Abstract
The aorta plays a central role in the modulation of blood flow to supply end organs and to optimize the workload of the left ventricle. The constant interaction of the arterial wall with protective and deleterious circulating factors, and the cumulative exposure to ventriculoarterial pulsatile load, with its associated intimal-medial changes, are important players in the complex process of vascular aging. Vascular aging is also modulated by biomolecular processes such as oxidative stress, genomic instability, and cellular senescence. Concomitantly with well-established cardiometabolic and sex-specific risk factors and environmental stressors, arterial stiffness is associated with cardiovascular disease, which remains the leading cause of morbidity and mortality in women worldwide. Sexual dimorphisms in aortic health and disease are increasingly recognized and explain—at least in part—some of the observable sex differences in cardiovascular disease, which will be explored in this review. Specifically, we will discuss how biological sex affects arterial health and vascular aging and the implications this has for development of certain cardiovascular diseases uniquely or predominantly affecting women. We will then expand on sex differences in thoracic and abdominal aortic aneurysms, with special considerations for aortopathies in pregnancy.
Graphical abstract
Résumé
L’aorte joue un rôle central dans la modulation du débit sanguin pour irriguer les organes cibles et optimiser la charge de travail du ventricule gauche. L’interaction constante entre la paroi artérielle et des facteurs protecteurs et délétères présents dans la circulation, ainsi que l’exposition cumulative à la charge pulsatile ventriculo-artérielle accompagnée des variations de l’épaisseur intima-média, sont des facteurs importants dans le processus complexe du vieillissement vasculaire. Le vieillissement vasculaire est également modulé par des processus biomoléculaires comme le stress oxydatif, l’instabilité génomique et la sénescence cellulaire. Conjointement avec les facteurs de risque cardiométaboliques et spécifiques au sexe bien établis et les sources de stress environnementales, la rigidité artérielle est associée aux maladies cardiovasculaires, qui demeurent la première cause de morbidité et de mortalité chez les femmes à l’échelle mondiale. Les dimorphismes sexuels en ce qui concerne la santé et les maladies de l’aorte sont de plus en plus reconnus et expliquent, du moins en partie, certaines des différences observables liées au sexe dans les maladies cardiovasculaires, ce qui a fait l’objet de cette analyse. Plus précisément, nous verrons le rôle que joue le sexe biologique dans la santé artérielle et le vieillissement vasculaire, et ce que cela implique dans l’évolution de certaines maladies cardiovasculaires qui touchent surtout ou uniquement les femmes. Nous élargirons ensuite l’étude des différences sexuelles aux anévrismes de l’aorte thoracique et abdominale, en accordant une attention particulière aux maladies de l’aorte pendant la grossesse.
Lay Summary
The aorta and the large central arteries are complex biological structures that exert important biochemical and hemodynamic effects to ensure adequate blood supply to end organs and to optimize left ventricular workload. Composed of 3 layers—intima, media, and adventitia—the arterial wall represents a dynamic infrastructure that is exposed to a variety of factors, some of which are protective—such as estrogen derivatives and their vasodilatory properties—and some of which are deleterious such as proinflammatory cytokines and prothrombotic factors. The balance among these factors varies with age and concomitant with well-established cardiometabolic and sex-specific cardiovascular risk factors—as well as environmental stressors—showcases sexual dimorphisms that alter the process of vascular aging between men and women. This, in turn, modulates the development and outcome of various cardiovascular diseases.
Cardiovascular disease remains the leading cause of morbidity and mortality in women worldwide, and incident myocardial infarction and cardiovascular death have increased in young and middle-aged women in the past 10 years.1 Furthermore, women remain under-represented in clinical trials, and sex-specific evaluation of the impact of common cardiovascular preventive strategies and pharmacotherapies is limited. As such, it is of paramount importance to understand the impact of biological sex on the pathogenesis of common cardiovascular pathologies, many of which are tightly related to aortic structure and function.
In this review, we will explore the sex differences in aortic health and disease. To begin, we will review the process of vascular aging and explore how biological sex affects the aortic pressure-buffering function. We will then explore the interplay between aortic/vascular health and select female-specific or female-predominant cardiovascular pathologies including hypertensive disorders of pregnancy, heart failure with preserved ejection fraction (HFpEF), and coronary microvascular dysfunction. Finally, we will compare the sex differences in pathogenesis, prevalence, management, and outcomes of thoracic aortic aneurysms (TAAs) and abdominal aortic aneurysms (AAAs), with a dedicated discussion on aortopathies in pregnancy and in the postpartum period.
Methods
The current article represents a narrative review aiming to synthesize the existing literature on sex differences in aortic health, function, aging, as well as the previously mentioned aortic pathologies. Common scientific databases were used to identify relevant studies, including PubMed, MEDLINE, Web of Science, and Google scholar. Key words were identified for each topic of interest and searched on the databases. After reviewing titles and abstracts of the results of each search, articles were selected for inclusion in the review based on overall relevance. No specific exclusion criteria were set a priori. Generally, articles published in journals with high-impact factors, cardiology societies’ guidelines and reviews, and high-quality evidence such as randomized controlled trials or large observational studies were favoured. Further details on the search strategies used can be found in Supplemental Appendix S1.
For this review, the term “sex” will be used to refer to biological sex, defined as the assigned sex at birth based on primary sexual characteristics of reproductive organs and genitalia, as well as sex-determining chromosomes (females being XX and males being XY). It is important to highlight that gender is a more complex concept that are related to socially constructed roles, behaviours, and actions, which also affect cardiovascular health. This review will focus on synthesizing the existing evidence of the impact of biological female sex on aortic health and function.
The Aorta Beyond a Conduit: Sex Differences in Aortic Health, Function, and Aging
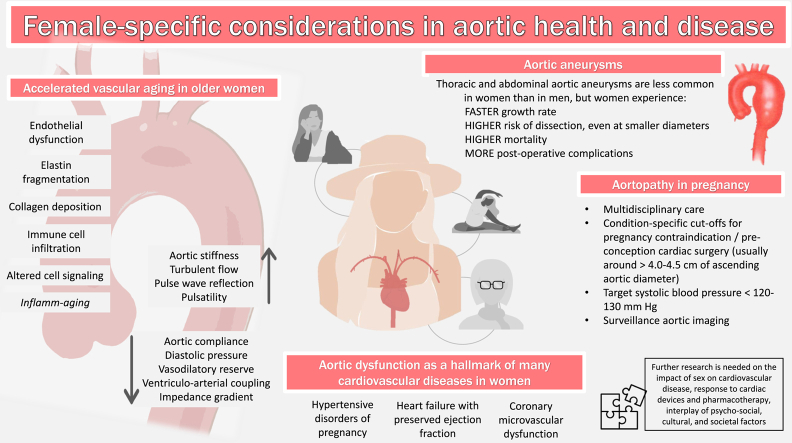
To understand the influence of sex on the complex process of accelerated or pathologic vascular aging, a review of normal aortic and vascular aging is needed. In broad terms, vascular aging is a normal biological process that occurs through time and begins in infancy. At the level of the aorta, it is characterized by structural changes—namely, increase in diameter, length, and tortuosity, and decrease in elasticity with associated stiffening—as well as functional changes, including reduced vasodilatory reserve and diminished arterial buffering capacity.2 In addition, myriad changes at the cellular level contribute to the dysregulated function of the components of the aortic infrastructure, mediated by the process of "inflamm-aging." Multiple age-related, proinflammatory mechanisms are at play, including genomic instability, oxidative stress, loss of proteostasis, altered metabolism, cellular senescence, and altered neurohormonal response to stress.3
To understand the changes that the aorta undergoes through time, it is useful to evaluate each component of the aortic wall separately. The intima is composed of a single layer of endothelial cells (ECs), a basement membrane, and the subendothelial space, and is the regulatory powerhouse of vascular tone and blood flow. because of their direct contact with circulating molecules, ECs are exposed to proaging factors that contribute to their dysfunction, by reducing their ability to produce and respond to nitric oxide and increasing their production of prothrombotic molecules such as endothelin-1, and proinflammatory cytokines, such as interleukin-1, interleukin-6, and tumour necrosis factor-alpha.4 The senescent, dysfunctional ECs also allow for infiltration of immune cells into deeper layers, contributing to disruption of other components of the extracellular matrix. Indeed, these aging-related intimal changes have important effects on the media, promoting fragmentation of elastin fibres and deposition of collagen fibres, which are 100 to 1000 times stiffer. The degraded elastin fibres lead to accumulation of elastin-derived peptides, which, in turn, have proinflammatory and procalcific properties, further contributing to arterial stiffening.4,5 Finally, age-associated dysregulation in the signalling pathways between the components of the adventitia—including stem-cell progenitor cells, fibroblasts, and collagen—contribute to aortic remodelling, although these processes are less well characterized. The summation of these changes contributes to age-related increase in aortic stiffness, aortic impedance, and increase in peripheral wave-reflection velocity, leading to increases in systolic and pulse pressures and increase in the workload of the left ventricle.2,5
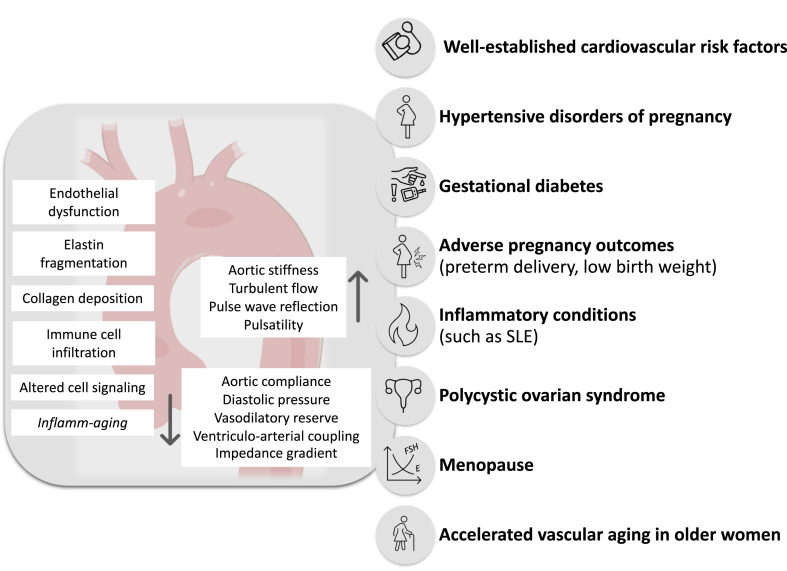
There is mounting evidence supporting the presence of a biochemical basis for sex differences in aging in general and at the level of the vasculature (Fig. 1). Intrinsically, there seems to be sex-related differences in arterial characteristics, demonstrated by the observable differences between prepubertal males and females, the latter showing stiffer large arteries and wider pulse pressures than age-matched males.6 Male subjects appear to have higher numbers of senescent cells, shorter telomeres, and greater biological age when compared with female subjects, who also display greater capacity for cellular regeneration and proliferation of stem cells in the premenopausal years.3 This is also found at the epigenetic level, in which female subjects present a more favourable age-related epigenetic methylation pattern compared with male subjects (which refers to the transfer of a methyl group onto cytosine, which has downstream effects on DNA expression, gene transcription, and more, without actually changing the DNA sequence): so much so, that the concept of feminization of the methylation profile has been seen in men who live into their 10th decade.3,5
Figure 1.
