Abstract
Background:
Substance use and related consequences (e.g., impaired driving, injuries, disease transmission) continue to be major public health concerns. Contingency management (CM) is a highly effective treatment for substance use disorders. Yet CM remains vastly underutilized, in large part due to implementation barriers to in-person delivery. If feasible and effective, remote delivery of CM may reduce barriers at both the clinic- and patient-level, thus increasing reach and access to effective care. Here, we summarize data from a systematic review of studies reporting remote delivery of CM for substance use treatment.
Methods:
We conducted a systematic review, reported according to PRISMA guidelines. The study team identified a total of 4358 articles after deduplication. Following title and abstract screening, full-text screening, and reference tracking, 39 studies met the eligibility criteria. We evaluated the methodological quality of the included studies using the Effective Public Health Practice Project Quality tool.
Results:
Of 39 articles included in the review, most (n = 26) targeted cigarette smoking, with others focusing on alcohol (n = 9) or other substance use or targeting multiple substances (n = 4). Most remotely delivered CM studies focused on abstinence (n = 29), with others targeting substance use reduction (n = 2), intervention engagement (n = 5), and both abstinence and intervention engagement (n = 3). CM was associated with better outcomes (either abstinence, use reduction, or engagement), with increasingly more remotely delivered CM studies published in more recent years. Studies ranged from moderate to strong quality, with the majority (57.5 %) of studies being strong quality.
Conclusions:
Consistent with in-person CM, remotely delivered CM focusing on abstinence or use reduction from substances or engagement in substance use treatment services improves outcomes at the end of treatment compared to control conditions. Moreover, remotely delivered CM is feasible across a variety of digital delivery platforms (e.g., web, mobile, and wearable), with acceptability and reduced clinic and patient burden as technological advancements streamline monitoring and reinforcer delivery.
Keywords: Smoking cessation, Alcohol, Substance use, Motivational incentives, Digital health, Contingency management, Systematic review
1. Introduction
Substance use, including tobacco and alcohol use, is the leading modifiable risk factor for preventable causes of death in the United States (Danaei et al., 2009; Mokdad et al., 2004; Yoon et al., 2014). Contingency management (CM), also termed motivational incentives, is a highly effective treatment for substance use (Lussier et al., 2006; Prendergast et al., 2006). Much of the CM work in the substance use field focuses on people with substance use disorders, but CM is increasingly being used in prevention interventions aiming to minimize risk of substance use progression (e.g., Neighbors et al., 2018). CM interventions remain vastly underutilized due in a large part to implementation barriers at the clinic and patient-level (Coughlin et al., 2022; Ledgerwood, 2008; Petry, 2010; Petry et al., 2001; Roll et al., 2009). Conventional, in-person delivery of CM is staff- and resource-intensive for clinics, requiring frequent abstinence or engagement verification and administration of reinforcers. The burden is even more considerable for patients who must present in-person at a clinic frequently, often multiple times a week or daily, for abstinence verification to be considered adherent to the treatment (Benishek et al., 2014; Davis et al., 2016; Lussier et al., 2006; Prendergast et al., 2006). Technological advances have facilitated remotely delivered CM interventions using digital platforms (Getty et al., 2019). Remotely delivered CM interventions alleviate much of the treatment burden by reducing, and in some cases completely eliminating, the need for clinic visits for abstinence verification and automating reinforcer delivery. Consequently, several recent studies address the effectiveness of remotely delivered CM interventions.
Studies published using remotely delivered CM to date largely focus on alcohol use or cigarette smoking, with more recent work focused on other substance use (e.g., opioid use). These studies also often combine remotely delivered CM with a variety of other psychosocial (e.g., counseling) or pharmacological (e.g., nicotine replacement therapy) treatments. In addition, the published literature is heterogeneous with regard to remote delivery modalities (e.g., internet, phone, mobile phone), subpopulations (e.g., women in the perinatal period, adolescents and young adults, adults), and contingent behaviors (e.g., substance use abstinence, substance use reduction, and intervention engagement). Therefore, a systematic review of the extant literature across remotely delivered CM interventions addressing substance use is warranted to crystalize the existing evidence for these interventions and to identify gaps in current work and areas in need of future study.
The current study aims to systematically review and synthesize research findings on the extant support for remotely delivered CM interventions for substance use, critically evaluate the methodological quality of studies, and identify gaps in the literature and directions for future research.
2. Methods
The current review is reported to be consistent with the PRISMA systematic review guidelines (Shamseer et al., 2015), and the PRISMA checklist appears in Supplemental Table 1. The protocol and templates of data collection forms may be requested from the corresponding author. This review was not prospectively registered.
2.1. Inclusion/exclusion criteria
Inclusion criteria involved studies with the following characteristics: (1) original primary research published in a peer-reviewed journal; (2) employed a CM intervention where the monitoring of the contingent behavior (e.g., abstinence) and/or delivery of the contingent reinforcers were remote (e.g., phone, web, app, wearable); (3) used a controlled trial–either a no/delayed treatment control group or an alternative therapy control group, or controlled by repeated measures participation in two or more treatment arms; (4) reported an intervention-related outcome (e.g. abstinence, treatment engagement, reduced risky behavior); and (5) used a research design that permitted isolation of effects to the CM intervention. We excluded reviews and conference abstracts at the point of full-text review.
2.2. Search strategy
An experienced medical librarian refined the search strategy, which included studies from the time of inception of each database. We conducted searches of PubMed.gov, Elsevier Embase (including Embase Classic), Elsevier Scopus, Web of Science Core Collection (SCI-EXPANDED; SSCI; A&HCI; CPCI-S; CPCI-SSH; BKCI-S; BKCI-SSH; ESCI; CCR-EXPANDED), EBSCOhost CINAHL Complete, EBSCOhost PsycInfo on September 4, 2020, and again on December 8, 2021, to identify relevant articles. Each search utilized controlled vocabulary whenever possible supplemented with title and abstract keywords. We did not apply any limits to the search. Prior to the search, we used a set of sentinel articles to generate search terms and test the effectiveness of the strategies in each database. We developed the original search strategies in Medline with translation as appropriate to the other databases using the Systematic Review Accelerator Polyglot tool (Clark et al., 2020). We employed a modified Bramer method to de-duplicate citations (Bramer et al., 2016). We also searched the reference sections from included articles to identify additional studies.
2.3. Data screening, collection, and synthesis strategy
After duplicate study removal, two independent and blinded reviewers screened studies based on the titles and abstracts using the Covidence software platform (N.S., M.L., S.S., C.J.). We conducted full text screening for all remaining studies with data extraction occurring for all studies meeting the inclusion criteria using a predetermined data table, with blinded, independent duplicate entry (N.S., M.L., S.S., C.J.). A third reviewer (L.C.) resolved discordant reviewer ratings and data extraction. Reviewers recorded the following information for each study: 1) Publication details (first author, publication year); 2) Study characteristics (study design, retention at end of intervention, longest follow-up); 3) demographic characteristics (enrolled sample size, eligible participant ages); 4) intervention description (target behavior, type of reinforcement, reinforcement schedule, maximum possible contingent reinforcers, length of intervention, reward delivery method); and 5) study outcomes (primary outcome of CM conditions, primary outcome of control condition). If any information was missing or could not be found, reviewers recorded it as not reported (N/R). The resulting studies were heterogeneous in their study designs, sampling techniques, sample size, contingent behaviors, and measurement of outcomes.
2.4. Quality assessment of the included literature
We used the Quality Assessment Tool for Quantitative Studies (Ciliska et al., 1998) to evaluate the internal and external validity of each of the included studies with quality ratings across six dimensions (i.e., selection bias, study design, confounds, blinding, data collection, and withdrawals/dropouts). Two independent reviewers rated study quality (selected from C.J., M.L., N.S., S.S.) as strong, moderate, or weak (Supplemental Table 2). A third reviewer (L.C.) resolved discordant quality ratings.
3. Results
Of the 3644 unique study records identified in the initial search in September 2020, 54 warranted full-text review, and 29 met the inclusion criteria, with one of these articles (Jarvis & Dallery, 2017a,b) including two studies (see Fig. 1). A subsequent search in December 2021 identified 714 unique study records, with 17 included for full-text review and six meeting inclusion criteria. During reference tracking of included studies, we identified three additional articles for inclusion for a total of 38 articles reporting 39 remotely delivered CM studies.
Fig. 1.
