Abstract
Oropharyngeal squamous cell carcinoma (OPSCC), a subset of head and neck squamous cell carcinoma (HNSCC), involves the palatine tonsils, soft palate, base of tongue, and uvula, with the ability to spread to adjacent subsites. Personalized treatment strategies for Human Papillomavirus-associated squamous cell carcinoma of the oropharynx (HPV+OPSCC) are yet to be established. In this article, we summarise our current understanding of the pathogenesis of HPV+OPSCC, the intrinsic role of the immune system, current ICI clinical trials, and the potential role of small molecule immunotherapy in HPV+OPSCC.
Keywords: head & neck cancer, immunotherapy, immune system, cancer of the oropharynx
1. Introduction
Oropharyngeal squamous cell carcinoma (OPSCC) is a subset of head and neck squamous cell carcinoma (HNSCC) involving the palatine tonsils, soft palate, base of tongue, and uvula, with a common capacity to spread to adjacent subsites. For decades, it was assumed that all OPSCC cases were aetiologically homogenous. However, in 1983, HPV antigens were discovered in a subset of OPSCC tumours [1]. Subsequent investigation has improved our understanding of this disease process and defined HPV+OPSCC as a disease process distinct from HPV−OPSCC, with differing epidemiological, genetic, and prognostic traits [2].
By way of example, genetic analyses have identified mutations in PIK3CA and FGFR pathways in HPV+OPSCC and overexpression of CDKN2, encoding for p16 [3,4,5]. In contrast, HPV−OPSCC is characterised by mutation of tumour suppressor genes (i.e., TP53), EGFR upregulation, and low p16 expression [3,4,5]. Furthermore, at the cellular level, there is a distinct difference between tumour microenvironments (TME) and associated immune cell infiltrates [6]. The clinical presentation of HPV+OPSCC patients is also very different. Simplistically, HPV+OPSCC patients are commonly younger, non-smokers/drinkers, presenting with enlarged non-tender cervical lymphadenopathy and an unknown primary (low T-stage, high N-stage tumours) [7,8,9,10,11], whilst HPV−OPSCC patients are older, smokers/drinkers, often presenting with a larger painful primary tumour causing dysphagia or odynophagia and late-stage cervical lymphadenopathy (high T-stage, low N-stage tumours) [12,13,14,15]. Importantly, this disparity has recently helped to drive clinical and radiomic diagnostic techniques [16,17]. Additionally, HPV+OPSCC is more sensitive to current standard of care therapies (specifically non-surgical regimes) and has a superior prognosis over HPV−OPSCC for any stage [18,19]. Consequently, the 8th edition of the AJCC TNM staging system acknowledged this and defined HPV+OPSCC as a distinct clinical entity [20].
Despite this, personalized treatment strategies specific for HPV+OPSCC are yet to be established. Currently, the standard of care intervention for OPSCC involves surgery, radiotherapy, or chemotherapy as either uni- or multi-modality therapy that is stage-dependent. As this is applied irrespective of HPV status, it does not account for the improved prognosis typically seen with HPV+OPSCC patients. This exposes patients to increased survivorship morbidity and a 15–40% risk of radiation-induced metachronous primary in a younger demographic [21]. Radiotherapy de-escalation trials are currently recruiting to ascertain the ability of dose reduction in HPV+OPSCC, which is exquisitely radiosensitive, to maintain high rates of cure, preserve quality of life, and reduce metachronous primary rates. Early trials are promising; however, study comparison has been difficult due to varied trial designs, further complicated by small samples sizes, treatment variability, non-uniform inclusion criteria, and long-term follow-up issues [22]. Despite this, these studies universally demonstrate that de-escalation is feasible and safe, helping to personalise the therapy of HPV+OPSCC [23]. It is important to note, however, that there is currently a recommendation away from dose de-escalation in heavy smokers and patients with increased alcohol consumption [24,25].
In a further step towards personalised and targeted treatment strategies, development of immune checkpoint inhibitors (ICI) has heralded a paradigm shift in HNSCC therapy. Targeting molecular “brakes” applied to the immune system, the release of these brakes can enable immunorecognition and promote immune-mediated tumour cytotoxicity, affording a small population of patients suffering recurrent or metastatic HNSCC (R/M HNSCC) a partial to complete response [26]. Trials are currently being completed to determine the efficacy of immunotherapy in HPV+OPSCC over standard-of-care regimes. This article explores our current insight into the pathogenesis of HPV+OPSCC, the intrinsic role of the immune system, current ICI clinical trials, and the potential role of small molecule immunotherapy to personalise treatment strategies further in HPV+OPSCC.
1.1. Epidemiology
The global incidence of OPSCC was over 3.7 million cases in 2020, 33% attributable to HPV [27,28], with the incidence of OPSCC rapidly increasing in developed countries when compared to all cancers [29,30]. This increase has been identified in the US, Europe, UK, Australia, New Zealand, and certain countries within Asia [28,31,32,33,34,35,36,37], with further studies confirming a rising trend across all countries [34,36,37,38,39,40,41,42]. HPV is the most common sexually transmitted infection (STI) and one of the most common viral infections in the world [43], and HPV+OPSCC has now achieved the notorious distinction of becoming the predominant HPV-related malignancy in the US and UK, superseding cervical cancer [44].
Early studies identified that HPV+OPSCC disproportionately affected males and a younger population when compared to HPV−OPSCC [30]. However, in a result likely to represent a ‘birth cohort effect’, recent studies have demonstrated a rise in the diagnostic age of HPV+OPSCC patients, with a significant increase over the age of 65 [30,45,46,47,48]. Specifically, one study found that the median age of diagnosis increased from 53 years in 1995–2000 to 58 years in 2001–2013 [49]. This demographic redistribution has important clinical implications for therapeutic delivery within the aging population.
1.2. Risk Factors
Risk factors for HNSCC have classically been identified to include tobacco smoking [50], alcohol consumption [51], betel quid chewing [52], and EBV infection [53]. Specific to the oropharyngeal subsite, oral infection with the high-risk HPV serotypes (HR-HPV), particularly serotypes 16 and 18, is highly correlated with OPSCC [54]. In comparison to HPV−OPSCC, HPV+OPSCC is not commonly associated with a history of tobacco smoking or alcohol use [18,55]. However, HPV+OPSCC in the setting of either heavy smoking or alcohol consumption is associated with a worse prognosis [25,56] that may be dose dependent in the setting of smoking [57]. Certainly, studies have identified that tobacco chemicals enhance viral oncogenic expression in HPV-infected cervical epithelium [58]. However, the relationship between these three risk factors (HPV, tobacco smoke, and alcohol) is poorly understood, with some studies demonstrating synergistic effects [59,60] while others have not identified this phenomenon [61,62].
While there was initially some evidence to suggest an association between high-risk sexual activity and development of HPV+OPSCC [54], these are not reproducible. Subsequent studies have found that neither the number of sexual partners nor oral sex practices are associated with OPSCC development when adjusted for potential confounders including age, gender, smoking, and alcohol consumption [63,64,65,66]. In contrast, in a finding universal to SCC, primary (i.e., epidermodysplasia verruciformis, WHIM Syndrome, DOCK8 mutations, GATA Binding protein mutations, and severe combined immunodeficiency, defects in NK-cells and lymphopenias) [67,68,69], acquired, or secondary immunodeficiencies (transplant recipients, iatrogenic immunosuppressant, and autoimmunity) are risk factors for HPV-driven malignancies [69,70,71]. Specifically, research has found that HIV-positive patients have 1.6–3.2 times higher risk of developing HPV+OPSCC [72,73].
1.3. Pathogenesis/Oncogenesis
HPV are 50–60 nm in diameter, non-enveloped, icosahedral double-stranded DNA (dsDNA) viruses comprising 8000 base pairs bound to cellular histones and contained in a protein capsid of 72 pentameric capsomers [74]. HPVs are highly epitheliotropic, infecting cutaneous and mucosal epithelial cells via an unconventional clathrin-independent endocytic pathway [75] and are strongly associated with cervical, head and neck, anogenital, and oesophageal cancers. Of the more than 200 HPV serotypes, 14 are characterised as HR-HPVs due to their carcinogenic potential, including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 [76]. For OPSCC specifically, HPV 16 has been identified in the majority of HPV+OPSCC cases, while HPV 18 and other HR-HPV serotypes have been identified in HPV+OPSCC cases, but are much less common [77]. This is distinct from the multiple oncogenic HPV serotypes associated with cervical cancer, specifically 16, 18, 31, 33, 45, and 52 [78].
The viral genome of all HPV serotypes contains eight open reading frames (ORFs) that are transcribed from a single strand of DNA. The ORF is composed of three functional sections: the early € region encoding for E1, E2, E3, E4, E5, E6, and E7 genes required for viral replication, the late gene section encoding for structural proteins (L1 and L2) required for virion assembly, and a large non-coding section known as the long control region (LCR), containing cis elements crucial for transcription and replication of viral DNA [79]. Extensive research has implicated the E5, E6, and E7 genes as primary oncoproteins in HPV-associated carcinogenesis and tumour progression [74]. These oncoproteins each play a unique and integral role in supporting cellular proliferation and inhibiting apoptosis (refer Figure 1: HPV Patho-oncogenesis). Specifically, E5 downregulates antiviral responses via inhibition of cGAS-STING pathway, immunoproteasome function, and antigen processing [80]. On the other hand, E6 and E7 predominantly promote the degradation of p53 and pRb, respectively [16], both representing tumour suppressor genes (TSG) critical for maintaining adequate regulation of the cell cycle in the context of DNA damage [81]. pRb dysfunction reprograms the host cell to become more dependent on HPV oncogenes and p16INK4A, which in this context helps to subvert oncogene-induced cellular senescence [82]. Although based upon cervical cancer, a recent study has shown that tumour progression is positively correlated with viral load and HPV E7 oncoprotein expression [83]. HPV has also been found to induce epigenetic alteration, such as DNA hypomethylation and tumour suppressor gene hypermethylation, which may contribute to tumorigenesis [84]. In summary, these mechanisms allow HPV-infected cells to accumulate the hallmark properties of transformation and facilitate carcinogenesis.
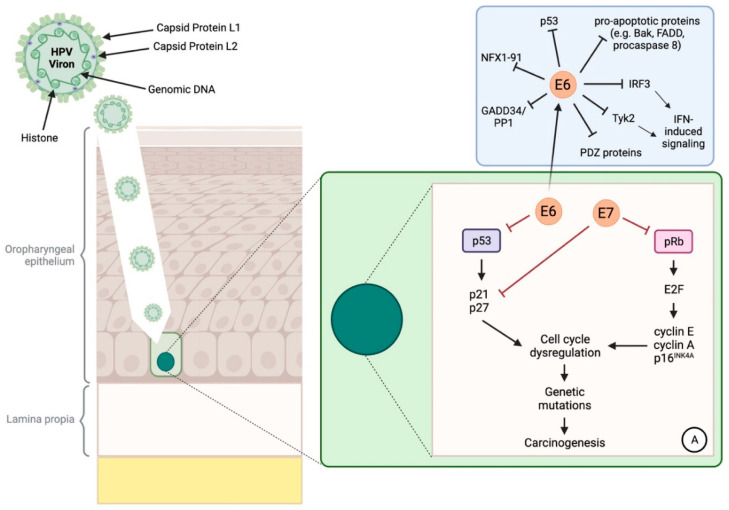
Figure 1.
HPV Patho-oncogenesis. The HPV virion invades the epithelium of the oropharynx, infecting the basal layer of cells. Within these cells, E6 and E7 inhibit p53 (tumour protein 53) and pRb (retinoblastoma protein) respectively, affording the cell the ability to replicate without regulation by these Tumor Suppressor Genes (TSGs). Further to this E6, promotes carcinogenesis in several ways: firstly, through inhibition of pro-apoptotic proteins (namely, GADD34/PP1 (growth arrest and DNA damage induced transcript 34/serine/threonine protein phosphatase), Procaspase 8, FADD (FAS-associated death domain protein), or Bak) [85,86,87,88]; secondly, by suppressing host–IFN (interferon) antiviral response by downregulating IRF3 (interferon regulatory factor 3) and Tyk2 (Tyrosine kinase 2); thirdly, by disrupting tissue integrity through degradation of PDZ proteins (PDZ proteins play an important role in anchoring receptor proteins in the cell membrane to cytoskeletal components; these proteins also play an integral role in signal transduction complexes. Interaction with certain proteins promotes oncogenic potential) via expression of a PDZ protein binding motif (PSD-95/D1g/ZO-1) [89]; and finally, by promoting cellular immortalisation by targeting NFX1-91, which is an endogenously expressed transcriptional regulator of human telomerase reverse transcription (hTERT) (active in stem cells) that promotes telomerase induction and cellular immortalisation [90].
