Abstract
Polyomavirus causes a broad spectrum of tumors as the result of the action of its early proteins. This work compares signaling from middle T antigen (MT), the major transforming protein, to that from small T antigen (ST). The abilities of MT mutants to promote cell cycle progression in serum-starved NIH 3T3 cells were compared. Transformation-defective mutants lacking association with SHC or with phosphatidylinositol 3-kinase (PI3-K) retained the ability to induce DNA synthesis as measured by bromodeoxyuridine incorporation. Only when both interactions were lost in the Y250F/Y315F double mutant was MT inactive. ST promoted cell cycle progression in a manner dependent on its binding of protein phosphatase 2A (PP2A). Since the Y250F/Y315F MT mutant was wild type for PP2A binding yet unable to promote cell cycle progression, while ST was capable of promoting cell cycle progression, these experiments revealed a functional difference in MT and ST signaling via PP2A. Assays testing the abilities of MT and ST to induce the c-fos promoter and to activate c-jun kinase led to the same conclusion. ST, but not Y250F/Y315F MT, was able to activate the c-fos promoter through its interaction with PP2A. In contrast, MT, but not ST, was able to activate c-jun kinase by virtue of its interaction with PP2A.
Polyomaviruses have proven to be valuable models for studying growth regulation. The viruses require the apparatus of cellular DNA synthesis for their own replication. To meet this need, these viruses have evolved many different ways to intervene in cellular growth regulation, which can cause a broad range of tumors in different types of cells (26, 31). Examining how these viruses work has provided repeated leads that can be applied to understanding normal and abnormal cell behavior. Tyrosine phosphorylation (30) and phosphatidylinositol 3-kinase (PI3-K) (99) signaling are examples of two avenues of investigation driven by studies of the polyomavirus middle T antigen (MT).
MT is the most important of the early gene products for transformation. MT is necessary (14, 87) and in many cases sufficient (89) for transformation in vitro. The importance of MT in tumor induction is also evident (3, 33). MT is associated with membranes and underlying cytoskeletal elements (1, 47, 77, 80). Its ability to transform depends upon those associations (14).
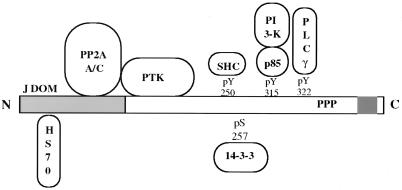
MT functions as a kind of adaptor on which a collection of cellular signaling proteins are assembled (Fig. 1). MT, like polyomavirus small T antigen (ST), binds the A and C subunits of protein phosphatase 2A (PP2A) (70, 92). PP2A is a heterotrimeric serine-threonine phosphatase present in most cell types that has been implicated in the regulation of cell cycle progression, transcription, and DNA replication and translation (60, 61, 79). The B subunit, which is replaced by MT or ST, confers substrate specificity (79) and localization (85). The viral proteins should provide useful insight into PP2A regulation. It is becoming clear that binding of cell proteins can also modulate PP2A activity (41, 51) and that cellular targets can be regulated by the binding of PP2A (96).
FIG. 1.
Schematic diagram of MT and its known associated proteins. The shaded area at the N terminus represents the 191 amino acids shared with the 195-amino-acid ST. The shaded area near the C terminus represents the membrane attachment site.
MT associates with protein tyrosine kinases (PTKs) of the src family (src, yes, and fyn) (19, 22, 44, 53). pp60c-src bound to MT is activated (9) and is unphosphorylated at the C terminus (16, 23). The association with PP2A is critical for the binding of PTKs (12), although the precise molecular basis of PP2A action is not known.
The association with PTKs is critical for transforming activity (30, 76, 81). In the PTK complex, MT is phosphorylated on tyrosine residues including 315, 322, and 250 (15, 40, 45, 74). Each of these residues serves as a binding site for well-known signal transduction molecules: 315 to PI3-K (50, 99), 250 to SHC (13, 27), and 322 to PLCγ1 (86). MT has been particularly revealing because, in contrast to single knock-outs in growth factor receptors, single MT mutations that affect individual associations have usually had clearly identifiable phenotypes. Mutation of Y322 has had a modest effect in some transformation assays (57), but a striking effect was observed on transformation in medium with a low concentration of serum (86). Mutation of tyrosine 250 has a dramatic effect on transforming ability (57) as do mutations in the regions amino terminal to 250 (the NPTY motif) (28, 29). The mutation of Y315, disrupting the association between MT and PI3-K, also has a serious effect on MT’s ability to transform (15). Tumor studies using animals have confirmed a role for the SHC and PI3-K interactions in polyomavirus tumorigenesis (11, 32, 95, 102).
Polyomavirus ST has been studied less intensively than MT. A virus carrying wild-type ST is not sufficient for transformation (55). However, ST can complement MT for tumor induction (2) and transformation (5). ST also induces lectin agglutinability, which is often used as a measure of cell transformation (55). Independent expression of ST in fibroblasts enables them to grow to a high cell density (20, 65). In serum-starved cells ST can contribute to S phase induction in conjunction with large T (7, 68). ST can also affect viral DNA synthesis (7, 64). For simian virus 40 (SV40) it has been shown that ST can transactivate (56, 62, 71, 94) or even repress (93) various exogenous promoters. Polyomavirus ST enhances viral DNA replication and has been implicated in virion assembly (58).
ST can be found in both nuclear and cytoplasmic compartments (66, 80, 103). Like MT, ST has been shown to bind PP2A (70). The ability of ST to activate promoters such as cyclin D has been linked genetically to its PP2A binding (94). In SV40, the ST-PP2A interaction also stimulates the mitogen-activated protein (MAP) kinase pathway and cell proliferation (82). Finally, ST is responsible for activation of PKCζ and NF-κB (83).
In this work, genetic analysis has been used to extend our understanding of MT signaling and to define differences between MT and ST in the regulation of PP2A. Bromodeoxyuridine (BrdU) incorporation assays demonstrated that MT sends multiple signals, each of which can promote S phase after serum withdrawal. However, while either interaction with SHC or PI3-K led to cellular DNA synthesis, interaction with PP2A was not enough for MT to promote S phase induction. In sharp contrast, the ST-PP2A interaction was sufficient to promote cellular DNA synthesis. Genetic analysis of c-fos transactivation confirmed the differing roles of MT and ST interactions with PP2A. Previous experiments showed a role for PP2A in recruiting tyrosine kinase into MT complexes. In addition, experiments here showed that the MT interaction with PP2A could lead to activation of the c-jun kinase.
