Abstract
INTRODUCTION
While studies report that sleep disturbance can have negative effects on brain vasculature, its impact on cerebrovascular diseases such as white matter hyperintensities (WMHs) in beta‐amyloid‐positive older adults remains unexplored.
METHODS
Sleep disturbance, WMH burden, and cognition in normal controls (NCs), and individuals with mild cognitive impairment (MCI) and Alzheimer's disease (AD), were examined at baseline and longitudinally. A total of 912 amyloid‐positive participants were included (198 NC, 504 MCI, and 210 AD).
RESULTS
Individuals with AD reported more sleep disturbances than NC and MCI participants. Those with sleep disturbances had more WMHs than those without sleep disturbances in the AD group. Mediation analysis revealed an effect of regional WMH burden on the relationship between sleep disturbance and future cognition.
DISCUSSION
These results suggest that WMH burden and sleep disturbance increase from aging to AD. Sleep disturbance decreases cognition through increases in WMH burden. Improved sleep could mitigate the impact of WMH accumulation and cognitive decline.
Keywords: Alzheimer's disease, amyloid positivity, cognitive decline, mild cognitive impairment, older adults, sleep disturbances, white matter hyperintensities
1. INTRODUCTION
Sleep disturbance refers to experiencing difficulties when falling and/or staying asleep throughout the night. Such disturbances may result in daytime fatigue and may also cause other related problems, such as concentration issues, memory loss, irritability, and depression. 1 Sleep disturbances such as insomnia or sleep apnea are a physiological hallmark of increasing age and become more prevalent in people with mild cognitive impairment (MCI) and Alzheimer's disease (AD). 2 , 3 , 4 , 5 Sleep disorders observed in people with AD 6 and MCI 7 are more severe than in healthy older adults. For example, people with AD and MCI tend to experience more disrupted nocturnal sleep, earlier bedtimes, more variable wake‐up times, and fragmented sleep compared to healthy older adults. 6 , 7 , 8 Sleep disturbances in aging, MCI, and AD have been observed to be related to increased cognitive change, pathology, and risk of and progression to AD. 2
With respect to cognition, several studies have noted a positive correlation between the severity of sleep disturbance and the severity of cognitive decline in healthy older adults 9 and individuals with MCI/AD. 4 , 5 , 10 , 11 These declines are often observed in global cognition and memory. 10 , 11 Additionally, sleep disturbances have been thought to be associated with the accumulation of amyloid‐beta (Aβ) 4 , 5 and neurodegeneration. 12 Higher levels of Aβ deposition have been observed in people with more sleep disruptions. 4 , 13 , 14 , 15 The presence of Aβ is observed in people with AD, and is used as a biomarker to identify individuals who will develop AD. 16 Therefore, the relationship between Aβ and sleep highlights the crucial role that sleep plays in the development of AD‐related pathology. Other pathological changes in the brain, such as white matter hyperintensities (WMHs), have also been associated with sleep disturbances. For example, insomnia and short sleep durations are associated with increased WMH burden. 17 , 18 , 19 However, the results examining this relationship have not been consistently reported, with some studies finding no association between sleep disturbance and WMH burden. 20 , 21 WMHs are observed as increased signals during T2‐weighted or fluid‐attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) scans. They are often used as a proxy for cerebrovascular disease, which is a well‐established contributor to cognitive decline and dementia. 22 , 23 , 24 WMHs are associated with increased cognitive decline in healthy older adults 25 , 26 , 27 and in MCI 26 , 28 , 29 , 30 as well as increased risk of conversion to MCI and dementia. 31
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (eg, PUBMED). While sleep has been associated with cognitive decline and dementia progression, few studies examined the impact sleep disturbances have on white matter hyperintensities (WMHs) and cognition.
Interpretation: Our findings suggest that people with Alzheimer's disease (AD) report more sleep disturbances than healthy older adults and people with mild cognitive impairment. AD patients who reported sleep disturbances had more WMHs than AD patients without sleep disturbances. Sleep disturbances increased cognitive deficits through increases in WMHs.
Future directions: Increased WMH burden was observed in people with AD who report sleep disturbances over people with AD without sleep disturbances. Furthermore, sleep disturbances impair cognition through WMH burden. These findings suggest that if sleep disturbances are managed, it may be possible to reduce WMH burden observed in people with AD, thus reducing cognitive change and slowing disease progression.
The current research investigating the relationship between sleep and WMHs in aging and dementia has several limitations that may account for conflicting results. The present study includes longitudinal follow‐ups of the participants, which is a notable strength, as it allows us to track changes over time and observe the progression of WMH burden in the same individuals, providing a more comprehensive understanding of the relationship between sleep and WMHs. Previous research has not yet examined regional WMH differences and sleep disturbance in individuals who are amyloid positive. Most studies have used a total brain WMH approach, which may not capture regional differences specific to sleep disturbances. It is possible that sleep may be associated with WMH accumulation in specific regions, therefore a regional approach would provide insight into the relationship between sleep and regional WMHs. Other findings have reported that posterior WMHs are associated with AD 32 , 33 while a widespread accumulation of WMH is associated with vascular dementia. 34 Thus, a regional approach may indicate whether sleep disturbances are more strongly associated with AD‐related WMH burden or vascular‐related WMH burden.
The goal of this study was to examine whether sleep disturbances influence WMH burden in healthy older adults, and people with MCI and AD, who are amyloid positive, and whether these sleep disturbances influence global cognition. To our knowledge, this would be the first study to examine the relationship between sleep disturbances and WMH burden and global cognition in older adults who are amyloid positive. Given that sleep disturbances may be modifiable using various intervention techniques (eg, medications, cognitive‐behavioral strategies, and circadian therapies), 35 , 36 cognitive decline and pathological brain changes may be mitigated by treating sleep disturbance.
