ABSTRACT
Dexmedetomidine (DEX) is a highly selective α2-adrenoceptor agonist that is widely used in intensive and anesthetic care for its sedative and anxiolytic properties. DEX has the capacity to alleviate inflammatory pain while limiting immunosuppressive glucocorticoid stress during major surgery, thus harboring therapeutic benefits for oncological procedures. Recently, the molecular mechanisms of DEX-mediated anticancer effects have been partially deciphered. Together with additional preclinical data, these mechanistic insights support the hypothesis that DEX-induced therapeutic benefits are mediated via the stimulation of adaptive anti-tumor immune responses. Similarly, published clinical trials including ancillary studies described an immunostimulatory role of DEX during the perioperative period of cancer surgery. The impact of DEX on long-term patient survival remains elusive. Nevertheless, DEX-mediated immunostimulation offers an interesting therapeutic option for onco-anesthesia. Our present review comprehensively summarizes data from preclinical and clinical studies as well as from ongoing trials with a distinct focus on the role of DEX in overcoming (tumor microenvironment (TME)-imposed) cancer therapy resistance. The objective of this update is to guide clinicians in their choice toward immunostimulatory onco-anesthetic agents that have the capacity to improve disease outcome.
KEYWORDS: Cancer, dexmedetomidine, immunity, opioid-free-anesthesia
Introduction
Dexmedetomidine (DEX) is an α2-adrenergic receptor (α2-AR) agonist broadly used in clinical practice for its sedative and analgesic effects. DEX is the dextrorotatory S-enantiomer of medetomidine (4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1 H-imidazole),1 an imidazole with high affinity and selectivity for the α2-AR as compared to α1-AR (α2:α1 affinity ratio of 1260:1) (Figure 1a). Similar to clonidine, DEX exhibits a 5 to 10 times higher specificity and selectivity for the α2-AR expressed in the central nervous system as compared to the periphery.2 The α2-AR belongs to the group of G-protein coupled receptors (GPCRs). Upon ligation, α2-AR agonists induce a conformational change from the GDP-bound to the GTP-bound state, inducing the efflux of potassium and a consequent hyperpolarization of the plasma membrane. Membrane hyperpolarization in turn inhibits the gating of voltage-dependent Ca2+ channels, thus blocking the release of neurotransmitters (Figure 1b). By binding to presynaptic α2-ARs, DEX also exerts a negative feedback compromising the release of norepinephrine and inhibiting neurons located in the locus coeruleus of the brainstem involved in the maintenance of consciousness3 (Figures 2a,c). In contrast to benzodiazepines and intravenous hypnotics such as propofol or etomidate, DEX has no direct action on gamma-aminobutyric acid (GABA) receptors, explaining its remarkable property of inducing sedative effects without forcing respiratory depression, but instead mimicking natural sleep, thus avoiding respiratory arrest and preventing post-anesthetic amnesia. However, DEX can disinhibit the ventrolateral preoptic nucleus (VLPN), thus indirectly promoting the release of GABA and activating downstream signaling such as the inhibition of the tuberomammillary nucleus involved in arousal4 (Figure 2a). DEX also acts on α2-ARs expressed in the peripheral system and the dorsal horn of the spinal cord, thus inhibiting the release of nociceptive molecules such as norepinephrine, substance P and calcitonin gene-related peptide, in turn impairing pain conduction5–7 (Figures 2a,d). Furthermore, DEX stimulates a systemic increase of acetylcholine, which acts on cholinergic receptors thus attenuating neuro-inflammation and hyperalgesia provoked by neuropathic pain8–11 (Figure 2d). Moreover, DEX-mediated non-narcotic analgesia does not impact on opioid receptors and is therefore non-addictive.12 Nevertheless, by acting on post-synaptic α2-ARs, DEX can modulate hemodynamic responses and consequently induce dose-dependent vasoconstriction- or vasodilatation-mediated side effects including transient hypertension, and hypotension, respectively. In addition, in some cases, DEX was reported to induce reflex bradycardia or cause an atrioventricular block, that can lead to asystole, especially after intravenous loading, altogether encouraging its administration by continuous and progressive infusion13,14 (Figure 2a).
Figure 1.
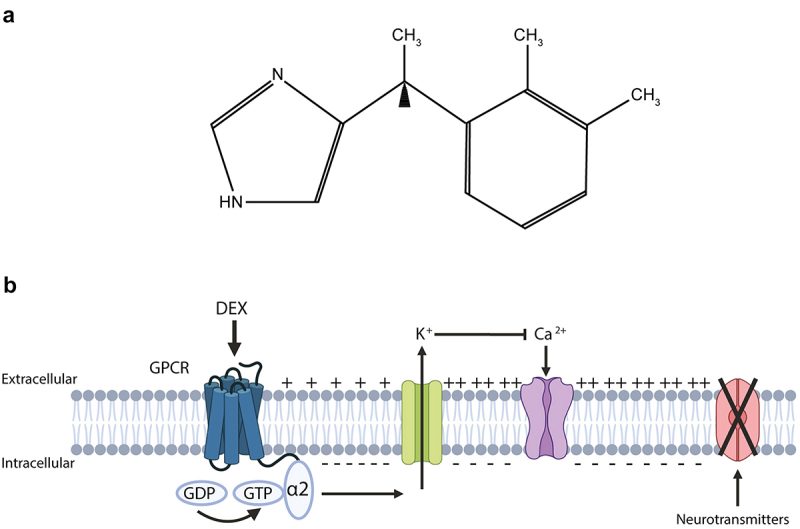
Dexmedetomidine: chemical formula and mode of action.