MATERIALS AND METHODS
Plasmids and mutagenesis.
pCMV-MT is a human cytomegalovirus (CMV) promoter-based eukaryotic expression vector (pCMV-NeoBam) in which MT cDNA is inserted at a unique BamHI site (25). During the course of the work presented here all MT mutants were introduced into pCMV-MT by standard PCR techniques. Site-directed mutagenesis of pCMV-MT was carried out with overlap extension PCR. 5′-GCGCGGATCCATGGATAGAGTTCTGAGC-3′ and 5′-GCGCGGATCCCTAGAAATGCCGGGAACGTT-3′ were used as outside primers. The deletion mutant del205-214 was created with 5′-CTGAGGAGAGCGGCCACAGTCATGGAAGCAAGTACTTCACAA-3′ and 5′-TTGTG AAGTACTTGCTTCCATGACTGTGGCCGCTCTCCTCAG-3′ as internal primers. The NG59 mutant was designed with 5′-ATTGCAAGCATGCCTATAATAAACTGGCTGGACCTGGATCTGCAC-3′ and 5′-CACATCCAGGTCCAGCCAGTTTATTATAGGCATGCTTGCAATGAAG-3′ for the internal primers. The Y250F mutant was created with the degenerate internal primers 5′-TCTGAGCAACC(C/T)GACCT(A/T)TTCTGTTATGA-3′ and 5′-TCATAACAGAA(T/A)AGGTCG/AGGTTGCTCAGA-3′. The Y315F mutant was made with the following degenerate oligonucleotides as internal primers: 5′-GGAGG AGGAGT(A/T)CATGCCAATGGAGGATCTGT(A/T)TTTGGACATC-3′ and 5′-GGATGTCCAAA(T/A)ACAGATCCTCCATTGGCATG(T/A)ACTCCTCCTCC3′. InsAL107 in pBD15, a kind gift of T. Roberts (Dana Farber Cancer Institute), was cloned into BamHI sites of the pCMV expression vector by PCR with the outside primers listed above.
Two different versions of ST were used with similar results. CMV-ST was constructed in the same CMV-NeoBam vector as used for MT. A second vector expressing ST was a kind gift of Marcel Bastin (4). Mutant insAL107 ST was made by PCR with the N-terminal outside primer and 5′-CGCGGATCCCTAGGGGGAGAGCCTTGGATTATACACGCTG-3′ on the ins107AL MT template. After treatment with BamHI, the fragment was cloned into CMV-NeoBam.
CMV–β-galactosidase used in the BrdU incorporation assays was a gift from E. Androphy (Tufts University). c-fos CAT promoter was kindly provided by W. Wang (93).
Cell lines and transient transfections.
ATCC NIH 3T3 cells, originally obtained from the American Type Culture Collection (ATCC), were kindly provided by Bruce Cohen. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% calf serum (CS) Gibco). 293T cells were maintained in DMEM with 10% iron-supplemented bovine calf serum. Transient transfections were performed by the method of Chen and Okayama (17). Cells were ordinarily used 48 h posttransfection. For serum starvation experiments, the precipitate was allowed to form for 5 h and was then replaced with 0.2% CS–DMEM.
Radiolabeling of cells.
Before labeling, 293T cells were rinsed once in Hanks balanced salt solution (Gibco). Cells were labeled by incubation in Hanks salts supplemented with 1% (vol/vol) of a 7.5% (wt/vol) sodium bicarbonate solution and [35S]methionine-[35S]cysteine mixture (NEN) for 2 h. For the isoelectric focusing (IEF) experiments 250 uCi of label was used in 1 ml of medium per dish.
Extraction and immunoprecipitation.
The protocols for T-antigen extraction and immunoprecipitation have been previously published (74, 75). Cells were extracted for 20 min at 4°C in TEB buffer (0.137 M NaCl, 0.01 M Tris-Cl [pH 9.0], 0.001 M MgCl2, 0.001 M CaCl2, 10% [vol/vol] glycerol, 1% [vol/vol] Nonidet P-40 [NP-40]). The supernatants were then incubated with 40 μl of Staphylococcus aureus protein A-Sepharose (Pharmacia) and 4 μl of polyclonal antiserum 45-1, directed against MT. If the immunoprecipitates were to be subjected to IEF they were resuspended in Garrels’ buffer and handled as described in “IEF” below.
Western blot analysis.
Western blot analysis was carried out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by blotting onto nitrocellulose (43). Blots were blocked in Tris-buffered saline-Tween (50 mM Tris-Cl [pH 7.5], 0.15 M NaCl, 0.05% [vol/vol] Tween 20) containing 5% (wt/vol) dried milk (Carnation) at room temperature for 1 h. Blots were then incubated with 1:50 PN-116 monoclonal antibody, which recognizes the N-terminal common domain, or with anti-FLAG antibody (0.5 μg/ml) followed by 1:10,000 horseradish peroxidase-conjugated anti-mouse secondary antibody (Amersham). Protein was detected by enhanced chemiluminescence.
IEF.
IEF samples were prepared by treatment with Garrels’ sample buffer (9.5 M urea, 2% ampholytes [pH 3 to 10-pH 5 to 7-pH 6 to 8 in a 1:2:2 ratio] [Pharmacia-LKB], 100 mM dithiothreitol, 4% NP-40) (35). After incubation on ice for 30 min the samples were loaded onto IEF cylinders. First-dimension IEF gels composed of 4% bisacrylamide (1.62-28.3), 8.0 M urea, 4% NP-40, and 2% ampholytes were poured to a height of 15 cm. Samples were run at 400 to 500 V per h for 9,000 V · h. At that point, the gels were turned up to 800 v for 1 h. Second-dimension electrophoresis of the focused proteins was on 5 or 7.5% acrylamide discontinuous buffer SDS slab gels.
BrdU assays.
ATCC NIH 3T3 cells grown on coverslips were cotransfected at a ratio of 3:1 with vector expressing β-galactosidase and either control pCMV vector or wild-type or mutant CMV-MT. Growing NIH 3T3 cells were transfected for 5 h at 35°C, 3% CO2, and then fed with 0.2% CS-DMEM and transferred to 37°C, 5% CO2. At 40 h after transfection, BrdU was added to a final concentration of 100 μM for 8 h. Cells were then fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 30 min at 37°C in a humidity chamber. After fixation cells were washed in PBS and permeabilized in ice-cold 3:7 methanol-acetone at −20°C. Cells were then stained for β-galactosidase with an anti-β-galactosidase polyclonal antibody (5′→3′) diluted 1:80 into the anti-BrdU monoclonal antibody BU-1 (Amersham) for 1 h at 37°C in a humidity chamber. Coverslips were washed in PBS and costained with the secondary antibodies, anti-rabbit fluorescein isothiocyanate (Cappel) and anti-mouse tetramethylrhodamine isothiocyanate (Sigma), each diluted 1:50 in PBS for 1 h at 37°C. Coverslips were rinsed with PBS and given a final wash with distilled water. The coverslips were mounted on 3 μl of 50% glycerol on a microscope slide and examined on a Zeiss fluorescence microscope. Results were calculated by scoring β-galactosidase-positive cells incorporating BrdU versus β-galactosidase-positive cells that did not incorporate BrdU.
CAT assays.
ATCC NIH 3T3 cells were transfected with precipitates that contained 4 μg of fos chloramphenicol acetyltransferase (CAT) (93) and 5 μg of various pCMV-MT- or pCMV-ST-based constructs. Five hours after transfection, cells were washed twice with PBS and fed with DMEM containing 0.2% CS in order to serum starve the transfected cells. Cells were harvested 48 h posttransfection. CAT activity was measured by standard chromatographic techniques (6). Thin-layer plates (EM Sciences) were quantitated with ImageQuant software (Molecular Dynamics) to determine the percentage of acetylated [14C]chloramphenicol versus all forms.
c-Jun NH2-terminal kinase (JNK1) assays.