1.4. Diagnostic Techniques
The gold standard diagnostic test for HPV+OPSCC is E6/E7 mRNA detection [91]; however, its labour-intensive nature and cost preclude it from widespread use. Instead, routine diagnosis makes use of p16 immunohistochemistry (IHC), utilising p16 protein expression as a surrogate marker for E7-mediated pRb degradation [92]. The 8th edition of the TNM staging system also relies on p16 expression to distinguish HPV+OPSCC [20]. While it is widely used for its high sensitivity and ease of use, p16 IHC has moderate specificity, which can produce false positives [93]. Hence, p16 IHC in combination with highly accurate HPV DNA PCR is recommended for optimised specificity [92].
1.5. Vaccination
Given the remarkable impact of HPV vaccines on cervical cancer rates in the last 17 years [94], the question has been raised as to whether this prophylactic intervention will confer similar protection for HPV+OPSCC. Certainly, recent studies have demonstrated that vaccinated patients are less likely to develop HPV associated oral infections, with resistance extending to serotype 16 [95,96]. Confirming the benefit of vaccination, a recent study indicated that unvaccinated patients have a 19 times greater risk of developing OPSCC [97].
Although gender neutral vaccination regimes are being implemented in many developed countries [98], there is a paucity of vaccination in developing countries, limited by resources and socioeconomic and cultural factors. In 2023, 140 countries registered implementation of HPV vaccination programs in females [99] and 43 with gender-neutral vaccination, all being developed countries except for Bhutan [100]. Globally, an estimated 17% of females and 5% of males aged 15 in 2022 had completed the full course of the vaccine, while 21% of females and 7% of males had received one dose [101]. In addition, even with the implementation of gender-neutral regimes, there remains a significantly lower proportion of males being vaccinated, 44% in developed and 5% in developing countries overall respectively [102]. Clearly, there is a significant gap between ideal and practical vaccination rates especially in the male population, who are more likely to develop OPSCC.
Additionally, due to the latency of OPSCC development at a median age of 58 years [49], it may take several decades for the recent changes to HPV vaccination to be reflected in the OPSCC incidence rates. In the United States, it is projected that HPV vaccination will have limited impact on OPSCC rates until 2045 given that the current vaccination programs do not encompass the older population, who remain at elevated risk [103]. This continues to be an evolving preventative strategy that should pay dividends in lowering OPSCC rates.
Unfortunately, prophylactic vaccines are ineffective against established HPV-infection. There is, therefore, a necessity for specifically targeted immunotherapy or therapeutic HPV vaccine for people who have already acquired HR-HPV [69]. It is also unclear whether prophylactic vaccination will provide ongoing efficacy in the immunocompromised population and further investigation in this area is needed.
2. Role of the Immune System
The immune system is a complex interwoven network of specialised cells and molecules, all working in synchrony as the body’s defence mechanism. Decades of cancer immunology research have identified inextricable links between the immune system and tumorigenesis, a ‘tug-of-war’ between ‘immunosurveillance’-guided tumour cell cytotoxicity versus tumour immune evasion mechanisms sustaining cellular escape and proliferation.
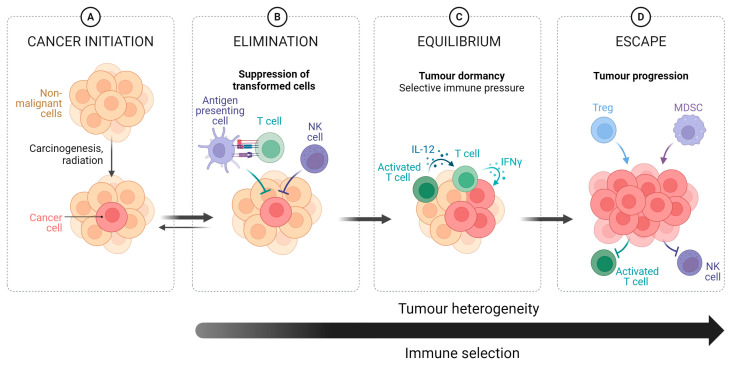
Dunn and colleagues [104] developed a model, known as “immunoediting”, to help explain this phenomenon (refer to Figure 2 for a graphical overview). It postulates three phases of ‘cancer immune’ system interaction: elimination, equilibrium, and escape. Elimination represents successful interactions between innate and adaptive immune systems to eradicate tumour cells. A complex system, it relies upon direct recognition and cell-mediated cytotoxicity of tumour cells by NK-cells, tumour antigen uptake by antigen-presenting cells (dendritic cells and macrophages), and subsequent presentation and activation of T and B lymphocytes mediating inflammatory and cellular cytotoxicity processes to promote tumour cell cytotoxicity [105]. Antigen activation of the adaptive immune system has the added benefit of producing long-standing immunity. Activating the adaptive immune system promotes various downstream effects to support the innate immune system, destroy the tumour cells, and support systemic immunity and memory [106].
Figure 2.
The different phases of the cancer immunoediting process. (A) Initiation of a carcinogenic process, producing cancer cells and development of a tumour. (B) Elimination is characterised by immunosurveillance leading to suppression of the transformed cancer cells directed by inflammatory and cellular cytotoxicity processes. (C) Equilibrium is characterised by a balance between the immune system and the tumour, leading to partial control of the tumour. This phase is characterised by high tumour mutational burden due to selective immune pressure. (D) Escape is caused by the increased selective pressure caused by the immune system leading the tumour to acquire immune evasion, resulting in uncontrolled tumour proliferation. Arrow indicates increasing Tumour heterogeneity and Immune selection. Figure adapted from “Cancer Immunoediting”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates, accessed on 10 February 2024.
Equilibrium represents a balance between the immune system and the tumour. Only partial control of the tumour can be achieved, commonly caused by high mutational burden contributing to immune resistance. Escape is associated with uncontrolled proliferation of tumour cells, inadvertently supported by the immune system to acquire evasive properties. The ability to evade immune-mediated cytotoxicity was recognised as a hallmark of cancer in 2011 [107].
2.1. HPV & Immune System Evasion
Like other virally-associated malignancies, the immune system responds to HPV viral proteins. L1 capsid protein, E6, and E7 are primary antigens recognised by the immune system in HPV [108]. However, cervical cancer research has helped identify several immune evasion strategies that HPV employs in both the innate and adaptative immune system, helping to establish prolonged infection and increase the risk of tumorigenesis [109,110].
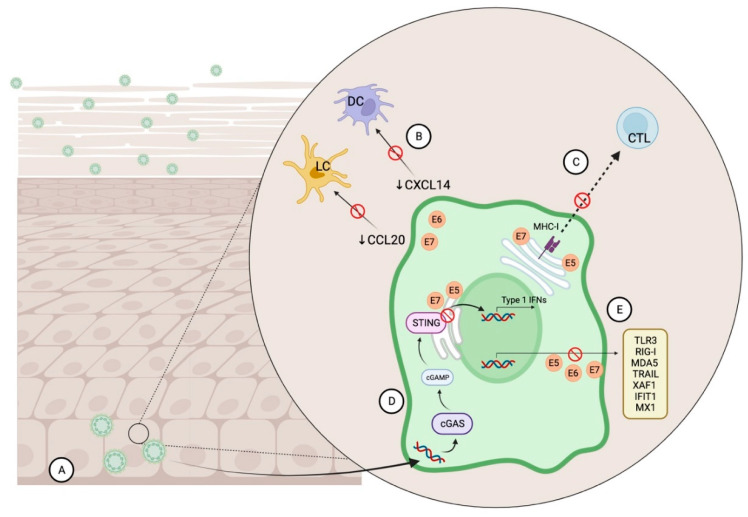
The first such mechanism involves minimising exposure to the immune system, starting from strategic infection of the basal layer of epithelium external to the basement membrane [108] (refer Figure 3: HPV’s Immune evasion strategies). HPV hijacks differentiating keratinocytes early in their evolution and completes its own viral replication cycle alongside this process. This means that the highest levels of viral gene expression and replication occur at the superficial epithelial layers, where immune cells are sparse [111]. Its virions are eventually shed alongside fully-differentiated keratinocytes during natural desquamation. This mechanism allows for HPV to infect host cells for a prolonged period without immune detection since it does not induce unexpected cell death, inflammation, or viraemia [110,112,113]. Because of this, poor anti-HPV humoral immunity and impaired T-cell immunity against HPV proteins are common in exposed individuals [114].
Figure 3.
HPV’s immune evasion strategies. (A) HPV infects basal epithelial cells and utilises the progressive differentiation of keratinocytes to conceal its viral replication process from the immune system. Immune detection is prevented since the most viral gene expression happens in superficial epithelial layers and since no unexpected cell death or inflammation occurs. (B) HPV E6 and E7 oncogenes reduce secretion of chemokines, CCL20 and CXCL14, which hinders Langerhan’s and dendritic cell recruitment. (C) HPV E5 and E7 oncogenes prevent antigen presentation to cytotoxic T cells by downregulating major histocompatibility complex (MHC) class I expression. (D) HPV E5 and E7 oncogenes inhibit the cGAS-STING (cyclic GMP–AMP synthase, stimulator of interferon genes) pathway, which responds to aberrant DNA from viruses by activating the innate immune system. (E) HPV E5, E6, and E7 oncogenes can also inhibit IFN-stimulated gene expression (e.g., TLR3, RIG-I, MDA5, TRAIL, XAF1, IFIT1, MX1). cGAMP = 2′3′ cyclic GMP–AMP, CTL = cytotoxic T cell, DC = dendritic cell, IFN = Interferon, LC = Langerhan’s cell, MHC-I = major histocompatibility complex-I.
Secondly, HPV evades immune recognition by downregulating keratinocyte-induced alarm signals which indicate the presence of the pathogen to immune cells. One of these signals occurs via the cGAS-STING pathway, where cyclic GMP–AMP synthase (cGAS) detects viral dsDNA, activating stimulator of interferon genes (STING) protein [115]. STING recruits the innate immune system via the Type-1 interferon (IFN) antiviral response. Multiple HPV studies support HPV 16, 18 and E7 and E5 oncogene antagonism of STING [116,117], including within HPV+HNSCC cells [118,119], which helps the virus prevent immune activation. In addition, E6 and E7 oncogenes from HPV 16 have the ability to prevent keratinocyte secretion of NF-κB-dependent CCL20 [120,121] and CXCL14 [122] chemokines, interfering with Langerhan and dendritic cell migration respectively.
Thirdly, E5 and E7 can suppress antigen presentation and CD8+ cytotoxic T-lymphocyte activity via downregulation of major histocompatibility complex (MHC) class I expression [123]. Finally, HPV 16 E5, E6, and E7 oncogenes also have the capacity to inhibit IFN-stimulated gene expression for pathogen recognition receptors (TLR3, RIG-I, MDA5), for apoptosis (TRAIL, XAF1), for antiviral responses (IFIT1, MX1), and genes involved in the IFN pathway (STAT1) [124,125]. In combination, these evasive mechanisms enable HPV to persist in host cells, increasing the potential for malignant change.
2.2. HPV+OPSCC and the Tumour Microenvironment
Once HPV+ tumour cells develop, the immune system has been shown to play an integral role in supporting tumour progression and modulating the tumour response to therapeutic intervention. Innate and adaptive immune cells form a substantial part of the tumour microenvironment (TME), in addition to a diverse collection of non-malignant cells (including, but not limited to endothelial cells, fibroblasts, and adipose cells) forming the tumour stroma. Previously defined as passive, recent research has demonstrated the active impact of the TME in facilitating tumour growth and invasion [126,127].
HNSCC is one of the most highly immune cell-infiltrated tumours [128], a feature that has opened new avenues for personalised treatment using immunotherapy. The composition of the HNSCC immune infiltrate varies substantially between HPV+OPSCC and HPV− OPSCC. HPV+OPSCC have greater densities of tumour-infiltrating lymphocytes (TIL), including CD3+ T cells, CD8+ T cells, Treg cells, B cells, and plasma cells, compared to HPV−OPSCC, but importantly and specifically B cells and CD8+ cytotoxic T-cells [129,130,131,132]. Additionally, HPV16 antigen-specific T cells with anti-tumour programs have been identified within immune infiltrates in 64% of HPV+OPSCC, providing a further potential personalised immunotherapy target in HPV+OPSCC [133].
