Key Points
-
•
Axi-cel demonstrated durable responses in patients with FL and MZL after 3 years of follow-up.
-
•
Elevated baseline total metabolic tumor volume and recent prior bendamustine use may affect durable remissions of patients with FL.
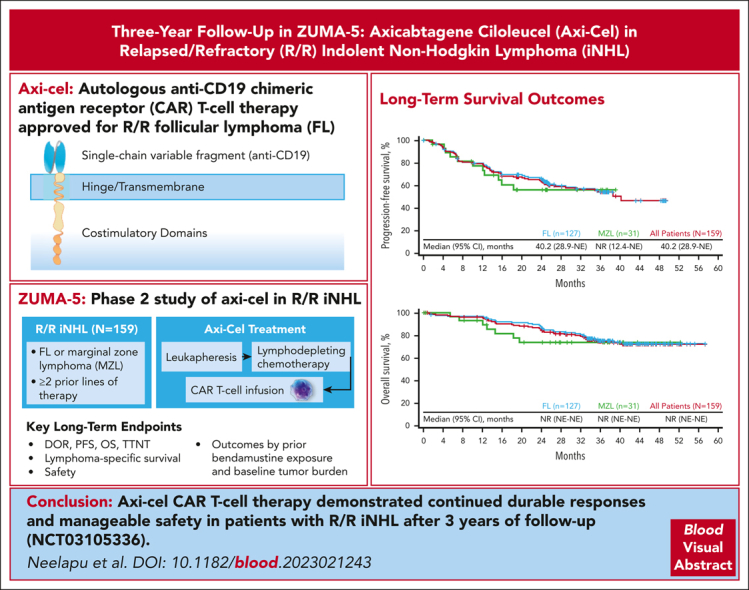
Visual Abstract
Abstract
Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for relapsed/refractory (R/R) follicular lymphoma (FL). Approval was supported by the phase 2, multicenter, single-arm ZUMA-5 study of axi-cel for patients with R/R indolent non-Hodgkin lymphoma (iNHL; N = 104), including FL and marginal zone lymphoma (MZL). In the primary analysis (median follow-up, 17.5 months), the overall response rate (ORR) was 92% (complete response rate, 74%). Here, we report long-term outcomes from ZUMA-5. Eligible patients with R/R iNHL after ≥2 lines of therapy underwent leukapheresis, followed by lymphodepleting chemotherapy and axi-cel infusion (2 × 106 CAR T cells per kg). The primary end point was ORR, assessed in this analysis by investigators in all enrolled patients (intent-to-treat). After median follow-up of 41.7 months in FL (n = 127) and 31.8 months in MZL (n = 31), ORR was comparable with that of the primary analysis (FL, 94%; MZL, 77%). Median progression-free survival was 40.2 months in FL and not reached in MZL. Medians of overall survival were not reached in either disease type. Grade ≥3 adverse events of interest that occurred after the prior analyses were largely in recently treated patients. Clinical and pharmacokinetic outcomes correlated negatively with recent exposure to bendamustine and high metabolic tumor volume. After 3 years of follow-up in ZUMA-5, axi-cel demonstrated continued durable responses, with very few relapses beyond 2 years, and manageable safety in patients with R/R iNHL. The ZUMA-5 study was registered at www.clinicaltrials.gov as #NCT03105336.
Neelapu et al report on long-term outcomes from ZUMA-5, a trial of axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor T-cell therapy, for relapsed/refractory follicular lymphoma (FL) and marginal zone lymphoma (MZL). At a median follow-up of 41.7 months, the overall response rates were 94% in FL and 77% in MZL, similar to what was seen in earlier reported results at 17.5 months. Median progression-free survival was 40.2 months in FL and not yet reached in MZL; median overall survival was not reached in either group. Clinical outcomes were worse following recent bendamustine therapy and for patients with high-tumor volume. After 3 years, axi-cel demonstrates durable responses with few relapses beyond 2 years.
Introduction
Relapsed or refractory (R/R) indolent non-Hodgkin lymphoma (iNHL), including follicular lymphoma (FL) and marginal zone lymphoma (MZL), is considered largely incurable, with most patients ultimately experiencing additional disease relapses.1,2 Among patients with FL, treatment at later lines is heterogeneous, but a commonality is that remissions are progressively shorter and survival is reduced after secondline and later therapies.3,4 In addition, patients with FL who progress within 24 months from initiating the first anti-CD20–containing chemoimmunotherapy (POD24) have an unfavorable prognosis and a shortened survival with the available R/R treatment options.5
Recent advances in therapeutic options for iNHL, including chimeric antigen receptor (CAR) T-cell therapy, have improved outcomes in patients with R/R disease.1 Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 CAR T-cell therapy, which includes a CD28 costimulatory domain to elicit rapid and robust expansion that results in target-specific cytotoxicity and helps to overcome the limitations of the immune system.6,7 Axi-cel is approved for the treatment of adults with R/R FL.6,8 Approval was supported by the primary analysis of the ZUMA-5 trial, a single-arm, international, phase 2 study of patients with iNHL (N = 104), in which the overall response rate (ORR) was 92% (complete response [CR] rate, 74%) after a median of 17.5 months of follow-up.9
Long-term follow-up analyses are particularly important in R/R indolent lymphomas because of the heterogeneity of pretreatment tumor characteristics and its long clinical course.10 Here, we report efficacy, safety, and biomarker assessments from ZUMA-5 after 3 years of follow-up, representing the longest follow-up analysis of a CAR T-cell therapy in iNHL, to our knowledge, to date. This analysis includes exploratory assessments of the association of clinical outcomes with baseline variables, including prior bendamustine exposure and baseline tumor burden based on total metabolic tumor volume (TMTV).
Methods
Patients and study design
ZUMA-5 is a multicenter, single-arm, registrational, phase 2 trial at 17 medical centers in the United States and France and is registered at Clinicaltrials.gov (NCT03105336). A full list of sites was reported previously.9 Enrolled patients provided written informed consent for participation and the study protocol was approved by the institutional review board at each site. ZUMA-5 was conducted in compliance with the principles of the Declaration of Helsinki. The study investigators and study sponsor (Kite, a Gilead Company) designed and performed the study. All authors had access to the data and contributed to the study conduct, data analysis and interpretation, and manuscript development.
Full patient eligibility criteria have been previously reported.9 Briefly, patients aged ≥18 years with R/R iNHL, including FL (grade 1-3a) and MZL (nodal or extranodal; both per World Health Organization 2016 criteria), who had ≥2 prior systemic therapies that must have included an anti-CD20 monoclonal antibody combined with an alkylating agent. Patients who had previous autologous stem cell transplantation within 6 weeks of axi-cel, any allogeneic stem cell transplantation, CD19-targeted therapy, or CAR T-cell therapy were excluded. Disease progression <6 months of completion of the most recent prior therapy was considered refractory.
