Abstract
Background
The effectiveness of using a spray nozzle to deliver lidocaine for superior topical airway anaesthesia during non-sedation flexible bronchoscopy (FB) remains a topic of uncertainty when compared with conventional methods.
Methods
Patients referred for FB were randomly assigned to receive topical lidocaine anaesthesia via the bronchoscope's working channel (classical spray (CS) group) or through a washing pipe equipped with a spray nozzle (SN group). The primary outcome was cough rate, defined as the total number of coughs per minute. Secondary outcomes included subjective perceptions of both the patient and operator regarding the FB process. These perceptions were rated on a visual analogue scale, with numerical ratings ranging from 0 to 10.
Results
Our study enrolled a total of 126 (61 CS group; 65 SN group) patients. The SN group exhibited a significantly lower median cough rate compared with the CS group (4.5 versus 7.1 counts·min−1; p=0.021). Patients in the SN group also reported less oropharyngeal discomfort (4.5±2.7 versus 5.6±2.9; p=0.039), better tolerance of the procedure (6.8±2.2 versus 5.7±2.7; p=0.011) and a greater willingness to undergo a repeat FB procedure (7.2±2.7 versus 5.8±3.4; p=0.015) compared with those in the CS group. From the operator's perspective, patient discomfort (2.7±1.7 versus 3.4±2.3; p=0.040) and cough scores (2.3±1.5 versus 3.2±2.4; p=0.013) were lower in the SN group compared with the CS group, with less disruption due to coughing observed among those in the SN group (1.6±1.4 versus 2.3±2.3; p=0.029).
Conclusions
This study illustrates that employing a spray nozzle for the delivery of lidocaine provides superior topical airway anaesthesia during non-sedation FB compared with the traditional method.
Shareable abstract
Using a spray nozzle to administer topical lidocaine provides superior cough control, less discomfort from oropharyngeal anaesthesia and improved tolerance of the procedure during non-sedation flexible bronchoscopy compared with the traditional method https://bit.ly/4b4l5lL
Introduction
Flexible bronchoscopy (FB), a common procedure used to examine, diagnose and treat abnormalities in the bronchopulmonary system, has seen significant advancements over the past two decades. The evolution of the bronchoscope itself, along with auxiliary techniques, has greatly expanded its utility. Techniques such as endobronchial ultrasound, which can be either convex or radial probe, navigation technologies, cone-beam computed tomography, cryobiopsy and others have broadened the diagnostic and therapeutic capabilities of FB [1]. As an invasive procedure, ensuring safety and patient comfort are paramount. Reports indicate that FB has a commendable safety profile with a low incidence of complications and mortality [2, 3]. Patient comfort can be enhanced through careful administration of sedatives, analgesics, antitussives and topical anaesthesia [4–6].
Although society guidelines recommend sedation for all bronchoscopic procedures, unless contraindicated [4, 7], several studies have established the feasibility of performing FB without sedation [8–15]. In fact, a non-sedation strategy remains a primary modality in many institutions and nations [14–17]. This approach can be well tolerated with the application of topical anaesthesia [18]. In this regard, a number of studies have been conducted to develop an optimal protocol for topical anaesthesia, with the goal of enhancing patient comfort. For example, a recent study by Dhooria et al. [12] found that 10 actuations of 10% lidocaine oropharyngeal spray were more effective than nebulised lidocaine or a combination of both during non-sedation FB [12]. Another study by Madan et al. [11] found no additional benefit from nebulised lidocaine when used in conjunction with pharyngeal lidocaine spray for non-sedation FB.
Research into the method of delivering topical lidocaine via the “spray-as-you-go” technique is also a noteworthy area in clinical research. When administered under general anaesthesia, the use of a multi-orifice epidural catheter for topical lidocaine delivery has proven superior to traditional methods in reducing both cough severity and lidocaine consumption during FB [19]. In a study conducted by Venkatnarayan et al. [20], the provision of sedation for FB was left to the operator's discretion. Their findings indicated that lidocaine instillation through a spray catheter, as opposed to the working channel, resulted in a reduction in coughing, less need for sedation and improved operator satisfaction [20].
