Significance
Protected areas are set to cover one-third of oceans by 2030 yet rigorous empirical estimates of the relative performance of different marine protected area (MPA) types are few. Using a quasi-experimental design and global dataset, we elucidate the role of context and management when comparing benefits from no-take vs. multiple-use MPAs, providing insights for locations where no-take restrictions may not be ethical or culturally acceptable. While multiple-use performance was on average lower than for no-take MPAs, in high-pressure areas they produced similar outcomes when adequately staffed and appropriately regulated. We also highlight potential biases in existing estimates of relative benefits from no-take vs. multiple-use MPAs in our study and in previous literature, and show how counterfactual-based approaches can address them.
Keywords: marine protected areas, fishing restrictions, conservation, quasi-experiment, causal inference
Abstract
Marine protected areas (MPAs) are widely used for ocean conservation, yet the relative impacts of various types of MPAs are poorly understood. We estimated impacts on fish biomass from no-take and multiple-use (fished) MPAs, employing a rigorous matched counterfactual design with a global dataset of >14,000 surveys in and around 216 MPAs. Both no-take and multiple-use MPAs generated positive conservation outcomes relative to no protection (58.2% and 12.6% fish biomass increases, respectively), with smaller estimated differences between the two MPA types when controlling for additional confounding factors (8.3% increase). Relative performance depended on context and management: no-take MPAs performed better in areas of high human pressure but similar to multiple-use in remote locations. Multiple-use MPA performance was low in high-pressure areas but improved significantly with better management, producing similar outcomes to no-take MPAs when adequately staffed and appropriate use regulations were applied. For priority conservation areas where no-take restrictions are not possible or ethical, our findings show that a portfolio of well-designed and well-managed multiple-use MPAs represents a viable and potentially equitable pathway to advance local and global conservation.
The deterioration of the world’s ecosystems has led to numerous calls for effective and scalable conservation solutions [e.g., (1, 2)]. While conservation can include a broad range of actions, the current centerpiece of most ocean conservation initiatives are marine protected areas (MPAs). Currently, MPAs cover over 29.5 million km2 (~8.2%) of the ocean (3) and often limit or prohibit destructive and extractive activities, most notably fishing, within their boundaries (4). High-profile international agreements are calling for increased MPA coverage, including the Sustainable Development Goals [SDGs; (5)] and Target 3 of the Kunming-Montreal Global Biodiversity Framework that aims for 30% of ocean area to be effectively protected by 2030 (“30 by 30”) (6).
Benefits and Costs of Total vs. Partial Fishing Restrictions
Scientists and conservationists often contend that areas with total fishing restrictions—typically called fully protected or no-take areas—should be the primary focus when expanding global conservation efforts (7–10). Proponents point to empirical studies of no-take MPAs that show ecological gains relative to unprotected areas (11–13), in addition to studies suggesting greater species density or biomass in no-take MPAs compared to multiple-use MPAs that allow some types of fishing (9, 14–17).
However, the social implications of total fishing restrictions can limit no-take MPA placement options and thus overall impact. High overlap between areas with high conservation value and high local resource dependency (18, 19) implies that no-take MPA expansion will likely impose significant social costs on some societal groups. This can generate opposition in some areas, particularly in poorer communities with lower capacity to adapt to MPA restrictions [e.g., (20, 21)], and make no-take MPAs more difficult to establish. Such political dynamics can bias the siting of MPAs away from heavily used areas and restrict MPA size, limiting global expansion (22–24). Furthermore, if no-take MPAs are implemented despite local opposition, they may experience low compliance (i.e., become "paper parks"), which in turn reduces conservation gains. All of these dynamics have been observed for terrestrial protected areas, where the probability of creation and likelihood of compliance decrease as socioeconomic opportunity costs increase (25, 26). Some marine empirical studies have documented similar ecological performance of MPAs with different levels of protection (27–31), including findings that low compliance can be a driver of poor no-take MPA performance [e.g., (15, 32, 33)].
Given potential social and ecological trade-offs associated with the level of fishing restrictions, there is an urgent need for rigorous evaluation of the relative conservation benefits of no-take vs. multiple-use MPAs, including the role of management and socio-environmental context in shaping outcomes (34, 35). Unfortunately, much of the existing empirical work on MPA impacts suffers from limitations in study design (36, 37). Few if any comparative studies account for biases in MPA placement or other social and ecological confounding factors that may explain observed outcomes. Those that do are limited to specific geographic contexts (17, 36–38). Thus, as decision-makers seek to balance conservation goals with sustainable and equitable use (6), they require more robust empirical evidence on the relative benefits of no-take and multiple-use MPAs to inform strategic placement.
Results
Comparing Conservation Impacts of No-Take and Multiple-Use MPAs.
We compiled a large global dataset of over 14,000 fish surveys and the social, environmental, and regulatory conditions within and around 216 MPAs in 43 countries and territories to examine the absolute and relative performance of both no-take and multiple-use MPAs (Fig. 1A and SI Appendix, Fig. S1 and Tables S1–S5). We used statistical matching and a Bayesian hierarchical inference framework to estimate MPA absolute (protection vs. no protection) and relative (no-take vs. multiple-use) impacts, controlling for confounding factors that influence MPA placement and fish biomass (SI Appendix, Eqs. S1 and S2, Figs. S1–S3 and Tables S6–S8). We estimated MPA impacts on fish biomass density (g/100 m2) as an indicator of ecosystem health and considered the critical roles of socio-environmental context (particularly the proximity to human pressure) and MPA management effectiveness in explaining differences across contexts in the relative performance of these two MPA types. Applying rigorous quasi-experimental, counterfactual approaches to this global dataset will not only shed light on absolute impacts but also the interactions between context, management, and the level of fishing restrictions globally.