Vascular aging and impact of female-specific factors. Women are exposed to a variety of risk factors that affect their vascular health across the lifespan (far right) and that contribute to vascular aging. Structural and functional changes (far left) mediate observable changes in the characteristics of the aorta and the large central arteries (centre). E, estradiol; FSH, follicle-stimulating hormone; SLE, systemic lupus erythematosus.
The impact of sex hormones on vascular aging is complex and not fully understood. In men, there is paucity of data and conflicting evidence on whether changes in testosterone and its derivatives are promoters—or surrogate markers—of cardiovascular aging.7 During early to mid-life, some of the previously mentioned sex differences are—at least in part—explained by the beneficial vasculoprotective effects of estrogen and its derivatives in women, including favourable vasodilatory reserve, reduction in reactive oxygen species and prevention of oxidative damage, attenuation of ischemia and reperfusion injury, reduction in collagen deposition by downregulation of metalloproteinases, and even modifying signalling pathways that attenuate response to inflammation.8 However, there appears to be sexual dimorphisms in many of the previously mentioned mechanisms of "inflamm-aging," some of which contribute to accelerated vascular aging in women later in life. Importantly, the expression of structural proteins that determine the biomechanical properties of the vascular system—particularly, the ratio of elastin and collagen in the extracellular matrix—is influenced by of sex steroids and greatly affects arterial stiffness. This is supported by previous in vitro studies in which components of the extracellular matrix were exposed to estrogen/progesterone and testosterone, demonstrating that in the presence of female sex steroids, there is a relative increase in elastin deposition, with an elastin-to-collagen ratio more than 11 times greater than in matrix exposed to male sex hormones.9
During and after the menopausal transition, the loss of estrogen and its protective vascular effects translates into increased endothelial dysfunction and arterial stiffening in females, which overall also translates into increase cardiovascular events.8 This applies to women in their mid-to-late life with naturally occurring menopause but also in women with abrupt menopause from other causes such as premenopausal oophorectomy.10 At the level of the systemic vasculature, compared with men, women entering the menopausal transition exhibit accelerated arterial stiffening, more pronounced intimal-medial thickening, and more abrupt increases in blood pressure,8,11 whereas men seem to have a steadier progression of vascular aging through time.5
In women, some important consequences of these vascular changes around the menopausal transition include higher burden of multivessel peripheral arterial disease, greater risk of AAA rupture, and greater risk of death from aortic dissection (despite overall lower prevalence of AAA and aortic dissection than in men).8 Moreover, previous work using echocardiography and arterial tonometry in men and postmenopausal women directly measured higher arterial impedance and lower arterial compliance in women, which were also found to be associated with surrogate markers of diastolic dysfunction, such as mitral inflow velocity E/A ratio.12
It is also important to recognize sex-specific risk factors for cardiovascular disease that are associated with—or a reflection of—vascular dysfunction, including adverse pregnancy outcomes (particularly history of hypertensive disorders of pregnancy, especially pre-eclampsia, preterm delivery, gestational diabetes, low birth weight), and polycystic ovarian syndrome.13 Overall, sex-specific changes to the vascular infrastructure and myocardium, as well as the effects of accumulating cardiometabolic risk factors and estrogen deprivation, are some of the key factors behind accelerated vascular aging in mid to late life in women and at the root of the female predominance of certain cardiovascular diseases, such as heart failure with preserved ejection fraction and coronary microvascular dysfunction, which will be explored in subsequent sections. Further research on the biomolecular effect of sex hormones on the pathogenesis of cardiovascular disease is needed, and sex-specific study and reporting of incident cardiovascular events, effects of cardiac devices, and pharmacotherapy is warranted.
In an effort to provide a comprehensive bio-psycho-social evaluation of the impact of sex on aortic health and disease, we must also consider the interplay of gender, gender identity, cultural and societal norms, as well as socioeconomic status that contribute to cardiovascular risk and that often go unnoticed. The impact of gender on cardiovascular health is beyond the scope of this review, but the process of vascular aging and cardiovascular trajectories of transgender individuals should be further studied, as the interactions of biological sex, sex-affirming hormone supplementation, and gender identity are complex and their impact on cardiovascular health is not yet fully understood. Moreover, there is paucity of data exploring the impact of culture and societal norms on aortic health and disease. A good example of how societal norms may modulate vascular aging in women is represented in the behaviours, beliefs, and access to regular physical activity. Indeed, there is strong evidence demonstrating that lifelong physical activity is associated with favourable vascular health in women,10 and psychosocial and cultural norms may affect a woman’s ability to engage in regular exercise and derive its benefit. It is well documented that cardiovascular disease is under-recognized in women living in socially deprived regions, communities with strong traditional or religious norms, women who are part of minorities or Indigenous populations, or with low socioeconomic status.14 How these sociocultural elements affect aortic health deserves further attention.14
Although the review of therapeutic interventions to prevent cardiovascular disease is outside of the scope of this review, it is worth mentioning that the use of menopausal hormone therapy is not recommended for primary prevention of cardiovascular disease in women and that regular physical activity has been shown to attenuate the negative cardiovascular impact of aging and of the menopausal transition.15 Finally, the use of testosterone replacement in older men for cardioprotection is insufficiently studied and currently not recommended.14
Future directions
The bio-psycho-social impact of biological sex on vascular health and cardiovascular disease is a fertile area of research. From a biomolecular perspective, whether variations in sex hormone levels and sex hormone receptor expression through the lifespan can be safely altered through lifestyle changes or pharmacotherapy—and, in turn, lead to vasculoprotective effects well into old age—remains to be assessed. Sex-specific data analysis and outcome reporting in cardiovascular clinical trials is essential. Exploration of cultural and societal influences on vascular aging patterns in women is interesting and may yield valuable insights into the effect of under-recognized social determinants of health.
Role of Aortic Aging and Dysfunction on Cardiovascular Diseases Uniquely or Predominantly Affecting Women
In the next section, we will describe how the health and function of the aorta play an important role in many diseases that predominantly affect women. Figure 2 summarizes these observations.
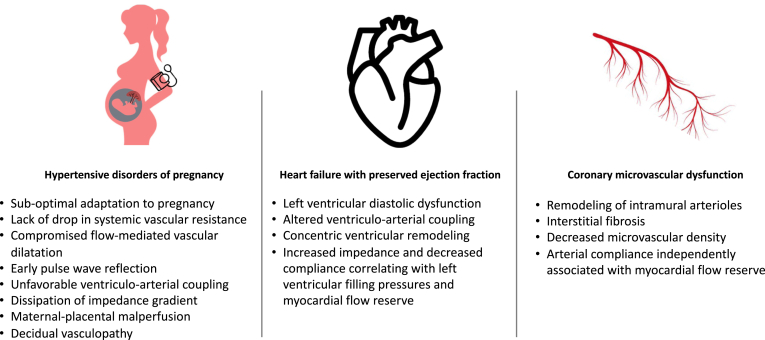
Figure 2.
Aortic dysfunction in female-predominant or female-specific conditions. Aortic dysfunction has been associated with many female-specific conditions and changes in the structure and function of the aorta and may contribute to their pathophysiology and explain—in part—the female predominance of some cardiovascular diseases. Women with hypertensive disorders of pregnancy exhibit pathologic changes in arterial function and increased arterial stiffness. Altered ventriculo-arterial coupling, increased impedance, and decreased compliance in the aorta is linked to high left ventricular filling pressure and diastolic dysfunction in heart failure with preserved ejection fraction. Coronary microvascular dysfunction is at least in part explained by decreased central artery compliance and remodelling of arteriolar beds.
Aortic dysfunction in hypertensive disorders of pregnancy
Hypertensive disorders of pregnancy (HDP) include gestational hypertension, pre-eclampsia and eclampsia, chronic hypertension, and pre-eclampsia and eclampsia superimposed on chronic hypertension, and are 1 of the leading causes of maternal and fetal adverse outcomes worldwide. HDP are reported to affect up to 10% of pregnancies and carry 20% to 50% risk of recurrence.16 Although initially thought to be solely placenta mediated, it has become clear that suboptimal maternal hemodynamic adaptation to pregnancy, particularly failure to drop systemic vascular resistance and arterial impairment,16 are key players in the development of HDP, particularly pre-eclampsia.