The TME immune profile of HPV+OPSCC vs. HPV−PSCC has also been correlated with prognosis, which may explain improved overall survival (OS) and disease-free survival (DFS) in HPV+OPSCC [18,19,134,135]. High-density TIL infiltrate, especially involving CD4+, CD8+, and CD3+ subsets, has been associated with greater OS rates in HPV+OPSCC [136,137,138,139,140]. Higher levels of infiltrating CD20+ B cells also correlates with improved prognosis, specifically those expressing FCER2 which were localised to the stroma in HPV+HNSCC tumours [141,142]. FCER2+ B-cells have been shown to inhibit HPV+ tumour migration in vitro. FCER2 is an Fc receptor specific to IgE, upregulated on haemopoietic and B cells. Additionally, the pre-treatment neutrophil-to-lymphocyte (NLR) ratio in peripheral blood has been shown to inversely correlate with OS and DFS in HPV+OPSCC [143,144,145]. NLR is also an independent risk factor for severe disease [146] and neutrophilia is associated with increased expression of neutrophil extracellular traps (NETs). Increasing evidence indicates that NETs play a critical role within the TME (tumour associated neutrophils or “TANs” undergo polarisation to either N1 or N2 phenotypes in a similar fashion to macrophages, playing an important role in immunoediting [147]) and have been implicated in tumour progression, metastatic dissemination, and therapy resistance [148,149,150,151,152,153,154,155,156,157,158,159,160,161]. A recent genetic profile study investigating the clinical prognostic value of NET-related genes and their correlation to immunotherapy response identified that NETs gene expression can predict clinical outcomes and therapeutic response in HNSCC [162], whilst stromal NET density has been identified as an independent prognostic factor for recurrence-free survival (RFS) in cervical cancer, although an association with HPV-associated cervical cancer was not explored. This led to the suggestion that NET expression be added to TNM staging systems to help with prognostic stratification [163]. To date, there is a paucity of literature exploring the role of NETs in OPSCC, specifically HPV+OPSCC. Therefore, NET expression may represent a prognostic tumour biomarker and a target for development of immunotherapy agents.
Recently, the number of macrophages infiltrating into the TME have also been reported as an independent prognostic factor in HNSCC [164]. Tumour-associated macrophages (TAMs) can exacerbate desmoplasia, angiogenesis, nutrient deprivation, and immune suppression to promote tumour growth and regulate therapy resistance. Like neutrophils, the TME milieu can polarise macrophages to form M1 or M2 phenotypes (although this may ultimately prove to be an over-simplification). M1 phenotypes, in comparison to M2 cells, demonstrate an anti-tumour program, activating cytotoxic CD8+ T-cells and supporting the differentiation of CD4+ T-cells towards a Th-1 effector subset. Recent research has identified that HPV+OPSCC is associated with reduced macrophage TME infiltration and improved prognosis [142].
Despite the high levels of immune infiltrate, many cases of HPV+OPSCC continue to progress, indicating continuous immune evasion. OPSCC cells do this primarily by taking advantage of immune checkpoints, which are natural inbuilt mechanisms for the host immune system to ensure self-tolerance [165]. Two prominent examples are CTLA-4 (cytotoxic T-lymphocyte associated protein 4) and PD-1 (programmed cell-death protein 1). CTLA-4, which is commonly expressed on Tregs to downregulate T cell-mediated responses and is stimulated by tumour cells to cause T cell exhaustion. PD-1, on the other hand, is expressed on activated T and B lymphocytes to similarly limit T cell function. The PD-1 and its ligand (PD-L1) seem to play a substantial role in HPV+OPSCC development since higher levels of PD-L1 have been found in HPV+OPSCC [129]. Both the PD-1 and CTLA-4 systems have been successfully targeted with directed humanised antibodies and have been the cornerstone for revolutionised HNSCC treatment.
2.3. Immune Checkpoint Inhibitors
ICIs combat the elements of the TME which subvert the immune system’s efforts to restrain tumour proliferation [166]. The vast heterogeneity in cancer somatic mutations creates challenges in designing targeted therapies aimed at individual mutations; in contrast, ICIs have a broad scope as targeted cancer therapeutics [167]. Indeed, ICIs targeting CTLA-4 and PD-1 have shown clinical activity against HNSCC, advanced melanoma, renal cell carcinoma, non-small-cell lung cancer, Hodgkin’s lymphoma, endometrial cancer, and bladder cancer [167,168]. Recently, the approval of relatlimab (an ICI targeting LAG-3) based on a study showing that combined relatlimab with anti-PD-1 doubled the progression free survival of advanced melanoma patients compared to patients receiving PD-1 monotherapy highlights the growing therapeutic potential of ICIs [169].
The introduction of ICIs was a landmark turning point for targeted therapy in HNSCC. Several key clinical trials, including KEYNOTE-012 [170], KEYNOTE-040 [171], and CHECKMATE-141 [172], established the benefit of pembrolizumab and nivolumab in treating R/M HNSCC refractory to platinum base chemotherapy regardless of HPV status. However, subgroup analysis by HPV status also revealed an improved objective response rate (ORR) in HPV+ when compared to HPV− tumours, such as in the Phase 1b KEYNOTE-012 trial when treated with pembrolizumab (25% to 14%) [170]. This was corroborated by KEYNOTE-012, which trialled fixed-dose pembrolizumab and demonstrated an ORR of 32% in HPV+ tumours and 14% in HPV− tumours [173]. KEYNOTE-40, a Phase 3 clinical trial of Pembrolizumab, failed to identify a difference in ORR based on HPV status [171], while CHECKMATE-141 concluded that nivolumab improved OS in HPV+ tumours [172].
KEYNOTE-48, a Phase-3 clinical trial comparing pembrolizumab as a first line treatment in R/M HNSCC against standard of care EXTREME regime (platinum-based chemotherapy, 5-fluorouracil, and cetuximab), confirmed the superiority of pembrolizumab as initial treatment for this condition. However, HPV+ tumours were matched across treatment arms, attenuating the ability to determine the benefit of pembrolizumab by HPV status [174].
Neoadjuvant delivery of ICIs is also being trialled. CHECKMATE-358, a Phase 1/2 trial, concluded that HPV+HNSCC tumours demonstrated improved response to neoadjuvant nivolumab compared to HPV−HNSCC (23.5% vs. 5.9%) [102]. Meta-analysis of 12 clinical trials found that HPV+ tumours had an overall 1.29-fold higher likelihood of responding to ICI therapy than HPV− tumours [175]. These results established a precedence for further research into the efficacy of ICIs for HPV+OPSCC treatment specifically, several of which are still ongoing (Table 1).
Table 1.
Ongoing clinical trials related to HPV+OPSCC.
| Phase | Trial | Population | Therapy | Objectives | Status |
|---|---|---|---|---|---|
| 2 | NCT03799445 | Advanced HPV+ HNSCC | Concurrent ipilimumab + nivolumab + RT |
|
Recruiting |
| 2 | NCT03383094 | Intermediate/high-risk HPV+ locoregionally-advanced HNSCC | Concurrent and adjuvant pembrolizumab + RT vs. RT + cisplatin |
|
Recruiting |
| 2 | NCT04988074 | Advanced HPV+OPSCC | Neoadjuvant cemiplimab + TORS/RT +/− chemotherapy |
|
Recruiting |
| 2 | NCT04867330 | HPV+OPSCC | Toripalimab + docetaxel/cisplatin |
|
Recruiting |
| 3 | NCT04116047 | Intermediate/high-risk HPV+OPSCC | Durvalumab vs. chemoradiotherapy |
|
Recruiting |
| 2 | NCT03410615 | Intermediate-risk HPV+ locoregionally-advanced OPSCC | Durvalumab + RT + adjuvant Durvalumab vs. Durvalumab + RT + adjuvant Tremelimumab and Durvalumab vs. Cisplatin + RT |
|
Active, not recruiting |
| 2 | NCT03829722 | High-risk HPV+OPSCC | Concurrent nivolumab + RT + carboplatin |
|
Active, not recruiting |
| 2 | NCT03107182 | Locoregionally-advanced HPV+OPSCC | Nivolumab/Nab-paclitaxel/Carboplatin Induction Chemotherapy followed by Response-stratified Locoregional Therapy |
|
Active, not recruiting |
| 2 | NCT03838263 | High-risk HPV+OPSCC | Neoadjuvant nivolumab + chemoradiotherapy vs. chemoradiotherapy alone |
|
Active, not recruiting |
| 2/3 | NCT03952585 | Early-stage HPV+OPSCC | Concurrent reduced-dose RT + either nivolumab or cisplatin |
|
Suspended |
| 3 | NCT03811015 | Intermediate risk locally-advanced HPV+OPSCC | Definitive chemoradiotherapy followed by maintenance nivolumab |
|
Recruiting |
RT = radiotherapy. TORS = transoral robotic surgery
While there may be potential benefits of ICIs in the HPV+OPSCC cohort, these therapies unfortunately also carry the risk of immune-related adverse effects (irAEs). Common adverse effects observed include gastrointestinal (diarrhoea, nausea, vomiting), dermatological (dermatitis, rash), endocrinological (hypothyroidism), and systemic effects (fever, fatigue, headache, myalgia, arthralgia) [176]. The incidence of any irAEs in HNSCC patients on ICIs is approximately 57–67%, while more severe irAEs occur in 8–17% of HNSCC patients [177]. Severe irAEs may impact cardiopulmonary, hepatic, renal, neurological, gastrointestinal, and haematological systems. The mortality rate from irAEs is reported to be 0.3–1.03%, predominantly associated with gastrointestinal toxicity associated with Ipilimumab and pulmonary toxicity in PD-1 inhibition. These present further important considerations in establishing the most appropriate immunotherapy regimen for HPV+OPSCC.
Additionally, these drugs are associated with financial toxicity, representing a significant burden to the healthcare system. A systematic review determined that nivolumab was not a cost-effective therapy in the setting of R/M HNSCC over standard of care therapy (cetuximab, docetaxel, or methotrexate) given the high cost per quality-adjusted life years ($140,672 per QALY) [178]. While there are limitations to the estimations and calculation methods employed, it is important to keep in mind the financial burden of novel therapies as they are introduced into the clinical environment.
3. Emerging Therapeutic Strategies
Several new immune targets have been identified that present new opportunities to develop HPV-specific therapies.
3.1. Small Molecule Immunotherapy
3.1.1. RTK
Receptor tyrosine kinases (RTKs) are a family of cell surface receptors which regulate a range of homeostatic cellular processes including intercellular communication, metabolic functions, and cell differentiation [179]. The majority of HNSCCs are found with overexpression of many RTKs, including HER, FGFR, and VEGFR families. EGFR (or HER1) has particularly been shown to be highly expressed in most HNSCCs, leading to the investigation and approval of cetuximab, an anti-EGFR monoclonal antibody, for locally-advanced and R/M HNSCC irrespective of HPV status [180,181]. The ORR to cetuximab as a single agent is limited (~13%) [182] but when used with PD-1 inhibitors, its efficacy improves to 45% in treating R/M HNSCC [183].
However, several studies have demonstrated cetuximab’s low efficacy in HPV+OPSCC (reviewed in [184]). Two major Phase 3 trials found cetuximab to be inferior to standard cisplatin treatment in combination with radiotherapy for HPV+OPSCC [185,186]. A recent meta-analysis further revealed that the combination of cetuximab and a PD-1 inhibitor improved the OS rate of HPV−OPSCC only compared to PD-1 inhibitor monotherapy, while patients with HPV+OPSCC did not experience the same benefit [187]. Another Phase 3 trial investigated adding panitumumab, another anti-EGFR monoclonal antibody, to chemotherapy and found improved OS only in HPV−OPSCC [188]. This may potentially be due to the reduced EGFR expression generally found in HPV+OPSCC [189,190,191]. However, a correlation between EGFR expression and therapeutic response has never been demonstrated in HNSCC [192,193]. Further research is required to fully elucidate the mechanism behind this poor response in HPV+OPSCC.
Interestingly, HPV+OPSCC has been found to express higher levels of HER2 and HER3 [194,195]. One study discovered that HPV+ cells relied on HER3 specifically for cellular proliferation and that HER3 expression was regulated by HPV E6 and E7 oncogenes, indicating that HER3 might be a viable therapeutic target [196]. HER3 signalling has also been shown to be important in HPV−OPSCC as upregulation contributes to cells acquiring cetuximab resistance [197,198].
An anti-HER3 monoclonal antibody, CDX-3379 (previously called KTN3379), initially demonstrated promising results in pre-clinical trials. In a Phase I window trial, tumour regression was noted in 42% of patients with newly diagnosed HNSCC, including two of the three HPV+ patients [199]. A Phase 1b trial found one patient with R/M HNSCC who had tumour progression on cetuximab to have a prolonged complete response when CDX-3379 was added to cetuximab [200]. However, in a Phase 2 clinical trial using CDX-3379 and cetuximab in patients with HPV− R/M HNSCC, the limited overall response rate (6.7%) and significant adverse effects observed necessitated closure of the trial [201]. Further research may be able to identify predictive biomarkers for responses to anti-HER3 therapy and explore other agents such as an emerging anti-HER3 antibody-drug conjugate, U3-1402, which has shown anti-tumour activity in breast and lung cancer [202,203].