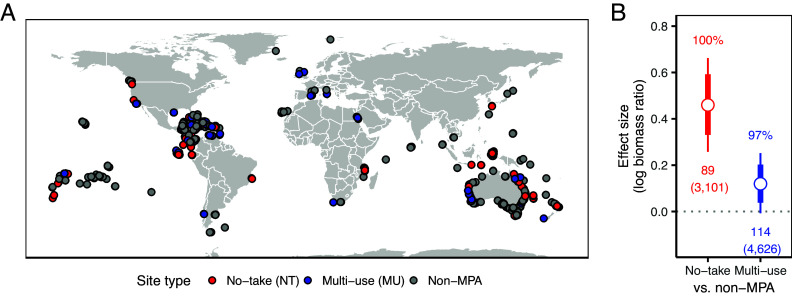
Fig. 1.
(A) Location of no-take (red), multiple-use (blue), and non-MPA (gray) sites (n = 216 MPAs) and (B) the absolute impacts of no-take and multiple-use MPAs on fish biomass. In (B), impacts are presented as effect sizes representing the expected percent difference in total fish biomass, comparing biomass in no-take (red) and multiple-use (blue) MPA sites to counterfactual unprotected sites. Thick and thin lines show the 80% and 95% credible intervals, respectively, around the median effect size (white dot). Probability of positive effects are shown above the estimates and number of MPAs (and number of sites, in parentheses) are shown below estimates.
Absolute Impacts: Protection vs. No Protection.
We estimated absolute impacts of each type of MPA using statistical matching to identify the most similar non-MPA site for each MPA site based on confounding factors that influence MPA placement and local fish biomass (e.g., likelihood of extractive uses, habitat, depth; SI Appendix, Table S7). We then used regression adjustments and Bayesian linear hierarchical models to estimate the average MPA-level effect (SI Appendix, Figs. S4–S12 and Tables S9–S13 for additional results, summary statistics, and diagnostics). Here, we report median effect sizes in percent biomass differences (SI Appendix, Eq. S3). Compared to no protection, both no-take and multiple-use MPAs increase fish biomass by about 58.2% and 12.6%, respectively (Fig. 1B and Table 1). Both no-take and multiple-use MPAs are over 97% likely to have a positive impact on fish biomass.
Table 1.
Summary posterior statistics for Bayesian models estimating MPA impacts for each comparison
| % biomass change | |||||
|---|---|---|---|---|---|
| Comparison type | MPA sample | # sites | #MPAs | Median (95% conf. int.) | P (positive effect) |
| No covariate predictors | |||||
| Multiple-use vs. no MPA | All | 4,626 | 114 | 12.6 (−0.8 to 28.7) | 97% |
| No-take vs. no MPA | 3,101 | 89 | 58.2 (29.4 to 93.9) | 100% | |
| No-take vs. Multiple-use | 3,261 | 79 | 8.3 (−7.1 to 26.4) | 84% | |
| Population center distance (Far >100 km; Near <100 km) | |||||
| Multiple-use vs. no MPA | Far | 742 | 41 | 44 (17.6 to 80.3) | 100% |
| Near | 3,884 | 76 | 4.0 (−9.6 to 19.9) | 71% | |
| No-take vs. no MPA | Far | 846 | 35 | 75.4 (30.8 to 135.3) | 100% |
| Near | 2,255 | 56 | 50.2 (19.5 to 89) | 100% | |
| No-take vs. Multiple-use | Far | 971 | 33 | 1.8 (−17.7 to 24.4) | 56% |
| Near | 2,290 | 49 | 15.7 (−4.9 to 42.9) | 92% | |
| Adequate staff capacity | |||||
| Multiple-use vs. no MPA | Inadequate | 1,896 | 20 | −5.7 (−24.9 to 19.6) | 29% |
| Adequate | 525 | 6 | 103.8 (−26.3 to 340.8) | 89% | |
| No-take vs. no MPA | Inadequate | 138 | 15 | 27.9 (−18.9 to 104.9) | 86% |
| Adequate | 201 | 7 | 77.5 (−18.3 to 325.5) | 92% | |
| No-take vs. Multiple-use | Inadequate | 544 | 13 | 120.8 (16.2 to 303.7) | 99% |
| Adequate | 505 | 6 | −27.4 (−61.2 to 34.4) | 14% | |
| Sustainable use regulations | |||||
| Multiple-use vs. no MPA | Weak | 1,788 | 7 | −10.7 (−38.6 to 25) | 22% |
| Strong | 629 | 18 | 8.9 (−22.2 to 61.8) | 68% | |
| No-take vs. no MPA | Weak | 9 | 4 | 11.8 (−56.7 to 196.5) | 59% |
| Strong | 325 | 17 | 50.6 (−2.7 to 146.4) | 97% | |
| No-take vs. Multiple-use | Weak | 470 | 4 | 189.7 (14.8 to 646.1) | 98% |
| Strong | 575 | 14 | 0.0 (−40.6 to 83.4) | 50% |
Rows display results from models for all MPA comparisons (no fixed effect covariate predictors), those near/far from population centers, with (in)adequate staff capacity, and weak/strong sustainable use regulations. Table also shows the median and 95% CIs in percent biomass increase, and the probability (P) of a positive effect. See SI Appendix, Table S9 for additional model results SI Appendix, Table S6 for comparison descriptions.
Relative Impacts: No-Take vs. Multiple-Use Restrictions.