(a) Chemical formula of dexmedetomidine (4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1 H-imidazole). (b) Upon ligation to α2-adrenoceptor, a G-protein coupled receptor (GPCR), dexmedetomidine (DEX) provokes a conformational change from the GDP-bound to the GTP-bound state, inducing the efflux of potassium and a hyperpolarization of the plasma membrane. Membrane hyperpolarization in turn inhibits the gating of voltage-dependent Ca2+ channels, blocking the release of neurotransmitters. Created with https://www.BioRender.com
Figure 2.
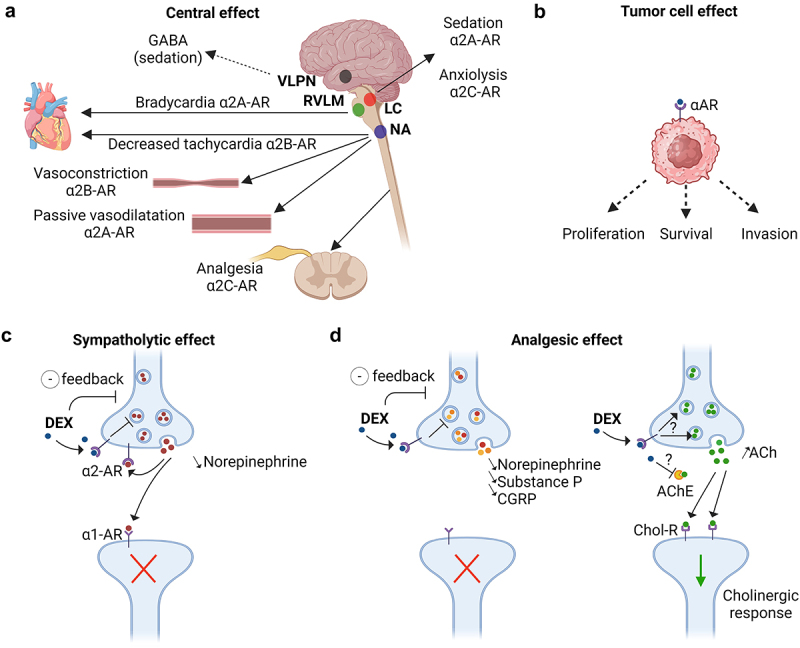
Sympatholytic and analgesic effects of dexmedetomidine through the α2-adrenoceptors.
(a, b, c) α2-adrenoceptors (α2-AR) are composed of four subtypes: α2A, α2B, α2C, and α2D. By acting on α2A-AR and α2C-AR in the locus coeruleus (LC), dexmedetomidine (DEX) decreases the release of norepinephrine from presynaptic neurons inducing sedative and anxiolytic effects. DEX could also disinhibit the ventrolateral preoptic nucleus (VLPN) promoting the release of GABA, which in turn suppresses the tuberomammillary nucleus involved in arousal. The sympatholytic action of DEX on α2A-AR in the rostral ventrolateral medulla (RVLM) and on α2B-AR in the nucleus ambiguous (NA) decreases the heart rate. On the vessels, DEX induces a transient vasoconstriction through α2B-AR, while the link to α2A-AR rather triggers a vasodilatation leading to hypotension. Moreover, DEX was described to act on α-ARs located on the surface of cancer cells, thereby exerting potential both pro- or antitumor effects. (d) DEX exhibits its analgesic properties by binding to the α2A-AR receptors in the dorsal horn of the spinal cord thus decreasing the release of nociceptive molecules such as norepinephrine, substance P and calcitonin gene-related protein (CGRP). In addition, DEX reduces neuropathic pain-induced inflammation and hyperalgesia by increasing the rate of acetylcholine (ACh) and cholinergic signaling through cholinergic receptors (Chol-R). The exact mechanism by which DEX increases the level of ACh, either through α2-AR mediated positive feedback or by inhibiting acetylcholinesterase (AChE) in the synaptic cleft, is unclear. Created with https://www.BioRender.com
DEX can be administered through various injection routes such as intranasal, sublingual, transmucosal, subcutaneous or intramuscular but is most often infused intravenously. In the circulation, DEX is tightly bound to plasma proteins (94%) and unfolds its sedative and analgesic effects within a few minutes after intravenous infusion, with an elimination half-life between 2–4 h. DEX is metabolized in the liver and eliminated by renal clearance, altogether necessitating particular precautions in case of preexisting hepatic and renal dysfunctions.15
At the beginning DEX was clinically employed for the treatment of acute agitation, schizophrenia and bipolar disorders. In addition, DEX was shown to be particularly useful to reverse the hyperactive effects of amphetamines and cocaine abuse. Following the approval by the Food and Drug Administration (FDA) in 1999 and by the European Medicines Agency (EMA) in 2011, DEX was employed for short sedation (less than 24 h) of critically ill patients under mechanical ventilation. In clinical intensive care practice, the use of DEX was extended to the maintenance of artificial coma with the aim to decrease the extubation time during the recovery phase. Currently, anesthesiologists also employ DEX as a sedative agent for awake procedures such as fibroscopic intubation or as a local adjuvant in spinal anesthesia or nerve block. In general anesthesia, DEX is employed for opioid-free-anesthesia (OFA) combining DEX, ketamine, lidocaine, and magnesium to decrease the requirement for the use of opioids, thus minimizing the occurrence of nausea and hallucinations during the post-operative period.16,17
Moreover, DEX was described to act on α-ARs located on the surface of cancer cells, thereby exerting both pro- and antitumor effects (Figure 2b). Recent publications indicate that DEX minimizes surgical stress responses, thus inducing immunostimulatory effects that positively impact anticancer immunity. Here, we summarized preclinical and clinical data as well as ongoing trials focusing on DEX-mediated anticancer effects with the main objective of guiding clinicians in their surge for the ideal oncological anesthesia protocols.
