Cytotoxic lymphocytes (CL) play a central role in immune responses against tumors and cells infected with viruses and intracellular bacteria. They also are of pivotal importance in immune regulation, transplant immunology, and autoimmunity. These functions have justified continued interest in the mechanisms of action of CL. Recent studies of CL have defined two major mechanisms of cytotoxicity. The first involves exocytosis of the contents of cytoplasmic granules from the CL toward the target cell (TC). The second involves engagement of a tumor necrosis factor receptor (TNFR)-like molecule on the TC (e.g., Fas [CD95] or TNFR) by its CL ligand (FasL or TNF, respectively). Evidence is emerging that the two different forms of lymphocyte-mediated cytotoxicity are quite distinct in vivo, although they both involve induction of an endogenous pathway of apoptosis in the targeted cell and they share many features with all other forms of physiological cell death. The focus of this review will be to comment on the relative role of each of these mechanisms of lymphocyte cytotoxicity in the pathophysiology of virus infection.
LYMPHOCYTES THAT KILL
Cellular immune responses require the direct participation of effector cells. The functional activities of both helper (predominantly CD4+) and cytotoxic (predominantly CD8+) T lymphocytes (CTL) are initiated by clonotypic T-cell receptors (TCR), associated with the invariant CD3 signaling complex, to recognize antigenic peptides bound to major histocompatibility complex (MHC) molecules on the TC (5). Activation of CTL results in direct killing of the TC. In addition, lymphocytes with innate cytolytic capacity, including natural killer (NK) cells, subsets of γδ T cells (10), and possibly also CD1-specific NK1 αβ T cells, may play a role in responses to intracellular pathogens. NK cells are CD3− CD56+ large granular lymphocytes and are important in the early phases of immune responses against certain viruses, parasites, and microbial pathogens (6, 84). The ability of NK cells to produce gamma interferon (IFN-γ) rapidly after infection, before the clonal expansion of antigen-specific T cells, plays a crucial role in the innate immune response (81). NK cells do not express somatically rearranged receptors and neither rearrange nor express surface TCR. Activation of NK cells in primary immune responses involves the production of and response to many cytokines, although the precise nature of their activation is unknown. Although the specific receptors involved in NK cell recognition and activation are not completely defined, it is becoming increasingly clear that both triggering and inhibitory molecules of two distinct superfamilies (type I transmembrane and type II transmembrane C-type lectin) dictate their lytic specificity (18, 53, 115). In general, it is likely that NK cells recognize and eliminate tumor or virus-infected TC because critical self class I molecules are absent from or altered on the TC surface (i.e., the absence of a negative signal) and NK cells refrain from killing when the TC expresses self class I molecules. The best-characterized membrane receptor responsible for NK cell activation is the low-affinity Fc receptor for immunoglobulin G, CD16 (51). Ligation of CD16 by anti-CD16 monoclonal antibody, immune complexes, or antibody-coated TC induces antibody-dependent cellular cytotoxicity and cytokine secretion (19, 52). Therefore, antibodies can confer exquisite antigen specificity to NK cells bearing CD16 receptors.
It has long been understood that several mechanisms of cytolysis are used by CL. Effector CL use distinct mechanisms that can be dissected in vitro on the basis of TC sensitivity, the presence or absence of extracellular Ca2+, the need for effector cell de novo protein synthesis, and requisite effector cell granule exocytosis (93). Although it is clear that the recognition of TC by CTL and NK cells is very different, there is abundant evidence to indicate that the lethal hit of both cell types involves components of their characteristic electron-dense cytoplasmic granules (35). The general properties of granule exocytosis- and receptor-mediated mechanisms of lymphocyte-mediated cell death are summarized in Table 1 and outlined below.
TABLE 1.
Dual mechanisms of lymphocyte-mediated cytotoxicity
| Mechanism | Nature | Effector cell(s) | Function | Ca2+ requirement | Kinetics | Morphology | TC protease involvement | Protein synthesis requirement | Nuclear requirement |
|---|---|---|---|---|---|---|---|---|---|
| Granule exocytosis | Exocytosis of granule proteins | Mainly CD8+ NK | Clearance of | Yes | Rapid (<4 h) | Nuclear DNA fragmentation; chromatin condensation; extensive membrane blebbing; vacuolation of cytoplasm | Yes | No | No |
| some virus-infected cells; | |||||||||
| intracellular bacteria-infected cells; | |||||||||
| malignant and/or transformed cells; | |||||||||
| transplanted cells | |||||||||
| FasL/TNF or Fas/TNFR | Signaling via death receptor | Mainly CD4+ Th1, but also CD4+ Th2, CD8+, and NK | Clonal deletion of autoreactive T | No | Rapid (FasL < 4 h; TNF > 4 h) | Nuclear DNA fragmentation; chromatin condensation; extensive membrane blebbing; vacuolation of cytoplasm | Yes | No | No |
| T cells in periphery | |||||||||
| Elimination of activated T cells | |||||||||
| responding to foreign antigen |
GRANULE-MEDIATED CELL DEATH
CL cytotoxic granules are vectorially secreted into the intercellular space formed during conjugation of the CL and TC (34), and lysis is often associated with the formation of membrane lesions on the TC (69). The granules of CL contain a number of proteins, including a pore-forming protein termed perforin (109), and a family of serine proteases coined granzymes (40). Perforin causes osmotic damage due to its binding of phosphorylcholine headgroups, polymerization, and subsequent pore formation in the lipid bilayer of the TC (57, 86, 109). Perforin is found essentially in CTL (including γδ T cells) and NK cell granules (66, 92), although macrophage precursors also appear to express perforin and may be cytolytic under the appropriate conditions (8, 56). CL-mediated TC death generally involves changes such as chromatin condensation, extensive membrane blebbing, and ultimately nuclear DNA fragmentation (apoptosis) (23, 79). These events clearly occur sometime before appreciable perforin-mediated cell lysis, and purified perforin alone is incapable of causing DNA fragmentation. Indeed, it is likely that a supplementary role is played by granzymes in TC killing, and this postulate is supported by much in vitro and in vivo experimental evidence (36, 85, 87). Granzymes are the major protein components of CTL and NK cell granules and they synergize with perforin to trigger an internal disintegration pathway in the TC (85, 94). Most notably, the killer cell serine protease granzyme B shares Asp-ase specificity with an increasingly large family of cell death cysteine proteases (termed caspases), including the interleukin-1β-converting enzyme (ICE)-like family members. Granzyme B appears to trigger an endogenous cell death cascade by activating key TC caspases (20, 28, 96, 99). Collectively, these data indicate that granzyme B can greatly amplify the activation of key signaling components shared by other forms of cell death (including the Fas/TNFR pathways) and directly contribute to apoptotic nuclear morphology. Key intracellular substrates for other granzyme family members are yet to be defined, although some data support a role for CL granzyme A and another granule tryptase serine protease in CL-mediated cytotoxicity (85, 87).