While matched counterfactual designs help to reduce biases in calculating absolute impacts, they do not directly explain the difference in impacts between the two MPA types (39, 40), which could be due to site-selection biases (24, 41). No-take MPAs may be less likely to be located in high-use contexts, for instance, because of social opposition to total fishing restrictions (42). Thus, absolute impact estimates may be confounded by the fact that one type of MPA is less likely than the other to be placed in certain contexts. We again used statistical matching and regression adjustments to directly match multiple-use with no-take MPA sites. This allowed us to estimate relative impacts in those locations where each MPA type (multiple-use or no-take) currently exists, while controlling for confounding factors (e.g., differences in MPA placement, age, or size; SI Appendix, Tables S7 and S8). More specifically, this approach provides estimates of the predicted effect of increasing restrictions to no-take within existing multiple-use MPAs. Using this approach, we observe an 8.3% median biomass increase from converting from multiple use to no-take regulations, with an 84% probability of biomass increasing (Fig. 2B and Table 1). This more rigorous and focused comparison suggests a markedly smaller difference in fish biomass impacts across types than is suggested by the absolute impacts (45.6% difference between multiple use and no-take MPAs absolute impacts; Fig. 1B and Table 1), which do not account for differences in site selection (see SI Appendix, Fig. S4 and Table S9 for results for other estimands).
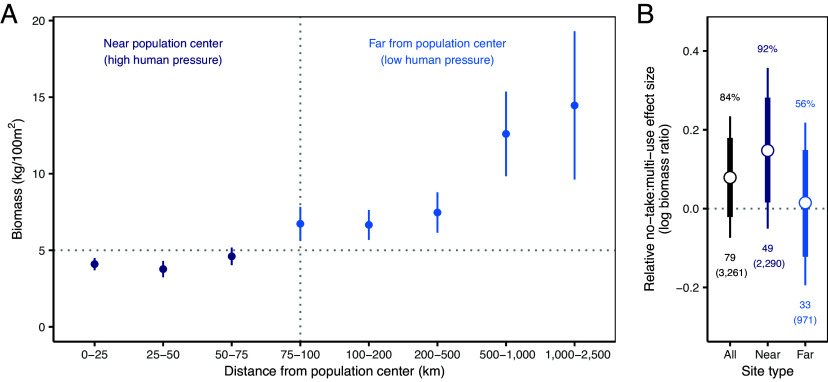
Fig. 2.
(A) Fish biomass in unprotected locations by distance to a population center, a proxy for human pressure; Horizontal dashed line (5 kg/100 m2) represents the level of fish biomass needed to maintain critical ecosystem functions (43, 44). (B) Relative no-take:multiple-use MPA impacts: effect sizes of the expected percent difference in total fish biomass from converting existing multiple-use to no-take MPAs for all sites (black) and those near (<100 km; dark blue) and far (>100 km; light blue) from population centers. Effect sizes are calculated by comparing multiple-use to counterfactual no-take MPA sites where greater values represent larger expected biomass increases from converting to no-take restrictions. Thick and thin lines show the 80% and 95% credible intervals, respectively, around the median (white dot). Probability of positive effects are shown above the estimates and number of MPAs (and number of sites, in parentheses) are shown below estimates.
Relative Impacts in High-Pressure Contexts.
We investigated the relative impacts in areas where fish populations are likely to be under high anthropogenic pressure and thus where conservation may be most needed. Previous work has suggested that total fish biomass must be at or above 5 kg/100 m2 to maintain critical ecosystem function in tropical marine ecosystems such as coral reefs (43, 44). As expected, fish biomass in unprotected sites close to population centers is considerably lower than in those further away (Fig. 2A), with biomass reaching or exceeding this critical 5 kg level at 100 km or further from population centers. We therefore used 100 km from the nearest population center as a threshold distance to distinguish between potentially high- and low-pressure locations.
We find larger gains in biomass if existing multiple-use MPAs were converted to no-take MPAs within high-pressure locations (15.7% median biomass increase with >92% certainty; Fig. 2B and Table 1). In contrast, if existing multiple-use MPAs located further from population centers (>100 km) were converted to no-take MPAs, we have only 56% certainty that fish biomass would increase—and only marginally if so (1.8% median biomass increase; Table 1). The generally stronger conservation performance of no-take MPAs (Figs. 1B and 2B) therefore seems to be driven in part by their superior performance when located closer to population centers. This finding also suggests that remoteness confers its own protection (29): if there is less threat to mitigate, then stricter protection may offer fewer conservation benefits. This does not negate, however, the potential longer-term benefits of protecting less disturbed locations from current or future threats (10).
Role of Management Effectiveness in High-Pressure Contexts.
The finding that multiple-use MPAs generate lower benefits than no-take MPAs for sites closer to population centers (Fig. 2B) raises questions about the role of MPA management and governance in high-pressure areas. In locations of high conservation importance where no-take implementation may not be feasible, ethical, or culturally preferred (e.g., areas with high local resource dependency), strong management institutions could play a key role in reducing the relative deficit in multiple-use MPA performance.
To evaluate this hypothesis, we employed a global dataset (45) that provides indicators of the adequacy and appropriateness of MPA management (SI Appendix, Tables S1 and S4). Here, we focused on the effects of converting multiple-use to no-take MPAs within high-pressure contexts (<100 km from population centers), evaluating specific management attributes previously shown to be associated with stronger ecological performance in protected areas: 1) the presence of adequate staff capacity to carry out critical management functions (“staff capacity”) and 2) the strength and contextual appropriateness of MPA regulations to control unsustainable activities (“sustainable-use regulations”) (10, 45, 46).
