Abstract
Background
Osteoarthritis (OA) is a common degenerative joint disease characterized by persistent articular cartilage degeneration and synovitis. Oxymatrine (OMT) is a quinzolazine alkaloid extracted from the traditional Chinese medicine, matrine, and possesses anti-inflammatory properties that may help regulate the pathogenesis of OA; however, its mechanism has not been elucidated. This study aimed to investigate the effects of OMT on interleukin-1β (IL-1β)-induced damage and the potential mechanisms of action.
Methods
Chondrocytes were isolated from Sprague-Dawley rats. Toluidine blue and Collagen II immunofluorescence staining were used to determine the purity of the chondrocytes. Thereafter, the chondrocytes were subjected to IL-1β stimulation, both in the presence and absence of OMT, or the autophagy inhibitor 3-methyladenine (3-MA). Cell viability was assessed using the MTT assay and SYTOX Green staining. Additionally, flow cytometry was used to determine cell apoptosis rate and reactive oxygen species (ROS) levels. The protein levels of AKT, mTOR, LC3, P62, matrix metalloproteinase-13, and collagen II were quantitatively analyzed using western blotting. Immunofluorescence was used to assess LC3 expression.
Results
OMT alleviated IL-1β-induced damage in chondrocytes, by increasing the survival rate, reducing the apoptosis rates of chondrocytes, and preventing the degradation of the cartilage matrix. In addition, OMT decreased the ROS levels and inhibited the AKT/mTOR signaling pathway while promoting autophagy in IL-1β treated chondrocytes. However, the effectiveness of OMT in improving chondrocyte viability under IL-1β treatment was limited when autophagy was inhibited by 3-MA.
Conclusions
OMT decreases oxidative stress and inhibits the AKT/mTOR signaling pathway to enhance autophagy, thus inhibiting IL-1β-induced damage. Therefore, OMT may be a novel and effective therapeutic agent for the clinical treatment of OA.
Keywords: Oxymatrine, interleukin-1β, Chondrocytes, AKT/mTOR pathway, Autophagy
Background
Osteoarthritis (OA) is a common degenerative disease characterized by the breakdown of the cartilage matrix, chondrocyte hypertrophy, inflammation of the synovial membrane, and osteophyte formation in joints [1]. As of 2021, more than 22% of individuals above 40 years of age have knee OA [2]. OA has a significant impact on functional impairment and disability, with 80% of having limitations in movement and 25% having difficulty in performing their major daily activities [3]. Historically, OA treatment has focused on managing pain and inflammation using nonsteroidal anti-inflammatory drugs and other medications. However, these therapeutic approaches have proven to be inadequate for providing satisfactory patient outcomes. Consequently, there is an urgent need to explore alternative treatment options for individuals with OA [4].
Chondrocytes are the main cells in articular cartilage and play an important role in maintaining normal physiological functions and cartilage morphology. In OA, the degradation of the extracellular matrix (ECM) and the apoptosis of chondrocytes are two crucial pathogenic events [5, 6]. Autophagy is a self-degradation process and an important protective mechanism against cartilage degeneration and apoptosis. Autophagy serves as a protective mechanism for chondrocytes, preventing apoptosis and cartilage degeneration. Additionally, it enhances the functionality of the cartilage [7–14]. Studies have linked the pathological processes of OA to reduced autophagy in chondrocytes [11]. Impaired autophagy is associated with the development and increased severity of OA [15], and improving autophagy can have therapeutic benefits in OA [16]. Inflammatory cytokines, such as interleukin-1β (IL-1β) can suppress autophagy and contribute to cartilage degeneration in OA [17].
Oxymatrine (OMT) is a quinzolazine alkaloid extracted from the traditional Chinese medicine matrine. It exhibits lower toxicity than matrine, making it an attractive option for further studies. It is currently used as an adjuvant drug for hepatitis, tumors, and other clinical diseases [18, 19]. In recent years, there has been significant interest in the use of OMT because of its effects on oxidative stress, inflammation, and apoptosis [20–22]. Studies have shown that OMT plays a protective role in OA by regulating chondrocyte homogeneity and inhibiting osteoclast generation [23, 24]. It also plays a protective role in chondrocytes by inhibiting NF-κB signaling [24]. These findings suggest that OMT has a potential therapeutic effect on OA. The AKT/mTOR pathway is a well-known autophagy-related signaling pathway that attenuates autophagy when activated [25]. OMT treatment of SW982 human synovial sarcoma cells resulted in reduced expression levels of phosphorylated AKT and phosphorylated mTOR. Based on these findings, we hypothesize that OMT induces autophagy through the same mechanism in OA. However, the mechanisms by which OMT may help regulate the pathogenesis of OA have not been elucidated.
This study aimed to investigate the protective effects of OMT on IL-1β induced chondrocytes and examine its impact on the AKT/mTOR signaling pathway and autophagy. Towards this goal, rat chondrocytes cultured in vitro were used to create a model of IL-1β-induced chondrocyte damage to mimic OA at the cellular level.
Methods
Chondrocyte isolation and cell culture
Articular cartilage was obtained from the knees of 8-week-old specific pathogen-free Sprague-Dawley rats. The cartilage was separated from the subchondral bone and cut into small pieces using sterile scissors and then stored in sterile phosphate-buffered saline (PBS) containing 1% penicillin–streptomycin solution. Primary chondrocytes were isolated by digestion with 0.25% Trypsin-EDTA Solution (Beyotime, Shanghai, China) for 0.5 h at 37 °C in a thermostatic shaker and Type IV collagenase (1 mg/mL, GenView) for 3 h at 37 °C. The cells were resuspended in DMEM/F12 containing 10% fetal bovine serum (GenView). Chondrocytes were cultured in incubator at 37 °C in 5% CO2. The cells were passed to 2–3 generations for subsequent experiments.
All animal experimental procedures and protocols were conducted in conformity with the principles of the Institutional Animal Care and Use Committee of Jinzhou Medical University, approval number 2022031001. All efforts were made to minimize the number of animals used and their suffering.