Procedures and end points
Enrolled patients underwent leukapheresis, followed by lymphodepleting chemotherapy with fludarabine (30 mg/m2 per day) and cyclophosphamide (500 mg/m2 per day) on days −5 to −3 before infusion, and axi-cel (2 × 106 CAR T cells per kg).9 Bridging therapy before lymphodepletion was per investigator discretion. Disease response assessments, as detailed previously, were performed by investigators and reviewed by an independent radiology review committee per Lugano classification at specified timepoints until the 24-month follow-up analysis, after which assessments were per investigator only.9,11 All adverse events (AEs) were monitored up to 3 months after infusion, and AEs of special interest (neurological, hematological, infectious, and autoimmune) were monitored up to 24 months; second primary malignancies were monitored up to 15 years. Serious events related to axi-cel were reported regardless of time period. The primary end point of ZUMA-5 was ORR. Secondary end points included CR, duration of response (DOR), progression-free survival (PFS), overall survival (OS), time to next treatment (TTNT), safety, and blood levels of CAR T cells. Exploratory end points included in this analysis were lymphoma-specific PFS and survival, in which progression events or deaths related to lymphoma, axi-cel, or lymphodepleting chemotherapy as assessed by the investigator were considered events of interest. Deaths owing to other or unknown causes were considered competing risks. An additional analysis of cumulative incidence of disease progression or death because of lymphoma was performed, with nonlymphoma related deaths (including death due to study treatment) as competing events. Clinical and pharmacokinetic outcomes were also assessed by key patient and clinical subgroups, including prior bendamustine use before leukapheresis and baseline TMTV (supplemental Methods, available on the Blood website).
Statistical analyses
The 3-year analysis of ZUMA-5 occurred when enrolled patients with FL had median follow-up of ≥36 months. Efficacy outcomes were assessed in all enrolled patients with iNHL (intent-to-treat); safety and translational assessments were in treated patients with iNHL (laboratory and biomarker assessments were previously described).9 Patients with FL who had ≥3 lines of therapy, excluding those with alternative histology on baseline central assessment, were assessed in a separate analysis (supplemental Methods). Patients treated again with axi-cel were also assessed separately (re-treatment criteria were previously reported).9
Descriptive statistics were used to summarize baseline characteristics, response, and incidence of AEs. Two-sided 95% confidence intervals (CIs) for response rates were assessed using the Clopper-Pearson method. Secondary end points involving time to event outcomes were assessed using Kaplan-Meier methodology. Lymphoma-specific PFS and survival were assessed using a competing risk approach in which events of interest were considered as main events, whereas deaths not attributed to lymphoma, axi-cel, or lymphodepleting chemotherapy were considered competing risks. Event rates over time were calculated for both main events and competing risks via the cumulative incidence function.
Propensity score matching (PSM) was performed to assess outcomes in patients with FL by prior bendamustine use, accounting for the distribution of baseline TMTV, Eastern Cooperative Oncology Group performance status score, Follicular Lymphoma International Prognostic Index score, number of prior chemotherapies, age, double refractory status, and whether the last systemic therapy was administered <12 months from leukapheresis (supplemental Methods). Wilcoxon rank-sum tests were used to assess associations between CAR T-cell levels and clinical outcomes.
Results
Patients
A total of 159 patients were enrolled (127 with FL, 31 with MZL, and 1 with diffuse large B-cell lymphoma) and underwent leukapheresis, including 6 additional patients with MZL who were enrolled after the data cutoff date for the 18-month analysis.9 Axi-cel was successfully manufactured for all enrolled patients. In addition to untreated patients previously described, 1 patient had disease transformation and 1 had no measurable disease.9 The patient diagnosed with diffuse large B-cell lymphoma did not receive axi-cel and discontinued the study. A total of 152 patients received lymphodepleting chemotherapy and axi-cel as of the data cutoff date of 31 March 2022 (FL, n = 124; MZL, n = 28).
Baseline characteristics among all 159 enrolled patients are reported in Table 1 (2-year results are reported in supplemental Table 1). The median age was 60 years for patients with FL (range, 34-79 years) and 64 years for those with MZL (range, 43-77 years). Among patients with FL, 56% had POD24 and 69% had prior bendamustine use. Baseline characteristics of patients with FL by prior bendamustine exposure before and after PSM are reported in supplemental Table 2. Baseline TMTV in patients with FL positively correlated with tumor burden as measured by the sum of product diameters (SPD; supplemental Figure 1), Follicular Lymphoma International Prognostic Index score, and tumor bulk by Groupe d Etude des Lymphomes Folliculaires criteria, although it was not correlated with baseline lactate dehydrogenase levels.
Table 1.
Baseline characteristics of all enrolled patients
| Characteristic | FL (n = 127) |
MZL (n = 31) |
All patients (N = 159)∗ |
|---|---|---|---|
| Age, median (range), y | 60 (34-79) | 64 (43-77) | 60 (34-79) |
| ≥65 y, n (%) | 40 (31) | 14 (45) | 54 (34) |
| Male sex, n (%) | 75 (59) | 15 (48) | 90 (57) |
| FL histological category, n (%) | |||
| Grade 1 | 34 (27) | — | — |
| Grade 2 | 63 (50) | — | — |
| Grade 3a | 30 (24) | — | — |
| MZL histological category, n (%) | |||
| Nodal | — | 10 (32) | — |
| Extranodal | — | 21 (68) | — |
| ECOG PS of 1, n (%) | 48 (38) | 16 (52) | 65 (41) |
| Stage III-IV disease, n (%) | 109 (86) | 29 (94) | 139 (87) |
| High-risk FLIPI (≥3), n (%) | 56 (44) | — | — |
| High tumor bulk (GELF criteria), n (%)† | 65 (51) | 16 (52) | 82 (52) |
| SPD, median (range), mm2 | 2604.15 (289.2-34 675.0) | 1746.45 (306.5-7 471.8) | 2449.50 (289.2-34 675.0) |
| TMTV, median (range), mL | 438.50 (11.21-5 576.58) | 368.83 (5.15-3 239.43) | 420.33 (5.15-5 576.58) |
| Number of prior therapies, median (range)‡ | 3 (1-10) | 3 (2-8) | 3 (1-10) |
| 3 prior lines of therapy, n (%) | 33 (26) | 10 (32) | 44 (28) |
| 4 prior lines of therapy, n (%) | 25 (20) | 1 (3) | 26 (16) |
| ≥5 prior lines of therapy, n (%) | 22 (17) | 9 (29) | 31 (19) |
| Prior PI3K inhibitor, n (%) | 36 (28) | 10 (32) | 46 (29) |
| Prior autologous SCT, n (%) | 30 (24) | 4 (13) | 34 (21) |
| Prior anti-CD20 mAb single agent, n (%) | 40 (31) | 11 (35) | 51 (32) |
| Prior lenalidomide, n (%) | 38 (30) | 9 (29) | 48 (30) |
| Prior bendamustine, n (%) | 88 (69) | 24 (77) | 113 (71) |
| ≤6 mo of leukapheresis | 8 (6) | 3 (10) | 11 (7) |
| ≥6 mo and <12 mo of leukapheresis | 10 (8) | 1 (3) | 12 (8) |
| >12 mo of leukapheresis | 70 (55) | 20 (65) | 90 (57) |
| R/R subgroup, n (%) | |||
| Refractory to last prior therapy | 87 (69) | 25 (81) | 113 (71) |
| Double refractory to prior anti-CD20 mAb and alkylating agent | 56 (44) | 13 (42) | 70 (44) |
| POD24 from initiating first anti-CD20 mAb–containing therapy§ | 70 (56) | 18 (60) | 89 (57) |
| Lymphoma present in bone marrow, n (%)‖ | 35 (28) | 14 (45) | 49 (31) |
| Received bridging therapy, n (%) | 4 (3) | 3 (10) | 7 (4) |
DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; GELF, Groupe d'Etude des Lymphomes Folliculaires; mAb, monoclonal antibody; PI3K, phosphatidylinositol-3-kinase; SCT, stem cell transplantation.