293T cells were transfected with 1.5 μg of plasmid PCDNA3-Flag-JNK1 and 2.5 μg of either wild type or different MT mutants. At 24 h after transfection the cells were incubated for 18 h in serum-free medium. Then, cells were scraped into ice-cold PBS and extracted for 20 min at 4°C in TEB buffer (pH 9). Extracts were then spun in an Eppendorf microcentrifuge at 10,000 rpm for 10 min at 4°C. The supernatants were then incubated with 40 μl of protein A-Sepharose (Pharmacia) and 1 μg of anti-FLAG antibody for 60 min at 4°C. The beads were then washed twice with PBS containing 1% (vol/vol) Triton X-100 and once with kinase buffer (50 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 1 mM dithiothreitol). The JNK activity present in the immunoprecipitates was determined by resuspension of the beads in 50 μl of kinase buffer containing 0.1 μCi of [γ32P]ATP/μl, 25 μM cold ATP, and 2.5 μg of glutathione S-transferase (GST)–c-Jun (amino acids 1 to 79) fusion protein/ml as a substrate for 20 min at room temperature. After the addition of 50 μl of dissociation buffer, samples were boiled for 5 min and analyzed by SDS-PAGE on 12% polyacrylamide gels. Dried gels were exposed to a PhosphorImager screen and quantified with ImageQuant software to determine the phosphorylation of the GST–c-Jun substrate.
RESULTS
Transformation defective MTs can promote cell cycle progression through PI3-K or SHC.
A series of mutations were created in MT that target the binding sites for the various associated cellular proteins. To avoid problems that could arise from comparing mutants expressed from different vectors, all mutant cDNAs were placed in the same vector, which used the CMV immediate-early promoter for expression. Most of the mutations have been previously documented, but some mutants were made to extend leads developed during the course of this work. Table 1 lists the mutants and describes the effect of each mutation on MT function.
TABLE 1.
Description of MT mutants
| Mutanta | Mutation | Description |
|---|---|---|
| WT | None | WT MT |
| del 205-214 | Deletion of aab 205-214 | PTK deficient |
| insAL107 | Insertion of ala-leu between aa 107-108 | PTK deficient/PP2A deficient |
| insAL107/del205-214 | Insertion of ala-leu between 107-108/deletion of aa 205-214 | PTK and PP2A negative |
| NG59 | asp 179 replaced by ile-asn | PTK and PP2A negative |
| Y250F | tyr 250 changed to phe | SHC negative |
| Y315F | tyr 315 changed to phe | PI3-K negative |
| Y250F/Y315F | tyr 250 and 315 changed to phe | SHC and PI3-K negative |
| Py1387T | Stop codon at 384 | PTK deficient, cytoplasmic |
WT, wild type. The wild-type MT cDNA was expressed from a human CMV promoter. References for the mutants used are as follows: del205-214, 10; insAL107, 12; NG59, 6a; Y250F, 13, 27, and 57; Y315F, 15; and Py1387T, 14.
aa, amino acids.
The ability to promote cellular DNA replication in the infected cell is critical to the life cycle of the virus, as polyomavirus utilizes the host cell DNA replication machinery. Large T and ST have been previously shown to possess the ability to promote cell cycle progression (68), yet this function has not been specifically studied in MT. Given the potent transforming ability of MT due to its association with proteins such as PP2A, pp60c-src, PI3-K, SHC, and PLCγ1, it seemed extremely likely that MT would possess this function as well. BrdU incorporation was used to assay stimulation of cellular DNA synthesis by MT. For this, a cotransfection protocol was used in which the β-galactosidase-expressing vector was transfected at a ratio of 3:1 with either wild-type MT, the mutant of interest, or control vector. ATCC NIH 3T3 cells in 10% CS were transfected and then added to medium containing 0.2% CS 5 h after transfection. At 40 h after transfection BrdU was added to a final concentration of 100 μM for 8 h. Cells were then immunostained for cytoplasmic β-galactosidase and nuclear BrdU incorporation.
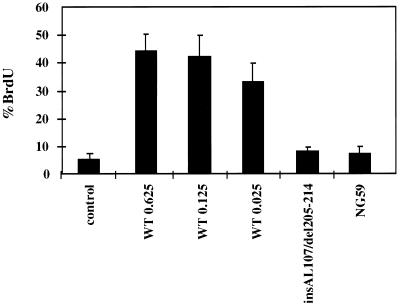
MT was a potent promoter of cell cycle progression. Figure 2 shows a dose response with wild-type MT. Results were calculated by scoring the percentage of β-galactosidase-positive cells incorporating BrdU. In the absence of cotransfection with MT, only 6% of the cells were BrdU labeled. However in the presence of the highest amount (0.625 μg) of MT, 44% of the cells incorporated BrdU. Interestingly, even when the amount of MT DNA transfected was approximately 25-fold less, i.e., 25 ng, the percentage of cells in S phase remained relatively similar at 33%. At the lowest DNA input MT was usually not detectable by Western blotting (data not shown). Polyomavirus large T has previously been shown by this assay to promote cell cycle progression (36). When MT was compared in the BrdU incorporation assay to polyomavirus large T, the two were equal in this ability (data not shown). Two highly transformation-defective MTs, NG59 and double mutant insAL107/del205-214, lacked the ability to promote cell cycle progression. In cells transfected with double mutant insAL107/del205-214 or NG59 only 7 to 8% of cells incorporated BrdU compared to 6% for the control and 42% for wild-type MT. These two mutants are nontransforming and defective in association with both PP2A and src family tyrosine kinases.
FIG. 2.
BrdU incorporation assays with wild-type MT, insAL107/del205-214, and NG59. Growing ATCC NIH 3T3 cells were transiently transfected with CMV–β-galactosidase and 0.625 μg of CMV-neoBam (control) or 0.625, 0.125, or 0.025 μg of CMV-MT (WT) or 0.625 μg of insAL107/del205-214 or NG59, placed in 0.2% CS-DMEM at 5 h posttransfection, and labeled with BrdU for 8 h starting at 40 h after transfection. Transfected cells were detected by staining for β-galactosidase; BrdU incorporation was measured by immunostaining. % BrdU, percentage of transfected cells (β-galactosidase positive) also staining for BrdU. Error bars represent standard deviations of the means.
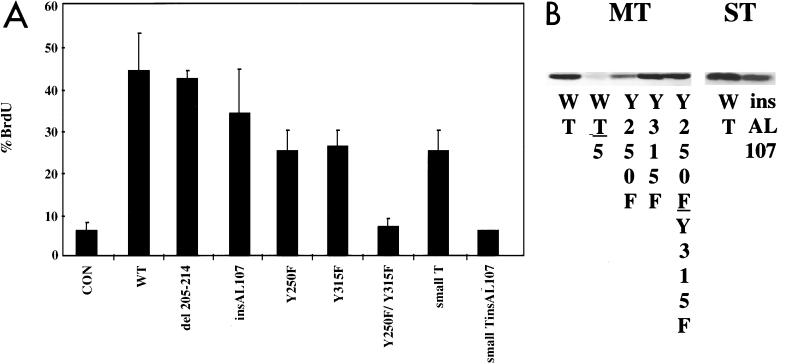
Examination of other transformation-defective mutants gave quite a different result (Fig. 3). Single mutants insAL107 (12), a mutant in the PP2A binding site, and del205-214 (10), a mutant in the src binding site, are known to be highly defective in transformation. However, when del205-214 and insAL107 were tested in the BrdU incorporation assay, 42 and 35% of the transfected cells incorporated BrdU, respectively (Fig. 3). The basis for their activity could have been leakiness, since both insAL107 and del205-214 retained some associated tyrosine kinase activity (references 10 and 12 and data not shown).
FIG. 3.