Toluidine blue staining
The second-generation chondrocytes were seeded into a 24-hole plate and cultured for 72 h. The cells were then washed with PBS, fixed in 4% neutral formalin for 30 min, and stained with 1% toluidine paraformaldehyde for 1 h at 25 °C followed by 2 washes with PBS. Subsequently, the cells were stained with 1% toluidine blue for 2 h at 25 ± 3 °C [26]. Images were captured using an inverted fluorescence microscope after the removal of the staining dye.
Immunohistochemical staining
The second-generation chondrocytes were fixed with 4% paraformaldehyde in 24-well plates for 30 min, washed three times with PBS, permeabilized for 20 min with 0.5% Triton X-100 in PBS at 4 °C, blocked for 30 min with 3% BSA, and probed overnight at 4 °C with anti-Collagen II (1:100) or LC3(1:100) in 3% BSA. After three rinses with 3% BSA, the cells were probed for 3 h with a secondary antibody (EarthOx, 1:50) in 3% BSA at room temperature, and the nuclei were stained with DAPI for 10 min [27]. Images were captured using an inverted fluorescence microscope (Nikon).
Experimental grouping and drug administration
To assess the effectiveness of OMT (Biopurify, Chengdu, China), the second-generation chondrocytes were divided into the control group (Con group), the IL-1β (ABclonal) group, and the IL-1β + OMT (0.25, 0.50, 1.00 mg/mL OMT) group. In the control group, the chondrocytes were cultured in complete medium for 24 h. In the IL-1β group, the chondrocytes were cultured in complete medium containing 10 ng/mL IL-1β for 24 h. In the IL-1β + OMT group, the chondrocytes were initially incubated in complete culture medium containing 0.25, 0.50, or 1.00 mg/mL OMT for 2 h. Subsequently, IL-1β was added to achieve a final concentration of 10 ng/mL in the culture medium, and the chondrocytes were further incubated for 24 h.
To investigate the role of autophagy in the effectiveness of OMT, chondrocytes were treated with 3-MA(5 mM) (MCE, Shanghai, China) for 2 h [28], followed by the addition of complete culture medium containing 1 mg/mL OMT for an additional 2 h. Subsequently, IL-1β was introduced into the complete culture medium to attain a final concentration of 10 ng/mL, and the cells were further incubated for a total of 24 h.
Cell viability assay
Cell viability was determined using the MTT assay. Chondrocytes were seeded in 96-well plates (5000 cells/well) and treated as experimental groups for drug administration. Chondrocytes were incubated with the MTT reagent (Solarbio, Beijing, China) at 37 °C for 4 h. Afterward, dimethyl sulfoxide (Solarbio) was added to dissolve the formazan product, and absorbance at 570 nm was examined using a microplate reader (Allsheng, Hangzhou, China). Three independent assays were performed.
SYTOX green staining
SYTOX Green (Baiao Laibo, Beijing, China) is an excellent green fluorescent nuclear and chromosome counterstain that is impermeant to live cells but penetrates the compromised membrane characteristics of dead cells, making it a useful indicator of dead cells within a population. Chondrocytes were cultured in 12-well plates for 24 h and subjected to the experiments and drug administration. Then, cells were washed three times with PBS, and 1 µM SYTOX Green dead cell stain was added to each hole and mixed in the dark for 10 min at room temperature.
Apoptosis assays
The apoptosis rate was evaluated using the Annexin V-FITC/PI (4 A Biotech, China) assay according to the manufacturer’s instructions. Chondrocytes were plated in 6-well plates and subjected to the experiments and drug administration. Following treatment, the cells were collected and washed with PBS and then resuspended in 1 mL binding buffer. Thereafter, 5 µL Annexin V-FITC was added to the cell suspension, and the cells were further incubated for 5 min at room temperature in the dark. Further, 10 µL PI and 400 µL PBS were added to the cell suspension. Cell fluorescence was assessed using flow cytometry within 1 h.
Detection of reactive oxygen species
Reactive oxygen species (ROS) levels were evaluated using an ROS Assay Kit (Beyotime, Shanghai, China). Chondrocytes were plated in 6-well plates and subjected to the experiments and drug administration. The cultured chondrocytes were initially washed twice with 1×PBS, and then ROS levels were detected according to the manufacturer’s instructions. Cell fluorescence within 1 h was assessed using flow cytometry.
Western blot
Cells were lysed in a lysis buffer containing phenylmethanesulfonyl fluoride (RIPA: PMSF = 100:1; Beyotime, Shanghai, China). The lysates were centrifuged at 12,000 ×g for 20 min at 4 °C, after which protein concentrations were measured using a Lowry method. Samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and sealed for 2 h. Blots were probed overnight at 4 °C with appropriate antibodies and then incubated for 1 h with appropriate secondary antibodies (ABclone, 1:8000). The proteins were visualized using enhanced chemiluminescence (Tanon, Beijing, China). The primary antibodies used were specific for β-actin (AC004, ABclone, 1:50000), matrix metalloproteinase-13 (MMP-13)(18,165,Proteintech, 1:1000), Collagen II (ABS130072, Absin, 1:1000), AKT (ABS130889, Absin, 1:1000), mTOR (AP0115, ABclone, 1:1000), p62 (A11483, ABclone, 1:1000), and LC3B (A19665, ABclone, 1:1000).
Statistical analysis
Data are expressed as the mean ± standard deviation. One-way analysis of variance followed by Tukey’s test was to compare among three or more groups. All experiments were performed at least three times. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software Inc, California, USA). P < 0.05 was considered statistically significant.
Results
Identification of chondrocytes
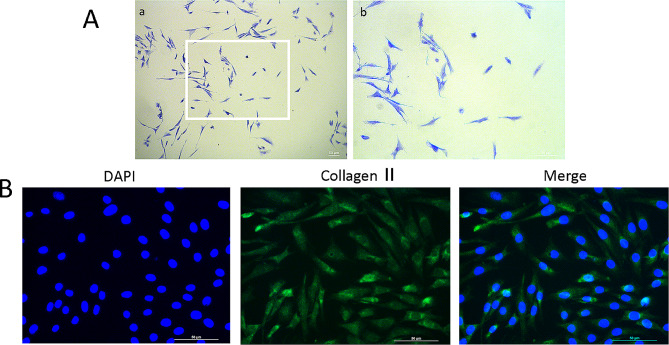
The main indicator of chondrocytes is proteoglycans, which can be stained with toluidine blue to appear blue-purple. In this study, the second-generation chondrocytes exhibited a blue-purple color, along with an elongated and spindle-shaped morphology, confirming their identity as chondrocytes (Fig. 1A). To further validate the isolated cells and assess their purity, we performed immunofluorescence staining for type II collagen, a chondrocyte-specific marker. Over 98% of cultured cells stained positive for collagen II, indicating their suitability for subsequent experiments (Fig. 1B).