2. METHODS
2.1. Participants
Data used in this article are from the Alzheimer's Disease Neuroimaging Initiative (ADNI; for details on this study see Appendix). Participant inclusion and exclusion criteria are available at www.adni‐info.org. Participants were included in the study if they were between the ages of 55 and 95, had MRIs from which WMHs could be extracted, and had completed the sleep questionnaires. Healthy older adults or normal controls (NCs) did not exhibit memory decline or impaired global cognition, as assessed by the Wechsler Memory Scale, Mini‐Mental State Examination (MMSE) and Clinical Dementia Rating (CDR). MCI participants had MMSE scores between 24 and 30, CDR scores of 0.5, and abnormal scores on the Wechsler Memory Scale. AD participants had abnormal memory function on the Wechsler Memory Scale, MMSE scores between 20 and 26, a CDR of 0.5 or 1.0, and met the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria. 37
To make sure that the participants in this study were on the AD trajectory, participants were only included if they were amyloid positive. Amyloid positivity was identified based on both positron emission tomography (PET) and cerebrospinal fluid (CSF) values using the following criteria: (1) a standardized uptake value ratio (SUVR) of >1.11 on AV45 PET 38 ; (2) an SUVR of >1.2 using Pittsburgh compound‐B PET 39 ; (3) an SUVR of ≥1.08 for florbetaben (FBB) PET 40 ; or (4) a cerebrospinal fluid Aß1‐42 of ≤980 pg/ml as per ADNI recommendations.
Participant sleep scores were downloaded from the ADNI website. Consistent with previous research, the presence of sleep disturbance was determined by caregiver or informant report on the Neuropsychiatric Inventory (NPI), a brief questionnaire form of the NPI (NPI‐Q), or Diagnosis and Symptoms Checklist (ADSXLIST). 41 , 42 , 43 The NPI is a caregiver‐informant interview that assess behavioral changes including sleep problems. The NPI‐sleep questionnaire pertains to recent changes in the patient's sleep behavior. In the present study, we focused on questions (from all three questionnaires) that were related to sleep and excluded questions that assessed erratic behavior. That is, we kept questions relating to difficulties in falling asleep (NPI‐K1), getting up during the night (NPI‐K2), awakening the caregiver during night (NPI‐K4), waking up too early in the morning (NPI‐K6), and excessive daytime napping (NPI‐K7). In the ADSXLIST, we extracted information on patient insomnia. For the NPI‐Q, three questions were included asking a caregiver if the patient awakens during night, arises too early in the morning, or takes excessive naps during the day. These questions were scored as either 0 for the absence or 1 for the presence of each symptom. This approach ensured that we were able to accurately identify and include participants who were experiencing sleep disturbance, while minimizing the inclusion of individuals who may have been experiencing other types of disruption.
Figure 1 summarizes participants included and follow‐up durations in each group. A total of 912 participants with amyloid positivity were included (198 NCs, 504 MCI, and 210 AD participants). Of the 912 participants, 292 had sleep disturbances (47 for the NC, 159 for the MCI, and 86 for the AD groups). The groups had the following number of average follow‐up years: (1) NC, 4.00 years for no sleep disturbance and 4.49 years with sleep disturbance; (2) MCI, 3.18 years for no sleep disturbance and 3.14 years with sleep disturbance; and (3) AD, 1.42 years for no sleep disturbance and 1.45 years with sleep disturbance. Participants that had at least two follow‐up visits were included in the study. To ensure that follow‐up time duration did not impact our study findings, the models were repeated with the groups matched based on sleep disturbance and follow‐up time (ie, excluding additional follow‐up time points from groups that had longer follow‐up durations).
FIGURE 1.
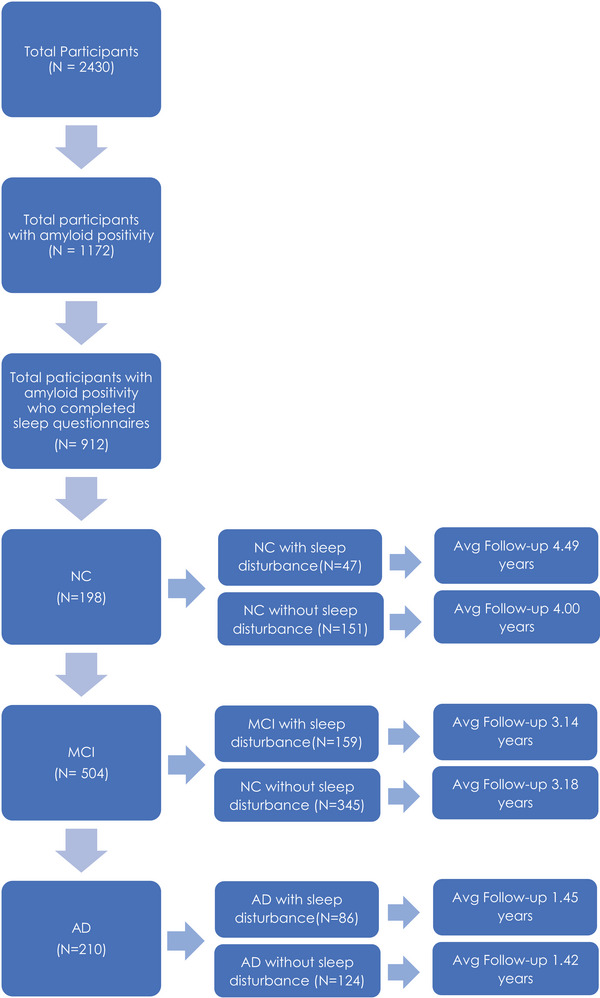
Flowchart summarizing participants included in each group and follow‐up duration from the ADNI database. AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; MCI, mild cognitive impairment; NC, normal control.