Indeed, suboptimal arterial hemodynamics have been observed in women with pre-eclampsia and deserve special review,17,18 The decrease in systemic vascular resistance in pregnancy is a critical adaptation to accommodate the increased cardiac output and plasma volume and optimize left ventricular and arterial coupling. In normal pregnancies, the drop in systemic vascular resistance is observed by a decrease in arterial stiffness, central aortic pressure, and pulse-wave reflection, combined with increase in flow-mediated dilation, compared with healthy, nonpregnant individuals. These adaptations appear to be lacking or suboptimal in women with pre-eclampsia, in a dose-dependent fashion, with more severe cases of pre-eclampsia exhibiting more profound arterial stiffness.18 Normal pregnancy is also associated with enhanced vascular reactivity, as observed by more robust flow-mediated dilatation on noninvasive vascular reactivity testing, which also appears compromised—or sometimes even absent—in pre-eclampsia.18 The left ventricle is therefore exposed to an inappropriate hemodynamic load in the setting of pre-eclampsia, with early pulse-wave reflection and increased arterial stiffness, which increase the left ventricular afterload in systole and contribute to unfavourable left ventricular-arterial coupling.18 Perfusion to low resistance systems, such as kidneys and placenta, is also further compromised by decreased diastolic pressure and the loss of the usual arterial “stiffness gradient” (as in normal physiological states, arterial resistance increases from central to peripheral arteries, as measurable by pulse-wave velocity, but in pre-eclampsia, dissipation of this gradient is observed, exposing low-resistance systems to higher pulsatile stress).18 Interestingly, markers of aortic stiffness, including increased carotid-femoral pulse-wave velocity (cfPWV), measured in the first trimester of pregnancy, have been shown to predict pre-eclampsia with better accuracy than blood pressure alone.19
Notwithstanding, the placenta remains a key player, and approximately one-half of patients with HDP exhibit some form of maternal-placental malperfusion, characterized by decidual vasculopathy with suboptimal or incomplete placental arterial remodelling and inadequate angiogenesis, as well as endothelial dysfunction. This contributes to ischemia and reperfusion injury within the placenta, which, in turn, initiates a cascade of antiangiogenic, proinflammatory, and oxidative reactions affecting not only the placenta and the fetus but also the maternal circulation and end organs through circulating factors (including FMS-like tyrosine kinase-1, transforming growth factor beta, and soluble endoglin).17,18,20 Through a process that mirrors atherosclerosis, the vasculopathy associated with maternal-placental syndromes likely unmasks a predisposition for cardiovascular disease later in life.21
Although the maternal symptoms in severe cases of pre-eclampsia and eclampsia improve with delivery of the placenta, the associated maternal cardiovascular risk remains in the short, middle, and long term.16,17 It has been proposed that a senescent cellular phenotype accompanies the pre-eclamptic state, persists after delivery, and promotes accelerated vascular aging, contributing to the accumulation of cardiometabolic comorbidity through time and the heightened lifelong cardiovascular risk.17 There is recent evidence reporting that women with history of pre-eclampsia are 9 times more likely to have early vascular aging (including greater aortic stiffness, central blood pressure, and wave reflections) than controls (P = 0.011).22 The impact of pre-eclampsia on vascular health was found, once again, to have a “dose-dependent” effect, with evidence of pre-eclampsia adding 6 years to the expected arterial age and 11 years in severe cases.22 Recent evidence demonstrated significant difference in premature myocardial infarction in women with history of pre-eclampsia compared with controls (P = 0.010), supporting the need of early and aggressive cardiovascular prevention in this population.23
Aortic dysfunction in women with heart failure with preserved ejection fraction (HFpEF)
The prevalence of HFpEF increases with age, and HFpEF is more common in women than men.24 Mechanisms accounting for this female predominance are not fully known, but differences in arterial structure and function may account for some of these observations. Our group has shown that in older hypertensive women without heart failure, proximal aortic stiffness and pulsatile arterial load are associated with impaired left ventricular diastolic function, altered ventricular-arterial coupling,12 and concentric left ventricular remodelling25: all hallmark characteristics of HFpEF. This suggests that arterial alteration in aging women can affect cardiac structure and function and potentially contribute to development of HFpEF.
In established HFpEF, arterial stiffness and pulsatile load are increased. Compared with controls, patients with HFpEF have significantly reduced total arterial compliance, increased arterial stiffness, and increased wave reflection-associated pressure load (augmentation index), the latter of which was shown to correlate with high pulmonary capillary wedge pressure and low cardiac output during exercise.26 Two studies have evaluated sex differences in invasive arterial hemodynamic measures in HFpEF. One study evaluating 190 participants with HFpEF found that women had significantly higher resting measures of arterial stiffness and load compared with men; for example, women with HFpEF had an augmentation pressure 10 mm Hg higher than men with HFpEF (P < 0.0001).27 The other study found that women with HFpEF had abnormalities in vascular function with exercise such as lower pulmonary and systemic compliance (P = 0.032 and P = 0.019, respectively) and higher arterial elastance (stiffness) (P < 0.001) compared with men with HFpEF.28 Our group has shown, using noninvasive techniques, that women with HFpEF have significantly greater arterial stiffness and pulsatile arterial load compared with similarly aged men with HFpEF and control female subjects. Furthermore, higher aortic characteristic impedance and low proximal arterial compliance correlated with high estimated left ventricular filling pressures and lower myocardial flow reserve on 82Rubidium positron emission tomography exclusively in women with HFpEF without obstructive epicardial coronary artery disease.29 Together, these studies highlight a distinct arterial phenotype unique to older women with HFpEF and support the correlation between arterial abnormalities and key pathologic features of HFpEF (filling pressures, microvascular dysfunction). Arterial stiffness could result in cardiac changes seen in HFpEF caused by increased cardiac workload and increased wall stress leading to increased left ventricular contractility, passive myocardial stiffness, and concentric remodelling. This helps maintain cardiac performance but comes at a cost of ventricular remodelling and impaired relaxation; thus, HFpEF develops.
Role of aortic dysfunction in coronary microvascular dysfunction
Coronary microvascular dysfunction is a predictor of adverse cardiovascular events and is more prevalent in women.30 Coronary microvascular dysfunction is mechanistically important in both ischemia with no obstructive coronary arteries and myocardial infarction with no obstructive coronary arteries.31 An important determinant of microvascular dysfunction in women is hypertension. Hypertension leads to vascular remodelling of intramural arterioles and interstitial fibrosis, which can result in a decrease in microvascular density.32 Specifically, isolated systolic hypertension has a more profound contribution to impairment of coronary microvascular function that combined systolic/diastolic hypertension.33 This mechanism is particularly relevant to women, as age-related increases in pulse pressure are greater in women than in men. Recently, Mitchell et al. determined, using longitudinal cohort data, that proximal aortic stiffening, as assessed by higher aortic characteristic impedance and larger forward wave amplitudes, was strongly associated with longitudinal increases in pulse pressure, especially in women.34 Higher central systolic pressure and lower central diastolic pressure (resulting from a stiffer dysfunctional aorta) may be deleterious to the coronary microvasculature, as the coronaries are predominantly perfused in diastole. In line with this, our group has shown that, in a population without epicardial coronary artery disease, lower arterial compliance was independently associated with abnormal myocardial flow reserve on 82Rubidium positron emission tomography in women.35 As such, aortic dysfunction associated with aging may contribute to abnormalities in coronary microvascular perfusion, with both aortic dysfunction and coronary microvascular dysfunction being more common in older women than men.
Future directions
As described, a dysfunctional aorta with increased stiffness and decreased ventriculo-arterial coupling is a hallmark feature of the pathologies discussed here. Further research should focus on identifying the mediators of such changes, by further exploring the role of sex hormones, inflammatory cytokines, and signalling pathways that may represent future therapeutic targets to slow down or reverse the effects of accelerated vascular aging in women and its downstream effects of incident cardiovascular disease.
Sex Differences in Thoracic Aortic Disease
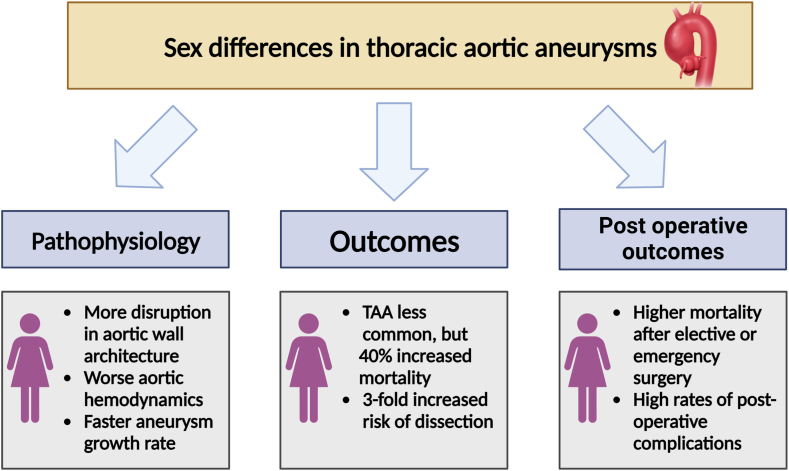
TAAs and acute aortic syndromes are the principal anatomic diseases of the thoracic aorta. TAAs include aneurysms at the aortic root, ascending aorta, aortic arch, and descending thoracic aorta. Acute aortic syndromes include aortic dissection, aortic rupture, penetrating aortic ulcer, and intramural hematoma. There are remarkable sex differences in the prevalence, pathogenesis, prognosis, and management of thoracic aortic diseases, as summarized in Figure 3. Overall, TAAs are nearly twice as common in men as in women. Over time, the detection of TAAs has increased in both sexes36,37; however, women with TAAs display a more aggressive form of the disease, characterized by faster aneurysm growth rate,38 greater predisposition to acute aortic syndromes,39 death,40,41 and worse perioperative outcomes42 than their male counterparts. Recognizing the significance of these sex-specific disease characteristics is crucial for optimizing patient care, reducing disparities, and refining therapeutic approaches.
Figure 3.
Summary of the sex differences in thoracic aortic aneurysms. Despite being nearly twice as common in men, thoracic aortic aneurysms in women display more disruption in the aortic wall architecture and a more aggressive form of the disease characterized by faster aneurysm growth rate, greater predisposition to acute aortic syndromes, death, worse perioperative outcomes, and higher postoperative complication rate compared with their male counterparts. TAA, thoracic aorta aneurysm.
