Abstract
Tumor immunity is a promising topic in the area of cancer therapy. The ‘soil’ function of the tumor microenvironment (TME) for tumor growth has attracted wide attention from scientists. Tumor-infiltrating immune cells in the TME, especially the tumor-infiltrating lymphocytes (TILs), serve a key role in cancer. Firstly, relevant literature was searched in the PubMed and Web of Science databases with the following key words: ‘Tumor microenvironment’; ‘TME’; ‘tumor-infiltrating immunity cells’; ‘gynecologic malignancies’; ‘the adoptive cell therapy (ACT) of TILs’; and ‘TIL-ACT’ (https://pubmed.ncbi.nlm.nih.gov/). According to the title and abstract of the articles, relevant items were screened out in the preliminary screening. The most relevant selected items were of two types: All kinds of tumor-infiltrating immune cells; and advanced research on TILs in gynecological malignancies. The results showed that the subsets of TILs were various and complex, while each subpopulation influenced each other and their effects on tumor prognosis were diverse. Moreover, the related research and clinical trials on TILs were mostly concentrated in melanoma and breast cancer, but relatively few focused on gynecological tumors. In conclusion, the present review summarized the biological classification of TILs and the mechanisms of their involvement in the regulation of the immune microenvironment, and subsequently analyzed the development of tumor immunotherapy for TILs. Collectively, the present review provides ideas for the current treatment dilemma of gynecological tumor immune checkpoints, such as adverse reactions, safety, personal specificity and efficacy.
Keywords: TME, TILs, gynecological malignancies, TIL-ACT
1. Introduction
With the development of tumor biology, tumor immunotherapy has gradually become the fourth emerging cancer treatment strategy after surgical resection, chemotherapy and radiotherapy (1). The tumor microenvironment (TME) refers to the local steady-state environment closely related to tumorigenesis, which is mainly composed of tumor cells, immune cells, endothelial cells, a variety of stromal cells and chemokines (1). As the ‘soil’ of tumor growth, the TME is involved in the occurrence, development and chemotherapy resistance of tumors (2). Increasing research on immune checkpoint-related antibody drugs has considered the immune cells in the TME as therapeutic targets, achieving the purpose of killing tumor cells by mobilizing the immune system of the patient (3,4).
On June 12, 2018, the U.S. Food and Drug Administration (FDA) approved the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab as a second-line treatment for programmed death-ligand 1 (PD-L1)-positive advanced and recurrent cervical cancer (5). The clinical practice guidelines for cervical, uterine and ovarian cancer issued by the National Comprehensive Cancer Network in 2018 also recommend the application of pembrolizumab (6). This indicated the formal arrival of the era of immunotherapy in the field of gynecological oncology. Immune checkpoint-related antibody drugs, including PD-L1, have achieved impressive therapeutic effects, although their efficacy is highly hindered by the existence of immunosuppression, tolerance and ineffective activation of an antitumor immune response (7). In terms of therapeutic effects, the survival and prognosis of patients with advanced cervical cancer, endometrial cancer and ovarian cancer have been improved to a certain extent. Compared with conventional radiotherapy and chemotherapy, immunotherapy can achieve precise treatment and avoid adverse reactions (8). For example, the efficacy of immune checkpoint inhibitors in the treatment of cervical cancer has been reported to range between 10 and 30%, whereas the 5-year survival rate of immune checkpoint inhibitor-treated patients with ovarian cancer was <50% and the survival rate was not greatly improved, compared with patients not treated with immunotherapy. In the treatment of endometrial cancer, problems, such as the small sample size of clinical trials and different inclusion criteria, have not yet been overcome (9,10).
In solid tumors, tumor-infiltrating lymphocytes (TILs) are usually the most abundant component of the infiltrated immune cells (11). In the TME, TILs are a heterologous group of immune cells in the tumor parenchyma and stroma. Under the influence of different cellular activation mechanisms and cytokines, TILs produce different immune responses, which can directly reflect the local immune response of the immune system to tumors (12).
Based on the aforementioned findings, relevant literature was searched according to the following key words: ‘Tumor microenvironment’; ‘TME’; ‘tumor-infiltrating immunity cells’; ‘gynecologic malignancies’; ‘the adoptive cell therapy (ACT) of TILs’; and ‘TIL-ACT’ in the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science databases (https://www.webofscience.com/). The present review subsequently focused on various TILs and advanced research on TILs in gynecological malignancies.
In the present review, the biological classifications of TILs, the mechanisms of their involvement in the regulation of the tumor immune microenvironment, as well as their association with the development and prognosis of gynecological tumors are discussed. Subsequently, tumor immunotherapy for TILs and their prognostic benefits were considered, which provides ideas for the current treatment dilemma of gynecological tumor immune checkpoints.
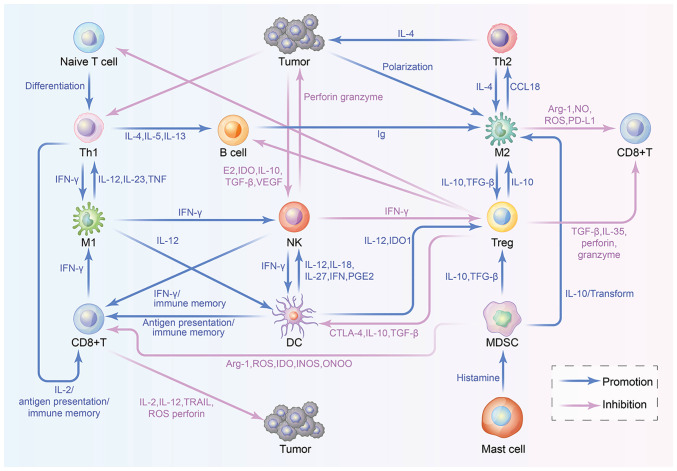
A description of the immune cells in the TME is shown in Table I. In addition, the crosstalk between immune cells in the TME is shown in Fig. 1 and each step is explored within the present review.
Table I.
Overview of immune cells in the tumor microenvironment.
| A, CD4+ T cells | |||||
|---|---|---|---|---|---|
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Szabo et al, 1997; Xu et al, 1998; Sallusto et al, 1998; Loetscher et al, 1998; Austrup et al, 1997 | Th1 | IFN-γ, IL-2, TNF-β | IL-12β2R, IL-18R, CXCR3, CXCR5, selectin ligand | Immune promotion, antitumor, pro-inflammatory cytokines | (16-20) |
| Journad et al, 1998; Sallusto et al, 1998; Zingoni et al, 1999; Nagata et al, 1999 | Th2 | IL-4, IL-5, IL-9, IL-10, IL-13, IL-25, dimodulin | CXCR4, CCR3, CCR8, CRTH2 | Immunosuppression, pro-tumor, anti-inflammatory cytokines | (73-77) |
| Hori et al, 2003; Kondrack et al, 2003; Dilek et al, 2013; Cohen et al, 2010; Hemon et al, 2011 | Tregs | IL-10, TGFβ, IL-35 | FoxP3, CD127, CTLA-4, GITR, LAG-3 | Immunosuppression, pro-tumor | (83-87) |
| B, CD8+ T cells | |||||
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Wongtrakoongate, 2015 | CD8+ T | IL-2, IL-12, IFN-γ | CD3+, CD8+ | Immune promotion, antitumor, cytotoxic effects | (38) |
| C, Macrophages | |||||
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Badylak et al, 2008; Spiller et al, 2014; Gordon et al, 2014; Wilson et al, 2014; Stöger et al, 2012 | M1 | IL-12, IL-23, IL-1β, IL-6, IL-12, IL-23, CCL10, CCL11, CCL2-5, CCL8, CCL9 | CD80, CD86, VEGF, SOCS3, CXCR7 | Immune promotion, antitumor, pro-inflammatory cytokines, cytotoxic effects | (28-32) |
| Vasconcelos et al, 2015; Porta et al, 2015 | M2 | IL10, IL12, CCL18 | CD163, IL10, SOCS1/2, CD206, CCL18, PDGF-BB, MMP | Immunosuppression, pro-tumor | (98,99) |
| D, NK cells | |||||
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Campbell et al, 2013; Caligiuri et al, 2008 | CD56brightNK | IFN-γ, TNF-α | CD16+/-, CD56, NKG2A, CCR7, CXCR, CXCR3 | Depends on the tumor type | (60,61) |
| CD56dimNK | IL-22, IL-10 | CD16hi, perforinhi | Depends on the tumor type | ||
| E, DCs | |||||
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Swiecki and Colonna, 2015; Guilliams et al, 2016; Ma et al, 2009 | cDC1 | IL-12, TNF-α, IFN-γ | TLR7, TLR9, RLRs | Depends on the tumor type | (45-47) |
| cDC2 | TGF-β, IL-6, IL-8, IL-1, IL-12, IL-23, IL-10, TNF-α | TLR1, TLR3, TLR6 | Depends on tumor type | ||
| pDCs | Type 1 IFN, TNF, IL-6 | CD11c, HLA-DR, CD304, CD303A | Depends on tumor type | ||
| MoDCs | TNF-α, IL-1, IL-12, IL-23 | CD11c, MHC-II+, CD11b+ | Immunepromotion antitumor Antigen recognition Th1 polarization | ||
| Tolerogenic DCs | TGF-β | CD11c, D14, Factor XIIIA, HLA-DR, CD62L, XCR3, D209, D1c, D80, D86, D64, AR-1 | Immunosuppression pro-tumor | ||
| F, MDSCs | |||||
| First author, year | Cell type | Cytokine/chemokine secretion | Markers | Effect | (Refs.) |
| Greten et al, 2011; Filipazzi et al, 2012 | PMN-MDSCs | NO, CCL3, CCL4, CCL5, Arg1, PGE2, IL4 | CCR2, CXCR4, CD11b+, CD13, CD15+, CD14- | Immunosuppression, pro-tumor, Treg promotion | (110,111) |
| M-MDSC | ROS, Arg1, PGE2, IL4 | CCR2, CXCR4, CD11b+, CD13, CD15-, CD14+ | |||
Figure 1.