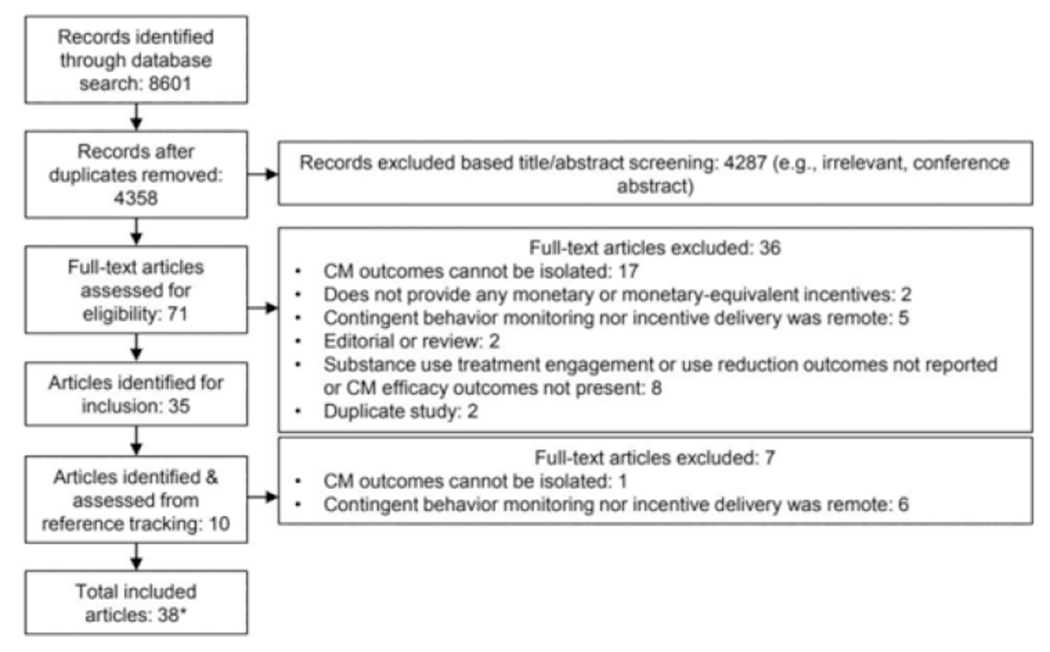
Flowchart on the literature search procedure.
* Jarvis et al. (2017) included two studies that are reported separately, for a total of 39 studies.CM = Contingency Management.
3.1. Participant characteristics
The 39 studies comprised 2333 enrolled participants with a target behavior of alcohol use, 4,281,296 enrolled participants with a target behavior of cigarette smoking, and 280 enrolled participants with a target behavior of other substance use or targeting more than one type of substance use (e.g., alcohol and opioid use). The studies included were all published between 2005 and 2021 (see Fig. 2). As Tables 1, 2, and 3 show, enrolled participants ranged in age from adolescence through older adulthood, with some studies focusing on specific subpopulations such as adolescents and pregnant women. The average percentage of female participants enrolled in all included studies is approximately 56 %.
Fig. 2.
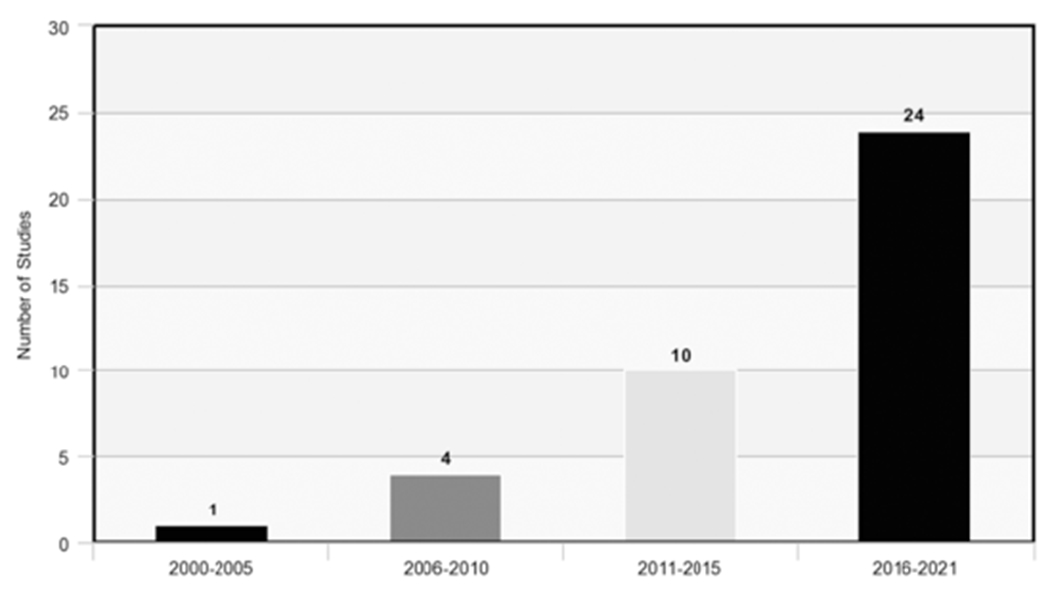
Remotely delivered contingency management studies by year published.
Table 1.
Remote contingency management studies in people who smoke cigarettes.
| Study author (year) | Design | Enrolled sample size (baseline) by condition | Target behavior | Retention at end of CM intervention | Eligible ages (mean) | Longest follow-up | Type of reinforcement | Reinforcement schedule | Maximum possible incentives for CM condition | Primary outcome: CM condition(s) Mean or % | Primary outcome: Control condition(s) Mean or % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alessi et al. (2017) | RCT | CM = 45; control = 45 | Smoking cessation | 100 % | 18+ (M = 45 %) | 24 weeks | Prizes | Escalating with reset | $502 expected value | PNS: 89.1 | PNS: 65.9 | ||||
| Anderson et al. (2018) | RCT | Counseling (n = ~3,841,826); counseling + patches (n = ~142, 148); counseling + gift card (n = ~142,148); counseling + patches + gift card (n = ~142,148); | Quitline engagement | N/A | N/R | N/A | Nicotine patches and/or gift card | 1-time constant rate | $20 gift card and/or 4 week supply of patches | % Calls: counseling + patches = 0.115; Counseling + gift card = 0.122; counseling + patches + gift card = 0.2 | % Calls: 0.029 | ||||
| Dallery (2005) | WS | Enrolled sample (n = 4) | Smoking cessation | 100 % | 18–60 (M = 39) | None | Vouchers | Escalating with reset | $171.50 | % CO reduction: 61.5 | N/A | ||||
| Dallery et al. (2007) | WS | Enrolled sample (n = 26) | Smoking cessation | 76.92 % | 18–60 (M = 38.5) | N/R | Vouchers | Shaping and thinning were constant rate; abstinence was escalating with reset | $171.50 | Significant decreases in CO, along with sustained periods of abstinence, were observed during the treatment phase relative to baseline | |||||
| Dallery et al. (2013) | RCT | CM = 39; control = 38 | Smoking cessation | N/R | 18–60 (M = 39.7) | 6 months | Vouchers | Tapering was constant rate; abstinence induction and thinning were escalating with reset | $530 | PNS: 66.7 | PNS: 25 | ||||
| Dallery et al. (2021) | WS | Enrolled sample (n = 14) | Smoking cessation | 78.6 % | 18–65 (M = 56.1) | None | Reloadable debit card | Escalating and escalating with reset | N/R | PNS: 89 | PNS: 4 | ||||
| Dan et al. (2016) | WS | Enrolled sample (n = 5) | Smoking cessation | 60 % (n = 3/5) | 18+ (M = 35.67) | 1 week | Vouchers | Tapering and thinning were constant rate; abstinence induction was escalating with reset | $171.50 | PNS tapering: 42, PNS abstinence induction: 53, PNS thinning: 42 | PNS baseline: 3 | ||||
| Glenn (2007) | WS | Enrolled sample (n = 15) | Smoking cessation | 93.33 % | 18–60 (M = 36.64) | N/R | Vouchers | Escalating with reset | $56.25 | PNS: 24.4 | PNS: 7.9 | ||||
| Halpern et al. (2018) | RCT | Usual care (n = 813); free cessation aids (n = 1588); free e-cigarettes (n = 1199); reward incentives + free cessation aids (n = 1198); redeemable deposits + free cessation aids (n = 1208) | Smoking cessation | N/R | 18+ (M = 43.98) | 6 months | Monetary deposit | Escalating | $600 | SSA: 2.0 % rewards, 2.9 % redeemable deposit | SSA: 0.1 % usual-care, 0.5 % free cessation aids, 1.0 % free e-cigarettes | ||||
| Harris (2015) | RCT | CM = 7; control = 10 | Smoking cessation | 100 % | Pregnant people 18+ (M = 24) | 2 follow-up sessions during remainder of pregnancy | Vouchers | Escalating with reset | N/R | % abstinent: 28.57 | % abstinent: 30 | ||||
| Harvanko et al. (2020) | RCT | CM = 87; control = 80 | Smoking cessation | N/R | Adolescents (M = 16.9) | 6 months | Vouchers | Escalating with reset | $875.25 | CO reduction from baseline to return to baseline: 5.0 ppm | CO reduction from baseline to return to baseline: 1.4 ppm | ||||
| Hertzberg et al. (2013) | RCT | CM = 11; control = 11 | Smoking cessation | 90.9 % | (M = 47.9) | 3 months | Monetary | Escalating with reset | $530 | QR at 4 weeks: 82 | QR at 4 weeks: 45 | ||||
| Jarvis (2017) | WS | Enrolled sample (n = 9) | Smoking cessation | 100.00 % | 18–60 (M = 41.22) | N/R | Monetary deposit | Constant rate | Based on deposit amount | PNS: 47 | PNS: 1 | ||||
| Jarvis (2017) | WS | Enrolled sample (n = 10) | Smoking cessation | 90 % | 18–60 (M = 39.9) | N/R | Monetary deposit + voucher | Constant rate | Based on deposit amount | PNS: 41.5 | PNS: 0 | ||||
| Koslovsky et al. (2018) | RCT | CM = 75; control = 71 | Smoking cessation | 97.26 % | 18+ (M = 52.3) | 4 weeks | N/R | N/R | N/R | Individuals in financial incentives group had decreased odds of smoking compared to those in usual care beginning 3 days before quit attempt and continuing throughout first week post-quit. | |||||
| Kurti et al. (2020) | N-RCT | CM = 31; control = 30 | Smoking cessation | 97 % | 18+ (M = 30.4) | 3 months | Gift card | Escalating with reset | $1620.00 | 7-day point prevalence abstinence: 36.7 | 7-day point prevalence abstinence: 10 | ||||
