Abstract
The eight biological hallmarks of health that we initially postulated (Cell. 2021 Jan 7;184(1):33-63) include features of spatial compartmentalization (integrity of barriers, containment of local perturbations), maintenance of homeostasis over time (recycling & turnover, integration of circuitries, rhythmic oscillations) and an array of adequate responses to stress (homeostatic resilience, hormetic regulation, repair & regeneration). These hallmarks affect all eight somatic strata of the human body (molecules, organelles, cells, supracellular units, organs, organ systems, systemic circuitries and meta-organism). Here we postulate that mental and socioeconomic factors must be added to this 8×8 matrix as an additional hallmark of health (“psychosocial adaptation”) and as an additional stratum (“psychosocial interactions”), hence building a 9×9 matrix. Potentially, perturbation of each of the somatic hallmarks and strata affects psychosocial factors and vice versa. Finally, we discuss the (patho)physiological bases of these interactions and their implications for mental health improvement.
Keywords: mental health, aging, psychiatry, psychology
INTRODUCTION
The comprehension of health –defined in a positive fashion rather than as the absence of disease–requires a theory. We recently launched the bases of such a health theory by enumerating the fundamental biological characteristics or “hallmarks” of healthy organisms [1]. We concluded that the hallmarks of health include features of spatial compartmentalization (Hallmark 1: integrity of internal and external barriers; Hallmark 2: containment of local perturbations), maintenance of homeostasis over time (Hallmark 3: recycling & turnover of building blocks of biological systems; Hallmark 4: integration of circuitries; Hallmark 5: rhythmic oscillations with supra-, circa- and infradian periodicity), and adequate responses to stress (Hallmark 6: homeostatic resilience; Hallmark 7: hormetic regulation; Hallmark 8: repair & regeneration) (Box1). We postulated that these eight hallmarks affected the organism through all eight organizational strata of the body including molecules, organelles, cells, supracellular units, organs, organ systems, systemic circuitries, and the meta-organism or holobiont [1].
BOX 1. The molecular and cellular hallmarks of health.
Three years ago, we tentatively launched the modern bases of a theoretical explanation of health in which we didactically enumerated the fundamental biological characteristics or “hallmarks” of healthy organisms (López-Otín & Kroemer, Hall-marks of Health, Cell 2021). To qualify as a “hallmark” of health, we postulated that a process would have to fulfil three basic criteria, namely (i) invariably manifest in the context of sustained health, (ii) inexorably cause the loss of the healthy state, if perturbed or disrupted, and (iii) vigorously maintain or improve health, if experimentally accentuated or restored. When applying these stringent criteria, we finally defined eight molecular and cellular hallmarks of health classified in three categories:
A. Spatial compartmentalization
Hallmark 1: integrity of internal and external biological barriers
Hallmark 2: containment of local perturbations in space and time
B. Maintenance of homeostasis over time
Hallmark 3: recycling and turnover of all major building blocks of biological systems
Hallmark 4: integration of molecular, cellular, and long-distance communication circuits
Hallmark 5: rhythmic oscillations with supra-, circa- and infradian periodicity
C. Adequate responses to stress
Hallmark 6: homeostatic resilience to maintain multiple biological parameters at adequate levels
Hallmark 7: hormetic regulation to acquire resilience against toxins and other stressors
Hallmark 8: repair and regeneration methods to sense and respond to the multiple body damages
In retrospect, we consider that our theory of health should incorporate one additional hallmark and one essential stratum, both of which are related to the psychosocial dimension of the human being. We postulate herein a ninth hallmark that we refer to as “psychosocial adaptation” (Fig. 1), as well as a ninth organizational stratum (“psychosocial interactions”), hence extending the biological/somatic 8×8 matrix to a larger 9×9 matrix (Fig. 2). In this article, we first define the ninth hallmark and evoke the impact of each of the eight biological hallmarks on mental health. Next, we show that perturbation of each of the somatic hallmarks and strata may affect psychosocial factors and vice versa. Finally, we discuss the (patho)physiological bases of these interactions and their potential applications for the improvement of mental health.
Figure 1. FIGURE 1: The psychosocial adaptation as a new hallmark of health.
The scheme represents the links between psychosocial adaptation and the eight previously proposed hallmarks of health: integrity of barriers, containment of local perturbations, recycling & turnover, integration of circuitries, rhythmic oscillations, homeostatic resilience, hormetic regulation, and repair & regeneration. These hallmarks are grouped into three categories: spatial compartmentalization, maintenance of homeostasis over time, and adequate responses to stress.
Figure 2. FIGURE 2: Square matrix representing the relationships between health hallmarks and body strata.
The 9×9 matrix depicts the bidirectional connections between the nine hallmarks of health and the nine organizational strata of the human body. The relationships between psychosocial adaptation and psychosocial interactions are specifically enhanced.
PSYCHOSOCIAL ADAPTATION AS AN ESSENTIAL HALLMARK OF HEALTH
According to the World Health Organization (WHO), “mental health is a state of mental well-being that enables people to cope with the stresses of life, realize their abilities, learn well and work well, and contribute to their community”. In this sense, mental health is a larger concept than the mere absence of psychiatric disease. Currently, the classification of mental disorders presents several challenges including genetic overlaps between different conditions such as autistic spectrum disorders (ASD), major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia (SCZ) [2, 3]. Such overlaps also involve alterations in brain anatomy, perturbations in gene expression signatures, symptomatic changes in mood and social behavior, and therapeutic responses to psychotropic drugs [4, 5]. In view of these uncertainties, the negative definition of mental health as the absence of mental disease appears imprecise.
The concept of “psychosocial adaptation” refers to the permanent tension between the individual and its social and socioeconomic context, which accompanies our personal trajectory from birth to death. This conflict has to be permanently resolved by adaptations that optimize the individual's capacity to cope with frustrations, to deal with the absence of positive social relationships, to avoid accidents and personal demolition, to successfully compete for resources and to contribute to the collective success of the social groups, while proactively taking the correct decisions [6]. Maladaptive reactions subvert subjective wellbeing and endanger the position and even the survival of the individual in the socioeconomic system [6], although sometimes the majority may take wrong decisions e.g. in a mass formation/mass psychosis situation. Our definition of psychosocial adaptation is not only circumscribed to the inner state of the individual and to the stressors that influence it. Previous studies have shown that positive social relationships are essential regulators of human physiology both in early and later life, as illustrated by works on maternal separation or maternal immune activation [7], and on interventions against the epidemic of loneliness [8]. Thus, psychosocial adaptation and development is not simply about the absence of social problems, but also requires the presence of stable and positive social interactions which represent critical sources of resilience to life stress [9–12]. Importantly, even extreme adversity does not necessarily undermine mental health [13]. While some individuals declare long-lasting mental problems after adverse experiences, others manage to cope with these events and maintain the state of “psychosocial adaptation”. This capacity is determined by genetic and non-genetic factors, as demonstrated by twin studies [14] and experiments on inbred mice [15], that have revealed interindividual differences between susceptibility and resistance/resilience to mental disease.
Social Stress Models in Rodents
A prototypic model of psychosocial stress in mice consists in the exposure of male mice to repeated aggression by male mice from an intruder strain (Fig. 3A). This model of chronic social defeat promotes behavioral alterations coupled to an activation of the sympathetic-adrenal medullary (SAM) and hypothalamic pituitary adrenal (HPA) axes, which culminates with an increase in circulating catecholamines and glucocorticoids, and pro-inflammatory reactions that contribute to the behavioral phenotype due to neuroinflammation (Fig. 3B) [16]. Models of acute stress including tube restrains, cage switching, and short-term social isolation cause an increase in circulating interleukin-6 (IL6) levels secondary to SAM activation (Fig. 3B). Then, IL6 stimulates hepatic gluconeogenesis and hyperglycemia to fuel the “fight or flight” response, but also increases the susceptibility to inflammation [17]. In yet another model of chronic isolation stress, HPA-independent brain-wide upregulation of tachykinin 2 (Tac2), a neuropeptide previously implicated in fear memory consolidation, induces enhanced aggression and other behavioral changes, which has suggested a role for Tac2 as an important mediator of the effects of chronic social isolation stress [18]. Social status among male mice can be studied in the dominance tube test (Fig. 3A) [19]. After repeated “forced loss” procedures (based on an experimental design in which a dominant mouse is repeatedly forced to back down and lose to a subordinate in the social hierarchy) the formerly dominant mouse loses its social rank and develops a depressive-like behavior coupled to activation of the lateral habenula. Likewise, loss of social status rather than a stable low rank constitutes a risk factor of MDD in primates including humans [20].
Figure 3. FIGURE 3: Mouse models of psychosocial stress and response pathways resulting in psychosocial adaptation.
(A) Prototypic models of psychosocial stress in mice include chronic social defeat and dominance tube test models, as well as models of acute stress such as tube restrains, cage switching and short-term social isolation. (B) Different stressors including psychosocial stress promote a compendium of physiological and behavioral alterations coupled to activation of the sympathetic-adrenal medullary (SAM) and the hypothalamic pituitary adrenal (HPA) axes, as well as additional pathways which need to be further characterized. Deconvoluting the molecular pathways linking social stress to compromised mental and physical health may lead to the introduction of intervention strategies for improving psychosocial adaptation to stress.
Social and Socioeconomic Determinants of Health in Humans
Social stress increases the probability of developing multiple somatic and psychiatric diseases [6, 21]. Similarly, perceived social isolation correlates with enhanced severity of symptoms after viral immune challenge, inflammatory reactions, mental and physical morbidity, as well as with higher mortality rates [22]. Conversely, individuals exhibiting a high degree of social integration are afflicted by a relatively low morbimortality [23]. Differences in socioeconomic status may translate in discrepancies of a decade or more of disability-free life expectancy, coupled to a reduction of the prevalence of most major diseases in favor of the rich [6]. Similarly, the poor are more likely than the affluent to experience MDD and anxiety disorders [24]. The reasons for this association are multifold with higher chances of early life adverse events, hardship-induced stress, poor nutrition, less exercise, higher exposure to tobacco, alcohol and drug abuse, environmental pollution, extreme temperatures, violence and crime, as well as shame, emotional abuse, bullying, discrimination and isolation. In addition, poor somatic and mental health may predispose to low socioeconomic status through a “poverty trap” mechanism that includes reduced cognitive functions, lack of motivation and fatigue, as well as poor economic choices, suggesting a bilateral relationship between poor mental health and low socioeconomic status [24].
The temporal order of events observed in patients as well as preclinical experimentation suggest that prevalent characteristics linked to low socioeconomic status may contribute to mental illness, as documented for obesity, which is associated with reduced cognitive functions in adults [25] and suppresses neurogenesis and causes anxiety in mice, as well as increased susceptibility to several mental pathologies including depression, psychosis, anxiety, and eating disorders in human [24, 26]. Most current evidence, in particular that drawn from animal models, support the “social causation” hypothesis, meaning that social interactions directly affect health outcomes [6].
Psychosocial Adaptation for the Improvement of Health
Loss of psychosocial adaptation often occurs as a correlate of deteriorating health conditions and interventions favoring this adaptation improve health outcomes in clinical trials [27–30]. Further, psychosocial interventions increase stress resilience [31] and improve the recovery from depression [32]. Several studies have suggested that inhibition of stress mediators such as inflammatory cytokines, glucocorticoids and catecholamines elicited by social stress can mediate effects on psychiatric diseases [33–37], while other works have shown that the presence of mental disorders amplifies morbidity or mortality due to somatic disease [38]. Accordingly, treatment of mental disorders with psychotropic drugs does not only reduce psychiatric symptoms, but also mitigates excessive somatic morbimortality [39].
In summary, the enfeeblement of somatic health impacts psychosocial adaptation and vice versa. Emerging evidence suggests that treatments designed to enhance psychosocial adaptation, to improve stress management, or to provide adequate psychopharmacological care, may have a positive impact on health outcomes. Based on this information, we aimed to unveil the connections between psychosocial adaptation and somatic hallmarks of health.
HALLMARK 1: INTEGRITY OF BARRIERS
The first hallmark of health consists in the integrity of biological barriers [1]. Such barriers must maintain strict compartmentalization to ensure the functional organization of the organism, but allow for the controlled exchange of solutes, electrolytes, soluble factors and mobile cells to sustain health. Here, we will examine how mental disorders compromise the integrity of barriers and how this integrity may impact psychosocial parameters (Fig. 4).
Figure 4. FIGURE 4: Psychosocial dimension of health hallmarks implicated in spatial compartmentalization.
Links between psychosocial adaptation and the mechanisms responsible for the integrity of biological barriers and the containment of local perturbations.
Mitochondrial Integrity
Mitochondrial structure determined by fusion/fission events as well as the expression of bioenergetically relevant pumps and enzymes determine the propensity of mitochondria to undergo permeabilization and hence to release pro-inflammmatory and pro-apoptotic molecules into the cytosol of stressed cells. Mitochondrial DNA (mtDNA) alterations are associated with human ASD [40] and autism-like phenotypes in mice [41]. Induced pluripotent stem cell (iPSC)-derived neural precursors from BD patients present a mitochondrial defect which can be reversed by lithium [42]. MDD has been associated with increased circulating levels of acylcarnitines – suggesting a defect in mitochondrial β-oxidation – [43] and of cell-free mtDNA derived from mitochondrial membrane permeabilization [44]. Notably, the 22q11.2 deletion syndrome, which often includes facets of ASD and SCZ [45], leads to mitochondrial disruption in mouse cortical neurons [46]. iPSC-derived neurons from 22q11.2-deleted patients with signs of SCZ manifest a defect in oxidative phosphorylation [47], while transplantation of normal mitochondria into the prefrontal cortex of adolescent rats suppresses SCZ phenotypes induced by maternal immune activation [48], further supporting the relationship between SCZ and mitochondrial dysfunction.
Cellular Integrity
In a mouse model of depression induced by chronic mild stress, astrocytic pyroptosis (an inflammatory form of cell death triggered by microbial infections and host factors) may contribute to this condition since knockout of pro-pyroptotic genes alleviates depression-like behavior [49]. Cell-free genomic DNA is increased in plasma from SCZ patients, supporting the possibility of increased cell death [50]. Brain-derived cell-free DNA, detected via epigenetic markers, is increased in SCZ patients with first psychotic episodes [51]. This may represent a sign of enhanced cell death by plasma membrane permeabilization, reduced clearance of dead cells, or deficient retention of cellular debris by the blood-brain barrier (BBB) in the central nervous system (CNS) from these patients. However, the literature on excessive cell death in mental disease is scarce, thus pointing to a higher importance of BBB integrity.
Blood-Brain Barrier Integrity
BBB assures the exclusion of mobile immune cells and pro-inflammatory factors, the transport mechanisms from the periphery into the brain and the active export of toxic metabolites and waste products from the brain [52]. Endothelial cells, which form the first barrier between blood and the brain, are connected by tight junction proteins such as claudin-5 (CLDN5). The local abundance of this protein is often a proxy to assess BBB intactness, which is perturbed in aging and psychiatric diseases [52]. Notably, the 22q11.2-deletion syndrome causes CLDN5 haploinsufficiency and leads to reduced CLDN5 levels in endothelial cells. In parietal lobes from non-syndromic SCZ patients, CLDN5 is discontinuously expressed in the BBB [53], while reduced expression of CLDN5 in the hippocampus of MDD or SCZ patients correlates with early onset and prolonged duration of disease [54]. Importantly, CLDN5 expression in specific brain areas correlates with susceptibility or resistance to stress-induced disorders [55, 56], supporting the implication of the CLDN5-dependent BBB integrity in mental health maintenance. Accordingly, chronic social defeat stress in mice causes Cldn5 downregulation in the nucleus accumbens with loss of BBB integrity [57]. This process is mediated by activation of TNFα/NFκB signaling and histone deacetylase 1 (HDAC1, that catalyzes the deacetylation of lysine residues of core histones), and by upregulation of the transcription factor forkhead box protein O1 (FOXO1) [58]. Of note, HDAC1 and FOXO1 are upregulated in the nucleus accumbens from untreated MDD patients, while CLDN5 is downregulated [58]. Similarly, female MDD patients who died from suicide present downregulation of CLDN5 in the prefrontal cortex [55]. Gain- and loss-of-function experiments in mice have provided causal support to the idea that BBB permeabilization is broadly neuropathogenic [55–57, 59]. Moreover, subtle alterations in BBB function that affect specific transport systems have been detected in neuropsychiatric patients [60, 61].
Choroid Plexus Barrier Integrity
The choroid plexus vascular barrier, which separates blood from cerebrospinal fluid, is usually permeable to molecules of up to 70 kDa, yet closes upon induction of intestinal inflammation due to sealing of the fenestration of endothelial cells. Genetically-driven closure of the choroid plexus induces anxiety-like behavior that was also observed in intestinal inflammation, suggesting a pathogenic role for these alterations that potentially link inflammatory bowel disease and psychosocial disturbances [62]. In both MDD and psychosis patients, the choroid plexus is enlarged compared to healthy controls, and this finding correlates with signs of neuroinflammation [63]. Moreover, the choroid plexus is histologically altered in SCZ patients [64]. However, it remains to be determined whether these macro- and microscopic alterations reflect changes in barrier function.
Intestinal Barrier Integrity
The gut vascular barrier protects from external insults through a multilayered structure that evolves in cooperation with the local microbiota. Numerous studies have suggested that general features of the unhealthy gut microbiota (dysbiosis) are non-specifically associated with multiple distinct disease states ranging through the entire spectrum of pathologies including oncological, metabolic, cardiovascular, and neuropsychiatric diseases. Hence, gut health is a common trait of general health. Accordingly, breaches in the mucus layer and the enterocyte epithelium [65] can lead to the translocation of microbes or microbe-derived molecules into the host, elicitation of inflammatory signals, and trafficking of gut-resident immune cells to other organs [66, 67]. Accordingly, “leaky gut” may trigger or modulate distinct diseases including mental disorders [66]. Psychological stress causing the activation of HPA or SAM impacts the gut. Thus, HPA-elicited chronic elevations in glucocorticoids have multiple effects on the gut due to the expansion of an inflammatory subset of enteric glia and the inhibition of acetylcholine responses by enteric neurons causing intestinal dysmotility [68]. In contrast, SAM activation accounts for a “stress ileopathy” with consequent shifts in the microbiota [69]. Reciprocally, the microbiota participates in HPA modulation, because its depletion exacerbates HPA activation in response to moderate stress [70]. Moreover, elevated levels of biomarkers of intestinal barrier permeability have been detected in patients with mood disorders [71]. Interestingly, fecal microbiota transplantation from MDD patients into rats induces a depressive-like phenotype [72], while lithium administration significantly increases species richness and diversity in the rat gut, likely contributing to the beneficial effects of this drug [73]. In mice, maternal immune activation or infection during pregnancy induces the differentiation of IL17–producing TH17 lymphocytes in the gut. IL17 then crosses the placental barrier and affects the fetal CNS, resulting in ASD-like behavior [74]. In this model, gavage with Lactobacillus reuteri corrects social impairments by signaling across the microbiota-gut-brain axis through vagal neurons [75]. In ASD patients, major shifts in the intestinal microbiota have been detected, but many of them are likely caused by altered food preferences of these patients [76]. Nonetheless, preclinical experimentation in mice indicates that bacterial L-tyrosine metabolites can induce anxiety-like behavior [77]. Moreover, an oral small-molecule sequestrant with affinity for microbiota metabolites mitigates anxiety and irritability in adolescents with ASD [78]. Altogether, it appears that some microbial metabolites (psychobiotics) can induce systemic effects on mental health. Such effects can be negative, as illustrated for ASD, but can also be positive, as exemplified by the microbiota-derived endocannabinoids that increase the motivation for physical exercise [79].