However, a study conducted by Tachihara et al. [21] did not find significant differences between the catheter spray and conventional syringe injection methods of lidocaine administration in terms of cough frequency, haemodynamic change or patient discomfort during non-sedation FB. The lack of significant results in their study may be attributed to the small number of participants and the relatively short observation period. Furthermore, the outcome measures of the study were only evaluated before certain techniques, such as brushing and biopsy, were performed. In light of these findings, we designed a randomised controlled trial to investigate whether the administration of topical lidocaine via a spray nozzle could effectively reduce coughing and patient discomfort and enhance the operator's experience during non-sedation FB compared with the traditional method.
Methods
Study design and setting
From May to August 2023, an assessor-blinded, randomised controlled trial was conducted at the bronchoscopy centre of the National Taiwan University Hospital (Taipei, Taiwan). The Research Ethics Committee of the National Taiwan University Hospital granted approval for the study protocol (202302060RINC) and all study participants provided written informed consent prior to the initiation of study procedures. The study adhered to the ethical principles outlined in the Declaration of Helsinki and was registered at ClinicalTrials.gov (NCT05970848).
At our bronchoscopy centre, the majority of FB procedures, which include airway inspection, bronchial washing and brushing, bronchoalveolar lavage, endobronchial biopsy, and transbronchial lung biopsy, are performed solely under topical anaesthesia [8–10, 13–15]. These procedures are carried out by chest fellows under the direct supervision of experienced pulmonologists. However, transbronchial needle aspiration is typically performed under sedation [22].
Study participants and randomisation
Patients aged ≥18 years, who were referred for FB, were considered for inclusion in this study. Those who met any of the following criteria were excluded: 1) those scheduled for sedation, 2) those with a tracheostomy, 3) those who declined to provide informed consent and 4) those known to be pregnant. Patients were randomised on a 1:1 basis into one of the two study groups using stratified randomisation. A randomisation sequence was generated using the National Cancer Institute Clinical Trial Randomisation Tool before the study began [23]. This sequence, created using MTI (maximum tolerated imbalance) randomisation, was then input into REDCap software [24]. Once entered into REDCap, no study personnel could access the sequence. Randomisation was stratified based on age (≥65 versus <65 years) and sex (male versus female), as these parameters were hypothesised to impact the outcome of interest and were available upon inclusion.
Study protocol
Prior to the insertion of the bronchoscope, patients were administered 0.05 mg of fentanyl intravenously or intramuscularly. Topical anaesthesia to the upper airway was achieved by spraying 10% lidocaine (Xylocaine Spray; AstraZeneca, Sodertalje, Sweden). Each patient received two actuations of 10% lidocaine in both nostrils and six actuations to the oropharynx. The bronchoscope was then introduced via the nasal route and additional lidocaine was administered using the “spray-as-you-go” technique. The standard application of 2% lidocaine (Xylocaine 2%; AstraZeneca) in this study involved administering 1 mL aliquots to specific areas as follows: two aliquots were applied to the vocal cords, and one aliquot each was applied to the trachea, carina, right second carina, left main bronchus and left second carina. Depending on the randomisation result, lidocaine was delivered either through the working channel of the bronchoscope (classical spray (CS) group) or through a washing pipe with a spray nozzle (SN group). A non-sterile, reusable spray catheter (PW-6P-1; Olympus, Tokyo, Japan) was used for this purpose (supplementary figures E1−E4). Additional aliquots of lidocaine (1 mL, 2%) were dispensed at the operator's discretion to alleviate patient cough. Throughout the procedure, patients were provided with oxygen via a nasal cannula, and continuous monitoring of oxygen saturation and ECG was conducted. Blood pressure measurements were taken every 5 min and as needed.
Outcome measures
The primary outcome of interest in this study was the cough rate, defined as the total number of coughs divided by the time (in minutes) from when the bronchoscope entered the vocal cords to when it was withdrawn. An audio recording was made during this period and a single study nurse, who was blinded to the study allocation, later calculated the cough count.