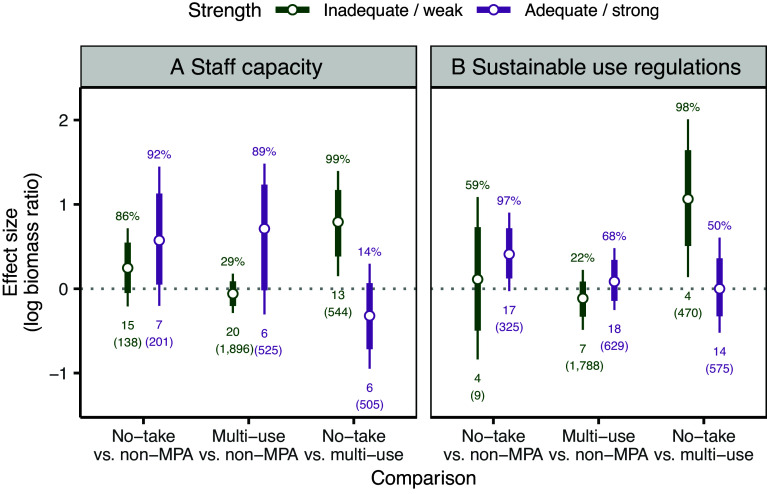
We find that in high-pressure locations, higher staff capacity results in better conservation performance for both MPA types (Fig. 3A and Table 1). This shift in impact is more important for multiple-use MPAs, which experience a more than three-fold increase in the likelihood of positive impacts (from 29% to 89%; Middle comparison, Fig. 3A and Table 1) and double the increase of biomass (median: from −5.7% to 103.8%; Table 1). Adequate staff capacity in multiple-use MPAs also appears to dramatically reduce the relative difference in the performance between the two MPA types when comparing multiple-use MPAs with (in)adequate capacity with no-take MPAs (Right comparison, Fig. 3A): at low capacity, no-take MPAs outperform multiple-use MPAs with 99% certainty, whereas with adequate staff capacity, there are little to no gains (and potentially even a cost) from increasing restrictions (median: −27.4% Fig. 3A). This aligns with earlier studies of the importance of MPA staff capacity (45), and the strong performance of no-take MPAs with both high and low staff capacity (Left comparison, Fig. 3A) suggests that no-take MPAs may be easier to enforce even with low capacity, perhaps because of simpler regulations (22, 47).
Fig. 3.
Absolute and relative no-take:multiple-use MPA impacts in high-pressure locations (<100 km from population center) by (A) staff capacity and (B) sustainable use regulations. Effect sizes of the expected percent difference in total fish biomass, comparing biomass in no-take (Left) and multiple-use (middle) MPA sites to unprotected sites, and multiple-use to counterfactual no-take MPA sites (Right) where greater values represent larger expected biomass increases from converting to no-take restrictions. Effect sizes are shown separately for (A) MPAs with adequate (purple) vs. inadequate (green) capacity to conduct critical management activities (staff capacity); and (B) MPAs with weak (green) vs. strong (purple) regulations to control unsustainable activities (sustainable use regulations). Thick and thin lines show the 80% and 95% credible intervals, respectively, around the median effect size (white dot). Probability of positive effects are shown above the estimates and number of MPAs (and number of sites, in parentheses) are shown below estimates. See SI Appendix, Table S4http://www.pnas.org/lookup/doi/10.1073/pnas.2313205121#supplementary-materials for indicator definitions and thresholds.
Similar patterns emerge when examining the facilitating role of sustainable-use regulations. In high-pressure locations, multiple-use MPAs with weak regulations offer little to no conservation benefit (Middle comparison, Fig. 3B and Table 1), but when regulations are strong, multiple-use MPAs produce similar impacts to comparable no-take MPA sites (0.0% median biomass increase, Right comparison, Fig. 3B). These results are consistent with common-pool resource and other governance theories that suggest that strong and contextually appropriate rules support more sustainable outcomes (46). Thus, while adequate capacity and appropriate local governance and management are important for all MPA types, they may be particularly important in reducing the potential loss of conservation benefits when implementing multiple-use or zoned MPAs instead of no-take MPAs within high-pressure contexts.
Discussion
Our research offers insights useful to marine conservation practitioners across multiple scales: from marine spatial planners and managers operating in places with both critical conservation needs and high local resource dependency to decision makers defining global conservation goals and policies. First, we find that both no-take and multi-use MPAs generate positive impacts (Fig. 1B) with no-take MPAs yielding the largest biomass gains in locations likely experiencing high anthropogenic pressures such as fishing. However, given their proximity to human population centers, these places may also be important for the livelihoods, food security, and culture of local and Indigenous groups, especially in high-poverty contexts where people may have limited capacity to adapt to restrictions (21). Top–down implementation of no-take MPAs in these areas can result in harmful and unjust outcomes for communities [e.g., (20, 21)] or simply make them functionally multiple-use MPAs due to low compliance (32, 48). Thus, as progress is made toward the 30 × 30 (6) and other conservation targets, the global conservation community may be facing an uphill battle in designating no-take MPAs in the locations where they can make the biggest conservation difference. Expansion efforts focused primarily on no-take MPAs could undermine local support for MPAs and conservation more generally. This could result in a lower total amount of area effectively conserved globally than could be achieved with more diverse yet sustainable approaches (22, 49).
Our results also point to the potential benefits of strengthening management capacity and local governance institutions within high-pressure locations, particularly for multiple-use MPAs (Fig. 3). Such MPAs could provide comparable conservation outcomes to no-take MPAs in contexts where banning all fishing might be inappropriate or infeasible. This could provide conservation gains without compromising local food security and overall well-being (22, 50)—key goals in the post-2020 Global Biodiversity Framework (6). Although not thoroughly assessed in this study, improvements in other aspects of governance and management in multiple-use MPAs (e.g., clear boundaries, inclusive decision making) could also support greater conservation efficacy and social equity (SI Appendix, Fig. S5). Furthermore, if managers are allowed greater flexibility in high-dependency contexts (e.g., ability to incorporate traditional sustainable fisheries management) and are able to improve both MPA management and conservation outcomes, there may be more support for continued MPA expansion (22, 27, 49, 51). This increased support could also lead to increased local involvement in management thereby increasing management capacity (52). Thus, the global conservation community should invest in enhancing capacity and contextually-appropriate management and governance, particularly for multiple-use MPAs. These findings are also relevant to the implementation of Other Effective Conservation Measures (OECMs). OECMs can be functionally similar to multiple-use MPAs as they represent a diverse suite of area-based management tools that produce conservation benefits whilst being designated for multiple purposes, with biodiversity conservation often not being the primary goal (49). These tools are receiving increasing global attention as a potential major contributor toward achieving global conservation coverage targets (e.g., 30 × 30). Strengthening overall area-based governance and management therefore represents a tremendous opportunity to advance conservation, given the thousands of multiple-use MPAs and OECMs that exist globally and that 90% and 30% of the world’s MPAs have low staff capacity and weak regulations, respectively (45).