3.1.2. STAT
The signal transducer and activator of transcription (STAT) proteins are a group of transcription factors with large influence on tumour proliferation and chemotherapy resistance [204]. These have been found to promote carcinogenesis by facilitating metabolic alterations which modulate gene expression and cytokine/growth factor signalling pathways. Additionally, they can reprogram immune cells within the TME to favour immunosuppression [205] and induce HNSCC resistance to standard therapies.
Both HPV+OPSCC and HPV−OPSCC have been found to have overactivated STAT protein signalling [206]. Hence, STAT inhibitors have long been a research focus for HNSCC regardless of HPV status. Several studies have demonstrated that the combination of STAT inhibitors with radiotherapy [207,208,209], chemotherapy [210], anti-CTLA-4 and anti-PD-L1 ICIs [211,212], and anti-EGFR therapies like cetuximab [213] can improve tumour response and ameliorate resistance. As a single agent, C188-9, a small-molecule STAT3 inhibitor, was found to have an in vitro anti-tumour effect on HNSCC cells [214].
In various HPV-related cancers, the JAK/STAT signalling pathway has also been found to be a primary pathway manipulated by HPV oncoproteins to facilitate ongoing viral replication [215]. Cervical cancer cells have been found to have high levels of STAT3 expression, and small molecule inhibition of STAT has been shown to facilitate cervical cancer cell death in vitro [216]. STAT inhibitors have not yet been trialled specifically in HPV+OPSCC but may prove to be an area of further interest.
3.1.3. STING
Stimulator of interferon genes (STING) is an important protein which, when activated, phosphorylates interferon regulatory factor 3 (IRF3) and promotes interferon production [205]. This has been found to be crucial in mediating innate immunity anti-viral and anti-tumour responses [217]. Recent research has found HPV oncogenes E7 and E5 to strongly antagonise the STING pathway in order to facilitate immune evasion [80,117,118,119]. Therefore, the development of STING activators may theoretically improve tumour susceptibility to immune destruction. Indeed, use of a STING activator in several cancer types, including cervical cancer [218], has demonstrated promising results in reducing tumour growth [219,220]. While it has not been used as a single agent in HPV+OPSCC, evidence suggests that preclinical co-administration of STING activators may improve tumour responses to ICIs [221] or cetuximab [222] in HPV+OSPCC cells.
3.1.4. PPAR
Peroxisome proliferator-activated receptors (PPARs) are gene transcription regulators belonging to the nuclear hormone receptor family. The three identified isoforms (PPARα, PPARβ/δ, and PPARγ) are uniquely expressed by distinct tissue types but all contribute to maintaining homeostatic metabolic activity [223]. They have also been implicated in the development of various diseases like atherosclerosis, Type 2 diabetes, and cancer. PPARs have also been identified also have a role in hepatocellular [224], pancreatic [225], lung [226], and breast carcinogenesis [227]. In cervical cancer, some evidence has found PPARγ to be anti-proliferative. Not only is it downregulated in cervical cancer cells [228], but treatment with a PPARγ agonist seems to enhance apoptosis [229] while PPARγ inhibition supports tumour progression [230]. One study posits that the mechanism for this involves HPV16 E7 inhibiting PPARγ expression via an increase in miR-27b to facilitate tumour proliferation [230].
Little research has gone into PPARs and HPV+OPSCC specifically. However, one paper found that fenofibrate demonstrated substantial anti-tumorigenic effects on HPV+ HNSCC cells, especially in combination with cisplatin administration [231]. Fenofibrate is an established drug for dyslipidaemia known to act on PPARα pathways; however, it is unclear if PPARα is similarly involved in its anti-tumour activity. The same study found increased p53 activation and immune cell infiltration with fenofibrate [231], which makes it an interesting research target for future HPV+OPSCC therapy.
3.1.5. AHR
Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that, when chronically activated, has a strong role in supporting tumour invasion, migration, and metastasis including in HNSCC [232,233,234,235]. Additionally, AHR can have immunosuppressive effects on CD8+ TILs by inducing PD-1 and antigen-presenting cells [236]. No research has been published on AHRs in HPV+OPSCC; however, when a natural AHR ligand, indole-3-carbinol, was administered to cervical cancer cells, it was found to hinder cell proliferation [237], indicating that this may be a target for future exploration.
3.1.6. NET Based Therapies
Polyanions
Potential polyanion agents aim to capitalise on electrostatic interaction between highly cationic components of NETs (i.e., histones) to inhibit pro-tumorigenic effects, namely tumour cell camouflage, migration, and dormant tumour cell reactivation. They may also interfere with pro-tumorigenic histone-dependent pathways, including TLR4/histone-dependent TME immunosuppression, histone-dependent endothelial, and platelet activation and thrombosis, all of which confer tumour cell survival and metastatic ability. Heparin particularly has been shown to degrade NETs. These polyanionic compounds include STC3141 (methyl β-cellobioside per-O-sulfate), unfractionated heparin, and enoxaparin [136,238,239].
3.1.7. NET Modulators or Preventors
Several agents have been shown to inhibit NET expression or production, including sivelestat, a neutrophil elastase inhibitor. NETosis and NET formation is dependent on neutrophil elastase and the pro-tumorigenic role of neutrophil elastase has been identified in several cancers [240,241,242]. In a murine model of colorectal cancer, sivelestat has been shown to suppress liver metastasis.
The COX-1 inhibitor, aspirin, has been shown to reduce neutrophil tissue invasion and NET production, believed to be caused by inhibition of CCL5 (RANTES) and CXCL4 (PF4) release, both shown to increase neutrophil chemotaxis. Several studies have demonstrated the anti-metastatic benefit produced through attenuation of NET production by COX-1 inhibition [243,244,245].
Studies have demonstrated that metformin decreases NET production, even in the presence of NET stimulants and recent evidence in hepatocellular carcinoma and pancreatic cancer confirmed that metformin reduced the production of NETs and the metastatic potential of these two cancers [246,247,248]. This has subsequently been corroborated in preclinical animal models [249].
3.1.8. NET Degraders
Dornase Alfa (rhDNase 1) is a recombinant human deoxyribonuclease that can selectively cleave DNA. Park and colleagues have demonstrated that ability of rhDNase 1 to reduce the 4T1 breast cancer metastatic burden in a preclinical model, while further preclinical studies have shown that it can reduce tumour progression in pancreatic cancer [157,247].
4. Conclusions
HPV+ and HPV−OPSCC are very different tumours and the immune system plays an integral role in this difference. Personalised treatment strategies remain to be determined; however, greater insight into the specific interaction between the HPV virus and the immune system for this cancer will help define immunotherapeutic strategy. As our understanding of this relationship improves, there exists an exciting potential for the development of novel small molecule immunotherapies that may help to de-escalate standard of care interventions, improving survival and attenuating functional deficits associated with treatment.
Acknowledgments
The figures were created using BioRender under private licence.
Abbreviations
| HPV+OPSCC | Human Papillomavirus Positive Oropharyngeal Squamous Cell Carcinoma |
| HPV−OPSCC | Human Papillomavirus Negative Oropharyngeal Squamous Cell Carcinoma |
Author Contributions
Conceptualization, A.K., L.M.K. and C.H.O.; writing—original draft preparation, A.K., C.H.O. and L.M.K.; writing—review and editing, A.K., C.H.O., M.B., Z.J., T.M., T.P., L.M.K., P.F., E.D., K.F., D.S.J. and J.G.; figure preparation, A.K., Z.J. and C.H.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data for this review article was sourced from reporting manuscripts.
Conflicts of Interest
There are no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Syrjänen K.J., Pyrhönen S., Syrjänen S.M., Lamberg M.A. Immunohistochemical demonstration of Human papilloma virus (HPV) antigens in oral squamous cell lesions. Br. J. Oral Surg. 1983;21:147–153. doi: 10.1016/0007-117X(83)90060-4. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira C.C. The relation between human papillomavirus (HPV) and oropharyngeal cancer: A review. PeerJ. 2023;11:e15568. doi: 10.7717/peerj.15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiwert T.Y., Zuo Z., Keck M.K., Khattri A., Pedamallu C.S., Stricker T., Brown C., Pugh T.J., Stojanov P., Cho J., et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung C.H., Guthrie V.B., Masica D.L., Tokheim C., Kang H., Richmon J., Agrawal N., Fakhry C., Quon H., Subramaniam R.M., et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann. Oncol. 2015;26:1216–1223. doi: 10.1093/annonc/mdv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welters M.J.P., Santegoets S.J., van der Burg S.H. The Tumor Microenvironment and Immunotherapy of Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2020;10:545385. doi: 10.3389/fonc.2020.545385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillison M.L., D’Souza G., Westra W., Sugar E., Xiao W., Begum S., Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 8.Gillison M.L., Broutian T., Pickard R.K., Tong Z.Y., Xiao W., Kahle L., Graubard B.I., Chaturvedi A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marur S., D’Souza G., Westra W.H., Forastiere A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellin H., Friesland S., Lewensohn R., Dalianis T., Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Posner M.R., Lorch J.H., Goloubeva O., Tan M., Schumaker L.M., Sarlis N.J., Haddad R.I., Cullen K.J. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann. Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIlwain W.R., Sood A.J., Nguyen S.A., Day T.A. Initial Symptoms in Patients with HPV-Positive and HPV-Negative Oropharyngeal Cancer. JAMA Otolaryngol. Head Neck Surg. 2014;140:441–447. doi: 10.1001/jamaoto.2014.141. [DOI] [PubMed] [Google Scholar]
- 13.Gillison M.L. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29:779–792. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 14.Gillison M.L., Zhang Q., Jordan R., Xiao W., Westra W.H., Trotti A., Spencer S., Harris J., Chung C.H., Ang K.K. Tobacco Smoking and Increased Risk of Death and Progression for Patients with p16-Positive and p16-Negative Oropharyngeal Cancer. J. Clin. Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tramacere I., Negri E., Bagnardi V., Garavello W., Rota M., Scotti L., Islami F., Corrao G., Boffetta P., La Vecchia C. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: Overall results and dose-risk relation. Oral Oncol. 2010;46:497–503. doi: 10.1016/j.oraloncology.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos P., van den Brekel M.W.M., Gouw Z.A.R., Al-Mamgani A., Waktola S., Aerts H., Beets-Tan R.G.H., Castelijns J.A., Jasperse B. Clinical variables and magnetic resonance imaging-based radiomics predict human papillomavirus status of oropharyngeal cancer. Head Neck. 2021;43:485–495. doi: 10.1002/hed.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H., Forastiere A., Gillison M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan B. Head and neck tumours. UICC TNM Classif. Malig. Tumours. 2017;8:17–54. [Google Scholar]
- 21.Morris L.G., Sikora A.G., Patel S.G., Hayes R.B., Ganly I. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol. 2011;29:739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen A.M. De-Escalation Treatment for Human Papillomavirus–Related Oropharyngeal Cancer: Questions for Practical Consideration. Oncology. 2023;37:281–287. doi: 10.46883/2023.25921000. [DOI] [PubMed] [Google Scholar]
- 23.Silver J.A., Turkdogan S., Roy C.F., Subramaniam T., Henry M., Sadeghi N. De-Escalation Strategies for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma—Where Are We Now? Curr. Oncol. 2022;29:3668–3697. doi: 10.3390/curroncol29050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhalawani H., Mohamed A.S.R., Elgohari B., Lin T.A., Sikora A.G., Lai S.Y., Abusaif A., Phan J., Morrison W.H., Gunn G.B., et al. Tobacco exposure as a major modifier of oncologic outcomes in human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma. BMC Cancer. 2020;20:912. doi: 10.1186/s12885-020-07427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Y.H., Su C.C., Wu S.Y., Hsueh W.T., Wu Y.H., Chen H.H.W., Hsiao J.R., Liu C.H., Tsai Y.S. Impact of Alcohol and Smoking on Outcomes of HPV-Related Oropharyngeal Cancer. J. Clin. Med. 2022;11:6510. doi: 10.3390/jcm11216510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskovitz J., Moy J., Ferris R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018;20:22. doi: 10.1007/s11912-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 28.Mariz B., Kowalski L.P., William W.N., Jr., de Castro G., Jr., Chaves A.L.F., Santos M., de Oliveira T.B., Araújo A.L.D., Normando A.G.C., Ribeiro A.C.P., et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020;156:103116. doi: 10.1016/j.critrevonc.2020.103116. [DOI] [PubMed] [Google Scholar]
