Abstract
DNA supercoiling factor (SCF) was first identified in silkworm as a protein that generates negative supercoils in DNA in conjunction with eukaryotic topoisomerase II. To analyze the in vivo role of the factor, we cloned a cDNA encoding Drosophila melanogaster SCF. Northern analysis revealed 1.6- and 1.8-kb mRNAs throughout development. The longer mRNA contains an open reading frame that shares homology with mouse reticulocalbin whereas the shorter one encodes a truncated version lacking the N-terminal signal peptide-like sequence. An antibody against SCF detected a 45-kDa protein in the cytoplasmic fraction and a 30-kDa protein in the nuclear fraction of embryonic extracts. Immunoprecipitation suggests that the 30-kDa protein interacts with topoisomerase II in the nucleus, and hence that it is a functional form of SCF. Immunostaining of blastoderm embryos showed that SCF is present in nuclei during interphase but is excluded from mitotic chromosomes. In larvae, the antibody stained the nuclei of several tissues including a posterior part of the salivary gland. This latter staining was associated with natural or ecdysteroid-induced puffs on polytene chromosomes. Upon heat treatment of larvae, the staining on the endogenous puffs disappeared, and strong staining appeared on heat shock puffs. These results implicate SCF in gene expression.
Many biological processes that require unwinding or writhing of the DNA helix are thought to be facilitated by negative supercoiling of DNA. These processes include replication and transcription that require the unwinding of DNA and formation of nucleosomes and certain protein complexes on DNA that stabilize the writhing of DNA (37).
Although the bulk of DNA in eukaryotic nuclei is not under superhelical tension (30), unconstrained supercoils occur locally in the chromatin DNA. Most of them appear to be produced by the tracking of processive enzyme complexes such as RNA polymerases along the DNA (38). However, recent studies have suggested the presence of unconstrained supercoils generated by mechanisms other than transcription (15, 17). It is possible that an enzymatic activity similar to that of bacterial DNA gyrase may also exist in eukaryotes. In support of this idea, we detected and purified a novel supercoiling activity from the silkworm Bombyx mori (21). The activity consists of the DNA supercoiling factor (SCF) and topoisomerase II. Cloning and characterization of a cDNA encoding B. mori SCF revealed a distinctive Ca2+-binding protein and Ca2+-dependent activation of the supercoiling reaction (22).
The silkworm B. mori is a useful organism for biochemical studies but it is far less suitable for the molecular genetic approach than the fly Drosophila melanogaster. To investigate the in vivo role of SCF, we initiated studies on a Drosophila homologue of the factor. We report here that Drosophila SCF interacts with topoisomerase II in nuclei and localizes to puffs on polytene chromosomes. These findings suggest a role of SCF in transcription on chromatin.
MATERIALS AND METHODS
Isolation of a cDNA encoding Drosophila SCF.
Two DNA fragments isolated from the B. mori SCF cDNA, one corresponding to nucleotide positions 1 to 794 and the other to positions 795 to 1095 as shown in Fig. 2 in an article by Ohta et al. (22) were used to screen a Drosophila genomic library in λEMBL3 (a gift of J. Tamkun and M. Scott). A clone that gave positive signals with both probes was chosen and designated λD2a. The hybridizing region was delimited to a 1-kb XhoI-XbaI fragment within the 13.5-kb insert of λD2a. This DNA fragment contained an open reading frame (ORF) with significant homology to B. mori SCF and was used to screen a Drosophila embryonic cDNA library in λZAPII (a gift of Y.-N. Jan). Individual cDNA inserts from positive clones were recovered as chimeric pBluescript SK(−) plasmids and showed a similar restriction pattern. The longest cDNA and the upstream region as well as the coding region of the genomic DNA were sequenced on both strands.
FIG. 2.
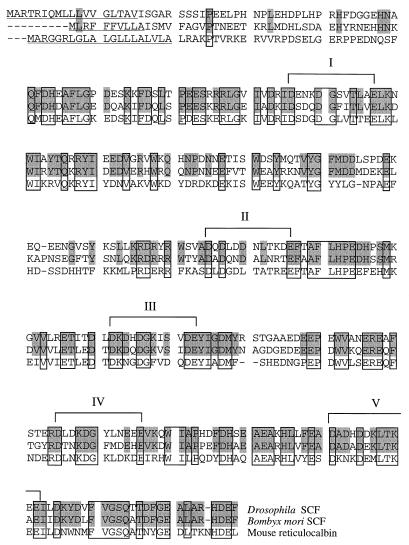
Sequence comparison of Drosophila SCF, silkworm SCF (22), and mouse reticulocalbin (23) deduced proteins. Amino acids identical between Drosophila and silkworm proteins are shaded, and those identical in all three proteins are boxed. Identity: Drosophila versus silkworm, 56%; Drosophila versus mouse, 43%; silkworm versus mouse, 45%. I to V represent loops of the EF-Hand domains. The presumptive signal peptides are underlined.
Preparation of cytosol and nuclei from embryos.
Dechorionated embryos (1 g) at 0 to 22 h after egg laying were homogenized and fractionated into cytosol (4 ml) and nuclear pellet as described by Ueda et al. (35) except that the homogenization buffer contained 0.5% Nonidet P-40. The nuclei were resuspended in 2 ml of 20 mM HEPES-KOH (pH 7.9)-50 mM NaCl-0.5 mM dithiothreitol-0.5 mM phenylmethylsulfonyl fluoride-20% glycerol.
Preparation of antibodies.