Fig. 1.
Morphological observations and identification of rat chondrocytes. (a) Toluidine blue-stained chondrocytes are observed under an inverted-phase contrast microscope. (b) Collagen II immunofluorescence-stained cultured chondrocytes are observed under fluorescence microscope. Scale bar = 50 μm
OMT improves IL‑1β treated chondrocyte viability
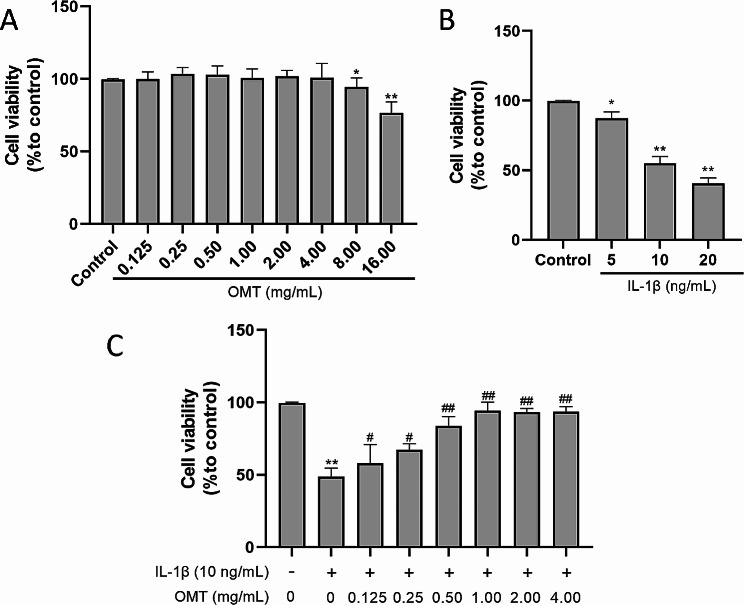
To verify the toxicity of OMT, chondrocytes were cultured with different concentrations of OMT, and cell viability was measured using the MTT assay. The results are shown in Fig. 2A. OMT exerted no significant cytotoxic effects at concentrations below 4 mg/mL. To determine the optimal concentration of IL-1β injury for chondrocytes, cell viability was assessed following a 24-hour culture with 5, 10, and 20 ng/mL of IL-1β. Notably, the results revealed that the cell viability was close to 50% in the group treated with 10 ng/mL of IL-1β, suggesting it to be the most suitable concentration. Subsequent experiments were performed using this concentration, and the corresponding results are shown in Fig. 2B. To assess the protective effects of OMT, chondrocytes were pretreated with varying concentrations of OMT (ranging from 0.125 mg/mL to 4.00 mg/mL) for 2 h. Thereafter, the chondrocytes were subjected to injury induced by 10 ng/mL IL-1β for 24 h. The results demonstrated that compared with the IL-1β group, the OMT group showed significantly higher cell viability that increased in parallel with the increasing concentration of OMT (P < 0.01 or 0.05). However, no further increase in cell viability was observed beyond a concentration of 1.00 mg/mL. To establish a meaningful dose-response relationship, subsequent experiments were conducted using three doses: 0.25, 0.50, and 1.00 mg/mL (Fig. 2C).
Fig. 2.
OMT improves IL-1β treated chondrocyte viability. (a) The cell viability of chondrocytes treated with different concentrations of OMT for 24 h as detected with the MTT assay. (b) The cell viability of chondrocytes treated with different concentrations of IL-1β for 24 h as detected with the MTT assay. (c) The effect of OMT on cell viability of chondrocytes treated with IL-1β for 24 h as detected with the MTT assay. Data are presented as the mean ± SD, n = 9 **P < 0.01, *P < 0.05 versus Con group; ##P < 0.01, #P < 0.05 versus IL-1β group
OMT mitigates IL-1β-induced damage in chondrocytes
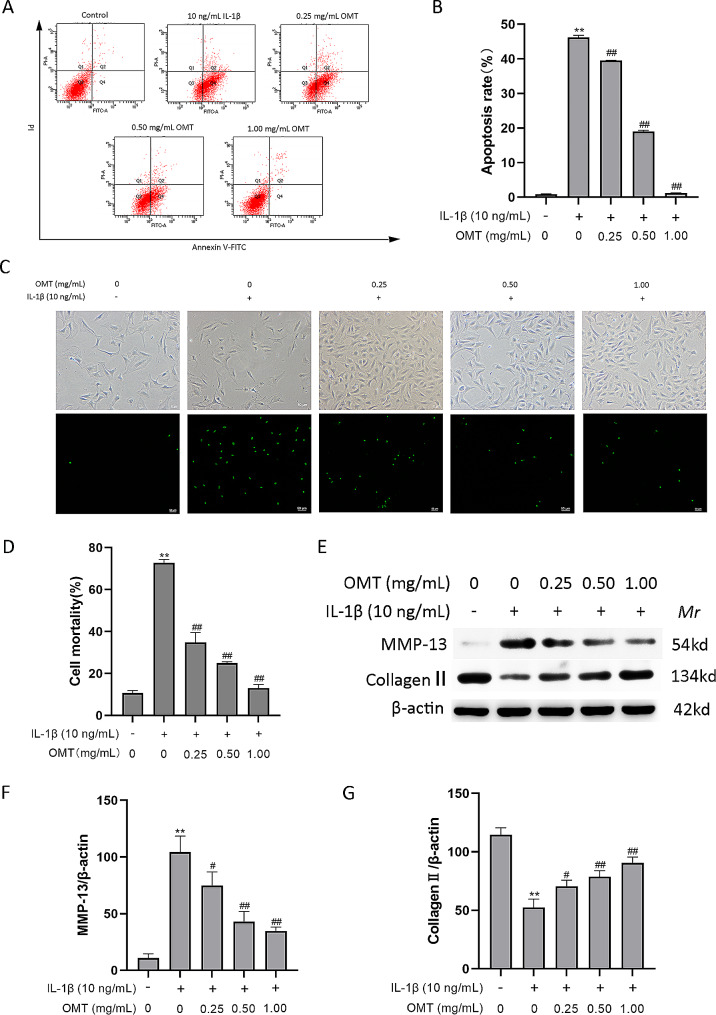
Cartilage damage caused by chondrocyte apoptosis plays a crucial role in OA [29]. To assess the extent of apoptosis, we conducted an Annexin V-FITC/PI assay using flow cytometry. The results indicate that the apoptosis rates of chondrocytes increased following IL-1β treatment (Fig. 3A and B). However, upon the addition of OMT at concentrations of 0.25, 0.50, and 1.00 mg/mL, the percentage of cells undergoing IL-1β-induced apoptosis decreased significantly from 46.23 ± 0.57% to 39.53 ± 0.06%, 19.03 ± 0.31%, and 1.23 ± 0.06%, respectively. Furthermore, SYTOX Green staining revealed increased chondrocyte damage in IL-1β-induced chondrocytes, and this was attenuated by OMT treatment (Fig. 3C, D). The degradation of the cartilage matrix caused by IL-1β is facilitated by catabolic enzymes such as MMPs, among which MMP-13 plays a significant role by breaking down the primary constituent of the extracellular matrix. Collagen II, a key component of the cartilage matrix, frequently undergoes degradation and reduction in cartilage affected by OA [30]. Western blot analysis in this study demonstrated that OMT increased the protein levels of collagen II and decreased those of MMP-13 (Fig. 3E-G).