Skin Barrier Integrity
The common manifestation of atopic dermatitis or psoriasis-two conditions with compromised skin barrier integrity- and different mental disorders might reflect common genetic causes simultaneously affecting the two tissues of ectodermal origin, skin and brain [80]. Independently of this speculative explanation, eczema and related atopic diseases are associated with more severe ASD manifestations. Hence, a hypothetical “skin-brain axis” has been proposed in which dermal inflammation would trigger mental disorders. Supporting this conjecture, experimental induction of dermatitis in mice increased anxiety- and depressive-like behaviors, along with elevated serum corticosterone levels [81]. More convincingly, in clinical trials, anti-IL17A [82] and anti-IL4R [83] antibodies which target inflammatory signaling in the skin, reduced psoriatic lesions and atopic dermatitis, respectively, as they simultaneously mitigated anxiety and depression.
HALLMARK 2: CONTAINMENT OF LOCAL PERTURBATIONS
Perturbations due to endogenous alterations, infectious agents and mechanical, chemical, physical or emotional trauma can cause focal damage to tissues and compromise barriers. Failure to confine such perturbations, avoiding their spread to a systemic level and their perpetuation, is intrinsically pathogenic. Hence, the containment of local perturbations is essential for the maintenance of somatic and mental health (Fig. 4). This is particularly well documented for inflammation, usually a local phenomenon that resolves. However, inflammation becomes broadly pathogenic if it acquires a system-wide, chronic dimension [1].
Self-limited Inflammation
Neuroinflammation is linked to systemic inflammation and likely contributes to the pathogenesis of mental disorders [84]. Orthopedic surgery performed in older adults often induces delirium as a result of systemic inflammation spurring neuroinflammation. Postoperative delirium can be reduced in patients by the α2-adrenergic receptor agonist dexmedetomidine [85] and in mice by inhibition of microgliosis by ω-3 fatty acids or resolvins [86]. CNS-specific inflammation due to autoimmunity or stroke can trigger MDD. Thus, relapsing multiple sclerosis is associated with depression that responds to fingolimod [87], an immunosuppressor which is also active against SCZ [88]. Anti-TNFα-based treatments mitigate depressive symptoms in rheumatic diseases patients [89]. Likewise, intracerebroventricular infusions of anti-TNFα antibody or several resolvins produce antidepressant-like effects in rodent models [90], while in MDD patients, oral supplementation with the ω-3 fatty acid eicosapentaenoic acid induces clinical responses associated with elevated plasma concentrations of pro-resolving lipid mediators [91]. These findings support the possibility to intervene on neuroinflammation in mental disorders. If inflammation resolves in a timely fashion after an acute phase, it facilitates tissue repair [92]. In the CNS, such repair reactions allow to restore BBB integrity and axon remyelination which are essential for the maintenance of CNS function.
Repair of the Blood-Brain Barrier
BBB permeabilization can be repaired through a process that involves the contribution of endothelial cells, pericytes, astrocytes and fibroblasts. BBB repair slows with aging [93], and can be stimulated by CNS-targeted gene therapy with engineered Wnt ligands [94] or by intracerebroventricular administration of protease inhibitors [95]. Traumatic brain injury in mice induces BBB permeabilization and axonal degeneration leading to progressive neurological and mental deficits. These long-term consequences of brain trauma can be prevented by activation of NAD+ biosynthesis by P7C3, an activator of NAMPT, and the NAD+ precursor nicotinamide riboside improved depressive- and anxiety-like behaviors in rats exposed to mild stress [96]. Similarly, nicotinamide, another NAD+ precursor, mitigates BBB damage and psychosis in rats chronically exposed to ketamine [97]. These observations suggest the use of NAD+ precursors in neuropsychiatric diseases linked to BBB permeabilization.
CNS Remyelination
Human SCZ is accompanied by reduced myelination of the medial frontal regions. In rodents, demyelination resulting in SCZ-like behaviors can be induced by administration of cuprizone, a copper chelator that paradoxically increases copper concentrations in the brain [98]. Remyelination can occur spontaneously after cuprizone withdrawal but is enhanced by some antipsychotics and by the histamine receptor H1 antagonist clemastine [98]. Of note, clemastine can reduce depressive-like behaviors in mice exposed to social defeat stress [99]. Interestingly, clemastine has other pharmacological effects (e.g., anticholinergic) that increase the activity of histone methyltransferases and may also contribute to the behavioral benefits of this drug. Clemastine and sobetirom - another promyelinating drug - also promote functional recovery in an ASD mouse model [100]. Multiple remyelination-inducing drugs are being developed, mostly for multiple sclerosis treatment, and it will be interesting to learn whether they mediate positive effects on patients with mental disorders [101].
Synaptic Pruning
Synaptic pruning is a fundamental neurodevelopmental process in which excess or weak synapses are eliminated to optimize neural circuitry [102]. Synaptic pruning also plays a crucial role in shaping circuits involved in learning and memory [103]. Both excessive and deficient synaptic pruning might contribute to mental disorder pathogenesis. Deficient pruning mechanisms during early brain development may result in atypical neural connectivity patterns, linked to the social and behavioral deficits observed in ASD patients. As compared to neurotypical controls, the frontal, temporal and parietal lobes from these patients exhibit increased synapse density, suggesting an underpruning phenotype [104]. Abnormalities in synaptic pruning have also been implicated in SCZ pathogenesis, which is associated with synaptic alterations suggestive of overpruning [105]. Dysregulated synaptic pruning during adolescence may underlie these structural changes and contribute to the cognitive deficits observed in SCZ [106]. Accordingly, SCZ is linked to allelic variants of the complement component 4A (C4A) locus that enhance C4a protein production, while overexpression of human C4A in mice causes excessive pruning in the cortex associated to SCZ-like behaviors [107].
HALLMARK 3: RECYCLING AND TURNOVER
Many of the building blocks of the organisms undergo spontaneous or stress-induced alterations that must be counterbalanced by their constant dismantling and rebuilding. Recycling and turnover hence are critical for the maintenance of the healthy state, as this is particular well documented for protein homeostasis (proteostasis), autophagy and cell replacement [1]. Alterations in these processes are also associated with mental illnesses (Fig. 5).
Figure 5. FIGURE 5: Psychosocial dimension of the hallmarks of health involved in maintenance of homeostasis.
Interconnectivity between psychosocial adaptation and mechanisms of recycling and turnover in tissues and cells, crosstalk among different circuitries (cell-tissue-organ-system), and their synchronization with circadian, infradian or ultradian rhythmic oscillations.
Proteostasis
Aberrant proteostasis is a hallmark of aging and neurodegenerative diseases [108, 109]. Proteostasis alterations in neuropsychiatric disorders do not result in massive neuronal death, but in diverse loss- and gain-of-function events converging in the disruption of synaptic and neural functions [110–112]. Specific proteases and E3 ubiquitin ligases are mutated in hereditary mental and neurodevelopmental disorders [113–117]. Conversely, loss of ErbB3-binding protein 1 (EBP1, a signaling molecule which is mutated in some SCZ patients) causes upregulation of the E3 ubiquitin ligase Fbxw7 and an SCZ-like behavior in mice [118]. Moreover, in fragile X syndrome – a leading monogenic cause of autism – loss of Fragile X Mental Retardation protein 1 (FMRP1) impairs proteostasis. This deficiency can be mitigated in mice by administration of proteasome inhibitors, which also attenuate hyperexcitability in response to auditory stimulation [119]. These findings point to the causal involvement of specific alterations of proteostasis in neuropsychiatric diseases.
Mitophagy
Mitochondrion-specific autophagy (mitophagy) involves several genes/proteins that mark dysfunctional mitochondria for autophagic destruction and hence serve as “autophagy adaptors” – such as PARK and PINK – that are mutated in Parkinson's disease patients [120]. Of note, Parkinson's disease is not limited to motor deficits, but is usually preceded and accompanied by neuropsychiatric alterations and cognitive impairment [121]. Disrupted-in-SCZ-1 (DISC1) – the mutation of which causes behavioral abnormalities – has been identified as a mitophagy receptor [122]. Defective mitophagy might also contribute to MDD-associated mitochondrial dysfunction [123]. In contrast, excessive Pink- and Park-dependent mitophagy in the basolateral amygdala from mice has been proposed to mediate disproportionate elimination of mitochondria in chronic social defeat stress, facilitating anxiety and aversive social behavior [124]. Hence, both deficient and excessive mitophagy may contribute to the pathogenesis of mental disorders.
Autophagy
Macroautophagy (to which we refer to as autophagy) can be activated in all relevant cell types of the CNS [125]. Neuron-specific knockout of the essential autophagy gene Atg5 results in increased excitatory neurotransmission [126]. Autophagy also contributes to synaptic remodeling, for instance in long-term synaptic depression induced by NMDAR activation [127]. In microglia, knockout of another essential autophagy gene, Atg7, impairs synaptic pruning and elicits ASD-like behaviors [128]. Moreover, genetic defects in specific autophagy genes cause neuropsychiatric disorders [125, 129, 130]. Tsc2+/- mice -which manifest constitutive overactivation of nutrient sensor mTorc1 and tonic inhibition of autophagy-exhibit ASD-like features [131]. Similarly, valproic acid administration to mice and vitamin B6 deficiency in rats are associated with autism-like behavior that can be reversed by mTorc1 inhibition with rapamycin [132, 133]. Obesity activates mTORC1 in microglia and astrocytes with disabled autophagy, while rapamycin treatment restores autophagy and reduces depressive and anxiety-like behaviors [134]. Autophagy inhibition caused by obesity might explain the link between excessive adiposity and ASD, MDD or SCZ [135, 136], but the observations suggesting that fasting may improve MDD must be replicated in large randomized studies [137]. These findings suggest that mTORC1 is hyperactivated in neuropsychiatric diseases, but there are exceptions to this rule. Thus, mTORC1 hypoactivity in the prefrontal cortex from men with BD and psychosis is pathogenic [138]. Accordingly, the antidepressant actions of ketamine depend on the neural activation of mTORC1 and its downstream effectors [139]. Moreover, systemic Gdf11 injection into aged mice alleviates depression-like symptoms through mTorc1 activation in hippocampal neurons [140]. Likewise, some of the neuroprotective mechanisms induced by lithium in BD and other neuropsychiatric conditions may be related to autophagy regulation, although the underlying mechanisms are still unclear.
Common antidepressants induce autophagy in circulating leukocytes from MDD patients [141]. as well as in mouse hippocampal neurons secondary to the accumulation of ceramide in the endoplasmic reticulum and sphingomyelin in lysosomes and Golgi apparatus [142]. Direct inhibition of sphingomyelin synthase with D609 enhances accumulation of ceramide and activation of autophagy, and reduces stress-induced MDD. Moreover, mice lacking acidic sphingomyelinase – which converts sphingomyelin into ceramide – exhibit depressive behavior, while the antidepressant effects of amitriptyline and D609 are abolished by the autophagy inhibitor spautin [142]. These findings suggest that antidepressants may exert some of their beneficial effects through the enhancement of autophagic flux.
Cell Replacement
Cell death requires a local response for efficient corpse removal by phagocytes, and for replacing the missing cell or palliating its absence. Neuronal cell death triggers an orchestrated reaction by astrocytes and microglia, which engulf dendritic arbors, and the soma from neurons, respectively [143]. In the CNS, different cell types undergo replacement at rather different rates. While most neurons are post-mitotic cells that should finish their existence when their host dies, there is evidence for postnatal neurogenesis in some human brain areas, and impaired neurogenesis has been involved in the pathogenesis of several neuropsychiatric conditions (see Hallmark 8).
Ablation of brain astrocytes can induce a compensatory proliferation of neighboring juxtavascular astrocytes [144]. Nerve growth factor receptor (p75NTR)-dependent astrocyte proliferation has also been observed after brain injury [145]. In contrast, astrocyte-specific knockout of Soc2 interferes with astrocyte proliferation and improves recovery from traumatic brain injury, yet causes a hyperactivity/hyperexcitability phenotype [146]. Hence, the role of astrocyte proliferation and differentiation in brain health requires further investigation. Microglial progenitor cells formed in the embryonic yolk sac enter the CNS before the BBB forms and then constitute a self-renewing population without further contribution by the hematopoietic system [147]. With age, microglia is characterized by an increase in senescent cells coupled to the activation of the pro-inflammatory senescence-associated secretory phenotype. Chemogenetic ablation and systemic senolysis of such senescent microglial cells improves cognition in aged mice [93], while forced turnover of microglia following colony stimulating factor 1 receptor (CSF1R) inhibition improves recovery from traumatic brain injury at the inflammatory and neuropsychiatric levels [148]. These results suggest that strategies for increasing microglial renewal might be useful for intervening on neuropsychiatric diseases.
HALLMARK 4: INTEGRATION OF CIRCUITRIES
The organism – including the nervous system – is built in a way that molecular, intracellular, extracellular and long-distance neuroendocrine communication systems constitute interwoven circuitries, favoring integration [1]. We posit that loss of integration is invariably pathogenic, including at the psychosocial level (Fig. 5).
The Synapse
Numerous genes whose mutations or variations are involved in mental diseases encode proteins acting at the level of synapses [149]. Such genes may also be modulated epigenetically by early-life experiences, environmental factors and stress [149]. A few examples underscore the implication of synaptic alterations in mental disorders. A mutation in the cell adhesion protein neuroligin-3 (NLGN3) linked to ASD induces an enhancement in excitatory synaptic transmission [150], while loss of synapses in SCZ disrupts pyramidal neuron function in the cortex to elicit cognitive symptoms and disinhibits mesostriatal projections to promote dopamine overactivity and psychosis [106]. In response to prolonged stress, prefrontal cortex and hippocampus undergo dendritic retraction and spine loss, while other regions including amygdala and lateral habenula manifest elevated spine density and potentiated activity [151]. Clinically active antidepressants reverse the effects of stress and depression on synapse function, augmenting neurotransmission, boosting plasticity, and favoring synaptogenesis [151].
Neuroplasticity
CNS is endowed with an unmatched capacity to simultaneously capture sensory, emotional and cognitive inputs and to stock, encrypt, retrieve and utilize information through an integrative learning process compatible with creativity, imagination and improvisation [152]. Neuroplasticity is achieved by a combination of three mechanisms. First, synaptic plasticity is shaped by coordinated neuronal activity, reinvigorating the strength and efficiency of synaptic connections by long-term potentiation or attenuating their function by long-term depression [153]. Second, structural plasticity involves reorganization of interneuronal connections by the pruning of dendritic spines [154]. Third, neurogenesis facilitates the generation of new neurons in specific brain regions. Beyond its role in neurodevelopment and the acquisition of specific talents, neuroplasticity is crucial in the brain's adaptive capacity to reorganize and compensate for deficits caused by stroke, trauma or sensory deprivation. Neuroplasticity can also be maladaptive in specific neurological conditions such as phantom limb sensations, chronic pain, hyperactivity and addiction. Maladaptive neuroplasticity can be tackled by several strategies including transcranial stimulation methods and biofeedback techniques [155]. In addition, ketamine and psychedelics may mediate their antidepressant effects by stimulating neuroplasticity [156].
Neuroendocrine Circuitries and Interoception
The nervous and endocrine systems are linked through intricate bidirectional interactions. Starting by neural inputs, these circuitries involve SAM and HPA, but also thyroid and sex hormones, and the pro-social hormone oxytocin [157, 158]. Conversely, stress hormones, classical hormones produced by endocrine organs and a long list of “tissue hormones” impact the CNS. These tissue hormones include adiponectin, which has neuroprotective effects but also increases susceptibility to social stress;[159]. diazepam-binding inhibitor, which stimulates central appetite centers but may also cause depression-like behavior;[160]. and glucagon-like peptide-1, which induces satiety but may also attenuate depression [161]. These examples illustrate a constant neuroendocrine communication between CNS and the endocrine system that allows for the coordination of mental and bodily functions.
Interoception permanently informs the brain on multiple physiological parameters to generate a representation of the internal state of the organism and to facilitate adequate reactive or proactive control [162]. Cardiac and gastric interoception involves parasympathetic and sympathetic signals, as well as subcortical relay nuclei including the nucleus tractus solitarius and parabrachial nucleus [163]. Dysfunctional interoception may compromise mental health and participate in the pathogenesis of anxiety and mood and eating disorders [164]. Of note, optogenetically induced extreme tachycardia causes anxiety in mice via activation of the posterior insular cortex [165], demonstrating that peripheral organs responding to stress may control the affective behavioral state, hence closing an anxiogenic feedforward loop [165]. The disruption of such a malicious circuitry may contribute to the anxiolytic and antidepressant effects of calcium channel blockers and β-adrenergic receptor antagonists used for the treatment of tachycardia and hypertension [166].
Neuroimmune Circuitries
Cytokines released by immune cells in response to pathogens may act as interoceptive signals since they transmit signals across BBB, including stimulation of the afferent (sensory) vagus nerve. This elicits a reciprocal response via HPA. In addition, the CNS can stimulate the efferent vagus nerve of the parasympathetic nervous system to exert a systemic anti-inflammatory effect termed as the “inflammatory reflex”. Signals communicated via the vagus and splenic nerves cause T cells to produce acetylcholine that acts on macrophages to dampen inflammation [167]. Disruption and mimicry of this reflex may exacerbate or suppress inflammation and depression [167]. Genetic models of immunodeficiency have supported the link between the cellular immune system and anxiety-like behaviors [168]. T cells can affect behavior in multiple ways. For example, adoptive transfer of CD4+ T lymphocytes from stressed mice to non-stressed recipients induces anxiety-like behavior [169]. In the chronic social defeat model, susceptible mice manifest the depletion of Lactobacillus johnsonii from their gut microbiota coupled to an increase in the frequency of IL17-producing γδ T cells in the colonic lamina propria, as well as in the meninges. In this model, supplementation with L. johnsonii or depletion of γδ T cells suppresses social avoidance [170]. Collectively, these observations suggest the existence of multiple yet-to-be-discovered circuitries connecting the brain to the peripheral immune system.