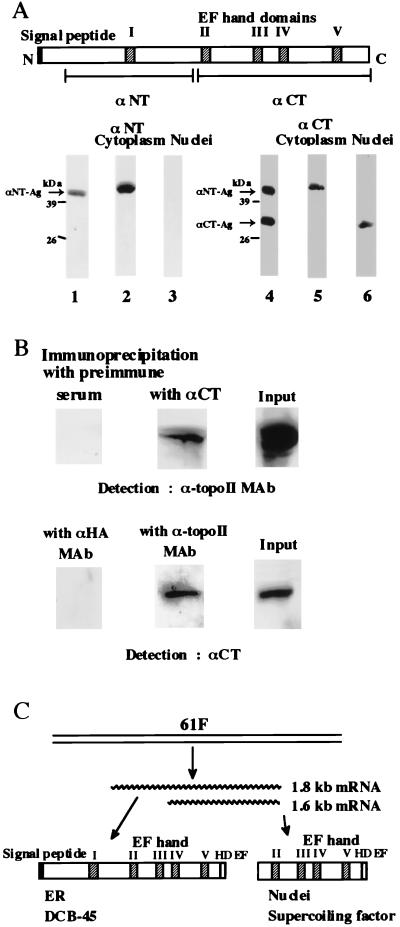
To produce a histidine-tagged full-length protein (his-tag SCFf) or its truncated version (his-tag SCFt) in Escherichia coli, an NdeI-BamHI-tagged ORF (amino acid positions 1 to 330 or 138 to 330; see Fig. 1) was subcloned into 6HisT-pET11d to give pSCFhf or pSCFht. The recombinant proteins were purified with Ni-immobilized resin (Novagen) followed by MonoQ column chromatography. A mouse monoclonal antibody against his-tag SCFf (αNT) was produced by K. Tamai (MBL, Nagoya, Japan) and donated to us. Its epitope was mapped between amino acid positions 37 and 141. His-tag SCFt was used to raise a polyclonal antibody in a rabbit (αCT). A mouse monoclonal antibody against topoisomerase II (6H8) was a gift of A. Kikuchi. The supernatants of culture media (αNT, 6H8) or serum (αCT) were used directly after appropriate dilution.
FIG. 1.
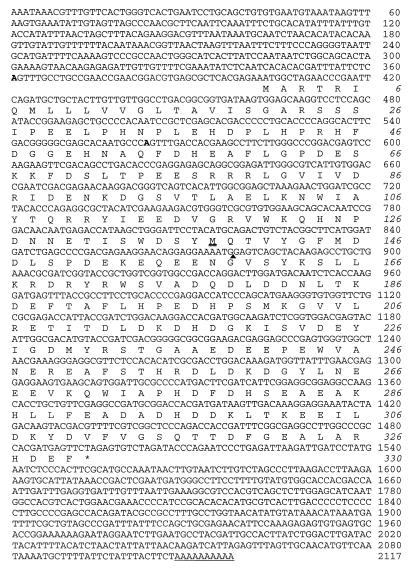
Nucleotide sequence of Drosophila SCF. Complete coding sequence and the 5′- and 3′-noncoding sequences are shown. The first 369 nucleotides represent the genomic sequence, and the following region was derived from cDNA. Comparison of the genomic and cDNA sequences revealed a 118-bp intron between nucleotide positions 877 and 878 (marked by a filled triangle). The predicted amino acid sequence of the entire ORF is shown in the single-letter code. The asterisk represents the stop codon. The boldfaced A residues and double underlining show the presumptive initiation sites for the 1.6- and 1.8-kb mRNA and the first in-frame methionine in the 1.6-kb mRNA, respectively. The single underlining represents the poly (A) tail.
Western blot analysis.
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Boehringer, Mannheim, Germany). After blocking with TBST (25 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.2% Tween 20) containing 5% skim milk powder, the membrane was incubated with αNT (100-fold dilution) or αCT (10,000-fold dilution), and signals were detected by using horseradish peroxidase-linked anti-mouse or anti-rabbit immunoglobulin G (IgG) (Cappel) and the ECL detection system (Amersham). To detect topoisomerase II, the monoclonal antibody 6H8 was used at a 1:10,000 dilution.
Immunostaining.
Immunostaining of salivary gland polytene chromosomes from third instar wandering larvae was carried out as described by Andrew and Scott (1). Isolated salivary glands were incubated with 20 μM 20-hydroxyecdysone in Grace medium at 25°C for 90 min when indicated. For heat shock, larvae were incubated at 37°C for 30 min before isolation of salivary glands. Staining of embryos and larvae with αNT (10-fold diluted) or αCT (1,000-fold diluted) was performed as described by Hayashi et al. (12). In the case of αNT, the staining was detected with either a combination of biotin-conjugated anti-mouse IgG (Jackson Laboratory), ABC complex (Vectastain Elite kit), and diaminobenzidine or Cy3-conjugated anti-mouse IgG (Chemicon International). αCT was visualized by using Cy3-conjugated anti-rabbit IgG (Chemicon International).
Immunoprecipitation.
For immunoprecipitation, 20 μl of anti-rabbit IgG-conjugated Dyna beads (Dynal, Oslo, Norway) containing 2 μg of IgG was incubated at 4°C for 2 h with 50 μl of αCT or the corresponding preimmune serum and then washed with phosphate-buffered saline (PBS). The nuclear suspension from embryos was quickly frozen in liquid nitrogen and then thawed in a water bath three times, and a 10-μl portion of it was added to the antibody-loaded beads. After incubation at 4°C for 2 h, the beads were washed with PBS containing 0.2% Triton X-100. Bound proteins were eluted from the beads with Laemmli buffer and resolved by SDS–10% PAGE, and topoisomerase II was detected on a Western blot with the monoclonal antibody 6H8. In a reciprocal experiment, 50 μl of 6H8 or a monoclonal antibody against hemagglutinin was incubated with 20 μl of anti-mouse IgG conjugated Dyna beads, and SCF that bound to the antibody-loaded beads was detected on a Western blot with αCT.