Improving Rigor of MPA Impact Evaluation.
Compared to several existing studies, our estimated magnitudes of absolute impacts (MPA vs. no MPA) are noticeably smaller, particularly for no-take MPAs, which have been reported to result in five-fold or higher increases in biomass relative to areas without MPAs [e.g., (9, 12)]. This could be attributed to two factors. First, our methodology utilizes statistical matching and other inference techniques that reduce potential biases and the influence of other confounding factors. Indeed, our results are in line with studies employing similarly rigorous methods in various contexts (15, 17, 31). Second, our study compiled a large number of monitoring datasets, resulting in a larger sample size and wider geographic coverage, thereby avoiding potential publication biases toward high-performing MPAs (53). Potential biases toward MPA studies with positive effects, combined with the lack of attention to confounding factors, are suspected to affect meta-analyses, literature reviews, and other synthetic research (36, 38).
When controlling for potential differences in placement and MPA attributes (e.g., MPA size and age), we also found smaller differences in relative impacts for multiple-use vs. no-take MPAs (8 to 18% median difference in performance; Fig. 2B and SI Appendix, Fig. S4 and Tables 1 and S9) than when simply comparing the absolute impacts of the two MPA types (i.e., MPA vs. no MPA: 45.6% difference; Fig. 1B and Table 1). While further research is needed to explain these disparate results, the smaller estimate for relative impacts is consistent with several studies showing similar performance across MPA types under varying contextual settings (17, 28, 31, 45, 54). Thus, without applying more rigorous methods for assessing relative performance, studies that compare absolute effects could be overestimating the relative benefits of no-take MPAs.
Despite the size of the fish survey dataset, we were limited by available management and baseline data and unable to apply more rigorous approaches to some analyses (e.g., directly matching high- and low-capacity multiple-use MPAs or high capacity multiple-use to high capacity no-take MPAs). These limitations highlight the need for more comprehensive and counterfactual-based monitoring programs to advance understanding of the varied impacts of MPAs. This includes assembling time-series data on MPA outcomes (e.g., changes in biomass, diversity, function, etc.) and governance, management, and socio-ecological trends (e.g., changes in fishing activity, oceanic conditions, local participation in management, etc.). Ideally monitoring should occur before and after MPA implementation at both MPA and comparable non-MPA sites (36, 55). More in-depth, rigorous assessments could facilitate investigations into the roles of other aspects of MPA management and governance (e.g., inclusive decision-making; SI Appendix, Fig. S5) (46, 56).
Another limitation of this study is using fish biomass to compare no-take and multiple-use MPA performance. Fish biomass is a well-established measure of MPA fish population recovery and thus MPA performance (10–17), however, multiple-use MPAs represent a broad array of conservation interventions with diverse management objectives (e.g., sustainable harvest, food security, recreational use). As a result, maximizing fish biomass may not be a primary management goal or the most appropriate performance indicator for all multiple-use MPAs.
Advancing Conservation and Equity through Diverse and Strong Management Portfolios.
The results suggest that well-managed and designed multiple-use MPAs represent a viable and potentially more equitable strategy to achieve conservation gains in locations where total fishing restrictions are not possible or appropriate. However, multiple-use MPAs (and similar OECMs) are not a panacea, given their lower average performance compared to no-take MPAs (especially when inadequately staffed or inappropriately regulated) and expectations should be adjusted based on the type and intensity of fishing that they allow (10, 57, 58) and the specific MPA management goals. In some instances, strict regulations might be the optimal solution to protect against current and future threats (10). For example, despite showing almost equivocal performance in remote areas, no-take MPAs can act as refugia for overfished species and against future threats. They may also be easier to implement in these locations now while opportunity costs for other uses are low. Furthermore, MPAs should be considered within a broader portfolio of policy options suitable to address local (and future) stressors and management priorities (59). A diverse suite of approaches, including MPAs with different types of restrictions (e.g., multiple-use MPAs and OECMs with no-take areas), is likely to be necessary to adapt to ongoing climate impacts (10, 22, 31).
Therefore, we recommend carefully considering the local and broader social, ecological, and governance context to inform the optimal configuration of MPA types and supporting governance and management to achieve conservation objectives (10, 27). This includes, when feasible, implementing robust monitoring and evaluation to inform adaptive management when conditions change (10, 31, 55). As conservation actors define local to global strategies for MPA design and implementation in the next decade and beyond (6), we urge decision-makers and the conservation community to avoid one-size-fits-all approaches and work towards more contextually appropriate and comprehensive solutions that consider the relative costs, benefits, and barriers to effective and equitable conservation.
Materials and Methods
Data Sample and Compilation.
Fish biomass outcomes.
Using a global dataset of underwater visual census surveys assembled by Gill et al. (45), we estimated total fish biomass from 15,978 surveys conducted in and around 287 MPAs in 58 countries (SI Appendix, Table S1). We averaged transect-level observations to calculate total fish biomass at each site in grams per 100 m2, using natural log values to reduce the effect of outliers.
Site level covariates.
We assembled covariate data on social and environmental conditions at each site (e.g., habitat type, wave energy, distance to population centers; SI Appendix, Table S7) to control for observable and unobservable factors that could bias the estimate of MPA impacts.