One patient was found to have disease type DLBCL after enrollment via pretreatment biopsy. This patient did not receive axi-cel and discontinued the study.
High tumor bulk, as defined by any of GELF criteria: involvement of ≥3 nodal sites, each with a diameter of ≥3 cm, any nodal or extranodal tumor mass with a diameter of ≥7 cm, B symptoms, splenomegaly, pleural effusions or peritoneal ascites, cytopenias, or leukemia.
One patient received prior therapy for DLBCL, not for the primary disease of FL.
Proportions are based on the number of patients who ever received anti-CD20–chemotherapy combination therapy.
Bone marrow was assessed by the investigator at baseline for lymphoma presence per Lugano11 bone marrow assessment/bone marrow assessment using aspirate or core biopsy at screening. If these were not available, lymphoma presence was based on diagnosis history of bone marrow involvement.
Efficacy in patients with FL
Median follow-up from leukapheresis for enrolled patients with FL was 41.7 months (range, 32.7-57.4 months). The investigator-assessed response among enrolled patients with FL was consistent with prior analyses (ORR, 94%; 95% CI, 88-97; CR rate, 79%; Table 2; supplemental Table 3).9 Thirty-two patients with initial partial response (PR) or stable disease later converted to CR. Median DOR for patients with FL was 38.6 months (Table 2; supplemental Figure 2). Median DOR was not reached for patients with a CR and was 4.9 months in those with a PR. At data cutoff, 67 of 127 enrolled patients with FL (53%) were in ongoing response; 65 of 127 (51%) were in ongoing CR. Among those who achieved a CR (n = 100), 65% were in ongoing response at data cutoff. Consistent with prior reporting, all 13 patients with FL re-treated with axi-cel responded (CR, 69%; PR, 31%). With a median follow-up of 23 months after re-treatment, the median post–re-treatment DOR was 5.0 months, with 46% of patients in ongoing response at data cutoff.
Table 2.
Investigator-assessed best response among all enrolled patients in the 3-year analysis
| FL (n = 127) |
MZL (n = 31) |
All patients (N = 159)∗ |
|
|---|---|---|---|
| ORR, n (%) | 119 (94) | 24 (77) | 143 (90) |
| CR | 100 (79) | 20 (65) | 120 (75) |
| PR | 19 (15) | 4 (13) | 23 (14) |
| SD, n (%) | 2 (2) | 3 (10) | 5 (3) |
| PD, n (%) | 2 (2) | 1 (3) | 3 (2) |
| Not done, n (%) | 4 (3) | 3 (10) | 8 (5) |
| DOR, median (95% CI), mo | 38.6 (29.0-NE) | NR (13.4-NE) | 38.6 (33.1-NE) |
| Estimate at 36 mo (95% CI), % | 57 (47-66) | 64 (40-80) | 58 (48-66) |
| Duration of CR, median (95% CI), mo | NR (35.4-NE) | NR (14.2-NE) | NR (35.4-NE) |
| Estimate at 36 mo (95% CI), % | 62 (48-72) | NR (NE-NE) | 61 (49-72) |
| Duration of PR, median (95% CI), mo | 4.9 (2.2-8.2) | 3.5 (1.9-NE) | 4.9 (2.1-6.2) |
| Estimate at 36 mo (95% CI), % | NR (NE-NE) | 0 (NE-NE) | NR (NE-NE) |
DLBCL, diffuse large B-cell lymphoma; NE, not estimable; NR, not reached; PR, partial response; SD, stable disease.
One patient was found to have disease type DLBCL after enrollment via pretreatment biopsy. This patient did not receive axi-cel and discontinued the study.