Preclinical studies
The expression of α2-ARs at the plasma membrane surface of many types of cancer cells such as breast carcinoma, non-small cell lung cancer and neuroglioma18,19 led to the hypothesis that DEX may exert direct pharmacological effects on malignant cells.
Initial preclinical investigations showed a cytoprotective effect of DEX in malignant cells under oxygen-glucose deprivation (OGD). DEX prevented OGD-induced cellular injuries by triggering anti-apoptotic PI3K-AKT signaling and by up-regulating the expression of hypoxia and DNA damage response genes including hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF).20 Furthermore, DEX was found to stimulate the hypoxia-induced proliferation of human lung and colorectal carcinoma cells by promoting the expression of survivin, matrix metalloproteinase (MMP) 2 and 9, HIF-1α21 and favoring tumor angiogenesis in human hepatocellular carcinoma cells.22 Under normoxic conditions, low-dose (10 µg/kg) of DEX induced HIF-1α/VEGF-dependent tumor angiogenesis in orthotopically established hepatocellular carcinoma in rodents, whereas high-dose (25 µg/kg) caused cytotoxic effects.22 Additional preclinical work showed that DEX (in a concentration range of 1 nM to 10 µM) promoted the proliferation, migration and survival of tumor cells18,19 by upregulating anti-apoptotic proteins such as BCL-2, BCL-XL, thus retarding cell death, as well as the cyclins A, D and E, that stimulate cell division.19 DEX was also reported to activate the migration of malignant cells through the signal transducer and activator of transcription 3 (STAT3)-mediated secretion of the transmembrane protease serine 2 (TTPRSS2), a member of the transmembrane serine protease family involved in tumor growth and proliferation.23 In addition, DEX was reported to promote the secretion of the proinflammatory cytokine IL-6 in vitro24 and to stimulate tumor growth and the occurrence of secondary lesions in several solid tumors established in rodents.24–26 DEX-induced effects were abolished by the co-injection of yohimbine, an α2-AR antagonist, while phenoxybenzamine, an α1-AR antagonist had no effect, suggesting that DEX-mediated specific protumor effects through α2-AR25,26 (Table 1). Based on these premises, DEX could trigger protumorigenic signaling pathways increasing the supplementation of oxygen to the tumor through neoangiogenesis, encouraging the invasion of distant organs by facilitating the migration of malignant cells through the extracellular matrix with the activation of the specific enzymes MMP, and sustaining survival and tumor cell proliferation through the activation of cyclins and anti-apoptotic molecules.
Table 1.
Preclinical research describing protumor effects of dexmedetomidine.
| Date | Cancer type | Cell lines Animal model |
Effects on cancer | Mode of action | Doses | Ref |
|---|---|---|---|---|---|---|
| 2012 | Glioma | Rat glioma C6 | Protected cell viability of cells exposed to OGD | Anti-apoptotic properties, I2 imidazoline receptor-PI3K/AKT pathway activation, up-regulation of HIF-1α, VEGF and RTP801 expression | In vitro (0.01–10 µM) | 20 |
| 2017 | Breast | Human breast cancer MDA-MB-231 | Increased cell proliferation, migration and invasion in a dose-dependent manner; increased volume of xenograft tumor; clonidine mimics the effects of DEX | Enhanced ERK phosphorylation; upregulated α2-AR | In vitro (0.01, 0.1, 1 µM) | 18 |
| 2018 | Breast Colon Lung |
Rat breast carcinoma MADB 106; mouse Lewis Lung carcinoma 3LL; mouse colon carcinoma CT26; MADB 106 lung metastases model in F344 rats; 3LL lung metastases model in C57Bl/6 mice; CT26 liver metastases model in BALB/c mice | Increased tumor-cell retention, growth of metastases and metastatic burden in different stress models | Effects reversed by yohimbine but not by phenoxybenzamine | In vivo (2, 5, 20, 100 µg/kg/h for 6–12 h) | 25 |
| 2018 | Lung Neuro-glioma | Human lung carcinoma A549; human neuroglioma H4 | Promoted cell proliferation and migration | Increase in cyclins A, D, E and Ki67; upregulated anti-apoptotic proteins (Bcl-2, Bcl-xl); effects reversed by atipamezole | In vitro (0.001–10 nM) | 19 |
| 2018 | Lung | Mouse Lewis Lung carcinoma LLC; 3LL lung metastases model in C57Bl/6 mice | Promoted lung metastases | Increased M-MDSC and promoted VEGF by α2-AR | In vivo (10 µg/kg/h) | 26 |