| Martner (2019) | WS | Enrolled sample (n = 12) | Smoking cessation | 83.33 % | 18–65 (M = 37.5) | N/A | Monetary vouchers | Escalating with reset | Mean: $32.04 (range = $18–52.95, SD = $11.30). | PNS: EC + CM: 30.4 | PNS: EC: 34.4 | ||||
| Meredith et al. (2011) | WS | Enrolled sample (n = 15) | Smoking cessation | 86.66 % | 18–60 (M = 33.15) | 1 week | Vouchers | Escalating with reset, tapering is constant rate, | $161.50 | PNS abstinence phase: 57 | PNS baseline phase: <1 | ||||
| Meredith (2013) | WS | Enrolled sample (n = 42) | Smoking cessation | 76 % | 18–60 (M = 39.6) | 1 week | Vouchers | Escalating with reset | $122.50 | PNS: independent contingency condition: 55.6; PNS interdependent contingency conditions: 52.8 | PNS: 34.7 | ||||
| Mundt et al. (2019) | RCT | CM = 948; control = 952 | Quitline engagement and smoking cessation | N/R | (M = 45) | 6 months | Monetary | Constant rate | $190 | # Calls: M = 3.8 calls; QR: 21.6 % at 6-month follow-up | # Calls: M = 2.9; QR: 13.8 % at 6-month follow-up | ||||
| Parks et al. (2019) | RCT | $20 + protective call = 621; $20, no protective call = 621; $10 + protective call = 621; $10, no protective call = 619, $0 + protective call = 620; $0, no protective call = 621 | Quitline engagement | CM only (no protective calls) - 89.35 % | 20–88 (M = 52.8) | N/A | Gift card | 1-time constant rate | $20 | # Calls: $10 + no call: 23; $20 + no call: 29 | $0 + no call: 11 | ||||
| Raiff et al. (2017) | WS | Enrolled sample = 12 | Smoking cessation | 83.3 % | 19–48 (M = 26.2) | 1 month | Voucher | Abstinence induction phase escalating with reset, tapering was constant rate | $35.25 | PNS abstinence induction: 35.5; tapering: 13.8 | PNS baseline: 1.25 | ||||
| Ramo et al. (2018) | RCT | CM = 251; control =249 | Comments on Facebook | 67 % | 18–25 (M = 20.9) | 12 months | Gift card | Constant rate | $90 | # Comments based on incentive frequency: monthly: 30; weekly: 31; daily: 11 | # Comments without incentive: 5 | ||||
| Reynolds et al. (2008) | WS | Enrolled sample (n = 4) | Smoking cessation | 100 % | Adolescents (M = 17.5) | N/A | Monetary | Escalating with reset with bonuses | $314.75 | All participants reduced CO levels during abstinence conditions relative to initial baseline | |||||
| Reynolds et al. (2015) | RCT | CM = 44; control = 37 | Smoking cessation | 70 % | Adolescents (M = 16.6) | 6 weeks | Voucher | Shaping and thinning were constant rate; abstinence was escalating with reset | $803 | Abstinence phase CO: 4.65 ppm | Abstinence phase CO: 9.49 ppm | ||||
| Stoops et al. (2009) | RCT | CM (n = 35); control (n = 33) | Smoking cessation | 82.86 % | 18+ (M = 39) | 1.5 months | Voucher | Escalating with reset | Approx. $800 | PNS for weeks 1, 2, 3, 4, 5 & 6: 30.4, 39.0, 39.8, 36.9, 39.8 & 33.9, respectively | PNS weeks 1, 2, 3, 4, 5 & 6: 18.4, 8.7, 6.7, 10.0, 11.3 & 12.6, respectively | ||||
Abbreviations: WS = Within Subjects Design; RCT = Randomized Control Trial Design; N-RCT = Non-Randomized Control Trial Design; CM = Contingency Management; M = Mean; N/R = Not Reported; N/A = Not Applicable; PNS = Percent Negative Samples; SSA = Sustained Smoking Abstinence; QR = Quit Rate; CO = Carbon Monoxide.
Table 2.
Remote contingency management studies in people who drink alcohol.
| Study author (year) | Design | Enrolled sample size (baseline) by condition | Target behavior | Retention at end of CM condition | Eligible ages (mean) | Longest follow-up | Type of reinforcement | Reinforcement schedule | Maximum possible incentives for CM condition | Primary outcome: CM condition(s) Mean or % | Primary outcome: Control condition(s) Mean or % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alessi (2013) | RCT | CM = 15; control =15 | Alcohol abstinence | 100 % | 21+ (M = 39.35) | N/R | Vouchers | Escalating with reset | $340 | PNS:87.1 | PNS:66.9 |
| Barnett et al. (2011) | WS | Enrolled sample (n = 20) | Alcohol use reduction | 65 % | 18+ (M = 32) | N/R | Monetary | Escalating with reset | $154 | PDA: 82.4, Percent days of self-reported abstinence in week 3: 75.8, TAC in weeks 2–3: 0.011 g/dL | PDA: 23.3, TAC in week 1: 0.044 g/dL |
| Dougherty et al. (2014) | WS | Enrolled sample (n = 29) | Alcohol abstinence | 89.7 % | 21–39 (M = 28.5) | N/R | Monetary | Constant rate | $300 | PNS $25: 87; PNS $50: 89 | PNS: 70 |
| Dougherty et al. (2015) | WS | Enrolled sample (n = 80) | Alcohol use reduction | 80 % | 21–54 (M = 30.20) | 3 months | Monetary | Constant rate | $600 | Odds of meeting contingency criteria were 7-fold greater for CM phase compared to observation phase | |
| Koffarnus et al. (2018) | RCT | CM = 20; control = 20 | Alcohol abstinence | 100 % | 18+ (M = 45.9) | 1 month | Monetary | Escalating with bonus and reset | $350 | PDA: 85 | PDA: 38 |
| Koffarnus et al. (2021) | RCT | CM = 18; control = 18 | Alcohol abstinence | 100 % | 18+ (M = 37.7) | 6 months | Reloadable debit card | Escalating with bonus and reset | $350 | PDA: 86 | PDA:40 |
| Oluwoye et al. (2020) | WS | Enrolled sample (n = 9) | Alcohol abstinence | 66.6 % | 21+ (M = 30.5) | 9 weeks | E-gift card | Escalating with reset | $369 | PNS: 49 | PNS: 27 |
| Neighbors et al. (2018) | RCT | In person $0 (n = 584); In person $30 (n = 655); Remote $0 (n = 490); Remote $30 (n = 330) | Intervention engagement | N/A | 18–26 (M = 22.14) | 6 months | Gift cards | 1-time constant | $30 | % engaged: 39.1 | % engaged: 24.5 |
| Barnett et al. (2017) | RCT | CM = 15; control = 15 | Alcohol abstinence | 100 % | 18+ (M = 28.9) | 1 month | Monetary | Escalating with reset | $231 | PDA: 54.3 | PDA: 31.2 |
Abbreviations: WS = Within Subjects Design; RCT = Randomized Control Trial Design; CM = Contingency Management; M = Mean; N/R = Not Reported; N/A = Not Applicable; PNS = Percent Negative Samples; PDA = Percent Days Abstinent; TAC = Transdermal Alcohol Concentration.
Table 3.