- 29.Lechner M., Jones O.S., Breeze C.E., Gilson R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 2019;19:131–132. doi: 10.1016/S1473-3099(18)30802-8. [DOI] [PubMed] [Google Scholar]
- 30.Faraji F., Rettig E.M., Tsai H.L., El Asmar M., Fung N., Eisele D.W., Fakhry C. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer. 2019;125:761–769. doi: 10.1002/cncr.31841. [DOI] [PubMed] [Google Scholar]
- 31.Carlander A.F., Jakobsen K.K., Bendtsen S.K., Garset-Zamani M., Lynggaard C.D., Jensen J.S., Grønhøj C., Buchwald C.V. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses. 2021;13:1326. doi: 10.3390/v13071326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y., Xie Z., Luo G., Yan H., Qian H.-Z., Fu L., Wang B., Huang R., Cao F., Lin H., et al. Global burden of oropharyngeal cancer attributable to human papillomavirus by anatomical subsite and geographic region. Cancer Epidemiol. 2022;78:102140. doi: 10.1016/j.canep.2022.102140. [DOI] [PubMed] [Google Scholar]
- 33.Ndon S., Singh A., Ha P.K., Aswani J., Chan J.Y.-K., Xu M.J. Human Papillomavirus-Associated Oropharyngeal Cancer: Global Epidemiology and Public Policy Implications. Cancers. 2023;15:4080. doi: 10.3390/cancers15164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argirion I., Zarins K.R., McHugh J., Cantley R.L., Teeramatwanich W., Laohasiriwong S., Kasemsiri P., Naruikon J., Srimanta P., Chinn S.B., et al. Increasing prevalence of HPV in oropharyngeal carcinoma suggests adaptation of p16 screening in Southeast Asia. J. Clin. Virol. 2020;132:104637. doi: 10.1016/j.jcv.2020.104637. [DOI] [PubMed] [Google Scholar]
- 35.Hwang T.Z., Hsiao J.R., Tsai C.R., Chang J.S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int. J. Cancer. 2015;137:395–408. doi: 10.1002/ijc.29330. [DOI] [PubMed] [Google Scholar]
- 36.Rietbergen M.M., van Bokhoven A., Lissenberg-Witte B.I., Heideman D.A.M., Leemans C.R., Brakenhoff R.H., Bloemena E. Epidemiologic associations of HPV-positive oropharyngeal cancer and (pre)cancerous cervical lesions. Int. J. Cancer. 2018;143:283–288. doi: 10.1002/ijc.31315. [DOI] [PubMed] [Google Scholar]
- 37.Hong A., Lee C.S., Jones D., Veillard A.S., Zhang M., Zhang X., Smee R., Corry J., Porceddu S., Milross C., et al. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck. 2016;38:743–750. doi: 10.1002/hed.23942. [DOI] [PubMed] [Google Scholar]
- 38.Zumsteg Z.S., Luu M., Rosenberg P.S., Elrod J.K., Bray F., Vaccarella S., Gay C., Lu D.J., Chen M.M., Chaturvedi A.K., et al. Global epidemiologic patterns of oropharyngeal cancer incidence trends. J. Natl. Cancer Inst. 2023;115:1544–1554. doi: 10.1093/jnci/djad169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2023;41:3081–3088. doi: 10.1200/JCO.22.02625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvajal L.J., Shing J.Z., Vanegas J.C., González E., Guillén D., Sierra M.S., Hildesheim A., Porras C., Herrero R., Torres G., et al. Trends in incidence rates of head and neck squamous cell carcinomas overall and by potential relatedness to human papillomavirus, Costa Rica 2006 to 2015. Int. J. Cancer. 2023;152:2052–2060. doi: 10.1002/ijc.34437. [DOI] [PubMed] [Google Scholar]
- 41.Wittekindt C., Wagner S., Bushnak A., Prigge E.S., von Knebel Doeberitz M., Würdemann N., Bernhardt K., Pons-Kühnemann J., Maulbecker-Armstrong C., Klussmann J.P. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev. Res. 2019;12:375–382. doi: 10.1158/1940-6207.CAPR-19-0098. [DOI] [PubMed] [Google Scholar]
- 42.Donà M.G., Rollo F., Pichi B., Spriano G., Moretto S., Covello R., Pellini R., Benevolo M. Evolving Profile of HPV-Driven Oropharyngeal Squamous Cell Carcinoma in a National Cancer Institute in Italy: A 10-Year Retrospective Study. Microorganisms. 2020;8:1498. doi: 10.3390/microorganisms8101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kombe Kombe A.J., Li B., Zahid A., Mengist H.M., Bounda G.A., Zhou Y., Jin T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health. 2021;8:552028. doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osazuwa-Peters N., Massa S.T., Simpson M.C., Boakye E.A., Varvares M.A. Survival of human papillomavirus-associated cancers: Filling in the gaps. Cancer. 2018;124:18–20. doi: 10.1002/cncr.30945. [DOI] [PubMed] [Google Scholar]
- 45.Rettig E.M., Zaidi M., Faraji F., Eisele D.W., El Asmar M., Fung N., D’Souza G., Fakhry C. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018;83:147–153. doi: 10.1016/j.oraloncology.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cline B.J., Simpson M.C., Gropler M., Bukatko A.R., Adjei Boakye E., Mohammed K.A., Osazuwa-Peters N. Change in Age at Diagnosis of Oropharyngeal Cancer in the United States, 1975–2016. Cancers. 2020;12:3191. doi: 10.3390/cancers12113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tota J.E., Best A.F., Zumsteg Z.S., Gillison M.L., Rosenberg P.S., Chaturvedi A.K. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J. Clin. Oncol. 2019;37:1538–1546. doi: 10.1200/JCO.19.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu D.J., Luu M., Mita A., Scher K., Shiao S.L., Yoshida E.P., Sittig M.P., Mallen-St Clair J., Ho A.S., Zumsteg Z.S. Human papillomavirus–associated oropharyngeal cancer among patients aged 70 and older: Dramatically increased prevalence and clinical implications. Eur. J. Cancer. 2018;103:195–204. doi: 10.1016/j.ejca.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Windon M.J., D’Souza G., Rettig E.M., Westra W.H., van Zante A., Wang S.J., Ryan W.R., Mydlarz W.K., Ha P.K., Miles B.A., et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer. 2018;124:2993–2999. doi: 10.1002/cncr.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyss A., Hashibe M., Chuang S.C., Lee Y.C., Zhang Z.F., Yu G.P., Winn D.M., Wei Q., Talamini R., Szeszenia-Dabrowska N., et al. Cigarette, Cigar, and Pipe Smoking and the Risk of Head and Neck Cancers: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013;178:679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Maso L.D., Daudt A.W., Fabianova E., Wünsch-Filho V. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 52.Guha N., Warnakulasuriya S., Vlaanderen J., Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer. 2014;135:1433–1443. doi: 10.1002/ijc.28643. [DOI] [PubMed] [Google Scholar]
- 53.Guidry J.T., Birdwell C.E., Scott R.S. Epstein–Barr virus in the pathogenesis of oral cancers. Oral Dis. 2017;24:497–508. doi: 10.1111/odi.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Souza G., Kreimer A.R., Viscidi R., Pawlita M., Fakhry C., Koch W.M., Westra W.H., Gillison M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 55.Andrews E., Seaman W.T., Webster-Cyriaque J. Oropharyngeal carcinoma in non-smokers and non-drinkers: A role for HPV. Oral Oncol. 2009;45:486–491. doi: 10.1016/j.oraloncology.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Alotaibi M., Valova V., HÄnsel T., Stromberger C., Kofla G., Olze H., Piwonski I., Albers A., Ochsenreither S., Coordes A. Impact of Smoking on the Survival of Patients with High-risk HPV-positive HNSCC: A Meta-analysis. Vivo. 2021;35:1017–1026. doi: 10.21873/invivo.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grønhøj C., Jensen J.S., Wagner S., Dehlendorff C., Friborg J., Andersen E., Wittekindt C., Würdemann N., Sharma S.J., Gattenlöhner S., et al. Impact on survival of tobacco smoking for cases with oropharyngeal squamous cell carcinoma and known human papillomavirus and p16-status: A multicenter retrospective study. Oncotarget. 2019;10:4655–4663. doi: 10.18632/oncotarget.27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muñoz J.P., Carrillo-Beltrán D., Aedo-Aguilera V., Calaf G.M., León O., Maldonado E., Tapia J.C., Boccardo E., Ozbun M.A., Aguayo F. Tobacco Exposure Enhances Human Papillomavirus 16 Oncogene Expression via EGFR/PI3K/Akt/c-Jun Signaling Pathway in Cervical Cancer Cells. Front. Microbiol. 2018;9:3022. doi: 10.3389/fmicb.2018.03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Z., Sun P., Dahlstrom K.R., Gross N., Li G. Joint effect of human papillomavirus exposure, smoking and alcohol on risk of oral squamous cell carcinoma. BMC Cancer. 2023;23:457. doi: 10.1186/s12885-023-10948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eldridge R.C., Pawlita M., Wilson L., Castle P.E., Waterboer T., Gravitt P.E., Schiffman M., Wentzensen N. Smoking and subsequent human papillomavirus infection: A mediation analysis. Ann. Epidemiol. 2017;27:724–730.e1. doi: 10.1016/j.annepidem.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arif R.T., Mogaddam M.A., Merdad L.A., Farsi N.J. Does human papillomavirus modify the risk of oropharyngeal cancer related to smoking and alcohol drinking? A systematic review and meta-analysis. Laryngoscope Investig. Otolaryngol. 2022;7:1391–1401. doi: 10.1002/lio2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Applebaum K.M., Furniss C.S., Zeka A., Posner M.R., Smith J.F., Bryan J., Eisen E.A., Peters E.S., McClean M.D., Kelsey K.T. Lack of Association of Alcohol and Tobacco with HPV16-Associated Head and Neck Cancer. J. Natl. Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 63.Farsi N.J., El-Zein M., Gaied H., Lee Y.C., Hashibe M., Nicolau B., Rousseau M.C. Sexual behaviours and head and neck cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2015;39:1036–1046. doi: 10.1016/j.canep.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Wichmann G., Rudolph J., Henger S., Engel C., Wirkner K., Wenning J.R., Zeynalova S., Wiegand S., Loeffler M., Wald T., et al. Is High-Risk Sexual Behavior a Risk Factor for Oropharyngeal Cancer? Cancers. 2023;15:3356. doi: 10.3390/cancers15133356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talamini R., Vaccarella S., Barbone F., Tavani A., La Vecchia C., Herrero R., Muñoz N., Franceschi S. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br. J. Cancer. 2000;83:1238–1242. doi: 10.1054/bjoc.2000.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenquist K., Wennerberg J., Schildt E.B., Bladström A., Göran Hansson B., Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 67.Leiding J.W., Holland S.M. Warts and all: Human papillomavirus in primary immunodeficiencies. J. Allergy Clin. Immunol. 2012;130:1030–1048. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Béziat V. Human genetic dissection of papillomavirus-driven diseases: New insight into their pathogenesis. Hum. Genet. 2020;139:919–939. doi: 10.1007/s00439-020-02183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hewavisenti R.V., Arena J., Ahlenstiel C.L., Sasson S.C. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front. Immunol. 2023;14:1112513. doi: 10.3389/fimmu.2023.1112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S.Y., Solomon D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 2010;6:165–174. doi: 10.1038/nrrheum.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S.C., Glynn R.J., Giovannucci E., Hernández-Díaz S., Liu J., Feldman S., Karlson E.W., Schneeweiss S., Solomon D.H. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: A population-based cohort study. Ann. Rheum. Dis. 2015;74:1360–1367. doi: 10.1136/annrheumdis-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaturvedi A.K., Madeleine M.M., Biggar R.J., Engels E.A. Risk of Human Papillomavirus–Associated Cancers among Persons with AIDS. JNCI J. Natl. Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beachler D.C., Abraham A.G., Silverberg M.J., Jing Y., Fakhry C., Gill M.J., Dubrow R., Kitahata M.M., Klein M.B., Burchell A.N., et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol. 2014;50:1169–1176. doi: 10.1016/j.oraloncology.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araldi R.P., Sant’Ana T.A., Módolo D.G., de Melo T.C., Spadacci-Morena D.D., de Cassia Stocco R., Cerutti J.M., de Souza E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018;106:1537–1556. doi: 10.1016/j.biopha.2018.06.149. [DOI] [PubMed] [Google Scholar]
