Abstract
Background:
Black men who have sex with men are at a disproportionate risk for sexually transmitted infections (STI). Understanding the drivers of those disparities can lead to culturally tailored interventions. We aimed to characterize the incidence and correlates of STI among Black individuals from HIV Prevention Trials Network 061, a multicity cohort study conducted from 2009 to 2011 in the United States.
Methods:
We used Cox proportional hazards regression to estimate adjusted hazard ratios (aHRs) accounting for within-participant correlation over multiple follow-up visits (enrollment, 6 and 12 months). We examined correlates of incident rectal and urethral STI as well as incident syphilis.
Results:
Among 1522 individuals, the incidences of urethral and rectal Neisseria gonorrhoeae infection were 1.0 (95% confidence interval, 0.6–1.8) and 4.6 (95% CI, 3.5–6.3) cases per 100 person-years, respectively. The incidences of urethral and rectal Chlamydia trachomatis infection were 2.5 (95% CI, 1.7–3.6) and 2.5 (95% CI, 1.7–3.7) cases per 100 person-years, respectively. The incidence of syphilis was 3.6 (95% CI, 2.7–4.9) cases per 100 person-years. Younger age was associated with increased odds of incident urethral (aHR, 5.1; 95% CI, 2.3–11.1) and rectal (aHR, 2.6; 95% CI, 1.6–4.3) STI. Diagnosis of a rectal STI at baseline (aHR, 2.3; 95% CI, 1.1–4.0) and use of saliva as lubricant (aHR, 1.7; 95% CI, 1.1–2.8) were associated with incident rectal STI. Diagnosis of syphilis at baseline was associated with incident syphilis during follow-up (aHR, 5.6; 95% CI, 2.5–12.2).
Conclusions:
Younger participants had the highest STI incidence. Use of saliva as lubricant may be a driver of rectal infection, which deserves further study.
Black men who have sex with men (MSM) are at an increased risk for bacterial sexually transmitted infections (STI) and HIV.1,2 Individual-level drivers such as high-risk sexual behaviors, including condomless anal intercourse and increased number of sex partners, do not fully explain the differences in HIV and STI incidence between Black and White MSM. Numerous studies have demonstrated those disparities independent of individual risk-taking behavior.2,3
Instead, a complex set of socioeconomic and structural factors interact to create those disparities. Income, for example, may be a more important predictor of incident STI than individual behavior when comparing across races.4 A higher proportion of HIV prevalence in sexual networks and deferral of screening for HIV and STI due to medical mistrust also play a role.5,6 Racial disparities in STI are particularly important because bacterial STI increases biologic susceptibility to HIV acquisition and transmission,7,8 and thus play an important and potentially modifiable role in potentiating racial disparities in HIV incidence.
Better understanding of the links between STI and HIV, and structural racial disparities could result in more effective policy and public health efforts to decrease the spread of HIV in at-risk populations. Thus, the current study was undertaken to delineate the incidence and potential drivers of bacterial STI among Black MSM recruited from 6 US cities participating in the HIV Prevention Trials Network (HPTN) trial 061.
METHODS
Study Sample and Procedures
We used an existing data set from HPTN 061, designed to assess the feasibility of a multicomponent intervention to reduce HIV infection among Black MSM.3,9 In summary, Black MSM were recruited from 6 US cities (Atlanta; Boston; Los Angeles; San Francisco; Washington, DC; and New York) between July 2009 and October 2010, either through the community or through referral by an individual already enrolled, and offered HIV and STI testing at baseline and 6 and 12 months. Participants were also screened for unmet needs and offered system navigation assistance by trained peer navigators.