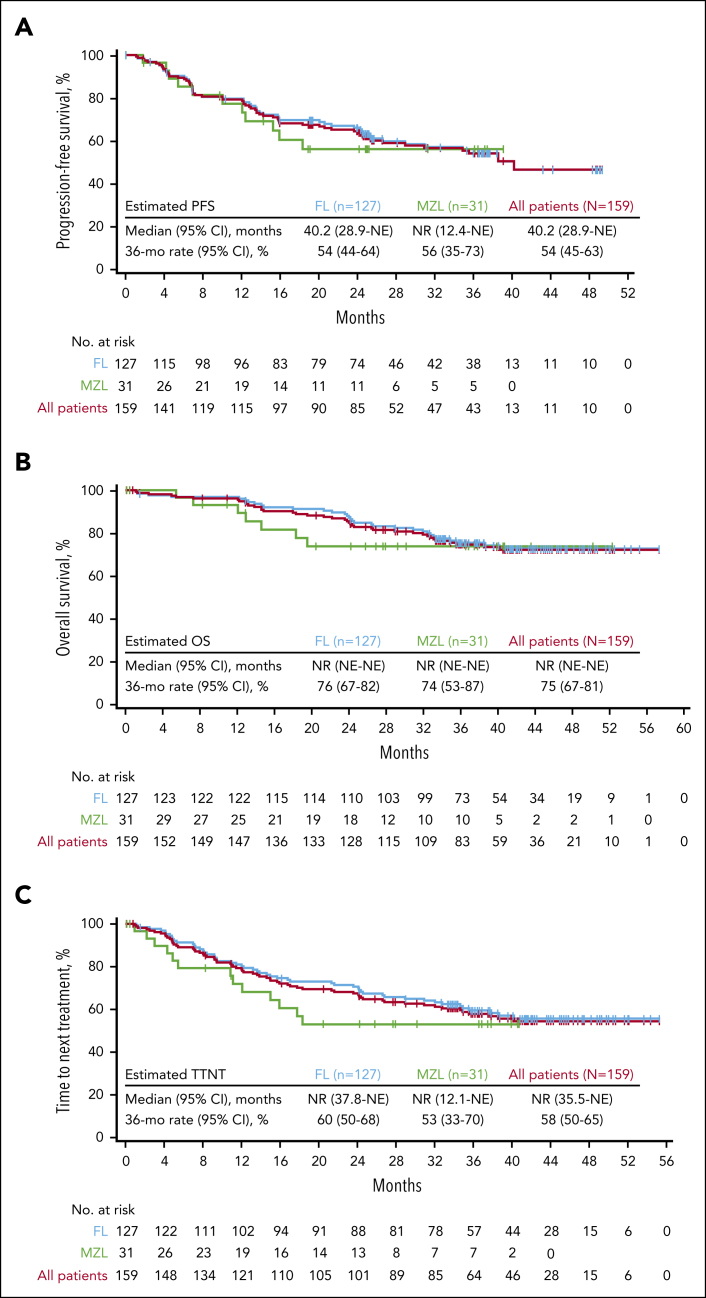
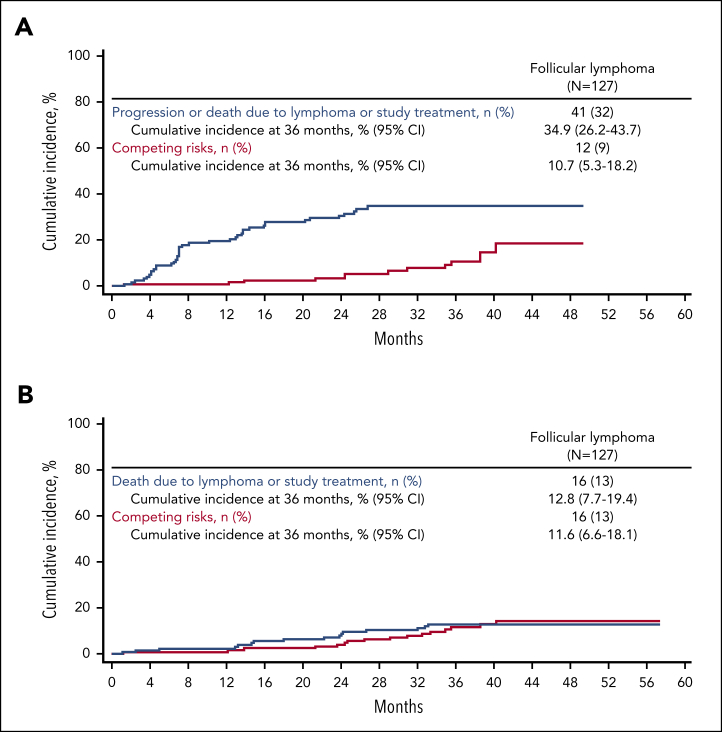
The median PFS for enrolled patients with FL was 40.2 months, with an estimated 36-month PFS rate of 54% (Figure 1). A total of 2 events of progression and 10 deaths occurred >24 months after leukapheresis. In a competing risk analysis of lymphoma-specific PFS in patients with FL, a total of 41 progression or death events (32%) due to lymphoma, lymphodepleting chemotherapy, or axi-cel occurred, of which 38 were progression events (Figure 2). The 36-month cumulative incidence rate of progression or lymphoma-specific death was 35%. Competing risks (deaths exclusive of progression or study treatment) occurred in 12 patients (9%), with most occurring after the 24-month timepoint. The cumulative incidence of competing risks at 36 months was 11% (Figure 2). In an analysis of cumulative incidence of disease progression or death due to lymphoma, with nonlymphoma related deaths (including death due to study treatment) as competing events, the cumulative incidence of progression at 36 months was 32% (no patients had death due to lymphoma before progression; supplemental Figure 3). The cumulative incidence of competing risks at 36 months was 14% (supplemental Figure 3). The median PFS among patients with (n = 70) or without POD24 (n = 41) was 40.2 months and not reached, respectively (supplemental Figure 4). The estimated 36-month PFS rate was largely consistent among patients with FL, regardless of other high-risk baseline characteristics (supplemental Figure 5).
Figure 1.
PFS, OS, and TTNT. Kaplan-Meier estimates of (A) PFS, (B) OS, and (C) TTNT by investigator assessment based on the disease type among the 159 enrolled patients with iNHL. mo, month; NE, not estimable; NR, not reached.
Figure 2.
Lymphoma-specific survival outcomes of patients with FL based in cumulative incidence and competing risk. Cumulative incidence plots of competing risk lymphoma-specific (A) PFS and (B) OS by investigator assessment for enrolled patients with FL. Main events included those due to lymphoma or study treatment complications. Events due to reasons other than lymphoma or study treatment complications were considered competing risks.
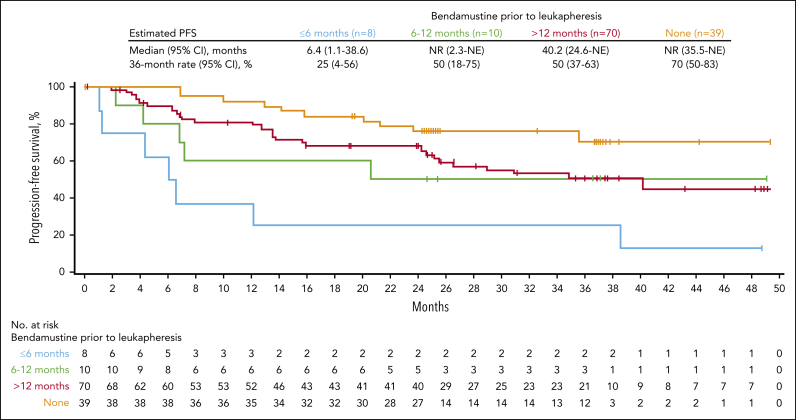
Patients with FL who received prior bendamustine had a numerically lower 36-month PFS rate than those who did not receive bendamustine; patients who received bendamustine ≤6 months from leukapheresis had notably numerically shorter PFS, although a small number of patients in this group may have limited comparison (Figure 3; supplemental Table 4) Further examination of prior bendamustine use after PSM demonstrated numerically higher CR and ongoing response at 36 months in patients without prior exposure to bendamustine compared with those with exposure ≤12 months from leukapheresis (supplemental Table 5). Of note, these analyses were descriptive and should only be viewed as hypothesis-generating.