2.2. Structural MRI acquisition and processing
All participants underwent MRI scanning using standardized acquisition protocols designed and implemented by ADNI. Further details about the MRI protocols and imaging parameters can be found at http://adni.loni.usc.edu/methods/mri‐tool/mri‐analysis/. MRI data from all participants (baseline and longitudinal) were downloaded from the ADNI public website.
T1w scans were preprocessed using our standard pipeline which includes noise reduction, 44 intensity inhomogeneity correction, 45 and intensity normalization into range (0 to 100). The preprocessed images were then linearly (nine parameters: three translation, three rotation, and three scaling) 46 registered to the MNI‐ICBM152‐2009c average template. 47
2.3. WMH measurements
A validated method for segmenting WMHs in aging and neurodegenerative diseases was used to obtain WMH measurements. 48 This technique has been used in the ADNI cohort 31 and other multicenter studies. 49 , 50 WMHs were automatically segmented using the T1w contrast, in addition to location and intensity features from a library of manually segmented scans (50 ADNI participants that were not part of the study) in combination with a random forest classifier to detect the WMHs in new images. 48 WMHs were segmented using T1w images (instead of FLAIR and T2w/PD scans) because ADNI1 only acquired T2w/PD images with resolutions of 1 × 1 × 3 mm3, whereas ADNI2/GO acquired only axial FLAIR images with resolutions of 1 × 1 × 5 mm3, and ADNI3 acquired sagittal FLAIR images with resolutions of 1 × 1 × 1.2 mm3. Due to the potential inconsistencies in WMH measurements across the ADNI cohorts, we opted to include consistently acquired T1w images to measure WMH burden. These T1w‐based WMH volumes have been previously observed to be very highly correlated with FLAIR and T2w‐based WMH loads in the ADNI dataset. 46 , 51 A visual assessment (performed by MD) was conducted to assess the quality of the registrations and WMH segmentations. Cases that did not meet the quality control criteria were removed from the analyses (N = 59). The WMH load was determined as the volume of all voxels identified as WMH in the standard space (in mm3) and was therefore normalized for head size. Regional and total WMH volumes were calculated based on Hammers Atlas. 51 , 52 Analyses were completed separately for frontal, temporal, parietal, occipital, and total WMHs. Regional WMH values (ie, frontal, temporal, parietal, and occipital) were summed across the right and left hemispheres to obtain one score for each region. To achieve normal distribution, All WMH volumes were log‐transformed.
2.4. Global cognition
The Clinical Dementia Rating–Sum of Boxes (CDR‐SB) was included as a measure of global cognitive functioning. CDR‐SB is measured and defined through a structured interview, which typically involves both the patient and a knowledgeable informant, usually a family member or caregiver. The CDR‐SB score is calculated by summing the scores in each of the six domains: memory, orientation, judgment and problem‐solving, community affairs, home and hobbies, and personal care. Each domain is scored on a 5‐point scale (0, 0.5, 1, 2, 3), with higher scores indicating more severe impairment. This cognitive score was also downloaded from the ADNI website.
2.5. Vascular risk factors
Hypertension was defined by the ADNI with each person being assigned 0 for no hypertension and 1 for hypertension. Diabetic status was completed using medication information. Medication lists were downloaded from ADNI and participants who took medications prescribed to manage diabetes were used as a proxy to determine diabetic status, 0 for no diabetes and 1 for diabetes. Smoking was defined by ADNI with each person being assigned 0 for no smoking and 1 for smoking. Alcohol abuse was defined by ADNI with each person being assigned 0 for no alcohol abuse and a 1 for alcohol abuse. Body mass index (BMI) was calculated for each person at each visit, using height and weight information provided by ADNI for the matching visit to the MRI visit.
2.6. Statistical analysis
2.6.1. Baseline assessments
Participant demographic information is presented in Table 1. Demographic data were analyzed with t‐tests and chi‐square tests and corrected for multiple comparisons using Bonferroni correction.
TABLE 1.
Demographic and clinical characteristics for NC, MCI, and AD participants.
| Demographic information | NC (n = 198) | MCI (n = 504) | AD (n = 210) | p |
|---|---|---|---|---|
| Baseline age | 75.0 ± 5.2 | 73.1 ± 7.1 | 74.4 ± 7.8 | N.S. |
| Education | 16.1 ± 2.6 | 16.0 ± 2.9 | 15.6 ± 2.7 | MCI vs. AD = 0.002 |
| APOE ԑ4+ | 78 (39%) | 313 (62%) | 158 (75%) |
NC vs. AD < 0.001 MCI vs. AD = 0.001 NC vs. MCI < 0.001 |
| Sleep disturbance | 47 (24%) | 159 (31%) | 86 (41%) | NC vs. AD < 0.001 |
| Male sex | 91 (46%) | 298 (59%) | 117 (55%) | NC vs. MCI = 0.002 |
| BMI | 26.5 ± 4.5 | 26.7 ± 4.7 | 25.3 ± 4.1 |
NC vs. AD < 0.001 MCI vs. AD < 0.001 |
| Hypertension | 96 (48%) | 238 (47%) | 103 (49%) | |
| Diabetes | 13 (7%) | 36 (7%) | 15(7%) | |
| Baseline CDR‐SB | .04 ± .2 | 1.55 ± .91 | 4.44 ± 1.61,2 |
NC vs. AD < 0.001 MCI vs. AD < 0.001 NC vs. MCI < 0.001 |
Notes: Values are expressed as mean ± standard deviation, or number of participants (percentage %). Statistically significant results reported if they survived Bonferroni correction and multiple comparisons.
Abbreviations: AD, Alzheimer's disease; APOE ԑ4+, number and percentage of people with at least one apolipoprotein E gene ԑ4 allele; BMI, body mass index; CDR‐SB, Clinical Dementia Rating–Sum of Boxes; MCI, mild cognitive impairment; NC, normal control.