(A) BrdU incorporation assays with wild-type MT, del205-214, insAL107, Y250F, Y315F, Y250F/Y315F, and ST. By using the same protocol described in the legend for Fig. 2, growing NIH 3T3 cells were transiently transfected with CMV–β-galactosidase and with 0.625 μg of either CMV-neo (control), CMV-MT (WT), del205-214, insAL107, Y250F, Y315F, Y250F/Y315F, ST, or STinsAL107, placed into 0.2% serum, and labeled with BrdU. Error bars represent standard errors of the means. (B) Extracts of cells transfected with equal amounts of wild-type or mutant MT DNAs, cells transfected with 1/5 the amount of wild-type MT, or cells transfected with wild-type or mutant STs were resolved in SDS-PAGE gels. The gels were then analyzed using Western blotting with the antibody PN116.
To test the idea that the activity of these mutants stemmed from leakiness, mutants lacking sites of tyrosine phosphorylation were examined. The most important associations for MT transformation are with SHC and PI-3K at tyrosines 250 and 315, respectively. When the double mutant Y250F/Y315F was tested for its effect on cell cycle progression, it was unable to promote BrdU incorporation, showing only a 1% increase over background (Fig. 3A). Western blotting showed that this failure did not result from a lack of protein production (Fig. 3B). The single mutants Y250F and Y315F were then tested in BrdU incorporation assays to evaluate the relative importance of each association. While each single mutant is highly defective in promoting focus formation in fibroblasts, both single mutants promoted cell cycle progression in 25 and 26% of the transfected cells, respectively (Fig. 3A). Furthermore, both mutants were active even at 25 ng of DNA input (data not shown). These data showed that MT binding to either SHC or PI3-K is sufficient to promote cell cycle progression in a substantial number of cells. It is worth noting that the level of BrdU incorporation (percent positive cells) with either Y250F or Y315F was somewhat lower than that achieved by wild-type MT. Coexpression of both single mutants did not restore BrdU incorporation to the level achieved by the wild type (data not shown).
Interactions of PP2A with ST, but not MT, promote cell cycle progression.
The failure of the Y250F/Y315F double mutant to promote BrdU incorporation was rather unexpected. The Y250F/Y315F double mutant is expected to bind PP2A like wild-type MT or ST. Data on SV40 ST have shown that it has the ability to stimulate cell proliferation in a manner dependent on the PP2A interaction (82). Polyomavirus ST, which also binds PP2A, was therefore tested for the ability to promote cell cycle progression. ST was shown to promote BrdU incorporation in 25% of the transfected cells (Fig. 3A). This value, similar to those obtained with the Y250F and Y315F single mutants, showed that ST promoted cell cycle progression. Introduction of the insAL107 mutation into ST abolishes PP2A binding (12). As shown in Fig. 3A, ST insAL107 lacked the ability to promote cell cycle progression. As reported previously (12), the mutation did not have a significant effect on the level of ST protein (Fig. 3B).
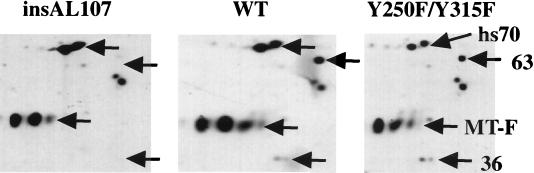
The differing results in the BrdU assays with ST and Y250F/Y315F suggest that the two proteins use PP2A differently. However, the discrepancy between ST and Y250F/Y315F required direct confirmation that the Y250F/Y315F binds PP2A. To confirm PP2A binding, IEF of a Y250F/Y315F immunoprecipitate was performed (Fig. 4). IEF is a well-established method for examining proteins associated with MT (21, 69, 70). Cells expressing wild-type or mutant MT were labeled with [35S]methionine-cysteine. After extraction and immunoprecipitation, MT-associated proteins were resolved by using two-dimensional IEF-SDS gels. Figure 4 shows the relevant portion of a typical pattern for wild-type MT. In this gel, hs70 and the 63- and 36-kDa subunits of PP2A can be observed. While full-length MT itself does not focus, a proteolytic fragment of MT can instead be seen (25). The multiple bands seen for the 36-kDa PP2A subunit and the MT fragment arise from modifications of those proteins. The association of PP2A with MT insAL107 or Y250F/Y315F was also measured in the same fashion (Fig. 4). The insAL107 MT mutant has been reported to be unable to associate with PP2A (12), and the focusing gel shown in Fig. 4 confirms the loss of PP2A binding. The 36- and 63-kDa subunits of PP2A associated with wild-type MT were not detected in the insAL107 immunoprecipitates. As expected, the 36- and 63-kDa subunits of PP2A were present in the Y250/Y315F immunoprecipitate. These data demonstrate that the failure of Y250F/Y315F to promote cell cycle progression is not due to an inability to associate with PP2A. It should also be noted that insAL107 retained the ability to bind hs70, indicating that the protein was not simply denatured.
FIG. 4.
35S-labeled MT immunoprecipitates from insAL107-, wild type-, and Y250F/Y315F-transfected cells were resolved by IEF and SDS-PAGE. The positions of heat shock 70 (hs 70), the 63- and 36-kDa subunits of PP2A, and a fragment of MT lacking the C terminus are shown.
Interaction of PP2A with ST, but not MT, is sufficient for c-fos transactivation.
The c-fos proto-oncogene is a member of a family of immediate-early genes that are induced in quiescent cells by stimuli such as the addition of serum or infection of polyomavirus (39, 104). MT is known to activate the transcription of fos, and the ability of mT to associate with both PP2A and src PTKs is thought to be required for this activation (37, 38). During the course of this work the contributions of SHC and PI3-K were also assessed, and both were found to contribute to the activation of the c-fos promoter by mT, with PI3-K binding being more critical (90). Additionally, it is known that signaling through the Ras-Raf-MAP pathway can stimulate activation of the c-fos promoter through TCF (42, 48, 54).
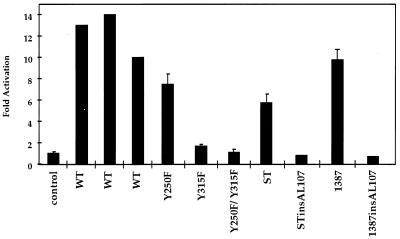
Wild-type MT and the tyrosine mutant Y250F, Y315F, and Y250F/Y315F were assayed for the ability to transactivate c-fos. Growing NIH 3T3 cells were transiently transfected for 5 h with the pfos-CAT plasmid and either decreasing amounts of wild-type mT or 5 μg of either Y250F, Y315F, or Y250F/Y315F and were then placed into 0.2% CS–DMEM (Fig. 5). The cells were then harvested 48 h after transfection. A range of fos activation from 9- to 14-fold was seen with wild-type MT. Figure 5 also shows that Y250F, which fails to associate with SHC, was only a little less active than the wild type. Y315F, which is deficient in PI3-K binding, was highly defective, and Y250F/Y315F was inactive in the fos assay. These results were similar to those obtained by others, where an association with PI3-K was more critical to fos activation than an association with SHC (90). These results are in contrast to those of Fig. 3, which demonstrated that Y315F was competent in the induction of BrdU incorporation. The ability to promote cell cycle progression without inducing fos has been seen before in the case of polyomavirus large T and ST (68).
FIG. 5.