Figure 3.
PFS of patients with FL based on the time point of bendamustine use before axi-cel infusion. Kaplan-Meier estimates of PFS among enrolled patients with FL by investigator assessment in those who had no prior bendamustine exposure, received bendamustine within 6 months of leukapheresis, received bendamustine between 6 and 12 months of leukapheresis, and received bendamustine >12 months before leukapheresis.
The median OS among enrolled patients with FL was not reached, and the estimated OS at 36 months was 76% (Figure 1). Median TTNT was also not reached, with a 36-month treatment-free estimate of 60% (Figure 1). A competing risk assessment of lymphoma-specific OS showed 16 deaths due to lymphoma, lymphodepleting chemotherapy, or axi-cel (13%). Competing risks (deaths due to other reasons) occurred in 16 patients (13%). The 36-month cumulative incidence of lymphoma-specific death was 13% (cumulative incidence of competing risks at 36 months, 12%; Figure 2).
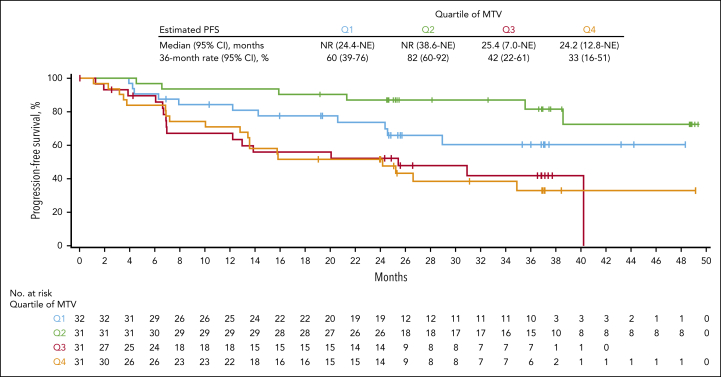
In an assessment of efficacy outcomes based on baseline TMTV in evaluable patients with FL (n = 125), the DOR and PFS were longer among patients with relatively low baseline TMTV, as observed below a historical threshold of 510 mL12 as well as below the study median and in lower quartiles (Figure 4; supplemental Tables 6 and 7). Notably, estimated PFS rate at 36 months was 71.2% for those whose baseline TMTV was below the study median and 37.3% among those with baseline TMTV above the median. No correlation between median baseline TMTV and ORR or CR was observed, likely because of the low number of nonresponders in this study. Patients with baseline TMTV below the study median were also more likely than those with baseline TMTV above the median to be in ongoing response at data cutoff. Association between baseline SPD and efficacy outcomes showed similar trends as those with TMTV, although none reached statistical significance.
Figure 4.
PFS of patients with FL based on the quartile of baseline metabolic tumor volume. Kaplan-Meier plot of PFS per investigator assessment based on the quartile of baseline metabolic tumor volume of evaluable enrolled patients with FL. MTV, metabolic tumor volume.
Efficacy results are reported separately in a subset of patients with FL with ≥3 prior lines of therapy after excluding patients whose central pathology assessment suggested alternative diagnoses other than FL. The outcomes were largely consistent with the overall cohort (supplemental Table 8).
Efficacy in patients with MZL
The median follow-up for enrolled patients with MZL from leukapheresis was 31.8 months (range, 8.3-52.3 months). The investigator-assessed ORR for enrolled patients with MZL was 77% (95% CI, 59-90), with a CR rate of 65% (Table 2; supplemental Table 3). Twelve patients converted to CR after initial PR or stable disease. Responses among subtypes of MZL (nodal and extranodal) are reported in supplemental Table 9. Median DOR in all patients with MZL was not yet reached, and 16 of 31 patients (52%) were in ongoing response as of data cutoff (Table 2; supplemental Figure 2).
Median PFS, OS, and TTNT were not yet reached among patients with MZL, and estimates at 24 months were 56%, 74%, and 53%, respectively (Figure 1). PFS estimates at 24 months were largely consistent among high-risk subgroups (supplemental Figure 6). No correlations were observed between baseline TMTV and efficacy outcomes among patients with MZL, possibly because of the small number of patients with this disease type in the study (supplemental Table 6).
Safety
No new safety signals were observed among treated patients with iNHL since the 18-month analysis (Table 3).9 AEs that occurred after the 18-month analysis (data cutoff date, 14 September 2020), including 1 grade 3 neurologic event, 2 infections of grade 3 to 4, and 5 cytopenias of grade 3 to 4, were largely among recently enrolled patients with MZL. Serious AEs occurred in 15 patients (10%; 11 with FL and 4 with MZL) since the 18-month analysis; events in 6 of those patients were considered related to axi-cel (3 in FL and 3 in MZL; Table 3). No new cases of grade ≥3 hypogammaglobulinemia occurred after the data cutoff date for the primary analysis (12 March 2020). During the study, 50 patients with iNHL (33%) received immunoglobulin therapy. In total, 18 patients had second primary malignancies (Table 4). No cases of axi-cel−related second primary malignancies, tumor lysis syndrome, or replication-competent retrovirus occurred at any time on study.
Table 3.
AEs occurring after the 18-month analysis among treated patients
| n (%) | FL (n = 124) |
MZL (n = 28) |
All patients (N = 152) |
|||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any AE | 20 (16) | 10 (8) | 10 (36) | 7 (25) | 30 (20) | 17 (11) |
| Serious AEs | 11 (9) | 10 (8) | 4 (14) | 3 (11) | 15 (10) | 13 (9) |
| Cytopenias | 3 (2) | 2 (2) | 5 (18) | 5 (18) | 8 (5) | 7 (5) |
| CRS | 0 | 0 | 3 (11) | 0 | 3 (2) | 0 |
| Neurologic events | 0 | 0 | 1 (4) | 1 (4) | 1 (1) | 1 (1) |
| Infections | 14 (11) | 6 (5) | 7 (25) | 2 (7) | 21 (14) | 8 (5) |
| Hypogammaglobulinemia | 1 (1) | 0 | 1 (4) | 0 | 2 (1) | 0 |
| Tumor lysis syndrome | 0 | 0 | 0 | 0 | 0 | 0 |
CRS, cytokine release syndrome. AEs of interest were reported up to 24 months after axi-cel infusion. Serious AEs related to axi-cel that occurred after the data cutoff for the 18-month analysis (14 September 2020) occurred in 6 patients: 1 with COVID-19 and COVID-19 pneumonia, 1 with pyrexia, 1 with cellulitis, 1 with myelodysplastic syndrome, 1 with febrile neutropenia, and 1 with pneumonia.9
Table 4.