Northern blot analysis.
Ten micrograms of total cellular RNA was electrophoresed in a 1% agarose gel after denaturation with glyoxal and dimethyl sulfoxide (28). Hybridization was performed as described by Sun et al. (32). When using probe 2, hybridization and washing temperatures were lowered to 55°C. Probe 1 was a 107-bp NdeI-XhoI fragment isolated from pSCFhf. Probes 2 and 3 were oligonucleotides complementary to nucleotide positions 866 to 888 and 1371 to 1395, respectively, as shown in Fig. 1. They were labeled with polynucleotide kinase and [γ-32P]ATP. Probe 4 was the full-length cDNA that was labeled with [α-32P]dCTP with a random primer DNA labeling kit (Boehringer).
Other methods.
Periodic acid-Schiff (PAS) staining of the salivary gland was carried out as previously described (31). The DNA supercoiling activity was assayed as described by Ohta et al. (22) except that Drosophila topoisomerase II (Amersham) was used in place of the human enzyme. 5′ rapid amplification of cDNA ends (RACE) was performed with a Marathon cDNA amplification kit (Clontech). Primers used were oligonucleotides complementary to nucleotide positions 877 to 898 (gene-specific primer for the first PCR) and 841 to 861, as seen in Fig. 1 (nested gene-specific primer for the second PCR).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the GenBank-EMBL-DDBJ database with accession no. AB011261. A part of the cDNA sequence has been deposited independently as an expressed-sequence tag with accession no. AA141884.
RESULTS
Cloning of a cDNA encoding Drosophila SCF.
Screening of a Drosophila genomic library by using the B. mori SCF cDNA as a probe yielded a clone that showed a significant sequence similarity to the probe DNA. After subcloning, this homologous region was employed to screen a Drosophila embryo cDNA library. The longest cDNA obtained was sequenced in its entirety and found to contain a sequence identical to that in the corresponding region of the genomic clone except for an intron (Fig. 1). The cDNA carries a single long ORF encoding 330 amino acids rich in acidic residues (Fig. 1). The amino acid sequence conceptually translated from the full-length ORF shares homology with that of B. mori SCF throughout the molecule except for the N-terminal region (Fig. 2). The conserved region includes five EF-Hand domains. The EF-Hand is a Ca2+-binding domain consisting of a helix-loop-helix motif, with the loop portion carrying a consensus sequence of DX(D or N) X (D, N or S) XXX(D, N, E, Q, S, or T)XXE (16).
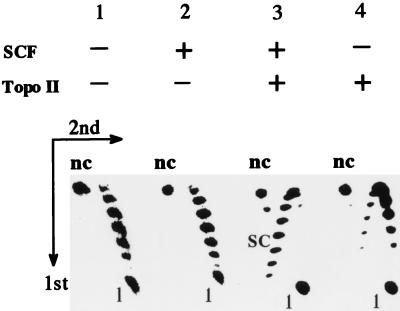
To test the DNA supercoiling activity of bacterially expressed recombinant protein from the full-length ORF, relaxed closed circular DNA was incubated with the recombinant protein and/or Drosophila topoisomerase II, and the reaction products were analyzed by two-dimensional gel electrophoresis (Fig. 3). We used pHSAR DNA containing the scaffold-associate region in the Drosophila histone gene cluster as the substrate because it enhanced the supercoiling activity (22). Under these electrophoresis conditions, input relaxed circular DNA migrated as topoisomers with several positive superhelical turns (Fig. 3, lane 1). Incubation with the recombinant protein alone did not alter the pattern (Fig. 3, lane 2). However, incubation with the recombinant protein and topoisomerase II resulted in negative supercoiling of DNA (Fig. 3, lane 3). A slight shift of topoisomers upon incubation with topoisomerase II alone (Fig. 3, lane 4) may be due to the difference in the reaction conditions between the supercoiling assay and preparation of the substrate DNA with topoisomerase I. As observed for B. mori SCF, the activity is dependent on Ca2+ and ATP, and the supercoils that formed were unconstrained because they were readily relaxed by further incubation with topoisomerase I (data not shown). From these results, we conclude that our cDNA encodes a Drosophila homologue of SCF.
FIG. 3.
Supercoiling activity of recombinant Drosophila SCF. 32P-labeled relaxed closed circular DNA of pHSAR was incubated with Drosophila topoisomerase II (topo II) and/or bacterially expressed and purified his-tag SCFf, and analyzed by two-dimensional agarose gel electrophoresis. The first dimension was normal gel electrophoresis from the top to the bottom, while the second dimension was in the presence of 5 ng of ethidium bromide/ml from the left to the right. nc, nicked circular DNA; SC, supercoiled circular DNA with several negative superhelical turns; 1, linear DNA. Supercoiled DNA with 10 or more negative superhelical turns migrated faster than linear DNA in the first dimension (19).
The Drosophila SCF gene was cytologically mapped by in situ hybridization to position 61F on the third chromosome (data not shown). Genomic Southern blot hybridization indicated that there is a single SCF gene within the genome (data not shown).
A homology search of the databases revealed that the amino acid sequences conceptually translated from the D. melanogaster and B. mori full-length ORFs share similarity with those of mouse reticulocalbin (Fig. 2) and human ERC-55 (comparison not shown). Reticulocalbin and ERC-55 are Ca2+-binding proteins present in the lumen of the endoplasmic reticulum (23, 41). They have signal peptide sequences in their N termini and an endoplasmic reticulum-retention signal HDEL in their C termini. We noticed that the D. melanogaster and B. mori ORFs also encode signal-peptide-like sequences in their N termini and a C-terminal sequence HDEF similar to HDEL (Fig. 2). However, SCF protein would not be able to function with the nuclear topoisomerase II if it were permanently restrained to the endoplasmic reticulum. This prompted us to examine subcellular localization of Drosophila SCF.