HALLMARK 5: RHYTHMIC OSCILLATIONS
Biological clocks establish the rhythms of life that orchestrate the complex mechanisms underlying organismal homeostasis, including those necessary for the maintenance of mental health [171]. The central component of this synchronization system is a circadian clock located in the suprachiasmatic nucleus of the hypothalamus. These neural pacemakers receive information on light cues from photoreceptive retinal cells and specialized retinal ganglion cells, and then confer circadian rhythmicity to the myriad of peripheral clocks present in the diverse body tissues [172]. Circadian oscillations are molecularly driven by intricate transcriptional-translational feedback loops involving the transcriptional activators BMAL1 and CLOCK, which transactivate the genes encoding cryptochromes CRY1 and CRY2, and the transcriptional repressors PER1 to PER3, which in turn inhibit BMAL1 and CLOCK expression [172]. Disruption of circadian rhythms due to alterations in core clock genes or to lifestyle changes is responsible for a variety of human pathologies -including sleep disorders, neurodevelopmental conditions, and neurodegenerative processes- which compromise mental health and interfere with social relationships [173] (Fig. 5).
Sleep Disorders
Circadian misalignment of environmental cues with the endogenous clock program due to artificial lighting, shift work or jet travel causes sleep disorders and is broadly pathogenic. Blue light that is continuously emitted by electronic devices used in daily life shifts the phase of neuronal and peripheral-tissue clocks [174]. Likewise, work scheduled during normal sleep time or frequent traveling across time zones desynchronizes sleep-wake rhythms from the light-dark natural cycle and leads to excessive sleepiness or insomnia. Additionally, intrinsic alterations of circadian function result in heritable early or late chronotypes characterized by an extremely advanced or delayed onset of sleep, which may impact on physical and cognitive performance, but also on mood status and social interactions [175]. Sleep disturbances together with inadequate eating schedules contribute to misalign clocks in metabolic organs, leading to obesity and other metabolic disorders, which in turn amplify mental perturbations. The early identification of individuals with extreme chronotypes, which are at risk of sleep dysfunctions, as well as behavioral and pharmacological interventions can reinforce circadian rhythmicity and improve the control of sleep-wake cycles [176].
The relevance of sleep timing and chronotypes for the maintenance of mental health has grown in the context of “social jetlag” [177]. This term defines the discrepancy between biological time, dictated by internal circadian clocks, and social time, determined by social activities. Epidemiological studies have identified associations between social jetlag and the prevalence and clinical onset of diverse disorders, ranging from depression to metabolic dysfunction and reduced cognition [178–180]. Accordingly, the social zeitgeber theory proposes that disruption in the timing of daily social routines increases the risk for mood disorders and exacerbates BD [181]. Hence, social jetlag may be considered as a public health risk.
Neurodevelopmental and Neuropsychiatric Diseases
Circadian clock disruption has been detected in psychiatric disorders characterized by temporal or seasonal changes [182]. Such disorders are commonly associated with alterations in different biological rhythms, including those controlling the sleep-wake cycle, cortisol and melatonin production, blood pressure, and circadian variations in the expression of clock genes and their transcriptional targets. Such changes occur across a large spectrum of neuropsychiatric disorders, likely contributing to their overlapping symptoms [183]. Genetic studies have identified circadian clock gene variants associated to mood disorders, although data are not univocal, probably due to the variability of environmental influences in diverse study populations [173]. Neuroimaging analysis has suggested that infradian fluctuations in the sensorimotor network and in subcortical 5-hydroxytryptamine projection regions explain the seasonality of psychiatric diseases [184]. Neurodevelopmental syndromes such as Prader-Willi syndrome are also associated with dysfunctional circadian rhythms [185], while studies with mutant mice deficient in clock genes have confirmed that the circadian clock modulates mood-related behaviors [186, 187]. Several molecular pathways (i.e., the HPA axis and monoaminergic neurotransmission) are involved in the circadian clock disruption observed in SCZ and mood disorders in preclinical models [188–190]. Substance abuse disorders are also associated with desynchronization of circadian rhythms occurring during the transition from recreational consumption to addictive behavior [191]. This process involves the activation of the dopamine D2 receptor, which then triggers a regulatory circuit that finally leads to the activation of the PPARγ nuclear receptor. Interestingly, administration of pioglitazone, a specific PPARγ agonist to D2r-deficient mice restores adequate rhythms of circadian genes [191], pointing to new opportunities for the treatment of drug addiction disorders.
Neurodegenerative Diseases
Experimental jet lag in mice inhibits adult neurogenesis and causes cognitive impairments [192]. The circadian clock is functionally disrupted in patients with different neurodegenerative disorders, such as Alzheimer's, Huntington's and Parkinson's diseases, which in turn are linked to deficient adult neurogenesis. Mammalian clock genes participate in the control of neurogenesis by restricting the expansion of rapidly dividing neural precursors and by regulating the entry of quiescent neural stem cells into the cell cycle. Circadian rhythms in neural stem cells are regulated by glucocorticoids through a balanced action on mineralocorticoid and glucocorticoid receptors. Mice deficient in clock genes lack the circadian gating of cell cycle and lose diurnal rhythmicity [192]. Likewise, patients with neurodegenerative diseases often exhibit a severe reduction in the robustness of the circadian clock that results in profound disturbances of sleep–wake cycles [193]. Moreover, polymorphisms in clock genes have been associated with an increased risk of Alzheimer's or Parkinson's disease, while preclinical and clinical data have correlated circadian disruption with the accumulation of neurotoxic proteins and neurodegeneration itself [173, 182]. Finally, the lack of appropriate light-dark cues, the presence of irregular sleep-wake cycles and the functional deterioration of circadian clocks contribute to the “sundown syndrome” which is prevalent in people with dementia or neurodegenerative illnesses.
All these circadian system-related alterations have been traditionally viewed as correlative rather than causal events, but recent studies indicate that signs of circadian disruption precede the manifestation of other clinical symptoms, reinforcing the idea that perturbation of biological rhythms contribute to disease pathogenesis [194]. Accordingly, chronotherapeutic interventions aimed at resynchronization of these rhythms have shown promising effects [183]. An illustrative example is the antidepressant agomelatine which directly targets the circadian system, acting as a melatonin-receptor agonist and also as a 5-hydroxytryptamine 2B/2C receptors antagonist. Agomelatine resynchronizes disrupted circadian rhythms and improves sleep patterns in patients with autism, attention-deficit/hyperactivity disorder, anxiety, and depression [195]. Therefore, circadian medicine and chronotherapy – which target specific clock components while carefully timing the administration of drugs – may improve the clinical outcome of psychiatric patients.
HALLMARK 6: HOMEOSTATIC RESILIENCE
Homeostatic regulation defines resilience and determines lifespan and healthspan by controlling and repairing internal damages, eliciting appropriate stress responses, minimizing biological noise, and facilitating constant tissue remodeling [1]. Homeostatic resilience involves the participation of complex neural mechanisms, which act in concert with a variety of genetic, epigenetic, metabolic, endocrine, immunological and microbial processes. Deficiencies in any of these resilience mechanisms may contribute to the development and progression of numerous human pathologies, including mental disorders (Fig. 6).
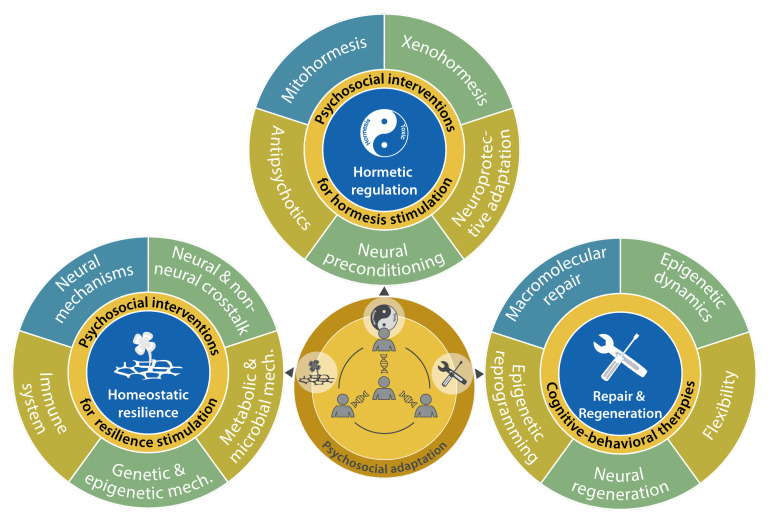
Figure 6. FIGURE 6: Psychosocial dimension of the hallmarks of health involved in responses to stress.
Interactions between psychosocial adaptation and mechanisms of homeostatic resilience, hormetic regulation and repair and regeneration strategies aimed at achieving biological stability and maintenance of health, including mental health.
Neural Mechanisms
Resilience results from adaptive changes in the functional activity of numerous brain circuits that control the psychobiological responses to acute or chronic stressors. These changes involve the participation of multiple hormones, neuropeptides, neurotransmitters, and their corresponding receptors and signaling pathways, which collectively elicit homeostatic responses to stress [196]. Acute actions of glucocorticoids are protective and elicit homeostatic resilience responses, whereas chronic exposure to high glucocorticoid levels causes neural damage and debilitates mental health [197]. Glucocorticoids function in close coordination with neurotransmitters and neurotrophic peptides to modulate stress resistance. Acute stressors increase the brain turnover of serotonin, a central component of the circuits that control mood and emotion. They also affect dopamine, which modulates reward and aversion, contributes to fear extinction and participates to stress resilience [198]. Neuropeptide Y (NPY) is another factor that induces anxiolytic effects under stressful conditions [199]. In patients with MDD or PTDS, plasma levels of NPY are reduced [200], again illustrating the diversity of neural mechanisms of homeostatic resilience.
Neural-Non-Neural Crosstalk
Homeostatic resilience is not only directly controlled by neuron-intrinsic mechanisms, but also involves non-neuronal cells (i.e., glial, myeloid and endothelial cells) that interact within the CNS to modulate neural networks and control stress behaviors. Non-neuronal cells from limbic regions of brain can interact at synapses, the neurovascular unit, and other sites of cell-cell communication to mediate both the pro-resilient and deleterious effects of chronic stress [201]. These brain regions play critical roles in the regulation of mood and emotional states through a fertile crosstalk between neural and non-neural components. For example, in response to aversive or rewarding stimuli, the nucleus accumbens integrates dopaminergic projections from the ventral tegmental area and glutamatergic inputs from the hippocampus, prefrontal cortex, amygdala and thalamus, and then determines resilience to stress, as well as reward-driven learning and motivation [202]. Similarly, a stress-sensitive projection connecting basolateral amygdala and nucleus accumbens plays a critical role in executing disrupted reward behaviors provoked by early-life adversity [203]. The correct function of the hippocampus-amygdala complex and its interaction with the prefrontal cortex are also essential for resilience mechanisms aimed at the control of intrusive memories caused by trauma [204]. Likewise, stress and glucocorticoid release decrease adult neurogenesis at the dentate gyrus, and increasing neurogenesis in mice indeed promotes resilience to social defeat stress by inhibiting the activity of mature granule cells [205]. A specific circuit in the midbrain involving GABA-somatostatin producing cells detects stress and induces restorative sleep [206], while overexpression of the zinc finger protein gene Zfp189 in the prefrontal cortex promotes behavioral resilience [207]. Also in this regard, Dong et al. have recently identified stress relief as a natural resilience mechanism against depression-like behaviors [208].
Alterations in these communication systems contribute to stress-related disorders by compromising a broad range of processes such as adequate differentiation and maturation of oligodendrocytes and astrocytes, limited trafficking of peripheral myeloid cells to the brain, maintenance of ionic and neurotransmitter homeostasis, proper dynamics at synapses and preservation of BBB integrity. Stress-related signals such as oxidative reactions and cytokine signaling from the periphery may induce the loss of myelin in brain areas related to emotional regulation and executive function. Neurovascular adaptations, endocannabinoid signaling and the kynurenine pathway also contribute to modulate mood and stress responses [209–211].
Immune System
Psychosocial stress strongly influences immune function and modulates the participation of immune cells in homeostatic resilience mechanisms. Different brain regions and neural circuits control the body trafficking and functional role of leukocytes in response to stress [212]. Motor circuits trigger the rapid mobilization of bone marrow neutrophils to peripheral tissues through the participation of neutrophil-attracting chemokines, while the hypothalamus controls the egression of lymphocytes and monocytes from blood and secondary lymphoid organs to the bone marrow via glucocorticoid signaling. These stress-induced changes in leukocyte distribution throughout the body are linked to the development of several disorders including mental illnesses. Some patients with stress-related disorders exhibit increased peripheral immune system activation and elevated levels of proinflammatory cytokines, which then activate the kynurenine pathway, depleting tryptophan and generating neuroactive catabolites that impinge on the main stress response pathways [211].
Chronic stress can also directly activate microglia and increase levels of several cytokines and chemokines through glucocorticoid and noradrenergic signaling or via the NLRP3 inflammasome. Notably, MDD exhibits significant comorbidity with autoimmune disorders and other chronic inflammatory illnesses. Accordingly, anti-inflammatory therapies elicit antidepressant effects in some patients [37]. Animal models of social stress have also shown important disturbances in peripheral myeloid cells, which are associated with activation of the innate immune system and relative suppression of the adaptive immune system [213]. Rodent studies of susceptibility to chronic stress are consistent with a pro-resilient neuroprotective effect of T cells. Immunization of rats with modified myelin basic protein before chronic mild stress induces autoreactive T cells and reduces depressive behaviors [214]. Resilience to stress can also be promoted by immunization against Mycobacterium [215], or by attenuation of inflammation via sphingosine-1-phosphate receptor 3 (S1PR3) overexpression in the medial prefrontal cortex of rats [216]. Finally, clinical studies have confirmed that emotion regulation strategies can attenuate inflammatory responses [217].
Genetic Mechanisms
Susceptibility or resilience to develop behavioral disorders in response to psychosocial stress is influenced by the interplay between genetic predisposition and environmental factors. Genomic investigations identified pro-resilience variants in genes encoding modulators of the HPA stress response axis. Thus, a polymorphism in FKBP51 – a negative modulator of glucocorticoid signalling – is linked to susceptibility (AT allele) or resilience (CG allele) to stress-related disorders. Pharmacological inhibition of FKBP51 promotes hippocampal neurogenesis and resilience to chronic psychosocial stress in mice [218]. Additionally, polymorphisms in NPY, BDNF, COMT and SLC6A4 associated with deficient resilience increase the risk of mental illness [219]. Notably, the same genomic variants (i.e., polymorphisms at the regulatory region of the serotonin transporter gene SLC6A4) that increase the risk of pathological responses to adversity may be beneficial in favorable environments [220]. This “pleiotropic antagonism” reflects the fact that, depending on the context, the same genetic variant can have positive or negative consequences.
Other Resilience Mechanisms
The initial findings linking epigenetic alterations and MDD were based on the observation that loss or inhibition of histone deacetylases and demethylases in several brain regions has antidepressant-like effects in stressed rodents [221]. Moreover, glucocorticoids suppress DNA methylation and upregulate the expression of the stress-response gene Fkbp5 in mouse neuronal cells, thus generating a negative feedback loop that limits glucocorticoid signaling and may contribute to stress-related mental disorders [222]. Several miRNAs, such as miR-25-3p, are induced in mice exposed to social defeat stress. Selective elimination in peripheral leukocytes of the miRNA cluster containing miR-25-3p reduces inflammation and promotes behavioral resilience to psychosocial stress [223]. miR-135 is necessary for maintaining intact serotonergic activity under normal conditions and confers resilience to social stress [224], whereas overexpression of miR-124 in hippocampal neurons enhances chronic stress resilience [225]. Likewise, systemic knockdown of miR-144-3p by subcutaneous administration of a specific antagomir reduces the depression-related phenotype in stress-susceptible mice [226]. Together, these works reinforce the role of epigenetic mechanisms in inflammatory and behavioral responses to psychosocial stress.
Hormonal and metabolic pathways also influence stress resilience, and patients with stress-induced psychiatric disorders exhibit metabolic phenotypes that substantially overlap with metabolic syndrome [227]. Glucocorticoids switch metabolism from anabolism to catabolism, thereby providing energy sources and building blocks for adequate stress responses. These glucocorticoid effects are modulated by other hormones, such as leptin and ghrelin [228, 229]. Somatostatin also contributes to resilience by reducing CRH release during chronic stress conditions. Sex hormones have a strong impact on homeostatic resilience and explain, at least in part, the sexual dimorphism in the responsiveness to chronic stressors [230]. Finally, microbiota-related mechanisms also contribute to stress resilience. The maintenance of a stable microbiota contributes protects from a variety of dysbiosis-related pathologies but is also critical for establishing the cognitive/emotional balance necessary to deal with psychosocial stress. In fact, alterations in the gut microbiota have been detected in a variety of mental disorders, such as MDD and ASD. The healthy microbiota contributes to homeostatic resilience against stress conditions through the production of biologically active metabolites impacting the microbiota-gut-brain axis [231]. Diverse prebiotics, probiotics and postbiotics may increase the resilience of gut bacterial communities, although for most of the currently available products there is no clear evidence yet to support beneficial effects on mental health [232].
In summary, highly interconnected body communication systems are organized in a way that allows them to elaborate a rapid and efficient response to virtually any kind of perturbation. These responses mostly involve negative feedback loops and are ultimately responsible for maintaining homeostatic resilience. Unfortunately, a wide range of chronic or excessive stressors cause the failure of resilience mechanisms and promote neuropsychiatric decompensation. Further studies of the mechanisms underlying homeostatic resilience and failure should help to design interventions that favor the maintenance of mental health.
HALLMARK 7: HORMETIC REGULATION
Hormesis is an evolutionary conserved phenomenon that leads to the development of acquired resilience against toxins and other stressors called hormetins [233]. Hormesis relies on biological processes in which low doses of potentially harmful agents elicit a protective response that prevents the organism from experiencing harm upon exposure to a higher dose of the same hormetins. Hormesis has been pinpointed in the context of mitochondrial function as “mitohormesis” to describe the beneficial effects of mild and transient mitochondrial stress on cells, tissues or organisms [234]. Mitohormesis inducers include physical exercise, caloric restriction, intermittent fasting, and dietary phytochemicals or xenohormetins [235]. The beneficial effect of hormesis may rely on direct short-range cytoprotection through the induction of ROS, heat shock proteins, thioredoxins and sirtuins, but may also involve long-range intercellular communication events via neural circuits, endocrine signals, metabolic pathways, and immune or inflammatory responses [233]. Hormesis is also involved in the maintenance of mental and brain health (Fig. 6).
Hormesis and Psychotropic Drugs
The concept of hormesis may offer a useful framework to improve neurological performance and brain health [236]. Embryonic, adult and induced-pluripotent stem cells of different sources, including those of neural origin, exhibit hormetic responses to low doses of noxious chemicals, ionizing radiation and hypoxia with respect to their capacities to proliferate, differentiate and resist inflammatory conditions [237, 238]. Dietary supplements reputed to improve human health, such as epigallocatechin-3-gallate and resveratrol, may also induce hormetic responses in neural stem cells [238], while lithium, a widely used drug for the treatment of BD and other mood-related disorders, elicits biphasic dose responses typical of hormesis [239].