Participants were enrolled if they indicated they were HIV uninfected or if they were HIV infected but not engaged in care and reported engaging in condomless anal intercourse. Additional inclusion criteria were as follows: self-identification as Black, African American, Caribbean Black, or multiracial Black, self-identification as a man or male at birth, being 18 years or older, residence in the metropolitan area of the study with no plans to move during the study period, and providing informed consent. Exclusion from the study occurred if individuals were enrolled concurrently in any other HIV research study, or they reported prior participation in an HIV vaccine trial. Furthermore, we excluded individuals identifying as transgender women from the analysis given that MSM and transgender women are increasingly recognized as 2 distinct populations with differing sexual practices and sociobehavioral contexts.10
Institutional review board approval was obtained from all participating institutions. HIV testing was performed via rapid HIV antibody tests with confirmatory Western blot (quality assurance testing to confirm HIV infections was performed retrospectively at the HPTN Laboratory Center, Baltimore, MD). All participants who were found to be infected with HIV also received CD4 cell count and HIV viral load testing. Testing for Neisseria gonorrhoeae and Chlamydia trachomatis was done by nucleic acid amplification testing (NAAT) with the Gene-Probe Aptima Combo 2 assay (Hologic, San Diego, CA). Participant's serum was tested for antibody to Treponema pallidum, the causative agent of syphilis, using rapid plasma reagin (RPR) and confirmatory treponemal antibody testing. Participants also filled out a standardized questionnaire via audio computer-assisted self-interview (ACASI). For the present analysis, we only included participants with complete ACASI and testing data.
Outcomes
Urine and serum samples were collected at every study visit. Rectal swabs were self- or clinician-collected at baseline and at 12 months. We assessed 3 outcomes in this study. First, urethral infection with either N. gonorrhoeae or C. trachomatis was defined as a positive urine NAAT result. Second, rectal infection with either N. gonorrhoeae or C. trachomatis was defined as a positive NAAT result from a rectal swab. Finally, a new syphilis diagnosis was defined as a newly reactive RPR with a positive confirmatory treponemal test result or a 4-fold increase in RPR titers. All STI diagnosed at baseline were promptly treated, so those with a STI diagnosed at baseline were included in the analysis.
Predictors of Interest
We assessed multiple variables that have been associated with incident STI in the literature. Sociodemographics included the following: age, sexual orientation, gender identity, ethnicity and race, country of birth, highest level of education, relationship and employment status, housing, and financial security, health care coverage, and history of incarceration.3 Sexual orientation was categorized into 1 of 3 groups. The first group “gay/alternative sexuality” included one of the following self-reported identities: gay, queer, same-gender-loving, pansexual, sexual, unsure, another sexual identity, or a combination of sexual identities. The second and third groups were exclusively bisexual and exclusively straight, respectively. Behavioral characteristics were modeled as time-varying covariates and included the following: a report in the preceding 6 months of the number of anal sex partners, receptive and insertive practices, condom use, use of saliva as lubricant, and drug use. The psychosocial measures were assessed at baseline and included report of intimate partner violence, internalized homophobia, experiences of racism or discrimination based on sexual orientation, depression, and religious affiliations during childhood and currently.
Two scales assessed participants' experience with a range of discriminatory behaviors based on perceived sexual orientation and race, which were based on previously developed scales.11 The items for the sexual orientation discrimination and racism scales had 5 answer choices scored 0 to 4 (has never happened to me/does not bother me at all, only bothers me a little, bothers me somewhat, bothers me a lot, bothers me extremely, respectively). The sexual orientation discrimination scale had a total of 25 items for a range of scores from 0 to 100. The racism scale had 28 items for a range of scores from 0 to 112. Because there were no defined cutoffs for these scores in the literature, we modeled these variables as flexibly as possible using restricted cubic splines with 3 knots.12
We created a variable indicating HIV status based on several factors; first, previously diagnosed HIV infection was based on self-reported infection at baseline as well as for individuals with a new positive HIV test result but had a viral load <400 copies/mL at baseline; second, individuals who were newly diagnosed at baseline, individuals who had a positive HIV test result after a prior documented negative HIV test result, and individuals who tested positive for HIV during the study period who did not have a documented prior negative HIV test result and did not self-report that they were living with HIV at baseline and who had an HIV viral load >400 copies/mL (i.e., new diagnosis); third, individuals who had a documented negative HIV test result during the study period without a subsequent positive test result (i.e., HIV uninfected throughout the study); and finally, those who declined testing during the study period (i.e., unknown HIV status).
Statistical Analysis
We calculated total person-time contributed by participants for each of the 3 outcomes and the incidence of urethral infection, rectal infection, and syphilis diagnosis. We used the first infection during follow-up as the outcome, as only one participant had a urethral infection at both 6- and 12-month visits, and no participant was diagnosed with syphilis at both the 6- or 12-month visits.