SCF gene encodes a nuclear and an endoplasmic reticulum-resident protein.
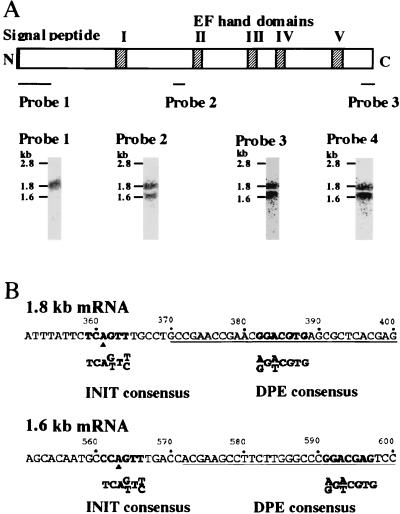
Northern analysis using the full-length cDNA as a probe revealed 1.6- and 1.8-kb mRNAs in Drosophila embryos, larvae, prepupae, pupae, and adults (data not shown). When a blot of RNA from prepupae was probed with a DNA fragment encoding the N-terminal region, only the longer mRNA was detected (Fig. 4A, probe 1). In contrast, if the same blot was reprobed with an oligonucleotide encoding the middle (probe 2) or the C-terminal (probe 3) regions, both mRNAs were detected, as was the case with the full-length cDNA probe (probe 4). A RACE protocol was employed to map the 5′ ends of these mRNAs with the total RNA from prepupae as the substrate. Two groups of PCR products that differed only in their 5′ ends and otherwise had the same sequence were found. The longest PCR product of the first group ended with nucleotide position 370 and that of the second group ended with nucleotide position 572 (Fig. 4B). (The nucleotide position corresponds to that shown in Fig. 1.) Around these positions, we found consensus sequences for initiator and downstream promoter elements that are present in most TATA-less promoters (6). It is most likely that the 1.8- and 1.6-kb mRNAs initiate at nucleotide positions 361 and 563, respectively. The results also suggest that the 1.8-kb mRNA carries the entire ORF whereas the 1.6-kb mRNA encodes its truncated version lacking the signal peptide-like sequence.
FIG. 4.
Transcripts from the SCF locus. (A) Northern blot hybridization with region-specific probes. A blot of total cellular RNA from prepupae was successively probed with the indicated DNA fragments (probes 1-3) and a full-length cDNA (probe 4). Size marker RNAs were run in a parallel lane on the same gel, and their positions are indicated. N and C represent the N and C termini of the full-length protein. Signal peptide denotes the signal-peptide-like sequence, and I to V represent the loops of the EF-Hand domains. (B) Mapping of the 5′ end of each mRNA by 5′ RACE. Underlining represents sequences of the longest PCR products from 1.6 and 1.8 kb mRNAs. INIT consensus, initiator consensus sequence; DPE consensus, downstream promoter element consensus. The filled triangles show the presumptive initiation sites for each mRNA.
To examine subcellular localization of proteins translated from these mRNAs, cytoplasmic and nuclear fractions were prepared from an embryonic extract, and Western blots of these fractions were probed with a monoclonal antibody against the N-terminal half of the full-length SCF gene product (αNT) or a polyclonal antibody against the C-terminal half (αCT). Using αNT, we observed a 45-kDa band in the cytoplasmic fraction, but no band was detectable in the nuclear fraction (Fig. 5A). In contrast, αCT detected a 30-kDa protein in the nuclear fraction in addition to the 45-kDa protein in the cytoplasm (Fig. 5A).
FIG. 5.
Identification of SCF. (A) Western blot analyses with region-specific antibodies. The proteins in 10 μl of the cytoplasmic (lanes 2 and 5) or 5 μl of the nuclear (lanes 3 and 6) fraction from 0- to 22-h-old embryos or 2.5 ng each of the recombinant proteins used for production of αNT and αCT (αNT-Ag and αCT-Ag, lanes 1 and 4) were resolved by SDS–10% PAGE. After blotting, the membrane was probed with αNT (lanes 1 to 3) or αCT (lanes 4 to 6). Positions of size marker proteins are indicated. Symbols are the same as those in Fig. 4A. (B) Coimmunoprecipitation of SCF and topoisomerase II. Upper panels: Western blots of nuclear proteins from the 0- to 22-h-old embryos incubated with αCT (middle) or the preimmune serum (left) were probed with antitopoisomerase II monoclonal antibody (α-topoII MAb). Lower panels: Western blots of the same nuclear proteins incubated with α-topoII monoclonal antibody (middle), or anti-hemagglutinin monoclonal antibody (αHA MAb, left) were probed with αCT. Western blots of input proteins are shown in the right column. Only regions of the blots containing the relevant bands are shown because each antibody detected only SCF (see panel A, lane 6) or topoisomerase II (data not shown) among the proteins in the nuclear fraction. (C) Scheme of expression of SCF and DCB-45. ER, endoplasmic reticulum. Other abbreviations are as described in the legend for Fig. 4A.
When nuclear proteins from embryos were subjected to immunoprecipitation, a small population of topoisomerase II was coprecipitated with αCT but not with the preimmune serum. In a reciprocal test, most of the 30-kDa protein was recovered with a monoclonal antibody against topoisomerase II but not with that against an unrelated protein hemagglutinin (Fig. 5B). These results suggest that most of the 30-kDa protein is present as a complex with a subpopulation of topoisomerase II in embryonic nuclei.