Workflow of crosstalk between immune cells in the TME. TILs within the TME include T lymphocytes, NK cells, DCs, macrophages and MDSCs, which regulate the tumor immune microenvironment and influence the proliferation of tumor cells via positively and negatively interacting with each other.
2. An overview of TILs
TILs are heterogeneous lymphocytes existing in the TME, including T lymphocytes, natural killer (NK) cells, dendritic cells (DCs), macrophages and myeloid-derived suppressor cells (MDSCs), which play various roles in shaping the tumor immune microenvironment (13). Understanding the characteristics and functions of immune cells is pivotal for targeting immune cells to reshape the TME to improve the antitumor effect of immunotherapy in the future.
Depending on the roles of TILs in the TME, they can be approximately divided into two types of cell subsets, which include immune cells that positively regulate the immune response [e.g. CD4+ T helper (Th)1 cells, CD8+ cytotoxic T cells (CTLs) and NK cells] and those that negatively regulate the immune response [e.g. tumor-associated macrophages (TAMs), regulatory T cells (Tregs) and MDSCs]. The former cell subset can recognize, kill and clear tumor cells to achieve a tumor immune response, whereas the latter subset secrete a large number of immunosuppressive factors and inhibit activation of the former subset, thus allowing tumor cells to escape immune surveillance, and leading to tumor formation, invasion and metastasis (14). The present review describes the aforementioned cell subtypes in detail from three aspects: Surface markers; functions; and crosstalk with other cells. Unfortunately, some of these aspects remain unclear due to the current immaturity of this research field.
Positive regulatory immune cells. Th1 cells
CD4+ T cells, as major organizers of cellular immunity, participate in all stages of the immune response. They differentiate into different Th subsets, including Th1 and Th2 cells, and present tumor antigen peptides on target cells (e.g. DCs and tumor cells) through the interaction between the T-cell receptor (TCR) and the major histocompatibility complex (MHC) class II molecules (15). It is well known that the surface markers of Th1 cells include interleukin (IL)-12 receptor β2 (β2R) (16), IL-18 receptor (IL-18R) (17), chemokine receptor (CXCR)3(18), CXCR5(19) and selectin ligand (20). Moreover, Th1 cells mainly mediate cellular immunity and function in tumor differentiation, immune regulation and development (21). The antitumor immune mechanisms are summarized as follows: i) Production of interferon-γ (IFN-γ) and IL-2 to activate CD8+ T cells and NK cells, as well as promotion of cellular immunity; and ii) activation of antigen-presenting cells (APCs) and induction of antibody production to further enhance tumor cell uptake of APCs (22). Evidence has shown that the differentiation and function of Th1 cells are closely related to those of other immune cells in the TME. For instance, DCs produce a large amount of IL-12, promoting CD4+ T cells to differentiate into Th1 cells and triggering a robust Th1 immune response (23). Similar to DCs, B cells and TAMs have been confirmed to enhance the differentiation of Th1 cells (24,25), whereas Tregs mediate immunosuppression by inhibiting the differentiation of Th1 cells (26).
M1 macrophages
TAMs differentiate under different conditions into two different subtypes, namely M1 and M2 macrophages. When macrophages are exposed to inflammatory cytokines produced by Th1 cells in the TME, such as tumor necrosis factor α (TNF-α) and IFN-γ, these differentiate into proinflammatory M1 macrophages (27) presenting several surface markers, such as CD80(28), CD86(29), vascular endothelial growth factor (VEGF) (30), suppressor of cytokine signaling 3 (SOCS3) (31) and CXCR7(32), useful to distinguish between M1 and M2 macrophages. Regarding their function in the TME, proinflammatory M1 macrophages stimulate the activation of mature T cells and amplify the Th1 response, thus exerting efficient antitumor activity (33). In addition, M1 macrophages are regulated by immune cells. Th1 cells and CD8+ T cells can induce M1 polarization of macrophages by secreting IFN-γ (34). However, due to the lack of in-depth research on M1 macrophages, the mechanism by which immune cells regulate M1 macrophages in the TME remains unclear.
CD8+ T cells
After tumor invasion, immature CD8+ T cells differentiate into effector CD8+ T cells under the influence of antigen peptide-MHC, cytokines and costimulatory signals produced by APCs (35), as well as the secretion, metabolism, epigenetic modification and transcription factors of extracellular cytokines (36). Furthermore, effector CD8+ T cells differentiate into cytotoxic and memory CD8+ T cells (37). The specific surface markers of CD8+ T cells include CD3+ and CD8+. Notably, CTLs in the TME can produce IL-2, IL-12 and IFN-γ, demonstrating the cytotoxic function of CD8+ T cells and mediating antitumor immunity by producing TNF-related apoptosis-inducing ligands, reactive oxygen species (ROS) and perforin (38). In return, CD8+ T cells can also be modulated by various immune cells in the TME. DCs can cross-present exogenous tumor-associated antigens to MHC-I molecules to activate CD8+ T cells, and participate in the regulation of CD8+ T-cell immunity and tumor antigen tolerance (39). In addition to the stimulatory signals derived from DCs, CD4+ T cells are also a necessary condition for the initiation of CTLs. CD4+ T cells directly promote the activation of CD8+ T cells and develop CD8+ T cells to memory CD8+ T cells by the interaction with CD40-CD40 ligand (40) and costimulatory molecule combinations (41).
DCs
DCs, known as the most powerful professional APC, activate primitive antigen-specific CD4+ and CD8+ T cells by ingesting, processing and presenting antigens, thus initiating adaptive immune responses. DCs originate from macrophages/DC progenitor cells in the bone marrow and then differentiate into DC subsets, including classical DCs (cDCs), plasma cell-like DCs (pDCs) and monocyte-derived inflammatory DCs (MoDCs) (42). cDCs can be further divided into cDC1s and cDC2s. The development of cDC1s is dependent on IFN regulatory factor 8 and basic leucine zipper transcription factor ATF-like 3, and they specifically present internalized exogenous antigens to MHC-I to activate CD8+ T cells (43). cDC2s depend on IFN regulatory factor 4 development, presenting internalized antigens on MHC-II to activate CD4+ T cells (44). pDCs are a multifunctional population that produces large amounts of type I IFN (45).
There are also differences in DC surface markers among the different subsets: cDC1s are characterized by TLR7, TLR9 and RIG-I-like receptors; cDC2s are characterized by TLR1, TLR3 and TLR6; pDCs are characterized by CD11c, human leukocyte DR antigen (HLA-DR), CD304 and CD303A; whereas MoDCs are characterized by CD11c+, MHC-II+ and CD11b+ (45-47). Studies have confirmed that DCs strengthen immune function in the TME through a series of mechanisms: i) Receptors identify DCs to activate T cells and regulate CD4+ T-cell differentiation (48); ii) DCs induce the transformation of effector T cells into memory T cells (49); and iii) DCs activate CD8+ T cells, Tregs and NK cells by secreting an array of cytokines and metabolism-related enzymes, such as IL-2, IL-12(50) and indoleamine-2 dioxygenase (IDO1) (51). However, since tumors of different tissues and stages have unique features, DC function will also change in different TMEs, which may suppress tumor-specific immunity under certain conditions (52).
Although DCs promote the antitumor immune effect of the TME, DCs in the TME are often functionally impaired or defective, and various cytokines and cells can inhibit their antitumor effects in the TME As follows: i) IL-6, IL-10, VEGF and transforming growth factor-β (TGF-β) can negatively regulate the function of DCs and induce a tolerant phenotype by inhibiting their maturation and migration (53-57); and ii) Tregs exhibit inhibitory effects on DCs, either by downregulating the expression of the costimulatory molecules CD80, CD86 and CTL-associated protein 4 (CTLA-4) on DCs (58), or by releasing cytokines, such as IL-10 and TGF-β (59).