We used time-to-event methods to assess associations between the variables and outcomes. We included participants with a baseline STI diagnosis, as baseline STI were promptly treated per study protocol. Participants were censored either at their first STI diagnosis or the last study visit at which they had complete ACASI and STI testing data, whichever came first. For each outcome, we used univariable Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for each predictor of interest. We used the global Wald tests to assess the statistical significance of each univariable model, including variables with a P value ≤0.1 in a multivariable Cox proportional hazards model to calculate adjusted HRs (aHRs). We used such a stringent criterion for model building to avoid overfit models in the context of low out-come event rates over the follow-up period. We then applied a stepwise, backward variable selection algorithm to create final models that minimized the Akaike information criterion.13
All models used robust standard error estimation that accounted for within-participant correlation over study visits. We plotted scaled Schoenfeld residuals to assess whether modeled variables violated the assumption of proportional hazards; none of the variables included in the models were found to violate this assumption. We stratified all univariable and multivariable models by study site. We used the last value carried forward method for missing time-varying behavioral variables.
All analyses were conducted using STATA 16.0 (StataCorp, College Station, TX).
RESULTS
Among 1522 individuals enrolled, 1518 completed the baseline ACASI. Overall, there were 4304 total participant visits and 3806 visits with ACASI data. Between baseline and the first follow-up visit, there were 88 (5.8%) individuals lost to follow-up; between the first and second visits, there were 81 (5.6%) individuals lost to follow-up. At the first follow-up visit, 1157 (76.0% of baseline population) individuals completed the ACASI, whereas at the second follow-up visit, 1131 (74.3% of baseline population) individuals completed the ACASI.
Of the 1157 participants who completed the ACASI at 6 months, 1139 (98.4%) were tested for urethral infection and 1152 (99.6%) were tested for syphilis. Of the 1131 participants who completed the ACASI at 12 months, 1101 (97.3%) were tested for urethral infection, 963 (85.1%) were tested for rectal infection, and 1129 (99.8%) were tested for syphilis.
The mean age at enrollment was 37.7 years, with 21.1% of participants between the ages of 18 and 24 years and 17.0% ≥50 years (Table 1). Sixty-seven percent identified as gay/alternative sexuality, and 28.4% identified as bisexual. Although 17.0% of the men had not completed high school, 13.2% had completed college. Unstable housing was reported by 13.3% participants.
TABLE 1.
Baseline Characteristic of a Population of Black Men Who Have Sex with Men from 6 US Cities (n = 1518)
| n (%) | |
|---|---|
| Age at enrollment, y | |
| 18–24 | 306 (21.2) |
| 24–29 | 168 (11.1) |
| 30–49 | 786 (51.8) |
| 50+ | 258 (17.0) |
| Sexual orientation | |
| Gay/alternative sexuality | 1018 (67.1) |
| Exclusively bisexual | 431 (28.4) |
| Exclusively straight | 68 (4.5) |
| Hispanic ethnicity | 114 (7.5) |
| Multiracial | 90 (5.9) |
| Born outside the United States | 67 (4.4) |
| Study site | |
| Boston, MA | 235 (15.5) |
| Georgia | 288 (19.0) |
| Los Angeles, CA | 279 (18.4) |
| New York, NY | 306 (20.2) |
| San Francisco, CA | 195 (12.8) |
| Washington, DC | 215 (14.2) |
| Highest level of education | |
| Less than high school | 258 (17.0) |
| High school or equivalency | 528 (34.8) |
| Some college/technical/vocational/2-y college | 525 (34.6) |
| College or greater | 200 (13.2) |
| Currently in school either full- or part-time | 307 (20.2) |
| Relationship status | |
| Single/divorced/widowed | 1347 (88.7) |
| Legal partnership/main partner | 164 (10.8) |
| Currently working full- or part-time | 474 (21.2) |