| 2019 | Colorectal Lung | Human lung carcinoma A549; human colorectal carcinoma HCT116 | Enhanced the progression of cancer cells | Upregulated survivin, MMP-2, MMP-9 and HIF-1α in response to hypoxia | In vitro (1 nM) | 21 |
| 2020 | Breast | Human breast cancer MCF-7, MDA-MB-231 | Increased migration | Secreted TMPRSS2 in exosomes through Rab11 in dose and time-dependent manner; upregulated TMPRSS2 via α2-AR/STAT3 signaling | In vitro (0.01–1 µM) | 23 |
| 2020 | Liver | Human hepatocarcinoma Huh7; mouse hepatocarcinoma Hepa1–6; human hepatic stellate LX-2; Hepa1–6 subcutaneous and orthotopic HCC model in C57Bl/6 mice; H22 and pHSC subcutaneous HCC in BALB/c mice | Promoted proliferation, metastasis, tumor growth and hepatic/pulmonary metastasis in liver fibrosis model | α2-AR expressed on activated hepatic stellate cells (aHSC) but rarely on hepatocellular carcinoma (HCC); induced IL-6 secretion from aHSC; no action on quiescent HSC cells; promoted STAT3 activation of HCC in presence of aHSC; | In vitro (10 µM); in vivo (10 µg/kg) | 24 |
| 2022 | Liver | Human hepatocellular carcinoma SMMC-7721, MHCC97-H; BALB/c nude mice (orthotopic hepatic carcinoma) | In vitro: promoted angiogenesis; in vivo: low dose 10 µg/kg favored angiogenesis but not high dose 25 µg/kg (opposite effect) | In vitro: activation of HIF-1α/VEGFA; in vivo: activation of HIF-1α/VEGFA pathway | In vitro (0.5 µg/ml); in vivo (10 µg/kg, 25 µg/kg for 14 days) | 22 |
Abbreviations: aHSC, activated hepatic stellate cells; AR, adrenoceptor; ERK, extracellular regulated kinase; HCC, hepatocellular carcinoma; HIF, hypoxia-inducible factor; IL, interleukin; MDSC, myeloid-derived suppressive cells; MMP, matrix metalloproteinase; OGD, oxygen-glucose deprivation; PI3K/Akt, phosphoinositide 3 kinase/protein kinase B; STAT3, signal transducer and activator of transcription 3; TMPRSS2, transmembrane protease serine 2; VEGF, vascular endothelial growth factor.
Conversely, more recent publications rather support the idea that DEX compromises tumor growth both in vitro and in vivo.27–39 Specific underlying molecular mechanisms have been unveiled. In vitro, DEX was found to upregulate pro-apoptotic BAX and activate effector caspase but downregulate the anti-apoptotic protein BCL-2.28,30–32,34,39 Furthermore, DEX was shown to promote oxidative stress and iron production in human gastric carcinoma cells leading to the induction of ferroptotic cell death.38,40,41 In addition, DEX impairs cellular migration by decreasing vimentin expression and increasing the adherence of tumor cells via increased expression of E-cadherin. Moreover, DEX inhibits the proliferation of malignant cells by provoking cell cycle arrest via a decrease in cyclin D1 expression.28,32 Some studies also reported an extracellular regulated kinase (ERK) 1/2-dependent inhibition of the c-MYC oncogene, which is amplified in various types of cancer and promotes invasiveness and proliferation.29,34 It is worth noting that most DEX-mediated antitumor effects are provoked by epigenetic changes such as the modification of the expression of non-coding RNAs including miRNAs, circRNAs and lncRNAs. Thus, DEX activates the miR-143-3p/epidermal growth factor receptor (EGFR) pathway substrate 8 axis,28 increases miR-493-5p, which targets RASL11B implicated in cancer development,30 decreases the expression of miR-1307,31 upregulates miR-185, which inactivates the Sry (sex determining region Y)-box 9 (SOX9)/Wnt/B-catenin pathway32 and miR-520a-3p that targets the metastases-inducing gene Yod1 coding for the deubiquitinating enzyme YOD1.33 DEX regulates circular RNAs, thereby affecting the circ -0003340/miR-198/HMGA235 and circ0008035/miR-302a/E2F7 axes.38 Moreover DEX reduces the expression of the long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), thus reducing cellular viability in vitro and decreasing tumor growth in vivo36 (Figure 3). Taken together, these recent data argue that DEX could also mediate direct anti-tumor effects through a variety of mechanisms including epigenetic modifications, the induction of apoptotic and ferroptotic cell death,42 a decrease in tumor cell migration via the expression of E-cadherin and the depletion of vimentin, and an arrest in cell cycle through the cessation of cyclin D1 synthesis.
Figure 3.
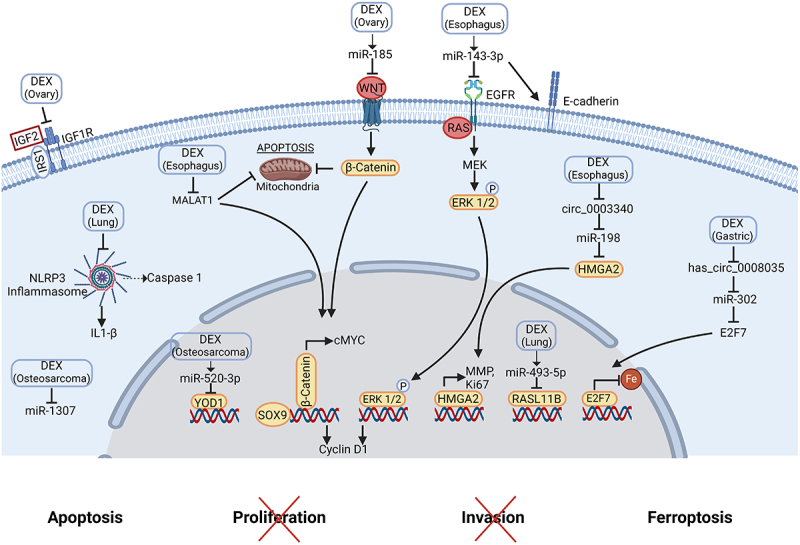
Molecular mechanisms of dexmedetomidine-mediated anti-tumor effects.