The following linear regression model was used to investigate the impact of sleep differences on regional (frontal, temporal, parietal, occipital) and total WMH loads within each diagnostic group at baseline. The model included age, years of education, sex, and apolipoprotein E (APOE) ε4 status, as covariates. The categorical variable of interest was sleep disturbance (ie, 0 = no, 1 = yes).
| (1) |
2.6.2. Longitudinal assessments
Longitudinal linear mixed‐effects models were used to investigate whether there was an interaction between diagnostic group and sleep status, impacting regional (frontal, temporal, parietal, occipital) and total WMH loads. The models included age, years of education, sex, and APOE4 status as covariates. The categorical variables of interest were sleep disturbance and its interaction with diagnosis, based on baseline diagnosis. Participant ID was included as a categorical random effect.
| (2) |
The following linear mixed effects model was conducted to examine whether sleep disturbance would influence global cognition (ie, CDR‐SB). The models included age, years of education, sex, and APOE4 status as covariates. The categorical variable of interest was sleep disturbance.
| (3) |
Finally, we used mediation analysis to test the hypothesis that WMH burden mediates the impact of sleep disturbance on future cognitive decline, including age, sex, years of education, and APOE4 status as covariates. Future cognitive decline was measured as yearly rate of change in CDR‐SB scores in participants that had at least three longitudinal time points. The mediation analysis was performed using the mediation toolbox (https://github.com/canlab/MediationToolbox). A 95% bootstrap confidence interval based on 10,000 bootstrap samples was used to estimate significance.
All continuous values were z‐scored within the population prior to the regression and mediation analyses. All results were corrected for multiple comparisons using false discovery rate (FDR), p‐values are reported as raw values with significance then determined by FDR correction. 53 All statistical analyses were performed using MATLAB version 2021a. To complete the baseline analysis we used function fitlm and for longitudinal assessments we used fitlme.
3. RESULTS
3.1. Demographics and cognitive scores
Table 1 presents demographic and clinical information for all participants. Differences in demographics are reported for those that survived correction for multiple comparisons. The AD group exhibited more sleep disturbances (41%) than both MCI (31%) and NC participants (24%), and MCI participants had significantly more sleep disturbances than the NC group. NC participants had significantly greater education levels than the AD group (t = 3.17, p = .001). The NC group had fewer APOE ɛ4+ people than both the MCI (χ 2 = 28.79, p < .001) and AD groups (χ 2 = 52.24, p < .001). The MCI group had more people with APOE ɛ4+ than the AD group (χ 2 = 10.81, p = .001). NC (χ 2 = 3.36, p < .001) and MCI participants (χ 2 = 3.50, p < .001) both had greater BMI than those in the AD group. There were no differences in the vascular risk factors of hypertension or diabetes between the groups. Baseline cognitive performance decreased with each stage of progressive decline for the CDR‐SB (NC:MCI, t = −35.41, p < .001; NC:AD, t = −39.75, p < .001; MCI:AD, t = −24.70, p < .001).
3.2. Baseline assessments
Figure 2 shows boxplots of baseline WMHs overall and separately for each lobe. As expected, the AD group had significantly greater WMH burden than the NC group overall and across all regions (t‐values ranging from 4.56 to 3.06, p < .01, Figure 2). MCI participants had significantly greater WMH burden than the NC group only in the frontal region (t = 2.15, p = .03). People with AD had significantly greater WMH burden than the MCI group in all regions (t‐values ranging from 3.46 to 2.21, p < .03). In addition, people with AD who reported sleep disturbance had significantly greater WMH loads at total, frontal, and occipital regions than people with AD without sleep disturbance (t‐values ranging from 2.11 to 3.12, p < .03). No significant differences were observed between the MCI and NC groups.
FIGURE 2.
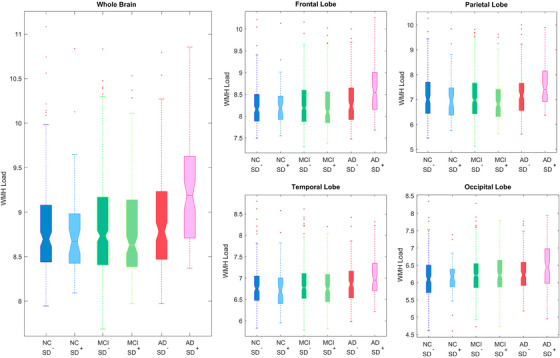
Boxplots showing baseline WMH distributions across diagnostic groups for each lobe. AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; SD–, without sleep disturbance; SD+, with sleep disturbance; WMH, white matter hyperintensity.
3.3. Longitudinal assessments
Table 2 summarizes the results of the longitudinal linear mixed effects models, revealing significant interactions between a diagnosis of AD and sleep disturbance. Specifically, people with AD who reported sleep disturbance had significantly increased WMH loads in all regions except occipital compared to both NC participants (t‐values ranging from 2.74 to 2.44, p < .02) and people with MCI (t‐values ranging from 3.18 to 2.28, p < .03) with sleep disturbances. NCs and MCI with sleep disturbance did not differ in WMH loads at any region.
TABLE 2.
Linear mixed model results showing the interaction between diagnostic group (NC, MCI, and AD) and sleep disturbance.
| Total | Frontal | Temporal | Parietal | Occipital | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T stat | p | T stat | p | T stat | p | T stat | p | T stat | p | |
| MCI vs NC: sleep disturbance | 0.46 | =0.654 | .12 | =0.902 | 0.368 | 0.071 | 0.75 | 0.450 | −0.11 | 0.910 |
| AD vs NC: sleep disturbance | 2.74 | =0.006 | 2.58 | =0.009 | 2.44 | =0.015 | 2.44 | =0.01 | 0.91 | 0.363 |
| AD vs MCI: sleep disturbance | 2.99 | =0.003 | 3.18 | =0.001 | 2.71 | =0.006 | 2.28 | =0.02 | 1.29 | 0.195 |
Note: Bolded values represent results that remained significant after correction for multiple comparisons. Secondary analyses incorporated additional risk factors that could influence WMH burden such as diabetes, hypertension, BMI, smoking, and alcohol abuse into our models. The inclusion of additional risk factors did not change our initial findings.