MPA spatial data.
We compiled spatial and regulatory information on MPA and MPA zone boundaries to identify the fishing regulations at each survey site, defining no-take as sites that did not allow any forms of fishing (subsistence, commercial, recreational) at any period of time. To accomplish this, we first compiled over one thousand documents that described the activities permitted or prohibited in each zone of each MPA. We then extracted spatial and attribute data for these MPAs from a larger spatial dataset of over 17,000 MPA and zone boundary shapefiles compiled by the authors and other research partners (SI Appendix, Table S2). After removing observations and sites that may impact estimation (e.g., insufficient covariate data or unclear regulations), the final dataset comprised 14,044 sites (89.9% of original dataset) from within 335 zones in 217 MPAs (Fig. 1A).
MPA management and governance.
We assessed MPA management and governance using a database compiled by Gill et al. (45) of management assessments completed in 433 MPAs in 70 countries (SI Appendix, Table S1). Here, MPA management staff and/or other stakeholders provided responses to Likert-scale questions on the adequacy and appropriateness of MPA management (e.g., staff capacity, appropriate regulations to support sustainable use; SI Appendix, Table S4). See SI Appendix, Fig. S1 for an overview of the major analytical steps and diagnostic tests used in this study and SI Appendix for more information on data sources, compilation, and processing.
Estimating MPA Treatment Effects.
Site-level treatment effects.
We estimated relative impacts of no-take and multiple-use MPAs by comparing fish biomass outcomes in each MPA type to two counterfactual outcomes: no MPA and the alternative MPA type, resulting in four treatment effects (SI Appendix, Table S6). Here, we define treatment as no-take or multiple-use MPA establishment and treatment effects (specifically, average treatment effect on the treated [ATT]) as the expected difference in total fish biomass in treated sites and the same sites if they were not treated (i.e., counterfactual outcomes). For example, we define the absolute impact of multiple-use MPA establishment as the expected difference in biomass at sites in protected multiple-use MPA/zones compared to the same sites if they were not protected at all (SI Appendix, Table S6). On the other hand, we define relative no-take: multiple use impacts as the expected difference between biomass in multiple-use MPA/zones compared to the same sites if they were under no-take regulations instead. We estimated these counterfactual outcomes used statistical matching, selecting comparable untreated (control) sites to pair with each treated site based on factors that affect both treatment (e.g., MPA placement, level of restrictions) and fish biomass outcomes (i.e., social and environmental site covariates; SI Appendix, Table S7). We also used post-matching regression adjustments to account for residual differences in covariate values between matched treated and control sites that could bias the impact estimate.
MPA-level treatment effects.
We used Bayesian linear hierarchical models to estimate MPA-level treatment effects for each comparison (SI Appendix, Table S6). These models included the site-level bias-adjusted treatment effects described above as the response variable, a binary fixed effect term for the population center distance, staff capacity, and regulations models, and random effects for MPA and matched control units to account for dependence between observations that shared the same MPA and control site (SI Appendix, Eqs. S5–S9). See SI Appendix for more information on the matching and model estimation procedures along with relevant sensitivity and model assumption tests and study limitations.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (R)
Dataset S02 (CSV)
Dataset S03 (CSV)
Dataset S04 (CSV)
Dataset S05 (CSV)
Acknowledgments
We thank the following data providers: Atlantic Gulf Rapid Reef Assessment contributors and data managers, Conservation International, Healthy Reefs Initiative, Ivor Williams (National Oceanic and Atmospheric Administration (NOAA) Coral Reef Ecosystem Program), NOAA Coral Reef Conservation Program, the Gulf-Caribbean Fisheries Institute, United Nations Environment Programme (UNEP) Caribbean Environment Programme, Caribbean Marine Protected Area Management network, other developers of the Caribbean Marine Protected Area Capacity Assessment Tool, MPAConnect, K. Knights (Global Database for Protected Area Management Effectiveness), R. Stuart-Smith (Reef Life Surveys), The Nature Conservancy, Wildlife Conservation Society, the UNEP World Conservation Monitoring Centre, World Wildlife Fund, Universtias Papua, and other data contributors to the Bird’s Head Seascape Ecological Impact Evaluation program, Isobel Pring, Erika Gress, and Isabel Silva. We would also like to thank the following advisors: Chris Kennedy, Louise Glew, and Megan Barnes, and research technicians: Mark Nepf, Matheus deNardo, Claire Poelking, Jeremy Raynal, Alexandra Dubel, Claire Atkins-Davis, Robert Jarrett, Gia Mancini, and Kyle Cornish. D.A.G. was partially funded by the David H. Smith Conservation Fellowship. C.M.F. was funded through the Arnhold University of California Santa Barbara-Conservation International Climate Solutions Collaborative. S.E.L. was partially funded by the Waitt Foundation. J.G. was funded by The Danish Independent Research council (Grant No. 0165-00018B). Initial data compilation supported by the National Socio-Environmental Synthesis Center under funding received from the NSF DBI-1052875, as part of the working group: Solving the Mystery of Marine Protected Area (MPA) Performance: Linking Governance, Conservation, Ecosystem Services, and Human Well Being.
Author contributions
D.A.G., S.E.L., C.M.F., G.A., D.A.A.-B., E.S.D., H.E.F., J.G., M.B.M., and P.J.M. designed research; D.A.G., C.M.F., R.M.-G., L.V., and L.M.W. performed research; D.A.G., C.M.F., E.I., B.J.R., and S.Y. contributed new reagents/analytic tools; D.A.G., C.M.F., E.I., B.J.R., and S.Y. analyzed data; G.J.E. provided data and reviewed paper; and D.A.G., S.E.L., C.M.F., A.P., E.I., B.J.R., S.Y., G.A., D.A.A.-B., E.S.D., G.J.E., H.E.F., J.G., D.T.L., M.B.M., R.M.-G., P.J.M., L.V., and L.M.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Although PNAS asks authors to adhere to United Nations naming conventions for maps (https://www.un.org/geospatial/mapsgeo), our policy is to publish maps as provided by the authors.