DEX suppresses esophagus cancer progression via miR-143-3p/EGFR, by repressing c-MYC, MALAT1 and ERK1/2 expression, by increasing E-cadherin expression, and by regulating circ -0003340/miR-198/HMGA2. Furthermore, DEX promotes ferroptosis in gastric adenocarcinoma through the inhibition of the circ0008035/miR-302a/E2F7 axis. It decreases the proliferation and migration of human osteosarcoma cells and triggers apoptosis via the up-regulation of miR-520a-3p that targets YOD1 and the inhibition of miR-1307. Moreover, DEX inhibits lung tumor growth and favors apoptosis via an up-regulation of miR-493-5p, which targets RASL11B and inhibits aberrant inflammasome activation. DEX induces ovarian cancer cell apoptosis via the up-regulation of miR-185 that inactivates SOX9/Wnt/B-catenin and decreases the invasion and migration by inhibiting IGF2 pathway. Created with https://www.BioRender.com
Abbreviations: DEX, dexmedetomidine; EGFR, epidermal growth factor receptor; ERK, extracellular regulated kinase; HMGA2, high mobility group AT-hook 2; IGF, insulin-like growth factor; IL, interleukin; IRS1, insulin receptor substrate 1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; MMP, matrix metalloproteinase; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; SOX9, Sry (Sex determining region Y)-box 9
In addition to its direct effect on cancer cells, DEX was shown to alleviate stress and inflammatory responses. In vitro, DEX exposure reduces OGD-induced inflammation by attenuating the release of IL-1β, IL-6 and tumor-necrosis factor (TNF)-α.43 In a murine model of surgical stress, DEX significantly reduced the level of cortisol and TNF-α in the postoperative period, correlating with lower tumor burden.44 In metastatic lung tumor xenografts, the continuous injection of DEX for 14 days significantly repressed the release of inflammatory cytokines including IL-1β, IL-18,TNF-α and inhibited inflammasome activation in tumor tissues by impinging on the expression of nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3), carnitine palmitoyltransferase 1A (CPT1A), thioredoxin (TRX)-interacting protein (sTXNIP), adapter protein apoptosis associated speck-like protein containing a CARD (ASC) and caspase 1.37 Interestingly, these readouts illustrate that DEX might induce indirect anti-tumor effects by decreasing surgical inflammatory stress, which favors the proliferation and survival of tumor cells. If confirmed in clinical practice, these results could suggest that DEX is an outstanding adjuvant for controlling protumor inflammation in the per-operative period optimally. DEX was also shown to increase IFN-γ expression and to stimulate effectors of both innate and adaptive anticancer immunity such as natural killer (NK) cells37,44 in ovarian and lung cancer in vivo. Moreover, DEX fosters tumor infiltration by CD4+, CD8+ and NK cells in murine colon adenocarcinoma.39 Finally, DEX could also elicit an immune anti-tumor response by stimulating specific anti-tumor immune effectors to recognize and kill malignant cells and infiltrate tumor bed and its microenvironment45,46 (Table 2).
Table 2.
Preclinical research describing antitumor effects of dexmedetomidine.
| Date | Cancer type | Cell lines Animal model |
Effects on cancer | Mode of action | Doses | Ref |
|---|---|---|---|---|---|---|
| 2019 | Ovary | Rat ovarian adenocarcinoma; NUTU-19; NUTU-19 subcutaneous ovarian model in F344 rats | Improved immune function; inhibited invasion and migration at high dose | Decreased TNF-α, IGF2, IGF1R, IRS1; increased IL-2, IFN-γ, CD4+ and CD8+, CD4+/CD8+ ratio; inhibited IGF2 pathway | In vivo (0.2, 1, 5 µg/kg/h for 15 days) | 27 |
| 2020 | Esophagus | Human esophageal carcinoma KYSE150, Eca-109; KYSE150 ×enograft model | Decreased proliferation and metastasis; suppressed cancer progression | Induction of apoptosis; regulation of miR-143-3p/EGFR pathway substrate 8 |
In vitro (1 µM) In vivo (NA) |
28 |
| 2020 | Glioma | Human glioma U251, U87MG | Decreased cell invasion at 50 nM (and not at 10 nM); cisplatin toxicity was attenuated by 10 nM DEX but enhanced by 50 nM DEX | Increased p-ERK1/2, PI3K and p-AKT; decreased in Ca2+ concentration |
In vitro (10, 50 nM) |
29 |