NK cells
NK cells are innate immune effector cells. Currently, two different NK cell subsets have been identified: i) CD56bright NK; and ii) CD56dim NK cells. Under the action of IL-12, IL-15 and IL-18, they can secrete cytokines and chemokines, and participate in the regulation of acquired immunity (60). Regarding surface markers, NK cells lack the phenotypic markers of B (CD19-) and T (CD3-) lymphocytes, but they can be defined by their unique CD56+ state (61). Notably, unlike T cells that recognize target cells in an MHC-restricted manner through TCRs, NK cells recognize target cells through the activation and inhibition signal combinations of cell surface receptors, thus playing an antitumor immune role (62). Briefly, the NK cells operate through the following mechanisms: i) NK-activated receptors, as well as the natural cytotoxic receptors NKp30, NKp44 and NKp46, mediate the release of intracellular perforin and granzyme from NK cells after activation, which directly kill tumor cells (63); ii) NK cells regulate the dynamic balance of the immune system by secreting cytokines and chemokines such as IFN-γ to promote the activation and effector function of CTLs and restrain Treg functions by increasing their activity (63,64); and iii) NK cells have memory, self-renewal, long-term proliferation and persistence abilities in vivo and memory NK cells show stronger tumor-specific cytotoxicity, compared with naive NK cells (65). It is worth noting that, similar to DCs, NK cells may play different tumor immune roles in different situations (66). Compared with NK cells in healthy non-tumor tissues, tumor-infiltrating NK cells exhibit weaker cytotoxic activity, accompanied by the downregulation of activated receptors and upregulation of inhibitory receptors. These observations suggest that the function of NK cells is impaired by the following immune cells in the TME (67): i) Tregs and MDSCs, which can secrete TGF-β to directly undermine the killing ability of NK cells, or indirectly inhibit the killing effect mediated by NK cells by reducing the expression of the activated receptors NKG2D and NKp30 through membrane binding to TGF-β (68-70); and ii) similar to Tregs and MDSCs, tumor-associated fibroblasts and tumor cells can secrete IDO, prostaglandin E2 and TGF-β to reduce the expression of NKG2D in NK cells to restrain the cytotoxicity of NK cells (71,72).
Negative regulatory immune cells. CD4+ Th2 cells
CD4+ Th2 cells are a type of CD4+ T cell that induce B cells to differentiate into antibody-secreting cells to participate in the humoral immune response, secreting a variety of cytokines, such as IL-4, IL-5, IL-9, IL-10, IL-13, IL-25 and dimodulin, to participate in the antitumor immune response in vivo. Different from Th1 cell surface markers, Th2 cell surface markers include CXCR4(73), C-C motif chemokine receptor (CCR)3(74), CCR8(75) and chemokine receptor homologous molecule 2(76). The role of Th2 cells in the TME is also opposite to that of Th1 cells. The number of Th2 cells infiltrated by the tumor stroma is significantly higher than that of Th1 cells, resulting in Th1/Th2 drift. The resulting immunosuppressive state seriously affects antitumor immunity, eventually leading to the occurrence and development of tumors (77). In detail, the secretion of the cytokines IL-4, IL-5 and IL-25, as well as the transcription factor GATA-3 by Th2 cells contribute to the increase in cytokine cascades, therefore inhibiting the proliferation of Th1 cells, DCs and eosinophils, as well as regulating the inflammatory response of mast cells and lymphocytes (78-80). However, it has been speculated that consistent with Th1 cells, immune cells and factors in the TME can affect the differentiation and function of Th2 cells based on the results of the current research, although the specific regulatory mechanism remains unclear (81).
Tregs
Tregs, also known as suppressor T cells, are a subgroup of CD4+ T cells, characterized by the expression of CD4, CD25 and forked/winged helix transcription factors (Foxp3) (82). Human Tregs can be classified according to the expression levels of Foxp3 and CD45RA: i) FoxP3loCD45RA+CD25lo-immature Tregs; ii) FoxP3hiCD45RA-CD25hi-effect Tregs; and iii) FoxP3loCD45RA-CD25lo-non-Tregs. Tregs mainly express a variety of activated cell surface markers, including Foxp3(83), CD127(84), CTLA-4(85), glucocorticoid-induced tumor necrosis factor receptor (86) and lymphocyte-activation gene 3 (LAG-3) (87).
Extensive research has been performed on Tregs, demonstrating the protumor effect of Tregs on the TME (88,89). The effects of Tregs are summarized as follows: i) Significant expression of CTLA-4 and LAG-3 on Tregs inhibits the proliferation of T cells and the release of cytokines, thus suppressing the maturation of T cells (90,91); ii) Treg-derived perforin and granzyme inhibit CTLs in the TME and prevent the death of tumor cells (92); iii) secretion of IL-10, IL-35 and TGF-β restrains the proliferation and optimal activation of T cells (93-95); and iv) Tregs directly inhibit the proliferation of B cells and increase cell death through cell-cell contact (96).
Tregs, as the main cells mediating immunosuppression in the tumor microenvironment, are also regulated by other immune cells; i) DCs promote the development of Tregs by presenting polypeptide-MHC to TCRs, and secreting IL-2 and IDO1, which play important immunosuppressive functions (50,51); and ii) MDSCs can transform antigen-specific CD4+CD25- cells into CD4+CD25-Foxp3 Tregs and mediate immunosuppression in the presence of IFN-γ and IL-10(97).
M2 macrophages
As mentioned previously, M2 macrophages are one of the subtypes of TAMs. Macrophages exposed to Th2 mediators (IL-4, IL-13, IL-10 and TGF-β) in the TME have an immunosuppressive phenotype, namely M2 macrophages (27). The M2 phenotype is triggered by a series of different transcription factor cascades, including interferon regulatory factor (IRF)/STAT family members (IRF4, STAT3 and STAT6), inhibitory NF-κB homodimer and hypoxia inducible factor 2, the surface markers of which are composed of CD163, IL-10, SOCS1/2, CD206, CCL-18, PDGF-BB and MMP (98,99). According to the current understanding, M2 macrophages can regulate the TME and mediate immune escape through the following mechanisms: i) By secreting factors, such as IL-10, IL-12 and CCL18(100), promoting the expression of STAT3, and inducing the release of Th2 cytokines, thus regulating immunosuppressive activity (101,102); and ii) by producing metabolic enzymes, such as human arginase 1 and nitric oxide synthase 2, undermining the T-cell immune response (103,104).
In recent years, it has become clear that TAMs differentiate and change under the regulation of the TME, such as through the secretion of tumor-related molecules by tumor cells, metabolic changes in the TME and the presence of other immune cells. The TME promotes the polarization of M2 macrophages via: i) IL-4 secreted from Th2 cells, IL-10 secreted from Tregs and immunoglobulin secreted from B cells; and ii) the regulation of Tregs and MDSCs (105,106). Tregs inhibit the secretion of IFN-γ by CD8+ T cells, thus preventing the metabolism of M2 macrophage fatty acids, and indirectly but selectively maintain the metabolic adaptability and survival rate of M2 macrophages (107), whereas MDSCs stimulate M2 macrophage differentiation and promote tumor proliferation by downregulating STAT3(108).
MDSCs
MDSCs are a specialized population of immature heterogeneous cells, consisting of myeloid cell precursors, immature granulocytes, monocytes and DCs. MDSCs can be divided into two types: Polymorphonuclear cells and monocytes, which have relatively common surface markers and the same immunosuppressive function. Generally, they aggregate in the TME to secrete a variety of cytokines and mediate immunosuppression (109). Human MDSCs express the myeloid common surface markers CD11b and CD33, but do not express the MHC class II molecule HLA-DR or mature myeloid or lymphocyte cell surface markers (110). However, MDSCs exhibit different phenotypes and functions in different patients with cancer (111). In the TME, MDSCs suppress the immune microenvironment by regulating the release of cytokines and inhibiting immune responses through several mechanisms: i) The MDSC surface receptor TGF-β mediates the downregulation of IFN-γ and the NK-cell receptor NKG2D, to reduce the cytotoxicity of NK cells, thus restraining the function of NK cells (112,113); ii) MDSCs produce IL-10 to promote the polarization of macrophages to the M2 phenotype and can differentiate into TAMs (114); iii) metabolic factors, such as a decrease in arginine (115) and cysteine levels (116), and increased NO (117), ROS and peroxynitrite (118,119), which are derived from MDSCs, have a negative impact on immune activity inside the TME, thus regulating T-cell function (120); iv) MDSCs can also prevent T cells from homing to the lymph, thus affecting their antigen stimulation and inhibiting activation, resulting in tumor-specific CD4+/8+ T cells that are unable to respond effectively to tumor antigens (121); and v) can induce the production of Tregs by transforming antigen-specific CD4+CD25- cells into CD4+CD25-Foxp3 Tregs and mediating immunosuppression in the presence of IFN-γ and IL-10(97). Furthermore, MDSCs can be modulated by other immune cells in the TME. It has been found that mast cells can enhance the immunosuppressive function of MDSCs by secreting histamine (122), but the specific regulatory mechanisms of other immune cells remain unclear and further research is needed.