Secondary outcomes included total lidocaine consumption, the lowest oxygen saturation level during the procedure, instances of oxygen desaturation and haemodynamic alteration, as well as the subjective perceptions of both the patient and operator regarding the FB process. A significant event of oxygen desaturation was defined as a transcutaneous oxygen saturation level dropping <90% for a duration of ≥5 s [25]. A relevant haemodynamic alteration was characterised by hypotension or tachy- or bradyarrhythmia that necessitated an intervention or led to the premature termination of the procedure [26]. Immediately following FB, patients were asked to complete a questionnaire (supplementary table E1) to assess their experiences. This included their discomfort from oropharyngeal anaesthesia and FB, the severity of their cough, their tolerance and satisfaction with the examination, and their willingness to undergo FB again. The operators also completed a questionnaire (supplementary table E2) regarding patient discomfort and coughing during FB, and how patient coughing interfered with their operation process. All questionnaire items were rated on a visual analogue scale (VAS), with numerical ratings assigned from 0 to 10.
In this study, we also collected additional variables including patient demographics, smoking status, prior experience with FB, the reasons for the procedure and the specific interventions performed during FB.
Sample size calculation
The sample size for this study was determined based on an assumed effect size of 0.5 for the difference in means of the primary outcome [20]. This calculation was performed using G*Power version 3.1.9.2 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). With a statistical power set at 0.8 and an α error set at 0.05, we estimated that a total of 126 patients would be required to detect a significant difference between the two groups using an independent samples t-test.
Statistical analysis
Data in this study were presented as either mean with standard deviation or median (interquartile range). Categorical variables were analysed using the Chi-squared or Fisher exact test, while continuous variables were compared using the independent samples t-test or Mann–Whitney–Wilcoxon test. All statistical analyses were conducted using SPSS for Windows version 20 (IBM, Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Results
Patient characteristics
During the study period, we enrolled a total of 126 patients, all of whom underwent randomisation (61 were assigned to the CS group and 65 to the SN group). Among the 68 patients who were excluded, 58 underwent sedation, eight had a tracheostomy and two did not provide consent (figure 1). The baseline characteristics were comparable across both study groups (table 1). The most prevalent indication for FB was tumour diagnosis (47%), followed by pulmonary infection (29%). The most frequently performed procedures (table 2) included bronchial washing (61%), followed by transbronchial lung biopsy (21%). Airway inspection alone was performed in 21% of the study subjects. The average time for conducting FB was 12±10 min. There were no significant differences in the procedures performed and their duration between the two patient groups.
FIGURE 1.
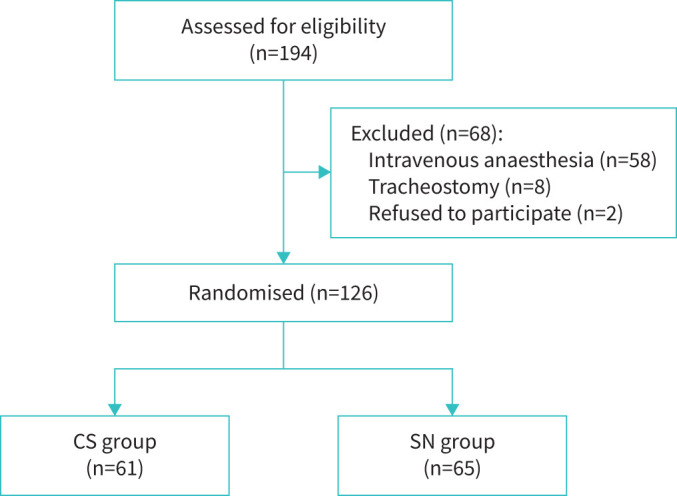
Study flow diagram. CS: classical spray; SN: spray nozzle.
TABLE 1.