LYMPHOCYTE RECEPTOR-MEDIATED CELL DEATH
Previous observations of TC death in the absence of Ca2+, granule exocytosis, or perforin suggested the existence of alternative pathways of CL-mediated cytotoxicity. Rouvier et al. demonstrated that Ca2+-independent lysis was accounted for by CTL cross-linking with the TC Fas receptor (78). Cloning and characterization of Fas indicated that it belonged to the TNFR superfamily of molecules (39). Mutational analysis of the cytoplasmic domains of these receptors has indicated a region in both Fas and TNFR1 that is conserved and necessary for transduction of the apoptotic signal (103). Signaling via Fas or TNFR leads to apoptotic cell death, with characteristic cytoplasmic and nuclear condensation and DNA fragmentation (39, 103, 108). This death process is rapid (within several hours), occurs in the absence of RNA or protein synthesis, and can also be triggered in TC by monoclonal antibodies to Fas (108). Cytoplasmic caspases, including those with adaptor function (i.e., association with Fas via adaptor Fas-associated death domains [FADD]) and Asp-ase activity (e.g., FADD-like ICE [FLICE] proteases), are involved in Fas and TNFR cell death pathways, and many of the signaling molecules in these apoptotic pathways have been characterized (60, 65, 117). Triggering of these pathways requires cross-linking of Fas or TNFR, and similar to TNF, the soluble form of FasL has a trimeric structure in solution. In addition, the FasL is a CL surface receptor of the TNF family (100). FasL expression appears constitutive in NK cells (4) and can be rapidly induced in T cells by TCR engagement (3). Among the classical CD4+ T helper (h) subpopulations, Th1 cells can express FasL and lyse in a Fas-based manner more readily than Th2 cells (41). By contrast, CD8+ T cells may predominantly display TNF-mediated cytotoxicity. Fas/FasL (primarily CD4+ T) and TNFR/TNF (primarily CD8+ T) interactions are involved in both the clonal deletion of autoreactive T cells in peripheral lymphoid organs and the elimination of activated T cells following their response to foreign antigens (21, 42, 119). In particular, in viral infection models, a general role for FasL/TNF in T-cell autoregulation remains unclear. For example, influenza virus hemagglutinin transgenic mice have been used to demonstrate that both FasL- and TNF-mediated cell death can contribute to the deletion of activated peripheral T cells (101). This is one of several studies that indicate that the major role of lymphocyte receptor-mediated cell death is immunoregulation of T cells. By contrast, down regulation of the CD8+ T cell response following acute lymphocytic choriomeningitis virus (LCMV) infection does not appear to be dependent on Fas/FasL interactions, although the role of TNF alpha (TNF-α) was not examined in this study (59).
MODELS OF CL FUNCTION
The expression of perforin and granzymes by CL infiltrating areas of inflammation has been recognized as a diagnostic tool (31). However, the major benefit of creating perforin (43) and granzyme (24, 36) gene knockout mice and achieving a better understanding of the mouse spontaneous mutants lpr (lymphoproliferation) and gld (generalized lymphoproliferative disease) have been the demonstration that lymphocyte cytotoxicity actually plays an indispensable role in a number of immune responses. We now appreciate that lpr is a loss-of-function mutation of the Fas gene and that gld represents a point mutation in the FasL gene, abolishing the ability of FasL to bind Fas (17). lpr/lpr and gld/gld mice develop lymphadenopathy and splenomegaly and produce large quantities of some autoantibodies (17). Importantly, functional deletion of the perforin or granzyme genes does not appear to affect the fertility, T/NK cell hematopoiesis, and cytotoxic granule formation in these mice. Experiments in perforin gene knockout mice (P0) indicate that perforin is critical for the cytotoxicity of lymphokine-activated killer and NK effector cells (43). The use of these mouse models to study various disease processes (outlined below) has provided a wealth of information about the biological role of these two basic forms of lymphocyte-mediated cell death.
ROLE OF CTL/NK CYTOTOXIC MECHANISMS UPON VIRUS INFECTION
The essential components of a host’s immune response to virus depends on the type and life cycle of the virus and may begin with virus-induced suicide of infected cells and progress to direct CL-mediated cytotoxicity and the secretion of soluble factors and antibodies. NK cells are involved in limiting viral replication during the initial stage of an infection (111), whereas CTL undergo clonal selection, expansion, and differentiation to competent effector cells that then are able to recognize and eliminate virus-infected cells. A discussion of the relative role of granule exocytosis and FasL/TNF mechanisms of lysis in the clearance and pathology of several viruses follows.
(i) Direct CTL/NK-mediated lysis. (a) Noncytopathic viruses.
It is currently not clear whether perforin alone is sufficient for the induction of TC death in vivo, but clearly of all the CL granule proteins characterized thus far, gene knockout/mutant models indicate that perforin is a critical component of host immune defense against some viruses. LCMV-specific CTL are responsible for both the eradication of the virus and the onset of pathology associated with the disease, depending on the timing, route of entry, and strain of the virus (12). Experiments performed on the antiviral immune responses of P0 or gld (FasL mutant) mice indicated that clearance of an acute infection with noncytopathic LCMV was mediated mainly by perforin-dependent cytotoxicity without measurable participation of the Fas-dependent pathway (43–45, 110). CD8+ T cells are activated by LCMV in P0 mice but fail to clear the virus effectively. The data suggest that control of an LCMV infection depends critically on perforin-mediated lysis of infected cells. Thus contact-dependent cytotoxicity appears to reduce viral proliferation by lysing infected cells before the assembly of virus particles is complete. Granzyme A-deficient mice readily recover from LCMV infection (24); however, the ability of other granzyme-deficient mice or those crossed to P0 mice to clear LCMV has yet to be reported. LCMV-specific CTL also exert their protective effects by secreting cytokines (e.g., IFN-γ) that inhibit viral replication, and the cytokines and products of cell lysis can also cause inflammation of tissue (22). Antiviral cytokines may be able to reduce, but not completely inhibit, intracellular virus proliferation, so elimination of infected cells may be the only way to clear a noncytopathic virus infection completely. Indeed, in the absence of perforin, persistent infection with LCMV often leads to the overproduction of cachectic cytokines, such as TNF-α and IFN-γ, and subsequent death (43).