Sex differences in the epidemiology and outcomes of TAA
The epidemiology of TAA is challenging to study because of the indolent nature of the disease, as TAAs typically remain unidentified unless they are detected incidentally, through family member screening, or present as an acute aortic syndrome. In a retrospective study from Ontario, Canada, between 2002 and 2014, the incidence proportion of TAAs was 7.6 per 100,000, with an annual incidence of aortic dissections of 4.6 per 100,000 individuals.41 Importantly, TAAs are associated with high morbidity and mortality.43 Research has shown that 22% of individuals experiencing acute aortic dissections will die before reaching hospitals, whereas among those who receive medical care, an additional 34% die within 30 days.37
TAA etiology can be broadly classified as heritable or degenerative. Approximately 20% of patients with TAA have positive family histories.44 Most often, heritable TAAs are caused by a highly penetrant pathogenic mutation, inherited in an autosomal dominant manner (such as the fibrillin-1 gene in Marfan syndrome [MFS]), meaning that men and women are equally likely to inherit the pathogenic variant. However, despite the autosomal dominant inheritance pattern in most of these syndromic disorders, there are sex differences in age of onset, aortic presentation, and outcomes. In a large cohort of patients with MFS, men were significantly more likely to have aortic root dilation (92% vs 84%) and to have undergone prophylactic aortic root replacement than women (47% vs 24%), P < 0.001 for each.45.In this study, men were more likely to have type B aortic dissections than women.45 In contrast, another study of patients with MFS and TAAs found that in those who were “fast dilators” the dissection rate was high—at 25%—and this disproportionately affected women.46 Other common genetic-congenital causes of TAA include Turner syndrome (TS) and bicuspid aortic valve (BAV) aortopathy. In female patients with TS, an X-linked disorder, TAA has been reported in up to 25% of patients.47 BAV aortopathy is more prevalent in men than women. Large registry studies have found that, after multivariable adjustment, aneurysms at the aortic root were larger in men,48 whereas differences in ascending aortic diameter were either not significantly different48 or were larger in women.49 There is a sex-specific mortality paradox in BAV disease, in which female patients, despite lower prevalence of TAA, have a higher relative risk of death than male patients. This may partially be explained by concomitant valve dysfunction, but sex differences in TAA mortality have been shown in other non-BAV cohorts40 and warrants further investigation. Finally, degenerative TAAs are similarly more prevalent in men than women, although in older adults this predisposition is attenuated. Male and female patients share similar risk factors for developing degenerative TAAs, including older age, history of smoking, and hypertension.50
Prophylactic aortic surgery is the principal intervention for preventing acute aortic syndromes and is generally indicated when an aneurysm reaches a size of 5.5 cm, or smaller in some genetic conditions.51 Aortic root or arch surgery is also the treatment for acute aortic syndromes when the ascending aorta is involved. A recent Canadian study evaluated the impact of sex on outcomes following elective and emergency surgery and found that women experienced higher mortality rates (11% vs 7.4%; P = 0.02) and more perioperative complications. Moreover, female sex was an independent predictor of death, stroke, and adverse events.42 Data from the International Registry of Acute Aortic Dissection found higher mortality among women who underwent surgery for acute type A aortic dissection (31.9% in women vs 21.9% in men; P = 0.013).40 Women were diagnosed later and had increased complications, likely contributing to poorer outcomes.
Finally, despite the lower prevalence of TAAs among women, the prognosis is worse than in men. Women have a 40% higher risk of mortality40 and a 3-fold increased risk of aortic dissection or rupture as compared with men.39 This may be partially explained by faster aneurysm expansion rates in women, particularly among those with degenerative forms of the disease, speaking to more aggressive disease activity.38 Although these differences are striking, the underlying reasons for the sex differences in TAA outcomes remain incompletely understood.
Sex differences in TAA pathogenesis that may account for differences in outcomes
One possibility for the observed sex differences in TAA outcomes is that women tend to be older than men, potentially indicating a more advanced stage of the disease.52 Further, women can experience acute aortic dissection or rupture even with relatively small aneurysms,53 suggesting that aneurysm size alone does not fully capture TAA-related risk in women. Research looking at type B aortic dissections has shown that the proportion of female patients with aortic dissection is higher at smaller aortic diameters (< 3.5 cm) compared with larger diameters.53 Indexing aneurysm diameter to body size,54, 55, 56 provides a more accurate prediction of TAA dissection and rupture in retrospective studies. However, even after adjusting for body size, female sex remains an independent risk factor for acute aortic syndromes, indicating that the sex differences in TAA outcomes cannot be solely attributed to body size.54
As discussed earlier in this review, the aorta remodels differently with natural aging in men and women, with women exhibiting more accelerated functional deterioration with aging compared with men after the age of 50.57 In a population with degenerative TAA, our group has demonstrated that aneurysm expansion is 3 times faster in female patients than in male patients.38 This was finding was subsequently confirmed by the Yale group.56 This may suggest a differential effect of arterial aging on adverse TAA behaviour in women. To this point, we have demonstrated that measures reflecting the health and function of the aorta, such as aortic stiffness as assessed by the cfPWV, is associated with aneurysm expansion in women.58 In TAA, the aortic wall undergoes structural alterations akin to those seen in natural arterial aging, and some experts suggest TAAs represent localized and intensified aging of the arterial wall.59 At the structural and molecular level, Sokolis et al.60 evaluated surgical degenerative TAA specimens and concluded that female TAAs have higher matrix metalloproteinase (MMP)-1 and -9 activity, with decreased activity of the inhibitory enzymes tissue inhibitor of metalloproteinase-1 and -2, culminating in greater extracellular matrix remodelling in the wall of female TAAs. This results in increased aortic stiffness and decreased aortic strength in female TAAs. Hemodynamic properties of the large arteries are largely conveyed by the extracellular matrix and their interaction with smooth-muscle cells.61 Thus, although women are generally protected from TAAs because of protective hormonal and molecular mechanisms during the reproductive years, those who go on to develop TAA may have sicker and more aged aortas to begin with. Development of TAAs in these compromised aortas further impairs aortic wall architecture and function, contributing to greater aneurysm expansion and a greater predisposition to complications.
Future directions
To address the hypothesis that women with TAAs may have worse outcomes because of impairments in aortic function, longitudinal studies will be needed to assess measures of aortic function and outcomes such as death and acute aortic syndromes. Using measures of aortic function to better estimate the sex-specific risk of negative outcome in a patient with TAA is also subject to future study. Finally, exploring the pathophysiologic mechanisms of thoracic aortic disease may open new avenues for treatment, with the goal of preserving aortic health and function.
Sex Differences in Abdominal Aortic Disease
AAA is the principal anatomic disease of the abdominal aorta. AAAs are located below the level of the diaphragm up to the iliac arteries. As with TAAs, there are important sex differences in the epidemiology, pathophysiology, outcomes, and screening considerations in AAAs. Figure 4 summarizes these findings.
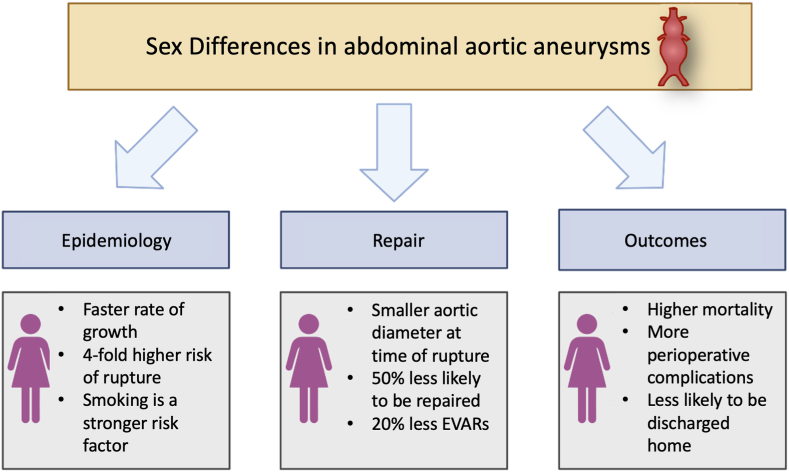
Figure 4.
Summary of the sex differences in abdominal aortic aneurysms. Despite being more common in men, women with abdominal aortic aneurysms have faster aneurysm growth rate, greater predisposition to aneurysm rupture, and rupture at smaller aneurysm size. Women have higher rates of death, worse perioperative outcomes, and higher postoperative complication rates compared with their male counterparts. EVARs, endovascular aneurysm repairs.
Epidemiology and risk factors for AAA
Similar to what is observed in TAA, abdominal aortic aneurysms are more common in men than in women.62, 63, 64 However, in women, AAAs have a faster rate of growth,65,66 up to a 4-fold higher risk of rupture,67,68 and experience rupture at smaller aneurysm diameters.69,70 Women with AAAs are, on average, 3 to 5 years older than men; have more comorbidities; and have smaller aneurysm sizes.71.As with men, hypertension, smoking, and older age are risk factors for AAAs, although smoking appears to be a stronger risk factor in women.72, 73, 74 Premature menopause and the reduction of circulating ovarian sex hormones may also contribute risk to developing AAA in women.75,76 Although only examined in a few studies, parity, use of oral contraceptive medications, previous gynecologic surgery, and breast cancer are not risk factors for development of AAAs in women.76
Sex differences in AAA biology and pathophysiology
Although the pathogenesis of AAA formation is complex and not fully understood, key histologic and pathologic characteristics include vascular inflammation, oxidative stress, degradation of elastin and collagen in the aortic wall, and loss of vascular smooth-muscle cells. Macrophage-derived MMPs and other proteases mediate the breakdown in components of the extracellular matrix of the aortic wall.77,78 The role of MMP-9 in formation of aneurysms has been observed in murine models to have sex-based differences, with lower levels of MMP-9 in female nonaneurysmal aortic smooth-muscle cells compared with male nonaneurysmal aortic smooth-muscle cells, and a reduction in plasma MMP-9 with smaller formation of aneurysms in estradiol-treated male rats compared with untreated male rats.79, 80, 81 Conversely, once an AAA has developed, a more adverse remodelling process is observed in female patients. In humans with AAAs, higher levels of MMP-9 have been found in female patients compared with male patients with equivalent AAA sizes, suggesting greater proteolytic activity of the aortic wall in women with overt AAA disease.82 These differences in protease expression may explain the observed increased rate of growth and risk of rupture in women with similarly sized aortic aneurysms.