Second primary malignancies
| FL (n = 124) |
MZL (n = 28) |
All patients (N = 152) |
|
|---|---|---|---|
| Any second primary malignancy, n (%) | 13 (10) | 5 (18) | 18 (12) |
| Nonmelanoma skin cancer | 3 (2) | 0 | 3 (2) |
| Melanoma | 1 (1) | 0 | 1 (1) |
| t-MDS/t-AML | 5 (4) | 3 (11) | 8 (5) |
| Colorectal cancer | 1 (1) | 0 | 1 (1) |
| B-ALL/AML | 1 (1) | 0 | 1 (1) |
| Anal/rectal cancer | 1 (1) | 0 | 1 (1) |
| Prostate cancer | 1 (1) | 0 | 1 (1) |
| Neuroendocrine tumor | 0 | 1 (4) | 1 (1) |
| Breast cancer | 0 | 1 (4) | 1 (1) |
ALL, acute myeloid leukemia; AML, acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; t, therapy-related. t-MDS/t-AML are events of MDS or AML that have been identified as being likely related to prior chemotherapy before the first axi-cel infusion.
Among patients with iNHL who had any grade ≥3 cytopenias on or after day 30 after infusion (n = 51), a total of 5 had cytopenias present 12 months after infusion and 4 had cytopenias 24 months after infusion (supplemental Table 10). No correlations were observed between baseline TMTV and either grade ≥3 cytokine release syndrome or neurologic events, possibly because of the low incidence of grade ≥3 toxicities. In total, 39 deaths occurred in ZUMA-5, of which 19 were lymphoma-related as assessed by investigators (15 from complications of underlying lymphoma and 4 because of AEs related to study treatment in patients with FL; supplemental Table 11). Among the treated patients, 8 died because of an AE and 5 died owing to second primary malignancy (unrelated to axi-cel). After the data cutoff date of the prior analysis, deaths because of AEs considered related to axi-cel included 1 due to COVID-19 pneumonia in a patient with FL and 1 due to progressive multifocal leukoencephalopathy in a patient with FL.9 Safety results in the subset of patients with FL with ≥3 prior lines of therapy excluding those with suggested alternative diagnosis are reported in supplemental Table 12.
Biomarkers
Among treated patients with iNHL, the median peak CAR T-cell levels were significantly higher in those with ongoing responses at 36 months (53.9 cells per μL) than in those who relapsed (29.6 cells per μL) or nonresponders (22.2 cells per μL; supplemental Figure 7). Most treated patients with FL had detectable B cells by month 12. By month 24, half of patients with ongoing response had low levels of detectable CAR gene–marked cells (supplemental Figure 8). The levels of CAR gene–marked cells were inversely correlated with that of the B cells at each timepoint after infusion.
Among 14 patients with iNHL (13 FL and 1 MZL) with evaluable tumor biopsy samples at progression, all patients had detectable B-cell antigens, CD19, and CD20. Although PFS was similar among patients with POD24 and those without, those with POD24 had higher pretreatment levels of macrophage-associated chemokines, CCL17 (TARC) and CCL22 (MDC), than those without POD24. These analytes have been previously associated with relapse in patients with FL.13
Treated patients with FL who received any prior bendamustine treatment appeared to have lower CAR T-cell expansion by peak and area under the curve, along with a lower proportion of naive (CCR7+CD45RA+) T cells in axi-cel product, vs those without bendamustine exposure (supplemental Table 13). In the PSM analysis, those with bendamustine exposure ≤12 months before leukapheresis demonstrated numerically lower CAR T-cell expansion and number of infused CCR7+CD45RA+ T cells than those with no bendamustine exposure, although small number of patients in the analysis limited comparison. Additionally, interferon gamma in coculture was significantly higher in those with no prior exposure to bendamustine (supplemental Table 5).
Discussion
To our knowledge, this analysis of ZUMA-5 represents the longest follow-up of a registrational trial of an anti-CD19 CAR T-cell therapy for patients with iNHL. With >3 years of follow-up for patients with FL, axi-cel demonstrated durable remissions in a substantial proportion of patients, with more than half of patients in ongoing response as of data cutoff. Durability of response appeared to be associated with best response, as demonstrated in aggressive lymphomas.14 Additionally, patients with MZL appeared to have improved PFS (not yet reached) with longer follow-up than in the prior analysis.9 These findings represent a considerable advancement in clinical outcomes for R/R indolent lymphomas, for which standard noncellular therapies provide limited durable remission off therapy.
After a median follow-up of 41.7 months in ZUMA-5, the median PFS in patients with FL was >3 years (40.2 months), comparing favorably with the bispecific antibody mosunetuzumab (median PFS, 17.9 months), recently approved for patients with FL in the third line, although additional follow-up is needed to determine long-term survival for this class of therapy and there are inherent limitations in comparing outcomes across different trials.15 However, corroborating these findings, the retrospective analysis comparing findings in ZUMA-5 with other standard treatment results for FL (SCHOLAR-5) after 2 years of follow-up demonstrated significant benefit in PFS with axi-cel compared with the standardized mortality-weighted control cohort (39.6 vs 12.7 months; hazard ratio, 0.28).16 Median OS was not yet reached in patients with FL in this study, even though most patients received ≥3 prior lines of therapy (exclusive of single-agent anti-CD20 antibody), an indicator of poor prognosis in the modern era.3 Additionally, more than half of the patients had POD24, and the PFS for these patients at high risk appeared largely similar to those without POD24. Similar to axi-cel, tisagenlecleucel appeared to have favorable survival in R/R FL, with PFS not yet reached after 28.9 months of follow-up in the ELARA study, further supporting the durability of CAR T-cell therapy in FL.17,18 Late progression events or deaths related to axi-cel or lymphodepleting therapy were uncommon. Indeed, competing risk analysis of lymphoma-specific PFS suggested that most events occurring after the 24-month timepoint were because of competing risks, and there was an emergence of a plateau beyond 2 years. This is consistent with prior studies in which deaths due to nonrelapse mortality have been observed in up to half of the patients with FL.19,20 However, longer follow-up will be needed to determine the curative potential of axi-cel in FL.
Bendamustine, a conventional treatment for both aggressive and indolent lymphomas, may attenuate T-cell fitness and has been shown to impair CAR T-cell expansion and thus its efficacy.21,22 Patients in ZUMA-5 with FL who had recent exposure to bendamustine, particularly within 6 months of leukapheresis, had worse efficacy outcomes after axi-cel relative to those with no prior exposure, similar to recent findings with brexucabtagene autoleucel in mantle cell lymphoma.21 CAR T-cell expansion was less robust, and CCR7+CD45RA+ T cells in axi-cel product were numerically lower with recent bendamustine exposure, which correlates to reduced efficacy with axi-cel in FL.13 Of note, median PFS for patients with bendamustine exposure >6 months of leukapheresis compared favorably with historical outcomes.16 Taken together, these results suggest that bendamustine-based therapies may be carefully considered in patients who are likely to need CAR T-cell therapy in the near future, especially those at high risk.21,22 Further assessments on larger patient cohorts are needed to determine whether and to what extent the inferior outcomes in this subset of patients are due to the unfavorable disease biology vs prior treatment with bendamustine and to identify an optimal washout period.