(b) Cytopathic viruses. (1) CMV.
In humans, CD8+ CTL provide immune protection against both cytopathic cytomegalovirus (CMV) infection and reactivation of quiescent CMV infection (76). In recipients of allogeneic bone marrow transplants, protection against CMV pneumonia correlated with the appearance of CD8+ CMV-specific CTL (76) and adoptive transfer of CD8 CMV-specific CTL from an immunocompetent bone marrow donor to an immunosuppressed recipient selectively reconstituted immunity against CMV (75). Longevity of the anti-CMV response after adoptive transfer was dependent on the CD4+ Th response (30a). NK cells and CD8+ CTL also have a protective role against cytopathic murine cytomegalovirus (MCMV) infection (72). Spleen NK cells control MCMV infection in a perforin-dependent manner (104); however, in the liver, production of IFN-γ by NK cells was the predominant mechanism that regulated MCMV synthesis. The finding that the optimal antiviral response varies from organ to organ is interesting and may reflect a balance between suppressing infection while inflicting minimal tissue damage (see also hepatitis virus [HV] below). A role for perforin and granzymes in CTL immunity against MCMV is likely but has not yet been reported in gene knockout mice.
(2) Retroviruses.
CD8+ T cells have previously been shown to be important in preventing lymphoproliferation and immunodeficiency following infection of murine AIDS (MAIDS)-resistant mice with murine leukemia viruses. The causative agent of MAIDS is the defective murine leukemia virus BM5d. Use of MAIDS-resistant B10.A mice that were also β2-microglobulin or perforin deficient demonstrated that expression of BM5d was enhanced in the spleens of the knockouts in comparison with wild-type mice (102). These and other data indicated that perforin-dependent functions of CD8+ T cells contributed to MAIDS resistance but that other, non-CD8-dependent, mechanisms were of equal or greater importance. Interestingly, after infection with BM5d, MAIDS appeared to be inducible in B6.lpr (Fas mutant) mice; however, the B6 strain is normally disease susceptible (37). Although a human immunodeficiency virus (HIV)-specific cellular immune response may provide immune protection against disease progression (68), a role for perforin in CD8+ T cell or NK activity against virus-infected cells has not yet been demonstrated in vivo.
(3) Poxviruses.
Previous studies have established that NK/CD8+ T cytotoxicity was not essential to resolve cytopathic poxvirus infections and that secretion of IFN-γ by CD4+ and CD8+ T cells was crucial in immunity against poxviruses (71, 98). For example, protection against vaccinia virus was not affected by the lack of either perforin- or FasL-dependent cytotoxicity (54) and the effect of IFN-γ and TNF-α on vaccinia virus replication was independent of NK cell cytotoxicity mediated by perforin (43, 45).
(4) HV.
Hepatitis C virus (HCV) and hepatitis B virus (HBV) are cytopathic hepatotropic viruses and are the major causative agents of chronic liver disease in humans. The control of HBV infection is thought to be mediated by class I-restricted CTL. Patients that successfully clear the virus in the acute phase mount a polyclonal CTL response to several HBV-encoded antigens. In mouse models of hepatitis infection, P0 mice have been infected with a neurotropic strain of mouse HV (JHMV) to analyze the role of perforin-mediated cytotoxicity in acute lethal and subacute central nervous system (CNS) infections (58). Virus was cleared from the P0 mice as in the controls; however, the rate of clearance was delayed in the P0 mice, indicating that perforin-mediated cytolysis was involved in viral clearance. These data confirmed the importance of cell-mediated cytotoxicity but also suggested that additional components of the immune response contribute to the clearance of JHMV from the CNS. As outlined below, transgenic mouse models of hepatitis infection in the liver indicate that direct lysis mediated by CTL granule exocytosis is a less-favorable immune response compared to cytokine release and that associated bystander FasL/TNF activity may greatly contribute to the immunopathology of the infection.
(5) Other cytopathic viruses.
Recent experiments with P0 mice have shown that cytotoxicity is not crucial for the resolution of infection with several cytopathic viruses, including vesicular stomatitis virus, Semliki Forest virus, and influenza virus (44, 46). These findings may reflect the general pattern that infections with cytopathic viruses are mainly controlled by soluble mediators such as antibodies and interferons (54, 64, 121).
(ii) CTL-mediated immunopathologies. (a) HV—a good example of bystander lysis.
Persistently infected patients with chronic hepatitis do not mount a polyclonal CTL response. Nonetheless, CTL play a crucial role in liver cell injury by HCV or HBV infection, but the exact mechanisms responsible for liver cell injury remain to be clarified. Kondo et al. (50) have demonstrated that FasL plays an essential role in the development of hepatitis by using a transgenic model in which hepatitis is induced by a single intravenous injection of cloned MHC-restricted CD8+ CTL targeted to the HBV envelope protein expressed by liver cells. Both soluble Fas and TNFR1 appeared to have a protective effect against hepatocyte apoptosis in this model. It is not clear how representative this model is for HV infection, but it is likely that granule exocytosis is the viral antigen-specific killing mechanism used by HV-specific CTL and that the death of Fas-expressing hepatocytes (immunopathology) may significantly be a result of bystander activity. Evidently, HCV-specific CTL clones kill non-antigen-bearing bystander cells primarily by FasL and TNF-α, and the killing of antigen-presenting sensitive cells is mediated predominantly by perforin at low effector/target cell ratios. Certainly, in chronic hepatitis C, Fas expression is up regulated in the hepatocytes, especially near liver-infiltrating lymphocytes, and FasL is expressed on these lymphocytes (2). An early efficient immune response involving CTL may result in the lysis of most HV-infected hepatocytes and the clearance of virus, while causing acute but transient hepatitis. By contrast, a weak CTL response may lead to incomplete lysis of infected hepatocytes and hence to continuing replication of HV in those cells. Chisari has proposed that CTL are unable to recognize HV-positive parenchymal cells outside of the liver (because of micovasculature barriers) and hence virus may survive a vigorous CTL response but contribute to the maintenance of memory CTL and a reservoir of antigen in chronic hepatitis (16). Persistent infection may lead to chronic hepatitis mediated by poorly focused CTL activity or, alternatively, as infected cells die the continuous degeneration and regeneration of liver tissue results in secondary inflammation, culminating in cirrhosis with an elevated risk of carcinoma. To limit CL-mediated destruction of infected hepatocytes, CTL-secreted IFN-γ and TNF-α evidently play an important role in suppressing HBV gene expression (32). These antiviral cytokines may limit virus replication and viral antigen presentation in hepatocytes without killing them, thereby minimizing potential CTL damage by direct lysis. However, as with noncytopathic LCMV, cytokines by themselves may be ineffective in completely clearing the virus.