The effect of sex hormones on the expression of extracellular matrix proteins and proteases may also explain the increased disease prevalence of AAA in men.83 Endogenous estrogen (estradiol) is generally thought to be vasoprotective in women through key antioxidant, antiapoptotic, and immunomodulatory pathways.84 In murine models, estradiol has been shown to downregulate MMP-2 in AAA tissue and slow AAA growth rates.85 In human aortic smooth-muscle cells, female sex hormones reduce collagen deposition to a greater extent than male sex hormones.9 Differences in sex hormone receptor expression are seen between in aneurysmal aortic walls compared with nonaneurysmal aortic walls.86 In another study, the density of estrogen receptor-alpha in aneurysmal aortic walls was 80% higher in samples from female patients compared with male patients.86,87 In male patients, low progesterone and estradiol levels and higher luteinizing hormone levels have been found to be associated with abdominal aortic aneurysms independently of risk factors.88,89 Finally, follicle-stimulating hormone has also been hypothesized to promote formation of AAAs, but this has not been conclusively confirmed.90 Together, these studies suggest a protective effect of female sex hormones and may explain why menopause and the resulting fall in estrogen and progesterone, may result in women losing this protective effect against formation of AAAs.75 Despite these associations, the effect of hormone replacement therapy on AAA outcomes is unclear, with some observational studies showing protective effects, whereas randomized trials have shown no consistent benefit.91,92
Sex differences in abdominal aortic biomechanical properties and vascular geometry have also been examined as potential explanations for the higher rates of growth and rupture in women with AAAs. Studies of excised aortic tissue at the time of open AAA repair demonstrate lower aortic tissue stiffness, lower dry weight percentages of collagen, a significant lower energy required to propagate a dissection in female patients compared with male patients,93 and a trend toward lower tensile strength.94 Other studies using computer and computerized tomography based geometric modelling have found increased peak wall stress, and wall stress/strength ratios in female compared with male subjects.95,96 AAAs in women more often have proximal angulation > 60˚, shorter and more diseased aortic necks, and smaller iliac arteries, which are unfavourable to endovascular repair.97 Elevated aortic stiffness is observed in patients with AAAs and is a risk factor for AAA progression and rupture.98,99 Although, in general, premenopausal women have less aortic stiffness, aortic stiffness increases in the postmenopausal stage and is compounded by smoking.100
Sex differences in AAA outcomes
Published data from large national registries consistently show significantly worse outcomes for women with AAAs. Although global mortality from AAAs is falling in both men and women, possibly related to decreased rates of smoking, women with AAAs still have lower survival compared with men with AAAs.101,102 In a large 15-year national registry of 16,040 patients followed after elective endovascular aneurysm repair, 5-year survival was found to be significantly lower among White and Black women compared with White men (hazard ratio [HR], 1.2; 95% confidence interval [CI], 1.2-1.3 and HR, 1.4; 95% CI, 1.1-1.8, respectively).103 Female sex has also been independently associated with significantly worse 30-day survival after elective repair after adjusting for repair type, age, aneurysm diameter, and comorbidities, but not aortic size index (odds ratio [OR],1.7; 95% CI, 1.1-2.6; P = 0.02).104
Global data demonstrates that women presenting with AAAs are 20% less likely to be deemed suitable for endovascular repair, and are nearly one-half as likely to be offered any repair.102,105 Women undergoing intact endovascular aneurysm repair suffer more major complications, intraoperative arterial complications, have longer hospital stays, and a lower likelihood of being discharged home.71,106 Operative mortality for either endovascular or open repair is higher in women.64,102,107 Following either endovascular or open repair of AAA, women continue to have significantly lower survival and increased incidence of recurrent formation and rupture of aneurysms than men.108 Recent data from a large North American registry found in-hospital mortality for ruptured aneurysm was 34.4% in women vs 26.6% in men: a difference that persisted despite adjustment for demographic, clinical, and procedural characteristics.107 Eight-year survival was 36.7% in women compared with 49.5% in men. These worse short-term and long-term outcomes in women has persisted from the early 2000s, when open repair of AAAs was most common, to more recently as endovascular aneurysm repair has become first line if feasible.109
AAA screening and management guidelines
The recent 2022 American College of Cardiology/American Heart Association guidelines now contain recommendations for ultrasound screening women ≥ 65 with first-degree relatives with AAAs (Class 1 Recommendation), or who have ever smoked (Class 2a Recommendation),51 whereas the 2019 European Society of Vascular Surgery guidelines continue to recommend against population screening for women without family histories.110 The Canadian Cardiovascular Society, Canadian Society of Vascular Surgery, and Society of Vascular Surgery recommend that women aged 65 to 80 years with histories of smoking or cardiovascular disease, and women aged > 55 with affected first-degree relatives, should be screened once for AAAs.111 The current definition of aortic aneurysm (> 3.0 cm) is sex-neutral, despite the smaller diameter of the aorta in women by 2 to 6 mm.106 One study found that if a threshold of the median aortic diameter plus 2 standard deviations is used to define AAA, the diameter for diagnosis would be 3.0 cm in male patients but 2.7 cm in female patients.112 As such, AAA may be underdiagnosed in women because of the absence of a sex-specific threshold.106
Current Society of Vascular Surgery Guidelines suggest elective AAA repair in women with aneurysms between 5.0 cm and 5.4 cm in diameter.113 Similarly, the European Society for Vascular Surgery suggests consideration of repair in women with aortic diameters of > 5.0 cm, but these recommendations are of weaker strength. A recent analysis of the North American Vascular Quality Initiative database of more than 55,000 patients with AAA (20% female), found that of female patients undergoing rupture repair, 14% had aneurysm diameters at or below 5 cm, compared with 12% of male patients, suggesting overall that current thresholds for elective repair may still miss a significant proportion of patients.
Future directions
Although advancements have been made in understanding sex differences in abdominal aortic disease, further research is needed to elucidate the interplay among hormonal influences, genetic factors, and environmental determinants in the context of AAA pathogenesis. Future directions include embracing larger and diverse cohorts in longitudinal studies, improving modelling of AAA fluid dynamics and geometry with advanced imaging modalities, and incorporation of artificial intelligence in large data sets to better understand risk factors for aneurysmal growth and dissection. These will allow more personalized treatment strategies, including medical therapy and timing of intervention, and—it is hoped—improve AAA outcomes in both men and women.
Special Considerations in Pregnant Women With Aortopathy
The physiological changes of pregnancy, particularly the increase in cardiac output, as well as the pregnancy-related, hormone-mediated changes to the composition of vascular beds, including fragmentation of the tunica media by increased metalloproteinases, contribute to aortic dilatation and increased risk of aortic dissection in women at risk.114 Normal pregnancy is associated with a small increase in aortic diameter, which is more pronounced in the setting of hypertension and is most significant toward the end of the third trimester.115 Aortic dissection in pregnancy is rare and occurs in 0.0004% of pregnancies.116 Type A aortic dissection during pregnancy carries high mortality: up to 70%. Type B aortic dissection during pregnancy may occur despite normal aortic diameters, and its associated mortality is approximately 9%.114 Identifying women at risk is of paramount importance, and individualized risk assessment guides counselling, monitoring, and intervention from preconception to late postpartum care and beyond. The “big 6” aortic conditions to be aware of include syndromic entities, namely MFS, Loeys-Dietz syndrome, and vascular-subtype Ehlers-Danlos syndrome, as well as nonsyndromic hereditary thoracic aortopathy, BAV aortopathy, and TS.117 Risk of pregnancy-associated aortic dissection varies but is generally considered to be 1% to 10% in these conditions and largely driven by aortic diameter (except for BAV, in which risk is usually less than 1%).116 Genes responsible for hereditary aortopathies involve important elements of the vascular infrastructure, including extracellular matrix elements such as microfibrils, vascular smooth-muscle cells, and their associated signalling pathways.118 Importantly, many women enter pregnancy without established aortopathy diagnoses. As such, health care providers must inquire vigilantly about malignant family history of aortic disease, including history of aortic aneurysms, dissection, or sudden death. These conditions may put patients at higher risk and warrant preconception, or at least antepartum evaluation of the aorta by imaging. Contemporary diagnosis and management of aortopathies involve genotyping; however, the presence of a pathogenic mutation is not necessary or sufficient to classify a patient as high or low risk for aortic complications during pregnancy. Indeed, women with strong family histories remain at high risk despite absence of pathogenic mutations, and those with known pathogenic variants remain at high risk even in the absence of aortic abnormalities on imaging. This highlights the importance of combining comprehensive vascular imaging with individualized risk assessment.114 Secondary causes of aortic dissection during pregnancy include trauma, infection, and drugs such as cocaine.
Multidisciplinary care is required and begins with the identification of high-risk individuals and preconception counselling. Avoidance of pregnancy is recommended for women with histories of aortic dissection and vascular-subtype Ehlers-Danlos syndrome (which not only carries risk of arterial dissection but also hollow viscus rupture including uterine rupture). Pregnancy should also be avoided in the setting of severe ascending aorta dilatation, and preconception aortic surgery should be offered if pregnancy is desired, depending on the underlying condition and the aortic diameter as outlined in Figure 5.51,117,119 Prepregnancy comprehensive imaging of the aorta can be achieved with transthoracic echocardiography, computed tomography, or magnetic resonance imaging.117 Imaging of other vascular beds may be indicated depending on the underlying suspected or confirmed etiology. Condition-specific cutoffs of aortic diameter exist to guide indications and timing of surgical intervention (Figure 5).51,117 All patients with known aortopathy must be counselled on the risk of dissection and may be offered preimplantation genetic diagnosis, as most familial aortopathies are of autosomal dominant inheritance.114 Surveillance imaging of the aortic root and ascending aorta during pregnancy is best done by serial maternal transthoracic echocardiography at least every trimester (at 4- to 12-week intervals, depending on the pathology and presentation); if comprehensive aortic assessment is needed, magnetic resonance imaging without gadolinium is preferred.119 In the setting of known aortopathy, important considerations include aortic diameter, rate of progression, family history of dissection, and coexisting aortic regurgitation and hypertension.
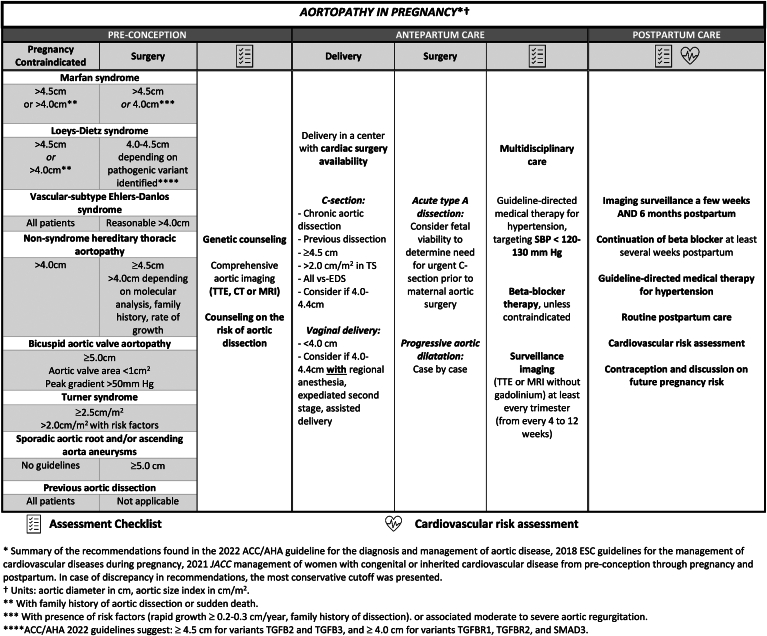
Figure 5.