- 75.Mikuličić S., Strunk J., Florin L. HPV16 Entry into Epithelial Cells: Running a Gauntlet. Viruses. 2021;13:2460. doi: 10.3390/v13122460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.IARC . Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- 77.Kreimer A.R., Clifford G.M., Boyle P., Franceschi S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 78.de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B., Tous S., Felix A., Bravo L.E., Shin H.R., et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 79.Fehrmann F., Laimins L.A. Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- 80.Miyauchi S., Kim S.S., Jones R.N., Zhang L., Guram K., Sharma S., Schoenberger S.P., Cohen E.E.W., Califano J.A., Sharabi A.B. Human papillomavirus E5 suppresses immunity via inhibition of the immunoproteasome and STING pathway. Cell Rep. 2023;42:112508. doi: 10.1016/j.celrep.2023.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Engeland K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022;29:946–960. doi: 10.1038/s41418-022-00988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLaughlin-Drubin M.E., Park D., Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA. 2013;110:16175–16180. doi: 10.1073/pnas.1310432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mir B.A., Ahmad A., Farooq N., Priya M.V., Siddiqui A.H., Asif M., Manzoor R., Ishqi H.M., Alomar S.Y., Rahaman P.F. Increased expression of HPV-E7 oncoprotein correlates with a reduced level of pRb proteins via high viral load in cervical cancer. Sci. Rep. 2023;13:15075. doi: 10.1038/s41598-023-42022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soto D., Song C., McLaughlin-Drubin M.E. Epigenetic Alterations in Human Papillomavirus-Associated Cancers. Viruses. 2017;9:248. doi: 10.3390/v9090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas M., Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. Pt 6J. Gen. Virol. 1999;80:1513–1517. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 86.Kazemi S., Papadopoulou S., Li S., Su Q., Wang S., Yoshimura A., Matlashewski G., Dever T.E., Koromilas A.E. Control of α Subunit of Eukaryotic Translation Initiation Factor 2 (eIF2α) Phosphorylation by the Human Papillomavirus Type 18 E6 Oncoprotein: Implications for eIF2α-Dependent Gene Expression and Cell Death. Mol. Cell. Biol. 2004;24:3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garnett T.O., Filippova M., Duerksen-Hughes P.J. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006;13:1915–1926. doi: 10.1038/sj.cdd.4401886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Storey A., Thomas M., Kalita A., Harwood C., Gardiol D., Mantovani F., Breuer J., Leigh I.M., Matlashewski G., Banks L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 89.Nagasaka K., Kawana K., Osuga Y., Fujii T. PDZ Domains and Viral Infection: Versatile Potentials of HPV-PDZ Interactions in relation to Malignancy. BioMed Res. Int. 2013;2013:369712. doi: 10.1155/2013/369712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katzenellenbogen R. Telomerase Induction in HPV Infection and Oncogenesis. Viruses. 2017;9:180. doi: 10.3390/v9070180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schache A.G., Liloglou T., Risk J.M., Jones T.M., Ma X.J., Wang H., Bui S., Luo Y., Sloan P., Shaw R.J., et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br. J. Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fauzi F.H., Hamzan N.I., Ab Rahman N., Suraiya S., Mohamad S. Detection of human papillomavirus in oropharyngeal squamous cell carcinoma. J. Zhejiang Univ. Sci. B. 2020;21:961–976. doi: 10.1631/jzus.B2000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prigge E.-S., Arbyn M., von Knebel Doeberitz M., Reuschenbach M. Diagnostic accuracy of p16INK4aimmunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer. 2017;140:1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 94.Brisson M., Kim J.J., Canfell K., Drolet M., Gingras G., Burger E.A., Martin D., Simms K.T., Bénard É., Boily M.-C., et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nielsen K.J., Jakobsen K.K., Jensen J.S., Grønhøj C., Von Buchwald C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses. 2021;13:1339. doi: 10.3390/v13071339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsentemeidou A., Fyrmpas G., Stavrakas M., Vlachtsis K., Sotiriou E., Poutoglidis A., Tsetsos N. Human Papillomavirus Vaccine to End Oropharyngeal Cancer. A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2021;48:700–707. doi: 10.1097/OLQ.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 97.Katz J. The impact of HPV vaccination on the prevalence of oropharyngeal cancer (OPC) in a hospital-based population: A cross-sectional study of patient’s registry. J. Oral Pathol. Med. 2021;50:47–51. doi: 10.1111/jop.13091. [DOI] [PubMed] [Google Scholar]
- 98.Bruni L., Saura-Lázaro A., Montoliu A., Brotons M., Alemany L., Diallo M.S., Afsar O.Z., LaMontagne D.S., Mosina L., Contreras M., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 99.World Health Organisation Global Partners Cheer Progress towards Eliminating Cervical Cancer and Underline Challenges. 2023. [(accessed on 19 January 2024)]. Available online: https://www.who.int/news/item/17-11-2023-global-partners-cheer-progress-towards-eliminating-cervical-cancer-and-underline-challenges.
- 100.Dykens J.A., Peterson C.E., Holt H.K., Harper D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health. 2023;11:1067299. doi: 10.3389/fpubh.2023.1067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.World Health Organization . Human Papillomavirus (HPV) Vaccination Coverage. World Health Organization; Geneva, Switzerland: 2023. [Google Scholar]
- 102.Ferris R.L., Spanos W.C., Leidner R., Gonçalves A., Martens U.M., Kyi C., Sharfman W., Chung C.H., Devriese L.A., Gauthier H., et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J. Immunother. Cancer. 2021;9:e002568. doi: 10.1136/jitc-2021-002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Fakhry C., D’souza G. Projected Association of Human Papillomavirus Vaccination with Oropharynx Cancer Incidence in the US, 2020–2045. JAMA Oncol. 2021;7:e212907. doi: 10.1001/jamaoncol.2021.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 105.Finn O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012;23:viii6–viii9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiam-Galvez K.J., Allen B.M., Spitzer M.H. Systemic immunity in cancer. Nat. Rev. Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 108.Stern P.L. Harnessing immunity for therapy in human papillomavirus driven cancers. Tumour Virus Res. 2021;11:200212. doi: 10.1016/j.tvr.2021.200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nunes R.A.L., Morale M.G., Silva G.F., Villa L.L., Termini L. Innate immunity and HPV: Friends or foes. Clinics. 2018;73:e549s. doi: 10.6061/clinics/2018/e549s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steinbach A., Riemer A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer. 2018;142:224–229. doi: 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 111.Manzo-Merino J., Del-Toro-Arreola S., Rocha-Zavaleta L., Peralta-Zaragoza Ó., Jiménez-Lima R., Madrid-Marina V. Immunology of cervical cancer. Rev. Investig. Clin. 2020;72:188–197. doi: 10.24875/RIC.20000057. [DOI] [PubMed] [Google Scholar]
- 112.Schiffman M., Doorbar J., Wentzensen N., de Sanjosé S., Fakhry C., Monk B.J., Stanley M.A., Franceschi S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 113.Stanley M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012;25:215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.HPV and Cervical Cancer Achievements in Prevention and Future Prospects. Anticancer Res. 2012;32:3595. [Google Scholar]
- 115.Cai X., Chiu Y.-H., Chen Zhijian J. The cGAS-cGAMP-STING Pathway of Cytosolic DNA Sensing and Signaling. Mol. Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 116.Lau L., Gray E.E., Brunette R.L., Stetson D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350:568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 117.Lou M., Huang D., Zhou Z., Shi X., Wu M., Rui Y., Su J., Zheng W., Yu X.F. DNA virus oncoprotein HPV18 E7 selectively antagonizes cGAS-STING-triggered innate immune activation. J. Med. Virol. 2023;95:e28310. doi: 10.1002/jmv.28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shaikh M.H., Bortnik V., McMillan N.A., Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019;132:162–165. doi: 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 119.Luo X., Donnelly C.R., Gong W., Heath B.R., Hao Y., Donnelly L.A., Moghbeli T., Tan Y.S., Lin X., Bellile E., et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020;130:1635–1652. doi: 10.1172/JCI129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karim R., Meyers C., Backendorf C., Ludigs K., Offringa R., van Ommen G.J., Melief C.J., van der Burg S.H., Boer J.M. Human Papillomavirus Deregulates the Response of a Cellular Network Comprising of Chemotactic and Proinflammatory Genes. PLoS ONE. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caberg J.H., Hubert P., Herman L., Herfs M., Roncarati P., Boniver J., Delvenne P. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: Role of CCL20. Cancer Immunol. Immunother. 2009;58:39–47. doi: 10.1007/s00262-008-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cicchini L., Westrich J.A., Xu T., Vermeer D.W., Berger J.N., Clambey E.T., Lee D., Song J.I., Lambert P.F., Greer R.O., et al. Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14. mBio. 2016;7:e00270-16. doi: 10.1128/mBio.00270-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou C., Tuong Z.K., Frazer I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019;9:682. doi: 10.3389/fonc.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reiser J., Hurst J., Voges M., Krauss P., Münch P., Iftner T., Stubenrauch F. High-Risk Human Papillomaviruses Repress Constitutive Kappa Interferon Transcription via E6 To Prevent Pathogen Recognition Receptor and Antiviral-Gene Expression. J. Virol. 2011;85:11372–11380. doi: 10.1128/JVI.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nees M., Geoghegan J.M., Hyman T., Frank S., Miller L., Woodworth C.D. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 2001;75:4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neophytou C.M., Panagi M., Stylianopoulos T., Papageorgis P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers. 2021;13:2053. doi: 10.3390/cancers13092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mandal R., Şenbabaoğlu Y., Desrichard A., Havel J.J., Dalin M.G., Riaz N., Lee K.W., Ganly I., Hakimi A.A., Chan T.A., et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tosi A., Parisatto B., Menegaldo A., Spinato G., Guido M., Del Mistro A., Bussani R., Zanconati F., Tofanelli M., Tirelli G., et al. The immune microenvironment of HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma: A multiparametric quantitative and spatial analysis unveils a rationale to target treatment-naïve tumors with immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2022;41:279. doi: 10.1186/s13046-022-02481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mito I., Takahashi H., Kawabata-Iwakawa R., Ida S., Tada H., Chikamatsu K. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 2021;11:16134. doi: 10.1038/s41598-021-95718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matlung S.E., van Kempen P.M.W., Bovenschen N., van Baarle D., Willems S.M. Differences in T-cell infiltrates and survival between HPV+ and HPV− oropharyngeal squamous cell carcinoma. Future Sci. OA. 2016;2:FSO88. doi: 10.4155/fso.15.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gurin D., Slavik M., Hermanova M., Selingerova I., Kazda T., Hendrych M., Shatokhina T., Vesela M. The tumor immune microenvironment and its implications for clinical outcome in patients with oropharyngeal squamous cell carcinoma. J. Oral Pathol. Med. 2020;49:886–896. doi: 10.1111/jop.13055. [DOI] [PubMed] [Google Scholar]
- 133.Welters M.J.P., Ma W., Santegoets S., Goedemans R., Ehsan I., Jordanova E.S., van Ham V.J., van Unen V., Koning F., van Egmond S.I., et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018;24:634–647. doi: 10.1158/1078-0432.CCR-17-2140. [DOI] [PubMed] [Google Scholar]
- 134.Whitmarsh A., Pring M., Thomas S.J., Waylen A., Ness A.R., Dudding T., Pawlita M., Brenner N., Waterboer T., Schroeder L. Survival advantage in patients with human papillomavirus-driven oropharyngeal cancer and variation by demographic characteristics and serologic response: Findings from Head and Neck 5000. Cancer. 2021;127:2442–2452. doi: 10.1002/cncr.33505. [DOI] [PubMed] [Google Scholar]
- 135.Kędzierawski P., Huruk-Kuchinka A., Radowicz-Chil A., Mężyk R., Rugała Z., Sadowski J. Human papillomavirus infection predicts better survival rate in patients with an oropharyngeal cancer. Arch. Med. Sci. 2021;17:1308–1316. doi: 10.5114/aoms.2019.83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Borsetto D., Tomasoni M., Payne K., Polesel J., Deganello A., Bossi P., Tysome J.R., Masterson L., Tirelli G., Tofanelli M., et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers. 2021;13:781. doi: 10.3390/cancers13040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pokrývková B., Grega M., Klozar J., Vencálek O., Nunvář J., Tachezy R. PD1(+)CD8(+) Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer. Biomedicines. 2022;10:2794. doi: 10.3390/biomedicines10112794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ward M.J., Thirdborough S.M., Mellows T., Riley C., Harris S., Suchak K., Webb A., Hampton C., Patel N.N., Randall C.J., et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baudouin R., Hans S., Lisan Q., Morin B., Adimi Y., Martin J., Lechien J.R., Tartour E., Badoual C. Prognostic Significance of the Microenvironment in Human Papillomavirus Oropharyngeal Carcinoma: A Systematic Review. Laryngoscope. 2023. Early View . [DOI] [PubMed]