Fig. 3.
OMT mitigates IL-1β-induced injury in chondrocytes. (a, b) The cell apoptosis rates of chondrocytes as detected with Annexin V-FITC/PI assay using flow cytometry. (c, d) The cell damage rate of chondrocytes as detected with SYTOX Green staining. (e–g) The protein levels of collagen II and MMP-13 as detected with Western blot. Data are presented as the mean ± SD. A, B: n = 3/group; D–G: n = 3/group. **P < 0.01, *P < 0.05 versus Con group; ##P < 0.01, #P < 0.05 versus IL-1β group
OMT decreases ROS levels in chondrocytes treated with IL‑1β
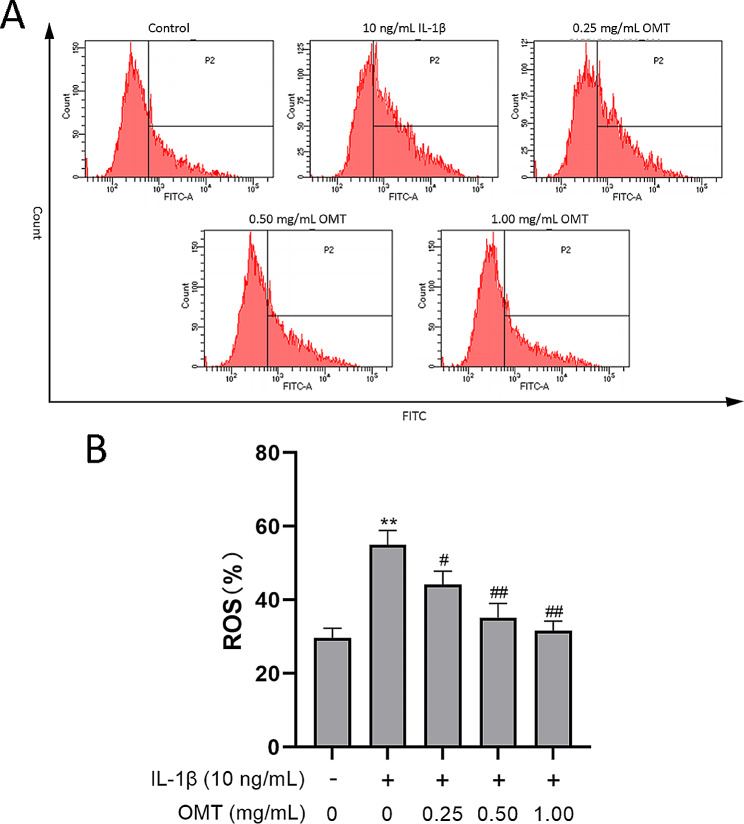
In this study, DCFH-DA was chosen to demonstrate whether OMT attenuated the levels of IL-1β-induced ROS in chondrocytes. The results showed that OMT at concentrations of 0.25, 0.50, and 1.00 mg/mL decreased ROS production (Fig. 4A) by 48.4%, 39.6%, and 34.6%, respectively (Fig. 4B). Further, OMT protected chondrocytes from an IL-1β-induced increase in ROS level.
Fig. 4.
OMT decreases ROS levels in chondrocytes treated with IL‑1β. (a, b) The ROS level as assessed with the DCFH-DA method using flow cytometry. Data are presented as the mean ± SD, n = 3/group. **P < 0.01, *P < 0.05 versus Con group; ##P < 0.01, #P < 0.05 versus IL-1β group
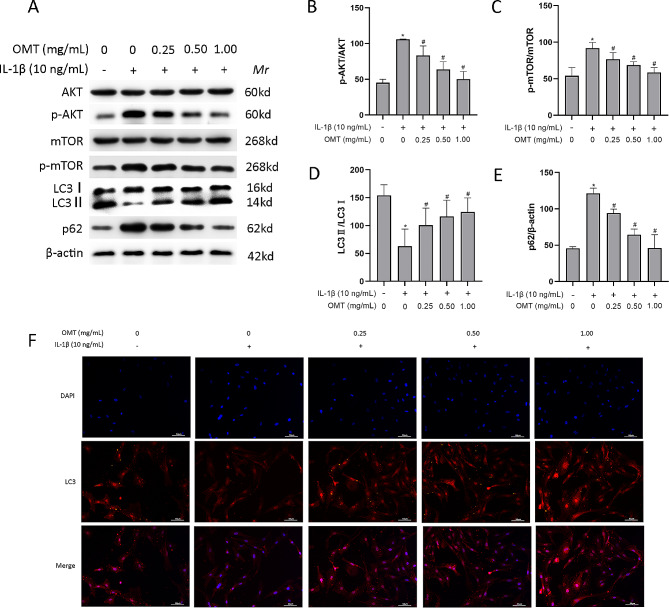
OMT decreases AKT/mTOR signaling pathway and increases autophagy in chondrocytes treated with IL‑1β
The AKT/mTOR signaling pathway is intricately linked to apoptosis and autophagy in chondrocytes. Western blot analysis was performed to assess the levels of AKT and mTOR in chondrocytes in each experimental group. The results showed that IL-1β increased the ratio of p-AKT/AKT and p-mTOR/mTOR, and OMT inhibited this increase. In addition, IL-1β decreased the ratio of LC3 II:I and increased the level of p62, while OMT exerted the opposite effect (Fig. 5A-E). This pattern was also observed for the fluorescence intensity of LC3(Fig. 5F). Collectively, these findings suggested that OMT played a protective role against osteoarthritis by activating autophagy.