Aortopathy in pregnancy. Women of childbearing age contemplating pregnancy who are at risk or have known aortopathy require multidisciplinary care from preconception to postpartum period and beyond. Pregnancy is contraindicated with severe aortic dilatation, and preconception aortic surgery may be offered. Antepartum aortic surveillance imaging is important, and delivery planning should account for aortic diameter. Beta blockade and blood pressure control are essential. Postpartum surveillance imaging and cardiovascular risk assessment is warranted. ACC/AHA, American College of Cardiology/American Heart Association; CT, computed tomography; ESC, European Society of Cardiology; JACC, Journal of the American College of Cardiology; TTE, transthoracic echocardiography; MRI, magnetic resonance imaging.
Preconception “prophylactic” aortic surgery may be considered, particularly in the presence of aortic diameter > 45 mm or rapid progression on serial imaging, along with appropriate counselling, as it does not eliminate the risk of aortic complications, given that dissections may occur distally or in other vascular beds.51,117 If semielective cardiac surgery is needed during pregnancy, the second trimester is thought to be “safer” compared with the first trimester, when organogenesis may be severely compromised during cardiopulmonary bypass, and compared with the third trimester, when peak hemodynamic changes make it a high-risk period overall.51,115 In the event of a type A aortic dissection during pregnancy with fetal viability, it is suggested to proceed with urgent delivery via cesarean section first, immediately followed by maternal cardiac surgery. If cardiac surgery is required before viability, careful preoperative planning is required, and intraoperative strategies to minimize fetal compromise may be employed (such as near-normothermia, pulsatile flow of bypass machine, close fetal monitoring) while prioritizing maternal well-being.115 Type B aortic dissections are best managed medically and with endovascular repair in the event of complications.51 As with many—if not all—cardiovascular complications of pregnancy, the risk to the mother persists after delivery. Indeed, dissection risk is highest in third trimester and early postpartum period, and surveillance imaging should be done after delivery and several weeks postpartum to monitor aortic diameter.
Blood pressure control is essential, and current guidelines support a target of 120 to 130 mm Hg of systolic blood pressure or less.114 Beta blockers were first shown to decrease the rates of aortic growth in MFS in the 1990s, and since then they have been widely prescribed in syndromic aortopathies, despite lack of condition-specific evidence of benefit.118 Labetalol is often the agent of choice for control of blood pressure and mitigation of aortic dilation during pregnancy. Although beta blockers have been associated with fetal growth restriction, recent data from the Registry of Pregnancy and Cardiac Disease did not yield significant results.51 Beta blockers should be continued postpartum for at least several weeks, as risk of dissection remains high in early postpartum period. Of note, calcium channel blockers have evidence of adverse aortic remodelling and increased dissection risk in MFS and should be avoided in all familial aortopathies.118
Moreover, delivery planning is important and should involve obstetricians, cardiologists, anaesthesiologists, cardiac surgeons, and possibly pediatric and neonatal intensivists. If the ascending aorta diameter is below 40 mm, vaginal delivery is favoured. Beyond 45 mm, or in the setting of chronic dissection, cesarean section is favoured, and women should be delivered in facilities with access to cardiac surgery. Placement of an arterial line may be considered for close blood pressure monitoring. For women with aortic diameters between 40 mm and 45 mm, a vaginal delivery with regional anaesthesia, expedited second stage of labour, and assisted delivery may be considered.51
A collaborative, patient-centred, multidisciplinary approach is required when caring for women with aortopathy from preconception to the late postpartum period. Preconception counselling and serial follow-up should be done jointly by obstetricians, cardiologists, and may also necessitate early input from specialists in genetics, cardiac surgery, and anaesthesia, as described in a recent review on pregnancy and thoracic aortic disease published in the Canadian Journal of Cardiology.120 The European Society of Cardiology provides a Class I recommendation for delivery of care for these women at an experienced centre, with a pregnancy heart team and access to cardiothoracic surgery.117 The feasibility and benefit of a pregnancy heart team was illustrated by the prospective, single-centre Standardized Outcomes in Reproductive Cardiovascular Care (STORCC) initiative, carried out at the Brigham and Women’s Hospital in Boston.121 In this study, pregnant women with cardiovascular disease were serially followed during pregnancy, and a monthly multidisciplinary meeting took place in which each participant’s cardiovascular risk was evaluated and assigned a colour code, which would then allow to streamline the required testing, frequency of follow-up, and delivery considerations. The assigned risk colour code was strongly correlated with maternal outcomes. Since then, a few studies have provided insights into the logistic considerations and required building blocks to create a strong pregnancy heart team,122,123 and we can expect further publications on the implementation and evaluation of such teams in the years to come.
Future directions
Recognizing malignant family history, appropriate detection of familial aortopathy, and individualized risk stratification is necessary to prevent aortic complications during pregnancy and in the postpartum period and requires a patient-centred, multidisciplinary approach. Implementation of pregnancy heart teams in tertiary-care institutions should be pursued and prospectively evaluated for its impact on maternal and offspring outcomes, cost effectiveness, as well as patient satisfaction.
Conclusions
In this review, we sought to explore female-specific consideration in aortic health and disease. We highlighted the influence of sex on accelerated and pathologic vascular aging. Although women initially benefit from the vasculo-protective effects of estrogen, menopause leads to increased endothelial dysfunction and arterial stiffening. Women entering menopause experience accelerated arterial stiffness and pulsatile arterial load, which are risk factors for cardiovascular disease. We reviewed how the abnormalities in arterial function are highly relevant to female-specific or female-predominant diseases including hypertensive disorders of pregnancy, HFpEF, and coronary microvascular dysfunction. We also discussed the notable sex differences in aneurysmal diseases of the thoracic and abdominal aorta. Although TAAs and AAAs are more prevalent in men, women have worse outcomes. For thoracic aortic aneurysms in particular, alterations in the health and function of the aorta likely contribute to the worse outcomes seen in women. Finally, we discussed aortic diseases in pregnancy. Pregnancy is a high-risk period for women with aortopathy because of the physiological changes in pregnancy, combined with hormone-mediated alterations to vascular composition, increasing the risk of acute aortic syndromes. Preconception counselling, surveillance imaging, blood pressure control, and careful delivery planning are essential components of a multidisciplinary approach to manage and reduce the risk of aortic complications during pregnancy and the postpartum period.
Acknowledgments
Ethics Statement
The research reported adhered to the relavent ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a review article with no patient data or information.
Funding Sources
No funding was provided for this paper.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 403 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.09.006.
Supplementary Material
References
- 1.Kazzi B., Shankar B., Elder-Odame P., Tokgözoğlu L.S., Sierra-Galan L.M., Michos E.D. A woman’s heart: improving uptake and awareness of cardiovascular screening for middle-aged populations. Int J Womens Health. 2023;15:1171–1183. doi: 10.2147/IJWH.S328441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins J.A., Munoz J.V., Patel T.R., Loukas M., Tubbs R.S. The anatomy of the aging aorta. Clin Anat. 2014;27:463–466. doi: 10.1002/ca.22384. [DOI] [PubMed] [Google Scholar]
- 3.Liberale L., Badimon L., Montecucco F., Lüscher T.F., Libby P., Camici G.G. Inflammation, aging, and cardiovascular disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:837–847. doi: 10.1016/j.jacc.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammoto A., Matus K., Mammoto T. Extracellular matrix in aging aorta. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.822561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahimastos A.A., Formosa M., Dart A.M., Kingwell B.A. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab. 2003;88:5375–5380. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 7.Yeap B.B. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202. doi: 10.1097/MED.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 8.Ji H., Kwan A.C., Chen M.T., et al. Sex differences in myocardial and vascular aging. Circ Res. 2022;130:566–577. doi: 10.1161/CIRCRESAHA.121.319902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natoli A.K., Medley T.L., Ahimastos A.A., et al. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension. 2005;46:1129–1134. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 10.Somani Y.B., Pawelczyk J.A., De Souza M.J., Kris-Etherton P.M., Proctor D.N. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol. 2019;317:H395–H404. doi: 10.1152/ajpheart.00430.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30:756–764. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho T., Borlaug B.A., Pellikka P.A., Turner S.T., Kullo I.J. Sex Differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins P., Maas A., Prasad M., Schierbeck L., Lerman A. Endothelial vascular function as a surrogate of vascular risk and aging in women. Mayo Clin Proc. 2020;95:541–553. doi: 10.1016/j.mayocp.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Vogel B., Acevedo M., Appelman Y., et al. The Lancet Women and Cardiovascular Disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 15.Tamariz-Ellemann A., Wickham K.A., Nørregaard L.B., Gliemann L., Hellsten Y. The time is now: regular exercise maintains vascular health in ageing women. J Physiol. 2023;601:2085–2098. doi: 10.1113/JP282896. [DOI] [PubMed] [Google Scholar]
- 16.Poon L.C., Nguyen-Hoang L., Smith G.N., et al. Hypertensive disorders of pregnancy and long-term cardiovascular health: FIGO Best Practice Advice. Int J Gynecol Obstet. 2023;160:22–34. doi: 10.1002/ijgo.14540. [DOI] [PubMed] [Google Scholar]