| 2021 | Bone | Human osteosarcoma MG-63 | Decreased proliferation and migration | Decrease in miR-1307 expression; triggered apoptosis in dose-dependent manner | In vitro (25, 50, 100 ng/ml) | 31 |
| 2021 | Bone | Human osteosarcoma HOS, U2OS | Decreased cell viability, proliferation and adhesion | Promoted apoptosis via up-regulation of miR-520a-3p that targets YOD1 | In vitro (1, 10, 100 ng/ml) | 33 |
| 2021 | Esophagus | Human esophageal carcinoma; Eca109 | Inhibited viability and proliferation | Promoted apoptosis by repressing c-MYC and ERK1/2 protein expression | In vitro (1, 10, 100, 1000 ng/ml) | 34 |
| 2021 | Lung | Human lung adenocarcinoma A549 | Inhibited tumor growth | Favored apoptosis via up-regulation of miR-493-5p, which targets RASL11B | In vitro (1 nM) | 30 |
| 2021 | Ovary | Human ovarian adenocarcinoma; SK-OV-3-Luc cells; BALB/c nude mice | In vivo model of surgery = lower tumor burden at 4 weeks in DEX group | In the DEX group = at day 3, faster NK cell activity, lower cortisol level, lower TNF-α level | In vitro (0.1, 0.5, 1, 5 µg/ml); in vivo (12 µg/kg/j for 4 weeks) | 44 |
| 2021 | Ovary | Human ovarian cancer SKOV3, HO-8910; BALB nude mice | Inhibited cell growth | Decrease in cyclin D1 and c-MYC and increased apoptosis (enhanced cleaved caspase 3 and Bax, decreased Bcl-2) in dose-dependent manner via the up-regulation of miR-185 that inactivates SOX9/Wnt/B-catenin pathway both in vitro and in vivo | In vitro (1–100 nM); in vivo (1 bolus, 100 nM) | 32 |
| 2022 | Esophagus | Esophageal cancer cells KYSE410, Eca109; BALB/c nude mice | Decreased proliferation and invasion | Regulation of circ -0003340/miR-198/HMGA2 | In vitro (50, 100, 200 ng/ml); in vivo (for 3 days) | 35 |
| 2022 | Esophagus | Human esophageal carcinoma Eca109, TE-1; nude mice | Inhibited the viability and decreased tumor growth | Decrease in MALAT1 expression (dose dependent) | In vitro 1 ng/ml-1 mg/ml; in vivo 0.5 mg/kg every 5 days | 36 |
| 2022 | Pheochro-mocytoma | Rat pheochromocytoma PC12 | Inhibited OGD reperfusion | Inhibited IL-1β, TNF-α and IL-6 release; decreased apoptosis (downregulated Bax and caspase-3 by increasing miR-17-5p) | In vitro (1, 10, 50 µM) | 43 |
| 2023 | Breast Colorectal Fibro-sarcoma Lung |
Murine breast cancer EO771, 4T1; murine lung cancer LAP0297; murine Lewis Lung Carcinoma LLC; murine fibrosarcoma MCA205; murine colon adenocarcinoma MCA38, CT26 | Inhibited tumor growth | Promoted apoptosis for MCA38 and CT26 tumors; increased tumor infiltration by CD4+, CD8+, NK in MCA38 tumors | In vivo (1 bolus 25 µg/kg) | 39 |
| 2023 | Gastric | Human gastric adenocarcinoma; SNU-1, AGS; BALB/c nude mice | Decreased cell viability and tumor growth | Increased apoptosis and ROS production; promoted ferroptosis through circ0008035/miR-302a/E2F7 axis; | In vitro (0.1–100 µM); in vivo (0.5, 1, 2 g/kg for 15 days) | 38 |
| 2023 | Lung | Human lung adenocarcinoma A549; BALB/c nude mice | Inhibited tumor growth | Decreased IL-1β, TNF-α, IL-10 and IL-18; increased NK cell activity and IFN-γ expression; suppressed inflammasome (decrease in NLRP3, CPT1A, TXNIP, ASC, IL-1β and caspase 1) | In vivo (20 µg/kg/j for 14 days) | 37 |
Abbreviations: ASC, adapter protein apoptosis associated speck-like protein containing a CARD; CPT1A, carnitine palmitoyltransferase 1A; DEX, dexmedetomidine; EGFR, epidermal growth factor receptor; ERK, extracellular regulated kinase; HMGA2, high mobility group AT-hook 2; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; IRS1, insulin receptor substrate 1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; NK, natural killer; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; OGD, oxygen-glucose deprivation; PI3K/Akt, phosphoinositide 3 kinase/protein kinase B; ROS, reactive oxygen species; SOX9, Sry (Sex determining region Y)-box 9; TNF, tumor necrosis factor; TXNIP, thioredoxin (TRX)-interacting protein.
In sum, these preliminary findings suggest a dual dose-dependent role of DEX. In thus far that low doses of DEX would trigger tumorigenic pathways able to support tumor growth, while clinically relevant concentrations of DEX would facilitate cytotoxic responses and the recruitment of immune effectors against malignant cells.