Fig. 5.
OMT decreases the AKT/mTOR signaling pathway and increases autophagy in chondrocytes treated with IL‑1β. (a–e) Western blot analysis and quantitative analysis of p-AKT, AKT, p-mTOR, mTOR, p62, and LC3 in chondrocytes. (f) LC3 expression is detected using the immunofluorescence assay. Data are presented as the mean ± SD, n = 3/group. **P < 0.01, *P < 0.05 versus Con group; ##P < 0.01, #P < 0.05 versus IL-1β group
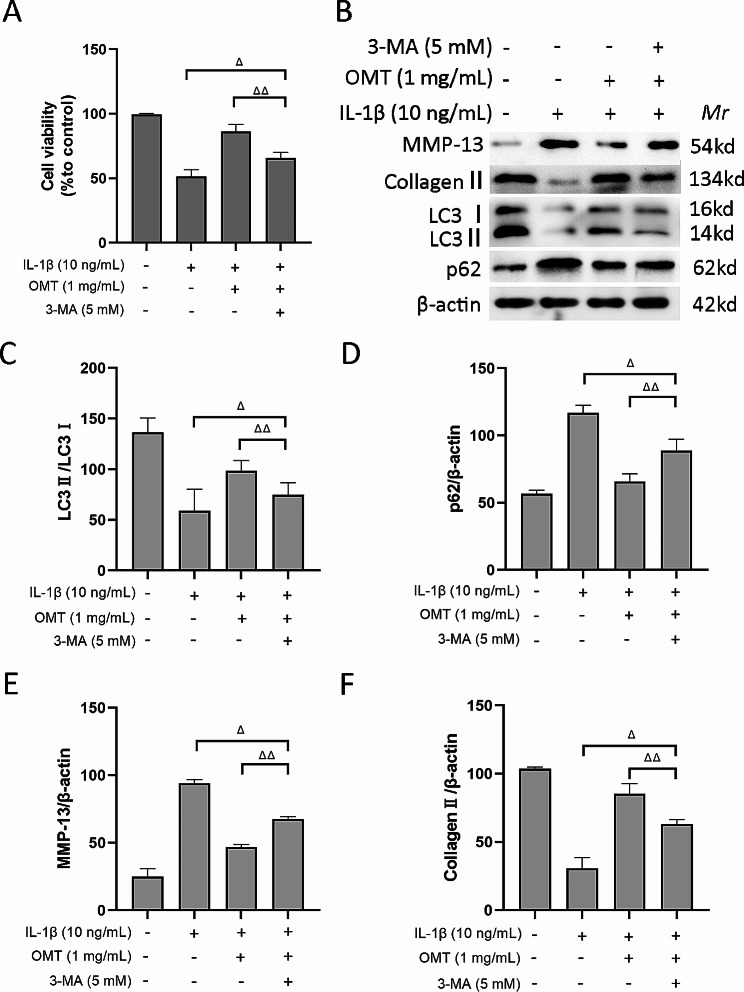
Effectiveness of OMT in improving chondrocyte viability under IL-1β treatment is limited in the presence of the autophagy inhibitor 3-MA
To further verify the role of autophagy in the protective effects of OMT, we performed experiments using 3-MA, a specific autophagy inhibitor. The results of the cell viability assay revealed that chondrocyte viability was significantly lower in the IL-1β + OMT + 3-MA group than in the IL-1β + OMT group (P < 0.01). However, chondrocyte viability was significantly higher in the IL-1β + OMT + 3-MA group than in the IL-1β group (P < 0.05) (Fig. 6A, B). These findings indicated that 3-MA effectively inhibited the protective effect of OMT in these cells. Additionally, Western blot analysis of the ECM and autophagy-related proteins demonstrated that the IL-1β + OMT + 3-MA group had a lower LC3 II:I ratio and a higher p62 level than the IL-1β + OMT group, suggesting that 3-MA could decrease autophagy (Fig. 6C, D). Furthermore, compared to the IL-1β + OMT group, the IL-1β + OMT + 3-MA group showed higher MMP-13 protein expression and lower collagen II expression (Fig. 6E, F), indicating that 3-MA significantly diminished the beneficial effects of OMT on the ECM.
Fig. 6.
Role of autophagy in OMT protection. (a) The cell viability of chondrocytes detected by MTT assay. (b-f) Western blot analysis and quantitative analysis of MMP-13, collagen II, p62, and LC3. Data are presented as the mean ± SD. A: n = 9/group; B-F: n = 3/group. △P < 0.05; △△P < 0.01
Discussion
The mechanism by which OMT may regulate the pathogenesis of OA is unclear. The current study found that OMT effectively alleviated IL-1β-induced damage in chondrocytes. The underlying mechanism involved the activation of autophagy through inhibition of the AKT/mTOR pathway in chondrocytes. These findings provide baseline evidence for future applications of OMT in OA treatment.