Some neurotoxic agents induce reactive oxygen species (ROS) that at low levels activate hormetic responses in stressed neurons and induce the expression of genes – such as BCL2 and SOD2 – which protect against apoptosis and detoxify ROS, respectively. Downstream of ROS, transcription factors – such as NRF2 – trigger efficient cytoprotective mechanisms [240]. The endogenous metabolite N-acetyl-L-tyrosine (formed in response to stress from its precursor tyrosine) triggers a mitohormetic process that implies an elevation of ROS levels and a subsequent retrograde response activating the transcription factor FoxO, which in turn transactivates anti-oxidant genes and KEAP1 to elicit neuroprotective mechanisms [241]. Atypical antipsychotic drugs may also act through hormetic mechanisms and mediate neuroprotection through the induction of superoxide dismutase 1 and p75 neurotrophin receptor [242, 243]. Peripheral modulation of the antidepressant targets MAO-B and GABAAR by β-carbolines induces mitohormesis and improves healthspan and lifespan in preclinical models [244]. These findings suggest that several classes of antipsychotic drugs elicit hormetic effects, although it is unclear whether this truly contributes to their mode of action.
Hormesis and Mental Stress
Mild and limited stress can result in a series of moderate cognitive benefits that facilitate the development of human resilience [245]. Accordingly, hormesis has been proposed to play a positive role in cognitive processes and behavioral responses [246]. In favor of this interpretation, cortisol concentrations measured in adolescents were the lowest in individuals experiencing moderate socioeconomic and psychosocial adversity, but higher in individuals reporting low or high adversity [247]. Moreover, low-to-moderate stress perceived by young adults correlates with optimal cognitive performance and reduced psychopathological symptoms [248]. Thus, in a well-tempered/medium range, negative life experiences and perceived stress may have a beneficial effect.
From an educational/psychological viewpoint, it appears important to change the valuation of stress by shifting the overarching objective of stress regulation from avoiding and minimizing stress to accepting and utilizing stress to achieve enhancing outcomes [31]. The subjective appraisal of stress as negative (distress) versus positive (eustress) has a profound impact on its consequences, which can be detrimental versus hormetic, respectively. Indeed, negative beliefs about stress constitute an independent risk factor for morbidity and mortality [249], in line with the well-established negative impact of pessimism on life expectancy [250]. Several randomized studies with adolescent have proven that psychological training designed to improve the acceptance of stress reduced cortisol levels and perceived anxiety [31]. Notably, improved stress management correlates with higher emotional intelligence [251], while optimal stress responses may explain “post-traumatic growth”, a phenomenon allowing individuals to develop increased skills and a deeper appreciation for life as a legacy of traumatic events [252].
In summary, the concept of hormesis has gained interest in the field of neural functions. The use of hormetic preconditioning strategies can enhance the functional performance of neural cells, including neural stem cells, with respect to their ability to improve metabolic functions and contribute to neuroplasticity, neurorepair or regeneration. Theoretically, knowledge on hormetic regulation may help to establish optimal schedules for administering drugs that favor brain health and cognitive performance.
HALLMARK 8: REPAIR AND REGENERATION
Organisms have developed complex mechanisms and signaling pathways able to sense and efficiently respond to the myriad of lesions suffered by all organizational strata of the body, from molecules to the meta-organism, and to activate repair and regeneration mechanisms [172]. Insufficient repair and regeneration entails a broad range of pathological perturbations, including those causing neuropsychiatric disorders (Fig. 6).
DNA Damage and Repair in Neural Systems
Nuclear and mitochondrial DNA are constantly subjected to genotoxic stress by exogenous and endogenous challenges. This causes a wide spectrum of DNA lesions, which are repaired by a network of systems collectively known as the DNA damage response (DDR) [253]. The effectors of this response, such as TP53 and various immune cells, drive cellular senescence or apoptosis and contribute to maintain homeostasis. DDR also engages the cGAS/STING pathway and stimulates a non-cell-autonomous response that facilitates homeostasis maintenance. However, deregulated DDR causes uncontrolled inflammation and tissue damage, including in the CNS [254, 255]. Impaired DNA damage repair in concert with mitochondrial dysfunction is a common feature of diverse psychiatric disorders [255, 256]. Elevated levels of oxidative DNA damage and altered DNA repair gene expression are found in GABAergic neurons in SCZ, while genomes from ASD patients are enriched for de novo mutations in genes expressed in striatal neurons [257]. Interestingly, selective serotonin reuptake inhibitors decrease the level of oxidative DNA damage in MDD patients [258]. Hence, it will be interesting to test pharmacological agents that stimulate oxidative DNA damage repair [259] in the context of neuropsychiatric disorders.
Epigenetic Dynamics, Reprogramming and Mental Health
Epigenetic factors contribute to the development and promotion of neurological and behavioral diseases [260]. The expression of the epigenetic reader BRD1 increases after periods of chronic stress, and Brd1+/- mice display cognitive deficits and behavioral phenotypes [261]. Chromatin profiling in neurons from SCZ patients has revealed aberrant roles for histone acetylation and BRD1 [262], while BRD1-interaction networks show enrichment for SCZ risk genes and enhanced binding to gene promoters associated with brain development and mental disorders [261]. Besides histone modifications, DNA methylation studies in postmortem SCZ brains have identified multiple differentially methylated sites between cases and controls. Genes in or near these sites tend to be involved in embryo development, cell fate commitment or nervous system differentiation, and are also modestly overrepresented in SCZ-associated loci. MDD patients exhibit higher global DNA methylation rates than healthy controls and a significant correlation of gene methylation changes in the blood and in MDD-relevant brain areas such as the prefrontal cortex [263]. DNA methylation-based epigenetic clocks that reflect biological aging indicate that MDD patients undergo accelerated aging compared to non-depressed controls [264], while individuals with different behavioral disorders exhibit an accelerated pace of DNA-methylation [265]. Additionally, correlative studies have found significant ncRNA alterations during stress-induced responses and in patients with mood disorders. Knockout of the SCZ-related miR-501-3p gene in mice impairs sociability and memory by enhancing mGluR5-mediated glutamatergic transmission, while treatment of these mir-501-3p-null mice with negative allosteric modulators of mGluR5 or NMDA receptor antagonists ameliorates their cognitive and behavioral deficiencies [266].
Collectively, these findings suggest that neuropsychiatric disorders are linked to epigenetic alterations, opening new therapeutic strategies aiming at restoring the epigenetic landscape [267]. Indeed, epigenomic editing at the enhancer region of the activity-regulated cytoskeleton-associated protein (Arc) gene in rats ameliorates adult anxiety and excessive drinking after adolescent alcohol exposure, a major risk factor for psychiatric disorders later in life. Conversely, dCas9-KRAB increases repressive histone methylation at this genomic region, decreases Arc expression, produces anxiety and stimulates alcohol drinking in control rats [268].
Neural Regeneration and Mental Health
Stem and progenitor cells can repair or regenerate damaged tissues and hence favor adaptive and compensatory responses. Stem cells are also present in the mammalian brain, an organ long-time considered to be irreparable. Adult neurogenesis has been well characterized in the dentate gyrus of the rodent hippocampus and has important implications for regenerative medicine in humans, although the possibility that this process is fully preserved in the adult human brain is still debated [269–272]. Neural stem cells can self-renew and generate terminally differentiated neurons and glial cells. Due to their persistence throughout life, stem cells are particularly susceptible to biological and environmental stress, and decline in number and proliferative and differentiation capacity with age, compromising tissue repair and regenerative potential [108, 109]. Several studies have suggested that psychosocial factors may contribute to stem cell loss [273]. Moreover, chemogenetic inhibition of neurogenesis in the ventral dentate gyrus promotes susceptibility to social defeat stress, while increasing neurogenesis confers resilience to chronic stress [205]. Notably, treatment with atypical antipsychotics increases hippocampal neurogenesis in adult mice [274]. In MDD patients, neurotrophic factors necessary for neural stem cells niche maintenance are reduced, while low levels of neurotrophic factors have been associated with poor treatment responses and cognitive impairment in MDD [275].
Physical exercise and dietary interventions may also contribute to neurological repair. Several signaling circuits including glutamatergic, serotonergic, dopaminergic, adrenergic, neurotrophin-receptor and tropomyosin-related kinase B pathways have been implicated in the exercise-stimulated enhancement of neurogenesis [276]. In addition, regulator of G protein signaling 6 stands out as a key mediator of exercise-induced neurogenesis [277]. The administration of exerkines (molecules released in response to physical exercise) [278]. and exercise mimetics (compounds that mimic the therapeutic effects of exercise) [279]. may represent emerging strategies for improving neurogenesis and synaptic plasticity [280]. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of the longevity gene Klotho [281]. Consistent with this, low-dose injections of Klotho increase synaptic plasticity in mice and improve cognition in aged nonhuman primates [282]. Similarly, caloric restriction and diets enriched with bioactive compounds, such as polyunsaturated fatty acids and polyphenols, improve neurogenesis, learning and memory performance in neuropsychiatric diseases [283].
Cognitive and Behavioral Flexibility
Flexibility is substantially impaired across many mental disorders irrespective of the age of onset [284]. Neuroimaging, behavioral, genetic and pharmacological studies [285] have identified large functional brain networks that support flexibility. Serotonergic and dopaminergic signaling, as well as striatal cholinergic systems play important roles in flexible cognition and behavior. Reduced neurogenesis, changes in dendritic morphology and density, and alterations in growth factor and neurotransmitter levels contribute to the loss of neuroplasticity and functional connectivity underlying cognitive and behavioral inflexibility in mood-related disorders [285]. Social isolation reinforces aging-related behavioral inflexibility in aging-prone SAMP8 mice by promoting neuronal necroptosis (an alternative mode of regulated cell death mimicking features of apoptosis and necrosis) in basolateral amygdala, a critical brain region for behavioral flexibility [286]. This flexibility impairment can be reversed by the necroptosis inhibitor necrostatin-1s, and involves inhibition of glycogen synthase kinase 3α (GSK-3α), a central regulator of age-related pathologies in mice [286]. Interestingly, infusion of young cerebrospinal fluid into brains of aged mice restores oligodendrogenesis and memory through a process involving Fgf17, thereby offering new possibilities to enhance cognitive flexibility [287]. Of note, and in the context of tissue damage, preemptive immunity to the microbiota directly promotes neuron regeneration via IL-17A [288]. Other strategies to improve behavioral flexibility include antidepressant drugs, lifestyle interventions, enrichment of the particular social environment and different methods of cognitive training [289, 290].
CONCLUSIONS AND PERSPECTIVES
To advance in the comprehension of human health, which does include essential mental facets, we have expanded our previous compendium of eight biological hallmarks [1] by adding a ninth determinant of health, psychosocial adaptation. In addition, we have analyzed the psychosocial, mostly neuropsychiatric, implication of each of the eight somatic hallmarks, unveiling numerous connections between all somatic hallmarks and psychosocial factors. We have also identified links between mental health and all eight somatic strata of the human body, from individual molecules through cells and tissues to the meta-organism, in addition to the stratum encompassing psychosocial interactions. Perturbations of each of these nine strata threaten mental health (Fig. 7).
Figure 7. FIGURE 7: Psychosocially relevant perturbations across health hallmarks and body strata.
The nine hallmarks of health integrate and leverage the multifunctionality of each hierarchical stratum and orchestrate the complex interactions across the nine body strata encompassing the distinct molecular, organellar, cellular, supracellular, tissular, systemic, organismal, meta-organismal and psychosocial components. Examples of specific psychosocial dysfunctions or disorders affecting the nine different hallmarks and strata are shown to illustrate the multidimensional basis of health, including mental health.
The inclusion of the psychosocial dimension as a further hallmark of health is in accordance with earlier models of health and disease [291], and with current definitions and concepts of health and well-being [292]. Nevertheless, our work has tried to go beyond these studies by integrating psychosocial and biological determinants of health. We have shown that there are so many intricate links between the somatic and psychosocial hallmarks of health that the contours of physical and mental health dissipate. We posit that virtually any disease, even pathologies with an infectious or mechanical cause have a major effect on the mental state, while all major mental disorders compromise general health. This holistic view transcends the unidirectional idea of “mens sana in corpore sano”, which correctly expresses the notion that physical fitness is required for mental agility but does not reflect the fact that, vice versa, psychosocial adaptation is indispensable for somatic health.
The historical distinction between physical and mental diseases has been fueled by the apparent absence of a clear anatomopathological substrate of the latter. However, progress in ever more refined “omics” technologies yielding a “brain atlas” [293], the incipient elucidation of the neural connectome [294], and the development of high-resolution functional neuroimaging tools, has yielded quantitative measurements of CNS perturbations. Ultimately, these objective measurements will create a mechanism-based classification of neuropsychiatric disorders and will drive the era of precision and preventive psychiatry [295], which will have to be integrated with cultural-ecosocial approaches aimed at person-centered mental health care [296]. The progress in analytical tools will be coupled to powerful experimental systems including multiplexed chemogenetic or optogenetic methods, and to the development of sophisticated iPSC-derived human cerebral organoids that are able to maturate and integrate with host circuits controlling behavior when implanted into rodent brains [297]. Such experimental approaches will also be indispensable for structuring AI-based algorithms and hence for interpreting the accumulating big data generated by large-scale “omic” efforts as well as by the monitoring of human behavior, either by observation or by means of specific tests. Systematic approaches will also be necessary to quantify the exposome that, besides the totality of physical, chemical, dietary, microbial and toxicological insults, should also integrate psychological and social stressors. Of note, there is evidence of association between exposure to environmental pollutants and the incidence of dementia and behavioral disorders, again linking somatic to psychosocial factors [24]. This question is of special concern in the context of the current climate change that is going to impose grand challenges for future human health, including mental health [298]. We also need to better understanding how psychological, social and economic stress permeates the human body. Beyond the frequently evoked SAM and HPA axes, additional communication systems linking the CNS to the periphery are likely to play a major role in this process. The elucidation of these pathways might guide neuropsychiatric research from the current CNS-centric to a body-wide exploration.
Today, around one billion people are affected by mental disorders [299] and dramatically, close to one million individuals decide to take their own lives each year [300]. However, the relative investment in mental health services is under-dimensioned compared to physical health systems [24]. Over the last decades, the extraordinary progress of science and medicine has resulted in remarkable advances in the prevention or treatment of most human pathologies, but progress has been more limited with regard to psychosocial health [155]. Several studies have provided evidence that certain psychosocial interventions improve physical health [27–30], but it is necessary to perform large-scale, long-term randomized clinical trials to assess the specific benefits provided by these interventions. Similarly, public campaigns to promote population health must expand their current focus on exercise, diet, environmental pollution, alcohol and drug abuse, and target additional lifestyle factors that favor psychosomatic health. Such neglected lifestyle factors are exemplified by the need of increased levels of social respect and education, the avoidance of digital addiction, the improvement of quantity and quality of sleep, the introduction of mind-body and stress management practices, and – utopically but necessarily – the reduction of violence and inequality.
Acknowledgments
We apologize for not citing all relevant papers due to space constraints. We acknowledge Y. Español, D. Carrero, M. Roca, S. Lamas, A. Tomás-Loba and L. Ortiz for helpful comments and advice during the elaboration of this manuscript. C.L-O. is supported by grants from the European Research Council (ERC Advanced Grant, DeAge, Grant No. 742067) and Ministerio de Ciencia e Innovación, Spain (Grant No. PDI2020-118394RB-100). The Instituto Universitario de Oncología is supported by Fundación Bancaria Caja de Ahorros de Asturias. G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council Advanced Investigator Award (ERC-2021-ADG, ICD-Cancer, Grant No. 101052444), European Union Horizon 2020 Projects Oncobiome, Prevalung (grant No. 101095604) and Crimson; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology ANR-18-IDEX-0001; a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Abbreviations:
- WHO
– world health organization,
- ASD
– autistic spectrum disorders,
- MDD
– major depressive disorder,
- BD
– bipolar disorder,
- SCZ
– schizophrenia,
- SAM
– sympathetic-adrenal medullary,
- HPA
– hypothalamic pituitary adrenal,
- IL6
– interleukin-6,
- Tac2
– tachykinin 2,
- mtDNA
– mitochondrial DNA,
- iPSC
– induced pluripotent stem cell,
- BBB
– blood-brain barrier,
- CNS
– central nervous system,
- CLDN5
– claudin-5,
- HDAC1
– histone deacetylase 1,
- FOXO1
– forkhead box protein O1,
- C4A
– component 4A,
- EBP1
– ErbB3-binding protein 1,
- FMRP1
– fragile X mental retardation protein 1,
- DISC1
– disrupted-in-SCZ-1,
- CSF1R
– colony stimulating factor 1 receptor,
- NLGN3
– neuroligin-3,
- NPY
– neuropeptide Y,
- S1PR3
– sphingosine-1-phosphate receptor 3,
- ROS
– reactive oxygen species,
- DDR
– DNA damage response,
- Arc
– activity-regulated cytoskeleton-associated protein,
- GSK-3α
– glycogen synthase kinase 3α.