Published clinical studies
Thirty-one clinical studies (19 randomized controlled trials (RCT), 5 prospective studies, 5 retrospective studies, 2 meta-analyses) investigated the biological effects of DEX administered during oncological surgeries. Among these, four trials attributed a potentially pro-tumorigenic role to DEX. In the study of Liu et al., 29 women undergoing simple or radical mastectomy with sentinel lymph node biopsy were randomly allocated to receive either DEX or normal saline in addition to general anesthesia. Then, human breast cancer MCF-7 cells were cultured and exposed to the serum of patients. The proliferation, migration and invasion were significantly accelerated in presence of sera from DEX-treated patients as compared to control sera.47 In a similar study, 124 patients who underwent radical mastectomy were randomly assigned to be anesthetized with or without an intravenous infusion of DEX. The serum levels of anti-tumor immune effector NK cells and CD8+ T lymphocytes were decreased, while an important increase in inflammatory markers (IL-6 and IL-10) were observed in the treatment group.48 In a prospective trial, Su et al. investigated whether DEX could favor metastases by promoting myeloid-derived suppressor cells (MDSC) after lung surgery. In this study, 103 patients scheduled for thoracotomy were enrolled. Patients treated with DEX during the procedure had more circulating MDSC. In addition, these MDSC were more capable of inducing the synthesis of the neoangiogenic factor VEGF and suppressing T cell proliferation. In vitro, the expansion of MDSC isolated from lung cancer patients was enhanced in the presence of exogenous DEX, while the addition of yohimbine, an α2-AR antagonist, inhibited the DEX-induced proliferative effect, suggesting the involvement of α2-AR in the DEX-mediated protumor effect.26 Finally, a propensity score-matched retrospective study evaluated the impact of intraoperative DEX administered during lung cancer surgery on survival. No association was observed between DEX and recurrence-free survival. However, multivariate analysis indicated that DEX could reduce overall survival.49 One additional retrospective study and two RCTs investigated whether DEX injected during hyperthermic intraperitoneal chemotherapy (HIPEC) for appendiceal carcinomatosis, prostatectomy or hysterectomy could improve postoperative oncological outcome, yet failed to detect improved progression-free, recurrence-free or overall survival.50–52
Conversely, 25 trials indicate that DEX exhibits beneficial effects on oncological patients. Notably, DEX has the ability to alleviate the immunosuppressive glucocorticoid stress produced by surgery. In the double-blind RCT of Kim et al., 143 patients were randomly assigned to receive continuous infusion of DEX or normal saline during thoracic surgery. Here, the inflammatory cytokines IL-8 and IL-10 were significantly lower 1 h after the intervention.53 Similarly, in the prospective study by Zhou et al., the group treated with a loading dose of DEX had significantly lower circulating level of IL-8 and TNF-α after lung cancer surgery. This effect was directly correlated to the downregulation of miR-10a.54 Three RCTs and one retrospective trial studied intraoperative immune effects of DEX during gastric, esophageal and ovarian cancer surgery. The authors observed a stable level55,56 or decline of C-reactive protein (CRP), IL-6, IL-10 or TNF-α in the treated group.57,58 Two meta-analyses including 17 RCTs and 11 RCTs focused on the employment of DEX during major digestive and lung cancer procedures respectively. Both meta-analyses concluded on a DEX-induced decrease in protumor and inflammatory cytokines such as CRP, IL-6, IL-8 and TNF-α.59,60 Many other studies dealing with the surgical removal of various solid primary tumors demonstrated that patients receiving intravenous DEX during the perioperative period had an attenuated increase in glucocorticoid stress compared to the control group.61–70 Interestingly, two trials observed that DEX affected the activity of the corticotropic axis by attenuating the concentrations of circulating catecholamines and adrenocorticotropic hormone (ACTH).53,71 DEX also compromised the synthesis of protumor biomarkers, and key regulators of invasiveness. In the RCT by Ren et al., 132 non-small cell lung cancer patients operated with video-assisted thoracic surgery were enrolled and received DEX, lidocaine, DEX+lidocaine or saline solution. Patients of the DEX group produced significantly less neutrophil extracellular traps (NETs), MMP-3, MMP-9 and VEGF involved in cancer-associated inflammation, angiogenesis and the migration of residual cancer cells to the distant organs. DEX-mediated effects were further potentiated by concomitant use of lidocaine.72–74 Similarly, in the peripheral blood of patients undergoing hysterectomy under DEX infusion, Cho et al. observed an increase in the cytolytic cytokine IFN-γ, which is produced by the T lymphocytes to kill residual cancer cells.52 In the RCT of Huang et al., the authors included 34 patients for oral cancer surgery, known to be highly painful and inflammatory. Intraoperative infusion of DEX hampered the production of circulating immunosuppressive MDSCs, thus decreasing escape from immunosurveillance.75–77 Some trials noticed that DEX limited the decrease of immune effectors such as dendritic cells (DC), B, T and NK cells and the increase of regulatory T cells (Treg) as compared to control groups.61,63,72,75,78,79 The phase 3 RCT of Mohamed et al. explored whether wound infiltration of DEX together with the local anesthetic bupivacaine could decrease consumption of opioids and minimize glucocorticoid stress by optimal pain control during hysterectomy compared to the combination of ketamine and bupivacaine. The combination of DEX with ketamine significantly attenuated stress responses and had an opioid-sparing effect.80 Taken together, these data can be interpreted to suggest that DEX impedes the immunosuppressive effects of surgical stress (Table 3). Conversely to preclinical investigations, accumulating clinical data reveal that during oncological procedures DEX alleviates surgical glucocorticoid stress and inflammation, both known to stimulate tumorigenic signaling and to impair the anticancer immune response. It is tempting to speculate that these characteristics might be employed to reinforce anticancer immunity during oncosurgery.81
Table 3.