| Homeless, shelter, transitional housing | 202 (13.3) |
| Income | |
| <$5000 | 375 (24.7) |
| $5000–$19,999 | 519(34.2) |
| $20,000–$49,999 | 433 (28.5) |
| ≥$50,000 | 171 (11.3) |
| How often do you feel that you do not have enough money for basic needs | |
| Never | 676 (44.5) |
| Once in a while | 489 (32.2) |
| Fairly or very often | 346 (22.8) |
| Health care coverage | 914 (60.2) |
| Ever incarcerated | 890 (58.6) |
| HIV status | |
| Previous HIV diagnosis | 237 (15.6) |
| New HIV diagnosis | 128 (8.4) |
| HIV uninfected | 1121 (73.8) |
| Unknown | 32 (2.1) |
| STI at baseline | |
| Rectal infections | |
| None | 1306 (86.0) |
| Neisseria gonorrhoeae only | 30 (2.0) |
| Chlamydia trachomatis only | 73 (4.8) |
| Both Neisseria gonorrhoeae and Chlamydia trachomatis | 23 (1.5) |
| Missing | 86 (5.7) |
| Urethral infections | |
| None | 1439 (94.8) |
| Neisseria gonorrhoeae only | 18 (1.2) |
| Chlamydia trachomatis only | 30 (2.0) |
| Both Neisseria gonorrhoeae and Chlamydia trachomatis | 1 (0.1) |
| Missing | 30 (2.0) |
| Syphilis | 50 (3.3) |
Urethral Infection
There were 40 incident urethral infections: 11 N. gonorrhoeae only, 28 C. trachomatis only, and 1 coinfection over 1155.5 years of person-time for an incidence of 3.5 (95% CI, 2.5–4.7) cases per 100 person-years. The incidence of urethral N. gonorrhoeae infection was 1.0 (95% CI, 0.6–1.8) cases per 100 person-years, whereas the incidence of urethral C. trachomatis infection was 2.5 (95% CI, 1.7–3.6) cases per 100 person-years. By univariable analyses, we found that incident urethral infection was associated with younger age, being foreign born, being a student, baseline urethral infection, condomless insertive anal sex, methamphetamine use, and experiences of racism (Supplemental Table 2, http://links.lww.com/OLQ/A684). Multivariable analysis showed that younger age groups (18–24 and 24–29 years) were at a higher risk for urethral STI (aHRs, 5.1 [95% CI, 2.3–11.1] and 3.9 [95% CI, 1.6–9.9], respectively) than those 30 years and older, as were participants with urethral infection at baseline (aHR, 3.5; 95% CI, 1.4–8.5) and participants who reported condomless insertive anal sex in the prior 6 months (aHR, 2.7; 95% CI, 1.3–5.7).
Rectal Infection
There were 64 incident rectal infections: 19 N. gonorrhoeae, 40 C. trachomatis, and 5 with coinfections, over 963 person-years of follow-up for an incidence of 6.6 (95% CI, 5.2–8.5) cases per 100 person-years. The incidence of rectal N. gonorrhoeae infection was 4.6 (95% CI, 3.5–6.3) cases per 100 person-years, whereas the incidence of rectal C. trachomatis infection was 2.5 (95% CI, 1.7–3.7) cases per 100 person-years. By univariable analyses, incident rectal infection was associated with younger age, gay/alternative sexuality identity, reporting a multiracial identity, being a student, having less than a college education, a history of incarceration, baseline rectal infection, condomless receptive anal sex, use of saliva as lubricant during anal sex, reporting travel for sex, and use of poppers (Supplemental Table 3, http://links.lww.com/OLQ/A684). Multivariable analysis showed that younger age (18–24 years compared with >25 years) was associated with incident rectal infection (aHR, 2.6; 95% CI, 1.6–4.3). A gay/alternative sexuality identity was associated with a 2.3-fold increase in the risk of rectal infection compared with those identifying as exclusively bisexual or straight (95% CI, 1.1–5.1). Participants who reported being incarcerated were 2 times more likely to experience a rectal infection compared with those without a history of incarceration (95% CI, 1.1–3.4). Having a rectal STI at baseline (aHR, 2.3; 95% CI, 1.1–4.0), a new anal sex partner (aHR, 1.8; 95% CI, 1.0–3.2), condomless receptive anal sex (aHR, 2.0; 95% CI, 1.2,−3.4), using saliva as lubricant for anal intercourse (aHR, 1.7; 95% CI, 1.1–2.8), and reporting travel for sex (aHR, 1.8; 95% CI, 1.1–2.9) increased the risk for incident rectal infection during follow-up.