REFERENCES
- 1.López-Otín C, Kroemer G. Hallmarks of Health. Cell. 2021;184(1):33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Brainstorm Consortium et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Als TD, et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat Med. 2023;29(7):1832–1844. doi: 10.1038/s41591-023-02352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson PM, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. 2020;10(1):100. doi: 10.1038/s41398-020-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie C, et al. A shared neural basis underlying psychiatric comorbidity. Nat Med. 2023;29(5):1232–1242. doi: 10.1038/s41591-023-02317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O'Rand A, Harris KM, Shively CA, Alberts SC, Tung J. Social determinants of health and survival in humans and other animals. Science. 2020;368(6493):eaax9553. doi: 10.1126/science.aax9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucknor MC, Gururajan A, Dale RC, Hofer MJ. A comprehensive approach to modeling maternal immune activation in rodents. Front Neurosci. 2022;16:1071976. doi: 10.3389/fnins.2022.1071976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeste DV, Lee EE, Cacioppo S. Battling the Modern Behavioral Epidemic of Loneliness: Suggestions for Research and Interventions. JAMA Psychiatry. 2020;77(6):553–554. doi: 10.1001/jamapsychiatry.2020.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen S, Sherrod DR, Clark MS. Social skills and the stress-protective role of social support. J Pers Soc Psychol. 1986;50(5):963–973. doi: 10.1037//0022-3514.50.5.963. [DOI] [PubMed] [Google Scholar]
- 10.Holt-Lunstad J. Social Connection as a Public Health Issue: The Evidence and a Systemic Framework for Prioritizing the “Social” in Social Determinants of Health. Annu Rev Public Health. 2022;43:193–213. doi: 10.1146/annurev-publhealth-052020-110732. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 12.Delgado MR, Fareri DS, Chang LJ. Characterizing the mechanisms of social connection. Neuron. S0896-6273(23)00699–2. 2023. [DOI] [PMC free article] [PubMed]
- 13.Meili I, Maercker A. Cultural perspectives on positive responses to extreme adversity: A playing field for metaphors. Transcult Psychiatry. 2019;56(5):1056–1075. doi: 10.1177/1363461519844355. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MJ, Martin J, Lu Y, Brikell I, Lundström S, Larsson H, Lichtenstein P. Association of Genetic Risk Factors for Psychiatric Disorders and Traits of These Disorders in a Swedish Population Twin Sample. JAMA Psychiatry. 2019;76(3):280–289. doi: 10.1001/jamapsychiatry.2018.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Biltz RG, Sawicki CM, Sheridan JF, Godbout JP. The neuroimmunology of social-stress-induced sensitization. Nat Immunol. 2022;23(11):1527–1535. doi: 10.1038/s41590-022-01321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. 2020;182(2):372–387.:e14. doi: 10.1016/j.cell.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, Beadle K, Gradinaru V, Deverman BE, Anderson DJ. The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell. 2018;173(5):1265–1279.:e19. doi: 10.1016/j.cell.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Z, Chang J, Liang Y, Zhu H, Zhang C, Zheng D, Wang J, Xu Y, Li Q-J, Hu H. Neural mechanism underlying depressive-like state associated with social status loss. Cell. 2023;186(3):560–576.:e17. doi: 10.1016/j.cell.2022.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 21.Bhutta ZA, Bhavnani S, Betancourt TS, Tomlinson M, Patel V. Adverse childhood experiences and lifelong health. Nat Med. 2023;29(7):1639–1648. doi: 10.1038/s41591-023-02426-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee CR, Chen A, Tye KM. The neural circuitry of social homeostasis: Consequences of acute versus chronic social isolation. Cell. 2021;184(10):2794–2795. doi: 10.1016/j.cell.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 24.Ridley M, Rao G, Schilbach F, Patel V. Poverty, depression, and anxiety: Causal evidence and mechanisms. Science. 2020;370(6522):eaay0214. doi: 10.1126/science.aay0214. [DOI] [PubMed] [Google Scholar]
- 25.Canadian Alliance of Healthy Hearts and Minds (CAHHM) and the Prospective Urban and Rural Epidemiological (PURE) Study Investigators. Anand SS, Friedrich MG, Lee DS, Awadalla P, Després JP, Desai D, de Souza RJ, Dummer T, Parraga G, Larose E, Lear SA, Teo KK, Poirier P, Schulze KM, Szczesniak D, Tardif J-C, Vena J, Zatonska K, Yusuf S, Smith EE. Evaluation of Adiposity and Cognitive Function in Adults. JAMA Netw Open. 2022;5(2):e2146324. doi: 10.1001/jamanetworkopen.2021.46324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019;29(5):1061–1077.:e8. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A. 2014;111(31):11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JMG, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71(4):366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brody GH, Gray JC, Yu T, Barton AW, Beach SRH, Galván A, MacKillop J, Windle M, Chen E, Miller GE, Sweet LH. Protective Prevention Effects on the Association of Poverty With Brain Development. JAMA Pediatr. 2017;171(1):46–52. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeager DS, Bryan CJ, Gross JJ, Murray JS, Krettek Cobb D, H F Santos P, Gravelding H, Johnson M, Jamieson JP. A synergistic mindsets intervention protects adolescents from stress. Nature. 2022;607(7919):512–520. doi: 10.1038/s41586-022-04907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scazufca M, Nakamura CA, Seward N, Moreno-Agostino D, van de Ven P, Hollingworth W, Peters TJ, Araya R. A task-shared, collaborative care psychosocial intervention for improving depressive symptomatology among older adults in a socioeconomically deprived area of Brazil (PROACTIVE): a pragmatic, two-arm, parallel-group, cluster-randomised controlled trial. Lancet Healthy Longev. 2022;3(10):e690–e702. doi: 10.1016/S2666-7568(22)00194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Block T, Petrides G, Kushner H, Kalin N, Belanoff J, Schatzberg A. Mifepristone Plasma Level and Glucocorticoid Receptor Antagonism Associated With Response in Patients With Psychotic Depression. J Clin Psychopharmacol. 2017;37(5):505–511. doi: 10.1097/JCP.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK. Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry. 2018;175(5):427–433. doi: 10.1176/appi.ajp.2017.17050481. [DOI] [PubMed] [Google Scholar]
- 35.Zamzow RM, Ferguson BJ, Stichter JP, Porges EC, Ragsdale AS, Lewis ML, Beversdorf DQ. Effects of propranolol on conversational reciprocity in autism spectrum disorder: a pilot, double-blind, single-dose psychopharmacological challenge study. Psychopharmacology (Berl). 2016;233(7):1171–1178. doi: 10.1007/s00213-015-4199-0. [DOI] [PubMed] [Google Scholar]
- 36.Smith CM, Santalucia M, Bunn H, Muzyk A. Sublingual Dexmedetomidine for the Treatment of Agitation in Patients with Schizophrenia and Bipolar Disorder. Clin Psychopharmacol Neurosci. 2023;21(2):215–221. doi: 10.9758/cpn.2023.21.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21(3):224–244. doi: 10.1038/s41573-021-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dragioti E, Radua J, Solmi M, Gosling CJ, Oliver D, Lascialfari F, Ahmed M, Cortese S, Estradé A, Arrondo G, Gouva M, Fornaro M, Batiridou A, Dimou K, Tsartsalis D, Carvalho AF, Shin JI, Berk M, Stringhini S, Correll CU, Fusar-Poli P. Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry. 2023;22(1):86–104. doi: 10.1002/wps.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correll CU, Solmi M, Croatto G, Schneider LK, Rohani-Montez SC, Fairley L, Smith N, Bitter I, Gorwood P, Taipale H, Tiihonen J. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. 2022;21(2):248–271. doi: 10.1002/wps.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalkia D, Singh LN, Leipzig J, Lvova M, Derbeneva O, Lakatos A, Hadley D, Hakonarson H, Wallace DC. Association Between Mitochondrial DNA Haplogroup Variation and Autism Spectrum Disorders. JAMA Psychiatry. 2017;74(11):1161–1168. doi: 10.1001/jamapsychiatry.2017.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross JM, Stewart JB, Hagström E, Brené S, Mourier A, Coppotelli G, Freyer C, Lagouge M, Hoffer BJ, Olson L, Larsson N-G. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature. 2013;501(7467):412–415. doi: 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osete JR, Akkouh IA, de Assis DR, Szabo A, Frei E, Hughes T, Smeland OB, Steen NE, Andreassen OA, Djurovic S. Lithium increases mitochondrial respiration in iPSC-derived neural precursor cells from lithium responders. Mol Psychiatry. 2021;26(11):6789–6805. doi: 10.1038/s41380-021-01164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ait Tayeb AEK, Colle R, Chappell K, El-Asmar K, Acquaviva-Bourdain C, David DJ, Trabado S, Chanson P, Feve B, Becquemont L, Verstuyft C, Corruble E. Metabolomic profiles of 38 acylcarnitines in major depressive episodes before and after treatment. Psychol Med. 2023. pp. 1–10. [DOI] [PubMed]
- 44.Ogata H, Higasa K, Kageyama Y, Tahara H, Shimamoto A, Takekita Y, Koshikawa Y, Nonen S, Kato T, Kinoshita T, Kato M. Relationship between circulating mitochondrial DNA and microRNA in patients with major depression. J Affect Disord. 2023;339:538–546. doi: 10.1016/j.jad.2023.07.073. [DOI] [PubMed] [Google Scholar]
- 45.Fiksinski AM, Hoftman GD, Vorstman JAS, Bearden CE. A genetics-first approach to understanding autism and schizophrenia spectrum disorders: the 22q11.2 deletion syndrome. Mol Psychiatry. 2023;28(1):341–353. doi: 10.1038/s41380-022-01783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, Radin EA, Lubin N, Bonner ER, Popratiloff A, Rothblat LA, Maynard TM, LaMantia A-S. Mitochondrial Dysfunction Leads to Cortical Under-Connectivity and Cognitive Impairment. Neuron. 2019;102(6):1127–1142.:e3. doi: 10.1016/j.neuron.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni P, Noh H, Park G-H, Shao Z, Guan Y, Park JM, Yu S, Park JS, Coyle JT, Weinberger DR, Straub RE, Cohen BM, McPhie DL, Yin C, Huang W, Kim H-Y, Chung S. iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry. 2020;25(11):2873–2888. doi: 10.1038/s41380-019-0423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ene HM, Karry R, Farfara D, Ben-Shachar D. Mitochondria play an essential role in the trajectory of adolescent neurodevelopment and behavior in adulthood: evidence from a schizophrenia rat model. Mol Psychiatry. 2023;28(3):1170–1181. doi: 10.1038/s41380-022-01865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, Li X, Song N, Ding J, Lu M, Hu G. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021;6(23):e146852. doi: 10.1172/jci.insight.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melamud MM, Buneva VN, Ermakov EA. Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2023;24(4):3402. doi: 10.3390/ijms24043402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubotzky A, Pelov I, Teplitz R, Neiman D, Smadja A, Zemmour H, Piyanzin S, Ochana B-L, Spalding KL, Glaser B, Shemer R, Dor Y, Kohn Y. Elevated brain-derived cell-free DNA among patients with first psychotic episode - a proof-of-concept study. Elife. 2022;11:e76391. doi: 10.7554/eLife.76391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knox EG, Aburto MR, Clarke G, Cryan JF, O'Driscoll CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. 2022;27(6):2659–2673. doi: 10.1038/s41380-022-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, Cassidy LM, Martiniano R, Shashi V, Hooper SR, Grant GA, Kenna PF, Norris K, Callaghan CK, Islam M dN, O'Mara SM, Najda Z, Campbell SG, Pachter JS, Thomas J, Williams NM, Humphries P, Murphy KC, Campbell M. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156–2166. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, Parise LF, Cathomas F, Samba N, Hudson N, Lebel M, Signature Consortium, Campbell M, Turecki G, Mechawar N, Menard C. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat Commun. 2022;13(1):164. doi: 10.1038/s41467-021-27604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Ding Z, Fan T, Wang K, Li S, Zhao J, Zhu W. Childhood social isolation causes anxiety-like behaviors via the damage of blood-brain barrier in amygdala in female mice. Front Cell Dev Biol. 2022;10:943067. doi: 10.3389/fcell.2022.943067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20(12):1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C, Turecki G, Mechawar N, Russo SJ, Menard C. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U S A. 2020;117(6):3326–3336. doi: 10.1073/pnas.1914655117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz-Ballester C, Mahmutovic D, Rafiqzad Y, Korot A, Robel S. Mild Traumatic Brain Injury-Induced Disruption of the Blood-Brain Barrier Triggers an Atypical Neuronal Response. Front Cell Neurosci. 2022;16:821885. doi: 10.3389/fncel.2022.821885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tărlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, Galluccio M, Tesulov M, Morelli E, Sonmez FM, Bilguvar K, Ohgaki R, Kanai Y, Johansen A, Esharif S, Ben-Omran T, Topcu M, Schlessinger A, Indiveri C, Duncan KE, Caglayan AO, Gunel M, Gleeson JG, Novarino G. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell. 2016;167(6):1481–1494.:e18. doi: 10.1016/j.cell.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tangeraas T, et al. BCKDK deficiency: a treatable neurodevelopmental disease amenable to newborn screening. Brain. 2023;146(7):3003–3013. doi: 10.1093/brain/awad010. [DOI] [PubMed] [Google Scholar]
- 62.Carloni S, Bertocchi A, Mancinelli S, Bellini M, Erreni M, Borreca A, Braga D, Giugliano S, Mozzarelli AM, Manganaro D, Fernandez Perez D, Colombo F, Di Sabatino A, Pasini D, Penna G, Matteoli M, Lodato S, Rescigno M. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374(6566):439–448. doi: 10.1126/science.abc6108. [DOI] [PubMed] [Google Scholar]
- 63.Senay O, Seethaler M, Makris N, Yeterian E, Rushmore J, Cho KIK, Rizzoni E, Heller C, Pasternak O, Szczepankiewicz F, Westin C-F, Losak J, Ustohal L, Tomandl J, Vojtisek L, Kudlicka P, Kikinis Z, Holt D, Lewandowski KE, Lizano P, Keshavan MS, Öngür D, Kasparek T, Breier A, Shenton ME, Seitz-Holland J, Kubicki M. A preliminary choroid plexus volumetric study in individuals with psychosis. Hum Brain Mapp. 2023;44(6):2465–2478. doi: 10.1002/hbm.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams MR, Macdonald CM, Turkheimer FE. Histological examination of choroid plexus epithelia changes in schizophrenia. Brain Behav Immun. 2023;111:292–297. doi: 10.1016/j.bbi.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 66.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 67.Fidelle M, et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science. 2023;380(6649):eabo2296. doi: 10.1126/science.abo2296. [DOI] [PubMed] [Google Scholar]
- 68.Schneider KM, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186(13):2823–2838.:e20. doi: 10.1016/j.cell.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yonekura S, et al. Cancer Induces a Stress Ileopathy Depending on β-Adrenergic Receptors and Promoting Dysbiosis that Contributes to Carcinogenesis. Cancer Discov. 2022;12(4):1128–1151. doi: 10.1158/2159-8290.CD-21-0999. [DOI] [PubMed] [Google Scholar]
- 70.Cryan JF, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 71.Kronsten VT, Tranah TH, Pariante C, Shawcross DL. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76(3):665–680. doi: 10.1016/j.jhep.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Knudsen JK, Michaelsen TY, Bundgaard-Nielsen C, Nielsen RE, Hjerrild S, Leutscher P, Wegener G, Sørensen S. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci Rep. 2021;11(1):21869. doi: 10.1038/s41598-021-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donoso F, Cryan JF, Olavarría-Ramírez L, Nolan YM, Clarke G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin Pharmacol Ther. 2023;113(2):246–259. doi: 10.1002/cpt.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101(2):246–259.:e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yap CX, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021;184(24):5916–5931.:e17. doi: 10.1016/j.cell.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 77.Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu W-L, Rabut C, Ladinsky MS, Hwang S-J, Guo Y, Zhu Q, Griffiths JA, Knight R, Bjorkman PJ, Shapiro MG, Geschwind DH, Holschneider DP, Fischbach MA, Mazmanian SK. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602(7898):647–653. doi: 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart Campbell A, Needham BD, Meyer CR, Tan J, Conrad M, Preston GM, Bolognani F, Rao SG, Heussler H, Griffith R, Guastella AJ, Janes AC, Frederick B, Donabedian DH, Mazmanian SK. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nat Med. 2022;28(3):528–534. doi: 10.1038/s41591-022-01683-9. [DOI] [PubMed] [Google Scholar]
- 79.Dohnalová L, et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature. 2022;612(7941):739–747. doi: 10.1038/s41586-022-05525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jameson C, Boulton KA, Silove N, Nanan R, Guastella AJ. Ectodermal origins of the skin-brain axis: a novel model for the developing brain, inflammation, and neurodevelopmental conditions. Mol Psychiatry. 2023;28(1):108–117. doi: 10.1038/s41380-022-01829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan H, Sun Y, Zhang S, Feng J, Tian Z, Liu J, Wang H, Gao Y, Tang Y, Zheng F. NLRP3 neuroinflammatory factors may be involved in atopic dermatitis mental disorders: an animal study. Front Pharmacol. 2022;13:966279. doi: 10.3389/fphar.2022.966279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.the SUPREME Study Group. Talamonti M, Malara G, Natalini Y, Bardazzi F, Conti A, Chiricozzi A, Mugheddu C, Gisondi P, Piaserico S, Pagnanelli G, Amerio P, Potenza C, Cantoresi F, Fargnoli MC, Balato A, Loconsole F, Offidani A, Bonifati C, Prignano F, Bartezaghi M, Rausa A, Aloisi E, Orsenigo R, Costanzo A. Secukinumab Improves Patient Perception of Anxiety and Depression in Patients with Moderate to Severe Psoriasis: A Post hoc Analysis of the SUPREME Study. Acta Derm Venereol. 2021;101(3):adv00422. doi: 10.2340/00015555-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cork MJ, Eckert L, Simpson EL, Armstrong A, Barbarot S, Puig L, Girolomoni G, de Bruin-Weller M, Wollenberg A, Kataoka Y, Remitz A, Beissert S, Mastey V, Ardeleanu M, Chen Z, Gadkari A, Chao J. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat. 2020;31(6):606–614. doi: 10.1080/09546634.2019.1612836. [DOI] [PubMed] [Google Scholar]