Characteristics of the study population
| CS group (n=61) | SN group (n=65) | p-value | |
| Age, years | 63±13 | 63±14 | 0.537 |
| Male | 36 (59) | 40 (62) | 0.772 |
| Ever-smoker | 33 (54) | 34 (52) | 0.840 |
| First-ever bronchoscopy | 45 (74) | 48 (74) | 0.992 |
| Indications | |||
| Tumour diagnosis | 31 (51) | 28 (43) | 0.325 |
| Oesophageal cancer staging | 4 (7) | 9 (14) | |
| Pulmonary infection | 15 (25) | 22 (34) | |
| Haemoptysis | 7 (12) | 2 (3) | |
| Airway inspection | 2 (3) | 1 (2) | |
| Diffuse lung disease | 1 (2) | 1 (2) | |
| Chronic cough | 1 (2) | 2 (3) |
Data are presented as mean±sd or n (%), unless otherwise stated. CS: classical spray; SN: spray nozzle.
TABLE 2.
Details of the bronchoscopic procedures
| CS group (n=61) | SN group (n=65) | p-value | |
| Procedure time, min | 13.6±10.4 | 11.3±9.7 | 0.188 |
| Procedures conducted | |||
| Inspection alone | 17 (28) | 22 (34) | 0.468 |
| Washing | 35 (57) | 42 (65) | 0.405 |
| Brushing | 13 (21) | 13 (20) | 0.856 |
| Bronchoalveolar lavage | 6 (10) | 1 (2) | 0.056 |
| Transbronchial lung biopsy | 15 (25) | 12 (19) | 0.402 |
| Endobronchial biopsy | 3 (5) | 3 (5) | 0.629 |
Data are presented as mean±sd or n (%), unless otherwise stated. CS: classical spray; SN: spray nozzle.
Primary outcome
The average cough rate was notably lower in the SN group (5.2±4.0 counts·min−1) compared with the CS group (9.0±10.5 counts·min−1), with the difference being statistically significant (p=0.01) (table 3). Given that the cough counts were not normally distributed, we also compared the median values between the groups. Consistent with the previous finding, patients in the SN group exhibited a lower median cough rate than those in the CS group (4.5 versus 7.1 counts·min−1; p=0.021).
TABLE 3.
Primary outcome
| CS group (n=61) | SN group (n=65) | p-value | |
| Total cough count, n | |||
| Mean±sd | 116±160 | 63±91 | 0.025 |
| Median (IQR) | 57 (27−144) | 27 (18−69) | 0.007 |
| Cough rate, counts·min−1 | |||
| Mean±sd | 9.0±10.5 | 5.2±4.0 | 0.010 |
| Median (IQR) | 7.1 (2.8−11.7) | 4.5 (2.6−6.3) | 0.021 |
CS: classical spray; SN: spray nozzle; IQR: interquartile range.
Secondary outcomes
The total doses of lidocaine administered during the FB procedure were similar between the two patient groups (table 4). Among the study participants, only one significant adverse event was observed. An 82-year-old female patient in the CS group experienced oxygen desaturation, with levels dropping to 88% during bronchoalveolar lavage. This necessitated the provision of additional oxygen to complete the procedure.
TABLE 4.
Secondary outcomes
| CS group (n=61) | SN group (n=65) | p-value | |
| Total lidocaine dose, mg | 248±16 | 244±11 | 0.198 |
| Nadir SpO2, % | 98±2 | 98±2 | 0.664 |
| Adverse events | |||
| Oxygen desaturation | 1 (2) | 0 | 0.484 |
| Haemodynamic change | 0 | 0 | NA |
| Patient experience | |||
| Discomfort from oropharyngeal anaesthesia | 5.6±2.9 | 4.5±2.7 | 0.039 |
| Discomfort during bronchoscopy | 5.4±2.8 | 5.0±2.5 | 0.408 |
| Cough during the procedure | 5.4±2.7 | 5.0±2.6 | 0.463 |
| Tolerance of the procedure | 5.7±2.7 | 6.8±2.2 | 0.011 |
| Overall rate of satisfaction | 7.9±2.0 | 8.2±1.7 | 0.423 |
| Consent to a re-examination | 5.8±3.4 | 7.2±2.7 | 0.015 |
| Operator experience | |||
| Patient discomfort | 3.4±2.3 | 2.7±1.7 | 0.040 |
| Cough during bronchoscopy | 3.2±2.4 | 2.3±1.5 | 0.013 |
| Interference by cough | 2.3±2.3 | 1.6±1.4 | 0.029 |
Data are presented as mean±sd or n (%), unless otherwise stated. CS: classical spray; SN: spray nozzle. SpO2: oxygen saturation measured by pulse oximetry; NA: not applicable.