(b) Other CTL-related viral immunopathologies.
Several of the aforementioned viral infections have immunopathologies that have been associated with various arms of the CTL/NK effector response. For example, a role for CD4+ T-cell Fas-mediated cytotoxicity in the onset of LCMV-induced immunopathology has been demonstrated in β2-microglobulin-deficient mice (118). By contrast, during both acute and subacute infections, the overall mortality of the P0 mice infected in the CNS with neurotropic JMHV (58) was not different from that of the controls and the absence of perforin-mediated cytolysis did not prevent encephalomyelitis or extensive demyelination. At this stage, examples of CL-FasL/TNF-related immunopathology have been more often reported, but interestingly, granzyme A-deficient mice exposed to influenza virus have an increased lung pathology and associated morbidity when compared with wild-type mice (90a). This data highlights that in some types of viral infections and local sites certain CL granule components may also have detrimental effects on the host.
A slightly different example of how CL may contribute to immunopathology occurs when viral infection alters the endogenous programmed cell death pathway of the infected cell. Upon infection of cells many viruses trigger the expression of death receptors (e.g., Fas). For example, both T cells and hematopoietic progenitor cells from mice infected with CMV undergo apoptosis via up regulation of surface Fas antigen (62, 116). This induced form of apoptosis is postulated to act as an antiviral immune mechanism by either directly inducing suicide or by flagging the infected cell to the cellular immune system before viral replication has occurred. However, in some cases (depending on the infected cells or tissue involved), this form of protection may be detrimental and CTL may contribute to the ultimate immunosuppression by lysing infected or uninfected cells (122). The depletion of CD4+ T cells in AIDS is correlated with high turnover of HIV-1, and the HIV-1 proteins Tat and gp120 accelerate Fas-mediated, activation-induced T-cell apoptosis (112). However, whether these molecular mechanisms are significant in AIDS pathology in HIV infection is largely unknown. Previous studies have demonstrated increased levels of Fas on T cells in individuals with HIV infection (91), leading many to believe that HIV destroys immune cells via the Fas-FasL system. Other researchers have noted that, compared to similar cells from healthy people, HIV-infected individuals have a monocyte FasL deficiency (90). It is possible therefore that CTL (bystander)-FasL and/or monocyte-FasL normally triggers the death of Fas-bearing cells. Thus, by down regulating FasL, HIV provides itself additional time to replicate. Of note, recent new evidence suggests that simian immunodeficiency virus Nef induction of FasL in the infected cell population may protect these cells from CTL attack by killing virus-specific CTL via their surface Fas (114). It is difficult to comprehend that HIV might rapidly up regulate FasL on infected cells in vivo given the early up regulation of Fas, and certainly infection of mice with recombinant vaccinia virus expressing FasL does not lead to death of the responding T cells (25). Determining how HIV regulates FasL on infected and immune cells in concert with the up regulated expression of Fas in infected T cells may be an important key to understanding AIDS pathology.
Other contributions of the granule exocytosis mechanism to virus clearance?
Using mice deficient for granzyme A, it has been shown that granzyme A plays a crucial role in recovery from the mouse poxvirus, ectromelia, by a mechanism(s) other than cytolytic activity (63). Granzyme A-deficient mice have an increased virus titer and increased mortality and morbidity. The mechanism(s) remains undefined, but highlights the possibility that in viral infection, granzymes may additionally act independently of perforin, a finding also supported by indications that adenovirus can substitute for perforin in enabling the entry of granzyme B into mammalian cells (29). Other evidence also supports additional functions for granzymes (13, 63, 97). These other effects include the triggering of inflammatory cytokines, the stimulation of monocyte phagocytic activity (recognizing infected and apoptotic cells), or extracellular processing of proteins for more effective antigen presentation. All of these actions in the context of antiviral function need greater attention.
VIRAL INHIBITORS OF LYMPHOCYTE CYTOTOXICITY
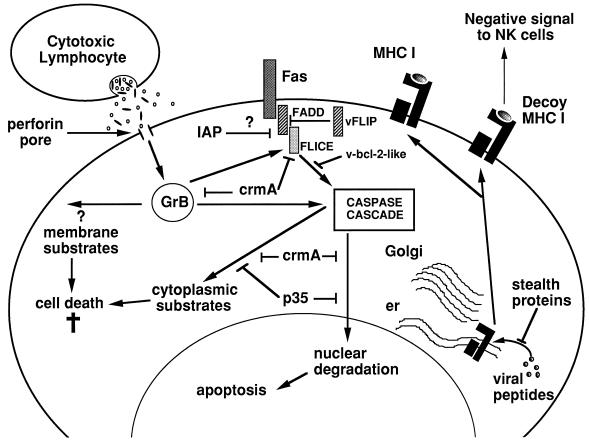
While T-cell-mediated cytotoxicity is an important means of defense against viral pathogens, most viruses possess mechanisms to disrupt immune recognition and subsequent CL-mediated lysis or the apoptosis of host cells, thereby allowing them to maintain a cellular environment but avoid immune clearance (Fig. 1). There are many examples in which viruses have been shown to inhibit cytotoxicity by interfering with the capacity of T lymphocytes to specifically recognize infected cells by interfering with MHC class I peptide expression at a number of distinct levels (e.g., blocking TAP-peptide translocation, exporting MHC class I to the cytosol, retaining MHC class I in the endoplasmic reticulum, etc.) (1, 30, 48, 61, 113, 120). In addition, more recent data indicate that viruses may even thwart NK cells by using their own decoy class I molecules (26, 74). Apoptosis is used as a host cellular defense against infection by viruses. To counter this protective mechanism, some viruses express proteins that inhibit the apoptotic process in the host cell. In some instances, viruses have hijacked TNFR sequences that are used to express receptors that can be secreted to bind extracellular TNF (autocrine or paracrine) or to inhibit TNF-mediated apoptosis within the host cell membrane (83). In addition, with the elucidation of caspase-dependent cell death pathways it has become apparent that many viruses produce proteins that interfere with a common endogenous cell death cascade (7, 49, 105). Examples of these caspase inhibitors are the poxvirus-encoded serpins CrmA and baculovirus p35, which have been demonstrated to inhibit Fas- and TNFR-mediated apoptosis. In addition, more recent identification of viral inhibitors of apoptosis proteins (IAP) that are related to RING finger TNFR2-associated proteins indicates that some of these viral IAP can indeed inhibit both Fas- and TNFR-mediated apoptosis (33, 77). Other viral proteins that can act at points upstream in the Fas/TNFR and related TNFR-like death pathways are the FLICE inhibitory proteins (FLIP) (9, 38, 106). Thus far these viral FLIP have been characterized in human molluscipox virus (MC159; 160) and in several gammaherpesviruses, including herpesvirus saimiri, equine herpesvirus 2 (E8), bovine