- 140.Näsman A., Romanitan M., Nordfors C., Grün N., Johansson H., Hammarstedt L., Marklund L., Munck-Wikland E., Dalianis T., Ramqvist T. Tumor Infiltrating CD8+ and Foxp3+ Lymphocytes Correlate to Clinical Outcome and Human Papillomavirus (HPV) Status in Tonsillar Cancer. PLoS ONE. 2012;7:e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamila H., Vladimír K., Jan B., Jan L., Marek G., Miroslav H., Michal Z., Milan V., Kateřina R., Hana V., et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. ImmunoTherapy Cancer. 2019;7:261. doi: 10.1186/s40425-019-0726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Z., Wang Q., Tao Y., Chen J., Yuan Z., Wang P. Characterization of immune microenvironment in patients with HPV-positive and negative head and neck cancer. Sci. Data. 2023;10:694. doi: 10.1038/s41597-023-02611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fanetti G., Alterio D., Marvaso G., Gandini S., Rojas D.P., Gobitti C., Minatel E., Revelant A., Caroli A., Francia C.M., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in HPV status era for oropharyngeal cancer. Oral Dis. 2020;26:1384–1392. doi: 10.1111/odi.13366. [DOI] [PubMed] [Google Scholar]
- 144.Rodrigo J.P., Sánchez-Canteli M., Triantafyllou A., de Bree R., Mäkitie A.A., Franchi A., Hellquist H., Saba N.F., Stenman G., Takes R.P., et al. Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2023;15:802. doi: 10.3390/cancers15030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Justesen M.M., Jakobsen K.K., Bendtsen S.K., Garset-Zamani M., Mordhorst C., Carlander A.F., Gothelf A.B., Grønhøj C., von Buchwald C. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for the Outcome of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Viruses. 2023;15:198. doi: 10.3390/v15010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaltenmeier C., Yazdani H.O., Morder K., Geller D.A., Simmons R.L., Tohme S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021;12:785222. doi: 10.3389/fimmu.2021.785222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shahzad M.H., Feng L., Su X., Brassard A., Dhoparee-Doomah I., Ferri L.E., Spicer J.D., Cools-Lartigue J.J. Neutrophil Extracellular Traps in Cancer Therapy Resistance. Cancers. 2022;14:1359. doi: 10.3390/cancers14051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teijeira Á., Garasa S., Gato M., Alfaro C., Migueliz I., Cirella A., de Andrea C., Ochoa M.C., Otano I., Etxeberria I., et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856–871.e8. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H., Wang Y., Onuma A., He J., Wang H., Xia Y., Lal R., Cheng X., Kasumova G., Hu Z., et al. Neutrophils Extracellular Traps Inhibition Improves PD-1 Blockade Immunotherapy in Colorectal Cancer. Cancers. 2021;13:5333. doi: 10.3390/cancers13215333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Y., Chandra V., Riquelme Sanchez E., Dutta P., Quesada P.R., Rakoski A., Zoltan M., Arora N., Baydogan S., Horne W., et al. Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020;217:e20190354. doi: 10.1084/jem.20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang Z.Z., Peng Z.P., Liu X.C., Guo H.F., Zhou M.M., Jiang D., Ning W.R., Huang Y.F., Zheng L., Wu Y. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. OncoImmunology. 2022;11:2052418. doi: 10.1080/2162402X.2022.2052418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee W., Ko S.Y., Mohamed M.S., Kenny H.A., Lengyel E., Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019;216:176–194. doi: 10.1084/jem.20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monti M., De Rosa V., Iommelli F., Carriero M.V., Terlizzi C., Camerlingo R., Belli S., Fonti R., Di Minno G., Del Vecchio S. Neutrophil Extracellular Traps as an Adhesion Substrate for Different Tumor Cells Expressing RGD-Binding Integrins. Int. J. Mol. Sci. 2018;19:2350. doi: 10.3390/ijms19082350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nie M., Yang L., Bi X., Wang Y., Sun P., Yang H., Liu P., Li Z., Xia Y., Jiang W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019;25:1867–1879. doi: 10.1158/1078-0432.CCR-18-1226. [DOI] [PubMed] [Google Scholar]
- 157.Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H., et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., Wang Y., Yang S., Liang C., Liang Y., et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–437.e7. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 160.Yang L., Liu Q., Zhang X., Liu X., Zhou B., Chen J., Huang D., Li J., Li H., Chen F., et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 161.Yang L.Y., Luo Q., Lu L., Zhu W.W., Sun H.T., Wei R., Lin Z.F., Wang X.Y., Wang C.Q., Lu M., et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020;13:3. doi: 10.1186/s13045-019-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen N., He D., Cui J. A Neutrophil Extracellular Traps Signature Predicts the Clinical Outcomes and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2022;9:833771. doi: 10.3389/fmolb.2022.833771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan B., Dai X., Ma Q., Wu X. Stromal Neutrophil Extracellular Trap Density Is an Independent Prognostic Factor for Cervical Cancer Recurrence. Front. Oncol. 2021;11:659445. doi: 10.3389/fonc.2021.659445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seminerio I., Kindt N., Descamps G., Bellier J., Lechien J.R., Mat Q., Pottier C., Journé F., Saussez S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget. 2018;9:11046–11059. doi: 10.18632/oncotarget.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mei Z., Huang J., Qiao B., Lam A.K.-Y. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020;12:16. doi: 10.1038/s41368-020-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Topalian S.L., Drake C.G., Pardoll D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alturki N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J. Clin. Med. 2023;12:4301. doi: 10.3390/jcm12134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Albrecht L.J., Livingstone E., Zimmer L., Schadendorf D. The Latest Option: Nivolumab and Relatlimab in Advanced Melanoma. Curr. Oncol. Rep. 2023;25:647–657. doi: 10.1007/s11912-023-01406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C., et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 171.Cohen E.E.W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.-J., Soria A., Machiels J.-P., Mach N., Mehra R., et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 172.Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chow L.Q.M., Haddad R., Gupta S., Mahipal A., Mehra R., Tahara M., Berger R., Eder J.P., Burtness B., Lee S.H., et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results from the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016;34:3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 175.Galvis M.M., Borges G.A., Oliveira T.B., Toledo I.P., Castilho R.M., Guerra E.N.S., Kowalski L.P., Squarize C.H. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020;150:102966. doi: 10.1016/j.critrevonc.2020.102966. [DOI] [PubMed] [Google Scholar]
- 176.Kessler R., Pandruvada S. Immune-related adverse events following checkpoint inhibitor treatment in head and neck cancers: A comprehensive review. Oral Oncol. Rep. 2023;6:100036. doi: 10.1016/j.oor.2023.100036. [DOI] [Google Scholar]
- 177.Wang H., Mustafa A., Liu S., Liu J., Lv D., Yang H., Zou J. Immune Checkpoint Inhibitor Toxicity in Head and Neck Cancer: From Identification to Management. Front. Pharmacol. 2019;10:1254. doi: 10.3389/fphar.2019.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Verma V., Sprave T., Haque W., Simone C.B., 2nd, Chang J.Y., Welsh J.W., Thomas C.R., Jr. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother. Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Du Z., Lovly C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., Jones C.U., Sur R., Raben D., Jassem J., et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 181.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.R., Cupissol D., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 182.Vermorken J.B., Trigo J., Hitt R., Koralewski P., Diaz-Rubio E., Rolland F., Knecht R., Amellal N., Schueler A., Baselga J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab as a Single Agent in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J. Clin. Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 183.Sacco A.G., Chen R., Worden F.P., Wong D.J.L., Adkins D., Swiecicki P., Chai-Ho W., Oppelt P., Ghosh D., Bykowski J., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PubMed] [Google Scholar]
- 184.Mirghani H., Amen F., Moreau F., Guigay J., Hartl D., Guily J.L.S. Oropharyngeal cancers: Relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur. J. Cancer. 2014;50:1100–1111. doi: 10.1016/j.ejca.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 185.Mehanna H., Robinson M., Hartley A., Kong A., Foran B., Fulton-Lieuw T., Dalby M., Mistry P., Sen M., O’Toole L., et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gillison M.L., Trotti A.M., Harris J., Eisbruch A., Harari P.M., Adelstein D.J., Jordan R.C.K., Zhao W., Sturgis E.M., Burtness B., et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang S., Zheng M., Nie D., Xu L., Tian H., Wang M., Liu W., Feng Z., Han F. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: A systematic review and meta-analysis. J. Immunother. Cancer. 2022;10:e005158. doi: 10.1136/jitc-2022-005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vermorken J.B., Stöhlmacher-Williams J., Davidenko I., Licitra L., Winquist E., Villanueva C., Foa P., Rottey S., Skladowski K., Tahara M. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 189.Hong A., Dobbins T., Lee C.S., Jones D., Jackson E., Clark J., Armstrong B., Harnett G., Milross C., O’Brien C., et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur. J. Cancer. 2010;46:2088–2096. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 190.Kong C.S., Narasimhan B., Cao H., Kwok S., Erickson J.P., Koong A., Pourmand N., Le Q.T. The Relationship Between Human Papillomavirus Status and Other Molecular Prognostic Markers in Head and Neck Squamous Cell Carcinomas. Endocrine. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reimers N., Kasper H.U., Weissenborn S.J., Stützer H., Preuss S.F., Hoffmann T.K., Speel E.J.M., Dienes H.P., Pfister H.J., Guntinas-Lichius O., et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 192.Cohen E.E.W., Lingen M.W., Martin L.E., Harris P.L., Brannigan B.W., Haserlat S.M., Okimoto R.A., Sgroi D.C., Dahiya S., Muir B., et al. Response of Some Head and Neck Cancers to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors May Be Linked to Mutation of ERBB2 rather than EGFR. Clin. Cancer Res. 2005;11:8105–8108. doi: 10.1158/1078-0432.CCR-05-0926. [DOI] [PubMed] [Google Scholar]
- 193.Licitra L., Störkel S., Kerr K.M., Van Cutsem E., Pirker R., Hirsch F.R., Vermorken J.B., Von Heydebreck A., Esser R., Celik I., et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: Analysis of data from the EXTREME and CRYSTAL studies. Eur. J. Cancer. 2012;49:1161–1168. doi: 10.1016/j.ejca.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 194.Pollock N.I., Wang L., Wallweber G., Gooding W.E., Huang W., Chenna A., Winslow J., Sen M., DeGrave K.A., Li H., et al. Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies. Clin. Cancer Res. 2015;21:4597–4606. doi: 10.1158/1078-0432.CCR-14-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mazibrada J., Longo L., Vatrano S., Cappia S., Giorcelli J., Pentenero M., Gandolfo S., Volante M., dell’Oste V., Lo Cigno I., et al. Differential expression of HER2, STAT3, SOX2, IFI16 and cell cycle markers during HPV-related head and neck carcinogenesis. New Microbiol. 2014;37:129–143. [PubMed] [Google Scholar]
- 196.Brand T.M., Hartmann S., Bhola N.E., Peyser N.D., Li H., Zeng Y., Isaacson Wechsler E., Ranall M.V., Bandyopadhyay S., Duvvuri U., et al. Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV(+) Patients. Clin. Cancer Res. 2017;23:3072–3083. doi: 10.1158/1078-0432.CCR-16-2203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 197.Baro M., Lopez Sambrooks C., Burtness B.A., Lemmon M.A., Contessa J.N. Neuregulin Signaling Is a Mechanism of Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2019;18:2124–2134. doi: 10.1158/1535-7163.MCT-19-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang D., Qian G., Zhang H., Magliocca K.R., Nannapaneni S., Amin A.R., Rossi M., Patel M., El-Deiry M., Wadsworth J.T., et al. HER3 Targeting Sensitizes HNSCC to Cetuximab by Reducing HER3 Activity and HER2/HER3 Dimerization: Evidence from Cell Line and Patient-Derived Xenograft Models. Clin. Cancer Res. 2017;23:677–686. doi: 10.1158/1078-0432.CCR-16-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Duvvuri U., George J., Kim S., Alvarado D., Neumeister V.M., Chenna A., Gedrich R., Hawthorne T., LaVallee T., Grandis J.R., et al. Molecular and Clinical Activity of CDX-3379, an Anti-ErbB3 Monoclonal Antibody, in Head and Neck Squamous Cell Carcinoma Patients. Clin. Cancer Res. 2019;25:5752–5758. doi: 10.1158/1078-0432.CCR-18-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Falchook G.S., Bauer T.M., LoRusso P., McLaughlin J.F., LaVallee T., Peck R.A., Eder J.P. Safety, pharmacokinetics (PK), pharmacodynamics (Pd), and antitumor activity in a phase 1b study evaluating anti-ErbB3 antibody KTN3379 in adults with advanced tumors alone and with targeted therapies. J. Clin. Oncol. 2016;34:2501. doi: 10.1200/JCO.2016.34.15_suppl.2501. [DOI] [Google Scholar]