From the patients’ perspective (table 4), those in the SN group reported less oropharyngeal discomfort, as indicated by a lower VAS score, compared with those in the CS group (4.5±2.7 versus 5.6±2.9; p=0.039). However, the severity of general discomfort and cough during the FB procedure was similar between the groups. Patients in the SN group reported better tolerance of the procedure than those in the CS group (6.8±2.2 versus 5.7±2.7; p=0.011) and were more likely to be willing to undergo a repeat FB procedure (7.2±2.7 versus 5.8±3.4; p=0.015). Despite these differences, the overall satisfaction scores were comparable between the two study groups.
According to the operators’ ratings on the VAS, the intensity of the patients’ general discomfort (2.7±1.7 versus 3.4±2.3; p=0.040) and cough (2.3±1.5 versus 3.2±2.4; p=0.013) was lower in the SN group compared with the CS group (table 4). Furthermore, the operators reported that coughing episodes interfered less with the procedure in the SN group than in the CS group (1.6±1.4 versus 2.3±2.3; p=0.029).
Discussion
This study's findings indicate that administering topical lidocaine through a spray nozzle offers superior cough control during non-sedation FB compared with the classical spray method. Patients in the SN group reported less discomfort from oropharyngeal anaesthesia and a better tolerance of the procedure than those in the CS group. Furthermore, operators observed lower patient discomfort and cough scores in the SN group compared with the CS group, and reported less disruption due to coughing among SN group patients. Taken together, a spray nozzle is a more effective method for delivering topical lidocaine anaesthesia during non-sedation FB than the traditional approach.
While the use of sedative agents in FB can help reduce patient anxiety and pain, and induce antegrade amnesia, it may also lead to potential complications such as haemodynamic instability, respiratory suppression and additional costs [27]. Factors such as limited anaesthesia resources, high patient volume, post-procedure monitoring requirements or patient preference can also hinder the widespread use of sedation for FB [17, 28]. Therefore, the significance of effectively administering topical lidocaine to the airway during non-sedation FB cannot be overstated. Consistent with previous studies [19, 20], our research indicates that the use of a spray nozzle to administer lidocaine results in superior cough suppression during non-sedation FB compared with delivery through the bronchoscope's working channel. This positive outcome is likely attributable to the aerosolised lidocaine from the nozzle creating a circumferential spray, which distributes the drug more evenly across the mucosa of the bronchial trees, thereby achieving more effective topical anaesthesia. These findings underscore the utility of a spray nozzle for topical anaesthesia during FB in a non-sedation setting and enrich the existing body of knowledge.
Our primary finding was further supported by several secondary outcomes, highlighting the benefits of using a spray nozzle for both patients and operators during FB. Patients reported less oropharyngeal discomfort, better tolerance and a greater willingness to repeat the procedure when a spray nozzle was used for topical anaesthesia. Previous studies have shown that patient comfort during FB procedures directly correlates with their willingness to undergo repeat procedures [29, 30]. From the operator's perspective, using a spray nozzle was found to be superior in reducing patient discomfort and coughing, and in minimising procedure interference due to coughing. Patients in the SN group also reported lower scores for general discomfort and coughing during the procedure, and higher scores for overall satisfaction, although these differences did not reach statistical significance. In summary, our findings consistently indicate that administering lidocaine via a spray nozzle is more effective in achieving superior topical anaesthesia compared with traditional methods.