IL-1β plays a significant role in the development of OA by promoting the degradation of the ECM of articular cartilage [31–33]. Furthermore, IL-1β is a key factor in triggering chondrocyte apoptosis, making it widely utilized as an apoptosis-inducing agent for chondrocyte studies [34, 35]. Chondrocytes treated with IL-1β offer a valuable in vitro model for studying OA chondrocytes [36, 37]. In OA, apoptosis and degradation of the chondrocyte ECM are significant pathological events [5]. Apoptosis has been observed in OA cartilage, indicating its role in the development of the disease [38]. Apoptosis is associated with cartilage damage and reduced cell density [39]. Therefore, apoptosis is a potential target for OA treatment, and understanding apoptosis is crucial for the development of new therapeutic strategies [40, 41].
Irreversible degradation of ECM is a central aspect of the pathological process of OA [42]. Chondrocytes are involved in ECM biosynthesis and collagen II degradation, which are important signals in OA [43, 44]. MMP-13 is the primary enzyme that contributes to cartilage degradation by cleaving type II collagen [45, 46]. The current study demonstrates that OMT significantly enhances the survival of chondrocytes, reduces apoptosis rates, and prevents IL-1β-induced degradation of the cartilage matrix. These findings suggest that OMT possesses a protective effect against chondrocyte damage induced by IL-1β.
Oxidative stress significantly contributes to the development of osteoarthritis. An imbalance between ROS production and the antioxidant capacity of chondrocytes leads to cartilage degradation and chondrocyte apoptosis [47–50]. In the current study, IL-1β was utilized to induce damage to chondrocytes, mimicking the cellular model of OA. The experimental results demonstrate that OMT has an inhibitory effect on IL-1β-induced elevation of ROS in chondrocytes, thereby suggesting that OMT may provide a protective effect by suppressing oxidative stress. Excessive ROS levels not only lead to oxidative damage but also disrupt cell signaling pathways, including the AKT/mTOR pathway [51]. The AKT/mTOR pathway, which involves more than 150 proteins, plays a crucial role in maintaining joint health and is thus involved in OA development [52, 53]. This study found that OMT effectively reduced the IL-1β-induced activation of the AKT/mTOR signaling pathway. This suggests that OMT exerts its effects by inhibiting the AKT/mTOR pathway.
mTOR is a crucial suppressor of autophagy and is primarily regulated by upstream signaling molecules involving AKT [53–55]. Autophagy, a vital regulator of energy utilization and nutrient metabolism, is involved in cellular homeostasis by eliminating dysfunctional and damaged macromolecules and organelles [56]. Autophagy failure can result in death at the cellular level [56]. The transition from autophagy to apoptosis plays a significant role in the progression of chondrocytes to OA [57]. mTOR upregulation in the OA cartilage is associated with increased chondrocyte apoptosis and reduced expression of autophagy-related genes. In mice, the cartilage knockdown of mTOR results in elevated autophagy, decreased apoptosis, and altered cartilage homeostasis [58]. Administration of the mTOR inhibitor rapamycin mitigates the severity of experimental OA by stimulating autophagy [59].
LC3 conversion (i.e., from LC3-I to LC3-II) reflects the progression of autophagy, and LC3 detection by immunoblotting is often used to monitor autophagic activity [60]. The p62 protein, also called sequestosome 1, is degraded during autophagy and serves as a marker for studying autophagic flux [61]. The present study found that IL-1β suppressed the level of autophagy as indicated by the reduced ratio of LC3 II:I and increased protein level of p62 in chondrocytes. After treatment with OMT, the levels of autophagy were enhanced in IL-1β-treated chondrocytes.
In further experiments using the autophagy inhibitor 3-MA to verify the role of autophagy in OMT protection, the effect of OMT on chondrocyte autophagy was significantly weakened after the addition of 3-MA. This was mainly manifested as a decreased ratio of LC3 II:I and an increased level of p62. Simultaneously, the protective effect of OMT on chondrocytes was significantly weakened, and this was mainly reflected as decreased rate of cell survival, increased protein level of ECM MMP-13, and decreased level of collagen II. These results suggest that autophagy plays an important role in OMT protection. We also notice that Autophagy serves dual roles in OA, acting both to protect cells and promote cell death [62, 63]. It can influence the survival and death of chondrocytes during different stages of osteoarthritis progression [62, 64]. So, we need to use difference model representing different stages of OA to demonstrate the effectiveness of OMT. This will help to identify candidates for benefiting from OMT treatment.
In summary, IL-1β can induce chondrocyte apoptosis and decrease ECM synthesis, increase ROS production, activate the AKT/mTOR pathway, and inhibit autophagy. OMT can alleviate chondrocyte apoptosis and ECM synthesis induced by IL-1β. The mechanism may be related to the inhibition of ROS production, inhibition of the AKT/mTOR pathway, and activation of autophagy, suggesting that OMT can be used to treat OA.
Our study had some limitations. First, the data obtained from in vitro experiments may differ from those obtained from in vivo experiments. Therefore, the curative effect of OMT in OA requires further investigation. Second, Studying the effects of combining 3-MA and OMT on the AKT-mTOR signaling pathway would improve the paper’s clarity.
Conclusions
OMT has a protective effect against IL-1β-induced chondrocyte damage. The potential mechanism involves the reduction of oxidative stress and inhibition of the AKT/mTOR signaling pathway, thereby promoting autophagy. These findings suggest that OMT is a promising and effective therapeutic option for the clinical management of OA.
Acknowledgements
None.
Abbreviations
- ECM
Extracellular matrix
- IL-1β
Interleukin-1β
- MMP-13
Matrix metalloproteinase-13
- OA
Osteoarthritis
- OMT
Oxymatrine
- PBS
Phosphate-buffered saline
- ROS
Reactive oxygen species
Author contributions
Yang J, Lu JY and Zhao XM concepted and designed the study, and wrote the main manuscript text; Lu JY and Bian J handled the materials and data acquisition; Zhao Y, Wang YT and Wang G analyzed and interpreted the data, and performed literature searches; All authors reviewed the manuscript.