- 17.Garovic V.D., White W.M., Vaughan L., et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira M.M., Torrado J., Sosa C., Zócalo Y., Bia D. Role of arterial impairment in preeclampsia: should the paradigm shift? Am J Physiol Heart Circ Physiol. 2021;320:H2011–H2030. doi: 10.1152/ajpheart.01005.2020. [DOI] [PubMed] [Google Scholar]
- 19.Phan K., Gomez Y.H., Gorgui J., et al. Arterial stiffness for the early prediction of pre-eclampsia compared with blood pressure, uterine artery Doppler and angiogenic biomarkers: a prospective cohort study. BJOG. 2023;130:932–940. doi: 10.1111/1471-0528.17430. [DOI] [PubMed] [Google Scholar]
- 20.Metoki H., Iwama N., Hamada H., et al. Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement. Hypertens Res. 2022;45:1298–1309. doi: 10.1038/s41440-022-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Countouris M.E., Villanueva F.S., Berlacher K.L., Cavalcante J.L., Parks W.T., Catov J.M. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77:1057–1068. doi: 10.1016/j.jacc.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werlang A., Paquin A., Coutinho T. The EVA study: early vascular aging in women with history of preeclampsia. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Countouris M.E., Koczo A., Reynolds H.R., et al. Characteristics of premature myocardial infarction among women with prior adverse pregnancy outcomes. JACC Adv. 2023;2 doi: 10.1016/j.jacadv.2023.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho J.E., Gona P., Pencina M.J., et al. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33:1734–1741. doi: 10.1093/eurheartj/ehs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coutinho T., Pellikka P.A., Kullo I.J. Sex differences in the associations of arterial hemodynamic load with left ventricular hypertrophy and remodeling. Eur Heart J. 2013;34:P4140. doi: 10.1093/ajh/hpv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy Y.N.V., Andersen M.J., Obokata M., et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau E.S., Panah L.G., Zern E.K., et al. arterial stiffness and vascular load in HFpEF: differences among women and men. J Card Fail. 2022;28:202–211. doi: 10.1016/j.cardfail.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beale A.L., Nanayakkara S., Segan L., et al. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail. 2019;7:239–249. doi: 10.1016/j.jchf.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Crosier R., Paquin A., Zhu T., et al. Sex differences in systemic and coronary arterial hemodynamics in heart failure with preserved ejection fraction. Am J Cardiol. 2023;205:87–93. doi: 10.1016/j.amjcard.2023.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Sara J.D., Widmer R.J., Matsuzawa Y., Lennon R.J., Lerman L.O., Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Smilowitz N.R., Toleva O., Chieffo A., Perera D., Berry C. coronary microvascular disease in contemporary clinical practice. Circ Cardiovasc Interv. 2023;16 doi: 10.1161/CIRCINTERVENTIONS.122.012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shome J.S., Perera D., Plein S., Chiribiri A. Current perspectives in coronary microvascular dysfunction. Microcirculation. 2017;24 doi: 10.1111/micc.12340. [DOI] [PubMed] [Google Scholar]
- 33.Bozbas H., Pirat B., Yildirir A., et al. Coronary microvascular function in patients with isolated systolic and combined systolic/diastolic hypertension. J Clin Hypertens. 2012;14:871–876. doi: 10.1111/j.1751-7176.2012.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell G.F., Rong J., Larson M.G., et al. longitudinal hemodynamic correlates of and sex differences in the evolution of blood pressure across the adult lifespan: the Framingham Heart Study. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coutinho T., Mielniczuk L.M., Srivaratharajah K., deKemp R., Wells G.A., Beanlands R.S. Coronary artery microvascular dysfunction: role of sex and arterial load. Int J Cardiol. 2018;270:42–47. doi: 10.1016/j.ijcard.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 36.Lodewyks C.L., Prior H.J., Hiebert B.M., et al. A province-wide analysis of the epidemiology of thoracic aortic disease: incidence is increasing in a sex-specific way. Can J Cardiol. 2020;36:1729–1738. doi: 10.1016/j.cjca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Olsson C., Thelin S., Ståhle E., Ekbom A., Granath F. Thoracic aortic aneurysm and dissection. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 38.Cheung K., Boodhwani M., Chan K., Beauchesne L., Dick A., Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies R.R., Goldstein L.J., Coady M.A., et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–28. doi: 10.1016/s0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 40.Nienaber C.A., Fattori R., Mehta R.H., et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 41.McClure R.S., Brogly S.B., Lajkosz K., Payne D., Hall S.F., Johnson A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: a population-based study. J Thorac Cardiovasc Surg. 2018;155:2254–2264.e4. doi: 10.1016/j.jtcvs.2017.11.105. [DOI] [PubMed] [Google Scholar]
- 42.Chung J., Stevens L.M., Ouzounian M., et al. Sex-related differences in patients undergoing thoracic aortic surgery. Circulation. 2019;139:1177–1184. doi: 10.1161/CIRCULATIONAHA.118.035805. [DOI] [PubMed] [Google Scholar]
- 43.Kuzmik G.A., Sang A.X., Elefteriades J.A. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. doi: 10.1016/j.jvs.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 44.Gillis E., Van Laer L., Loeys B.L. Genetics of thoracic aortic aneurysm. Circ Res. 2013;113:327–340. doi: 10.1161/CIRCRESAHA.113.300675. [DOI] [PubMed] [Google Scholar]
- 45.Roman M.J., Devereux R.B., Preiss L.R., et al. Associations of age and sex with Marfan phenotype. Circ Cardiovasc Genet. 2017;10 doi: 10.1161/CIRCGENETICS.116.001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meijboom L.J., Timmermans J., Zwinderman A.H., Engelfriet P.M., Mulder B.J.M. Aortic root growth in men and women with the Marfan’s syndrome. Am J Cardiol. 2005;96:1441–1444. doi: 10.1016/j.amjcard.2005.06.094. [DOI] [PubMed] [Google Scholar]
- 47.Sachdev V., Matura L.A., Sidenko S., et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol. 2008;51:1904–1909. doi: 10.1016/j.jacc.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Kong W.K.F., Regeer M.V., Ng A.C.T., et al. Sex differences in phenotypes of bicuspid aortic valve and aortopathy. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005155. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Chen X., Qi Y., et al. Gender differences in bicuspid aortic valve Sievers types, valvulopathy, aortopathy, and outcome of aortic valve replacement. Echocardiography. 2022;39:1064–1073. doi: 10.1111/echo.15405. [DOI] [PubMed] [Google Scholar]
- 50.Lo R.C., Schermerhorn M.L. Abdominal aortic aneurysms in women. J Vasc Surg. 2016;63:839–844. doi: 10.1016/j.jvs.2015.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isselbacher E.M., Preventza O., Hamilton Black J., et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334–e482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beller C.J., Farag M., Wannaku S., et al. Gender-specific differences in outcome of ascending aortic aneurysm surgery. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trimarchi S., Jonker F.H.W., Froehlich J.B., et al. Acute type B aortic dissection in the absence of aortic dilatation. J Vasc Surg. 2012;56:311–316. doi: 10.1016/j.jvs.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 54.Davies R.R., Gallo A., Coady M.A., et al. novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Masri A., Kalahasti V., Svensson L.G., et al. aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–1737. doi: 10.1161/CIRCULATIONAHA.116.022995. [DOI] [PubMed] [Google Scholar]
- 56.Zafar M.A., Li Y., Rizzo J.A., et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. doi: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell G.F., Wang N., Palmisano J.N., et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boczar K.E., Cheung K., Boodhwani M., et al. Sex differences in thoracic aortic aneurysm growth. Hypertension. 2019;73:190–196. doi: 10.1161/HYPERTENSIONAHA.118.11851. [DOI] [PubMed] [Google Scholar]
- 59.Humphrey J.D., Schwartz M.A., Tellides G., Milewicz D.M. Role of mechanotransduction in vascular biology. Circ Res. 2015;116:1448–1461. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokolis D.P., Iliopoulos D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J Mech Behav Biomed Mater. 2014;34:154–164. doi: 10.1016/j.jmbbm.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Dingemans K.P., Teeling P., Lagendijk J.H., Becker A.E. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14. doi: 10.1002/(SICI)1097-0185(20000101)258:1<1::AID-AR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Tang W., Yao L., Roetker N.S., et al. lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the ARIC study (Atherosclerosis Risk in Communities) Arterioscler Thromb Vasc Biol. 2016;36:2468–2477. doi: 10.1161/ATVBAHA.116.308147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X., Zhao G., Zhang J., Duan Z., Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population:a meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz D.J., Stanley J.C., Zelenock G.B. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25:561–568. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 65.Mofidi R., Goldie V.J., Kelman J., Dawson A.R., Murie J.A., Chalmers R.T. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94:310–314. doi: 10.1002/bjs.5573. [DOI] [PubMed] [Google Scholar]
- 66.Solberg S., Singh K., Wilsgaard T., Jacobsen B.K. Increased growth rate of abdominal aortic aneurysms in women: the Tromsø study. Eur J Vasc Endovasc Surg. 2005;29:145–149. doi: 10.1016/j.ejvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 67.RESCAN collaborators. Sweeting M.J., Thompson S.G., Brown L.C., et al. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 68.Talvitie M., Stenman M., Roy J., Leander K., Hultgren R. Sex differences in rupture risk and mortality in untreated patients with intact abdominal aortic aneurysms. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo R.C., Lu B., Fokkema M.T., et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59:1209–1216. doi: 10.1016/j.jvs.2013.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skibba A.A., Evans J.R., Hopkins S.P., et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429–1436. doi: 10.1016/j.jvs.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 71.Lo R.C., Bensley R.P., Hamdan A.D., Wyers M., Adams J.E., Schermerhorn M.L. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg. 2013;57:1261–1268.e1-5. doi: 10.1016/j.jvs.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stackelberg O., Bjorck M., Larsson S.C., Orsini N., Wolk A. Sex differences in the association between smoking and abdominal aortic aneurysm. Br J Surg. 2014;101:1230–1237. doi: 10.1002/bjs.9526. [DOI] [PubMed] [Google Scholar]
- 73.Rodin M.B., Daviglus M.L., Wong G.C., et al. Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension. 2003;42:61–68. doi: 10.1161/01.HYP.0000078829.02288.98. [DOI] [PubMed] [Google Scholar]
- 74.Lederle F.A., Johnson G.R., Wilson S.E. Aneurysm Detection Management Veterans Affairs Cooperative Study. Abdominal aortic aneurysm in women. J Vasc Surg. 2001;34:122–126. doi: 10.1067/mva.2001.115275. [DOI] [PubMed] [Google Scholar]