Remote contingency management studies in people who use other substances.
| Study author (year) | Design | Enrolled sample size (baseline) by condition | Target behavior | Retention at end of CM condition | Eligible ages (mean) | Longest follow-up | Type of reinforcement | Reinforcement schedule | Maximum possible incentives for CM condition | Primary outcome: CM condition(s) Mean or % | Primary outcome: Control condition (s) Mean or % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DeFulio et al. (2021) | N-RCT | Enrolled CM = 85, Matched control = 85 | Drug and alcohol abstinence, treatment engagement | 100 % | N/R | None | Reloadable debit card | Escalating with reset and constant rate | $436 | CM vs. control: Months 1, 2, 3, 4: 14.12, 17.65, 16.47 & 20 percentage point increases in consistent samples, respectively; 7.28, 9.55, 13.62 & 18.01 percentage point increases in attendance, respectively. | |
| Hammond et al. (2021) | RCT | CM = 29; control = 32 | Drug and alcohol abstinence, treatment engagement | N/R | 18+ (M = 39.6) | 1 month | Reloadable debit card | Escalating with reset | $600 | Mean days in usual care treatment: 29.8; Treatment retention rate: 24 %; Rate of recent abstinence: 33 % | Mean days in usual care treatment: 22.2; Treatment retention rate: 3 %; Rate of recent abstinence: 16 % |
| Holtyn et al. (2021) | RCT | CM = 21; control = 20 | Intervention engagement | 100 % | 18+ (M = 47.1) | None | Reloadable credit card | Constant rate | $350 | % enrolled in treatment: 71 % | % enrolled in treatment: 30 % |
| Raiff et al. (2021) | WS | Enrolled sample (n = 8) | Nicotine vaping abstinence | 100 % | 18–35 (M = 19.9) | None | Reloadable debit card | Escalating with reset | $140 | PNS (<30 % ng/ml cotinine): 77 % | PNS (<30 % ng/ml cotinine): 0 % |
Abbreviations: WS = Within Subjects Design; RCT = Randomized Control Trial Design; N-RCT = Non-Randomized Control Trial Design; CM = Contingency Management Condition; M = mean; N/R = Not Reported; PNS = Percent Negative Samples.
3.2. Remotely delivered CM for cigarette smoking
Of the 26 studies focused on cigarette smoking (Table 1), 22 focused on smoking cessation as the target behavior, three targeted intervention engagement, and one focused on both smoking cessation and intervention engagement. Nineteen studies included adults aged 18 and up (Alessi et al., 2017; Dallery et al., 2007, 2013, 2021; Dallery & Glenn, 2005; Dan et al., 2016; Glenn & Dallery, 2007; Halpern et al., 2018; Harris & Reynolds, 2015; Jarvis & Dallery, 2017a- experiment 2, Jarvis & Dallery, 2017b- experiment 1; Kurti et al., 2020; Martner & Dallery, 2019; Meredith et al., 2011; Meredith & Dallery, 2013; Parks et al., 2019; Raiff et al., 2017; Stoops et al., 2009). Three studies targeted adolescents (Harvanko et al., 2020; Reynolds et al., 2008, 2015), and one study targeted college-aged students specifically, ranging from age 18 to 25 (Ramo et al., 2018). Two studies did not report an age range and only reported the mean age of participants as 45 (Mundt et al., 2019) and 47.9 years (Hertzberg et al., 2013). One did not report age eligibility criteria, but noted that 71.7 % of participants were older than 45 (Anderson et al., 2018).
Of the 22 studies focused on smoking cessation, 20 remotely monitored cessation using biochemical verification methods (Alessi et al., 2017; Dallery et al., 2007, 2013, 2021; Dallery & Glenn, 2005; Dan et al., 2016; Glenn & Dallery, 2007; Harris & Reynolds, 2015; Harvanko et al., 2020; Hertzberg et al., 2013; Jarvis & Dallery, 2017a- experiment 2; Kurti et al., 2020; Martner & Dallery, 2019; Meredith et al., 2011; Meredith & Dallery, 2013; Mundt et al., 2019; Raiff et al., 2017; Reynolds et al., 2008, 2015; Stoops et al., 2009). These studies utilized carbon monoxide (CO) monitors along with video recording, facial recognition software, and/or mobile applications. One study used blood and urine sampling to verify smoking cessation (Halpern et al., 2018), and one relied on a self-report (Koslovsky et al., 2018). Of the studies that targeted intervention engagement, three measured engagement in quitline services (Anderson et al., 2018; Mundt et al., 2019; Parks et al., 2019) and one measured intervention engagement via comments in hidden Facebook groups designed to help people quit smoking (Ramo et al., 2018).
Incentivization varied greatly between studies. Six studies used cash or check reinforcers (Halpern et al., 2018; Hertzberg et al., 2013; Jarvis & Dallery, 2017b- experiment 1; Martner & Dallery, 2019; Mundt et al., 2019; Reynolds et al., 2008), three used reloadable gift cards (Kurti et al., 2020; Parks et al., 2019; Ramo et al., 2018), one used reloadable debit cards (Dallery et al., 2021), 12 used vouchers (e.g., emailed vouchers redeemable at a variety of internet vendors) (Dallery et al., 2007, 2013; Dallery & Glenn, 2005; Dan et al., 2016; Glenn & Dallery, 2007; Harris & Reynolds, 2015; Harvanko et al., 2020; Meredith et al., 2011; Meredith & Dallery, 2013; Raiff et al., 2017; Reynolds et al., 2015; Stoops et al., 2009), one used prize-based reinforcers (i.e., a raffle where participants earned drawings for a prize) (Alessi et al., 2017), one used a combination of monetary reinforcers and vouchers (Jarvis & Dallery, 2017a- experiment 2), and one used a combination of nicotine patches and gift cards (Anderson et al., 2018). One study used financial reinforcers for biochemically verified abstinence but did not report their method of reinforcer delivery (Koslovsky et al., 2018).
Schedules of reinforcement also varied across studies. Ten of the studies employed an escalating schedule of incentivization with a reset, where the reinforcer value reset to the original amount based on negative or missed samples, with maximum total reported contingency-based reinforcers ranging from $52.95 to $1620 (Alessi et al., 2017; Dallery et al., 2007; Harris & Reynolds, 2015; Harvanko et al., 2020; Hertzberg et al., 2013; Kurti et al., 2020; Martner & Dallery, 2019; Meredith & Dallery, 2013; Stoops et al., 2009). One study used an escalating schedule but did not include the reset component, and had a maximum total contingency-based reinforcer of $600 (Halpern et al., 2018). Four studies used constant rate (i.e., non-escalating) reinforcement throughout the incentivization period. Two studies provided reward reinforcers with a maximum total contingency-based value of $90 (Ramo et al., 2018) and $190 (Mundt et al., 2019), while the other two studies utilized a deposit-based reinforcer program (i.e., where the participant deposits a sum of money that they then can earn back by providing evidence of smoking cessation) (Jarvis & Dallery, 2017a- experiment 2, Jarvis & Dallery, 2017b- experiment 1). Two studies used a one-time reinforcer for enrolling or connecting to a quitline, both with a maximum contingency-based reinforcer of $20 (Anderson et al., 2018; Parks et al., 2019). Seven studies used a combination of escalating with reset and constant rate reinforcement, with maximum contingency-based total reinforcers ranging from $35.25 to $803 (Dallery et al., 2007, 2013; Dallery & Glenn, 2005; Dan et al., 2016; Meredith et al., 2011; Raiff et al., 2017; Reynolds et al., 2015). One study used an escalating with reset reinforcement schedule along with additional bonuses for consecutive samples meeting contingent criteria (Reynolds et al., 2008), with maximum earnings of up to $314.75. One study did not report a reinforcement schedule, or their maximum value of contingency-based reinforcer (Koslovsky et al., 2018).
Studies reported on CM effectiveness using various outcomes (e.g., percent negative samples, quit rate, quitline engagement). Four studies incentivized for intervention engagement measured by connections to a quitline (Anderson et al., 2018 a; Mundt et al., 2019; Parks et al., 2019) or social media comments (Ramo et al., 2018). The remaining studies incentivized based on verified smoking cessation as the target behavior measured CM effectiveness through percentage of negative samples (PNS) (Alessi et al., 2017; Dallery et al., 2013, 2021; Dan et al., 2016; Glenn & Dallery, 2007; Jarvis & Dallery, 2017a- experiment 2, Jarvis & Dallery, 2017b- experiment 1; Martner & Dallery, 2019; Meredith et al., 2011; Meredith & Dallery, 2013; Raiff et al., 2017; Stoops et al., 2009), quit rate (Hertzberg et al., 2013; Mundt et al., 2019), smoking abstinence (Halpern et al., 2018; Harris & Reynolds, 2015), seven-day point prevalence rates (Kurti et al., 2020), and reduction in CO values (Dallery & Glenn, 2005; Harvanko et al., 2020; Reynolds et al., 2008, 2015). One study compared between independent CM, based on the individual’s smoking cessation status, and interdependent CM, based on an individual’s smoking cessation status along with that of two teammates (Meredith & Dallery, 2013).