Wild-type MT, insAL107, Y250F, Y315F, Y250F/Y315F, Py1387T, insAL107/Py1387T, ST, and STins107AL in the activation of the fos promoter in starved cells. Growing NIH 3T3 cells were transfected with pfos-CAT reporter plasmid and either CMV-neo (control), 5, 1, or 0.2 μg of CMV-MT (WT), or 5 μg of insAL107, Y250F, Y315F, Y250F/Y315F, Py1387T, insAL107/Py1387T, ST, or STins107AL. After transfection cells were placed into 0.2% CS-DMEM. CAT assays were performed as described in Materials and Methods. Error bars represent standard deviations of the means.
Polyomavirus ST is also known to induce the c-fos promoter after infection (39); presumably it does so by binding to PP2A. In the case of SV40 ST, for instance, the binding to PP2A results in the activation of the MAP kinase pathway in CV-1 cells (82). As with BrdU incorporation, measuring c-fos promoter activation also showed functional differences between the PP2A association with MT versus ST. Figure 5 shows the results of an experiment in which growing NIH 3T3 cells were transiently transfected for 5 h with the pfos-CAT plasmid and either decreasing amounts of wild-type mT, 5 μg of ST, or 5 μg of ST insAL107 and then placed into 0.2% CS–DMEM. The cells were then harvested 48 h after transfection. While less active than MT, ST showed a significant ability (five- to sixfold over that of the control) to activate the c-fos promoter. This activity depended on PP2A binding, since the ST insAL107 mutant was inactive.
The failure of PP2A bound to Y250F/Y315F to activate the c-fos promoter compared to the activation by PP2A bound to ST could mean that the interactions are intrinsically different. However, one obvious difference is that Y250F/Y315F, like wild-type MT, is localized to membranes, while ST is soluble. Py1387T is a tailless MT that is found in soluble fractions (14). Figure 5 shows that Py1387T, like ST, has the ability to activate the fos promoter. This result is reminiscent of early observations that a truncated version of MT, like ST, can complement a large T virus for growth (88). Mutation of the PP2A binding site by the addition of the insAL107 mutation to the Py1387T mutation abolished activity. These results argue that the interaction of PP2A with MT is potentially functional and that the failure of Y250F/Y315F to activate fos is likely a result of miscompartmentalization of the MT-PP2A complex.
MT interaction with PP2A leads to activation of JNK1.
The interaction of ST with PP2A is important for the ability of ST to activate fos and to promote cell cycle progression. However, the failure in these same assays of the MT Y250F/Y315F double mutant that associates with PP2A suggests that the MT-PP2A interaction is insufficient for these activities. What functions then can be ascribed to the PP2A interaction in the MT complex? One important role is in the recruitment of pp60c-src (12). In a search for other MT-induced pathways that could be responsive to the PP2A interaction, the activation of the c-Jun N-terminal kinase/stress-activated protein kinase (jnk/SAPK) was studied.
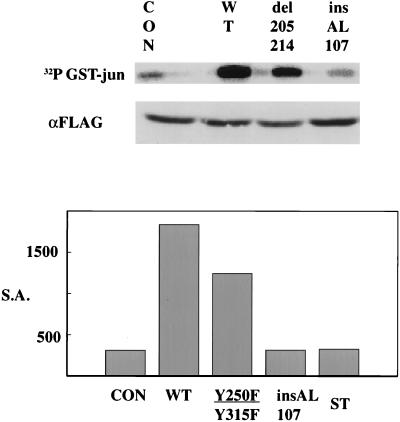
A number of studies have implicated jnk in signaling in response to cytokines or to cell stress (46, 100). Activation of jnk is a well-known consequence of tumor necrosis factor alpha (TNF-α) treatment (97). MT is known to sensitize cells to TNF-α (8). The effect of MT on jnk activity was measured in cotransfection experiments. 293T cells were transiently transfected with epitope-tagged (FLAG) jnk together with wild-type or mutant pCMV-MT constructs. At 48 h after transfection, jnk was immunoprecipitated with anti-FLAG antibody. Activity of the immunoprecipitated FLAG-jnk was determined in an immune complex kinase assay using GST–c-JUN (amino acid residues 1 to 79) as a substrate (Fig. 6). The results showed that MT increased the specific activity of jnk sevenfold without changing the amount of transfected protein. When MT del205-214, a mutant in src binding, was analyzed, activation of jnk was slightly reduced although jnk was still clearly active. Binding of PP2A seemed necessary for jnk activation since the MT insAL107 mutant was completely defective for jnk activation. The key issue was Y250F/Y315F. Figure 6 shows that, in contrast to the assays with BrdU or of the c-fos promoter, this mutant retained an activity similar to that of the wild type. This suggests a specific role for PP2A binding to MT that is more than just recruitment of src. ST was not active in this assay, suggesting that the localization of PP2A to membranes is a critical issue in jnk activation.
FIG. 6.
MT activates jnk. (Top) Jnk activation. 293T cells were transfected with FLAG-JNK1 and control or MT as indicated. At 48 h posttransfection, JNK1 was harvested with anti-FLAG antibody. JNK expression was determined with an anti-FLAG blot. JNK activity was assayed by using GST–c-JUN and [γ-32P]ATP as described in Materials and Methods. (Bottom) Comparison of the abilities of wild type, insAL107 MT, Y250F/Y315F MT, and ST to activate jnk in 293T cells. Specific activities (S.A.) were determined by dividing activity determined by PhosphorImager by the level of JNK determined by densitometry of an anti-FLAG blot.
DISCUSSION
From the data presented here, the ability of MT to transform cells is clearly more than the simple ability to promote cellular DNA synthesis. Mutants in SHC or PI3-K binding, as well as mutants with reduced tyrosine kinase activity such as del205-214 or insAL107, were all known previously to be substantially defective in their ability to transform fibroblasts; we repeated those results (data not shown). However, each of these mutants retained substantial ability to stimulate cell cycle progression as measured by BrdU incorporation. In this regard, MT is somewhat like the platelet-derived growth factor (PDGF) receptor, for which the presence of single phosphorylation sites mediating association of PI3-K or PLCγ1 could be sufficient for mitogenesis (91). In contrast to the PDGF receptor, the presence of the 322 site for PLCγ1 binding on MT was not sufficient to promote cell cycle progression, as shown by the inactive mutant Y250F/Y315F. An unexplained result is the observation that the percentage of cells incorporating BrdU with either the SHC or PI3-K single mutant was less than that with the wild type. This suggests that some cells are resistant to the effect of PI3-K or activation of the ras pathway. Surprisingly, cotransfection of Y250F and Y315F did not restore the frequency of BrdU incorporation to the level seen with the wild type (data not shown). It is possible that the presence of SHC and PI3-K on the same molecule of MT results in a more robust signal.
If MT prevents cell cycle withdrawal when either the SHC or PI3-K binding site is present, why are single mutants at either site not transforming? Before considering that question, it should first be noted that these mutants can cause tumors in some but not all cell types (11, 32, 102). Further, in some cell backgrounds, the single mutants show modest effects and partial phenotypes (57). In such cell backgrounds where the single mutants are transforming, the ability to promote cell cycle progression as measured in these experiments may be directly important. The strength of the MT signal in the fibroblasts used here may determine the likelihood of transformation, as it is known to determine the transformed phenotype (72). In the case of growth factors, down regulation of the receptors is often invoked to explain the limitation of the signal. There is no evidence that this applies to MT. It is possible that more stimulation, above that necessary for one round of BrdU incorporation, is needed to cause cells to undergo multiple rounds of replication. In F111 cells, for example, polyomavirus expressing only large T stimulates a single round of DNA replication, while wild-type virus expressing all three early proteins gives multiple rounds (78). An alternative explanation is that additional levels of growth suppression are imposed by cell-cell contact in transformation assays. In the transfection experiments carried out here, the cells are subconfluent so this type of growth control would not apply.