In summary, TILs can exert an immune response in several ways, which can not only promote antitumor immune effects but also enhance immunosuppression to aid tumor cell immune escape. In retrospect, the research on TILs in solid tumors, such as melanoma, colorectal cancer and breast cancer, has been more extensive, but there are few related studies on gynecological malignant tumors, such as ovarian cancer, endometrial cancer and cervical cancer. Moreover, immunotherapy for gynecological malignant tumors is facing new dilemmas regarding personal specificity, effectiveness and, adverse effects. Therefore, the present review focused on the association between TILs and the clinical prognosis of gynecological tumors, as well as the mechanism of the immune response. Based on the aforementioned summary, immunotherapy will hopefully be developed further in the future to improve the survival prognosis of patients.
3. TILs and gynecological malignancies
Based on the overview of each subpopulation of TILs, therapeutic strategies targeting TILs may be considered very promising in the treatment of gynecological malignancies. The theoretical basis is as follows: i) Gynecological malignant tumor cells express tumor-associated antigens that can be recognized by TILs; ii) the content of TILs in the TME, especially CD8+ T lymphocytes, has been demonstrated to be positively associated with patient prognosis (123); and iii) T lymphocytes can recognize the signaling peptides/MHC expressed by tumor cells (124). However, the progress of clinical research on TILs in gynecological malignancies is restricted due to the inconsistent preparation techniques of TILs, which requires the development of future technology. The present review describes the limited research progress into different TIL subtypes in various gynecological malignancies is reviewed.
To the best of our knowledge, MDSCs have not been widely studied in ovarian cancer. A 2013 study revealed a significant negative association between increased MDSCs and overall, as well as disease-free, survival in patients with ovarian cancer (125). Horikawa et al (126) demonstrated in a mouse model that MDSCs can inhibit local immunity and promote the development of ovarian cancer. In endometrial cancer tissues, MDSC population expression has been shown to be markedly increased, illustrating that MDSCs are also involved in the occurrence and progression of endometrial cancer (127). In addition, the number of MDSCs in the peripheral blood of patients with cervical cancer has been demonstrated to be significantly higher than that in healthy individuals. Moreover, the greater the number of MDSCs, the worse the response to platinum chemotherapy and radiotherapy (128), which is consistent with the research results in ovarian cancer.
A previous study has shown that in patients with ovarian cancer (129), Th2-type cytokines have obvious advantages over Th1-type cytokines and the immune response of the body is biased toward the Th2-type, which may serve a pivotal role in the pathogenesis of ovarian cancer. Similarly, in the serum of patients with endometrial cancer, an increase in Th2 cytokines (IL-4 and IL-5), a decrease in the Th1 cytokine (TNF-α) and a reduction in the Th1/Th2 ratio have been reported to return to normal following surgical resection. Moreover, with the increase in aggressiveness and malignancy, a significant decrease in Th1 content and IL-2 response rate was detected in patients with cervical cancer (130). All of these studies indicated that the immune status of patients with gynecological malignant tumors is mainly based on the increase in Th2 type, which can inhibit the antitumor immune ability of Th1 cytokines, thus leading to the occurrence and development of cancer.
Regarding Tregs, studies have demonstrated that CD4+CD25+ Tregs are negatively correlated with ovarian cancer progression and prognosis, and aggregate in blood, lymph nodes and local tumors (131). Meanwhile, the higher the proportion of Tregs, the worse the prognosis of chemotherapy, suggesting that Treg levels may help to assess the sensitivity of patients to chemotherapy drugs (132). In studies related to cervical cancer, there were obvious differences in the number of CD4+CD25+CD127-Tregs among healthy women, patients with cervical intraepithelial neoplasia (CIN), patients with different grades of cancer, and patients with or without lymphatic metastasis. These findings suggested that the malignant degree and prognosis of cervical cancer may be related to the infiltration degree of Tregs (133,134). While regulating immune overreaction, Tregs inhibit immune effects to form immune tolerance and promote the further deterioration of tumors.
Various studies have demonstrated the pro-oncogenic role of TAMs in gynecological malignancies. Upregulation of cytokines, such as IL-6 and IL-10, produced by TAMs in ovarian cancer tissues was revealed to be associated with higher tumor grade and poor prognosis (135). Patients with a high TAM M1/M2 ratio had longer survival (136) and CD163+ M2 TAMs were an independent factor in poor prognosis (137). In endometrial cancer, the levels of CD68+M2 TAMs were shown to be associated with myometrial invasion, microvascular density, angiogenesis, lymphovascular invasion and lymph node metastasis, as well as higher FIGO stage and histological grade (138). Likewise, in cervical cancer, TAMs were related to human papillomavirus (HPV) infection, the grade of the lesion in CIN, lymphatic metastasis and chemotherapy reactivity (139,140).
NK cells are an integral part of the innate immune surveillance system and play an essential role in the host defense system. Changes in the number and function of NK cells may affect the ability of intraperitoneal tumor cells to proliferate and spread in patients with advanced ovarian cancer, as reported in a 1997 study (141). Enhanced infiltration was again shown to predict a favorable prognosis in patients in 2015(142). An increased number of activated NK cells in endometrial cancer is associated with better overall survival (OS) of patients, while a decrease in NK cell subsets contributes to disease progression (143).
The presence of pDCs, the most abundant subgroup of DCs in ovarian cancer and malignant ascites, has been reported to be correlated with early recurrence and may serve as a prognostic marker for high-grade serous ovarian cancer (144). Early and highly differentiated endometrial carcinoma tissues have been shown to contain large numbers of tumor-infiltrating DCs (TIDCs), which can induce an intense antitumor immune response. The decrease in the number of infiltrating TIDCs and the absence of antigen presentation function may contribute to the occurrence of immune escape in endometrial cancer (145). Compared with in normal tissues, the number of CD1A+ and S100+ DCs in cervical cancer tissues have been shown to be significantly decreased, and to be negatively correlated with clinical stage, degree of malignancy and distant metastasis. This conclusion is also applicable to cervical cancer: the more DC infiltration there is, the better the patient prognosis (146).
In terms of CD8+ T cells, high ratios of CD8+/CD4+ and CD8+/Treg cells have been shown to predict a higher survival rate in patients with ovarian cancer (147). This conclusion was also identified in endometrial cancer. High CD8+ T-cell density was revealed to be an independent prognostic factor in endometrial (148) and cervical cancer (149). The 5-year survival rate was significantly improved in patients with cervical cancer with CD8+ TILs compared with that in patients without CD8+ TILs, and it also served as an independent prognostic predictor (150).
In conclusion, the existence of TILs is closely related to the development and prognosis of gynecological malignancies, which is crucial to the regulation of host immune function and local immune state.
4. Targeting of TILs to treat gynecological malignancies
The human immune system is capable of recognizing and eliminating damaged, infected and cancerous cells under normal conditions. This precise clearance is due to killer T cells, a type of immune cell that can recognize antigenic markers on the surface of diseased cells. In the early cancer stages, the immune system attacks the tumor by mobilizing TILs. In the 1980s, Rosenberg first demonstrated the antitumor activity of TILs in a mouse model and perceived that TILs were the strongest subtype of immune cells penetrating the tumor (151). However, since PD1 is expressed in the TME, the function of TILs is depressed and they cannot kill tumor cells effectively. Accordingly, it is considered that the antitumor effect of TILs can be enhanced by enriching and expanding TILs in tumor tissues in vitro and then transplanting them back to patients. Therefore, adoptive cell therapy (ACT) of TILs has emerged (152,153).
TIL-ACT for the treatment of gynecological malignant tumors
TIL-ACT takes advantage of the natural ability of TILs to recognize and eliminate tumor cells. The main steps include isolation, induction of differentiation, modification, amplification and infusion back into the body (154). Rather than simply amplifying T cells, it identifies T cells that effectively target specific mutations in different patients, and then screens and amplifies them. This strategy not only activates antitumor immunity in the host but also induces a more targeted and specific immune response.
To improve efficacy, in previous studies, patients have received lymphodepleting chemotherapy prior to infusion, which is designed to consume endogenous T cells and Tregs, and to provide the optimal environment for injected TILs over competing cell populations. This type of steady-state expansion ensures that TILs have persistent stability (155,156). In addition, to further activate TILs, patients are given a high dose of IL-2 intravenously until maximum tolerance is achieved (157).
The presence of TILs in tumor tissues has been associated with clinical outcomes in several tumor types, particularly in melanoma, in which TIL-ACT was revealed to be highly successful in phase I/II clinical trials (158). In addition, different degrees of studies have been performed in nonmelanoma types of cancer, including cervical (159), lung (160), ovarian (161), triple-negative breast (162) and gastrointestinal cancer (163). In all of these studies, TILs have been shown to affect prognosis (155) and studies have also proposed to use TILs as potential prognostic markers (164). Nevertheless, due to the presence of a high mutation load and high neoantigen rates, the efficacy of TIL-ACT therapy is highly variable (165). Therefore, further research and reliable clinical trials on TIL-ACT are warranted.