Syphilis
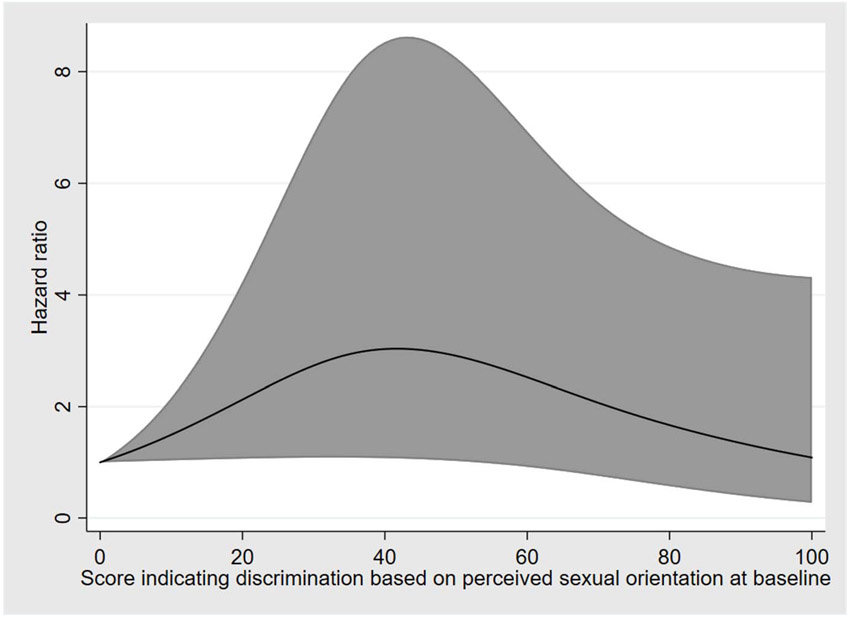
There were 43 incident syphilis diagnoses over 1180 years of person-time for an incidence of 3.6 (95% CI, 2.7–4.9) cases per 100 person-years. By univariable analyses, incident syphilis was associated with incomes greater than $5000 per year, HIV infection status, syphilis at baseline, receptive anal sex, use of poppers in the prior 6 months, and experiences of discrimination based on perceived sexual orientation (Supplemental Table 4, http://links.lww.com/OLQ/A684). Multivariable analysis showed that a new diagnosis of HIV infection (aHR, 3.7; 95% CI, 1.9–7.3), syphilis infection at baseline (aHR, 5.6; 95% CI, 2.5–12.2), and use of poppers (aHR, 2.1; 95% CI, 1.0–4.5) were associated with incident syphilis. Experiences of discrimination based on perceived sexual orientation were associated with incident syphilis in a non-linear fashion where lower levels and higher levels of reported discrimination were associated with a lower risk of syphilis compared with intermediate levels (Fig. 1). For example, with a score of zero as the referent, a score of 25 was associated with an HR of 2.4 (95% CI, 1.1–5.6), a score of 50 was associated with an HR of 2.9 (95% CI, 1.0–8.2), and a score of 75 was associated with an HR of 1.8 (95% CI, 0.7–5.2).
Figure 1.
Association between incident syphilis and experienced discrimination based on perceived sexual orientation among Black MSM, 6 US cities, 2009 to 2011. The figure represents the aHR and 95% CI for incident syphilis based on scores of reported experienced discrimination, with higher scores reflecting more severe experienced discrimination.
DISCUSSION
Our results demonstrate a high incidence of rectal and urethral STI, as well as syphilis, among Black MSM recruited from 6 US cities. Notably, incident infection was associated with being diagnosed with an STI at baseline. That finding may suggest a higher burden of infections among sexual networks; thus, network interventions may be important for reducing the incidence of STI as has been suggested for HIV.14 Similarly, insufficient partner therapy may also be contributing to the perpetual risk seen in those individuals. Alternatively, those with baseline infection may represent a further subpopulation with increased risk (either behavioral or structural) for reasons not clear in our data.
Similar to prior studies,15 incident urethral and rectal STI were more common among younger participants. Specific drivers of STI among youth are likely multifactorial and include increased risk-taking behaviors.16 Adolescents and young adults are also less likely to seek care compared with older adults,17 potentially driven by lower rates of health insurance and perceived discrimination by and stigma from providers.18 Such factors may result in avoidance of the health care system resulting in lower rates of STI screening and treatment, as well as decreased viral suppression due to suboptimal antiretroviral therapy adherence19,20 and retention in care.21 Such individuals may also be less likely to receive health education and counseling, which otherwise might help decrease risk-taking behavior.