herpesvirus 4 (E1.1), and Kaposi’s sarcoma associated-human herpesvirus 8 (K13). These viral FLIP contain two death-effector domains which interact with the adaptor protein FADD to inhibit recruitment of FLICE to the Fas receptor upon activation (106). Protection of virus-infected cells against death receptor-induced apoptosis may lead to a higher virus production and contribute to the persistence and oncogenicity of FLIP-containing viruses. An alternative strategy is for viruses to use Bcl-2-like molecules to inhibit various forms of apoptosis, including Fas- and TNF-mediated death. Epstein-Barr virus BHRF1 (47), adenovirus E1B 19-kilodalton protein (27), African swine fever virus A179L (73), herpesvirus saimiri ORF16, and other gammaherpesvirus proteins (15, 67) share homology with Bc1-2 in the BH1 domain and often heterodimerize with other Bc1-2 family members. Another alternative strategy used by viral proteins is their ability to affect gene transcription by direct interaction with transcription factors. Direct interaction of viral proteins with p53 (70, 82) can subsequently cause effects on apoptosis at two levels: (i) on their direct function in apoptosis and (ii) on their ability to transcriptionally activate key molecules in the effector arm of apoptotic pathways (55). An interesting recent finding was the selective inhibition of granzyme B mRNA transcription by the negative-stranded RNA virus parainfluenza virus type 3 (88). Viral proteins can also affect key apoptotic proteins posttranslationally, such as the effects of the human papillomavirus E6/E6-AP complex on p53 ubiquitination and degradation (82) or that of herpes simplex virus type 2 infection on plasma membrane FasL expression (89).
FIG. 1.
Viral inhibition of endogenous and CL-induced apoptotic pathways. Viruses can subvert the intrinsic apoptotic pathway by interfering with virtually every aspect of the cascade so far defined. Many viral strategies involve protease inhibitors (CrmA, p35, IAP, etc.) that can block a spectrum of cysteine protease caspases or inhibit signal transduction through Fas, FADD, or FLICE. Viral Bc1-2-like molecules (v-bc1-2) can also block activation of the caspase cascade by interfering with normal Bc1-2-like molecules that control apoptosis at the outer mitochondrial membrane. In addition, viruses evade immune detection by a variety of means, including inhibiting the loading of viral peptides onto MHC by TAP transporters, inducing degradation of MHC (by stealth proteins), or producing their own decoy MHC (HLA) molecules that bind NK cell inhibitory receptors and inhibit NK cytotoxicity. As yet, a specific viral inhibitor of perforin has not been described. Viral inhibitors can, in principle, block all Fas-mediated apoptotic events; however, CL do trigger target cell death through putative cytoplasmic and/or cell membrane granzyme substrates that do not depend on caspase activation. Abbreviations: er, endoplasmic reticulum; GrB, granzyme B; HLA, human leukocyte antigen.
The extensive list of potential blocks to apoptosis might at first glance suggest that host defenses should be easily defeated by many viral pathogens. This is not the case, and in fact the coevolution of viruses and higher organisms over the milennia has resulted in a delicate balance between the ability of viruses to subvert apoptosis and that of the host to survive infection. There is no advantage to the virus in killing its natural host, and the virus-host relationship is most obviously advantageous to the virus if it delays apoptosis, thus facilitating replication and spread to uninfected cells. Not surprisingly, viruses have evolved ways of interfering with every facet of immune recognition and effector function, often by expressing their own versions of mammalian genes that control cell death. In some instances (CrmA and FLIPs are good examples), the discovery of viral escape mechanisms has even preceded elucidation of the corresponding host pathways! It is noteworthy that all of the escape mechanisms elucidated so far appear to affect the Fas pathway preferentially, with little or no effect on granule exocytosis. CrmA might be one exception, but it can block ICE and FLICE far more effectively than granzyme B in vitro, and there is no convincing evidence that it can block granzyme B-mediated cytolysis in intact cells. This paucity of known inhibitors for perforin and granzyme B suggests that the granule mechanism may be the way in which the CL can have the final say on the death of a virus-infected cell. It has recently been hypothesized that perforin exerts its action by enabling granzyme B to disrupt and therefore escape from endocytic vesicles in a manner akin to that used by adenovirus (29). Like key adenovirus proteins, the granzyme B or other granule proteins might then access the cytoplasm, where they can trigger both caspase-dependent and -independent cell death pathways (80). If perforin mimics the endosmolytic effects of viruses, it might be problematic for viruses to inhibit perforin without also preventing their own access to the cell’s interior. Furthermore, even in the absence of perforin, granule proteins may still independently enter target cells (107).
CONCLUSIONS
Whether CTL/NK cell granule exocytosis plays a broader role in virus clearance remains to be determined. Certainly, with the availability of perforin- and granzyme-deficient mice a larger number of noncytopathic and cytopathic viruses needs to be examined. Furthermore, the breeding of mice deficient in both perforin and granzymes will greatly aid our understanding of the synergy between granule components and of how broad the antiviral protection afforded by granule exocytosis might be. For example, strict comparisons between infections in P0 and P0 × granzyme-deficient mice should answer the question of whether granzymes can be protective in the absence of perforin. In vitro, CTL, NK, and lymphocyte-activated killer cells derived from the granzyme B knockout mice are unable to induce rapid DNA fragmentation in allogeneic TCs. The defect is kinetic in nature and can be rescued with longer incubation times, again implying that other granule proteins may also play a supplementary role. In addition, the absence of granzyme B has never been reported to be of consequence for infection in vivo. At this stage, a role for other granule proteins, including granzymes, in mediating apoptosis in vivo is comparatively weak (24). Although, among granzymes, granzyme B appears to be the only CL protease with Asp-ase activity, CL granzymes do appear to enter the TC cytoplasm and nucleus in the presence of perforin and could quite possibly play other intracellular antiviral roles (107). Certainly, their nuclear localization and possible function there should be a topic of interest for virologists and cell biologists in the future.