- 84.Lozano-Vicario L, García-Hermoso A, Cedeno-Veloz BA, Fernández-Irigoyen J, Santamaría E, Romero-Ortuno R, Zambom-Ferraresi F, Sáez de Asteasu ML, Muñoz-Vázquez ÁJ, Izquierdo M, Martínez-Velilla N. Biomarkers of delirium risk in older adults: a systematic review and meta-analysis. Front Aging Neurosci. 2023;15:1174644. doi: 10.3389/fnagi.2023.1174644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fondeur J, Escudero Mendez L, Srinivasan M, Hamouda RK, Ambedkar B, Arzoun H, Sahib I, Mohammed L. Dexmedetomidine in Prevention of Postoperative Delirium: A Systematic Review. Cureus. 2022;14(6):e25639. doi: 10.7759/cureus.25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang T, Velagapudi R, Kong C, Ko U, Kumar V, Brown P, Franklin NO, Zhang X, Caceres AI, Min H, Filiano AJ, Rodriguiz RM, Wetsel WC, Varghese S, Terrando N. Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice. Br J Anaesth. 2023;130(2):e370–e380. doi: 10.1016/j.bja.2022.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunter SF, Agius M, Miller DM, Cutter G, Barbato L, McCague K, Meng X, Agashivala N, Chin P, Hollander E. Impact of a switch to fingolimod on depressive symptoms in patients with relapsing multiple sclerosis: An analysis from the EPOC (Evaluate Patient OutComes) trial. J Neurol Sci. 2016;365:190–198. doi: 10.1016/j.jns.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 88.Karbalaee M, Jameie M, Amanollahi M, TaghaviZanjani F, Parsaei M, Basti FA, Mokhtari S, Moradi K, Ardakani M-RK, Akhondzadeh S. Efficacy and safety of adjunctive therapy with fingolimod in patients with schizophrenia: A randomized, double-blind, placebo-controlled clinical trial. Schizophr Res. 2023;254:92–98. doi: 10.1016/j.schres.2023.02.020. [DOI] [PubMed] [Google Scholar]
- 89.Baghdadi LR, Alhassan MK, Alotaibi FH, Alsuwaida AA, Shehadah AE, Alzahrani MT. Effect of type of disease-modifying antirheumatic drugs on depression and anxiety of patients with rheumatoid arthritis in Saudi Arabia: a cross-sectional study. Front Psychiatry. 2023;14:1184720. doi: 10.3389/fpsyt.2023.1184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deyama S, Kaneda K, Minami M. Resolution of depression: antidepressant actions of resolvins. Neurosci Res. S0168-0102(22)00266–8. 2022. [DOI] [PubMed]
- 91.Lamon-Fava S, Liu M, Dunlop BW, Kinkead B, Schettler PJ, Felger JC, Ziegler TR, Fava M, Mischoulon D, Rapaport MH. Clinical response to EPA supplementation in patients with major depressive disorder is associated with higher plasma concentrations of pro-resolving lipid mediators. Neuropsychopharmacology. 2023;48(6):929–935. doi: 10.1038/s41386-022-01527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL, Jurk D. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20(2):e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin M, Vermeiren S, Bostaille N, Eubelen M, Spitzer D, Vermeersch M, Profaci CP, Pozuelo E, Toussay X, Raman-Nair J, Tebabi P, America M, De Groote A, Sanderson LE, Cabochette P, Germano RFV, Torres D, Boutry S, de Kerchove d'Exaerde A, Bellefroid EJ, Phoenix TN, Devraj K, Lacoste B, Daneman R, Liebner S, Vanhollebeke B. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science. 2022;375(6582):eabm4459. doi: 10.1126/science.abm4459. [DOI] [PubMed] [Google Scholar]
- 95.Xu L, Nirwane A, Xu T, Kang M, Devasani K, Yao Y. Fibroblasts repair blood-brain barrier damage and hemorrhagic brain injury via TIMP2. Cell Rep. 2022;41(8):111709. doi: 10.1016/j.celrep.2022.111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, Sun R, Xia L, Zhu X, Zhang Q, Ye Y. Potential Therapeutic Effects of NAMPT-Mediated NAD Biosynthesis in Depression In Vivo. Brain Sci. 2022;12(12):1699. doi: 10.3390/brainsci12121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ibrahim WW, Sayed RH, Kandil EA, Wadie W. Niacin mitigates blood-brain barrier tight junctional proteins dysregulation and cerebral inflammation in ketamine rat model of psychosis: Role of GPR109A receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110583. doi: 10.1016/j.pnpbp.2022.110583. [DOI] [PubMed] [Google Scholar]
- 98.Li Z, He Y, Fan S, Sun B. Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci Bull. 2015;31(5):617–625. doi: 10.1007/s12264-015-1555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimizu T, Ishida A, Hagiwara M, Ueda Y, Hattori A, Tajiri N, Hida H. Social Defeat Stress in Adolescent Mice Induces Depressive-like Behaviors with Reduced Oligodendrogenesis. Neuroscience. 2020;443:218–232. doi: 10.1016/j.neuroscience.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Bohlen JF, Cleary CM, Das D, Sripathy SR, Sadowski N, Shim G, Kenney RF, Buchler IP, Banerji T, Scanlan TS, Mulkey DK, Maher BJ. Promyelinating drugs promote functional recovery in an autism spectrum disorder mouse model of Pitt-Hopkins syndrome. Brain. 2023;146(8):3331–3346. doi: 10.1093/brain/awad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franklin RJM, Simons M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron. 2022;110(21):3549–3565. doi: 10.1016/j.neuron.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 102.Faust TE, Gunner G, Schafer DP. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat Rev Neurosci. 2021;22(11):657–673. doi: 10.1038/s41583-021-00507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Germann M, Brederoo SG, Sommer IEC. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr Opin Psychiatry. 2021;34(3):222–227. doi: 10.1097/YCO.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 105.Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel M-C, Wells L, Creeney H, Bonsall D, Rogdaki M, Shatalina E, Reis Marques T, Rabiner EA, Gunn RN, Natesan S, Vernon AC, Howes OD. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11(1):246. doi: 10.1038/s41467-019-14122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howes OD, Onwordi EC. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol Psychiatry. 2023. [DOI] [PMC free article] [PubMed]
- 107.Yilmaz M, Yalcin E, Presumey J, Aw E, Ma M, Whelan CW, Stevens B, McCarroll SA, Carroll MC. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 2021;24(2):214–224. doi: 10.1038/s41593-020-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186(4):693–714. doi: 10.1016/j.cell.2022.12.032. [DOI] [PubMed] [Google Scholar]
- 109.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Hui KK, Endo R, Sawa A, Tanaka M. A Perspective on the Potential Involvement of Impaired Proteostasis in Neuropsychiatric Disorders. Biol Psychiatry. 2022;91(4):335–345. doi: 10.1016/j.biopsych.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bulovaite E, Qiu Z, Kratschke M, Zgraj A, Fricker DG, Tuck EJ, Gokhale R, Koniaris B, Jami SA, Merino-Serrais P, Husi E, Mendive-Tapia L, Vendrell M, O'Dell TJ, DeFelipe J, Komiyama NH, Holtmaat A, Fransén E, Grant SGN. A brain atlas of synapse protein lifetime across the mouse lifespan. Neuron. 2022;110(24):4057–4073.:e8. doi: 10.1016/j.neuron.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lorenzo EC, Kuchel GA, Kuo C-L, Moffitt TE, Diniz BS. Major depression and the biological hallmarks of aging. Ageing Res Rev. 2023;83:101805. doi: 10.1016/j.arr.2022.101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang K, Shi Y, Du X, Wang J, Zhang Y, Shan S, Yuan Y, Wang R, Zhou C, Liu Y, Cai Z, Wang Y, Fan L, Xu H, Yu J, Cheng J, Li F, Qiu Z. SENP1 in the retrosplenial agranular cortex regulates core autistic-like symptoms in mice. Cell Rep. 2021;37(5):109939. doi: 10.1016/j.celrep.2021.109939. [DOI] [PubMed] [Google Scholar]
- 114.Yoshikawa A, Kushima I, Miyashita M, Suzuki K, Iino K, Toriumi K, Horiuchi Y, Kawaji H, Ozaki N, Itokawa M, Arai M. Exonic deletions in IMMP2L in schizophrenia with enhanced glycation stress subtype. PLoS One. 2022;17(7):e0270506. doi: 10.1371/journal.pone.0270506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buiting K, Williams C, Horsthemke B. Angelman syndrome - insights into a rare neurogenetic disorder. Nat Rev Neurol. 2016;12(10):584–593. doi: 10.1038/nrneurol.2016.133. [DOI] [PubMed] [Google Scholar]
- 116.Amar M, Pramod AB, Yu N-K, Herrera VM, Qiu LR, Moran-Losada P, Zhang P, Trujillo CA, Ellegood J, Urresti J, Chau K, Diedrich J, Chen J, Gutierrez J, Sebat J, Ramanathan D, Lerch JP, Yates JR, Muotri AR, Iakoucheva LM. Autism-linked Cullin3 germline haploinsufficiency impacts cytoskeletal dynamics and cortical neurogenesis through RhoA signaling. Mol Psychiatry. 2021;26(7):3586–3613. doi: 10.1038/s41380-021-01052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sleyp Y, et al. De novo missense variants in the E3 ubiquitin ligase adaptor KLHL20 cause a developmental disorder with intellectual disability, epilepsy, and autism spectrum disorder. Genet Med. 2022;24(12):2464–2474. doi: 10.1016/j.gim.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 118.Hwang I, Kim B-S, Ko HR, Cho S, Lee HY, Cho S-W, Ryu D, Shim S, Ahn J-Y. Cerebellar dysfunction and schizophrenia-like behavior in Ebp1-deficient mice. Mol Psychiatry. 2022;27(4):2030–2041. doi: 10.1038/s41380-022-01458-1. [DOI] [PubMed] [Google Scholar]
- 119.Louros SR, Seo SS, Maio B, Martinez-Gonzalez C, Gonzalez-Lozano MA, Muscas M, Verity NC, Wills JC, Li KW, Nolan MF, Osterweil EK. Excessive proteostasis contributes to pathology in fragile X syndrome. Neuron. 2023;111(4):508–525.:e7. doi: 10.1016/j.neuron.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 120.Youle RJ. Mitochondria-Striking a balance between host and endosymbiont. Science. 2019;365(6454):eaaw9855. doi: 10.1126/science.aaw9855. [DOI] [PubMed] [Google Scholar]
- 121.González-Usigli HA, Ortiz GG, Charles-Niño C, Mireles-Ramírez MA, Pacheco-Moisés FP, Torres-Mendoza BM de G, Hernández-Cruz J de J, Delgado-Lara DLDC, Ramírez-Jirano LJ. Neurocognitive Psychiatric and Neuropsychological Alterations in Parkinson's Disease: A Basic and Clinical Approach. Brain Sci. 2023;13(3):508. doi: 10.3390/brainsci13030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z-T, Lu M-H, Zhang Y, Ji W-L, Lei L, Wang W, Fang L-P, Wang L-W, Yu F, Wang J, Li Z-Y, Wang J-R, Wang T-H, Dou F, Wang Q-W, Wang X-L, Li S, Ma Q-H, Xu R-X. Disrupted-in-schizophrenia-1 protects synaptic plasticity in a transgenic mouse model of Alzheimer's disease as a mitophagy receptor. Aging Cell. 2019;18(1):e12860. doi: 10.1111/acel.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tripathi A, Scaini G, Barichello T, Quevedo J, Pillai A. Mitophagy in depression: Pathophysiology and treatment targets. Mitochondrion. 2021;61:1–10. doi: 10.1016/j.mito.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duan K, Gu Q, Petralia RS, Wang Y-X, Panja D, Liu X, Lehmann ML, Zhu H, Zhu J, Li Z. Mitophagy in the basolateral amygdala mediates increased anxiety induced by aversive social experience. Neuron. 2021;109(23):3793–3809.:e8. doi: 10.1016/j.neuron.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Griffey CJ, Yamamoto A. Macroautophagy in CNS health and disease. Nat Rev Neurosci. 2022;23(7):411–427. doi: 10.1038/s41583-022-00588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuijpers M, Kochlamazashvili G, Stumpf A, Puchkov D, Swaminathan A, Lucht MT, Krause E, Maritzen T, Schmitz D, Haucke V. Neuronal Autophagy Regulates Presynaptic Neurotransmission by Controlling the Axonal Endoplasmic Reticulum. Neuron. 2022;110(4):734. doi: 10.1016/j.neuron.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan Y, Zhou G, Li W, He X, Li C, Li Y, Li T, Hu H, Ma H. Excitation-transcription coupling via synapto-nuclear signaling triggers autophagy for synaptic turnover and long-lasting synaptic depression. Autophagy. 2021;17(11):3887–3888. doi: 10.1080/15548627.2021.1964888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim H-J, Cho M-H, Shim WH, Kim JK, Jeon E-Y, Kim D-H, Yoon S-Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry. 2017;22(11):1576–1584. doi: 10.1038/mp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mitjans M, Begemann M, Ju A, Dere E, Wüstefeld L, Hofer S, Hassouna I, Balkenhol J, Oliveira B, van der Auwera S, Tammer R, Hammerschmidt K, Völzke H, Homuth G, Cecconi F, Chowdhury K, Grabe H, Frahm J, Boretius S, Dandekar T, Ehrenreich H. Sexual dimorphism of AMBRA1-related autistic features in human and mouse. Transl Psychiatry. 2017;7(10):e1247. doi: 10.1038/tp.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Al Eissa MM, Fiorentino A, Sharp SI, O'Brien NL, Wolfe K, Giaroli G, Curtis D, Bass NJ, McQuillin A. Exome sequence analysis and follow up genotyping implicates rare ULK1 variants to be involved in susceptibility to schizophrenia. Ann Hum Genet. 2018;82(2):88–92. doi: 10.1111/ahg.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pagani M, Barsotti N, Bertero A, Trakoshis S, Ulysse L, Locarno A, Miseviciute I, De Felice A, Canella C, Supekar K, Galbusera A, Menon V, Tonini R, Deco G, Lombardo MV, Pasqualetti M, Gozzi A. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun. 2021;12(1):6084. doi: 10.1038/s41467-021-26131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lieberman OJ, Cartocci V, Pigulevskiy I, Molinari M, Carbonell J, Broseta MB, Post MR, Sulzer D, Borgkvist A, Santini E. mTOR Suppresses Macroautophagy During Striatal Postnatal Development and Is Hyperactive in Mouse Models of Autism Spectrum Disorders. Front Cell Neurosci. 2020;14:70. doi: 10.3389/fncel.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L, Li J, Liu X, Zhao Z, Jin Y, Fu Y, Zhou A, Wang C, Zhou Y. Vitamin B6 Deficiency Induces Autism-Like Behaviors in Rats by Regulating mTOR-Mediated Autophagy in the Hippocampus. Behav Neurol. 2023;2023:6991826. doi: 10.1155/2023/6991826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, Cheng Y, Zhou Y, Du H, Zhang C, Zhao Z, Chen Y, Zhou Z, Mei J, Wu W, Chen M. High fat diet-induced obesity leads to depressive and anxiety-like behaviors in mice via AMPK/mTOR-mediated autophagy. Exp Neurol. 2022;348:113949. doi: 10.1016/j.expneurol.2021.113949. [DOI] [PubMed] [Google Scholar]
- 135.McWhinney SR, et al. Obesity and brain structure in schizophrenia - ENIGMA study in 3021 individuals. Mol Psychiatry. 2022;27(9):3731–3737. doi: 10.1038/s41380-022-01616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arinami H, Watanabe Y, Suzuki Y, Tajiri M, Tsuneyama N, Someya T. Serum cortisol and insulin-like growth factor 1 levels in major depressive disorder and schizophrenia. Sci Rep. 2023;13(1):1148. doi: 10.1038/s41598-023-28449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stapel B, Fraccarollo D, Westhoff-Bleck M, Bauersachs J, Lichtinghagen R, Jahn K, Burkert A, Buchholz V, Bleich S, Frieling H, Ding X-Q, Kahl KG. Impact of fasting on stress systems and depressive symptoms in patients with major depressive disorder: a cross-sectional study. Sci Rep. 2022;12(1):7642. doi: 10.1038/s41598-022-11639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vanderplow AM, Eagle AL, Kermath BA, Bjornson KJ, Robison AJ, Cahill ME. Akt-mTOR hypoactivity in bipolar disorder gives rise to cognitive impairments associated with altered neuronal structure and function. Neuron. 2021;109(9):1479–1496.:e6. doi: 10.1016/j.neuron.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul M, Torres-Berrio A, Bermudez S, Rurak GM, Simard S, Salmaso N, Gobbi G, Lacaille J-C, Sonenberg N. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature. 2021;590(7845):315–319. doi: 10.1038/s41586-020-03047-0. [DOI] [PubMed] [Google Scholar]
- 140.Moigneu C, Abdellaoui S, Ramos-Brossier M, Pfaffenseller B, Wollenhaupt-Aguiar B, de Azevedo Cardoso T, Camus C, Chiche A, Kuperwasser N, Azevedo da Silva R, Pedrotti Moreira F, Li H, Oury F, Kapczinski F, Lledo P-M, Katsimpardi L. Systemic GDF11 attenuates depression-like phenotype in aged mice via stimulation of neuronal autophagy. Nat Aging. 2023;3(2):213–228. doi: 10.1038/s43587-022-00352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alcocer-Gómez E, Casas-Barquero N, Williams MR, Romero-Guillena SL, Cañadas-Lozano D, Bullón P, Sánchez-Alcazar JA, Navarro-Pando JM, Cordero MD. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol Res. 2017;121:114–121. doi: 10.1016/j.phrs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 142.Gulbins A, Schumacher F, Becker KA, Wilker B, Soddemann M, Boldrin F, Müller CP, Edwards MJ, Goodman M, Caldwell CC, Kleuser B, Kornhuber J, Szabo I, Gulbins E. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol Psychiatry. 2018;23(12):2324–2346. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, Grutzendler J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv. 2020;6(26):eaba3239. doi: 10.1126/sciadv.aba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 145.Chen M, Guo L, Hao J, Ni J, Lv Q, Xin X, Liao H. p75NTR Promotes Astrocyte Proliferation in Response to Cortical Stab Wound. Cell Mol Neurobiol. 2022;42(4):1153–1166. doi: 10.1007/s10571-020-01006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Y, Zhang S, Lan Z, Doan V, Kim B, Liu S, Zhu M, Hull VL, Rihani S, Zhang C-L, Gray JA, Guo F. SOX2 is essential for astrocyte maturation and its deletion leads to hyperactive behavior in mice. Cell Rep. 2022;41(12):111842. doi: 10.1016/j.celrep.2022.111842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017;20(4):779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bray CE, Witcher KG, Adekunle-Adegbite D, Ouvina M, Witzel M, Hans E, Tapp ZM, Packer J, Goodman E, Zhao F, Chunchai T, O'Neil S, Chattipakorn SC, Sheridan J, Kokiko-Cochran ON, Askwith C, Godbout JP. Chronic Cortical Inflammation, Cognitive Impairment, and Immune Reactivity Associated with Diffuse Brain Injury Are Ameliorated by Forced Turnover of Microglia. J Neurosci. 2022;42(20):4215–4228. doi: 10.1523/JNEUROSCI.1910-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fries GR, Saldana VA, Finnstein J, Rein T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023;28(1):284–297. doi: 10.1038/s41380-022-01806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang L, Mirabella VR, Dai R, Su X, Xu R, Jadali A, Bernabucci M, Singh I, Chen Y, Tian J, Jiang P, Kwan KY, Pak C, Liu C, Comoletti D, Hart RP, Chen C, Südhof TC, Pang ZP. Analyses of the autism-associated neuroligin-3 R451C mutation in human neurons reveal a gain-of-function synaptic mechanism. Mol Psychiatry. 2022. [DOI] [PMC free article] [PubMed]
- 151.Parekh PK, Johnson SB, Liston C. Synaptic Mechanisms Regulating Mood State Transitions in Depression. Annu Rev Neurosci. 2022;45:581–601. doi: 10.1146/annurev-neuro-110920-040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luciana M, Collins PF. Neuroplasticity, the Prefrontal Cortex, and Psychopathology-Related Deviations in Cognitive Control. Annu Rev Clin Psychol. 2022;18:443–469. doi: 10.1146/annurev-clinpsy-081219-111203. [DOI] [PubMed] [Google Scholar]