Abbreviations: AD, Alzheimer's disease; BMI, body mass index; MCI, mild cognitive impairment; NC, normal control.
Exploratory analyses were completed within the AD group, comparing those with sleep disturbance to those without sleep sdisturbance. These analyses investigated whether the longitudinal mixed effects model results (Model 2) remained consistent without contrasting AD patients against the NC and MCI groups. The within‐group models also showed significantly greater WMH loads in AD patients with sleep disturbance than AD patients without sleep disturbance in total, frontal, temporal, and parietal lobes (t‐values ranging from 2.56 to 3.99, p < .01).
3.3.1. Global cognition and regional WMH
The longitudinal models showed a significant impact of sleep disturbance on global cognition, as measured by CDR‐SB (t = 2.71, p = .007), suggesting that sleep disturbance (compared to no sleep disturbance) is associated with increased CDR‐SB scores (ie, worse cognition). Figure 3 and Table 3 summarize the results of the mediation analysis investigating whether the relationship between sleep disturbance and future cognitive decline is mediated by WMH burden. The results supported the existence of a mediation effect of WMH load on the relationship between sleep disturbance and future cognitive decline for total, frontal, and occipital WMHs, suggesting that sleep disturbance increases cognitive deficits through increases in WMH burden.
FIGURE 3.
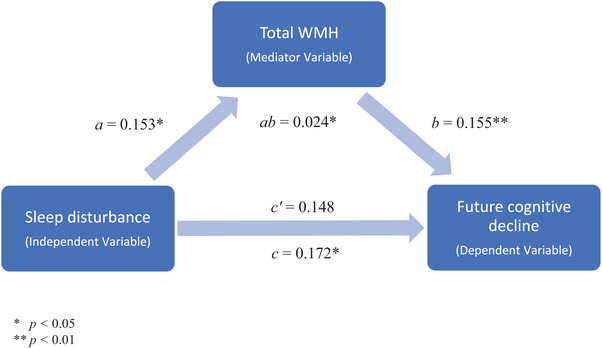
Mediation analysis results showing a mediation effect of total WMH burden on the relationship between sleep disturbance and future cognition. WMH, white matter hyperintensity.
TABLE 3.
Mediation analysis results showing a mediation effect of WMH burden on the relationship between sleep disturbance and future cognition.
| Total | Frontal | Temporal | Parietal | Occipital | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | p | Mean | p | Mean | p | Mean | p | Mean | p | |
| a | 0.153 | 0.019 | 0.176 | 0.009 | 0.082 | 0.230 | 0.074 | 0.291 | 0.189 | 0.012 |
| b | 0.155 | <0.001 | 0.162 | <0.001 | 0.123 | <0.001 | 0.123 | 0.001 | 0.104 | 0.003 |
| c | 0.172 | 0.018 | 0.174 | 0.018 | 0.173 | 0.021 | 0.174 | 0.022 | 0.173 | 0.024 |
| c’ | 0.148 | 0.042 | 0.145 | 0.054 | 0.163 | 0.029 | 0.165 | 0.030 | 0.153 | 0.047 |
| ab | 0.024 | 0.012 | 0.028 | 0.005 | 0.010 | 0.009 | 0.009 | 0.206 | 0.019 | 0.007 |
Note: Bolded values represent results that remained significant after correction for multiple comparisons. Leftmost column items a and b confirm the underlying conditions of the mediation analysis, showing that the mediator variable (ie, WMH) is significantly related to both independent (item a, sleep disturbance) and dependent (item b, future cognitive decline) variables. The item ab denotes the average causal mediation effect, while c’ is the average direct effect and c is the average indirect effect. Secondary analyses incorporating additional risk factors that could influence WMH burden such as diabetes, hypertension, body mass index, smoking, and alcohol abuse into our models. The inclusion of additional risk factors did not change our initial findings.
3.3.2. Additional risk factors
In the interest of refining our analysis and ensuring robustness, we conducted secondary analyses incorporating additional risk factors that could influence WMH burden such as diabetes, hypertension, BMI, smoking, and alcohol abuse into our models. The inclusion of additional risk factors did not change our initial findings. Additional analyses were also carried out including depression and anxiety as covariates to ensure results were not influenced by these factors. Depression (1 = depression and 0 = no depression) and anxiety (1 = anxiety and 0 = no anxiety) were determined via caregiver/informant report on the NPI and NPI‐Q from the ADNI database. The results remained the same in terms of effect size and significance suggesting depression and anxiety were not significantly impacting these findings.
4. DISCUSSION
Sleep disturbances are known to increase cognitive decline, AD‐related pathology, and risk for AD. 2 However, the relationship between sleep disturbances and WMHs, a known risk factor of cognitive decline and AD, is relatively unexplored. The current study investigated the association between sleep disturbances and WMHs in amyloid‐positive older adults. Furthermore, the impact of sleep disturbances on cognitive functioning was also examined. At baseline, we observed that AD participants with sleep disturbance had significantly larger WMH burden than those without sleep disturbance. Longitudinal results revealed that people with AD and sleep disturbance had increased WMHs compared to NC and MCI participants with sleep disturbance at all regions, except occipital. Furthermore, longitudinal analysis also revealed that people with AD with sleep disturbance had increased WMHs than AD participants with no sleep disturbances. Sleep disturbance was also related to global cognition (as measured by the CDR‐SB). Finally, we found a mediation effect of total, frontal, and occipital WMH burden on the relationship between sleep disturbance and future cognition. Taken together, these findings suggest that sleep disturbances are positively associated with WMH accumulation in older adults on the AD trajectory and lead to cognitive deficits through increase in WMH burden.