A key strength of our study lies in the diversity of our patient cohort, which included individuals referred for a variety of FB procedures such as bronchoalveolar lavage, transbronchial lung biopsy and endobronchial biopsy. All participants underwent these interventions in a non-sedation setting, ensuring that our results are applicable to a heterogeneous patient population and affirming the efficacy of a spray nozzle for topical lidocaine airway anaesthesia. In contrast, the study by Venkatnarayan et al. [20] only included patients referred for bronchoalveolar lavage and some of their subjects received intravenous sedation during FB. Similarly, Cai et al. [19] investigated the utility of a spray catheter in sedated patients and did not include those referred for transbronchial lung biopsy. Furthermore, Tachihara et al. [21] counted cough events prior to conducting any FB procedures. These differences highlight the broader applicability and comprehensive approach of our study.
Coughing is one of the most distressing symptoms during FB, potentially leading to significant changes in haemodynamics and an increased risk of post-lung biopsy pneumothorax [31, 32]. Despite our patients in the SN group exhibiting a significantly lower cough rate than those in the CS group, we observed only one instance of oxygen desaturation among our study participants, with no discernible differences in adverse events between the groups. These findings align with previous studies [19, 20] and may be attributed to the excellent safety profile of FB [2, 3].
Lidocaine, due to its favourable safety and pharmacokinetic profiles, is the most commonly used agent for topical anaesthesia [4, 7]. Despite its widespread use, it is important to note that lidocaine can cause rare but potentially serious adverse events such as seizures, hypotension and asystole [33]. Therefore, it is crucial to adhere to the manufacturer's instructions and not exceed a cumulative dose of 400 mg [34]. Research on the use of a spray catheter during FB has demonstrated its effectiveness in reducing total lidocaine consumption while maintaining superior or comparable cough control to conventional lidocaine delivery methods. Contrary to previous studies, our study did not observe a similar effect on lidocaine dose reduction. However, it is noteworthy that the total amount of lidocaine administered to both of our study groups was significantly below the recommended dose limit. This discrepancy could be attributed to our conservative approach towards supplementary lidocaine administration during the FB procedure.
Our study does have a few limitations that warrant discussion. First, the sample size was designed to detect a difference in the primary outcome, which may explain why statistical significance was not achieved for some secondary outcomes. Despite the observed consistency between primary and secondary outcomes, further research involving a larger participant pool may be necessary to address any remaining uncertainties. Second, this study was conducted at a single centre with extensive experience in FB and the results may not be generalisable to institutions with less FB experience. However, as FB is a procedure that can be easily learned and mastered, we anticipate that our findings should be broadly applicable. Lastly, due to inherent procedural limitations, the operators could not be blinded, potentially introducing bias into their evaluation of patient symptoms. However, it is worth noting that the operators’ assessment of patient coughing was consistent with the objective cough rate counted by the blinded study nurse.
In summary, our study demonstrates that the application of lidocaine via a spray nozzle provides superior topical anaesthesia for the airway during non-sedation FB compared with the traditional delivery method.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00913-2023.SUPPLEMENT (375.2KB, pdf)
Acknowledgements
We thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital (Taipei, Taiwan) for technical support during the study. We also thank Yu-Chen Hsieh (Department of Internal Medicine, National Taiwan University Hospital) for assistance with the study.
Provenance: Submitted article, peer reviewed.
This study is registered at ClinicalTrials.gov with identifier number NCT05970848. De-identified data are available from the corresponding author upon reasonable request.
Author contributions: C-T. Huang and C-C. Ho had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. C-T. Huang and H-C. Chou conceived this study. H-C. Chang, C-Y. Yang, S-Y. Lin, L-C. Chang, T-H. Tsai, C-L. Hsu and J-Y. Chien contributed to study design, data analysis and interpretation. C-T. Huang prepared the initial draft of the manuscript. All authors contributed to critical revision of the paper for intellectual content. The authors read and approved the final manuscript.
Conflict of interest: None declared.
Ethics statement: The Research Ethics Committee of the National Taiwan University Hospital granted approval for the study protocol (202302060RINC) and all study participants provided written informed consent prior to the initiation of study procedures.