Collectively, these data suggest the following scheme (Fig. 5C). Two types of mRNA are transcribed from the Drosophila SCF locus, presumably using different promoters. The 1.8-kb mRNA encodes the 45-kDa protein with N-terminal signal peptide-like sequence. We termed it DCB-45 for Drosophila Ca2+-binding protein with an apparent molecular mass of 45 kDa as determined by SDS-PAGE. DCB-45, similar to reticulocalbin and ERC-55, is transported into the endoplasmic reticulum and serves an unknown function. The 1.6-kb mRNA is translated into the 30-kDa truncated version that is transported into the nucleus, forms a complex with topoisomerase II, and exerts its function as the SCF. It remains to be determined whether the 30-kDa nuclear protein starts from the first in-frame methionine codon in the 1.6-kb mRNA (the underlined methionine at amino acid position 138 in Fig. 1). A bacterially expressed recombinant protein starting from this methionine (αCT-Ag) migrated to almost the same position as the 30-kDa nuclear protein during SDS-PAGE (Fig. 5A) and was functional in the DNA supercoiling reaction (data not shown). As was the case with B. mori SCF (22), both DCB-45 and Drosophila SCF are highly negatively charged and migrated more slowly during SDS-PAGE than expected from their molecular mass.
Expression of SCF in embryos and larval tissues.
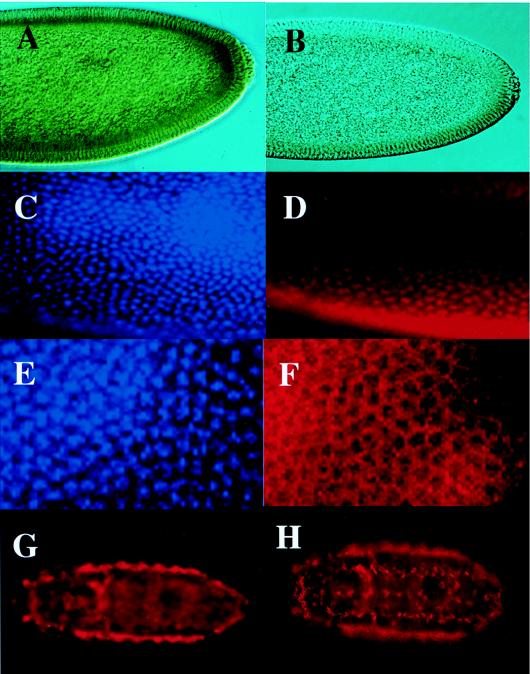
To analyze expression of SCF and DCB-45, we stained embryos and larval tissues with αCT or αNT. In all immunostaining experiments described below, no staining was obtained when αNT or αCT was replaced with monoclonal antibody against hemagglutinin or preimmune serum, respectively. Staining also disappeared if samples were incubated with the primary antibody in the presence of the recombinant protein used as the antigen. In blastoderm embryos, DCB-45 was detected with αNT in the cytoplasm just under the layer of nuclei (Fig. 6A). No such staining was detectable without primary antibody (Fig. 6B). Using αCT, SCF was found in the nuclei in addition to the cytoplasmic staining of DCB-45 (Fig. 6C and D). These results are in good agreement with those of the Western analyses described above. In these early embryos, nuclear division proceeds almost synchronously, and we can discriminate irregularly shaped metaphase chromosomes (Fig. 6E) from smooth interphase nuclei (Fig. 6C). The nuclear staining was restricted to interphase chromatin, and the staining was excluded from metaphase chromosomes (Fig. 6D and F). Both SCF and DCB-45 seem to be supplied maternally, as we were unable to detect any signals of mRNA by in situ hybridization in these early embryos: zygotic expression of mRNA started after gastrulation (data not shown). Moreover, strong staining of oocytes in female adults with these antibodies supports the maternal deposition of these proteins (data not shown). In late embryos, strong cytoplasmic staining of DCB-45 was detected in the fat body along the side line of the embryo (Fig. 6G) and macrophages (Fig. 6H) with both antibodies. Essentially the same zygotic expression patterns were observed by in situ hybridization of mRNA (data not shown).
FIG. 6.
Immunostaining of embryos. (A) Blastoderm embryo stained with αNT. (B) Another blastoderm embryo processed as just described but without primary antibody. (C) Part of a blastoderm embryo stained with DAPI. (D) The same embryo as in panel C but stained with αCT. (E) Part of a blastoderm embryo in metaphase stained with DAPI. (F) The same embryo as in panel E but stained with αCT. (G) Late embryo stained with αCT. A similar staining pattern was observed with αNT (data not shown). (H) Late embryo stained with αNT. Similar staining was obtained with αCT (data not shown). In panels C through F, parts of the embryos are shown at higher magnifications to show the shape of nuclei. In panels C to F and in panel H, the focus was on the upper surface of the embryos, while in panels A, B, and G, it was on the inside. All embryos are oriented anterior to the left.
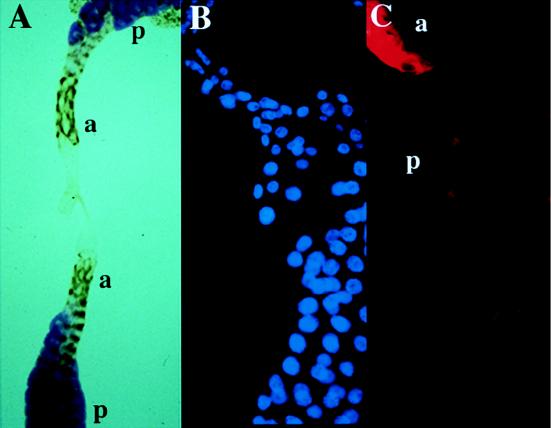
In larvae, both αNT and αCT stained DCB-45 in the cytoplasm of macrophages, garland cells, pericardial cells (data not shown), and an anterior part of the salivary gland (Fig. 7A and C). Interestingly, no staining of the salivary gland with αNT was detectable in posterior cells, where some carbohydrates were accumulating as revealed by the PAS staining (Fig. 7A). In addition to this cytoplasmic staining, αCT stained SCF in nuclei of the fat body, trachea, the intestine (data not shown), and the posterior part of the salivary gland (Fig. 7B and C).