For some of the cytopathic viruses described above, the intact resistance of mice lacking either the perforin- or FasL-dependent pathway of cytotoxicity could be explained by a complete redundancy of the two mechanisms. This hypothesis is unlikely, since cytopathic viruses infect many cell types not expressing Fas; however, the challenge of P0 × gld mice with the same cytopathic viruses should determine whether either pathway plays a role in virus clearance. Recognition and lysis of infected cells by CL probably proceeds too slowly in the immediate postinfection period to abrogate cytopathic viral replication efficiently, and thus antiviral cytokine and antibody mechanisms have predominated.
The role of CL-FasL and/or host infected-cell-Fas in the clearance of virus is best exemplified by models of viral hepatitis. Clearly, there are evolutionary reasons why the liver does not appear to be a preferred site of action of CL granule exocytosis. Liver infections (as noted above) are generally cleared by cytokines such as IFN-γ, and evidently when foreign antigen persists (as in a transgenic setting) antiviral CL immune attack can induce harmful inflammation in tissues that also express functional Fas. Recent findings of FasL/TNF-mediated bystander lysis by CL responding to allogeneic or viral antigen stimulus (2, 95) and the normal physiological role of the Fas/FasL interactions in the homeostatic control of T cells responding to antigen suggest that the role of FasL expressed on CL is not to destroy host cells expressing foreign antigen but rather to destroy altered (high Fas-expressing) bystander cells. Possibly then, bystander cells that have been (i) infected and/or (ii) induced to express Fas but (iii) do not display foreign antigen in the context of MHC can still be eliminated. Importantly, CTL secretion of IFN-γ and TNF-α not only suppresses viral gene expression (32), but these cytokines may also directly (TNF-α) or indirectly (IFN-γ increases sensitivity to Fas-mediated death) enhance bystander lysis, thus limiting effective virus infection in neighboring cells. Cells in a zone around the infection may be killed simply on suspicion of harboring the virus, and this rough justice in the acute setting is clearly beneficial, by limiting infection. However, in chronic widespread persistent infection, this bystander lysis may be either uncontrolled or simply detrimental as the scale of tissue damage is too great. This bifurcation of granule exocytosis and death receptor pathways of cytolysis into antigen-specific and self lysis may also be supported by the interesting findings of Brossart and Bevan (11) and Cao et al. (14). These studies described the ability of CTL recognizing the germline/self peptide to lyse only TC in a FasL-dependent fashion, while CTL recognizing foreign peptide (one amino acid distinct from self peptide) lysed the same TC by either perforin- or FasL-dependent pathways. Notably, self peptides also appeared to induce secretion of IFN-γ. Furthermore, the apparent ability of a CTL to trigger FasL-mediated lysis in the absence of perforin-mediated lysis may go a long way to explaining how self bystander cells can be lysed exclusively by a FasL-dependent mechanism.
Recent advances in the understanding of the basic biology of lymphocyte-mediated cytolysis and its physiological relevance have been greatest in the field of viral immunology. Virus infection models in vitro and in vivo have helped elucidate the relative role of CL granule exocytosis- and death receptor-mediated cytolysis in immune responses and continue to uncover new intracellular pathways and sites of action integral to cell death. Viruses remain one of our most powerful tools for continued elaboration of the CL lytic mechanism. Further dissection of how the immune state determines the CL’s decision to utilize one or another of these cytolytic mechanisms will enable novel, specific, and powerful therapeutic agents to control CL function in the antiviral immune response.
ACKNOWLEDGMENTS
We thank Ricky Johnstone, Bruce Wines, and Damian Purcell for helpful discussions and Michael Kershaw for assistance with the illustration.
M.J.S. is currently supported by a Wellcome Trust Senior Research Fellowship in Medical Science, and J.A.T. is supported by a National Health and Medical Research Council of Australia (NH&MRC) Senior Research Fellowship. This project was also funded by the NH&MRC and the Anti-Cancer Council of Victoria.
REFERENCES
- 1.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P A, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 3.Anel A, Buferne M, Boyer C, Schmitt-Verhulst A-M, Golstein P. T cell receptor-induced Fas ligand expression in cytotoxic T lymphocyte clones is blocked by protein tyrosine kinase inhibitors and cyclosporin A. Eur J Immunol. 1994;24:2469–2476. doi: 10.1002/eji.1830241032. [DOI] [PubMed] [Google Scholar]
- 4.Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashwell J D, Klausner R D. Genetic and mutational analysis of the T cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 7.Beidler D R, Tewari M, Friesen P D, Poirier G, Dixit V M. The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995;270:16526–16528. doi: 10.1074/jbc.270.28.16526. [DOI] [PubMed] [Google Scholar]
- 8.Berthou C, Marolleau J P, Lafaurie C, Soulie A, Dal Cortivo L, Bourge J F, Benbunan M, Sasportes M. Granzyme B and perforin lytic proteins are expressed in CD34+ peripheral blood progenitor cells mobilized by chemotherapy and granulocyte-colony stimulating factor. Blood. 1995;86:3500–3506. [PubMed] [Google Scholar]
- 9.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Yang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boismenu R, Havran W L. An innate view of gamma delta T cells. Curr Opin Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 11.Brossart P, Bevan M J. Selective activation of Fas/Fas ligand-mediated cytotoxicity by a self-peptide. J Exp Med. 1996;183:2449–2458. doi: 10.1084/jem.183.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 13.Carter C R, Sayers T J, Wiltrout R H, Turcovski-Corrales S M, Taub D D. Generation of antigenic peptides by lymphocyte granule serine proteases (granzymes) Cell Immunol. 1996;172:235–245. doi: 10.1006/cimm.1996.0238. [DOI] [PubMed] [Google Scholar]
- 14.Cao W, Tykodi S S, Esser M T, Braciale V L, Braciale T J. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 15.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bc1-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisari F V. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr Top Microbiol Immunol. 1996;206:149–173. doi: 10.1007/978-3-642-85208-4_9. [DOI] [PubMed] [Google Scholar]