References
- 1.Chandrika S, Yarmus L. Recent developments in advanced diagnostic bronchoscopy. Eur Respir Rev 2020; 29: 190184. doi: 10.1183/16000617.0184-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis 2009; 71: 8–14. doi: 10.4081/monaldi.2009.370 [DOI] [PubMed] [Google Scholar]
- 3.Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995; 107: 430–432. doi: 10.1378/chest.107.2.430 [DOI] [PubMed] [Google Scholar]
- 4.Wahidi MM, Jain P, Jantz M, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest 2011; 140: 1342–1350. doi: 10.1378/chest.10-3361 [DOI] [PubMed] [Google Scholar]
- 5.José RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev 2013; 22: 106–116. doi: 10.1183/09059180.00006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strohleit D, Galetin T, Kosse N, et al. Guidelines on analgosedation, monitoring, and recovery time for flexible bronchoscopy: a systematic review. BMC Pulm Med 2021; 21: 198. doi: 10.1186/s12890-021-01532-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013; 68: Suppl. 1, i1–i44. doi: 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Ruan SY, Liao WY, et al. Risk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesions. PLoS One 2012; 7: e49125. doi: 10.1371/journal.pone.0049125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CT, Tsai YJ, Ho CC, et al. Atypical cells in pathology of endobronchial ultrasound-guided transbronchial biopsy of peripheral pulmonary lesions: incidence and clinical significance. Surg Endosc 2019; 33: 1783–1788. doi: 10.1007/s00464-018-6452-1 [DOI] [PubMed] [Google Scholar]
- 10.Huang CT, Tsai YJ, Liao WY, et al. Endobronchial ultrasound-guided transbronchial biopsy of peripheral pulmonary lesions: how many specimens are necessary? Respiration 2012; 84: 128–134. doi: 10.1159/000339412 [DOI] [PubMed] [Google Scholar]
- 11.Madan K, Biswal SK, Tiwari P, et al. Nebulized lignocaine for topical anaesthesia in no-sedation bronchoscopy (NEBULA): a randomized, double blind, placebo-controlled trial. Lung India 2019; 36: 288–294. doi: 10.4103/lungindia.lungindia_348_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhooria S, Chaudhary S, Ram B, et al. A randomized trial of nebulized lignocaine, lignocaine spray, or their combination for topical anesthesia during diagnostic flexible bronchoscopy. Chest 2020; 157: 198–204. doi: 10.1016/j.chest.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 13.Huang CT, Tsai YJ, Ho CC, et al. The value of repeat radial-probe endobronchial ultrasound-guided transbronchial biopsy after initial non-diagnostic results in patients with peripheral pulmonary lesions. BMC Pulm Med 2017; 17: 132. doi: 10.1186/s12890-017-0478-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology 2009; 14: 859–864. doi: 10.1111/j.1440-1843.2009.01585.x [DOI] [PubMed] [Google Scholar]
- 15.Huang CT, Ruan SY, Tsai YJ, et al. Experience improves the performance of endobronchial ultrasound-guided transbronchial biopsy for peripheral pulmonary lesions: a learning curve at a medical centre. PLoS One 2017; 12: e0179719. doi: 10.1371/journal.pone.0179719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano F, Aoe M, Ohsaki Y, et al. Bronchoscopic practice in Japan: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2013; 18: 284–290. doi: 10.1111/j.1440-1843.2012.02273.x [DOI] [PubMed] [Google Scholar]