FIG. 7.
Immunostaining of the salivary gland. (A) Anterior parts of salivary glands from a third instar wandering larva stained with αNT (brown). Note that posterior cells with large nuclei were not stained with αNT but were positive for the PAS staining (purple). (B) Posterior part of a salivary gland from a third instar wandering larva stained with DAPI. (C) The same gland as in panel B but stained with αCT. a and p indicate the anterior and posterior parts of the salivary gland, respectively.
SCF localizes to puffs on polytene chromosomes.
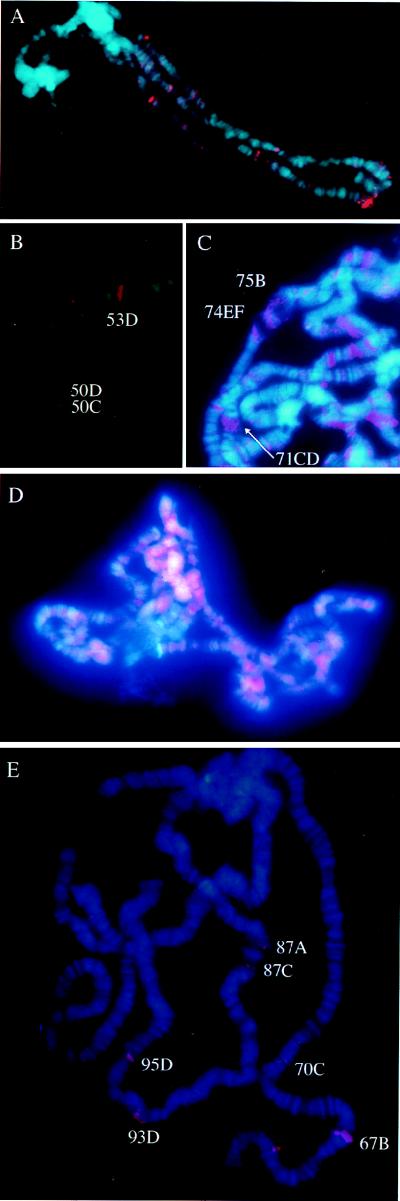
Having detected SCF in the large nuclei of the salivary glands, we examined distribution of SCF on polytene chromosomes. Immunostaining of SCF on polytene chromosomes isolated from third instar wandering-stage larvae revealed that the factor is not distributed evenly but rather is associated with a limited set of chromosomal sites (Fig. 8A). Most of them corresponded to puffs, e.g., sites 50C, 50D, and 53F (Fig. 8A and B). Some staining appeared as dots that for an unknown reason did not span the chromosome. No staining was detected in the centromeric heterochromatin. This staining pattern was reproducible as long as the glands were isolated at the same developmental stage. In contrast to SCF, staining of polytene chromosomes with antitopoisomerase II antibody showed a ubiquitous pattern with a few strongly stained sites (Fig. 8D; see also reference 4).
FIG. 8.
Immunostaining of SCF on salivary gland polytene chromosomes. (A) Polytene chromosomes from a third instar wandering larva stained with αCT (red) and DAPI (blue). (B) Part of the second chromosome stained with αCT and DAPI. (C) Polytene chromosomes from an ecdysteroid-treated gland stained with αCT and DAPI. (D) Polytene chromosomes as in panel A but stained with the antitopoisomerase II monoclonal antibody (red) and DAPI (blue). Most regions of the chromosomes appear purple due to overlapping staining. (E) Polytene chromosomes from a heat-treated wandering larva stained with αCT and DAPI. The numbers in panels B, C, and E represent the chromosomal sites.
To confirm the localization of SCF to puffs, we analyzed polytene chromosomes from ecdysteroid-treated glands or from heat-treated larvae. Many puffs are generated, and small endogenous puffs are expanded at distinct sites on polytene chromosomes when isolated salivary glands are incubated with ecdysteroid (3). These ecdysteroid-induced puffs were stained with αCT, e.g., the large puffs at sites 71CD, 74EF, and 75B (Fig. 8C). Upon heat shock, the staining at endogenous puffs disappeared and instead, strong staining was detected on heat shock puffs at 67B, 70C, 87A, 87C, 93D, and 95D (Fig. 8E). In these experiments, the staining was abolished when αCT was replaced with the preimmune serum or when polytene chromosomes were incubated with αCT in the presence of excess antigen protein (data not shown). These results indicate that SCF is associated with transcriptionally active chromatin.
DISCUSSION
Both SCF and DCB-45 are products of the Drosophila SCF locus.
Two different sizes of mRNA are transcribed from the Drosophila SCF locus. The longer mRNA codes for cytoplasmic protein DCB-45 that shares similarity with mouse reticulocalbin and human ERC-55. The shorter one encodes its truncated version that interacts with nuclear protein topoisomerase II and serves as SCF. Thus, subcellular localization appears to destine the two SCF gene products to different functions. No shorter version of mouse reticulocalbin has been reported, but a truncated form of human ERC-55 lacking the N-terminal 107 amino acids has been detected as a binding partner of papillomavirus E6 oncoprotein (7). Interestingly, E6 protein was also identified in both the nuclear and membrane fractions (2).
