries. EuroIntervention. 2021;17(5):e380-e387. doi: 10.4244/EIJ-D-19-01031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magalhaes MA, Minha S, Chen F, et al. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ Cardiovasc Interv. 2014;7(6):768-776. doi: 10.1161/CIRCINTERVENTIONS.114.001341 [DOI] [PubMed] [Google Scholar]
- 26.Yabushita H, Kawamoto H, Fujino Y, et al. Clinical outcomes of drug-eluting balloon for in-stent restenosis based on the number of metallic layers. Circ Cardiovasc Interv. 2018;11(8):e005935. doi: 10.1161/CIRCINTERVENTIONS.117.005935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. AGENT IDE Investigators, Study Support, and Enrollment by Site Name
eTable 2. AGENT IDE Committees
eTable 3. Agent IDE Study Inclusion Criteria
eTable 4. Agent IDE Study Exclusion Criteria
eTable 5. Procedural and Postprocedural Outcomes
eTable 6. Antiplatelet Medication Usage Through 1-Year
eTable 7. One-Year Primary End Point Results in the Analysis Cohort
eTable 8. Stratified Primary End Point Results
eTable 9. Competing Risk Analysis for the Primary Endpoint
eTable 10. EQ-5D Scores Through 1-Year
eReferences
Nonauthor Collaborators. AGENT IDE Investigators
Data Sharing Statement