Of the studies focusing on smoking, 24 reported the CM condition had a greater reduction in smoking behavior and/or engagement in intervention relative to the comparator group. Twelve of the studies used a within-subjects control condition (Dallery et al., 2007, 2021; Dallery & Glenn, 2005; Dan et al., 2016; Glenn & Dallery, 2007; Jarvis & Dallery, 2017a- experiment 2, Jarvis & Dallery, 2017b- experiment 1; Martner & Dallery, 2019; Meredith et al., 2011; Meredith & Dallery, 2013; Raiff et al., 2017; Reynolds et al., 2008). The majority (13 studies) used a between-person randomized controlled design (Alessi et al., 2017; Anderson et al., 2018; Dallery et al., 2013; Halpern et al., 2018; Harris & Reynolds, 2015; Harvanko et al., 2020; Hertzberg et al., 2013; Koslovsky et al., 2018; Mundt et al., 2019; Parks et al., 2019; Ramo et al., 2018; Reynolds et al., 2015; Stoops et al., 2009), where participants were randomized to either an incentivized or nonincentivized condition, and one study utilized a nonrandomized control design (Kurti et al., 2020). The majority of studies had an intervention duration between two weeks and seven weeks, although one lasted five months (Mundt et al., 2019) and another lasted six months (Halpern et al., 2018). Two studies did not show a greater reduction in the CM condition compared to the comparator condition, these included: 1) comparing electronic cigarettes alongside CM versus electronic cigarettes alone (Martner & Dallery, 2019), and 2) comparing CM during pregnancy to smoking cessation counseling specifically for pregnancy (i.e., Smoking Cessation for Healthy Births) (Harris & Reynolds, 2015). Notably, both of these studies had small sample sizes of <20 total participants.
The two studies that targeted quitline engagement incentivized for engagement one time (Anderson et al., 2018; Parks et al., 2019), where the outcome was if the participant called (or did not call) the quitline. Potential moderators included that age was positively related to quitline engagement in Parks et al. (2019). In another study that used deposit-based reinforcers, one moderator was that people with larger deposit amounts had more abstinent samples, longer durations of meeting the cutoff, and submitted more samples overall (Jarvis & Dallery, 2017b- experiment 1).
3.3. Remotely delivered CM for alcohol use
Of the nine included alcohol-focused studies, six focused on alcohol abstinence as the target behavior, two focused on alcohol use reduction, and one focused on intervention engagement (see Table 2). All nine compared CM alone to a control condition, with no other combined interventions. The populations targeted in each study were adults with ages either 18 and up (Barnett et al., 2011; Koffarnus et al., 2018, 2021) or 21 and up (Alessi & Petry, 2013; Dougherty et al., 2014, 2015; Oluwoye et al., 2020), with one study restricted to college-aged students (ages 18–26) (Neighbors et al., 2018).
Of the eight studies targeting alcohol abstinence or use reduction, four remotely monitored abstinence with remote transdermal alcohol monitors (Barnett et al., 2011, 2017; Dougherty et al., 2014, 2015). Four remotely monitored abstinence through breath alcohol concentration with cell phone video recordings (Alessi & Petry, 2013), an app with facial recognition (Oluwoye et al., 2020), or a breathalyzer with built-in facial recognition (Koffarnus et al., 2018, 2021). Neighbors et al. (2018) targeted intervention engagement and thus measured completion of a single-session personalized normative feedback (PNF) intervention for alcohol misuse.
The reinforcers for each study included vouchers redeemable for gift cards/checks (Alessi & Petry, 2013), e-gift cards (Neighbors et al., 2018; Oluwoye et al., 2020), mailed money orders or cash (Barnett et al., 2011), reloadable debit cards (Koffarnus et al., 2018, 2021), cash paid during weekly clinic visits (Dougherty et al., 2014, 2015), and cash given at a post intervention session (Barnett et al., 2017). Six of the alcohol studies used an escalating reinforcer schedule with reset (Alessi & Petry, 2013; Barnett et al., 2011, 2017; Oluwoye et al., 2020), with some also providing bonuses for consecutive negative samples (Koffarnus et al., 2018, 2021). The other three studies used a constant rate reinforcer structure, with the intervention engagement study using a one-time reinforcer for PNF completion (Dougherty et al., 2014, 2015; Neighbors et al., 2018).
Studies measured CM effectiveness using various outcomes including percentage of days abstinent (Barnett et al., 2011; Dougherty et al., 2014; Koffarnus et al., 2018, 2021), percentage of days meeting contingency criteria (Dougherty et al., 2015), PNS (Alessi & Petry, 2013; Barnett et al., 2011; Dougherty et al., 2014; Koffarnus et al., 2018; Oluwoye et al., 2020), and PNF engagement (Neighbors et al., 2018).
Of the eight studies targeting reductions in alcohol use (Alessi & Petry, 2013; Barnett et al., 2011, 2017; Dougherty et al., 2014, 2015; Koffarnus et al., 2018, 2021; Oluwoye et al., 2020), all eight reported a reduction in alcohol in the CM condition compared to the comparator condition. Seven of these eight studies reported a statistically significant reduction (Alessi & Petry, 2013; Barnett et al., 2011, 2017; Dougherty et al., 2014, 2015; Koffarnus et al., 2018, 2021; Oluwoye et al., 2020). Study design among the eight alcohol reduction-focused studies varied, with four utilizing a within-subject control condition (Barnett et al., 2011; Dougherty et al., 2014, 2015; Oluwoye et al., 2020), and four randomizing participants to abstinence monitoring control conditions, with noncontingent reinforcers (Alessi & Petry, 2013; Barnett et al., 2017; Koffarnus et al., 2018, 2021). Neighbors et al. (2018), which focused on PNF engagement, used a comparator group where participants were not incentivized for PNF completion. Five of the nine included alcohol-focused studies provided maximum reinforcers between $300 and $400 (Alessi & Petry, 2013; Dougherty et al., 2014; Koffarnus et al., 2018, 2021; Oluwoye et al., 2020). The other studies targeting alcohol reduction provided up to $154 (Barnett et al., 2011), $231 (Barnett et al., 2017), and $600 (Dougherty et al., 2015). The study targeting intervention engagement provided up to $30 (Neighbors et al., 2018). Of the eight studies targeting alcohol abstinence or use reduction, the length of CM interventions ranged over several weeks. CM interventions in six of the studies lasted between two to four weeks (Alessi & Petry, 2013; Barnett et al., 2011, 2017; Koffarnus et al., 2018, 2021; Oluwoye et al., 2020), while the other studies had CM interventions that lasted eight weeks (Dougherty et al., 2014) and 12 weeks (Dougherty et al., 2015).
3.4. Remotely delivered CM for other substance use or multiple substances
Of the four included studies focused on other substance use or multiple types of substance use, one study focused on both alcohol and drug abstinence and treatment engagement in people with an alcohol use disorder (Hammond et al., 2021), one focused on engagement in buprenorphine treatment for opioid use (Holtyn et al., 2021), one focused on nicotine vaping abstinence (Raiff et al., 2021), and one focused on drug and alcohol abstinence and treatment engagement among people with an opioid use disorder (DeFulio et al., 2021). The targeted populations in each study were adults ages 18 and older (Hammond et al., 2021; Holtyn et al., 2021) or young adults between 18 and 35 (Raiff et al., 2021), with one study not specifying the included age range of adult participants (DeFulio et al., 2021).
Studies verified abstinence using breath alcohol concentration with cell phone video recordings and remote 9-panel oral fluid testing for drug use in one study (Hammond et al., 2021); breath alcohol concentration with facial recognition was measured with a smartphone application whereas drug abstinence was verified with in-person urine screening (DeFulio et al., 2021). One study measured nicotine vaping abstinence with remote saliva tests (Raiff et al., 2021). Treatment engagement was measured through appointment attendance measured with GPS and start/stop time stamps (DeFulio et al., 2021) and buprenorphine treatment engagement through prescription documentation and cellphone video recordings of participants taking the medication (Holtyn et al., 2021).
The reinforcer for each study included reloadable debit cards (DeFulio et al., 2021; Hammond et al., 2021; Holtyn et al., 2021; Raiff et al., 2021). Two of the studies focused on other substance use used an escalating reinforcer schedule with reset (Hammond et al., 2021; Raiff et al., 2021). One study used a combination of escalating with reset and constant rate reinforcement (DeFulio et al., 2021). The other study used a constant rate reinforcer structure (Holtyn et al., 2021).
CM effectiveness was measured by treatment retention and recent abstinence (Hammond et al., 2021), percent enrolled in treatment (Holtyn et al., 2021), PNS (Raiff et al., 2021), and percent of consistent samples (DeFulio et al., 2021).