As early as 1991, Aoki et al (166) used TIL-ACT to treat seven patients with advanced or recurrent epithelial ovarian cancer and found a high response rate. Notably, it was considered that TIL-ACT combined with cisplatin holds promise for improving cure rates and long-term survival. Another clinical study in 1995 verified this conclusion, demonstrating that TIL-ACT may be a promising method for achieving a complete cure for advanced epithelial ovarian cancer (167). A 2015 review on TILs in ovarian cancer concluded that combining other therapies, such as immune checkpoint inhibitors, can better improve the clinical efficacy of TIL-ACT (168). In 2017, a large trial evaluating TILs in ovarian epithelial neoplasms demonstrated that higher levels of intratumor TILs were associated with improved prognosis and may serve as a clinically useful immunological prognostic indicator (161).
In a study of cervical cancer, researchers administered TIL-ACT to patients with HPV E6 and E7 reactivity, and found permanent regression of metastatic cervical cancer (154). More importantly, in a phase II clinical trial, 27 patients with advanced cervical cancer were treated with a novel TIL-ACT, LN145, and achieved 85% disease control and 44% objective response rates, without any serious side effects. The efficacy of LN145 in the treatment of advanced cervical cancer is promising compared with the response rate of chemotherapy or immunotherapy, which ranges between 4 and 14% for second-line treatment. LN145 has been approved by the FDA as a breakthrough therapy for advanced cervical cancer (169). As for ovarian cancer, a clinical trial demonstrated promising clinical benefit in patients treated with a maintenance TIL-ACT therapy after primary cytoreduction and platinum-based chemotherapy, showing significantly improved 3-year OS and 3-year disease-free interval (170). In addition, a TIL-ACT clinical trial is currently being performed on patients with endometrial cancer (NCT01174121), in which patients receive TILs and IL-2 after lymphocyte-depleting chemotherapy with cyclophosphamide and fludarabine; however, there is no published research data yet. Completed trials on ovarian, cervical and endometrial cancer are summarized in Table II. Regarding the selection of treatment strategies, some scholars tend to use combination therapy, e.g., combining radiation therapy with TIL-ACT (154). The sensitivity of immune effectors after tumor cells have received a lethal dose of radiation has been shown to be enhanced. In this case, the combination of TIL-ACT may result in the promotion of antitumor effects. However, this combination therapy still needs further study to balance the immune response in the host.
Table II.
Ongoing clinical studies via TIL therapy in gynecological cancers.
| Trial identifier | Cancer | ACT type | No. of patients | (Refs.) |
|---|---|---|---|---|
| NCT04072263 | Ovarian cancer | Ex vivo-expanded autologous TILs | 12 | N/Aa |
| NCT00003887 | Ovarian cancer | Peripheral blood lymphocyte therapy | Not applicable | N/Aa |
| NCT00228358 | Ovarian cancer | Ex vivo-expanded HER2-specific T cells | 8 | (182) |
| NCT00101257 | Ovarian cancer | Autologous CD4+ antigen-specific T-cell clones | 18 | N/Aa |
| NCT00562640 | Ovarian cancer | Wilms' tumor gene peptide-sensitized autologous T cells | 21 | (183) |
| NCT01174121 | Ovarian cancer | Re-stimulated TILs | 332 | (184) |
| NCT02482090 | Ovarian cancer | Re-stimulated TILs | 6 | (185) |
| NCT01883297 | Ovarian cancer | Re-stimulated TILs | 9 | N/Aa |
| NCT02876510 | Ovarian cancer | Endogenous CD8+ T cells | 31 | (186) |
| NCT03412526 | Ovarian cancer | Ex vivo-expanded autologous TILs | 15 | N/Aa |
| NCT02096614 | Ovarian cancer | MAGE-A4-specific TCR gene-transferred T lymphocytes | Not applicable | N/Aa |
| NCT02366546 | Ovarian cancer | NY-ESO-1-specific TCR gene-transduced T lymphocytes | Not applicable | N/Aa |
| NCT01312376 | Ovarian cancer | Vaccine-primed CD3/CD28-costimulated autologous T cells combined with vaccine boost and bevacizumab | Not applicable | N/Aa |
| NCT02277392 | Ovarian cancer | Recombinant human interleukin-18 (Sb-485232) combined with adoptive transfer of vaccine-primed CD3/CD28-costimulated autologous T cells following lymphodepletion | Not applicable | N/Aa |
| NCT01212887 | Ovarian cancer | MFE23 scFv-expressing autologous anti-CEA MFEz T lymphocytes, aldesleukin, cyclophosphamide and fludarabine phosphate | Not applicable | N/Aa |
| NCT01567891 | Ovarian cancer | Cytoreductive surgery followed by infusion with NYESO-1 (C259) transduced autologous T cells | Not applicable | N/Aa |
| NCT03108495 | Cervical cancer | Ex vivo-expanded autologous TILs | 18 | N/Aa |
| NCT01585428 | Cervical cancer | Ex vivo-expanded autologous TILs | 9 | (187) |
| NCT01174121 | Uterine cancer | CD8+-enriched eutologous TILs | 1 | N/Aa |
TILs, tumor-infiltrating lymphocytes; TCR, T-cell receptor; N/A, not applicable.
aSome clinical trials have not been completed; therefore, the literature cannot be retrieved. In addition, some clinical trials have not been indexed and retrieved. Details of these clinical trials can be found at https://www.wuxuwang.com.
Overall, the aim of TIL-ACT is to create an individualized treatment that targets only certain tumors in one patient. Over the past few decades, TIL-ACT has made great progress on a technical level as an anticancer therapy. There is also an increasing number of studies focusing on using surface antigens, such as CD137(171) and PD-1(172), to screen TILs with high tumor responsiveness. Among them, PD-1 monoclonal antibody blocks the binding between PD-L1 expressed by tumor cells and PD-1 on the surface of T cells, and promotes a large-scale release of TILs in the TME, contributing to tumor regression (173). Although immunotherapy for gynecological malignancies is receiving significant attention, only a few studies and clinical trials have focused on TIL-ACT; therefore, further studies need to be conducted.
Issues with TIL-ACT
TIL-ACT is becoming popular because of its high specificity and efficiency in killing tumor cells and minimal side effects. However, the cytotoxicity associated with TIL-ACT is the major hurdle in its therapeutic applications (174). Adverse events (AEs) can be classified into two types: Mild and severe. Mild AEs may be due to contamination during the expansion and preparation of TILs in vitro, resulting in subsequent infusion allergy and infection (175). Patients have been reported to exhibit mild fever, chills and other mild inflammatory reactions. Severe AEs include two subcategories: Autoimmune toxicity and cytokine release syndrome (CRS) (176).
Since some tumor-associated antigens are also expressed in normal tissues, once TILs recognize them, an enhanced immune response from the host is triggered, resulting in graft vs. host disease. Adoptive T cells have been shown to target not only antigen-specific tumor cells but also normal skin cells and uveal cells, leading to vitiligo and uveitis (177). It is important to note that with the development of T-cell sorting and modification techniques, autoimmune toxicity-related AEs have become very rare (154).
The extensive nonantigen-related inflammation resulting from TIL infusion is known as CRS or cytokine storm, which is a severe overreaction of the immune system induced by a positive feedback loop between cytokines and immune cells (178). When CRS occurs, T cells in patients are activated and proliferate rapidly, causing an excessive cascading release of cytokines. These cytokines mediate a variety of immune responses, leading to clinical symptoms such as fever, hypotension, heart problems, dyspnea, fatigue, nausea and clotting disorders. In severe cases, they may introduce great damage to body tissues and organs, and even death (179). CRS is not specific to TIL-ACT and may be induced by the use of certain monoclonal antibody drugs and other types of ACT cell therapies, such as chimeric antigen receptor T-cell immunotherapy (180). In addition to the two types of severe AEs described above, Rohaan et al (154) classified whole-blood cytopenia and febrile neutropenia caused by lymphatic depletion as toxicity due to the lymphodepletion preparative regimen.
Despite the risk of AEs, the combination of lymphoid depletion before TIL infusion (181) and the high dose of IL-2 after infusion makes the current TIL-ACT quite safe and serious adverse reactions have rarely been reported.
5. Discussion
With the rapid development of informatics technology and the increased understanding of the tumor immune microenvironment, the identification of tumor-specific targets and analysis of individual differences are more accurate and precise, and thus great progress has been made in tumor immunotherapy over the past 10 years. Significant research on immune checkpoint-related antibody drugs is being performed. The TME, specifically referring to the microenvironment with immunosuppressive properties, contributes to the low response rate to immunotherapy. This phenomenon is associated with the result of the interplay among various subgroups of TILs, accounting for the majority of immune-infiltrating cells (179).
Enhancing the antitumor activity of TILs and reducing the associated toxicity will further optimize the efficacy of TIL-ACT. Over 100 related clinical trials have been initiated since 2015, which should provide new insights into the future development of this therapeutic approach. Through a systematic evaluation of the relationship between TILs and gynecological malignancies, the role of TILs can be better understood to improve the application of TIL-ACT in the treatment of gynecological tumors.