Expression patterns of DCB-45 and SCF in salivary glands are intriguing. DCB-45 was detected only in the anterior cells, whereas SCF was found only in large nuclei of the posterior cells where PAS-staining-positive materials were accumulating. We were unable to find salivary gland cells that expressed both proteins. Two mRNAs seem to be synthesized in a mutually exclusive manner to allow the space-specific expression of these proteins in salivary glands.
DCB-45 has the C-terminal tetrapeptide HDEF that is similar to the endoplasmic reticulum-retention signal K/HDEL (25). This HDEF sequence appears to be functional for the retention, since a portion of DCB-45 lacking HDEF but not the intact molecule was secreted into the culture medium when expressed from cDNAs in transformed Schneider cell line S2 (unpublished observation). Judging from its strong expression in macrophages, garland cells, and pericardial cells, it might be involved in the regulation of endo- or exocytosis, but the precise function of DCB-45 remains unknown.
Possible role of SCF in transcription.
Immunostaining of SCF on polytene chromosomes revealed its localization to puffs. A trivial cause of this could be a mere deposition of SCF to the open chromatin structure. However, this possibility is highly unlikely because staining intensities were not necessarily correlated with the size of puffs. The staining was more prominent in the average-sized heat-shocked puffs at 67B, 93D, and 95D than in the large heat-shocked puffs at 87A and 87C (Fig. 8E). Moreover, extended chromatin was not stained homogeneously, but rather only limited regions within the puffs were stained (see the puffs at 74EF and 75B in Fig. 8C, and those at 87A and 87C in Fig. 8E). These observations suggest that SCF plays some specific role in chromatin transcription. However, whether it has a role in transcriptional regulation or some structural role remains to be determined.
Several lines of evidence support the proposal that unconstrained negative supercoils may be required for optimal transcription in eukaryotes. First, covalently closed circular DNA templates are transcribed more efficiently than linear DNA when introduced into cultured cells or Xenopus oocytes (11, 26, 27, 40). Second, negative supercoiling of DNA has been reported to enhance transcription in vitro from certain promoters (10, 19, 20, 24, 29), while linear templates are fully active for transcription from other promoters (18, 20). It is possible that SCF exerts its function by generating unconstrained negative supercoils to activate certain promoters.
Alternatively, unconstrained supercoils produced by SCF and topoisomerase II might be required to induce chromatin to a dynamic state. Approximately one negative superhelical turn is constrained in each nucleosome (9). Therefore, nucleosomes are thought to jump or slide more easily to a DNA region under negative superhelical tension than to a relaxed DNA. This dynamic state might facilitate remodeling of chromatin. Still another possibility is that negative superhelical tension may stimulate a putative DNA translocating activity of ISWI protein (8, 34) to achieve chromatin remodeling.
Topoisomerase II forms a complex with other proteins to execute its specific function.
Eukaryotic topoisomerase II is essential for condensation and segregation of chromosomes (14, 36) and appears to play roles in DNA replication and transcription (38). In vitro, it is a multifunctional enzyme that catalyzes relaxation of supercoiled DNA, decatenation of interlinked DNA, and unknotting of intramolecularly linked DNA by passing a DNA helix through a transient double-strand break in a second helix (37). Therefore, its particular roles in vivo are thought to be specified by auxiliary factors. Consistent with this notion are recent findings regarding proteins that associate with the enzyme. First, yeast Sgs1, a eukaryotic homologue of E. coli Rec Q, interacts with topoisomerase II and is necessary for faithful chromosome segregation at anaphase (39). Second, proper segregation of chromosomes requires Drosophila Barren protein that interacts with topoisomerase II and modulates its activity (5). Furthermore, Xenopus 13S condensin that is essential for chromosome condensation in vitro contains topoisomerase II and a Xenopus homologue of the Barren protein in addition to SMC family proteins (13). Finally, the present study suggests that SCF is involved in transcription on chromatin by conferring the supercoiling activity to topoisomerase II.
We have found that SCF is present in blastoderm nuclei during interphase but is excluded from metaphase chromosomes. Topoisomerase II has been shown to exist in different pools in blastoderm embryos (33). One of the pools leaves from nuclei into cytoplasm during prophase and another detaches from chromosomes during anaphase. These observations suggest that the former pool may be responsible for transcription on the interphase chromatin and the chromosome condensation at prophase and that the latter may be responsible for segregation of chromosomes at anaphase.
ACKNOWLEDGMENTS
We thank J. Tamkun, M. Scott, and Y.-N. Jan for Drosophila libraries, K. Tamai and A. Kikuchi for antibodies, M. Yamamoto for assignment of the chromosomal sites, M. Jindra and H. Ueda for critical reading of the manuscript, and M. Yanagida for discussion.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan to S. Hirose and S. Hayashi.
M.K. and N.A. contributed equally to this work.