- 153.Monday HR, Younts TJ, Castillo PE. Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu Rev Neurosci. 2018;41:299–322. doi: 10.1146/annurev-neuro-080317-062155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wong FK, Favuzzi E. The brain's polymath: Emerging roles of microglia throughout brain development. Curr Opin Neurobiol. 2023;79:102700. doi: 10.1016/j.conb.2023.102700. [DOI] [PubMed] [Google Scholar]
- 155.Scangos KW, State MW, Miller AH, Baker JT, Williams LM. New and emerging approaches to treat psychiatric disorders. Nat Med. 2023;29(2):317–333. doi: 10.1038/s41591-022-02197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci. 2021;42(11):929–942. doi: 10.1016/j.tips.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 157.Liu Y, Li S, Lin W, Li W, Yan X, Wang X, Pan X, Rutledge RB, Ma Y. Oxytocin modulates social value representations in the amygdala. Nat Neurosci. 2019;22(4):633–641. doi: 10.1038/s41593-019-0351-1. [DOI] [PubMed] [Google Scholar]
- 158.O'Connor DB, Thayer JF, Vedhara K. Stress and Health: A Review of Psychobiological Processes. Annu Rev Psychol. 2021;72:663–688. doi: 10.1146/annurev-psych-062520-122331. [DOI] [PubMed] [Google Scholar]
- 159.Formolo DA, Cheng T, Yu J, Kranz GS, Yau S-Y. Central Adiponectin Signaling - A Metabolic Regulator in Support of Brain Plasticity. Brain Plast. 2022;8(1):79–96. doi: 10.3233/BPL-220138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Joseph A, et al. Metabolic and psychiatric effects of acyl coenzyme A binding protein (ACBP)/diazepam binding inhibitor (DBI). Cell Death Dis. 2020;11(7):502. doi: 10.1038/s41419-020-2716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang T, Perkins MH, Chang H, Han W, de Araujo IE. An inter-organ neural circuit for appetite suppression. Cell. 2022;185(14):2478–2494.:e28. doi: 10.1016/j.cell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tallon-Baudry C. Interoception: Probing internal state is inherent to perception and cognition. Neuron. 2023;111(12):1854–1857. doi: 10.1016/j.neuron.2023.04.019. [DOI] [PubMed] [Google Scholar]
- 163.Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, Gnadt JW, Nielsen L, Hillaire-Clarke CS, Spruance V, Horowitz TS, Vallejo YF, Langevin HM. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021;44(1):3–16. doi: 10.1016/j.tins.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Interoception Summit 2016 participants. Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, Meuret AE, Nemeroff CB, Oppenheimer S, Petzschner FH, Pollatos O, Rhudy JL, Schramm LP, Simmons WK, Stein MB, Stephan KE, Van den Bergh O, Van Diest I, von Leupoldt A, Paulus MP. Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(6):501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hsueh B, Chen R, Jo Y, Tang D, Raffiee M, Kim YS, Inoue M, Randles S, Ramakrishnan C, Patel S, Kim DK, Liu TX, Kim SH, Tan L, Mortazavi L, Cordero A, Shi J, Zhao M, Ho TT, Crow A, Yoo A-CW, Raja C, Evans K, Bernstein D, Zeineh M, Goubran M, Deisseroth K. Cardiogenic control of affective behavioural state. Nature. 2023;615(7951):292–299. doi: 10.1038/s41586-023-05748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kessing LV, Rytgaard HC, Ekstrøm CT, Torp-Pedersen C, Berk M, Gerds TA. Antihypertensive Drugs and Risk of Depression: A Nationwide Population-Based Study. Hypertension. 2020;76(4):1263–1279. doi: 10.1161/HYPERTENSIONAHA.120.15605. [DOI] [PubMed] [Google Scholar]
- 167.Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep Med. 2022;3(7):100696. doi: 10.1016/j.xcrm.2022.100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fernandes DJ, Spring S, Corre C, Tu A, Qiu LR, Hammill C, Vousden DA, Spencer Noakes TL, Nieman BJ, Bowdish DME, Foster JA, Palmert MR, Lerch JP. Mouse models of immune dysfunction: their neuroanatomical differences reflect their anxiety-behavioural phenotype. Mol Psychiatry. 2022;27(7):3047–3055. doi: 10.1038/s41380-022-01535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fan K-Q, Li Y-Y, Wang H-L, Mao X-T, Guo J-X, Wang F, Huang L-J, Li Y-N, Ma X-Y, Gao Z-J, Chen W, Qian D-D, Xue W-J, Cao Q, Zhang L, Shen L, Zhang L, Tong C, Zhong J-Y, Lu W, Lu L, Ren K-M, Zhong G, Wang Y, Tang M, Feng X-H, Chai R-J, Jin J. Stress-Induced Metabolic Disorder in Peripheral CD4+ T Cells Leads to Anxiety-like Behavior. Cell. 2019;179(4):864–879.:e19. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 170.Zhu X, Sakamoto S, Ishii C, Smith MD, Ito K, Obayashi M, Unger L, Hasegawa Y, Kurokawa S, Kishimoto T, Li H, Hatano S, Wang T-H, Yoshikai Y, Kano S-I, Fukuda S, Sanada K, Calabresi PA, Kamiya A. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat Immunol. 2023;24(4):625–636. doi: 10.1038/s41590-023-01447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dollish HK, Tsyglakova M, McClung CA. Circadian rhythms and mood disorders: Time to see the light. Neuron. S0896-6273(23)00710–9. 2023. [DOI] [PMC free article] [PubMed]
- 172.Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021;371(6530):eabd0951. doi: 10.1126/science.abd0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Allada R, Bass J. Circadian Mechanisms in Medicine. N Engl J Med. 2021;384(6):550–561. doi: 10.1056/NEJMra1802337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.LeGates TA, Altimus CM, Wang H, Lee H-K, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lane JM, Qian J, Mignot E, Redline S, Scheer FAJL, Saxena R. Genetics of circadian rhythms and sleep in human health and disease. Nat Rev Genet. 2023;24(1):4–20. doi: 10.1038/s41576-022-00519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meyer N, Harvey AG, Lockley SW, Dijk D-J. Circadian rhythms and disorders of the timing of sleep. Lancet. 2022;400(10357):1061–1078. doi: 10.1016/S0140-6736(22)00877-7. [DOI] [PubMed] [Google Scholar]
- 177.Roenneberg T. How can social jetlag affect health? Nat Rev Endocrinol. 2023;19(7):383–384. doi: 10.1038/s41574-023-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taillard J, Sagaspe P, Philip P, Bioulac S. Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol. 2021;191:114438. doi: 10.1016/j.bcp.2021.114438. [DOI] [PubMed] [Google Scholar]
- 179.Caliandro R, Streng AA, van Kerkhof LWM, van der Horst GTJ, Chaves I. Social Jetlag and Related Risks for Human Health: A Timely Review. Nutrients. 2021;13(12):4543. doi: 10.3390/nu13124543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Long Z, Zhao A, Chen Y, Li R, Xia Y, Guo Y, Li S. The associations of chronotype and social jetlag with prosocial behavior problems among Chinese adolescents. Chronobiol Int. 2022;39(11):1498–1507. doi: 10.1080/07420528.2022.2127362. [DOI] [PubMed] [Google Scholar]
- 181.Meng J, Xiao X, Wang W, Jiang Y, Jin Y, Wang H. Sleep quality, social rhythms, and depression among people living with HIV: a path analysis based on social zeitgeber theory. Front Psychiatry. 2023;14:1102946. doi: 10.3389/fpsyt.2023.1102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang R, Volkow ND. Seasonality of brain function: role in psychiatric disorders. Transl Psychiatry. 2023;13(1):65. doi: 10.1038/s41398-023-02365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alachkar A, Lee J, Asthana K, Vakil Monfared R, Chen J, Alhassen S, Samad M, Wood M, Mayer EA, Baldi P. The hidden link between circadian entropy and mental health disorders. Transl Psychiatry. 2022;12(1):281. doi: 10.1038/s41398-022-02028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maruani J, Geoffroy PA. Bright Light as a Personalized Precision Treatment of Mood Disorders. Front Psychiatry. 2019;10:85. doi: 10.3389/fpsyt.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zahova SK, Humby T, Davies JR, Morgan JE, Isles AR. Comparison of mouse models reveals a molecular distinction between psychotic illness in PWS and schizophrenia. Transl Psychiatry. 2021;11(1):433. doi: 10.1038/s41398-021-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oliver PL, Sobczyk MV, Maywood ES, Edwards B, Lee S, Livieratos A, Oster H, Butler R, Godinho SIH, Wulff K, Peirson SN, Fisher SP, Chesham JE, Smith JW, Hastings MH, Davies KE, Foster RG. Disrupted circadian rhythms in a mouse model of schizophrenia. Curr Biol. 2012;22(4):314–319. doi: 10.1016/j.cub.2011.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200(1):3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 189.Kiss B, Laszlovszky I, Krámos B, Visegrády A, Bobok A, Lévay G, Lendvai B, Román V. Neuronal Dopamine D3 Receptors: Translational Implications for Preclinical Research and CNS Disorders. Biomolecules. 2021;11(1):104. doi: 10.3390/biom11010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Imamura K, Takumi T. Mood phenotypes in rodent models with circadian disturbances. Neurobiol Sleep Circadian Rhythms. 2022;13:100083. doi: 10.1016/j.nbscr.2022.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brami-Cherrier K, Lewis RG, Cervantes M, Liu Y, Tognini P, Baldi P, Sassone-Corsi P, Borrelli E. Cocaine-mediated circadian reprogramming in the striatum through dopamine D2R and PPARγ activation. Nat Commun. 2020;11(1):4448. doi: 10.1038/s41467-020-18200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kott J, Leach G, Yan L. Direction-dependent effects of chronic “jet-lag” on hippocampal neurogenesis. Neurosci Lett. 2012;515(2):177–180. doi: 10.1016/j.neulet.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 193.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71(5):589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Toccaceli Blasi M, Valletta M, Trebbastoni A, D'Antonio F, Talarico G, Campanelli A, Sepe Monti M, Salati E, Gasparini M, Buscarnera S, Salzillo M, Canevelli M, Bruno G. Sundowning in Patients with Dementia: Identification, Prevalence, and Clinical Correlates. J Alzheimers Dis. 2023. [DOI] [PubMed]
- 195.Savino R, Polito AN, Marsala G, Ventriglio A, Di Salvatore M, De Stefano MI, Valenzano A, Marinaccio L, Bellomo A, Cibelli G, Monda M, Monda V, Messina A, Polito R, Carotenuto M, Messina G. Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders. Brain Sci. 2023;13(5):734. doi: 10.3390/brainsci13050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 197.Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228–240. doi: 10.1016/j.neuroscience.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin J-P, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339(6117):332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- 199.Reichmann F, Holzer P. Neuropeptide Y: A stressful review. Neuropeptides. 2016;55:99–109. doi: 10.1016/j.npep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tural U, Iosifescu DV. Neuropeptide Y in PTSD, MDD, and chronic stress: A systematic review and meta-analysis. J Neurosci Res. 2020;98(5):950–963. doi: 10.1002/jnr.24589. [DOI] [PubMed] [Google Scholar]
- 201.Cathomas F, Holt LM, Parise EM, Liu J, Murrough JW, Casaccia P, Nestler EJ, Russo SJ. Beyond the neuron: Role of non-neuronal cells in stress disorders. Neuron. 2022;110(7):1116–1138. doi: 10.1016/j.neuron.2022.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mohebi A, Pettibone JR, Hamid AA, Wong J-MT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570(7759):65–70. doi: 10.1038/s41586-019-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.House PH. Postoperative astigmatism using the McIntyre needle. Ophthalmic Surg. 1987;18(9):654–657. [PubMed] [Google Scholar]
- 204.Mary A, Dayan J, Leone G, Postel C, Fraisse F, Malle C, Vallée T, Klein-Peschanski C, Viader F, de la Sayette V, Peschanski D, Eustache F, Gagnepain P. Resilience after trauma: The role of memory suppression. Science. 2020;367(6479):eaay8477. doi: 10.1126/science.aay8477. [DOI] [PubMed] [Google Scholar]
- 205.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559(7712):98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu X, Zhao G, Wang D, Wang S, Li R, Li A, Wang H, Nollet M, Chun YY, Zhao T, Yustos R, Li H, Zhao J, Li J, Cai M, Vyssotski AL, Li Y, Dong H, Franks NP, Wisden W. A specific circuit in the midbrain detects stress and induces restorative sleep. Science. 2022;377(6601):63–72. doi: 10.1126/science.abn0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lorsch ZS, Hamilton PJ, Ramakrishnan A, Parise EM, Salery M, Wright WJ, Lepack AE, Mews P, Issler O, McKenzie A, Zhou X, Parise LF, Pirpinias ST, Ortiz Torres I, Kronman HG, Montgomery SE, Loh Y-HE, Labonté B, Conkey A, Symonds AE, Neve RL, Turecki G, Maze I, Dong Y, Zhang B, Shen L, Bagot RC, Nestler EJ. Stress resilience is promoted by a Zfp189-driven transcriptional network in prefrontal cortex. Nat Neurosci. 2019;22(9):1413–1423. doi: 10.1038/s41593-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dong Y, Li Y, Xiang X, Xiao Z-C, Hu J, Li Y, Li H, Hu H. Stress relief as a natural resilience mechanism against depression-like behaviors. Neuron. S0896-6273(23)00668–2. 2023. [DOI] [PubMed]
- 209.Dion-Albert L, Dudek KA, Russo SJ, Campbell M, Menard C. Neurovascular adaptations modulating cognition, mood, and stress responses. Trends Neurosci. 2023;46(4):276–292. doi: 10.1016/j.tins.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 210.Bluett RJ, Báldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, Baechle J, Marcus DJ, Mardam-Bey R, Shonesy BC, Uddin MJ, Marnett LJ, Mackie K, Colbran RJ, Winder DG, Patel S. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. 2017;8:14782. doi: 10.1038/ncomms14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meier TB, Savitz J. The Kynurenine Pathway in Traumatic Brain Injury: Implications for Psychiatric Outcomes. Biol Psychiatry. 2022;91(5):449–458. doi: 10.1016/j.biopsych.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Poller WC, Downey J, Mooslechner AA, Khan N, Li L, Chan CT, McAlpine CS, Xu C, Kahles F, He S, Janssen H, Mindur JE, Singh S, Kiss MG, Alonso-Herranz L, Iwamoto Y, Kohler RH, Wong LP, Chetal K, Russo SJ, Sadreyev RI, Weissleder R, Nahrendorf M, Frenette PS, Divangahi M, Swirski FK. Brain motor and fear circuits regulate leukocytes during acute stress. Nature. 2022;607(7919):578–584. doi: 10.1038/s41586-022-04890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tsyglakova M, McDaniel D, Hodes GE. Immune mechanisms of stress susceptibility and resilience: Lessons from animal models. Front Neuroendocrinol. 2019;54:100771. doi: 10.1016/j.yfrne.2019.100771. [DOI] [PubMed] [Google Scholar]
- 214.Han Y, Sun C-Y, Meng S-Q, Tabarak S, Yuan K, Cao L, Yan W, Xu L-Z, Deng J-H, Zhu W-L, Li J-L, Lu L, Xue Y-X, Shi J. Systemic immunization with altered myelin basic protein peptide produces sustained antidepressant-like effects. Mol Psychiatry. 2020;25(6):1260–1274. doi: 10.1038/s41380-019-0470-9. [DOI] [PubMed] [Google Scholar]
- 215.Hassell JE, Baratta MV, Fallon IP, Siebler PH, Karns BL, Nguyen KT, Gates CA, Fonken LK, Frank MG, Maier SF, Lowry CA. Immunization with a heat-killed preparation of Mycobacterium vaccae NCTC 11659 enhances auditory-cued fear extinction in a stress-dependent manner. Brain Behav Immun. 2023;107:1–15. doi: 10.1016/j.bbi.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 216.Corbett BF, Luz S, Arner J, Pearson-Leary J, Sengupta A, Taylor D, Gehrman P, Ross R, Bhatnagar S. Sphingosine-1-phosphate receptor 3 in the medial prefrontal cortex promotes stress resilience by reducing inflammatory processes. Nat Commun. 2019;10(1):3146. doi: 10.1038/s41467-019-10904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ospina LH, Beck-Felts K, Ifrah C, Lister A, Messer S, Russo SJ, Gross JJ, Kimhy D. Inflammation and emotion regulation: Findings from the MIDUS II study. Brain Behav Immun Health. 2022;26:100536. doi: 10.1016/j.bbih.2022.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Codagnone MG, Kara N, Ratsika A, Levone BR, van de Wouw M, Tan LA, Cunningham JI, Sanchez C, Cryan JF, O'Leary OF. Inhibition of FKBP51 induces stress resilience and alters hippocampal neurogenesis. Mol Psychiatry. 2022;27(12):4928–4938. doi: 10.1038/s41380-022-01755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou Z, Zhu G, Hariri AR, Enoch M-A, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu X-Z, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen P-H, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta J-K, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Reiss D, Leve LD, Neiderhiser JM. How genes and the social environment moderate each other. Am J Public Health. 2013;103(Suppl 1(Suppl 1)):S111–121. doi: 10.2105/AJPH.2013.301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Walsh RM, Shen EY, Bagot RC, Anselmo A, Jiang Y, Javidfar B, Wojtkiewicz GJ, Cloutier J, Chen JW, Sadreyev R, Nestler EJ, Akbarian S, Hochedlinger K. Phf8 loss confers resistance to depression-like and anxiety-like behaviors in mice. Nat Commun. 2017;8:15142. doi: 10.1038/ncomms15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cox OH, Song HY, Garrison-Desany HM, Gadiwalla N, Carey JL, Menzies J, Lee RS. Characterization of glucocorticoid-induced loss of DNA methylation of the stress-response gene Fkbp5 in neuronal cells. Epigenetics. 2021;16(12):1377–1397. doi: 10.1080/15592294.2020.1864169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pfau ML, Menard C, Cathomas F, Desland F, Kana V, Chan KL, Shimo Y, LeClair K, Flanigan ME, Aleyasin H, Walker DM, Bouchard S, Mack M, Hodes GE, Merad MM, Russo SJ. Role of Monocyte-Derived MicroRNA106b∼25 in Resilience to Social Stress. Biol Psychiatry. 2019;86(6):474–482. doi: 10.1016/j.biopsych.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, Awatramani R, Binder EB, Deneris ES, Lowry CA, Chen A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83(2):344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 225.Higuchi F, Uchida S, Yamagata H, Abe-Higuchi N, Hobara T, Hara K, Kobayashi A, Shintaku T, Itoh Y, Suzuki T, Watanabe Y. Hippocampal MicroRNA-124 Enhances Chronic Stress Resilience in Mice. J Neurosci. 2016;36(27):7253–7267. doi: 10.1523/JNEUROSCI.0319-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.van der Zee YY, Eijssen LMT, Mews P, Ramakrishnan A, Alvarez K, Lardner CK, Cates HM, Walker DM, Torres-Berrío A, Browne CJ, Cunningham A, Cathomas F, Kronman H, Parise EM, de Nijs L, Shen L, Murrough JW, Rutten BPF, Nestler EJ, Issler O. Blood miR-144-3p: a novel diagnostic and therapeutic tool for depression. Mol Psychiatry. 2022;27(11):4536–4549. doi: 10.1038/s41380-022-01712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Raue S, Wedekind D, Wiltfang J, Schmidt U. The Role of Proopiomelanocortin and α-Melanocyte-Stimulating Hormone in the Metabolic Syndrome in Psychiatric Disorders: A Narrative Mini-Review. Front Psychiatry. 2019;10:834. doi: 10.3389/fpsyt.2019.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tomiyama AJ. Stress and Obesity. Annu Rev Psychol. 2019;70:703–718. doi: 10.1146/annurev-psych-010418-102936. [DOI] [PubMed] [Google Scholar]