Of the four studies focused on other substance use, all four reported substance use reduction and/or increased treatment engagement in the CM condition compared to the comparator condition. Two of the four studies reported a statistically significant increase in treatment retention or treatment enrollment, respectively (Hammond et al., 2021; Holtyn et al., 2021). One study reported a statistically significant difference in appointment attendance and percent consistent samples (DeFulio et al., 2021). Study design among the four studies varied, with two using a between-person randomized control design where participants were randomized to either a reinforced or non-reinforced condition (Hammond et al., 2021; Holtyn et al., 2021), one using a within-subjects control condition (Raiff et al., 2021), and the remaining study using a nonrandomized retrospective matched control design (DeFulio et al., 2021). Two of the four studies provided maximum reinforcers between $400 and $600 (DeFulio et al., 2021; Hammond et al., 2021). The other studies provided up to $140 (Raiff et al., 2021) and $1890 (Holtyn et al., 2021). The length of CM interventions ranged from three to six months for three of the studies focused on other substance use (DeFulio et al., 2021; Hammond et al., 2021; Holtyn et al., 2021), and two weeks for the remaining study (Raiff et al., 2021).
3.5. Quality assessment
Four reviewers (C.J., M.L., N.S., S.S.) independently assessed the quality of included studies using the Quality Assessment Tool for Quantitative Studies (Ciliska et al., 1998). Two reviewers completed the assessment for each included study. After resolving discrepancies across reviewers, the reviewers rated each study as strong, moderate, or weak. Of the 26 studies focused on cigarette smoking, 13 were rated as strong and 13 as moderate quality. Of the nine studies focused on alcohol use, the reviewers rated eight as strong and one as moderate quality. Of the remaining four studies focused on other substance use, one was rated as strong and three were rated as moderate quality. No studies received a weak quality rating.
4. Discussion
The goal of this systematic review was to identify and synthesize the extant literature on remotely delivered CM interventions for people who use substances. The systematic literature search resulted in 39 included studies, of which 66.7 % focused on smoking, 23.1 % targeted alcohol use outcomes, and 10.3 % focused on other substance use or multiple substances. Of particular importance, the vast majority of studies (37 out of 39) demonstrated the clinical benefit of remotely delivered CM across the variety of comparator conditions and study designs. This provides considerable and consistent support for the feasibility and benefits of remotely delivered CM interventions in populations who smoke cigarettes, use alcohol, or consume other substances.
All included studies were of moderate or better quality, indicative of rigorous research designs and methods. For studies rated as moderate quality, as opposed to high quality, decreased quality ratings resulted from either nonrepresentative sampling (i.e., potential selection bias) or high or unreported attrition. Future work in this area can improve on prior studies by ensuring the reporting of relevant information (e.g., number of participants who withdraw their participation) and also prioritizing intervention features that promote continued treatment engagement. For example, personalizing or tailoring the intervention to increase personal relevance (Bidargaddi et al., 2018) or incentivizing intervention engagement in addition to other behavior change targets.
A prior systematic review evaluated only mobile phone–delivered CM interventions in samples that use substances (Getty et al., 2019); however, due to the more limited use of mobile phone platforms to date this review only captured seven studies. The current systematic review extends and synthesizes work on the broader array of remotely delivered CM interventions targeting substance use outcomes, including those using landline phones and computers to deliver the intervention. Consideration of remote intervention delivery platforms beyond mobile phones is essential to reach underserved populations where the digital divide can limit the reach of mobile and sensing technologies (“Mobile Fact Sheet,” 2021). Furthermore, the current review captures the growth of remotely delivered CM interventions over the past two decades and shows the progression of digital technologies to streamline CM delivery while maintaining efficacy, minimizing patient, clinic, and administrative burden, and increasing acceptability.
To date, remotely delivered CM has largely focused on cigarette smoking and alcohol use, with a few recent investigations into other substance use and targeting polysubstance use (see Table 3). This may, in part, be due to the lag in digital and sensing technology to biologically verify recent substance use for substances other than alcohol and nicotine. With recent improvements in these technologies, such as wearables to detect cocaine, opioid, and cannabis use, there is potential to enhance future remotely delivered CM treatments by further reducing patient burden (Goldfine et al., 2020; Holtyn et al., 2019; Mahmud et al., 2018; Mishra et al., 2020). Furthermore, the COVID-19 pandemic and associated national public health emergency potentiated a rapid transition to remote health care. This transition has resulted in evidence of substance use treatment outcomes being maintained via remote treatment delivery (Lin, Fortney, et al., 2022) across multiple remote-delivery platforms (e.g., telephone delivered care) (Lin, Zhang, et al., 2022), with calls for enhancing access and reach to effective substance use prevention and treatment, such as CM, using remote delivery platforms (Coughlin et al., 2021), and acknowledgement that digital delivery methods confer benefits beyond traditional in-person health care models for substance use (McDonnell et al., 2021). Thus, the current health care environment, along with regulatory policies, have warmed to remotely delivered care models in recent years, opening the door for wide-scale adoption of remotely delivered CM for substance use.
Given the clear and considerable evidence of the effectiveness of remotely delivered CM in promoting health behavior change across a variety of types of substance use, future work should focus on enhancing the scalability of remotely delivered CM interventions without sacrificing efficacy, and advocating for payer support. One potential option to address costs of delivering CM is incorporating nonmonetary reinforcers (e.g., verbal reinforcement, token economies) to potentially substitute for some monetary reinforcers, both to reinforce behavior change as well as to encourage adherence to rigorous behavioral monitoring schedules (e.g., multiple breath samples a day). Nonmonetary reinforcement could be incorporated probabilistically, such as in the variable magnitude of reinforcement procedure that uses fishbowl draws with some portion of draws being nonmonetary (e.g., “Good Job!”) (Petry et al., 2001); non-monetary reinforcement could also be incorporated adaptively based on time-varying individual factors (e.g., risk for relapse) to decide the type of reinforcer (e.g., monetary, nonmonetary) necessary to promote or sustain effective behavior change in the moment (Coughlin et al., 2022; Nahum-Shani et al., 2018).
Another approach to addressing upfront costs of remotely delivered CM is identifying the minimum effective dose of reinforcer magnitude and intervention duration, which may vary between people. Studies had a wide variation in the total reinforcers available, ranging from $20 to over $1500. Although some of this variability may be due to different behavioral targets (e.g., sustained abstinence vs. one-time call to a quitline), the science of what is an effective reinforcer dose to achieve each behavioral target (e.g., alcohol abstinence, smoking cessation) remains under-investigated. Similarly, studies varied in the duration of the intervention, with most lasting between two and six weeks, although a few lasted up to six months. To the extent that the duration of monetary reinforcers can be optimized, in combination with providing the minimum effective magnitude of reinforcers, the upfront costs of these interventions could be reduced, significantly increasing opportunities for scalable implementation. Future investigations incorporating nonmonetary reinforcers and refining the minimum effective magnitude and duration of treatment may help to further minimize barriers to remotely delivered CM interventions and to facilitate wide-scale implementation, namely by reducing costs, minimizing patient, provider, and administrative burden, and maximizing intervention engagement. Despite these recommendations for continued improvement and optimization of remotely delivered CM interventions for substance use, the findings reported here support implementation of remotely delivered CM as an effective intervention to reduce use and consequences from substance use. Future work to further optimize these interventions should consider hybrid effectiveness-implementation models (Curran et al., 2012), so as to facilitate the rapid translation of remotely delivered CM interventions into real-world settings while continuing to enhance effectiveness and minimize barriers to wide-scale adoption (including payer support).
Considerations for health insurance coverage for remotely delivered CM are at least two-fold. The recent Department of Health and Human Services Office of Inspector General Advisory Opinion (OIG Advisory Opinion No. 22-04) (DeConti, 2022) clarifies the opportunity for provision and coverage of CM for substance use disorder. This, coupled with the National Drug Control 2022 report (Office of National Drug Control Policy, 2022), which calls for states to expand CM services to Medicaid-recipients using 1115 Medicaid Demonstration Authority, shows cross-agency federal support for widespread CM adoption. To date, the Substance Abuse and Mental Health Services Administration (SAMHSA) has maintained a $75 cap on CM incentives through State Opioid Relief grants (FY 2022 State Opioid Response Grants, n.d.), creating restrictive, nonempirically supported limits in the provision of CM. However, ongoing advocacy occurs through the Motivational Incentive Advocacy Group to increase SAMHSA incentive caps to align them with other federal agency recommendations (The Motivational Incentives Policy Group, 2022). In addition, remote delivery of CM often includes the use of digital therapeutics, or software-driven, evidence-based platforms such as apps (Makin, 2019). Digital therapeutics have in some instances sought approval through the Food and Drug Administration (FDA). For example, DynamiCare Health Inc. recently received Breakthrough Device designation (U.S. Food and Drug Administration, 2019) for the provision of remotely-delivered CM to help people who are pregnant stop smoking cigarettes (DynamiCare Health Digital Therapeutic Receives FDA Breakthrough Device Designation for Treatment of Smoking During Pregnancy, 2022). However, the costs in time and money to receive these approvals are often prohibative and a barrier to ensuring effective treatments are accessible. Continued efforts are necessary to increase coverage of CM interventions and to ensure remotely delivered CM reaches the many people who could benefit from this intervention.