Genetic analysis demonstrated that MT binding to PP2A can be connected to the activation of jnk. Since ST was not active in the jnk assay, MT apparently acts by bringing PP2A to the membrane. As noted above, jnk activation has been connected with TNF-α and with apoptosis. However, both the regulation of jnk and the biology of jnk are complex. For example, while no significant contribution of PI3-K was seen in the effect of MT on jnk in 293T cells, it has been shown in other systems that activated PI3-K can activate jnk (52). Srinivas and colleagues (83a) have shown that MT can modulate c-Jun phosphorylation and activity in a manner that can be blocked by dominant negative ras or dominant negative raf-1. Similarly, jnk activation can induce apoptosis in 293T cells (18). However, the connection between jnk and apoptosis is controversial (73) in that jnk can mediate both pro- and antiapoptotic signals (46). The biological significance of the activation of jnk by MT requires further study, and the report (8) that MT sensitizes cells to TNF-α treatment suggests that those studies will be important. In 293T cells, the effect of MT is proapoptotic (25a).
The most important new insights in the work presented here concern the interactions of PP2A with the polyomavirus T antigens. ST stimulated BrdU incorporation and activated the c-fos promoter in a manner dependent on an intact PP2A binding site. The Y250/Y315F MT mutant did neither, even though it had an association with PP2A that was indistinguishable from that of the wild type by IEF. The genetics connecting DNA synthesis and fos or jnk activation to binding of PP2A demonstrate that MT and ST use PP2A differently. It might be imagined that some of the differences between MT and ST function could arise from something other than the PP2A interaction. For example, for SV40 ST some functions can be genetically dissociated from PP2A binding (71). These functions seem to come from the N-terminal J domain (84). However, the polyomavirus ST functions studied here were abolished by the same insAL107 mutation that abolished binding of PP2A to MT. While it is conceivable that the insAL107 mutation had a global effect on ST function, for MT at least, the same mutation is not globally disruptive because the ability to bind hs70 is preserved in the insAL107 mutant (Fig. 4). Further, soluble Py1387T MT could activate fos in a manner dependent on PP2A binding. It therefore appears that wild-type ST and wild-type MT use PP2A differently.
Another important conclusion is that the effect of ST on PP2A measured in the BrdU and c-fos promoter assays must be positive. Most of the literature on SV40 focuses on the notion that ST inhibits the activity of PP2A (34, 49, 59, 82, 98). For example, ST can be used to enhance Ca-calmodulin-dependent protein kinase signaling by blocking PP2A (96). However, if its action were simply inhibitory, then the Y250F/Y315F MT mutant should have the same activity as ST. Immunoblotting results have shown that MT is expressed at equal or greater levels than ST. The difference between MT and ST did not arise because greater ST expression was titrating the cellular PP2A. The conclusion that ST can act positively could solve a puzzle that has confronted the polyomavirus field for some time. PP2A is an abundant protein. While titration by ST seems possible for SV40, where protein expression is greater, it is unlikely that the lower levels of polyomavirus MT and ST expression could titrate out the PP2A in transformation. If the effect of polyomavirus MT and ST on PP2A is positive, then titration is no longer a critical issue. We suggest that the MT-PP2A and ST-PP2A complexes may be responsible for dephosphorylating certain critical targets.
How could ST affect PP2A? There have been reports that ST can change the apparent intrinsic specificity of the AC complex, activating it towards H1 histone (101). The interaction of PP2A with another protein, casein kinase 2 alpha, results in its altered activity (41). Alternatively, ST could function by changing the targeting of PP2A. The common hypothesis to explain the many forms of B subunits of PP2A is that they spatially or functionally target the enzyme (24). It is known, for example, that PP2A activity associated with microtubules changes during the cell cycle (67); ST, which an old report showed could bind tubulin (63), could in principle deliver PP2A to such a target by replacing the B subunit. It is perhaps more likely that targets are presented by the N-terminal J domain of ST (84). The failure of Y250/Y315F MT to function in BrdU and fos assays may simply be one of localization; PP2A on the membrane may not be able to reach the same substrates as PP2A in the cytoplasm. Alternatively, the four unique amino acids at the C terminus of ST could be functionally important in a positive way or the unique MT C terminus could obstruct access to the relevant substrates. Sorting out these possibilities will be a goal of future experiments.
ACKNOWLEDGMENTS
This work was supported by NIH grants PO-CA50661 and R37-CA34722.
REFERENCES
- 1.Andrews D W, Gupta J, Abisdris G. Evidence that the middle T antigen of polyomavirus interacts with the membrane skeleton. Mol Cell Biol. 1993;13:4703–4713. doi: 10.1128/mcb.13.8.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselin C, Gelinas C, Bastin M. Role of the three polyoma virus early proteins in tumorigenesis. Mol Cell Biol. 1983;3:1451–1459. doi: 10.1128/mcb.3.8.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin C, Gelinas C, Branton P, Bastin M. Polyoma middle T antigen requires cooperation from another gene to express the malignant phenotype in vivo. Mol Cell Biol. 1984;4:755–760. doi: 10.1128/mcb.4.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselin C, Sullivan M, Bastin M. Introns enable the polyomavirus middle and small T antigens to stimulate the growth of primary rat embryo fibroblasts. Gene. 1997;203:175–181. doi: 10.1016/s0378-1119(97)00511-8. [DOI] [PubMed] [Google Scholar]
- 5.Asselin C, Vass-Marengo J, Bastin M. Mutation in the polyomavirus genome that activates the properties of large T associated with neoplastic transformation. J Virol. 1986;57:165–172. doi: 10.1128/jvi.57.1.165-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1989. [Google Scholar]
- 6a.Benjamin T L. Host range mutants of polyoma virus. Proc Natl Acad Sci USA. 1970;73:394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger H, Wintersberger E. Polyomavirus small T antigen enhances replication of viral genomes in 3T6 mouse fibroblasts. J Virol. 1986;60:768–770. doi: 10.1128/jvi.60.2.768-770.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergqvist A, Soderbarg K, Magnusson G. Altered susceptibility to tumor necrosis factor alpha-induced apoptosis of mouse cells expressing polyomavirus middle and small T antigens. J Virol. 1997;71:276–283. doi: 10.1128/jvi.71.1.276-283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolen J B, Thiele C J, Israel M A, Yonemoto W, Lipsich L A, Brugge J S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984;38:767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 10.Brewster C E, Glover H R, Dilworth S M. pp60c-src binding to polyomavirus middle T-antigen (MT) requires residues 185 to 210 of the MT sequence. J Virol. 1997;71:5512–5520. doi: 10.1128/jvi.71.7.5512-5520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronson R, Dawe C, Carroll J, Benjamin T. Tumor induction by a transformation-defective polyoma virus mutant blocked in signaling through shc. Proc Natl Acad Sci USA. 1997;94:7954–7958. doi: 10.1073/pnas.94.15.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell K, Auger K, Hemmings B, Roberts T, Pallas D. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell K S, Ogris E, Burke B, Su W, Auger K R, Druker B J, Schaffhausen B S, Roberts T M, Pallas D C. Polyoma middle T antigen interacts with SHC via the NPTY motif in middle T. Proc Natl Acad Sci USA. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael G, Schaffhausen B, Dorsky D, Oliver D, Benjamin T. The role of the carboxy terminus of middle T antigen of polyoma virus. Proc Natl Acad Sci USA. 1982;79:3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael G, Schaffhausen B S, Mandel G, Liang T J, Benjamin T L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci USA. 1984;81:679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartwright C A, Kaplan P L, Cooper J A, Hunter T, Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986;6:1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C A, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques. 1988;6:632–637. [PubMed] [Google Scholar]
- 18.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 19.Cheng S H, Harvey R, Espino P C, Semba K, Yamamoto T, Toyoshima T K, Smith A E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988;7:3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherington V, Morgan B, Spiegelman M, Roberts T M. Recombinant retroviruses that transduce individual polyoma tumor antigens: effects on growth and differentiation. Proc Natl Acad Sci USA. 1986;83:4307–4311. doi: 10.1073/pnas.83.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen B, Yoakim M, Piwnica-Worms H, Roberts T, Schaffhausen B. Tyrosine phosphorylation is a signal for the trafficking of pp85, a polypeptide associated with phosphatidylinositol kinase activity. Proc Natl Acad Sci USA. 1990;87:4458–4462. doi: 10.1073/pnas.87.12.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtneidge S, Smith A E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 23.Courtneidge S A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985;4:1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csortos C, Zolnierowicz S, Bako E, Durbin S, DePaoli-Roach A. High complexity in the expression of the B′ subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J Biol Chem. 1996;271:2578–2588. doi: 10.1074/jbc.271.5.2578. [DOI] [PubMed] [Google Scholar]
- 25.Culleré X, Rose P, Thathamangalam U, Chatterjee A, Mullane K, Pallas D, Benjamin T, Roberts T, Schaffhausen B. Serine 257 phosphorylation regulates association of polyoma middle T antigen with 14-3-3 proteins. J Virol. 1998;72:558–565. doi: 10.1128/jvi.72.1.558-563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Culleré, X. Unpublished data.