Baseline TMTV that was above the median correlated negatively with efficacy outcomes, including DOR and PFS, for patients with FL. These findings corroborate similar outcomes differentiated by baseline TMTV among patients with aggressive lymphomas in the ZUMA-7 randomized controlled trial.23 Similar to this analysis, findings from the ZUMA-7 trial showed that baseline SPD was not predictive of event-free survival, whereas baseline TMTV was, suggesting that TMTV was a more accurate measure of baseline tumor burden and corroborating prior preliminary analyses.23, 24, 25 Although outcomes were numerically inferior in patients in ZUMA-5 with relatively high baseline TMTV, most achieved a CR (71%), and median DOR was ∼2 years. Additional correlative studies on tumor biology are needed to better understand the reasons for inferior outcomes in patients with high tumor burden. These data collectively aim to establish TMTV as a predictor of outcomes with CAR T-cell therapy, identifying patients who may benefit from treatment enhancement strategies in future clinical trials with additional unmet need.
Durable responses emerged in patients with MZL in this long-term follow-up analysis, with enrollment of ∼25% more patients since the prior analysis and more mature follow-up.9 Patients with MZL in ZUMA-5 had PFS and OS not yet reached after 31.8 months of median follow-up. Their PFS compared favorably with those with secondline ibrutinib in the phase 2 PCYC-1121 trial in R/R MZL with similar follow-up (median PFS, 15.7 months), although OS was also not reached in this trial.26 Continued follow-up to determine long-term durability in this population is needed.
No new safety signals were observed among treated patients with either FL or MZL since the prior analysis.9 AEs that emerged in this analysis were those in recently enrolled patients, and as in the primary analysis, events were generally of low grade and reversible.9 Notably, few patients had prolonged high-grade cytopenias. Consistent with the prior analysis, durability of response correlated with early CAR T-cell expansion, and functional CAR T-cell persistence did not appear to be required for such durability in patients with FL.9 Though baseline biomarkers associated with relapse were elevated in patients with POD24, survival with axi-cel was not impacted by it.
In conclusion, long-term results demonstrated the continued durable clinical benefit of axi-cel among patients with indolent lymphomas and a manageable long-term safety profile. A substantial proportion of patients with R/R iNHL remained alive without progression; further analyses of survival are warranted to assess the curative potential of CAR T-cell therapy in this disease. Additionally, a phase 3 randomized trial has launched to assess the benefit of axi-cel compared with standard-of-care therapy for R/R FL (ZUMA-22; NCT05371093).
Conflict-of-interest disclosure: S.S.N. reports a consulting/advisory role for Kite/Gilead, Merck, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, Bristol Myers Squibb, Bluebird Bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, Orna Therapeutics, Takeda, and Synthekin; research funding from Kite, Bristol Myers Squibb, Allogene, Precision Biosciences, Adicet Bio, and Sana Biotechnology; stock options from Longbow Immunotherapy; and patents, royalties, or other intellectual property related to cell therapy. J.C.C. reports a consulting/advisory role for ADC Therapeutics, AdiCet, AstraZeneca, Bristol Myers Squibb, Genentech, Genmab, Kite, and Novartis; speakers’ bureau participation for BeiGene and Lilly; and research funding from ADC Therapeutics, AstraZeneca, Janssen, and Merck. A.R.S. reports grants, contracts, or research funding from Juno and Kite. N.E. reports a consulting/advisory role and honoraria with ADC Therapeutics, Lilly, Merck, and Novartis; speakers’ bureau participation for Incyte and BeiGene; and research funding from BeiGene. M.U. reports consulting/advisory role for Gilead Sciences and Stemline. E.B. reports honoraria from Kite. P.N.M. reports a consulting/advisory role for Incyte and speakers' bureau participation for Incyte and Kite. C.C. reports research funding from Bristol Myers Squibb, Genentech, Gilead Sciences, and Verastem. D.G.M. reports consulting or honoraria fees from A2 Biotherapeutics, Amgen, Bristol Myers Squibb, Celgene, Genentech, Gilead Sciences, Incyte, Janssen, Juno Therapeutics, Kite, Legend Biotech, MorphoSys, Mustang Bio, Navan Technologies, Novartis, Pharmacyclics, and Umoja; participation on a data safety monitory board or advisory board for Bioline Rx and Celgene; research funding paid directly to institution from Celgene, Juno, and Kite; rights to royalties from Fred Hutch for patents licensed to Juno; and stock options from A2 Biotherapeutics and Navan Technologies. S.d.V. reports participation on a data safety advisory board for BeiGene. R.R. reports honoraria from Gilead and Bristol Myers Squibb; consulting/advisory role for Atara Biotherapeutics, Gilead Sciences, Takeda, Incyte, Instil Bio, Regeneron, TScan, Synthekine, Orca, MidaTech, Capstan and Jasper; research funding (to the institution) from Atara Biotherapeutics, Incyte, Sanofi, Immatics, TCR2 Therapeutics, Takeda, Gilead Sciences, CareDx, TScan, Synthekine, Bristol Myers Squibb, Johnson & Johnson and Precision Biosciences; expert testimony to Bayer; and travel support from Gilead. L.A.L. reports a consulting/advisory role for AbbVie, AstraZeneca, BeiGene, Eli Lilly, Epizyme, Janssen/Johnson & Johnson, Kite, Merck, Pharmacyclics, Seagen, and TG Therapeutics; speakers’ bureau participation for and travel support from AbbVie, AstraZeneca, BeiGene, Celgene/Bristol Myers Squibb, Eli Lilly, Epizyme, Kite, Janssen/Pcyc, Pharmacyclics, Seagen, and TG Therapeutics. O.O.O. reports honoraria from Gilead and Pfizer; a consultancy/advisory role for AbbVie, ADC, Curio Science, Epizyme, Gilead, Janssen, Kite, Pfizer, Nektar, Novartis, Syncopation, and TGR BioSciences; other research funding to institution from Allogene, Daichi Sankyo, Kite, and Pfizer. I.Y.-A. reports honoraria from Bristol Myers Squibb, Janssen, Kite, a Gilead Company, and Novartis; consulting/advisory role for Kite and Novartis; and travel support from Kite. J.R. reports research funding from Biograph 55; patents, royalties, or intellectual property from University of Miami Miller School of Medicine; and other relationships with Synergys. R. Korn reports employment with Imaging Endpoints; leadership at Imaging Endpoints; and stock or other ownership in Globavir, Renibus, and Verve. W.P. and J.W. report employment with Kite. C.L. reports employment with, stock or other ownership in, and travel support from Kite. R.S. reports employment with and stock or other ownership in Kite; and patents, royalties and other intellectual property from Atara and Kite. S.P. reports employment with and travel support from Kite; and patents, royalties, or other intellectual property from University of California Los Angeles. A.S.J. reports employment with Kite; and stock or other ownership in Amgen, Gilead Sciences, Kura, and Turning Point. H.M. reports employment with Kite; and stock or ownership in Gilead Sciences. S.B. reports employment with, stock or other ownership in, and travel support from Kite. C.A.J. reports honoraria from Kite, Novartis, Bristol Myers Squibb/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, BlueBird Bio, and Daiichi-Sankyo; consulting/advisory role for Kite, Novartis, Bristol Myers Squibb/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme BlueBird Bio, and Daiichi-Sankyo; and research funding from Kite and Pfizer. R. Khanal declares no competing financial interests.