Further analysis of the distribution, number and status of immune cell subsets in the TME may lay a solid foundation for novel tumor immunotherapy. Therefore, the combination of targeted tumor immune cell therapy and existing therapies is a promising development for future clinical therapy.
In conclusion, tumor immunity has been a promising topic in the cancer therapy area. The ‘soil’ function of the TME for tumor growth has attracted wide attention from scientists. Tumor-infiltrating immune cells in the TME, especially the TILs, play a key role in the response against cancer. Elucidating the role of TILs in tumorigenesis and development is the basis for the development of tumor immunotherapy in the future. The present review summarizes the biological classification of TILs, the mechanisms of their involvement in the regulation of the immune microenvironment, and subsequently analyzed the development of tumor immunotherapy for TILs in gynecological tumors, providing ideas for the current treatment dilemma of gynecological tumor immune checkpoints to a certain extent.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by the China Medical Association Clinical Medical Research Special Fund Project (grant no. 17020310700); the National Natural Science Foundation of China (grant nos. 82071655 and 81860276); the Key Research and Development Program of Hubei Province (grant no. 2020BCB023); the Fundamental Research Funds for the Central Universities (grant no. 2042020kf1013); the Educational and Teaching Reform Research Project (grant no. 413200095); and the Graduate Credit Course Projects (grant no. 413000206).
Availability of data and materials
Not applicable.
Authors' contributions
ZW and ZD collected and initially screened the literature. FD, MY and SL wrote, integrated and modified the sentences in the review. BL and YC guided the research ideas and designed the structure of the review. ZW performed a visual analysis of the flow of the article, and generated the figure and was the main contributor to the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Naito Y, Yoshioka Y, Yamamoto Y, Ochiya T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell Mol Life Sci. 2017;74:697–713. doi: 10.1007/s00018-016-2346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18:359–376. doi: 10.1038/s41568-018-0006-7. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–959. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 5.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L, Zeigenfuss S, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 Study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 6.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, et al. Cervical Cancer, Version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:64–84. doi: 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11(31) doi: 10.1186/s13045-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matanes E, Gotlieb WH. Immunotherapy of gynecological cancers. Best Pract Res Clin Obstet Gynaecol. 2019;60:97–110. doi: 10.1016/j.bpobgyn.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Duranti S, Pietragalla A, Daniele G, Nero C, Ciccarone F, Scambia G, Lorusso D. Role of immune checkpoint inhibitors in cervical cancer: From preclinical to clinical data. Cancers (Basel) 2021;13(2089) doi: 10.3390/cancers13092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventriglia J, Paciolla I, Pisano C, Cecere SC, Di Napoli M, Tambaro R, Califano D, Losito S, Scognamiglio G, Setola SV, et al. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat Rev. 2017;59:109–116. doi: 10.1016/j.ctrv.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Sasada T, Suekane S. Variation of tumor-infiltrating lymphocytes in human cancers: Controversy on clinical significance. Immunotherapy. 2011;3:1235–1251. doi: 10.2217/imt.11.106. [DOI] [PubMed] [Google Scholar]
- 14.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 16.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D, Chan WL, Leung BP, Hunter D, Schulz K, Carter RW, McInnes IB, Robinson JH, Liew FY. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 20.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 21.Basu A, Ramamoorthi G, Albert G, Gallen C, Beyer A, Snyder C, Koski G, Disis ML, Czerniecki BJ, Kodumudi K. Differentiation and Regulation of TH cells: A balancing Act for cancer immunotherapy. Front Immunol. 2021;12(669474) doi: 10.3389/fimmu.2021.669474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The Central Role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim WS, Shin MK, Shin SJ. MAP1981c, a Putative Nucleic Acid-Binding Protein, Produced by Mycobacterium avium subsp. paratuberculosis, Induces Maturation of Dendritic Cells and Th1-Polarization. Front Cell Infect Microbiol. 2018;8(206) doi: 10.3389/fcimb.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongiorno EK, Garcia SA, Sauma S, Hooper DC. Type 1 immune mechanisms driven by the response to infection with attenuated rabies virus result in changes in the immune bias of the tumor microenvironment and necrosis of mouse GL261 brain tumors. J Immunol. 2017;198:4513–4523. doi: 10.4049/jimmunol.1601444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Wang X, Wang J, Lin J, Cong Y, Qiao G. CD21lo/medCD27+ proinflammatory B cells are enriched in breast cancer patients and promote antitumor T cell responses. Exp Cell Res. 2017;361:149–154. doi: 10.1016/j.yexcr.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Zou JY, Su CH, Luo HH, Lei YY, Zeng B, Zhu HS, Chen ZG. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J Cell Biochem. 2018;119:1420–1428. doi: 10.1002/jcb.26302. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences. Blood. 2013;121:e57–e69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 28.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 29.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson HM. SOCS proteins in macrophage polarization and function. Front Immunol. 2014;5(357) doi: 10.3389/fimmu.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol. 2015;6(212) doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang W, Shi R, Kang X, Zhang X, Chen P, Zhang L, Hou A, Wang R, Zhao Y, Zhao K, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun. 2018;9(2574) doi: 10.1038/s41467-018-04999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168–7174. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 36.Mellor AL, Munn DH. Creating immune privilege: Active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 37.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wongtrakoongate P. Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries. World J Stem Cells. 2015;7:137–148. doi: 10.4252/wjsc.v7.i1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Racioppi L, Nelson ER, Huang W, Mukherjee D, Lawrence SA, Lento W, Masci AM, Jiao Y, Park S, York B, et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat Commun. 2019;10(2450) doi: 10.1038/s41467-019-10424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HL, Hou SY, Li HB, Qiu JP, Bo L, Mao CP. Biological function and mechanism of long noncoding RNAs nuclear-enriched abundant transcript 1 in development of cervical cancer. Chin Med J (Engl) 2018;131:2063–2070. doi: 10.4103/0366-6999.239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordon Y. Dendritic cells: Sorting, sorted! Nat Rev Immunol. 2016;16(657) doi: 10.1038/nri.2016.115. [DOI] [PubMed] [Google Scholar]
- 43.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasqual G, Chudnovskiy A, Tas JMJ, Agudelo M, Schweitzer LD, Cui A, Hacohen N, Victora GD. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature. 2018;553:496–500. doi: 10.1038/nature25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, Kagan JC. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zitvogel L, Kroemer G. CD103 + Dendritic Cells Producing Interleukin-12 in Anticancer Immunosurveillance. Cancer Cell. 2014;26:591–593. doi: 10.1016/j.ccell.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, Li Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J Hematol Oncol. 2018;11(100) doi: 10.1186/s13045-018-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52(101481) doi: 10.1016/j.smim.2021.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 54.Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. 2010;185:3248–3255. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- 55.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–2157. [PubMed] [Google Scholar]
- 56.Shi Y, Yu P, Zeng D, Qian F, Lei X, Zhao Y, Tang B, Hao Y, Luo H, Chen J, Tan Y. Suppression of vascular endothelial growth factor abrogates the immunosuppressive capability of murine gastric cancer cells and elicits antitumor immunity. FEBS J. 2014;281:3882–3893. doi: 10.1111/febs.12923. [DOI] [PubMed] [Google Scholar]
- 57.Huang LY, Reis e Sousa C, Itoh Y, Inman J, Scott DE. IL-12 induction by a TH1-inducing adjuvant in vivo: Dendritic cell subsets and regulation by IL-10. J Immunol. 2001;167:1423–1430. doi: 10.4049/jimmunol.167.3.1423. [DOI] [PubMed] [Google Scholar]