- 201.Bauman J.E., Julian R., Saba N.F., Wise-Draper T.M., Adkins D.R., O’Brien P., Fidler M.J., Gibson M.K., Duvvuri U., Heath-Chiozzi M., et al. Phase II Trial of CDX-3379 and Cetuximab in Recurrent/Metastatic, HPV-Negative, Cetuximab-Resistant Head and Neck Cancer. Cancers. 2022;14:2355. doi: 10.3390/cancers14102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kogawa T., Yonemori K., Masuda N., Takahashi S., Takahashi M., Iwase H., Nakayama T., Saeki T., Toyama T., Takano T. Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in breast cancer patients: Phase 1 dose escalation study. J. Clin. Oncol. 2018;36:2512. doi: 10.1200/JCO.2018.36.15_suppl.2512. [DOI] [Google Scholar]
- 203.Janne P.A., Yu H.A., Johnson M.L., Steuer C.E., Vigliotti M., Iacobucci C., Chen S., Yu C., Sellami D.B. Safety and preliminary antitumor activity of U3-1402: A HER3-targeted antibody drug conjugate in EGFR TKI-resistant, EGFRm NSCLC. J. Clin. Oncol. 2019;37:9010. doi: 10.1200/JCO.2019.37.15_suppl.9010. [DOI] [Google Scholar]
- 204.Li Y.-J., Zhang C., Martincuks A., Herrmann A., Yu H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer. 2023;23:115–134. doi: 10.1038/s41568-022-00537-3. [DOI] [PubMed] [Google Scholar]
- 205.Zou S., Tong Q., Liu B., Huang W., Tian Y., Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Geiger J.L., Grandis J.R., Bauman J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol. 2016;56:84–92. doi: 10.1016/j.oraloncology.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moreira D., Sampath S., Won H., White S.V., Su Y.L., Alcantara M., Wang C., Lee P., Maghami E., Massarelli E., et al. Myeloid cell–targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell–mediated immunity. J. Clin. Investig. 2021;131:e137001. doi: 10.1172/JCI137001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bharadwaj U., Eckols T.K., Xu X., Kasembeli M.M., Chen Y., Adachi M., Song Y., Mo Q., Lai S.Y., Tweardy D.J. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget. 2016;7:26307–26330. doi: 10.18632/oncotarget.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Adachi M., Cui C., Dodge C.T., Bhayani M.K., Lai S.Y. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2012;48:1220–1226. doi: 10.1016/j.oraloncology.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bu L.L., Zhao Z.L., Liu J.F., Ma S.R., Huang C.F., Liu B., Zhang W.F., Sun Z.J. STAT3 blockade enhances the efficacy of conventional chemotherapeutic agents by eradicating head neck stemloid cancer cell. Oncotarget. 2015;6:41944–41958. doi: 10.18632/oncotarget.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yu G.T., Mao L., Wu L., Deng W.W., Bu L.L., Liu J.F., Chen L., Yang L.L., Wu H., Zhang W.F., et al. Inhibition of SRC family kinases facilitates anti-CTLA4 immunotherapy in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 2018;75:4223–4234. doi: 10.1007/s00018-018-2863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cohen E.E.W., Harrington K.J., Hong D.S., Mesia R., Brana I., Perez Segura P., Wise-Draper T., Scott M.L., Mitchell P.D., Mugundu G.M., et al. A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Ann. Oncol. 2018;29:viii372. doi: 10.1093/annonc/mdy287. [DOI] [Google Scholar]
- 213.Bonner J.A., Yang E.S., Trummell H.Q., Nowsheen S., Willey C.D., Raisch K.P. Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother. Oncol. 2011;99:339–343. doi: 10.1016/j.radonc.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 214.Di J.-X., Zhang H.-Y. C188-9, a small-molecule STAT3 inhibitor, exerts an antitumor effect on head and neck squamous cell carcinoma. Anti-Cancer Drugs. 2019;30:846–853. doi: 10.1097/CAD.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 215.Morgan E.L., Macdonald A. Manipulation of JAK/STAT Signalling by High-Risk HPVs: Potential Therapeutic Targets for HPV-Associated Malignancies. Viruses. 2020;12:977. doi: 10.3390/v12090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kohut A., Martincuks A., Santiago N.L., Austria T., Zhao Q., Lee S., Tergas A., Dellinger T., Han E., Rodriguez-Rodriguez L., et al. STAT3 is a potential therapeutic target in cervical cancer (257) Gynecol. Oncol. 2022;166:S137–S138. doi: 10.1016/S0090-8258(22)01478-0. [DOI] [Google Scholar]
- 217.Woo S.R., Fuertes M.B., Corrales L., Spranger S., Furdyna M.J., Leung M.Y., Duggan R., Wang Y., Barber G.N., Fitzgerald K.A., et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Huang X., Huo L., Xiao B., Ouyang Y., Chen F., Li J., Zheng X., Wei D., Wu Y., Zhang R., et al. Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ. 2023;31:78–89. doi: 10.1038/s41418-023-01242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chin E.N., Yu C., Vartabedian V.F., Jia Y., Kumar M., Gamo A.M., Vernier W., Ali S.H., Kissai M., Lazar D.C., et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369:993–999. doi: 10.1126/science.abb4255. [DOI] [PubMed] [Google Scholar]
- 220.Huang R., Ning Q., Zhao J., Zhao X., Zeng L., Yi Y., Tang S. Targeting STING for cancer immunotherapy: From mechanisms to translation. Int. Immunopharmacol. 2022;113:109304. doi: 10.1016/j.intimp.2022.109304. [DOI] [PubMed] [Google Scholar]
- 221.Dorta-Estremera S., Hegde V.L., Slay R.B., Sun R., Yanamandra A.V., Nicholas C., Nookala S., Sierra G., Curran M.A., Sastry K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer. J. Immunother. Cancer. 2019;7:252. doi: 10.1186/s40425-019-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lu S., Concha-Benavente F., Shayan G., Srivastava R.M., Gibson S.P., Wang L., Gooding W.E., Ferris R.L. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncol. 2018;78:186–193. doi: 10.1016/j.oraloncology.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mirza A.Z., Althagafi I.I., Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 224.Katoch S., Sharma V., Patial V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: Experimental and clinical scenarios. World J. Gastroenterol. 2022;28:3535–3554. doi: 10.3748/wjg.v28.i28.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li Y., Zhang D.-W., Lin D.-Q., Cao L.-Q. Peroxisome proliferator-activated receptor-γ inhibits pancreatic cancer cell invasion and metastasis via regulating MMP-2 expression through PTEN. Mol. Med. Rep. 2015;12:6255–6260. doi: 10.3892/mmr.2015.4224. [DOI] [PubMed] [Google Scholar]
- 226.Lakshmi S.P., Reddy A.T., Banno A., Reddy R.C. PPAR Agonists for the Prevention and Treatment of Lung Cancer. PPAR Res. 2017;2017:8252796. doi: 10.1155/2017/8252796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao B., Xin Z., Ren P., Wu H. The Role of PPARs in Breast Cancer. Cells. 2022;12:130. doi: 10.3390/cells12010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jung T.I., Baek W.K., Suh S.I., Jang B.C., Song D.K., Bae J.H., Kwon K.Y., Bae J.H., Cha S.D., Bae I., et al. Down-regulation of peroxisome proliferator-activated receptor gamma in human cervical carcinoma. Gynecol. Oncol. 2005;97:365–373. doi: 10.1016/j.ygyno.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 229.Chen H.M., Zhang D.G., Wu J.X., Pei D.S., Zheng J.N. Ubiquitination of p53 is Involved in Troglitazone Induced Apoptosis in Cervical Cancer Cells. Asian Pac. J. Cancer Prev. 2014;15:2313–2318. doi: 10.7314/APJCP.2014.15.5.2313. [DOI] [PubMed] [Google Scholar]
- 230.Zhang S., Liu F., Mao X., Huang J., Yang J., Yin X., Wu L., Zheng L., Wang Q. Elevation of miR-27b by HPV16 E7 inhibits PPARγ expression and promotes proliferation and invasion in cervical carcinoma cells. Int. J. Oncol. 2015;47:1759–1766. doi: 10.3892/ijo.2015.3162. [DOI] [PubMed] [Google Scholar]
- 231.O’Neill W.Q., Xie X., Gui S., Yu H., Davenport J., Cartwright T., Storl-Desmond M., Ryu E., Chan E.R., Cao S., et al. Repositioning Fenofibrate to Reactivate p53 and Reprogram the Tumor-Immune Microenvironment in HPV+ Head and Neck Squamous Cell Carcinoma. Cancers. 2022;14:282. doi: 10.3390/cancers14020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.John K., Lahoti T.S., Wagner K., Hughes J.M., Perdew G.H. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol. Carcinog. 2014;53:765–776. doi: 10.1002/mc.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.DiNatale B.C., Smith K., John K., Krishnegowda G., Amin S.G., Perdew G.H. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol. Cancer Res. 2012;10:1369–1379. doi: 10.1158/1541-7786.MCR-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stanford E.A., Ramirez-Cardenas A., Wang Z., Novikov O., Alamoud K., Koutrakis P., Mizgerd J.P., Genco C.A., Kukuruzinska M., Monti S., et al. Role for the Aryl Hydrocarbon Receptor and Diverse Ligands in Oral Squamous Cell Carcinoma Migration and Tumorigenesis. Mol. Cancer Res. 2016;14:696–706. doi: 10.1158/1541-7786.MCR-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hu A., Zhang J.W., Yang L.Y., Qiao P.P., Lu D. AHRR contributes to inflammatory lymphangiogenesis by activating the EPAS1/VEGFD signaling axis in head and neck cancer. Am. J. Cancer Res. 2022;12:537–548. [PMC free article] [PubMed] [Google Scholar]
- 236.Kenison J.E., Wang Z., Yang K., Snyder M., Quintana F.J., Sherr D.H. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl. Acad. Sci. USA. 2021;118:e2012692118. doi: 10.1073/pnas.2012692118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Arellano-Gutiérrez C.V., Quintas-Granados L.I., Cortés H., González Del Carmen M., Leyva-Gómez G., Bustamante-Montes L.P., Rodríguez-Morales M., López-Reyes I., Padilla-Mendoza J.R., Rodríguez-Páez L., et al. Indole-3-Carbinol, a Phytochemical Aryl Hydrocarbon Receptor-Ligand, Induces the mRNA Overexpression of UBE2L3 and Cell Proliferation Arrest. Curr. Issues Mol. Biol. 2022;44:2054–2068. doi: 10.3390/cimb44050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Meara C.H.O., Coupland L.A., Kordbacheh F., Quah B.J.C., Chang C.W., Simon Davis D.A., Bezos A., Browne A.M., Freeman C., Hammill D.J., et al. Neutralizing the pathological effects of extracellular histones with small polyanions. Nat. Commun. 2020;11:6408. doi: 10.1038/s41467-020-20231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Stürzl M., Staats L., Mahajan A., Schauer C., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vaguliene N., Zemaitis M., Lavinskiene S., Miliauskas S., Sakalauskas R. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol. 2013;14:366. doi: 10.1186/1471-2172-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kistowski M., Dębski J., Karczmarski J., Paziewska A., Olędzki J., Mikula M., Ostrowski J., Dadlez M. A Strong Neutrophil Elastase Proteolytic Fingerprint Marks the Carcinoma Tumor Proteome. Mol. Cell. Proteom. 2017;16:213–227. doi: 10.1074/mcp.M116.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Akizuki M., Fukutomi T., Takasugi M., Takahashi S., Sato T., Harao M., Mizumoto T., Yamashita J. Prognostic Significance of Immunoreactive Neutrophil Elastase in Human Breast Cancer: Long-Term Follow-Up Results in 313 Patients. Neoplasia. 2007;9:260–264. doi: 10.1593/neo.06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Patrignani P., Patrono C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016;68:967–976. doi: 10.1016/j.jacc.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 244.Guillem-Llobat P., Dovizio M., Bruno A., Ricciotti E., Cufino V., Sacco A., Grande R., Alberti S., Arena V., Cirillo M., et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7:32462–32477. doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bruno A., Dovizio M., Tacconelli S., Patrignani P. Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2012;26:e1–e13. doi: 10.1016/j.bpg.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 246.Menegazzo L., Scattolini V., Cappellari R., Bonora B.M., Albiero M., Bortolozzi M., Romanato F., Ceolotto G., Vigili de Kreutzeberg S., Avogaro A., et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018;55:593–601. doi: 10.1007/s00592-018-1129-8. [DOI] [PubMed] [Google Scholar]
- 247.Wang G., Gao H., Dai S., Li M., Gao Y., Yin L., Zhang K., Zhang J., Jiang K., Miao Y., et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice. Cancer Lett. 2023;562:216155. doi: 10.1016/j.canlet.2023.216155. [DOI] [PubMed] [Google Scholar]
- 248.Yang L.Y., Shen X.T., Sun H.T., Zhu W.W., Zhang J.B., Lu L. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic. J. Cancer. 2022;13:1261–1271. doi: 10.7150/jca.64170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen C.J., Wu C.C., Chang C.Y., Li J.R., Ou Y.C., Chen W.Y., Liao S.L., Wang J.D. Metformin Mitigated Obesity-Driven Cancer Aggressiveness in Tumor-Bearing Mice. Int. J. Mol. Sci. 2022;23:9134. doi: 10.3390/ijms23169134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this review article was sourced from reporting manuscripts.