- 17.Madan K, Mohan A, Agarwal R, et al. A survey of flexible bronchoscopy practices in India: the Indian bronchoscopy survey (2017). Lung India 2018; 35: 98–107. doi: 10.4103/lungindia.lungindia_417_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De S. Assessment of patient satisfaction and lidocaine requirement during flexible bronchoscopy without sedation. J Bronchology Interv Pulmonol 2009; 16: 176–179. doi: 10.1097/LBR.0b013e3181afca25 [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, Chen L, Dong D, et al. The utility of a multi-orifice epidural catheter when using the “spray-as-you-go” technique for topical airway anesthesia during flexible bronchoscopy, a randomised trial. J Clin Monit Comput 2023; 37: 55–62. doi: 10.1007/s10877-022-00856-8 [DOI] [PubMed] [Google Scholar]
- 20.Venkatnarayan K, Devaraj U, Krishnaswamy UM, et al. Comparison of spray catheter with “spray-as-you-go” technique for airway anesthesia during flexible bronchoscopy – a randomized trial. Lung India 2020; 37: 384–388. doi: 10.4103/lungindia.lungindia_528_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachihara M, Ishida T, Fukuhara N, et al. Catheter spray of lidocaine: a randomized study of topical anesthesia for flexible bronchoscopy. J Jpn Soc Respir Endosc 2014; 36: 359–363. doi: 10.18907/jjsre.36.4_359 [DOI] [Google Scholar]
- 22.Lin CK, Keng LT, Lim CK, et al. Diagnosis of mediastinal tuberculous lymphadenitis using endobronchial ultrasound-guided transbronchial needle aspiration with rinse fluid polymerase chain reaction. J Formos Med Assoc 2020; 119: 509–515. doi: 10.1016/j.jfma.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute . Clinical Trial Randomization Tool. 2023. https://ctrandomization.cancer.gov Date last accessed: 26 May 2023.
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darie AM, Schumann DM, Laures M, et al. Oxygen desaturation during flexible bronchoscopy with propofol sedation is associated with sleep apnea: the PROSA-Study. Respir Res 2020; 21: 306. doi: 10.1186/s12931-020-01573-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiten EO, Eagan TML, Martinsen EMH, et al. Complications and discomfort after research bronchoscopy in the MicroCOPD study. BMJ Open Respir Res 2020; 7: e000449. doi: 10.1136/bmjresp-2019-000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lentini C, Granlund B. Anesthetic Considerations for Bronchoscopic Procedures. Treasure Island, StatPearls, 2023. [PubMed] [Google Scholar]
- 28.Smyth CM, Stead RJ. Survey of flexible fibreoptic bronchoscopy in the United Kingdom. Eur Respir J 2002; 19: 458–463. doi: 10.1183/09031936.02.00103702 [DOI] [PubMed] [Google Scholar]
- 29.Mitsumune T, Senoh E, Adachi M. Prediction of patient discomfort during fibreoptic bronchoscopy. Respirology 2005; 10: 92–96. doi: 10.1111/j.1440-1843.2005.00642.x [DOI] [PubMed] [Google Scholar]
- 30.Putinati S, Ballerin L, Corbetta L, et al. Patient satisfaction with conscious sedation for bronchoscopy. Chest 1999; 115: 1437–1440. doi: 10.1378/chest.115.5.1437 [DOI] [PubMed] [Google Scholar]
- 31.Kar Kurt Ö, Talay F, Karğı A, et al. Fiberoptik bronkoskopide sedasyon: literatürün gözden geçirilmesi. [Sedation for fiberoptic bronchoscopy: review of the literature.] Tuberk Toraks 2015; 63: 42–47. doi: 10.5578/tt.8849 [DOI] [PubMed] [Google Scholar]
- 32.Stolz D, Chhajed PN, Leuppi JD, et al. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: a randomised, double blind, placebo controlled trial. Thorax 2004; 59: 773–776. doi: 10.1136/thx.2003.019836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose AA, Colt HG. Lidocaine in bronchoscopy: practical use and allergic reactions. J Bronchol Intervent Pulmonol 2008; 15: 163–166. doi: 10.1097/LBR.0b013e31817df77e [DOI] [Google Scholar]
- 34.AstraZeneca . Xylocaine Package Insert. 2013. www.astrazeneca.com/content/dam/az/Country-Sites/Country-sites-single-pagers/Taiwan/Medicines/Xylocaine-Injection-2.pdf Date last accessed: 28 September 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00913-2023.SUPPLEMENT (375.2KB, pdf)