- 75.Chou E.L., Pettinger M., Haring B., et al. Association of premature menopause with risk of abdominal aortic aneurysm in the Women’s Health Initiative. Ann Surg. 2022;276:e1008–e1016. doi: 10.1097/SLA.0000000000004581. [DOI] [PubMed] [Google Scholar]
- 76.Villard C., Swedenborg J., Eriksson P., Hultgren R. Reproductive history in women with abdominal aortic aneurysms. J Vasc Surg. 2011;54:341–345.e1-2. doi: 10.1016/j.jvs.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 77.Howatt D.A., Dajee M., Xie X., et al. Relaxin and matrix metalloproteinase-9 in angiotensin ii-induced abdominal aortic aneurysms. Circ J. 2017;81:888–890. doi: 10.1253/circj.CJ-17-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabkin S.W. The role matrix metalloproteinases in the production of aortic aneurysm. Prog Mol Biol Transl Sci. 2017;147:239–265. doi: 10.1016/bs.pmbts.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Woodrum D.T., Ford J.W., Ailawadi G., et al. Gender differences in rat aortic smooth muscle cell matrix metalloproteinase-9. J Am Coll Surg. 2005;201:398–404. doi: 10.1016/j.jamcollsurg.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Ailawadi G., Eliason J.L., Roelofs K.J., et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24:2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 81.Grootenboer N., Bosch J.L., Hendriks J.M., van Sambeek M.R. Epidemiology, aetiology, risk of rupture and treatment of abdominal aortic aneurysms: does sex matter? Eur J Vasc Endovasc Surg. 2009;38:278–284. doi: 10.1016/j.ejvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Villard C., Wågsäter D., Swedenborg J., Eriksson P., Hultgren R. Biomarkers for abdominal aortic aneurysms from a sex perspective. Gend Med. 2012;9:259–266.e2. doi: 10.1016/j.genm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Makrygiannis G., Courtois A., Drion P., Defraigne J.O., Kuivaniemi H., Sakalihasan N. Sex differences in abdominal aortic aneurysm: the role of sex hormones. Ann Vasc Surg. 2014;28:1946–1958. doi: 10.1016/j.avsg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Yang X.P., Reckelhoff J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:133–138. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X.F., Zhang J., Paskauskas S., Xin S.J., Duan Z.Q. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg. 2009;197:49–54. doi: 10.1016/j.amjsurg.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 86.Villard C., Eriksson P., Kronqvist M., et al. Differential expression of sex hormone receptors in abdominal aortic aneurysms. Maturitas. 2017;96:39–44. doi: 10.1016/j.maturitas.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Laser A., Ghosh A., Roelofs K., et al. Increased estrogen receptor alpha in experimental aortic aneurysms in females compared with males. J Surg Res. 2014;186:467–474. doi: 10.1016/j.jss.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohlsson C., Langenskiöld M., Smidfelt K., et al. low progesterone and low estradiol levels associate with abdominal aortic aneurysms in men. J Clin Endocrinol Metab. 2022;107:e1413–e1425. doi: 10.1210/clinem/dgab867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeap B.B., Hyde Z., Norman P.E., Chubb S.A.P., Golledge J. associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab. 2010;95:1123–1130. doi: 10.1210/jc.2009-1696. [DOI] [PubMed] [Google Scholar]
- 90.Tedjawirja V.N., Nieuwdorp M., Yeung K.K., Balm R., de Waard V. A novel hypothesis: a role for follicle stimulating hormone in abdominal aortic aneurysm development in postmenopausal women. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsia J., Criqui M.H., Herrington D.M., et al. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J. 2006;152:170–176. doi: 10.1016/j.ahj.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Hsia J., Criqui M.H., Rodabough R.J., et al. estrogen plus progestin and the risk of peripheral arterial disease. Circulation. 2004;109:620–626. doi: 10.1161/01.CIR.0000115309.63979.92. [DOI] [PubMed] [Google Scholar]
- 93.Tong J., Schriefl A.J., Cohnert T., Holzapfel G.A. gender differences in biomechanical properties, thrombus age, mass fraction and clinical factors of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2013;45:364–372. doi: 10.1016/j.ejvs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Vande Geest J.P., Dillavou E.D., Di Martino E.S., et al. Gender-related differences in the tensile strength of abdominal aortic aneurysm. Ann NY Acad Sci. 2006;1085:400–402. doi: 10.1196/annals.1383.048. [DOI] [PubMed] [Google Scholar]
- 95.Larsson E., Labruto F., Gasser T.C., Swedenborg J., Hultgren R. Analysis of aortic wall stress and rupture risk in patients with abdominal aortic aneurysm with a gender perspective. J Vasc Surg. 2011;54:295–299. doi: 10.1016/j.jvs.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 96.Fillinger M.F., Marra S.P., Raghavan M.L., Kennedy F.E. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37:724–732. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 97.Forbes T.L., Lawlor D.K., DeRose G., Harris K.A. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006;20:564–568. doi: 10.1007/s10016-006-9079-y. [DOI] [PubMed] [Google Scholar]
- 98.van Disseldorp E.M.J., Petterson N.J., van de Vosse F.N., van Sambeek M.R.H.M., Lopata R.G.P. Quantification of aortic stiffness and wall stress in healthy volunteers and abdominal aortic aneurysm patients using time-resolved 3D ultrasound: a comparison study. Eur Heart J Cardiovasc Imaging. 2019;20:185–191. doi: 10.1093/ehjci/jey051. [DOI] [PubMed] [Google Scholar]
- 99.Kadoglou N.P.E., Papadakis I., Moulakakis K.G., et al. Arterial stiffness and novel biomarkers in patients with abdominal aortic aneurysms. Regul Pept. 2012;179:50–54. doi: 10.1016/j.regpep.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Mozos I., Maidana J.P., Stoian D., Stehlik M. Gender differences of arterial stiffness and arterial age in smokers. Int J Environ Res Public Health. 2017;14:565. doi: 10.3390/ijerph14060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Png C.Y.M., Wu J., Tang T.Y., Png I.P.L., Sheng T.J., Choke E. Editor’s Choice – Decrease in mortality from abdominal aortic aneurysms (2001 to 2015): is it decreasing even faster? Eur J Vasc Endovasc Surg. 2021;61:900–907. doi: 10.1016/j.ejvs.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Ulug P., Sweeting M.J., von Allmen R.S., Thompson S.G., Powell J.T., SWAN Collaborators Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet. 2017;389:2482–2491. doi: 10.1016/S0140-6736(17)30639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marcaccio C.L., Patel P.B., de Guerre L., et al. Disparities in 5-year outcomes and imaging surveillance following elective endovascular repair of abdominal aortic aneurysm by sex, race, and ethnicity. J Vasc Surg. 2022;76:1205–1215.e4. doi: 10.1016/j.jvs.2022.03.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deery S.E., Soden P.A., Zettervall S.L., et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;65:1006–1013. doi: 10.1016/j.jvs.2016.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McPhee J.T., Hill J.S., Eslami M.H. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45:891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 106.Patel P.B., De Guerre L., Marcaccio C.L., et al. Sex-specific criteria for repair should be utilized in patients undergoing aortic aneurysm repair. J Vasc Surg. 2022;75:515–525. doi: 10.1016/j.jvs.2021.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li B., Eisenberg N., Witheford M., Lindsay T.F., Forbes T.L., Roche-Nagle G. Sex differences in outcomes following ruptured abdominal aortic aneurysm repair. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Guerre L., Varkevisser R.R.B., Swerdlow N.J., et al. Sex differences in perioperative outcomes after complex abdominal aortic aneurysm repair. J Vasc Surg. 2020;71:374–381. doi: 10.1016/j.jvs.2019.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bulder R.M.A., Talvitie M., Bastiaannet E., Hamming J.F., Hultgren R., Lindeman J.H.N. Long-term prognosis after elective abdominal aortic aneurysm repair is poor in women and men: the challenges remain. Ann Surg. 2020;272:773–778. doi: 10.1097/SLA.0000000000004182. [DOI] [PubMed] [Google Scholar]
- 110.Wanhainen A., Verzini F., Van Herzeele I., et al. European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 111.Varun K., Prasad J., Doug W., Vic V., Luc D. Screening for abdominal aortic aneurysms in Canada: 2020 review and position statement of the Canadian Society for Vascular Surgery. Can J Surg. 2021;64:E461. doi: 10.1503/cjs.009120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wanhainen A., Themudo R., Ahlström H., Lind L., Johansson L. Thoracic and abdominal aortic dimension in 70-year-old men and women: a population-based whole-body magnetic resonance imaging (MRI) study. J Vasc Surg. 2008;47:504–512. doi: 10.1016/j.jvs.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Chaikof E.L., Dalman R.L., Eskandari M.K., et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 114.Curtis S.L., Swan L. Aortopathy in pregnancy. Heart. 2022;108:1851. doi: 10.1136/heartjnl-2021-319828. [DOI] [PubMed] [Google Scholar]
- 115.Yates M.T., Soppa G., Smelt J., et al. Perioperative management and outcomes of aortic surgery during pregnancy. J Thorac Cardiovasc Surg. 2015;149:607–610. doi: 10.1016/j.jtcvs.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 116.Braverman A.C., Mittauer E., Harris K.M., et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021;6:58–66. doi: 10.1001/jamacardio.2020.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the Task Force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 118.Zentner D., James P., Bannon P., Jeremy R. Familial aortopathies: State of the Art Review. Genet Heart Dis. 2020;29:607–618. doi: 10.1016/j.hlc.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 119.Lindley K.J., Bairey Merz C.N., Asgar A.W., et al. Management of women with congenital or inherited cardiovascular disease from pre-conception through pregnancy and postpartum: JACC Focus Seminar 2/5. J Am Coll Cardiol. 2021;77:1778–1798. doi: 10.1016/j.jacc.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wanga S., Silversides C., Dore A., de Waard V., Mulder B. Pregnancy and thoracic aortic disease: managing the risks. Can J Cardiol. 2016;32:78–85. doi: 10.1016/j.cjca.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 121.Valente A.M., Landzberg M.J., Gauvreau K., et al. Standardized outcomes in reproductive cardiovascular care: the STORCC initiative. Am Heart J. 2019;217:112–120. doi: 10.1016/j.ahj.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 122.Easter S.R., Valente A.M., Economy K.E. Creating a multidisciplinary pregnancy heart team. Curr Treat Options Cardiovasc Med. 2020;22:3. doi: 10.1007/s11936-020-0800-x. [DOI] [PubMed] [Google Scholar]
- 123.Lucà F., Colivicchi F., Parrini I., et al. The role of the pregnancy heart team in clinical practice. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1135294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.