- 17.Cohen P, Eisenberg R A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 18.Colonna M. Specificity and function of immunoglobulin superfamily NK cell inhibitory and stimulatory receptors. Immunol Rev. 1997;155:127–134. doi: 10.1111/j.1600-065x.1997.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 19.Cuturi M C, Anegon I, Sherman F, Loudon R, Clark S C, Perussia B, Trinchieri G. Production of hematopoietic colony-stimulating factors by human natural killer cells. J Exp Med. 1989;169:569–583. doi: 10.1084/jem.169.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darmon A J, Nicholson D W, Bleackley R C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell derived granzyme B. Nature. 1996;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 21.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer P H. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 22.Doherty P C, Allan J E, Lynch F, Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990;11:55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- 23.Duke R C, Chervenak R, Cohen J J. Endogenous endonuclease-induced DNA fragmentation. Proc Natl Acad Sci USA. 1983;80:6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebnet K, Hausmann M, Lehmann-Grube F, Mullbacher A, Kopf M, Lamers M, Simon M M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehl S, Hoffman-Rohrer U, Nagata S, Hengartner H, Zinkernagel R. Different susceptibility of cytotoxic T cells to CD95 (Fas/Apo-1) ligand-mediated cell death after activation in vitro versus in vivo. J Immunol. 1996;156:2357–2360. [PubMed] [Google Scholar]
- 26.Farrell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 27.Farrow S N, White J H, Martinou I, Raven T, Pun K T, Grinham C J, Marinou J C, Brown R. Cloning of a bc1-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Armstrong R, Krebs J, Srinivasula S M, Wang L, Bullrich F, Fritz L C, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. Activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froelich C J, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah G M, Bleackley R C, Dixit V M, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 30.Galocha B, Hill A, Barnett B C, Dolan A, Raimondi A, Cook R F, Brunner J, McGeoch D J, Ploegh H L. The active site of ICP47, a herpes simplex virus-encoded inhibitor of the major histocompatibility complex (MHC)-encoded peptide transporter associated with antigen processing (TAP), maps to the NH2-terminal 35 residues. J Exp Med. 1997;185:1565–1572. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Greenberg P. Abstract. 1997. EMBO Cell Mediated Cytotoxicity Workshop. Kerkrade, The Netherlands. [Google Scholar]
- 31.Griffiths G M, Mueller C. Expression of perforin and granzymes in vivo: potential diagnostic markers for activated cytotoxic cells. Immunol Today. 1991;12:415–419. doi: 10.1016/0167-5699(91)90145-J. [DOI] [PubMed] [Google Scholar]
- 32.Guidotti L G, Ando K, Hobbs M V, Ishikawa T, Runkel L, Schreiber R D, Chisari F V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacker G, Hawkins C J, Smith K G, Vaux D L. Effects of viral inhibitors of apoptosis in models of mammalian cell death. Behring Inst Mitt. 1996;97:118–126. [PubMed] [Google Scholar]
- 34.Henkart P A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- 35.Henkart P A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 36.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 37.Hiromatsu K, Usami J, Aoki Y, Makino M, Yoshikai Y. Accelerated progression of a murine retrovirus-induced immunodeficiency syndrome in Fas mutant C57BL/6lpr/lpr mice. Microbiol Immunol. 1997;41:221–227. doi: 10.1111/j.1348-0421.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 38.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 39.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Has A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 40.Jenne D E, Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T-lymphocytes upon T-cell receptor stimulation. Immunol Rev. 1988;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 41.Ju S T, Cui H, Panka D J, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ju S-T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 43.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 44.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 45.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Immunol Rev. 1995;146:95–115. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 46.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 47.Kawanishi M. Epstein-Barr virus BHRF1 protein protects intestine 407 epithelial cells from apoptosis induced by tumor necrosis factor alpha and anti-Fas antibody. J Virol. 1997;71:3319–3322. doi: 10.1128/jvi.71.4.3319-3322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerkau T, Bacik I, Bennink J R, Yewdell J W, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith G L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1 beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1 beta-induced fever. J Gen Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 50.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 51.Lanier L L, Le A M, Phillips J H, Warner N L, Babcock G F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789–1796. [PubMed] [Google Scholar]
- 52.Lanier L L, Ruitenberg J J, Phillips J H. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–3485. [PubMed] [Google Scholar]
- 53.Lanier L L. Natural killer cells: from no receptors to too many. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 54.Lefrancois L, Lyles D S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982;121:168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- 55.Levine A. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Pohler U, Strehlow I, Hertig S, Baccarini M, Emmendorffer A, Tschopp J, Lohmann-Matthes M L. Macrophage precursor cells produce perforin and perform Yac-1 lytic activity in response to stimulation with interleukin-2. J Leukocyte Biol. 1994;56:117–123. doi: 10.1002/jlb.56.2.117. [DOI] [PubMed] [Google Scholar]
- 57.Lichtenheld M G, Olsen K J, Lu P, Lowrey D M, Hameed A, Hengartner H, Podack E R. Structure and function of human perforin. Nature. 1988;335:448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- 58.Lin M T, Stohlman S A, Hinton D R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohman B L, Razvi E S, Welsh R M. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Los M, Van de Craen M, Penning L C, Schenk H, Westendorp M, Baeuerle P A, Droge W, Krammer P H, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 61.Machold R P, Wiertz E J, Jones T R, Ploegh H L. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori T, Ando K, Tanaka K, Ikeda Y, Koga Y. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood. 1997;89:3565–3573. [PubMed] [Google Scholar]
- 63.Mullbacher A, Ebnet K, Blanden R V, Hla R T, Stehle T, Museteanu C, Simon M M. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 65.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 66.Nakata M, Smyth M J, Norihisa Y, Kawasaki A, Shinkai Y, Okumura K, Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma delta T cells: Implication for their cytotoxic role in vivo. J Exp Med. 1990;172:1777–1784. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nava V E, Cheng E H, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bc1-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pantaleo G, Graziosi C, Fauci A S. The immunopathogenesis of human immunodeficiency virus infection. N Eng J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 69.Podack E R, Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. Nature. 1983;302:442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- 70.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 72.Reddehase M J, Mutter W, Munch K, Buhring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Revilla Y, Cebrian A, Baixeras E, Martinez C, Vinuela E, Salas M L. Inhibition of apoptosis by the African swine fever virus Bc1-2 homologue: role of BH1 domain. Virology. 1997;228:400–404. doi: 10.1006/viro.1996.8395. [DOI] [PubMed] [Google Scholar]