4.1. Study limitations
The current systematic review of remotely delivered CM for substance use should be considered within the context of its weaknesses. First, the vast majority of studies focused on adults, limiting the generalizability of findings for adolescents who may benefit from remotely delivered CM interventions given they tend to be early adopters of digital technologies. Sample sizes varied across studies, with many studies including only modest sample sizes. This may be reflective of many of the studies to date being preliminary in nature, often with a focus on establishing feasibility, prior to more fully powered investigations. In addition, included studies varied with regard to design (e.g., within-subjects, randomized controlled trials), reinforcer magnitudes and schedules, target behaviors (e.g., service engagement, abstinence, use reduction), and reporting of quantitative outcomes. Given the rapidly increasing focus on remotely delivered CM (see Fig. 2) and expectation that the coming years will bring more empirical work in this field, meta-analyses to establish magnitudes of effects of different variations of remotely delivered CM will help to determine where limited resources are best directed. Furthermore, this systematic review may be limited by the search strategy and terms included. Though every attempt was made to conduct a thorough search, including the use of MeSH and preliminary testing against sentinel articles, the possibility that relevant literature may not have been captured always exists. Finally, remotely delivered CM is being increasingly included within multi-component interventions. In some cases, these study designs preclude isolating CM effects and thus were not included in the current review (e.g., Carpenter et al., 2015).
4.2. Future directions
Delivering CM interventions using digital technology creates the opportunity to extend CM in ways not feasible through in-person delivery, such as to reach people who otherwise would not be able to attend frequent clinic visits for behavior verification and reinforcer delivery, and to apply contemporary intervention designs (e.g., just-in-time adaptive interventions) to build on the existing science of CM interventions. As technology for remote monitoring of other substances (e.g., cocaine, cannabis) develops, the use of remotely delivered CM to promote reduction in or abstinence from a wide variety of substance use is likely to become increasingly feasible. Future work focused on maximizing impact of remotely delivered CM is critical as we move toward more digitally focused treatment modalities. Future work may also consider minimizing monetary reinforcers without sacrificing efficacy, and establishing necessary dosing recommendations (i.e., reinforcer magnitude, duration) to achieve proximal and distal goals around substance use.
Supplementary Material
Acknowledgements
We thank Mr. Nicholas Stewart (University of Michigan) for providing screening and review of articles.
Funding
LNC’s time was funded through NIAAA K23 AA028232.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.josat.2023.208977.
CRediT authorship contribution statement
Lara N. Coughlin: Conceptualization, methodology, writing; Sarah Salino: Data extraction and curation, writing; Claudia Jennings: Data extraction and curation, writing; Madelyn Lacek: Data extraction and curation; Whitney Townsend: Systematic search; Mikhail N. Koffarnus: Review and editing; Erin E. Bonar: Review and editing.
Declaration of competing interest
None.
References
- Alessi SM, & Petry NM (2013). A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction, 108(5), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Rash CJ, & Petry NM (2017). A randomized trial of adjunct mHealth abstinence reinforcement with transdermal nicotine and counseling for smoking cessation. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 19(3), 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Kirby CA, Tong EK, Kohatsu ND, & Zhu S-H (2018). Effects of offering nicotine patches, incentives, or both on quitline demand. American Journal of Preventive Medicine, 55(6 Suppl 2), S170–S177. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, & Swift RM (2017). A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction, 112(6), 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, & Colby SM (2011). Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug and Alcohol Dependence, 118(2–3), 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, & Festinger DS (2014). Prize-based contingency management for the treatment of substance abusers: A meta-analysis: Prize-based contingency management meta-analysis. Addiction, 109(9), 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidargaddi N, Almirall D, Murphy S, Nahum-Shani I, Kovalcik M, Pituch T, … Strecher V (2018). To prompt or not to Prompt? A microrandomized trial of time-varying push notifications to increase proximal engagement with a Mobile health app. JMIR mHealth and uHealth, 6(11), e10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer WM, Giustini D, de Jonge GB, Holland L, & Bekhuis T (2016). De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association, 104(3), 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter VL, Hertzberg JS, Kirby AC, Calhoun PS, Moore SD, Dennis MF, … Beckham JC (2015). Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. The Journal of Clinical Psychiatry, 76(7), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliska D, Miccouci S, Dobbins M, et al. (1998). Effective public health practice project. Quality assessment tool for quantitative studies. Hamilton, On: Effective Public Health Practice Project. [Google Scholar]
- Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, … Beller E (2020). Improving the translation of search strategies using the polyglot search translator: A randomized controlled trial. Journal of the Medical Library Association, 108(2), 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin LN, Bonar EE, & Bickel WK (2021). Considerations for remote delivery of behavioral economic interventions for substance use disorder during COVID-19 and beyond. Journal of Substance Abuse Treatment, 120, 108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin LN, Bonar EE, Walton MA, Fernandez AC, Duguid I, & Nahum-Shani I (2022). New directions for motivational incentive interventions for smoking cessation. Frontiers in Digital Health, 4. 10.3389/fdgth.2022.803301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran GM, Bauer M, Mittman B, Pyne JM, & Stetler C (2012). Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care, 50(3), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, & Glenn IM (2005). Effects of an internet-based voucher reinforcement program for smoking abstinence: A feasibility study. Journal of Applied Behavior Analysis, 38(3), 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, & Raiff BR (2007). An internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence, 86(2–3), 230–238. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, & Grabinski MJ (2013). Internet-based contingency management to promote smoking cessation: A randomized controlled study. Journal of Applied Behavior Analysis, 46(4), 750–764. 10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Stinson L, Bolívar H, Modave F, Salloum RG, Viramontes TM, & Rohilla P (2021). mMotiv8: A smartphone-based contingency management intervention to promote smoking cessation. Journal of Applied Behavior Analysis, 54(1), 38–53. [DOI] [PubMed] [Google Scholar]
- Dan M, Grabinski MJ, & Raiff BR (2016). Smartphone-based contingency management for smoking cessation with smokers diagnosed with attention-deficit/hyperactivity disorder. Translational Issues in Psychological Science, 2(2), 116–127. [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, & Ezzati M (2009). The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Medicine, 6(4), e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, & Higgins ST (2016). A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive Medicine. 10.1016/j.ypmed.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeConti RK Office of Inspector General Advisory Opinion No. 22-04. Department of Health and Human Services. https://oig.hhs.gov/documents/advisory-opinions/1024/AO-22-04.pdfhttps://oig.hhs.gov/documents/advisory-opinions/1024/AO-22-04.pdf. 2022. [Google Scholar]
- DeFulio A, Rzeszutek MJ, Furgeson J, Ryan S, & Rezania S (2021). A smartphone-smartcard platform for contingency management in an inner-city substance use disorder outpatient program. Journal of Substance Abuse Treatment, 120, 108188. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, … Roache JD (2014). Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug and Alcohol Dependence, 142, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns TE, Mullen J, & Roache JD (2015). Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcoholism, Clinical and Experimental Research, 39(4), 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DynamiCare Health Digital Therapeutic Receives FDA Breakthrough Device Designation for Treatment of Smoking During Pregnancy. PRWeb. https://www.prweb.com/releases/2022/2/prweb18504522.htm. 2022, February 22.