Consistent with prior research, this study demonstrated a link between sleep disturbances and WMHs. 17 , 18 , 19 It should be noted that some studies report no association between sleep disturbance and WMHs. 20 , 21 Li et al. 20 did not specifically investigate the relationship between sleep and WMHs but rather focused on the mediating effect of WMHs on the relationship between sleep and depression. Therefore, the lack of significant association between sleep and WMHs in that study may be due to the use of a sample of older adults with depression who may have different underlying brain changes than older adults without depression. Additionally, the study did not examine longitudinal findings but instead only investigated cross‐sectional data. Similarly, Zitser et al. 21 did not observe an association between sleep duration and WMHs in middle‐aged and older individuals over a period of 28 years. However, the study did not examine regional WMHs but rather looked at global WMH burden, which may have masked potential regional differences. The authors also noted that their sample had a relatively small proportion of individuals with poor sleep, which may have limited the statistical power to detect associations. 21 The present study utilizes longitudinal data to provide precise estimates of the association between Aβ pathology and self‐reported sleep duration on WMH burden. This study builds upon smaller baseline studies that have observed this association inconsistently to date.
The results of this study are also consistent with previous reports which found more severe sleep disturbances in people with AD 6 and MCI 7 compared to healthy older adults. However, we did not observe a diagnosis by sleep disturbance interaction for MCI (vs healthy older adults), indicating that the relationship between sleep disturbance and WMH burden is not significantly different in people with MCI compared to healthy older adults. On the other hand, we did observe that people with AD with sleep disturbance had increased WMH burden at baseline compared to healthy older adults with and without sleep disturbances. Over time, we also observed that people with AD and sleep disturbance had increased WMHs compared to both healthy older adults and people with MCI who had sleep disturbances. These findings follow previous reports indicating that WMH burden is more strongly associated with sleep disturbances in AD.
Sleep disturbance was also observed to be associated with changes in global cognition (as measured by the CDR‐SB). This relationship is consistent with previous reports indicating that sleep disturbances are negatively associated with cognition. 10 , 11 These findings indicate that sleep disturbances are associated with future cognitive decline in older adults on the AD trajectory. Thus, it may be possible to reduce cognitive decline in older adults by managing sleep disturbances. However, it is important to note that future research is needed to examine whether these sleep changes contribute to AD progression or simply reflect early symptoms. In addition, it is possible that different sleep disturbances (eg, sleep fragmentation, abnormal sleep duration, insomnia, excessive daytime sleepiness, sleep‐disordered breathing) are associated with varying risks of pathological changes and may influence AD in different manners.
Previous conflicting findings regarding the association of sleep with WMHs could be attributed to an inconsistent definition of sleep disturbance as well as subjective reports of sleep disturbance. For instance, research examining sleep disturbance typically incorporates a wide range of day and night‐time abnormalities from self‐reported sleep characteristics. This limitation could lead to the inclusion of individuals who do not actually experience sleep disturbance, which would contribute to the conflicting findings reported in the literature. The present study relies heavily on caregiver‐reported data, which may introduce bias. This method of data collection may not capture the full spectrum of sleep disturbances experienced by the individuals. Future research should incorporate more objective measures of sleep, such as polysomnography, which includes electroencephalography, electro‐oculography, and electromyography. These techniques would indeed enhance the reliability of sleep assessments as they provide a more comprehensive evaluation of sleep by capturing a range of physiological parameters during sleep. Another limitation is the lack of detailed measurement of other sleep problems separately such as insomnia, sleep apnea, or restless legs syndrome, which could limit the generalizability of our findings to the broader population of individuals with sleep disorders. Future research may also benefit from examining severity and frequency of sleep disturbances.
Sleep disturbances increase in prevalence in individuals who experience cognitive decline, particularly in AD. These sleep disturbances are associated with decreased quality of life for both patients and caregivers. There is also growing evidence that sleep disturbances may be linked to Aβ accumulation and increased atrophy. The current study adds to this literature by observing that sleep disturbances are associated with WMH burden in older adults on the AD trajectory. This finding suggests that individuals on the AD trajectory, who experience faster WMH progression, 30 may also develop a more widespread accumulation of WMHs if they experience sleep disturbances. Therefore, if sleep disturbances are managed, it may be possible to reduce the WMH burden observed in people with AD (in particular for frontal WMHs which have a more vascular origin), 54 thus reducing cognitive change and slowing disease progression. Identifying and treating sleep disturbances in individuals with dementia may have important implications for disease management and may help develop new interventions to help mitigate disease progression.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests. Author disclosures are available in the supporting information
CONSENT STATEMENT
Written informed consent was obtained from participants or their study partner
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; Euroimmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Alzheimer's Disease Neuroimaging Initiative. This research was supported by a grant from the Canadian Institutes of Health Research (CIHR). Dr. Kamal is supported by a scholarship from Fonds de Recherche duQuébec ‐ Santé (FRQS). Dr. Dadar reports receiving research funding from the QuebecBio‐Imaging Network and Fonds de Recherche du Québec ‐ Santé (FRQS), Natural Sciences and Engineering Research Council of Canada (NSERC), Healthy Brains for Healthy Lives (HBHL), Alzheimer Society Research Program (ASRP), CIHR, and Douglas Research Centre (DRC). Dr. Morrison is supported by CIHR.
1. COLLABORATORS
Alzheimer's Disease Neuroimaging Initiative.