Funding
This work was supported by the General Project of the Liaoning Provincial Department of Education (2021LJKZ0823).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All animal experimental procedures and protocols were conducted in accordance with the principles of the Institutional Animal Care and Use Committee of Jinzhou Medical University (approval number: 2022031001). All efforts were made to minimize the number of animals used and their suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–25. doi: 10.1016/j.joca.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quicke JG, Conaghan PG, Corp N, Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthritis Cartilage. 2022;30(2):196–206. doi: 10.1016/j.joca.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Oo WM, Little C, Duong V, Hunter DJ. The development of Disease-Modifying therapies for Osteoarthritis (DMOADs): the evidence to date. Drug Des Devel Ther. 2021;15:2921–45. doi: 10.2147/DDDT.S295224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Feng K, Li J, Yu D, Fan Q, Tang T, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 Signaling pathways Induced Autophagy. Nutrients. 2017;9(4):414. doi: 10.3390/nu9040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue H, Tu Y, Ma T, Liu X, Wen T, Cai M, et al. Lactoferrin inhibits IL-1β-Induced Chondrocyte apoptosis through AKT1-Induced CREB1 activation. Cell Physiol Biochem. 2015;36(6):2456–65. doi: 10.1159/000430206. [DOI] [PubMed] [Google Scholar]
- 6.Cai C, Min S, Yan B, Liu W, Yang X, Li L, et al. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging. 2019;11(16):6371–84. doi: 10.18632/aging.102194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Han G, Zhang Y, Li H. The combination treatment of Curcumin and Probucol protects chondrocytes from TNF-α Induced inflammation by enhancing autophagy and reducing apoptosis via the PI3K-Akt-mTOR pathway. Oxid Med Cell Longev. 2021;2021:5558066. doi: 10.1155/2021/5558066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai S, Fan J, Zhang Y, Hao Z, Yu H, Zhang Z. Celastrol promotes chondrocyte autophagy by regulating mTOR expression. Chin Med J (Engl) 2022;135(1):92–4. doi: 10.1097/CM9.0000000000001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67(4):966–76. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotz MK, Caramés B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7(10):579–87. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng NT, Guo A, Meng H. The protective role of autophagy in experimental osteoarthritis, and the therapeutic effects of Torin 1 on osteoarthritis by activating autophagy. BMC Musculoskelet Disord. 2016;17:150. doi: 10.1186/s12891-016-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng K, Chen H, Xu C. Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition. Inflamm Res. 2020;69(4):385–400. doi: 10.1007/s00011-020-01327-z. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZM, Du SH, Huang LG, Li JH, Xiao L, Tong P. Leptin promotes apoptosis and inhibits autophagy of chondrocytes through upregulating lysyl oxidase-like 3 during osteoarthritis pathogenesis. Osteoarthritis Cartilage. 2016;24(7):1246–53. doi: 10.1016/j.joca.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Jeon H, Im GI. Autophagy in osteoarthritis. Connect Tissue Res. 2017;58(6):497–508. doi: 10.1080/03008207.2016.1240790. [DOI] [PubMed] [Google Scholar]
- 15.Vinatier C, Domínguez E, Guicheux J, Caramés B. Role of the Inflammation-Autophagy-Senescence Integrative Network in Osteoarthritis. Front Physiol. 2018;9:706. doi: 10.3389/fphys.2018.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Mo Y, Xin D, Zeng L, Yue Z, Xu C. β-ecdysterone alleviates osteoarthritis by activating autophagy in chondrocytes through regulating PI3K/AKT/mTOR signal pathway. Am J Transl Res. 2020;12(11):7174–86. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Li G, Wang Y, Jiang R, Li Y, Wang H, et al. Microbial Metabolite Sodium Butyrate attenuates cartilage degradation by restoring impaired Autophagy and Autophagic Flux in Osteoarthritis Development. Front Pharmacol. 2021;12:659597. doi: 10.3389/fphar.2021.659597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, et al. Biomarkers of Chondrocyte apoptosis and autophagy in Osteoarthritis. Int J Mol Sci. 2015;16(9):20560–75. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Sang W, Wang C, Lu H, Zhang T, Wang Z, et al. Oxymatrine exerts protective effects on osteoarthritis via modulating chondrocyte homoeostasis and suppressing osteoclastogenesis. J Cell Mol Med. 2018;22(8):3941–54. doi: 10.1111/jcmm.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi HJ, Zhou H, Ma AL, Wang L, Gao Q, Zhang N, et al. Oxymatrine therapy inhibited epidermal cell proliferation and apoptosis in severe plaque psoriasis. Br J Dermatol. 2019;181(5):1028–37. doi: 10.1111/bjd.17852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge XH, Shao L, Zhu GJ. Oxymatrine attenuates brain hypoxic-ischemic injury from apoptosis and oxidative stress: role of p-Akt/GSK3β/HO-1/Nrf-2 signaling pathway. Metab Brain Dis. 2018;33(6):1869–75. doi: 10.1007/s11011-018-0293-4. [DOI] [PubMed] [Google Scholar]
- 22.Lan X, Zhao J, Zhang Y, Chen Y, Liu Y, Xu F. Oxymatrine exerts organ- and tissue-protective effects by regulating inflammation, oxidative stress, apoptosis, and fibrosis: from bench to bedside. Pharmacol Res. 2020;151:104541. doi: 10.1016/j.phrs.2019.104541. [DOI] [PubMed] [Google Scholar]
- 23.Adamczyk M. Transglutaminase 2 in cartilage homoeostasis: novel links with inflammatory osteoarthritis. Amino Acids. 2017;49(3):625–33. doi: 10.1007/s00726-016-2305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohetaer D, Cao L, Wang Y. Oxymatrine protects chondrocytes against IL-1β-triggered apoptosis in Vitro and inhibits osteoarthritis in mice Model. Evid Based Complement Alternat Med. 2022;2022:2745946. doi: 10.1155/2022/2745946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Sun X, Gong X, Wang G. Human umbilical cord mesenchymal stem cells derived exosomes exert antiapoptosis effect via activating PI3K/Akt/mTOR pathway on H9C2 cells. J Cell Biochem. 2019;120(9):14455–64. doi: 10.1002/jcb.28705. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Du M, Liu X, Chen W, Wu M, Lin J, et al. Millimeter wave treatment promotes chondrocyte proliferation by upregulating the expression of cyclin-dependent kinase 2 and cyclin A. Int J Mol Med. 2010;26(1):77–84. doi: 10.3892/ijmm.2013.1559. [DOI] [PubMed] [Google Scholar]