- 58.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallois A, Bhardwaj N. Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol. 2013;4(436) doi: 10.3389/fimmu.2013.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell KS, Hasegawa J. Natural killer cell biology: An update and future directions. J Allergy Clin Immunol. 2013;132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrow AD, Martin CJ, Colonna M. The Natural Cytotoxicity Receptors in Health and Disease. Front Immunol. 2019;10(909) doi: 10.3389/fimmu.2019.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goding SR, Yu S, Bailey LM, Lotze MT, Basse PH. Adoptive transfer of natural killer cells promotes the anti-tumor efficacy of T cells. Clin Immunol. 2017;177:76–86. doi: 10.1016/j.clim.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, et al. Interferon-γ Drives Treg fragility to promote anti-tumor immunity. Cell. 2017;169:1130–1141.e11. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, et al. Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol. 2019;4(eaat8116) doi: 10.1126/sciimmunol.aat8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong L, Jiménez-Cortegana C, Tay AHM, Wickström S, Galluzzi L, Lundqvist A. NK cells and solid tumors: Therapeutic potential and persisting obstacles. Mol Cancer. 2022;21(206) doi: 10.1186/s12943-022-01672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. 2019;16:415–422. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris JH, Noman MZ, Chouaib S. Critical role of tumor microenvironment in shaping NK cell functions: Implication of hypoxic stress. Front Immunol. 2015;6(482) doi: 10.3389/fimmu.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA. 2009;106:20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 73.Jourdan P, Abbal C, Noraz N, Hori T, Uchiyama T, Vendrell JP, Bousquet J, Taylor N, Pène J, Yssel H. IL-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol. 1998;160:4153–4157. [PubMed] [Google Scholar]
- 74.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 75.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 76.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, Abe H, Tada K, Nakamura M, Sugamura K, Takano S. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 77.Lauerova L, Dusek L, Simickova M, Kocák I, Vagundová M, Zaloudík J, Kovarík J. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–166. [PubMed] [Google Scholar]
- 78.Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, García Nores GD, Hespe GE, Nitti MD, Kataru RP, Mehrara BJ. Th2 cytokines inhibit lymphangiogenesis. PLoS One. 2015;10(e0126908) doi: 10.1371/journal.pone.0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. THE IL-4 RECEPTOR: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 80.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: Increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29:1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 81.Liu Z, Yang L, Cui Y, Wang X, Guo C, Huang Z, Kan Q, Liu Z, Liu Y. Il-21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1133–1144. doi: 10.1002/ibd.20923. [DOI] [PubMed] [Google Scholar]
- 82.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 83.Hori S, Nomura T, Sakaguchi S. Control of Regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 84.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dilek N, Poirier N, Hulin P, Coulon F, Mary C, Ville S, Vie H, Clémenceau B, Blancho G, Vanhove B. Targeting CD28, CTLA-4 and PD-L1 Costimulation Differentially Controls Immune Synapses and Function of Human Regulatory and Conventional T-Cells. PLoS One. 2013;8(e83139) doi: 10.1371/journal.pone.0083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, et al. Agonist Anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5(e10436) doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F, Al-Daccak R, Michel L. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol. 2011;186:5173–5183. doi: 10.4049/jimmunol.1002050. [DOI] [PubMed] [Google Scholar]
- 88.Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10(2453) doi: 10.3389/fimmu.2019.02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shan F, Somasundaram A, Bruno TC, Workman CJ, Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. 2022;8:944–961. doi: 10.1016/j.trecan.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 93.Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem. 2015;290:27158–27167. doi: 10.1074/jbc.M115.682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: Implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 95.Pylayeva-Gupta Y. Molecular pathways: Interleukin-35 in autoimmunity and cancer. Clin Cancer Res. 2016;22:4973–4978. doi: 10.1158/1078-0432.CCR-16-0743. [DOI] [PubMed] [Google Scholar]
- 96.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: Direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 97.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 98.Vasconcelos DP, Costa M, Amaral IF, Barbosa MA, Águas AP, Barbosa JN. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials. 2015;37:116–123. doi: 10.1016/j.biomaterials.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 99.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27:237–248. doi: 10.1016/j.smim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Che F, Heng X, Zhang H, Su Q, Zhang B, Chen Y, Zhang Z, Du Y, Wang L. Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol Immunother. 2017;66:717–729. doi: 10.1007/s00262-017-1961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q, Tang Y, Yu H, Yin Q, Li M, Shi L, Zhang W, Li D, Li L. CCL18 from tumor-cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol Carcinog. 2016;55:1688–1699. doi: 10.1002/mc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi: 10.1016/j.cellimm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: Granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 105.Dallavalasa S, Beeraka NM, Basavaraju CG, Tulimilli SV, Sadhu SP, Rajesh K, Aliev G, Madhunapantula SV. The role of tumor associated macrophages (TAMs) in cancer progression, chemoresistance, angiogenesis and metastasis-current status. Curr Med Chem. 2021;28:8203–8236. doi: 10.2174/0929867328666210720143721. [DOI] [PubMed] [Google Scholar]
- 106.Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated macrophages in tumor immunity. Front Immunol. 2020;11(583084) doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, Brunazzi EA, Vignali KM, Sun M, Stolz DB, et al. Treg cells promote the SREBP1-Dependent metabolic fitness of tumor-promoting macrophages via repression of CD8 + T cell-derived interferon-γ. Immunity. 2019;51:381–397.e6. doi: 10.1016/j.immuni.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, Hockstein N, Guarino M, Masters G, Penman E, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 113.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez PC, Quiceno DG, Ochoa AC. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des. 2004;10:893–898. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 119.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 120.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saleem SJ, Martin RK, Morales JK, Sturgill JL, Gibb DR, Graham L, Bear HD, Manjili MH, Ryan JJ, Conrad DH. Cutting edge: Mast cells critically augment myeloid-derived suppressor cell activity. J Immunol. 2012;189:511–515. doi: 10.4049/jimmunol.1200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oshi M, Asaoka M, Tokumaru Y, Yan L, Matsuyama R, Ishikawa T, Endo I, Takabe K. CD8 T cell score as a prognostic biomarker for triple negative breast cancer. Int J Mol Sci. 2020;21(6968) doi: 10.3390/ijms21186968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peiper M, Goedegebuure PS, Linehan DC, Ganguly E, Douville CC, Eberlein TJ. The HER2/neu-derived peptide p654-662 is a tumor-associated antigen in human pancreatic cancer recognized by cytotoxic T lymphocytes. Eur J Immunol. 1997;27:1115–1123. doi: 10.1002/eji.1830270511. [DOI] [PubMed] [Google Scholar]
- 125.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M, Konishi I. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation ofMyeloid-Derived Suppressor Cells. Clinical Cancer Research. 2017;23 doi: 10.1158/1078-0432.CCR-16-0387. [DOI] [PubMed] [Google Scholar]
- 127.Vanderstraeten A, Luyten C, Verbist G, Tuyaerts S, Amant F. Mapping the immunosuppressive environment in uterine tumors: Implications for immunotherapy. Cancer Immunol Immunother. 2014;63:545–557. doi: 10.1007/s00262-014-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K, et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep. 2015;5(18217) doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kusuda T, Shigemasa K, Arihiro K, Fujii T, Nagai N, Ohama K. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol Rep. 2005;13:1153–1158. [PubMed] [Google Scholar]
- 130.Tsukui T, Hildesheim A, Schiffman MH, Lucci J III, Contois D, Lawler P, Rush BB, Lorincz AT, Corrigan A, Burk RD, et al. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: Correlation with cervical pathology. Cancer Res. 1996;56:3967–3974. [PubMed] [Google Scholar]
- 131.Chang WC, Li CH, Huang SC, Chang DY, Chou LY, Sheu BC. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer. 2010;116:5777–5788. doi: 10.1002/cncr.25371. [DOI] [PubMed] [Google Scholar]
- 132.Dutsch-Wicherek MM, Szubert S, Dziobek K, Wisniewski M, Lukaszewska E, Wicherek L, Jozwicki W, Rokita W, Koper K. Analysis of the treg cell population in the peripheral blood of ovarian cancer patients in relation to the long-term outcomes. Ginekol Pol. 2019;90:179–184. doi: 10.5603/GP.2019.0032. [DOI] [PubMed] [Google Scholar]
- 133.Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang T, Kong B, Cui B. Distribution of Th17 cells and Foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin Chim Acta. 2012;413:1848–1854. doi: 10.1016/j.cca.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 134.Visser J, Nijman HW, Hoogenboom BN, Jager P, van Baarle D, Schuuring E, Abdulahad W, Miedema F, van der Zee AG, Daemen T. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol. 2007;150:199–209. doi: 10.1111/j.1365-2249.2007.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mustea A, Braicu EI, Koensgen D, Yuan S, Sun PM, Stamatian F, Lichtenegger W, Chen FC, Chekerov R, Sehouli J. Monitoring of IL-10 in the serum of patients with advanced ovarian cancer: Results from a prospective pilot-study. Cytokine. 2009;45:8–11. doi: 10.1016/j.cyto.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 136.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7(19) doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–267. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- 138.Hashimoto I, Kodama J, Seki N, Hongo A, Miyagi Y, Yoshinouchi M, Kudo T. Macrophage infiltration and angiogenesis in endometrial cancer. Anticancer Res. 2000;20 (6C):853–856. [PubMed] [Google Scholar]