The data used for this article was from the ADNI database (adni.loni.usc.edu), which was established in 2003 as a public‐private venture with Michael W. Weiner, MD as the Principal Investigator. ADNI's primary aim is to determine if serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be utilized to measure the progression of mild cognitive impairment and Alzheimer's disease. The research was granted ethical approval from the review boards of all the involved institutions, and written consent was obtained from participants or their study partner. The participants for this study were taken from all the ADNI cohorts (ADNI‐1, ADNI‐2, ADNI‐GO, and ADNI‐3).
Kamal F, Morrison C, Dadar M. Investigating the relationship between sleep disturbances and white matter hyperintensities in older adults on the Alzheimer's disease spectrum. Alzheimer's Dement. 2024;16:e12553. 10.1002/dad2.12553
Data used in preparation of this article were obtained from Alzheimer's Disease.
Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Contributor Information
Farooq Kamal, Email: farooq.kamal@mail.mcgill.ca.
Mahsa Dadar, Email: mahsa.dadar@mcgill.ca.
REFERENCES
- 1. Medic G, Wille M, Hemels MEH. Short‐ and long‐term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151‐161. doi: 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 2016;39(8):552‐566. doi: 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36(7):1027‐1032. doi: 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ju YES, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587‐593. doi: 10.1001/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology‐a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115‐119. doi: 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bombois S, Derambure P, Pasquier F, Monaca C. Sleep disorders in aging and dementia. J Nutr Heal Aging. 2010;14(3):212‐217. doi: 10.1007/s12603-010-0052-7 [DOI] [PubMed] [Google Scholar]
- 7. D'Rozario AL, Chapman JL, Phillips CL, et al. Objective measurement of sleep in mild cognitive impairment: a systematic review and meta‐analysis. Sleep Med Rev. 2020;52:101308. doi: 10.1016/j.smrv.2020.101308 [DOI] [PubMed] [Google Scholar]
- 8. Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40‐81. doi: 10.1093/sleep/16.1.40 [DOI] [PubMed] [Google Scholar]
- 9. Dzierzewski JM, Dautovich N, Ravyts S. Sleep and cognition in older adults. Sleep Med Clin. 2018;13(1):93‐106. doi: 10.1016/j.jsmc.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Silva RAPC. Sleep disturbances and mild cognitive impairment: a review. Sleep Sci. 2015;8(1):36‐41. doi: 10.1016/j.slsci.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mecca AP, Michalak HR, McDonald JW, et al. Sleep disturbance and the risk of cognitive decline or clinical conversion in the ADNI cohort. Dement Geriatr Cogn Disord. 2018;45(3‐4):232‐242. doi: 10.1159/000488671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348‐355. doi: 10.1016/j.nbd.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol. 2012;69(1):51‐58. doi: 10.1001/archneurol.2011.235.Effects [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cordone S, Annarumma L, Rossini PM, De Gennaro L. Sleep and β‐amyloid deposition in Alzheimer disease: insights on mechanisms and possible innovative treatments. Front Pharmacol. 2019;10(JUN):1‐12. doi: 10.3389/fphar.2019.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Insel PS, Mohlenhoff BS, Neylan TC, Krystal AD, Mackin RS. Association of sleep and β‐amyloid pathology among older cognitively unimpaired adults. JAMA Netw Open. 2021;4(7):1‐12. doi: 10.1001/jamanetworkopen.2021.17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535‐562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grumbach P, Opel N, Martin S, et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum Brain Mapp. 2020;41(15):4397‐4405. doi: 10.1002/hbm.25132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kocevska D, Tiemeier H, Lysen TS, et al. The prospective association of objectively measured sleep and cerebral white matter microstructure in middle‐aged and older persons. Sleep. 2019;42(10):1‐8. doi: 10.1093/sleep/zsz140 [DOI] [PubMed] [Google Scholar]
- 19. Kang JM, Joo SW, Son YD, et al. Low white‐matter integrity between the left thalamus and inferior frontal gyrus in patients with insomnia disorder. J Psychiatry Neurosci. 2018;43(6):366‐374. doi: 10.1503/jpn.170195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li C, Schreiber J, Bittner N, et al. White matter microstructure underlies the effects of sleep quality and life stress on depression symptomatology in older adults. Front Aging Neurosci. 2020;12(November):1‐11. doi: 10.3389/fnagi.2020.578037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zitser J, Anatürk M, Zsoldos E, et al. Sleep duration over 28 years, cognition, gray matter volume, and white matter microstructure: a prospective cohort study. Sleep. 2020;43(5):1‐7. doi: 10.1093/sleep/zsz290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 23. Abraham HMA, Wolfson L, Moscufo N, Guttmann CRG, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab. 2016;36(1):132‐142. doi: 10.1038/jcbfm.2015.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura Y, Araki A. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int. 2015;15:34‐42. doi: 10.1111/ggi.12666 [DOI] [PubMed] [Google Scholar]
- 25. Bangen KJ, Preis SR, Delano‐Wood L, et al. Baseline white matter hyperintensities and hippocampal volume are associated with conversion from normal cognition to mild cognitive impairment in the Framingham offspring study. Alzheimer Dis Assoc Disord. 2018;32(1):50‐56. doi: 10.1097/WAD.0000000000000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim S, Choi SH, Lee YM, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatrics. 2015;27(12):2069‐2077. doi: 10.1017/S1041610215001076 [DOI] [PubMed] [Google Scholar]
- 27. Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192‐2198. doi: 10.1212/01.wnl.0000249119.95747.1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirao K, Yamashita F, Tsugawa A, et al. Association of white matter hyperintensity progression with cognitive decline in patients with amnestic mild cognitive impairment. J Alzheimer's Dis. 2021;80(2):877‐883. doi: 10.3233/JAD-201451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li JQ, Tan L, Wang HF, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer's disease: a systematic review and meta‐analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87(5):476‐484. doi: 10.1136/jnnp-2014-310095 [DOI] [PubMed] [Google Scholar]