- FY 2022 State Opioid Response Grants, n.d.FY 2022 State Opioid Response Grants. (n.d.). Department of Health and Human Services. https://www.samhsa.gov/sites/default/files/grants/pdf/fy-22-sor-nofo.pdf (Original work published 2022) [Google Scholar]
- Getty CA, Morande A, Lynskey M, & Weaver T (2019). Mobile telephone-delivered contingency management interventions promoting behaviour change in individuals with substance use disorders: A meta-analysis. 10.1111/add.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn IM, & Dallery J (2007). Effects of internet-based voucher reinforcement and a transdermal nicotine patch on cigarette smoking. Journal of Applied Behavior Analysis, 40 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine C, Lai JT, Lucey E, Newcomb M, & Carreiro S (2020). Wearable and wireless mHealth technologies for substance use disorder. Current Addiction Reports, 7(3), 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, & Volpp KG (2018). A pragmatic trial of E-cigarettes, incentives, and drugs for smoking cessation. The New England Journal of Medicine, 378(24), 2302–2310. [DOI] [PubMed] [Google Scholar]
- Hammond AS, Sweeney MM, Chikosi TU, & Stitzer ML (2021). Digital delivery of a contingency management intervention for substance use disorder: A feasibility study with DynamiCare health. Journal of Substance Abuse Treatment, 126, 108425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, & Reynolds B (2015). A pilot study of home-based smoking cessation programs for rural, Appalachian, pregnant smokers. Journal of Obstetric, Gynecologic & Neonatal Nursing, 44(2), 236–245. [DOI] [PubMed] [Google Scholar]
- Harvanko A, Slone S, Shelton B, Dallery J, Fields S, & Reynolds B (2020). Web-based contingency management for adolescent tobacco smokers: A clinical trial. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 22(3), 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, … Beckham JC (2013). Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 15(11), 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Bosworth E, Marsch LA, McLeman B, Meier A, Saunders EC, … Ghitza UE (2019). Towards detecting cocaine use using smartwatches in the NIDA clinical trials network: Design, rationale, and methodology. Contemporary Clinical Trials Communications, 15, 100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Toegel F, Novak MD, Leoutsakos J-M, Fingerhood M, & Silverman K (2021). Remotely delivered incentives to promote buprenorphine treatment engagement in out-of-treatment adults with opioid use disorder. Drug and Alcohol Dependence, 225, 108786. 10.1016/j.drugalcdep.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, & Dallery J (2017). Internet-based self-tailored deposit contracts to promote smoking reduction and abstinence. Journal of Applied Behavior Analysis, 50(2), 189–205. 10.1002/jaba.377. experiment 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, & Dallery J (2017). Internet-based self-tailored deposit contracts to promote smoking reduction and abstinence. In. Journal of Applied Behavior Analysis, 50(2), 189–205. 10.1002/jaba.377. experiment 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK, & Kablinger AS (2018). Remote alcohol monitoring to facilitate incentive-based treatment for alcohol use disorder: A randomized trial. Alcoholism: Clinical and Experimental Research, 42(12), 2423–2431. 10.1111/acer.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Kablinger AS, Kaplan BA, & Crill EM (2021). Remotely administered incentive-based treatment for alcohol use disorder with participant-funded incentives is effective but less accessible to low-income participants. Experimental and Clinical Psychopharmacology, 29(5), 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslovsky MD, Hébert ET, Swartz MD, Chan W, Leon-Novelo L, Wilkinson AV, … Businelle MS (2018). The time-varying relations between risk factors and smoking before and after a quit attempt. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 20(10), 1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti AN, Tang K, Bolivar HA, Evemy C, Medina N, Skelly J, … Higgins ST (2020). Smartphone-based financial incentives to promote smoking cessation during pregnancy: A pilot study. Preventive Medicine, 140, 106201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM (2008). Contingency management for smoking cessation: Where do we go from here? Current Drug Abuse Reviews, 1(3), 340–349. [DOI] [PubMed] [Google Scholar]
- Lin LA, Fortney JC, Bohnert ASB, Coughlin LN, Zhang L, & Piette JD (2022). Comparing telemedicine to in-person buprenorphine treatment in U.S. veterans with opioid use disorder. Journal of Substance Abuse Treatment, 133, 108492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LA, Zhang L, Kim HM, & Frost MC (2022). Impact of COVID-19 telehealth policy changes on buprenorphine treatment for opioid use disorder. The American Journal of Psychiatry, appiajp21111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST (2006). A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction, 101(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Mahmud MS, Fang H, Wang H, Carreiro S, & Boyer E (2018). Automatic detection of opioid intake using wearable biosensor. International conference on computing, networking and communications: [Proceedings] (2018, pp. 784–788). International Conference on Computing, Networking and Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin S. (2019). The emerging world of digital therapeutics. Nature, 573(7775), S106–S109. [DOI] [PubMed] [Google Scholar]
- Martner SG, & Dallery J (2019). Technology-based contingency management and e-cigarettes during the initial weeks of a smoking quit attempt. Journal of Applied Behavior Analysis, 52(4), 928–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell A, MacNeill C, Chapman B, Gilbertson N, Reinhardt M, & Carreiro S (2021). Leveraging digital tools to support recovery from substance use disorder during the COVID-19 pandemic response. Journal of Substance Abuse Treatment, 124, 108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, & Dallery J (2013). Investigating group contingencies to promote brief abstinence from cigarette smoking. Experimental and Clinical Psychopharmacology, 21(2), 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, & Dallery J (2011). Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence, 118(1), 23–30. 10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra RK, Sempionatto JR, Li Z, Brown C, Galdino NM, Shah R, … Wang J (2020). Simultaneous detection of salivary Δ9-tetrahydrocannabinol and alcohol using a wearable electrochemical ring sensor. Talanta, 211, 120757. [DOI] [PubMed] [Google Scholar]
- Mobile Fact Sheet. Pew Research Center. www.pewinternet.org/fact-sheet/mobile/. 2021, April 7.
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA: TheJournal of the American Medical Association, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Mundt MP, Baker TB, Fraser DL, Smith SS, Piper ME, & Fiore MC (2019). Paying low-income smokers to Quit? The cost-effectiveness of incentivizing tobacco quit line engagement for medicaid recipients who smoke. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research, 22(2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, & Murphy SA (2018). Just-in-time adaptive interventions (JITAIs) in Mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 52(6), 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C, Rodriguez LM, Garey L, & Tomkins MM (2018). Testing a motivational model of delivery modality and incentives on participation in a brief alcohol intervention. Addictive Behaviors, 84, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. National Drug Control Strategy. The White House. https://www.whitehouse.gov/wp-content/uploads/2022/04/National-Drug-Control-2022Strategy.pdf. 2022. [Google Scholar]
- Oluwoye O, Reneau H, Herron J, Alcover KC, McPherson S, Roll J, & McDonell MG (2020). Pilot study of an integrated smartphone and breathalyzer contingency management intervention for alcohol use. Journal of Addiction Medicine, 14 (3), 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MJ, Hughes KD, Keller PA, Lachter RB, Kingsbury JH, Nelson CL, & Slater JS (2019). Financial incentives and proactive calling for reducing barriers to tobacco treatment among socioeconomically disadvantaged women: A factorial randomized trial. Preventive Medicine, 129, 105867. [DOI] [PubMed] [Google Scholar]
- Petry NM (2010). Contingency management treatments: Controversies and challenges. Addiction, 105(9), 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Petrakis I, Trevisan L, Wiredu G, Boutros NN, Martin B, & Kosten TR (2001). Contingency management interventions: From research to practice. The American Journal of Psychiatry, 158(5), 694–702. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J (2006). Contingency management for treatment of substance use disorders: A meta-analysis. Addiction, 101(11), 1546–1560. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Arena A, Meredith SE, & Grabinksi MJ (2017). Feasibility of a Mobile group financial-incentives intervention among pairs of smokers with a prior social relationship. The Psychological Record, 67(2), 231–239. [Google Scholar]
- Raiff BR, Newman ST, Upton CR, & Burrows CA (2021). The feasibility, acceptability, and initial efficacy of a remotely delivered, financial-incentive intervention to initiate vaping abstinence in young adults. Experimental and Clinical Psychopharmacology. 10.1037/pha0000468. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Thrul J, Delucchi KL, Hall S, Ling PM, Belohlavek A, & Prochaska JJ (2018). A randomized controlled evaluation of the tobacco status project, a Facebook intervention for young adults. Addiction. 10.1111/add.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, & Leraas K (2008). A web-based contingency management program with adolescent smokers [Review of A web-based contingency management program with adolescent smokers]. Journal of Applied Behavior Analysis, 41(4), 597–601. Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, & Lewis R (2015). A feasibility study of home-based contingency management with adolescent smokers of rural Appalachia. Experimental and Clinical Psychopharmacology, 23(6), 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Madden GJ, Rawson R, & Petry NM (2009). Facilitating the adoption of contingency management for the treatment of substance use disorders. Behavior Analysis in Practice, 2(1), 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, … Stewart LA, & the PRISMA-P Group. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ, 349(1), g7647. 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, … Wong CJ (2009). An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug and Alcohol Dependence, 105(1–2), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Motivational Incentives Policy Group. Letter to The Honorable Xavier Becerra May 12. https://www.naadac.org/assets/2416/mipg_letterto_hhs_sec_becerra_on_cm_12_may_2022.pdf. 2022.
- U.S. Food and Drug Administration. Breakthrough Devices Program. https://www.fda.gov/medical-devices/how-study-and-market-your-device/breakthrough-devices-program. 2019.
- Yoon PW, Bastian B, Anderson RN, Collins JL, & Jaffe HW, & Centers for Disease Control and Prevention (CDC). (2014). Potentially preventable deaths from the five leading causes of death--United States, 2008-2010. MMWR. Morbidity and Mortality Weekly Report, 63(17), 369–374. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