Acknowledgments
The authors thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and health care staff at each site; Wangshu Zhang of Kite, for support of statistical analyses; and Danielle Fanslow of Nexus Global Group Science for medical writing assistance.
Authorship
Contribution: S.S.N. and C.A.J. designed the trial; S.S.N., J.C.C., A.R.S., N.E., M.U., E.B., P.N.M., C.C., D.G.M., S.d.V., R.R., L.A.L., O.O.O., I.Y.-A., R. Khanal, J.R., and R. Korn provided study materials, collected, and assembled data; S.S.N., W.P., C.L., J.W., R.S., S.P., A.S.J., H.M., S.B., and C.A.J. analyzed and interpreted the data; and all authors contributed to the assessment of data and writing of the manuscript, and all approved the final version.
Footnotes
∗S.S.N. and J.C.C. contributed equally to this work.
Clinical trial data access is available to external medical experts and scientific researchers in the interest of advancing public health on request from Kite (medinfo@kitepharma.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–327. doi: 10.1002/ajh.25696. [DOI] [PubMed] [Google Scholar]
- 2.Leslie LA. Novel therapies for follicular lymphoma and other indolent non-Hodgkin lymphomas. Curr Treat Options Oncol. 2021;22(12):111. doi: 10.1007/s11864-021-00909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10(7):74. doi: 10.1038/s41408-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casulo C, Larson MC, Lunde JJ, et al. Treatment patterns and outcomes of patients with relapsed or refractory follicular lymphoma receiving three or more lines of systemic therapy (LEO CReWE): a multicentre cohort study. Lancet Haematol. 2022;9(4):e289–e300. doi: 10.1016/S2352-3026(22)00033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.YESCARTA (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2022. [Google Scholar]
- 7.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.YESCARTA (Axicabtagene Ciloleucel). Summary of product characteristics. Kite Pharma EU B.V.; 2022. [Google Scholar]
- 9.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
- 10.Weibull CE, Wasterlid T, Wahlin BE, et al. Survival by first-line treatment type and timing of progression among follicular lymphoma patients: a national population-based study in Sweden. Hemasphere. 2023;7(3):e838. doi: 10.1097/HS9.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meignan M, Cottereau AS, Versari A, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34(30):3618–3626. doi: 10.1200/JCO.2016.66.9440. [DOI] [PubMed] [Google Scholar]
- 13.Plaks V, Chou J, Goyal L, et al. Axicabtagene ciloleucel (axi-cel) product attributes and immune biomarkers associated with clinical outcomes in patients with relapsed/refractory indolent non-Hodgkin lymphoma in ZUMA-5. Cancer Res. 2021;81(13 suppl) [Google Scholar]
- 14.Neelapu SS, Jacobson CA, Ghobadi A, et al. 5-Year follow-up supports curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1) Blood. 2023;141(19):2307–2315. doi: 10.1182/blood.2022018893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–1065. doi: 10.1016/S1470-2045(22)00335-7. [DOI] [PubMed] [Google Scholar]
- 16.Palomba ML, Ghione P, Patel AR, et al. A 24-month updated analysis of the comparative effectiveness of ZUMA-5 (axi-cel) vs. SCHOLAR-5 external control in relapsed/refractory follicular lymphoma. Expert Rev Anticancer Ther. 2023;23(2):199–206. doi: 10.1080/14737140.2023.2171994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 18.Dreyling M, Dickinson M, Martinez Lopez J, et al. Long-term clinical outcomes and correlative efficacy analyses in patients (pts) with relapsed/refractory follicular lymphoma (r/r FL) treated with tisagenlecleucel in the ELARA trial [abstract] Blood. 2022;140(suppl 1):1459–1463. [Google Scholar]
- 19.Bachy E, Seymour JF, Feugier P, et al. Sustained progression-free survival benefit of rituximab maintenance in patients with follicular lymphoma: long-term results of the PRIMA study. J Clin Oncol. 2019;37(31):2815–2824. doi: 10.1200/JCO.19.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozas P, Nadeu F, Rivas-Delgado A, et al. Patterns of change in treatment, response, and outcome in patients with follicular lymphoma over the last four decades: a single-center experience. Blood Cancer J. 2020;10(3):31. doi: 10.1038/s41408-020-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555–567. doi: 10.1200/JCO.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacoboni G, Martin Lopez AA, Jalowiec KA, et al. Recent bendamustine treatment before apheresis has a negative impact on outcomes in patients with large B-cell lymphoma receiving chimeric antigen receptor T-cell therapy [abstract] Blood. 2022;140(suppl 1):1592–1594. doi: 10.1200/JCO.23.01097. [DOI] [PubMed] [Google Scholar]
- 23.Locke FL, Oluwole OO, Kuruvilla J, et al. Association of metabolic tumor volume (MTV) and clinical outcomes in second-line (2L) relapsed/refractory (R/R) large B-cell lymphoma (LBCL) following axicabtagene ciloleucel (axi-cel) versus standard-of-care (SOC) therapy in ZUMA-7 [abstract] Blood. 2022;140(suppl 1):638–640. [Google Scholar]
- 24.Hong R, Tan Su Yin E, Wang L, et al. Tumor burden measured by 18F-FDG PET/CT in predicting efficacy and adverse effects of chimeric antigen receptor T-cell therapy in non-Hodgkin lymphoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.713577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268–3276. doi: 10.1182/bloodadvances.2020001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noy A, de Vos S, Coleman M, et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv. 2020;4(22):5773–5784. doi: 10.1182/bloodadvances.2020003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.