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data used in the MPA impact analysis are included as supporting information. JAGs code used in analysis is also included. Additional code available upon request. For those interested in original, survey-level data, please contact the organizations and researchers listed in SI Appendix, Table S1.
Supporting Information
References
- 1.Worm B., et al. , Impacts of biodiversity loss on ocean ecosystem services. Science (New York, N.Y.) 314, 787–790 (2006). [DOI] [PubMed] [Google Scholar]
- 2.McCauley J., et al. , Marine defaunation: Animal loss in the global ocean. Science 347, 1255641 (2015). [DOI] [PubMed] [Google Scholar]
- 3.UNEP-WCMC, IUCN, Marine Protected Planet [Online] (2023).
- 4.Lubchenco J., Palumbi S. R., Gaines S. D., Andelman S., Plugging a hole in the ocean: The emerging science of marine reserves. Ecol. Appl. 13, S3–S7 (2003). [Google Scholar]
- 5.UNGA, Transforming our World: The 2030 agenda for sustainable development A/RES/70/1 (2015). 10.1891/9780826190123.ap02 (March 3, 2021). [DOI]
- 6.CBD, Kunming-Montreal Global Biodiversity Framework (Convention on Biological Diversity, 2022). [Google Scholar]
- 7.Lubchenco J., Grorud-Colvert K., Making waves: The science and politics of ocean protection. Science 350, 382–383 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Sala E., et al. , Assessing real progress towards effective ocean protection. Mar. Policy 91, 11–13 (2018). [Google Scholar]
- 9.Sala E., Giakoumi S., No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 75, 1166–1168 (2018). [Google Scholar]
- 10.Grorud-Colvert K., et al. , The MPA Guide: A framework to achieve global goals for the ocean. Science 373, eabf0861 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Halpern B. S., The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 13, 117–137 (2003). [Google Scholar]
- 12.Lester S. E., et al. , Biological effects within no-take marine reserves: A global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46 (2009). [Google Scholar]
- 13.Rolim F. A., et al. , Network of small no-take marine reserves reveals greater abundance and body size of fisheries target species. PLoS ONE 14, e0204970 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester S., Halpern B., Biological responses in marine no-take reserves versus partially protected areas. Mar. Ecol. Prog. Ser. 367, 49–56 (2008). [Google Scholar]
- 15.Edgar G. J., et al. , Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Sciberras M., et al. , Evaluating the relative conservation value of fully and partially protected marine areas. Fish Fish. 16, 58–77 (2015). [Google Scholar]
- 17.Thiault L., et al. , Ecological evaluation of a marine protected area network: A progressive-change BACIPS approach. Ecosphere 10, e02576 (2019). [Google Scholar]
- 18.Selig E. R., et al. , Global priorities for marine biodiversity conservation. PLoS ONE 9, e82898 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selig E. R., et al. , Mapping global human dependence on marine ecosystems. Conserv. Lett. 12, e12617 (2019). [Google Scholar]
- 20.Bennett N. J., Dearden P., Why local people do not support conservation: Community perceptions of marine protected area livelihood impacts, governance and management in Thailand. Mar. Policy 44, 107–116 (2014). [Google Scholar]
- 21.Kamat V., “The Ocean is our Farm”: Marine conservation, food insecurity, and social suffering in Southeastern Tanzania. Human Org. 73, 289–298 (2014). [Google Scholar]
- 22.Andradi-Brown D. A., et al. , Diversity in marine protected area regulations: Protection approaches for locally appropriate marine management. Front. Mar. Sci. 10, 1099579 (2023). [Google Scholar]
- 23.Bennett N. J., et al. , An appeal for a code of conduct for marine conservation. Mar. Policy 81, 411–418 (2017). [Google Scholar]
- 24.Kuempel C. D., Jones K. R., Watson J. E. M., Possingham H. P., Quantifying biases in marine-protected-area placement relative to abatable threats. Conserv. Biol. 33, 1350–1359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joppa L. N., Pfaff A., High and far: Biases in the location of protected areas. PLoS ONE 4, e8273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaff A., Robalino J., Lima E., Sandoval C., Herrera L. D., Governance, location and avoided deforestation from protected areas: Greater restrictions can have lower impact, due to differences in location. World Dev. 55, 7–20 (2014). [Google Scholar]
- 27.Bartlett C. Y., et al. , Comparison of outcomes of permanently closed and periodically harvested coral reef reserves. Conserv. Biol. 23, 1475–1484 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Campbell S. J., Edgar G. J., Stuart-Smith R. D., Soler G., Bates A. E., Fishing-gear restrictions and biomass gains for coral reef fishes in marine protected areas. Conserv. Biol. 32, 401–410 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Campbell S. J., et al. , Fishing restrictions and remoteness deliver conservation outcomes for Indonesia’s coral reef fisheries. Conserv. Lett. 13, e12698 (2020). [Google Scholar]
- 30.Ulate K., et al. , Conventional MPAs are not as effective as community co-managed areas in conserving top–down control in the Gulf of California. Biol. Conserv. 228, 100–109 (2018). [Google Scholar]
- 31.Zupan M., et al. , Marine partially protected areas: Drivers of ecological effectiveness. Front. Ecol. Environ. 16, 381–387 (2018). [Google Scholar]
- 32.McClanahan T. R., Graham N. A. J., Wilson S. K., Letourneur Y., Fisher R., Effects of fisheries closure size, age, and history of compliance on coral reef fish communities in the western Indian Ocean. Mar. Ecol. Prog. Ser. 396, 99–109 (2009). [Google Scholar]