- 139.Ding H, Cai J, Mao M, Fang Y, Huang Z, Jia J, Li T, Xu L, Wang J, Zhou J, et al. Tumor-associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS. 2014;122:1059–1069. doi: 10.1111/apm.12257. [DOI] [PubMed] [Google Scholar]
- 140.Chen XJ, Han LF, Wu XG, Wei WF, Wu LF, Yi HY, Yan RM, Bai XY, Zhong M, Yu YH, et al. Clinical Significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer. 2017;8:3868–3875. doi: 10.7150/jca.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal transducing proteins in tumor-associated T and natural killer cells in ovarian carcinoma. Biochem Soc Trans. 1997;25(218S) doi: 10.1042/bst025218s. [DOI] [PubMed] [Google Scholar]
- 142.Gras Navarro A, Björklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6(202) doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu Y, Ye S, Goswami S, Pei X, Xiang L, Zhang X, Yang H. Clinical significance of peripheral blood and tumor tissue lymphocyte subsets in cervical cancer patients. BMC Cancer. 2020;20(173) doi: 10.1186/s12885-020-6633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iva T, Lenka K, Michal H, Petr S, Jan L, Ladislav P, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. Journal for Immunotherapy of Cancer. 2018;6 doi: 10.1186/s40425-018-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lijun Z, Xin Z, Danhua S, Xiaoping L, Jianliu W, Huilan W, Lihui W. Tumor-Infiltrating Dendritic Cells May Be Used as Clinicopathologic Prognostic Factors in Endometrial Carcinoma. Int J Gynecol Cancer. 2012;22:836–841. doi: 10.1097/IGC.0b013e31825401c6. [DOI] [PubMed] [Google Scholar]
- 146.Mendoza L, Bubeník J, Símová J, Korb J, Bieblová J, Vonka V, Indrová M, Mikysková R, Jandlová T. Tumour-inhibitory effects of dendritic cells administered at the site of HPV 16-induced neoplasms. Folia Biol (Praha) 2002;48:114–119. doi: 10.14712/fb2002048030114. [DOI] [PubMed] [Google Scholar]
- 147.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 149.Ordoñez R, Henríquez-Hernández LA, Federico M, Valenciano A, Pinar B, Lloret M, Bordón E, Rodríguez-Gallego C, Lara PC. Radio-induced apoptosis of peripheral blood CD8 T lymphocytes is a novel prognostic factor for survival in cervical carcinoma patients. Strahlenther Onkol. 2014;190:210–216. doi: 10.1007/s00066-013-0488-x. [DOI] [PubMed] [Google Scholar]
- 150.Miyasaka Y, Yoshimoto Y, Murata K, Noda SE, Ando K, Ebara T, Okonogi N, Kaminuma T, Yamada S, Ikota H, et al. Treatment outcomes of patients with adenocarcinoma of the uterine cervix after definitive radiotherapy and the prognostic impact of tumor-infiltrating CD8+ lymphocytes in pre-treatment biopsy specimens: A multi-institutional retrospective study. J Radiat Res. 2020;61:275–284. doi: 10.1093/jrr/rrz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spiess PJ, Yang JC, Rosenberg SA. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987;79:1067–1075. [PubMed] [Google Scholar]
- 152.Krishna S, Lowery FJ, Copeland AR, Bahadiroglu E, Mukherjee R, Jia L, Anibal JT, Sachs A, Adebola SO, Gurusamy D, et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. 2020;370:1328–1334. doi: 10.1126/science.abb9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.J SP, C YJ, RS. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. Journal of the National Cancer Institute. 1987;79 [PubMed] [Google Scholar]
- 154.Kumar A, Watkins R, Vilgelm AE. Cell therapy with TILs: Training and taming T cells to fight cancer. Front Immunol. 2021;12(690499) doi: 10.3389/fimmu.2021.690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: The current landscape. Virchows Arch. 2018;474:449–461. doi: 10.1007/s00428-018-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gudiol C, Lewis RE, Strati P, Kontoyiannis DP. Chimeric antigen receptor T-cell therapy for the treatment of lymphoid malignancies: Is there an excess risk for infection? Lancet Haematol. 2021;8:e216–e228. doi: 10.1016/S2352-3026(20)30376-8. [DOI] [PubMed] [Google Scholar]
- 157.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4457. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Hölmich LR, Hendel HW, Met Ö, Andersen MH, et al. Long-Lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 159.Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Donnem T, Hald SM, Paulsen E, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M, Poehl M, et al. Stromal CD8+ T-cell Density-A promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 161.James FR, Jiminez-Linan M, Alsop J, Mack M, Song H, Brenton JD, Pharoah PDP, Ali HR. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer. 2017;17(657) doi: 10.1186/s12885-017-3585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(59) doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sideras K, Biermann K, Yap K, Mancham S, Boor PPC, Hansen BE, Stoop HJA, Peppelenbosch MP, van Eijck CH, Sleijfer S, et al. Tumor cell expression of immune inhibitory molecules and tumor-infiltrating lymphocyte count predict cancer-specific survival in pancreatic and ampullary cancer. Int J Cancer. 2017;141:572–582. doi: 10.1002/ijc.30760. [DOI] [PubMed] [Google Scholar]
- 164.Clemente O, Ottaiano A, Di Lorenzo G, Bracigliano A, Lamia S, Cannella L, Pizzolorusso A, Di Marzo M, Santorsola M, De Chiara A, et al. Is immunotherapy in the future of therapeutic management of sarcomas? J Transl Med. 2021;19(173) doi: 10.1186/s12967-021-02829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Radvanyi LG. Tumor-Infiltrating lymphocyte therapy: Addressing prevailing questions. Cancer J. 2015;21:450–464. doi: 10.1097/PPO.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 166.Aoki Y, Takakuwa K, Kodama S, Tanaka K, Takahashi M, Tokunaga A, Takahashi T. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 1991;51:1934–1939. [PubMed] [Google Scholar]
- 167.Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1:501–507. [PubMed] [Google Scholar]
- 168.Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807–820. doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, Slomovitz BM, Walther A, Thomas SS, Chesney JA, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;37 (15_suppl)(S2538) [Google Scholar]
- 170.KF HI, KT SK, AT TT, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. J Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 1, 1995. [PubMed] [Google Scholar]
- 171.Seliktar-Ofir S, Merhavi-Shoham E, Itzhaki O, Yunger S, Markel G, Schachter J, Besser MJ. Selection of shared and neoantigen-reactive T cells for adoptive cell therapy based on CD137 separation. Front Immunol. 2017;8(1211) doi: 10.3389/fimmu.2017.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol. 2016;27:1199–1206. doi: 10.1093/annonc/mdw181. [DOI] [PubMed] [Google Scholar]
- 174.Wolf B, Zimmermann S, Arber C, Irving M, Trueb L, Coukos G. Safety and tolerability of adoptive cell therapy in cancer. Drug Saf. 2019;42:315–334. doi: 10.1007/s40264-018-0779-3. [DOI] [PubMed] [Google Scholar]
- 175.Burger SR. Current regulatory issues in cell and tissue therapy. Cytotherapy. 2003;5:289–298. doi: 10.1080/14653240310002324. [DOI] [PubMed] [Google Scholar]
- 176.Cruz CR, Hanley PJ, Liu H, Torrano V, Lin YF, Arce JA, Gottschalk S, Savoldo B, Dotti G, Louis CU, et al. Adverse events following infusion of T cells for adoptive immunotherapy: A 10-year experience. Cytotherapy. 2010;12:743–749. doi: 10.3109/14653241003709686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(56) doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu D, Zhao J. Cytokine release syndrome: Grading, modeling, and new therapy. J Hematol Oncol. 2018;11(121) doi: 10.1186/s13045-018-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao BF, Zhang JT, Zhu YG, Cui XR, Lu ZM, Yu BT, Wu N. Chimeric antigen receptor T-Cell therapy in lung cancer: Potential and challenges. Front Immunol. 2021;12(782775) doi: 10.3389/fimmu.2021.782775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, Hinrichs CS. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200–205. doi: 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Disis ML, Dang Y, Coveler AL, Marzbani E, Kou ZC, Childs JS, Fintak P, Higgins DM, Reichow J, Waisman J, Salazar LG. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol Immunother. 2014;63:101–109. doi: 10.1007/s00262-013-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kyi C, Doubrovina E, Zhou Q, Kravetz S, Iasonos A, Aghajanian C, Sabbatini P, Spriggs D, O'Reilly RJ, O'Cearbhaill RE. Phase I dose escalation safety and feasibility study of autologous WT1-sensitized T cells for the treatment of patients with recurrent ovarian cancer. J Immunother Cancer. 2021;9(e002752) doi: 10.1136/jitc-2021-002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zacharakis N, Huq LM, Seitter SJ, Kim SP, Gartner JJ, Sindiri S, Hill VK, Li YF, Paria BC, Ray S, et al. Breast cancers are immunogenic: Immunologic analyses and a phase II pilot clinical trial using mutation-reactive autologous lymphocytes. J Clin Oncol. 2022;40:1741–1754. doi: 10.1200/JCO.21.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Westergaard MCW, Andersen R, Chong C, Kjeldsen JW, Pedersen M, Friese C, Hasselager T, Lajer H, Coukos G, Bassani-Sternberg M, et al. Tumour-reactive T cell subsets in the microenvironment of ovarian cancer. Br J Cancer. 2019;120:424–434. doi: 10.1038/s41416-019-0384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tsimberidou AM, Guenther K, Andersson BS, Mendrzyk R, Alpert A, Wagner C, Nowak A, Aslan K, Satelli A, Richter F, et al. Feasibility and safety of personalized, multi-target, adoptive cell therapy (IMA101): First-In-Human clinical trial in patients with advanced metastatic cancer. Cancer Immunol Res. 2023;11:925–945. doi: 10.1158/2326-6066.CIR-22-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stevanović S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong MLM, Somerville RPT, Klebanoff CA, Kammula US, Sherry RM, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25:1486–1493. doi: 10.1158/1078-0432.CCR-18-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.