The association of incident HIV infection with incident STI is likely due to several factors and has been demonstrated previously.22 Beyond common behavioral risk factors, bacterial STI increase mucosal inflammation and recruitment of cells susceptible to HIV8 and increase the concentration of HIV in mucosal fluids of infected individuals,23 theoretically increasing transmissibility. Furthermore, proinflammatory cytokines produced in response to N. gonorrhoeae infection enhance HIV replication in vitro.24 One study estimated that preventing an antecedent rectal STI could prevent 15% of HIV infections,25 whereas a modeling study concluded that increased screening for rectal STI in MSM would be a cost-effective method for preventing HIV transmission.26 Our study, notably, did not find an independent association between rectal infection and incident HIV infection, but may have been underpowered to do so.
Use of saliva as lubrication was associated with incident rectal infection. N. gonorrhoeae can be transmitted via saliva.27 Pharyngeal N. gonorrhoeae infection may drive urethral and rectal infections via oropharyngeal contact.28,29 The prevalence of antimicrobial-resistant N. gonorrhoeae is higher among isolates from the pharynx.30 The association demonstrated is not definitively causal. In addition, the survey did not specify in what manner saliva was used; thus, we are unable to say in which context transmission occurred. Furthermore, given restrictions in sample size, our analysis included rectal infection with either N. gonorrhoeae or C. trachomatis, where evidence is lacking for salivary transmission of the latter. Therefore, further research is necessary to better understand the transmission dynamics of rectal STI. In the meantime, safer sex counseling should include discussion of the potential role of saliva in facilitating STI transmission.
We also found a notable association between receptive condomless anal intercourse and incident rectal STI, supporting urethral-rectal transmission of infection. Incident rectal infection was also associated with report of a history of incarceration, a finding consistent with prior research31 and likely reflecting higher-risk sexual practices among incarcerated individuals compounded by a lack of access to testing and care. Finally, rectal STI was also associated with report of travel for sex, possibly reflecting differing sexual practices and higher-risk exposures for STI upon travel.
It is important to note that there remain multiple complex systemic and structural factors that affect the social determinants of sexual health in Black MSM. Factors such as decreased social mobility associated with poverty, limited access to health care either because of a lack of health insurance or because of alienation as a result of perceived prejudice, and lower average levels of education and health literacy all likely contribute to such a disparity.1,2,9 Experience of prejudice was associated in a complex way with incident syphilis among our population. Lower levels of discrimination based on perceived sexual orientation were associated with incident syphilis compared with no perceived prejudice and higher levels of perceive prejudice. That finding may reflect social network limitations that arise as a consequence of higher levels of prejudice, or possibly changes in risk behavior because of perceived dangers. Further research is needed to better understand the role that perceived prejudice plays in impacting the risk of STI.
Notably, our results did not find associations between socioeconomic status and other structural factors with incident STI; however, that is likely a result of how relatively homogeneously disenfranchized the population enrolled was. Thus, the absence of association in our analysis does not reflect a lack of importance in addressing such factors. Much more work is needed to address those underlying drivers of STI, and it is our hope that our findings assist future efforts.
Although the sample size was large overall and the population was recruited from a diverse subset of the United States, the enrollment criteria were specific to Black MSM from large urban cities; thus, our findings may not be generalizable to other populations. Furthermore, even among that subset of the population, baseline characteristics reflected a somewhat homogeneous sample of individuals of lower socioeconomic status; thus, our findings may not be generalizable to other groups. In addition, based on the design of the study, the differences in HIV prevalence may reflect in part differences in recruitment efforts. Among the subset of individuals who were newly diagnosed with HIV at baseline, we used viral load as a surrogate for prior treatment, which may underestimate slightly the number of incident infections at baseline and thus the impact of HIV infection on the incidence of STI. We were unable to obtain data for serum levels of antiretroviral medications. Furthermore, as evidenced by the wide confidence intervals in our multivariable model, the sample size was not sufficiently robust to determine aHRs precisely; thus, further research may be able to better refine the aforementioned results. Overall, however, we feel that such limitations do not negate the relevance of our findings.
Our findings identified younger age as a risk factor for STI. A history of incarceration and report of receptive anal intercourse were both associated with rectal STI. Use of saliva as lubricant may be an important driver of incident rectal infection. Overall, further work is needed to address the complex and multifaceted structural barriers that drive the significant discrepancy in incidence of bacterial STI among Black MSM.