- 33.Campbell S. J., et al. , Weak compliance undermines the success of no-take zones in a large government-controlled marine protected area. PLoS ONE 7, e50074 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spalding M. D., et al. , Building towards the marine conservation end-game: Consolidating the role of MPAs in a future ocean. Aquat. Conserv.: Mar. Freshwater Ecosyst. 26, 185–199 (2016). [Google Scholar]
- 35.Woodcock P., O’Leary B. C., Kaiser M. J., Pullin A. S., Your evidence or mine? Systematic evaluation of reviews of marine protected area effectiveness. Fish Fish. 18, 668–681 (2017). [Google Scholar]
- 36.Ferraro P. J., Sanchirico J. N., Smith M. D., Causal inference in coupled human and natural systems. Proc. Natl. Acad. Sci. U.S.A. 116, 5311–5318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smallhorn-West P. F., Weeks R., Gurney G., Pressey R. L., Ecological and socioeconomic impacts of marine protected areas in the South Pacific: Assessing the evidence base. Biodivers. Conserv. 29, 349–380 (2020). [Google Scholar]
- 38.Osenberg C., Shima J., Miller S., Stier A., "Assessing effects of marine protected areas: confounding in space and possible solutions" in Marine Protected Areas: A Multidisciplinary Approach, Claudet J., Ed. (Cambridge University Press, 2011), pp. 143–167. [Google Scholar]
- 39.Nolte C., Agrawal A., Linking management effectiveness indicators to observed effects of protected areas on fire occurrence in the Amazon rainforest. Conserv. Biol. 27, 155–165 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Ferraro P. J., et al. , More strictly protected areas are not necessarily more protective: Evidence from Bolivia, Costa Rica, Indonesia, and Thailand. Environ. Res. Lett. 8, 025011 (2013). [Google Scholar]
- 41.Rasolofoson R. A., Statistical matching for conservation science revisited: Response to Schleicher et al. 2020. Conserv. Biol. 36, e14006 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Rico-Straffon J., et al. , Forest concessions and eco-certifications in the Peruvian Amazon: Deforestation impacts of logging rights and logging restrictions. J. Environ. Econ. Manage. 118, 102780 (2023). [Google Scholar]
- 43.McClanahan T. R., Graham N. A. J., MacNeil M. A., Cinner J. E., Biomass-based targets and the management of multispecies coral reef fisheries. Conserv. Biol. 29, 409–417 (2015). [DOI] [PubMed] [Google Scholar]
- 44.MacNeil M. A., et al. , Recovery potential of the world’s coral reef fishes. Nature 520, 341–344 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Gill D. A., et al. , Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543, 665–669 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Ostrom E., Governing the Commons: The Evolution of Institutions for Collective Action (Cambridge University Press, 1990). [Google Scholar]
- 47.Iacarella J. C., Clyde G., Bergseth B. J., Ban N. C., A synthesis of the prevalence and drivers of non-compliance in marine protected areas. Biol. Conservation 255, 108992 (2021). [Google Scholar]
- 48.Advani S., Rix L. N., Aherne D. M., Alwany M. A., Bailey D. M., Distance from a fishing community explains fish abundance in a no-take zone with weak compliance. PLoS ONE 10, e0126098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurney G. G., et al. , Biodiversity needs every tool in the box: Use OECMs. Nature 595, 646–649 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Purwanto, et al. , The Bird’s head seascape marine protected area network—Preventing biodiversity and ecosystem service loss amidst rapid change in Papua, Indonesia. Conserv. Sci. Pract. 3, e393 (2021). [Google Scholar]
- 51.Jupiter S. D., Cohen P. J., Weeks R., Tawake A., Govan H., Locally-managed marine areas: Multiple objectives and diverse strategies. Pacific Conserv. Biol. 20, 165–179 (2014). [Google Scholar]
- 52.Bergseth B. J., Gurney G. G., Barnes M. L., Arias A., Cinner J. E., Addressing poaching in marine protected areas through voluntary surveillance and enforcement. Nat. Sustain. 1, 421–426 (2018). [Google Scholar]
- 53.Drucker A. M., Fleming P., Chan A.-W., Research techniques made simple: Assessing risk of bias in systematic reviews. J. Invest. Dermatol. 136, e109–e114 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Cinner J. E., et al. , Meeting fisheries, ecosystem function, and biodiversity goals in a human-dominated world. Science 368, 307–311 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Ahmadia G. N., et al. , Integrating impact evaluation in the design and implementation of monitoring marine protected areas. Philos. Trans. R Soc. B Biol. Sci. 370, 20140275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mascia M. B., et al. , A novel framework for analyzing conservation impacts: evaluation, theory, and marine protected areas. Ann. N.Y. Acad. Sci. 1399, 93–115 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Goetze J., Langlois T., Claudet J., Januchowski-Hartley F., Jupiter S. D., Periodically harvested closures require full protection of vulnerable species and longer closure periods. Biol. Conserv. 203, 67–74 (2016). [Google Scholar]
- 58.Dureuil M., Boerder K., Burnett K. A., Froese R., Worm B., Elevated trawling inside protected areas undermines conservation outcomes in a global fishing hot spot. Science 362, 1403–1407 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Cinner J. E., et al. , Gear-based fisheries management as a potential adaptive response to climate change and coral mortality. J. Appl. Ecol. 46, 724–732 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (R)
Dataset S02 (CSV)
Dataset S03 (CSV)
Dataset S04 (CSV)
Dataset S05 (CSV)
Data Availability Statement
All study data used in the MPA impact analysis are included as supporting information. JAGs code used in analysis is also included. Additional code available upon request. For those interested in original, survey-level data, please contact the organizations and researchers listed in SI Appendix, Table S1.