- 74.Reyburn H T, Mandelboim O, Vales-Gomez M, Davis D M, Pazmany L, Strominger J L. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature. 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 75.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 76.Riddell S R, Gilbert M J, Greenberg P D. CD8+ cytotoxic T cell therapy of cytomegalovirus and HIV infection. Curr Opin Immunol. 1993;5:484–491. doi: 10.1016/0952-7915(93)90027-p. [DOI] [PubMed] [Google Scholar]
- 77.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 78.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell J H, Dubos C B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980;125:1256–1261. [PubMed] [Google Scholar]
- 80.Sarin A, Williams M S, Alexander-Miller M A, Berzofsky J A, Zacharchuk C M, Henkart P A. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- 81.Scharton T M, Scott P. Natural killer cells are a source of interferon-γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 83.Schreiber M, Sedger L, McFadden G. Distinct domains of M-T2, the myxoma virus tumor necrosis factor (TNF) receptor homolog, mediate extracellular TNF binding and intracellular apoptosis inhibition. J Virol. 1997;71:2171–2181. doi: 10.1128/jvi.71.3.2171-2181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott P, Trinchieri G. The role of natural killer cells in host-parasite interactions. Curr Opin Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 85.Shi L, Kam C-M, Powers J C, Aebersold R, Greenberg A H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinkai Y, Takio K, Okumura K. Homology of perforin to the ninth component of complement (C9) Nature. 1988;334:525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- 87.Shiver J W, Su L, Henkart P A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71:315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- 88.Sieg S, Xia L, Huang Y, Kaplan D. Specific inhibition of granzyme B by parainfluenza virus type 3. J Virol. 1995;69:3538–3541. doi: 10.1128/jvi.69.6.3538-3541.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sieg S, Yildirim Z, Smith D, Kayagaki N, Yagita H, Huang Y, Kaplan D. Herpes simplex virus type 2 inhibition of Fas ligand expression. J Virol. 1996;70:8747–8751. doi: 10.1128/jvi.70.12.8747-8751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sieg S, Smith D, Yildirim Z, Kaplan D. Fas ligand deficiency in HIV disease. Proc Natl Acad Sci USA. 1997;94:5860–5865. doi: 10.1073/pnas.94.11.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90a.Simon M. Abstract. 1997. EMBO Cell Mediated Cytotoxicity Workshop. Kerkrade, The Netherlands. [Google Scholar]
- 91.Sloand E M, Young N S, Kumar P, Weichold F F, Sato T, Maciejewski J P. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood. 1997;89:1357–1363. [PubMed] [Google Scholar]
- 92.Smyth M J, Ortaldo J R, Shinkai Y-I, Yagita H, Nakata M, Okumura K, Young H A. Interleukin-2 induction of pore-forming protein gene expression in human peripheral blood CD8+ T cells. J Exp Med. 1990;171:1269–1281. doi: 10.1084/jem.171.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smyth M J, Norihisa Y, Ortaldo J R. Multiple cytolytic mechanisms displayed by activated human peripheral blood T cell subsets. J Immunol. 1992;148:55–62. [PubMed] [Google Scholar]
- 94.Smyth M J, Trapani J A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;19:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 95.Smyth M J. FasL-mediated bystander lysis of syngeneic cells in response to an allogeneic stimulus. J Immunol. 1997;158:5765–5772. [PubMed] [Google Scholar]
- 96.Song Q, Burrows S R, Smith G, Lees-Miller S P, Kumar S, Chan D W, Trapani J A, Alnemri E, Litwack G, Lu H, Moss D J, Jackson S, Lavin M F. Interleukin-1 beta converting enzyme-like protease cleaves DNA-dependent protein kinase in cytotoxic T cell killing. J Exp Med. 1996;184:619–626. doi: 10.1084/jem.184.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sower L E, Froelich C J, Allegretto N, Rose P M, Hanna W D, Klimpel G R. Extracellular activities of human granzyme A. Monocyte activation by granzyme A versus alpha-thrombin. J Immunol. 1996;156:2585–2590. [PubMed] [Google Scholar]
- 98.Spriggs M K, Koller B H, Sato T, Morrissey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszewski C R. Beta 2-microglobulin, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Srinivasula S M, Fernandes-Alnemri T, Zangrilli J, Robertson N, Armstrong R C, Wang L, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J Biol Chem. 1996;271:27099–27106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- 100.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sytwu H K, Liblau R S, McDevitt H O. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 102.Tang Y, Hugin A W, Giese N A, Gabriele L, Chattopadhyay S K, Frederickson T N, Kagi D, Hartley J W, Morse H C., III Control of immunodeficiency and lymphoproliferation in mouse AIDS: studies of mice deficient in CD8+ T cells or perforin. J Virol. 1997;71:1808–1813. doi: 10.1128/jvi.71.3.1808-1813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tartaglia L A, Ayres T M, Wong G H W, Goeddel D V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 104.Tay C H, Welsh R M. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 106.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 107.Trapani J A, Browne K A, Smyth M J, Jans D A. Localization of granzyme B in the nucleus. A putative role in the mechanism of cytotoxic lymphocyte-mediated apoptosis. J Biol Chem. 1996;271:4127–4133. doi: 10.1074/jbc.271.8.4127. [DOI] [PubMed] [Google Scholar]
- 108.Trauth B C, Klas C, Peters A M J, Matzku S, Moller P, Falk W, Debatin K M, Krammer P H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 109.Tschopp J, Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- 110.Walsh C M, Matloubian M, Liu C-C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Welsh R M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- 112.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 113.Wiertz E J H J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 114.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yokoyama W M, Seaman W E. The Ly-49 and NKR-P1 gene families encoding lectin like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 116.Yoshida H, Sumichika H, Hamano S, He X, Minamishima Y, Kimura G, Nomoto K. Induction of apoptosis of T cells by infecting mice with murine cytomegalovirus. J Virol. 1995;69:4769–4775. doi: 10.1128/jvi.69.8.4769-4775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan J. Transducing signals of life and death. Curr Opin Cell Biol. 1997;9:247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- 118.Zajac A J, Quinn D G, Cohen P L, Frelinger J A. Fas-dependent CD4+ cytotoxic T-cell-mediated pathogenesis during virus infection. Proc Natl Acad Sci USA. 1996;93:14730–14735. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M L. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 120.Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski U H. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]
- 121.Zinkernagel R M, Rosenthal K L. Experiments and speculation on antiviral specificity of T and B cells. Immunol Rev. 1981;58:131–155. doi: 10.1111/j.1600-065x.1981.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 122.Zinkernagel R M, Hengartner H. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol Today. 1994;15:262–268. doi: 10.1016/0167-5699(94)90005-1. [DOI] [PubMed] [Google Scholar]