- 229.Montégut L, Lopez-Otin C, Magnan C, Kroemer G. Old Paradoxes and New Opportunities for Appetite Control in Obesity. Trends Endocrinol Metab. 2021;32(5):264–294. doi: 10.1016/j.tem.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 230.Kropp DR, Hodes GE. Sex differences in depression: An immunological perspective. Brain Res Bull. 2023;196:34–45. doi: 10.1016/j.brainresbull.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Teichman EM, O'Riordan KJ, Gahan CGM, Dinan TG, Cryan JF. When Rhythms Meet the Blues: Circadian Interactions with the Microbiota-Gut-Brain Axis. Cell Metab. 2020;31(3):448–471. doi: 10.1016/j.cmet.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 232.MooDFOOD Prevention Trial Investigators. Bot M, Brouwer IA, Roca M, Kohls E, Penninx BWJH, Watkins E, van Grootheest G, Cabout M, Hegerl U, Gili M, Owens M, Visser M. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults With Subsyndromal Depressive Symptoms: The MooDFOOD Randomized Clinical Trial. JAMA. 2019;321(9):858–868. doi: 10.1001/jama.2019.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Calabrese EJ. Hormesis Mediates Acquired Resilience: Using Plant-Derived Chemicals to Enhance Health. Annu Rev Food Sci Technol. 2021;12:355–381. doi: 10.1146/annurev-food-062420-124437. [DOI] [PubMed] [Google Scholar]
- 234.Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med Hypotheses. 2006;66(4):832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 235.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 236.Calabrese EJ, Calabrese V, Giordano J. Brain health promotion: Tactics within a strategic approach based upon valid, yet evolving scientific evidence. Mech Ageing Dev. 2022;201:111605. doi: 10.1016/j.mad.2021.111605. [DOI] [PubMed] [Google Scholar]
- 237.Gopi IK, Rattan SIS. Biphasic Dose-Response and Hormetic Effects of Stress Hormone Hydrocortisone on Telomerase-Immortalized Human Bone Marrow Stem Cells In Vitro. Dose Response. 2019;17(4):1559325819889819. doi: 10.1177/1559325819889819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Calabrese EJ, Calabrese V, Dhawan G, Kapoor R, Giordano J. Hormesis and neural stem cells. Free Radic Biol Med. 2022;178:314–329. doi: 10.1016/j.freeradbiomed.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 239.Calabrese EJ, Pressman P, Hayes AW, Dhawan G, Kapoor R, Agathokleous E, Calabrese V. Lithium and hormesis: Enhancement of adaptive responses and biological performance via hormetic mechanisms. J Trace Elem Med Biol. 2023;78:127156. doi: 10.1016/j.jtemb.2023.127156. [DOI] [PubMed] [Google Scholar]
- 240.Calabrese EJ, Kozumbo WJ. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol Res. 2021;167:105526. doi: 10.1016/j.phrs.2021.105526. [DOI] [PubMed] [Google Scholar]
- 241.Matsumura T, Uryu O, Matsuhisa F, Tajiri K, Matsumoto H, Hayakawa Y. N-acetyl-l-tyrosine is an intrinsic triggering factor of mitohormesis in stressed animals. EMBO Rep. 2020;21(5):e49211. doi: 10.15252/embr.201949211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bai O, Wei Z, Lu W, Bowen R, Keegan D, Li X-M. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J Neurosci Res. 2002;69(2):278–283. doi: 10.1002/jnr.10290. [DOI] [PubMed] [Google Scholar]
- 243.Calabrese EJ, Calabrese V, Giordano J. Putative hormetic mechanisms and effects of atypical antipsychotic agents: Implications for study design and clinical psychopharmacotherapeutics. Chem Biol Interact. 2021;333:109327. doi: 10.1016/j.cbi.2020.109327. [DOI] [PubMed] [Google Scholar]
- 244.Costa-Machado LF, Garcia-Dominguez E, McIntyre RL, Lopez-Aceituno JL, Ballesteros-Gonzalez Á, Tapia-Gonzalez A, Fabregat-Safont D, Eisenberg T, Gomez J, Plaza A, Sierra-Ramirez A, Perez M, Villanueva-Bermejo D, Fornari T, Loza MI, Herradon G, Hofer SJ, Magnes C, Madeo F, Duerr JS, Pozo OJ, Galindo M-I, Del Pino I, Houtkooper RH, Megias D, Viña J, Gomez-Cabrera MC, Fernandez-Marcos PJ. Peripheral modulation of antidepressant targets MAO-B and GABAAR by harmol induces mitohormesis and delays aging in preclinical models. Nat Commun. 2023;14(1):2779. doi: 10.1038/s41467-023-38410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim S-A, Lee Y-M, Choi J-Y, Jacobs DR, Lee D-H. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ Pollut. 2018;233:725–734. doi: 10.1016/j.envpol.2017.10.124. [DOI] [PubMed] [Google Scholar]
- 246.Calabrese EJ, Calabrese V, Giordano J. The role of hormesis in the functional performance and protection of neural systems. Brain Circ. 2017;3(1):1–13. doi: 10.4103/2394-8108.203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ouellet-Morin I, Cantave C, Lupien S, Geoffroy M-C, Brendgen M, Vitaro F, Tremblay R, Boivin M, Côté S. Cumulative exposure to socioeconomic and psychosocial adversity and hair cortisol concentration: A longitudinal study from 5 months to 17 years of age. Psychoneuroendocrinology. 2021;126:105153. doi: 10.1016/j.psyneuen.2021.105153. [DOI] [PubMed] [Google Scholar]
- 248.Oshri A, Cui Z, Carvalho C, Liu S. Is perceived stress linked to enhanced cognitive functioning and reduced risk for psychopathology? Testing the hormesis hypothesis. Psychiatry Res. 2022;314:114644. doi: 10.1016/j.psychres.2022.114644. [DOI] [PubMed] [Google Scholar]
- 249.Keller A, Litzelman K, Wisk LE, Maddox T, Cheng ER, Creswell PD, Witt WP. Does the perception that stress affects health matter? The association with health and mortality. Health Psychol. 2012;31(5):677–684. doi: 10.1037/a0026743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Krittanawong C, Maitra NS, Khawaja M, Wang Z, Fogg S, Rozenkrantz L, Virani SS, Levin M, Storch EA, Tobler PN, Charney DS, Levine GN. Association of pessimism with cardiovascular events and all-cause mortality. Prog Cardiovasc Dis. 2023;76:91–98. doi: 10.1016/j.pcad.2022.11.018. [DOI] [PubMed] [Google Scholar]
- 251.Ahmadpanah M, Keshavarz M, Haghighi M, Jahangard L, Bajoghli H, Sadeghi Bahmani D, Holsboer-Trachsler E, Brand S. Higher emotional intelligence is related to lower test anxiety among students. Neuropsychiatr Dis Treat. 2016;12:133–136. doi: 10.2147/NDT.S98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9(3):455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 253.Dabin J, Mori M, Polo SE. The DNA damage response in the chromatin context: A coordinated process. Curr Opin Cell Biol. 2023;82:102176. doi: 10.1016/j.ceb.2023.102176. [DOI] [PubMed] [Google Scholar]
- 254.Mueller FS, Amport R, Notter T, Schalbetter SM, Lin H-Y, Garajova Z, Amini P, Weber-Stadlbauer U, Markkanen E. Deficient DNA base-excision repair in the forebrain leads to a sex-specific anxiety-like phenotype in mice. BMC Biol. 2022;20(1):170. doi: 10.1186/s12915-022-01377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Aditi null, McKinnon PJ. Genome integrity and inflammation in the nervous system. DNA Repair (Amst). 2022;119:103406. doi: 10.1016/j.dnarep.2022.103406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Czarny P, Bialek K, Ziolkowska S, Strycharz J, Sliwinski T. DNA damage and repair in neuropsychiatric disorders. What do we know and what are the future perspectives? Mutagenesis. 2020;35(1):79–106. doi: 10.1093/mutage/gez035. [DOI] [PubMed] [Google Scholar]
- 257.Pugsley K, Scherer SW, Bellgrove MA, Hawi Z. Environmental exposures associated with elevated risk for autism spectrum disorder may augment the burden of deleterious de novo mutations among probands. Mol Psychiatry. 2022;27(1):710–730. doi: 10.1038/s41380-021-01142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jorgensen A, Köhler-Forsberg K, Henriksen T, Weimann A, Brandslund I, Ellervik C, Poulsen HE, Knudsen GM, Frokjaer VG, Jorgensen MB. Systemic DNA and RNA damage from oxidation after serotonergic treatment of unipolar depression. Transl Psychiatry. 2022;12(1):204. doi: 10.1038/s41398-022-01969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Michel M, et al. Small-molecule activation of OGG1 increases oxidative DNA damage repair by gaining a new function. Science. 2022;376(6600):1471–1476. doi: 10.1126/science.abf8980. [DOI] [PubMed] [Google Scholar]
- 260.Singh G, Singh V, Schneider JS. Post-translational histone modifications and their interaction with sex influence normal brain development and elaboration of neuropsychiatric disorders. Biochim Biophys Acta Mol Basis Dis. 2019;1865(8):1968–1981. doi: 10.1016/j.bbadis.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 261.Qvist P, Christensen JH, Vardya I, Rajkumar AP, Mørk A, Paternoster V, Füchtbauer E-M, Pallesen J, Fryland T, Dyrvig M, Hauberg ME, Lundsberg B, Fejgin K, Nyegaard M, Jensen K, Nyengaard JR, Mors O, Didriksen M, Børglum AD. The Schizophrenia-Associated BRD1 Gene Regulates Behavior, Neurotransmission, and Expression of Schizophrenia Risk Enriched Gene Sets in Mice. Biol Psychiatry. 2017;82(1):62–76. doi: 10.1016/j.biopsych.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 262.Farrelly LA, Zheng S, Schrode N, Topol A, Bhanu NV, Bastle RM, Ramakrishnan A, Chan JC, Cetin B, Flaherty E, Shen L, Gleason K, Tamminga CA, Garcia BA, Li H, Brennand KJ, Maze I. Chromatin profiling in human neurons reveals aberrant roles for histone acetylation and BET family proteins in schizophrenia. Nat Commun. 2022;13(1):2195. doi: 10.1038/s41467-022-29922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Aberg KA, Dean B, Shabalin AA, Chan RF, Han LKM, Zhao M, van Grootheest G, Xie LY, Milaneschi Y, Clark SL, Turecki G, Penninx BWJH, van den Oord EJCG. Methylome-wide association findings for major depressive disorder overlap in blood and brain and replicate in independent brain samples. Mol Psychiatry. 2020;25(6):1344–1354. doi: 10.1038/s41380-018-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Protsenko E, Yang R, Nier B, Reus V, Hammamieh R, Rampersaud R, Wu GWY, Hough CM, Epel E, Prather AA, Jett M, Gautam A, Mellon SH, Wolkowitz OM. “GrimAge,” an epigenetic predictor of mortality, is accelerated in major depressive disorder. Transl Psychiatry. 2021;11(1):193. doi: 10.1038/s41398-021-01302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wertz J, Caspi A, Ambler A, Broadbent J, Hancox RJ, Harrington H, Hogan S, Houts RM, Leung JH, Poulton R, Purdy SC, Ramrakha S, Rasmussen LJH, Richmond-Rakerd LS, Thorne PR, Wilson GA, Moffitt TE. Association of History of Psychopathology With Accelerated Aging at Midlife. JAMA Psychiatry. 2021;78(5):530–539. doi: 10.1001/jamapsychiatry.2020.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liang W, Hou Y, Huang W, Wang Y, Jiang T, Huang X, Wang Z, Wu F, Zheng J, Zhang J, Ou H, Li S, Ping J, Zhang Y, Ye J, Li Z, Yang Q, Zhang J, Zheng X, Li S, Zhu X-H, Chen R, Zhao C. Loss of schizophrenia-related miR-501-3p in mice impairs sociability and memory by enhancing mGluR5-mediated glutamatergic transmission. Sci Adv. 2022;8(33):eabn7357. doi: 10.1126/sciadv.abn7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 268.Bohnsack JP, Zhang H, Wandling GM, He D, Kyzar EJ, Lasek AW, Pandey SC. Targeted epigenomic editing ameliorates adult anxiety and excessive drinking after adolescent alcohol exposure. Sci Adv. 2022;8(18):eabn2748. doi: 10.1126/sciadv.abn2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, Page CE, Sandoval K, Knox A, Connolly A, Huang EJ, Garcia-Verdugo JM, Oldham MC, Yang Z, Alvarez-Buylla A. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J Neurosci. 2021;41(12):2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Terreros-Roncal J, Moreno-Jiménez EP, Flor-García M, Rodríguez-Moreno CB, Trinchero MF, Cafini F, Rábano A, Llorens-Martín M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2021;374(6571):1106–1113. doi: 10.1126/science.abl5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Franjic D, Skarica M, Ma S, Arellano JI, Tebbenkamp ATN, Choi J, Xu C, Li Q, Morozov YM, Andrijevic D, Vrselja Z, Spajic A, Santpere G, Li M, Zhang S, Liu Y, Spurrier J, Zhang L, Gudelj I, Rapan L, Takahashi H, Huttner A, Fan R, Strittmatter SM, Sousa AMM, Rakic P, Sestan N. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022;110(3):452–469.:e14. doi: 10.1016/j.neuron.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tosoni G, Ayyildiz D, Bryois J, Macnair W, Fitzsimons CP, Lucassen PJ, Salta E. Mapping human adult hippocampal neurogenesis with single-cell transcriptomics: Reconciling controversy or fueling the debate? Neuron. 2023;111(11):1714–1731.:e3. doi: 10.1016/j.neuron.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 273.Berger T, Lee H, Young AH, Aarsland D, Thuret S. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer's Disease. Trends Mol Med. 2020;26(9):803–818. doi: 10.1016/j.molmed.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 274.Chikama K, Yamada H, Tsukamoto T, Kajitani K, Nakabeppu Y, Uchimura N. Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 2017;1676:77–82. doi: 10.1016/j.brainres.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 275.Molendijk ML, Spinhoven P, Polak M, Bus B a. A, Penninx BWJH, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 276.Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, Xiao J. Exercise sustains the hallmarks of health. J Sport Health Sci. 2023;12(1):8–35. doi: 10.1016/j.jshs.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gao Y, Shen M, Gonzalez JC, Dong Q, Kannan S, Hoang JT, Eisinger BE, Pandey J, Javadi S, Chang Q, Wang D, Overstreet-Wadiche L, Zhao X. RGS6 Mediates Effects of Voluntary Running on Adult Hippocampal Neurogenesis. Cell Rep. 2020;32(5):107997. doi: 10.1016/j.celrep.2020.107997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee C-H, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gubert C, Hannan AJ. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. 2021;20(11):862–879. doi: 10.1038/s41573-021-00217-1. [DOI] [PubMed] [Google Scholar]
- 280.Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369(6500):167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dias GP, Murphy T, Stangl D, Ahmet S, Morisse B, Nix A, Aimone LJ, Aimone JB, Kuro-O M, Gage FH, Thuret S. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol Psychiatry. 2021;26(11):6365–6379. doi: 10.1038/s41380-021-01102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Castner SA, Gupta S, Wang D, Moreno AJ, Park C, Chen C, Poon Y, Groen A, Greenberg K, David N, Boone T, Baxter MG, Williams GV, Dubal DB. Longevity factor klotho enhances cognition in aged nonhuman primates. Nat Aging. 2023. [DOI] [PMC free article] [PubMed]
- 283.Melgar-Locatelli S, de Ceglia M, Mañas-Padilla MC, Rodriguez-Pérez C, Castilla-Ortega E, Castro-Zavala A, Rivera P. Nutrition and adult neurogenesis in the hippocampus: Does what you eat help you remember? Front Neurosci. 2023;17:1147269. doi: 10.3389/fnins.2023.1147269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Uddin LQ. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat Rev Neurosci. 2021;22(3):167–179. doi: 10.1038/s41583-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tartt AN, Mariani MB, Hen R, Mann JJ, Boldrini M. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689–2699. doi: 10.1038/s41380-022-01520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang J, Liu D, Fu P, Liu Z-Q, Lai C, Yang C-Q, Chen K, Bao W-D, Hu F, Du H-Y, Yang W, Wang J, Man H-Y, Lu Y, Zhu L-Q. Social isolation reinforces aging-related behavioral inflexibility by promoting neuronal necroptosis in basolateral amygdala. Mol Psychiatry. 2022;27(10):4050–4063. doi: 10.1038/s41380-022-01694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Iram T, Kern F, Kaur A, Myneni S, Morningstar AR, Shin H, Garcia MA, Yerra L, Palovics R, Yang AC, Hahn O, Lu N, Shuken SR, Haney MS, Lehallier B, Iyer M, Luo J, Zetterberg H, Keller A, Zuchero JB, Wyss-Coray T. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature. 2022;605(7910):509–515. doi: 10.1038/s41586-022-04722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Enamorado M, Kulalert W, Han S-J, Rao I, Delaleu J, Link VM, Yong D, Smelkinson M, Gil L, Nakajima S, Linehan JL, Bouladoux N, Wlaschin J, Kabat J, Kamenyeva O, Deng L, Gribonika I, Chesler AT, Chiu IM, Le Pichon CE, Belkaid Y. Immunity to the microbiota promotes sensory neuron regeneration. Cell. 2023;186(3):607–620.:e17. doi: 10.1016/j.cell.2022.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Serafini G, Costanza A, Aguglia A, Amerio A, Placenti V, Magnani L, Escelsior A, Sher L, Amore M. Overall goal of Cognitive-Behavioral Therapy in Major Psychiatric Disorders and Suicidality: A Narrative Review. Med Clin North Am. 2023;107(1):143–167. doi: 10.1016/j.mcna.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 290.Köder F, Sharma C, Cameron S, Garraffa M. The effects of bilingualism on cognition and behaviour in individuals with attention deficits: A scoping review. Front Psychol. 2022;13:1057501. doi: 10.3389/fpsyg.2022.1057501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 292.Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2(Suppl 2)):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kaiser J. Next goal for U.S. initiative: A human brain atlas. Science. 2022;377(6613):1369. doi: 10.1126/science.ade9955. [DOI] [PubMed] [Google Scholar]
- 294.Axer M, Amunts K. Scale matters: The nested human connectome. Science. 2022;378(6619):500–504. doi: 10.1126/science.abq2599. [DOI] [PubMed] [Google Scholar]
- 295.Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20(2):200–221. doi: 10.1002/wps.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gómez-Carrillo A, Paquin V, Dumas G, Kirmayer LJ. Restoring the missing person to personalized medicine and precision psychiatry. Front Neurosci. 2023;17:1041433. doi: 10.3389/fnins.2023.1041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li M-Y, Birey F, Yang X, Saw NL, Baker SW, Amin ND, Kulkarni S, Mudipalli R, Cui B, Nishino S, Grant GA, Knowles JK, Shamloo M, Huguenard JR, Deisseroth K, Paşca SP. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610(7931):319–326. doi: 10.1038/s41586-022-05277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Campbell-Lendrum D, Neville T, Schweizer C, Neira M. Climate change and health: three grand challenges. Nat Med. 2023;29(7):1631–1638. doi: 10.1038/s41591-023-02438-w. [DOI] [PubMed] [Google Scholar]
- 299.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Fazel S, Runeson B. Suicide. N Engl J Med. 2020;382(3):266–274. doi: 10.1056/NEJMra1902944. [DOI] [PMC free article] [PubMed] [Google Scholar]