- 26.Dawe C J, Freund R, Mandel G, Ballmer-Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 27.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature. 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 28.Druker B J, Ling L E, Cohen B, Roberts T M, Schaffhausen B S. A completely transformation-defective point mutant of polyomavirus middle T antigen which retains full associated phosphatidylinositol kinase activity. J Virol. 1990;64:4454–4461. doi: 10.1128/jvi.64.9.4454-4461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Druker B J, Sibert L, Roberts T M. Polyomavirus middle T-antigen NPTY mutants. J Virol. 1992;66:5770–5776. doi: 10.1128/jvi.66.10.5770-5776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckhart W, Hutchinson M A, Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979;18:925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- 31.Eddy B E, Stewart S E, Grubbs G E. Influence of tissue culture passage, storage, temperature and drying on viability of SE polyoma virus. Proc Soc Exp Biol. 1958;99:289–292. doi: 10.3181/00379727-99-24326. [DOI] [PubMed] [Google Scholar]
- 32.Freund R, Dawe C J, Carroll J P, Benjamin T L. Changes in frequency, morphology, and behavior of tumors induced in mice by a polyoma virus mutant with a specifically altered oncogene. Am J Pathol. 1992;141:1409–1425. [PMC free article] [PubMed] [Google Scholar]
- 33.Freund R, Sotnikov A, Bronson R T, Benjamin T L. Polyoma virus middle T is essential for virus replication and persistence as well as for tumor induction in mice. Virology. 1992;191:716–723. doi: 10.1016/0042-6822(92)90247-m. [DOI] [PubMed] [Google Scholar]
- 34.Frost J, Alberts A, Sontag E, Guan K, Mumby M, Feramisco J. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrels J I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979;254:7961–7977. [PubMed] [Google Scholar]
- 36.Gjørup O, Rose P, Holman P, Bockus B, Schaffhausen B. Protein domains connect cell cycle stimulation directly to initiation of DNA replication. Proc Natl Acad Sci USA. 1994;91:12125–12129. doi: 10.1073/pnas.91.25.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glenn G M, Eckhart W. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J Virol. 1995;69:3729–3736. doi: 10.1128/jvi.69.6.3729-3736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glenn G M, Eckhart W. Mutation of a cysteine residue in polyomavirus middle T antigen abolishes interactions with protein phosphatase 2A, pp60c-src, and phosphatidylinositol-3 kinase, activation of c-fos expression, and cellular transformation. J Virol. 1993;67:1945–1952. doi: 10.1128/jvi.67.4.1945-1952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glenn G M, Eckhart W. Transcriptional regulation of early-response genes during polyomavirus infection. J Virol. 1990;64:2193–2201. doi: 10.1128/jvi.64.5.2193-2201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey R, Oostra G L, Belsham G J, Gillett P, Smith A E. An antibody to a synthetic peptide recognizes polyomavirus middle-T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol Cell Biol. 1984;4:1334–1342. doi: 10.1128/mcb.4.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heriche J K, Lebrin F, Rabilloud T, Leroy D, Chambaz E M, Goldberg Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science. 1997;276:952–955. doi: 10.1126/science.276.5314.952. [DOI] [PubMed] [Google Scholar]
- 42.Hill C S, Marias R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 43.Holman P, Gjoerup O, Davin T, Schaffhausen B. Characterization of an immortalizing N-terminal domain of polyomavirus large T antigen. J Virol. 1994;68:668–673. doi: 10.1128/jvi.68.2.668-673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horak I D, Kawakami T, Gregory F, Robbins K C, Bolen J B. Association of p60fyn with middle tumor antigen in murine polyomavirus-transformed rat cells. J Virol. 1989;63:2343–2347. doi: 10.1128/jvi.63.5.2343-2347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter T, Hutchinson M A, Eckhart W. Polyoma middle-T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 1984;3:73–80. doi: 10.1002/j.1460-2075.1984.tb01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ip Y, Davis R. Signal transduction by the c-Jun N-terminal kinase from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 47.Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important cell transformation. Virology. 1979;98:261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- 48.Janknecht R, Nordheim A. Regulation of the c-fos promoter by the ternary complex factor Sap-1a and its coactivator CBP. Oncogene. 1995;12:1961–1969. [PubMed] [Google Scholar]
- 49.Kamibayashi C, Estes R, Lickteig R L, Yang S I, Craft C, Mumby M C. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 50.Kaplan D R, Whitman M, Schaffhausen B, Raptis L, Garcea R L, Pallas D, Roberts T M, Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci USA. 1986;83:3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawabe T, Muslin A, Korsmeyer S. HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature. 1997;385:454–458. doi: 10.1038/385454a0. [DOI] [PubMed] [Google Scholar]
- 52.Klippel A, Reinhard C, Kavanaugh W M, Appel G, Escobedo M-A, Williams L. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kornbluth S, Sudol M, Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987;325:171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- 54.Kortenjann M, Thomae O, Shaw P. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994;14:4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang T J, Carmichael G G, Benjamin T L. A polyoma mutant that encodes small T antigen but not middle T antigen demonstrates uncoupling of cell surface and cytoskeletal changes associated with cell transformation. Mol Cell Biol. 1984;4:2774–2783. doi: 10.1128/mcb.4.12.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loeken M R. Simian virus 40 small t antigen trans activates the adenovirus E2A promoter by using mechanisms distinct from those used by adenovirus E1A. J Virol. 1992;66:2551–2555. doi: 10.1128/jvi.66.4.2551-2555.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markland W, B. A. O, Harvey R, Markham A F, Colledge W H, Smith A E. Site-directed mutagenesis of polyomavirus middle-T antigen sequences encoding tyrosine 315 and tyrosine 250. J Virol. 1986;59:384–391. doi: 10.1128/jvi.59.2.384-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martens I, Nilsson S A, Linder S, Magnusson G. Mutational analysis of polyomavirus small-T-antigen functions in productive infection and in transformation. J Virol. 1989;63:2126–2133. doi: 10.1128/jvi.63.5.2126-2133.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moens U, Seternes O M, Johansen B, Rekvig O P. Mechanisms of transcriptional regulation of cellular genes by SV40 large T- and small T-antigens. Virus Genes. 1997;15:135–154. doi: 10.1023/a:1007962908248. [DOI] [PubMed] [Google Scholar]
- 60.Mumby M. Regulation by tumour antigens defines a role for PP2A in signal transduction. Semin Cancer Biol. 1995;6:229–237. doi: 10.1006/scbi.1995.0030. [DOI] [PubMed] [Google Scholar]