- 27.Cai L, Ye H, Yu F, Li H, Chen J, Liu X. Effects of Bauhinia Championii (Benth.) Benth. Polysaccharides on the proliferation and cell cycle of chondrocytes. Mol Med Rep. 2013;7(5):1624–30. doi: 10.3892/mmr.2013.1368. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Zhang X, Li Z, Wen Z, Lin Y. Activation of autophagy contributes to the protective effects of lycopene against oxidative stress-induced apoptosis in rat chondrocytes. Phytother Res. 2021;35(7):4032–45. doi: 10.1002/ptr.7127. [DOI] [PubMed] [Google Scholar]
- 29.Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16(3):6093–112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. doi: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Moreno D, Jiménez G, Gálvez-Martín P, Rus G, Marchal JA. Cartilage biomechanics: a key factor for osteoarthritis regenerative medicine. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1067–75. doi: 10.1016/j.bbadis.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Chevalier X, Conrozier T, Richette P. Desperately looking for the right target in osteoarthritis: the anti-IL-1 strategy. Arthritis Res Ther. 2011;13(4):124. doi: 10.1186/ar3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yudoh K, Karasawa R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (OA) Aging. 2010;2(12):990–8. doi: 10.18632/aging.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, et al. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14(7):660–9. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad N, Ansari MY, Bano S, Haqqi TM. Imperatorin suppresses IL-1β-induced iNOS expression via inhibiting ERK-MAPK/AP1 signaling in primary human OA chondrocytes. Int Immunopharmacol. 2020;85:106612. doi: 10.1016/j.intimp.2020.106612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai H, Zhang Z, Li Y, Song X, Ma T, Liu C, et al. L-Theanine reduced the development of knee osteoarthritis in rats via its anti-inflammation and anti-matrix degradation actions: in vivo and in Vitro Study. Nutrients. 2020;12(7):1988. doi: 10.3390/nu12071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu W, He Z, Shi J, Wang Z, Wu W, Liu J, et al. AMD3100 attenuates post-traumatic osteoarthritis by maintaining transforming growth Factor-β1-Induced expression of tissue inhibitor of Metalloproteinase-3 via the phosphatidylinositol 3-Kinase/Akt Pathway. Front Pharmacol. 2019;10:1554. doi: 10.3389/fphar.2019.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galanti C, Musumeci G, Valentino J, Giunta S, Castorina S. A role for apoptosis in temporomandibularjoint disc degeneration. A contemporary review. Ital J Anat Embryol. 2013;118(1):151–8. [PubMed] [Google Scholar]
- 39.Zamli Z, Adams MA, Tarlton JF, Sharif M. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int J Mol Sci. 2013;14(9):17729–43. doi: 10.3390/ijms140917729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlier E, Relic B, Deroyer C, Malaise O, Neuville S, Collée J et al. Insights on Molecular mechanisms of chondrocytes Death in Osteoarthritis. Int J Mol Sci 2016;17(12). [DOI] [PMC free article] [PubMed]
- 41.Li G, Tan W, Fang Y, Wu X, Zhou W, Zhang C, et al. circFADS2 protects LPS-treated chondrocytes from apoptosis acting as an interceptor of miR-498/mTOR cross-talking. Aging. 2019;11(10):3348–61. doi: 10.18632/aging.101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malemud CJ. Biologic basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27(3):289–94. doi: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Q, Ecker M. Overview of MMP-13 as a Promising Target for the treatment of Osteoarthritis. 2021;22(4):1742. [DOI] [PMC free article] [PubMed]
- 44.Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Z, Xu L, He Y, Xu K, Chen Z, Moqbel SAA, et al. DUSP5 suppresses interleukin-1β-induced chondrocyte inflammation and ameliorates osteoarthritis in rats. Aging. 2020;12(24):26029–46. doi: 10.18632/aging.202252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins JA, Wood ST, Nelson KJ, Rowe MA, Carlson CS, Chubinskaya S, et al. Oxidative stress promotes Peroxiredoxin Hyperoxidation and attenuates Pro-survival Signaling in Aging chondrocytes. J Biol Chem. 2016;291(13):6641–54. doi: 10.1074/jbc.M115.693523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlo MD, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–30. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 49.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–20. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Litherland GJ, Dixon C, Lakey RL, Robson T, Jones D, Young DA, et al. Synergistic collagenase expression and cartilage collagenolysis are phosphatidylinositol 3-kinase/Akt signaling-dependent. J Biol Chem. 2008;283(21):14221–9. doi: 10.1074/jbc.M710136200. [DOI] [PubMed] [Google Scholar]
- 53.Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28(4):400–9. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 54.Dalle Pezze P, Ruf S, Sonntag AG, Langelaar-Makkinje M, Hall P, Heberle AM, et al. A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nat Commun. 2016;7:13254. doi: 10.1038/ncomms13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S, Li B. MiR-128-3p Post-transcriptionally inhibits WISP1 to suppress apoptosis and inflammation in human articular chondrocytes via the PI3K/AKT/NF-κB signaling pathway. Cell Transpl. 2020;29:963689720939131. doi: 10.1177/0963689720939131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte JH, Osteoarthritis Autophagy prevents age-related OA. Nat Rev Rheumatol. 2015;11(12):683. doi: 10.1038/nrrheum.2015.145. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–40. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 59.Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575–81. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang P, Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods. 2015;75:13–8. doi: 10.1016/j.ymeth.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 61.Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 2012;66(6):457–62. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Chang J, Wang W, Zhang H, Hu Y, Wang M, Yin Z. The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int J Mol Med. 2013;32(6):1311–8. doi: 10.3892/ijmm.2013.1520. [DOI] [PubMed] [Google Scholar]
- 63.Lee SW, Song YS, Lee SY, Yoon YG, Lee SH, Park BS, et al. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS ONE. 2011;6(4):e19163. doi: 10.1371/journal.pone.0019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adrien JL, Ornitz E, Barthelemy C, Sauvage D, Lelord G. The presence or absence of certain behaviors associated with infantile autism in severely retarded autistic and nonautistic retarded children and very young normal children. J Autism Dev Disord. 1987;17(3):407–16. doi: 10.1007/BF01487069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.