- 30. Kamal F, Morrison C, Maranzano J, Zeighami Y, Dadar M. Topographical differences in white matter hyperintensity burden and cognition in aging, MCI, and AD. GeroScience. http://medrxiv.org/content/early/2022/04/21/2022.04.20.22274087.abstract. Published online 2022:22274087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dadar M, Maranzano J, Ducharme S, Collins DL. White matter in different regions evolves differently during progression to dementia. Neurobiol Aging. 2019;76:71‐79. doi: 10.1016/j.neurobiolaging.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 32. Brickman AM. Contemplating Alzheimer's disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13(12). doi: 10.1007/s11910-013-0415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habes M, Erus G, Toledo JB, et al. Regional tract‐specific white matter hyperintensities are associated with patterns to aging‐related brain atrophy via vascular risk factors, but also independently. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2018;10:278‐284. doi: 10.1016/j.dadm.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bombois S, Debette S, Bruandet A, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39(7):2046‐2051. doi: 10.1161/STROKEAHA.107.505206 [DOI] [PubMed] [Google Scholar]
- 35. Wade AG, Farmer M, Harari G, et al. Add‐on prolonged‐release melatonin for cognitive function and sleep in mild to moderate Alzheimer's disease: a 6‐month, randomized, placebo‐controlled, multicenter trial. Clin Interv Aging. 2014;9:947‐961. doi: 10.2147/CIA.S65625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCurry SM, Logsdon RG, Teri L, et al. Characteristics of sleep disturbance in community‐dwelling Alzheimer's disease patients. J Geriatr Psychiatry Neurol. 1999;12(2):53‐59. doi: 10.1177/089198879901200203 [DOI] [PubMed] [Google Scholar]
- 37. Tierney MC, Fisher RH, Lewis AJ, et al. The NINCDS‐ADRDA work group criteria for the clinical diagnosis of probable alzheimer's disease: a clinicopathologic study of 57 cases. Neurology. 1988;38(3):359‐364. doi: 10.1212/wnl.38.3.359 [DOI] [PubMed] [Google Scholar]
- 38. Landau SM, Lu M, Joshi AD, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β‐amyloid. Ann Neurol. 2013;74(6):826‐836. doi: 10.1002/ana.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villeneuve S, Rabinovici GD, Cohn‐Sheehy BI, et al. Existing Pittsburgh Compound‐B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(7):2020‐2033. doi: 10.1093/brain/awv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Landau S, Koeppe R, Jagust W. Florbetaben processing and positivity threshold derivation. Alzheimer's Dis Neuro‐imaging Initiat. https://adni.bitbucket.io/reference/docs/UCBERKELEYFBB/UCBerkeley_FBB_Methods_04.11.19.pdf. Published online 2011. [Google Scholar]
- 41. Devanand DP, Lee S, Huey ED, Goldberg TE. Associations between neuropsychiatric symptoms and neuropathological diagnoses of Alzheimer disease and related dementias. JAMA Psychiatry. 2022;79(4):359‐367. doi: 10.1001/jamapsychiatry.2021.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shokouhi S. Associations of informant‐based sleep reports with Alzheimer's disease pathologies. Clin Interv Aging. 2019;14:1631‐1642. doi: 10.2147/CIA.S215208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cummings J. The neuropsychiatric inventory: development and applications. J Geriatr Psychiatry Neurol. 2020;33(2):73‐84. doi: 10.1177/0891988719882102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. IEEE Trans Med Imaging. 2008;27(4):425‐441. doi: 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in mri data. IEEE Trans Med Imaging. 1998;17(1):87‐97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 46. Dadar M, Fonov VS, Collins DL. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage. 2018;174(March):191‐200. doi: 10.1016/j.neuroimage.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 47. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age‐appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313‐327. doi: 10.1016/j.neuroimage.2010.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dadar M, Maranzano J, Misquitta K, et al. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. Neuroimage. 2017;157(April):233‐249. doi: 10.1016/j.neuroimage.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anor CJ, Dadar M, Collins DL, Tartaglia MC. The longitudinal assessment of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease and their association with white matter hyperintensities in the national Alzheimer's coordinating center's uniform data set. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(1):70‐78. doi: 10.1016/j.bpsc.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dadar M, Camicioli R, Duchesne S, Collins DL. The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2020;12(1):1‐11. doi: 10.1002/dad2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dadar M, Maranzano J, Ducharme S, Carmichael OT, Decarli C, Collins DL. Validation of T1w‐based segmentations of white matter hyperintensity volumes in large‐scale datasets of aging. Hum Brain Mapp. 2018;39(3):1093‐1107. doi: 10.1002/hbm.23894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dadar M, Pascoal TA, Manitsirikul S, et al. Validation of a regression technique for segmentation of white matter hyperintensities in Alzheimer's disease. IEEE Trans Med Imaging. 2017;36(8):1758‐1768. doi: 10.1109/TMI.2017.2693978 [DOI] [PubMed] [Google Scholar]
- 53. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to mutliple testing. J R Stat Soc Ser B. 1995;57(1):289‐300. http://www.stat.purdue.edu/~doerge/BIOINFORM.D/FALL06/Benjamini/and/Y/FDR.pdf%5C. http://engr.case.edu/ray_soumya/mlrg/controlling_fdr_benjamini95.pdf [Google Scholar]
- 54. McAleese KE, Miah M, Graham S, et al. Frontal white matter lesions in Alzheimer's disease are associated with both small vessel disease and AD‐associated cortical pathology. Acta Neuropathol. 2021;142(6):937‐950. doi: 10.1007/s00401-021-02376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