Supplementary Material
Conflict of Interest and Sources of Funding:
K.H.M. has received unrestricted research grants to study antiretrovirals for prevention from Gilead Sciences and Merck, Inc. The work is partially supported by the Bio-behavioral and Community Science Core of the Harvard Center for AIDS Research (NIAID P30AI060354).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Hall HI, Song R, Tang T, et al. HIV trends in the United States: Diagnoses and estimated incidence. JMIR Public Health Surveill 2017; 3:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: A multilevel approach. PLoS One 2014; 9:e90514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koblin BA, Mayer KH, Eshleman SH, et al. Correlates of HIV acquisition in a cohort of Black men who have sex with men in the United States: HIV Prevention Trials Network (HPTN) 061. PLoS One 2013; 8:e70413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owusu-Edusei K Jr., Chesson HW, Leichliter JS, et al. The association between racial disparity in income and reported sexually transmitted infections. Am J Public Health 2013; 103:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clermont D, Gilmer T, Burgos JL, et al. HIVand sexual health services available to sexual and gender minority youth seeking care at outpatient public mental health programs in two California counties. Health Equity 2020; 4:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickson DA, Mena LA, Wilton L, et al. Sexual networks, dyadic characteristics, and HIV acquisition and transmission behaviors among Black men who have sex with men in 6 US cities. Am J Epidemiol 2017; 185:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53:537–543. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis GA, Chang TL. Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr HIV Res 2012; 10:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer KH, Wang L, Koblin B, et al. Concomitant socioeconomic, behavioral, and biological factors associated with the disproportionate HIV infection burden among Black men who have sex with men in 6 U.S. cities. PLoS One 2014; 9:e87298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: A review. Lancet 2016; 388:412–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell S, Merchant MA, Young SA. Psychometric properties of the Racism and Life Experiences Scales (RaLES). Presented at: the 105th Annual Convention of the American Psychological Association; Chicago, IL; August 15–19, 1997. [Google Scholar]
- 12.Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant 2020; 55:675–680. [DOI] [PubMed] [Google Scholar]
- 13.Portet S. A primer on model selection using the Akaike Information Criterion. Infect Dis Model 2020; 5:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagkas-Bather J, Young LE, Chen YT, et al. Social network interventions for HIV transmission elimination. Curr HIV/AIDS Rep 2020; 17:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon CL, Klausner JD. The growing epidemic of sexually transmitted infections in adolescents: A neglected population. Curr Opin Pediatr 2018; 30:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widman L, Choukas-Bradley S, Helms SW, et al. Adolescent susceptibility to peer influence in sexual situations. J Adolesc Health 2016; 58:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sexually Transmitted Disease Surveillance, 2016. Atlanta, GA: Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, 2017. [Google Scholar]
- 18.Kerr JC, Valois RF, Diclemente RJ, et al. HIV-related stigma among African-American youth in the Northeast and Southeast US. AIDS Behav 2014; 18:1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandwani S, Koenig LJ, Sill AM, et al. Predictors of antiretroviral medication adherence among a diverse cohort of adolescents with HIV. J Adolesc Health 2012; 51:242–251. [DOI] [PubMed] [Google Scholar]
- 20.Williams PL, Storm D, Montepiedra G, et al. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics 2006; 118:e1745–e1757. [DOI] [PubMed] [Google Scholar]
- 21.Israelski D, Gore-Felton C, Power R, et al. Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Prev Med 2001; 33:470–475. [DOI] [PubMed] [Google Scholar]
- 22.Zetola NM, Bernstein KT, Wong E, et al. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr 2009; 50:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieg G, Butler DM, Smith DM, et al. Seminal plasma HIV levels in men with asymptomatic sexually transmitted infections. Int J STD AIDS 2010;21:207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobson-Belaire WN, Cochrane A, Ostrowski MA, et al. Differential response of primary and immortalized CD4+ T cells to Neisseria gonorrhoeae–induced cytokines determines the effect on HIV-1 replication. PLoS One 2011; 6:e18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of Black and White men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015; 31:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chesson HW, Bernstein KT, Gift TL, et al. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV infection. Sex Transm Dis 2013; 40: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow EP, Fairley CK. The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: New insights into transmission. J Int AIDS Soc 2019; 22(Suppl 6):e25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbee LA, Khosropour CM, Dombrowski JC, et al. An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: A case-control study. Sex Transm Infect 2016; 92:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornelisse VJ, Zhang L, Law M, et al. Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia. BMC Infect Dis 2018; 18:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect 2015; 91:234–237. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JC, Torrone E. Incarceration as forced migration: effects on selected community health outcomes. Am J Public Health 2008; 98(9 Suppl):S181–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.