- 61.Mumby M C, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Phys Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 62.Mungre S, Enderle K, Turk B, Porras A, Wu Y Q, Mumby M C, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy C, Bikel I, Livingston D. Cellular proteins which can specifically associate with simian virus 40 small t antigen. J Virol. 1986;59:692–702. doi: 10.1128/jvi.59.3.692-702.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilsson S V, Magnusson G. T-antigen expression by polyoma mutants with modified RNA splicing. EMBO J. 1983;2:2095–2101. doi: 10.1002/j.1460-2075.1983.tb01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noda T, Satake M, Robins T, Ito Y. Isolation and characterization of NIH 3T3 cells expressing polyomavirus small T antigen. J Virol. 1986;60:105–113. doi: 10.1128/jvi.60.1.105-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noda T, Satake M, Yamaguchi Y, Ito Y. Cooperation of middle and small T antigens of polyomavirus in transformation of established fibroblast and epithelial-like cell lines. J Virol. 1987;61:2253–2263. doi: 10.1128/jvi.61.7.2253-2263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunbhakdi-Craig V, Bloom G, Mumby M. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogris E, Mudrak I, Wintersberger E. Polyomavirus large and small T antigens cooperate in induction of the S phase in serum-starved 3T3 mouse fibroblasts. J Virol. 1992;66:53–61. doi: 10.1128/jvi.66.1.53-61.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pallas D C, Cherington V, Morgan W, DeAnda J, Kaplan D, Schaffhausen B, T. M R. Cellular proteins that associate with the middle and small T antigens of polyomavirus. J Virol. 1988;62:3934–3940. doi: 10.1128/jvi.62.11.3934-3940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 71.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raptis L, Lamfrom H, Benjamin T L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985;5:2476–2485. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roulston A, Reinhard C, Amiri P, Williams L. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 74.Schaffhausen B, Benjamin T L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981;40:184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaffhausen B, Silver J, Benjamin T. Tumor antigen(s) in cells productively infected by wild-type polyoma virus and mutant NG18. Proc Natl Acad Sci USA. 1978;75:79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaffhausen B S, Benjamin T L. Phosphorylation of polyoma T antigens. Cell. 1979;18:935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- 77.Schaffhausen B S, Dorai H, Arakere G, Benjamin T L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982;2:1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlegel R, Benjamin T L. Cellular alterations dependent upon the polyoma virus hr-t function: separation of mitogenic from transforming capacities. Cell. 1978;14:587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- 79.Shenolikar S. Protein serine/threonine phosphatases—new avenues for cell regulation. Ann Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 80.Silver J, Schaffhausen B, Benjamin T. Tumor antigens induced by non-transforming mutants of polyoma virus. Cell. 1978;15:485–496. doi: 10.1016/0092-8674(78)90018-1. [DOI] [PubMed] [Google Scholar]
- 81.Smith A E, Smith R, Griffin B, Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979;18:915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- 82.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 83.Sontag E, Sontag J M, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83a.Srinivas S, Schönthal A, Eckhart W. Polyoma middle-sized tumor antigen modulates c-Jun phosphorylation and transcriptional activity. Proc Natl Acad Sci USA. 1994;91:10064–10068. doi: 10.1073/pnas.91.21.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strack S, Zaucha J, Ebner F, Colbran R, Wadzinski B. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–527. [PubMed] [Google Scholar]
- 86.Su W, Liu W, Schaffhausen B, Roberts T. Association of polyomavirus middle tumor antigen with phospholipase C-gamma 1. J Biol Chem. 1995;270:12331–12335. doi: 10.1074/jbc.270.21.12331. [DOI] [PubMed] [Google Scholar]
- 87.Templeton D, Eckhart W. Mutation causing premature termination of the polyoma virus medium T antigen blocks cell transformation. J Virol. 1982;41:1014–1024. doi: 10.1128/jvi.41.3.1014-1024.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Templeton D, Simon S, Eckhart W. Truncated forms of the polyomavirus middle T antigen can substitute for the small T antigen in lytic infection. J Virol. 1986;57:367–370. doi: 10.1128/jvi.57.1.367-370.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Treisman R, Novak U, Favaloro J, Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle T protein. Nature. 1981;292:595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- 90.Urich M, Senften M, Shaw P E, Ballmer-Hofer K. A role for the small GTPase Rac in polyomavirus middle-T antigen-mediated activation of the serum response element and in cell transformation. Oncogene. 1997;14:1235–1241. doi: 10.1038/sj.onc.1200982. [DOI] [PubMed] [Google Scholar]
- 91.Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 92.Walter G, Ruediger R, Slaughter C, Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci USA. 1990;87:2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Bikel I, Marsilio E, Newsome D, Livingston D. Transrepression of RNA polymerase II promoters by the simian virus 40 small t antigen. J Virol. 1994;68:6180–6187. doi: 10.1128/jvi.68.10.6180-6187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watanabe G, Howe A, Lee R J, Albanese C, Shu I W, Karnezis A N, Zon L, Kyriakis J, Rundell K, Pestell R G. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webster M, Hutchinson J, Rauh M, Muthuswamy S, Anton M, Tortorice C, Cardiff R, Graham F, Hassell J, Muller W. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Westphal R, Anderson K, Means A, Wadzinski B. A signaling complex of Ca2+ calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science. 1998;280:1258–1261. doi: 10.1126/science.280.5367.1258. [DOI] [PubMed] [Google Scholar]
- 97.Westwick J, Weitzel C, Minden A, Karin M, Brenner D. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 98.Wheat W, Roesler W, Klemm D. Simian virus 40 small tumor antigen inhibits dephosphorylation of protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1994;14:5881–5890. doi: 10.1128/mcb.14.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 100.Whitmarsh A, Davis R. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 101.Yang S I, Lickteig R L, Estes R, Rundell K, Walter G, Mumby M C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yi X, Peterson J, Freund R. Transformation and tumorigenic properties of a mutant polyomavirus containing a middle T antigen defective in Shc binding. J Virol. 1997;71:6279–6286. doi: 10.1128/jvi.71.9.6279-6286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Z Y, Veldman G M, Cowie A, Carr A, Schaffhausen B, R K. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984;51:170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zullo J, Stiles C D, Garcea R L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci USA. 1987;84:1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]