REFERENCES
- 1.Andrew D J, Scott M T. Immunological methods for mapping protein distribution on polytene chromosomes. Methods Cell Biol. 1994;14:353–370. doi: 10.1016/s0091-679x(08)60923-1. [DOI] [PubMed] [Google Scholar]
- 2.Androphy E J, Schiller J T, Lowy D R. Identification of the protein encoded by the E6 transforming gene of bovine papillomavirus. Science. 1985;230:442–445. doi: 10.1126/science.2996134. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of Drosophila melanogaster cultured in vitro. Chromosoma. 1972;38:255–281. doi: 10.1007/BF00290925. [DOI] [PubMed] [Google Scholar]
- 4.Berrios M, Osheroff N, Fisher P A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci USA. 1985;82:4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat M A, Philp A V, Glover D M, Bellen H J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell. 1996;87:1103–1114. doi: 10.1016/s0092-8674(00)81804-8. [DOI] [PubMed] [Google Scholar]
- 6.Burke T W, Kadonaga J T. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- 7.Chen J J, Reid C E, Band V, Androphy E J. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- 8.Elfring L K, Deuring R, McCallum C M, Peterson C L, Tamkun J W. Identification and characterization of Drosophila relatives of the yeast transcriptional activator SNF2/SWI2. Mol Cell Biol. 1994;14:2225–2234. doi: 10.1128/mcb.14.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germond J E, Hirt B, Oudet P, Gross-Bellard M, Chambom P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci USA. 1975;72:1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodrich J A, Tjian R. Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell. 1994;77:145–156. doi: 10.1016/0092-8674(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 11.Harland R M, Weintraub H, McKnight S L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983;302:38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Hirose S, Metcalfe T, Shirras A. Control of imaginal cell development by the escargot gene of Drosophila. Development. 1993;118:105–115. doi: 10.1242/dev.118.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 14.Holm C, Golo T, Wang J C, Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- 15.Jupe E R, Sinden R R, Cartwright I L. Stably maintained microdomain of localized unrestrained supercoiling at a Drosophila heat shock gene locus. EMBO J. 1993;12:1067–1075. doi: 10.1002/j.1460-2075.1993.tb05748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretsinger R H. Calcium coordination and the calmodulin fold: divergent versus convergent evolution. Cold Spring Harbor Symp Quant Biol. 1987;52:499–510. doi: 10.1101/sqb.1987.052.01.057. [DOI] [PubMed] [Google Scholar]
- 17.Leonard M W, Patient R K. Evidence for torsional stress in transcriptionally activated chromatin. Mol Cell Biol. 1991;11:6128–6138. doi: 10.1128/mcb.11.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang C-P, Garrard W T. Template topology and transcription: chromatin templates relaxed by localized linearization are transcriptionally active in yeast. Mol Cell Biol. 1997;17:2825–2834. doi: 10.1128/mcb.17.5.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizutani M, Ohta T, Watanabe H, Handa H, Hirose S. Negative supercoiling of DNA facilitates an interaction between transcription factor IID and the fibroin gene promoter. Proc Natl Acad Sci USA. 1991;88:718–722. doi: 10.1073/pnas.88.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizutani M, Ura K, Hirose S. DNA superhelicity affects the formation of transcription preinitiation complex on eukaryotic genes differently. Nucleic Acids Res. 1991;19:2907–2911. doi: 10.1093/nar/19.11.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta T, Hirose S. Purification of a DNA supercoiling factor from the posterior silk gland of Bombyx mori. Proc Natl Acad Sci USA. 1990;87:5307–5311. doi: 10.1073/pnas.87.14.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta T, Kobayashi M, Hirose S. Cloning of a cDNA for DNA supercoiling factor reveals a distinctive Ca2+-binding protein. J Biol Chem. 1995;270:15571–15575. doi: 10.1074/jbc.270.26.15571. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa M, Muramatsu T. Reticulocalbin, a novel endoplasmic reticulum resident Ca2+-binding protein. J Biol Chem. 1993;268:699–705. [PubMed] [Google Scholar]
- 24.Parvin J D, Sharp P A. DNA topology and minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993;73:533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 25.Pelham H R B. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- 26.Pina B, Bruggemeier U, Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990;60:719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- 27.Pruitt S C, Reeder R H. Effect of topological constraint on transcription of ribosomal DNA in Xenopus oocytes. J Mol Biol. 1984;174:121–139. doi: 10.1016/0022-2836(84)90368-1. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schultz M C, Brill S J, Ju Q D, Sternglanz R, Reeder R H. Topoisomerases and yeast rRNA transcription: negative supercoiling stimulates initiation and topoisomerase activity is required for elongation. Genes Dev. 1992;6:1332–1341. doi: 10.1101/gad.6.7.1332. [DOI] [PubMed] [Google Scholar]
- 30.Sinden R R, Carlson J O, Pettijohn D E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980;21:773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- 31.Sumner B E H. Basic histochemistry. New York, N.Y: John Wiley & Sons; 1988. [Google Scholar]
- 32.Sun G-C, Hirose S, Ueda H. Intermittent expression of BmFTZ-F1, a member of the nuclear hormone receptor superfamily during development of the silkworm Bombyx mori. Dev Biol. 1994;162:426–437. doi: 10.1006/dbio.1994.1099. [DOI] [PubMed] [Google Scholar]
- 33.Swedlow J R, Sedat J W, Agard D A. Multiple chromosomal populations of topoisomerase II detected in vivo by time-lapse, three-dimensional wide-field microscopy. Cell. 1993;73:97–108. doi: 10.1016/0092-8674(93)90163-k. [DOI] [PubMed] [Google Scholar]
- 34.Tsukiyama T, Daniel C, Tamkun J, Wu C. ISWI, a member of the SWI2/SNF2 ATPase family, encodes the 140 kDa subunit of the nucleosome remodeling factor. Cell. 1995;83:1021–1028. doi: 10.1016/0092-8674(95)90217-1. [DOI] [PubMed] [Google Scholar]
- 35.Ueda H, Sonoda S, Brown J L, Scott M P, Wu C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990;4:624–635. doi: 10.1101/gad.4.4.624. [DOI] [PubMed] [Google Scholar]
- 36.Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987;50:917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- 38.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 39.Watt P M, Louis E J, Borts R H, Hickson I D. Sgs1: A eukaryotic homolog of E. coli RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell. 1995;81:253–260. doi: 10.1016/0092-8674(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 40.Weintraub H, Chenk P F, Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986;46:115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- 41.Weis K, Griffiths G, Lamond A I. The endoplasmic reticulum calcium-binding protein of 55 kDa is a novel EF-hand protein retained in the endoplasmic reticulum by a carboxyl-terminal His-Asp-Glu-Leu motif. J Biol Chem. 1994;269:19142–19150. [PubMed] [Google Scholar]