Published clinical trials studying the dexmedetomidine-induced biological effects potentially related to oncological outcome.
| Cancer surgery | Study type | Study design | Biological effects of DEX | Ref |
|---|---|---|---|---|
| Appendiceal carcinoma-tosis | Retrospective | After matching: -study group DEX 0.075–0.3 µg/kg/h (n = 107); -control group NaCl + volatile (n = 107) |
No association with improved PFS or OS | 50 |
| Brain | RCT | Forty children randomly allocated in: -study group DEX 0.5 µg/kg/h (n = 20); -control group NaCl (n = 20) |
Decrease in CD3+, CD4+, CD4+/CD8+, NK and B cells at 1 h and at POD1 compared to pre-anesthesia but less than control group (p < 0.05) | 78 |
| Breast | RCT | Twenty-nine women randomly allocated in: -study group DEX total dose 2 µg/kg (n = 16); -control group NaCl (n = 13) |
Increase in proliferation, migration, and invasion of human breast cancer MCF-7 cells exposed to the serum of treated patients (p < 0.001) | 47 |
| Breast | RCT | One hundred and twenty-four patients randomly allocated in: -study group DEX 0.1 µg/kg/min (n = 62); -control group NaCl (n = 62) |
Decrease in NK, CD8+ (p < 0.05); increase in CD4+, CD4+/CD8+, INF-γ, IL-2, IL-6, IL-10 (p < 0.05) | 48 |
| Colon | RCT | One hundred and forty-one patients randomly allocated in: -study group DEX 1 µg/kg/h (n = 72); -control group NaCl (n = 69) |
Increase in CRP, IL-6 and IL-8 but less than control group Decrease in CD4+, CD4+/CD8+, Th1 and increase in Treg but less than control group (p < 0.05) |
61 |
| Colon | Prospective | One hundred and seventy-six patients allocated in: -study group DEX 200 µg (n = 92); -control group NaCl (n = 84) |
Decrease in CD3+, CD4+, CD4+/CD8+ but less than control group (p < 0.05) | 79 |
| Colon | Prospective | One hundred and forty patients randomly allocated in: -study group DEX 1 µg/kg (loading dose) then 0.2–0.7 µg/kg/h (continuous) (n = 80); -control group NaCl (n = 60) |
Increase in IL-6 but less than control group (p < 0.05) | 62 |
| Digestive | Meta-analysis | Seventeen studies (RCTs) = 1,619 patients | Decrease in CRP, TNF-α, and IL-6 (p < 0.01); increase in IL-10, CD4+, CD4+/CD8+ (p < 0.05) |
59 |
| Esophagus | RCT | Sixty-two patients randomly allocated in: -study group DEX 0.5 µg/kg (loading dose) then 0.2–0.4 µg/kg/h (continuous) then 0.06 µg/kg/h for 5 days (n = 31); -control group NaCl (n = 31) |
No changes in leucocytes, IL-6, IL-10, CRP | 55 |
| Gastric | RCT | Fifty-five patients randomly allocated in: -study group DEX 0.2–0.7 µg/kg/h (n = 18); -control group Remifentanil (n = 18); -control group Sufentanil (n = 19) |
Increase in serum levels of glucose, IL-6, β-EP and decrease in IFN-γ but less than other groups. Significant decrease in IL-10 and increase in IL-18 compared to other groups (p < 0.05) | 70 |
| Gastric | RCT | Seventy-four patients randomly allocated in: -study group DEX 0.2 µg/kg/h (n = 37); -control group NaCl (n = 37) |
Increase in IL-1β, IL-6, TNF-α, NF-κB and CRP but less than control group (p < 0.05); decrease in T cells, CD4+/CD8+ but less than control group (p < 0.05) | 63 |
| Gastric | Retrospective | One hundred and two patients allocated in: -study group DEX 0.5 µg/kg (loading dose) then 0.2–0.4 µg/kg/h (continuous) (n = 52); -control group NaCl (n = 50) |
Increase in serum levels of cortisol, ACTH, TNF-α, IL-6 but less than control group (p < 0.05) | 64 |
| Gastric | RCT | Forty patients randomly allocated in: -study group DEX 0.5 µg/kg (loading dose) then 0.4 µg/kg/h (continuous) (n = 20); -control group NaCl (n = 20) |
Increase in Th1/Th2 (p < 0.05); no significant increase in IL-6 and TNF-α | 56 |
| Gastric | RCT | Seventy-eight patients randomly allocated in: -study group DEX 0.1 µg/kg (loading dose) (n = 39); -control group NaCl (n = 39) |
Decrease in IL-1β, IL-6, TNF-α, CRP (p < 0.05) |
57 |
| Lung | Prospective | One hundred and three patients allocated in: -study group DEX (median 122 µg [118–146] i.v.) (n = 51); -control group NaCl (n = 52) |
Increase in M-MDSC (p < 0.0001) by α2-AR; MDSC more efficient in producing VEGF and in suppressing T cells proliferation (p < 0.01) |
26 |
| Lung | RCT | One hundred and sixteen patients randomly allocated in: -study group DEX 0.3 µg/kg/h (n = 58); -control group NaCl (n = 58) |
Increase in serum levels of IL-6, IL-8, TNF-α, MDA but less than control group (p < 0.05) | 65 |
| Lung | RCT | Ninety patients randomly allocated in: -study group DEX 1 µg/kg/h (n = 30); -study group lidocaine (n = 30); -control group NaCl (n = 30) |
Increase in IL-6 and TNF-α but less than control group (p < 0.05) | 66 |
| Lung | Retrospective | Ninety patients allocated in: -study group DEX 0.4 µg/kg/h (n = 48); -control group NaCl (n = 42) |
Increase in serum levels of IL-8, IL-10, TNF-α but less than control group (p < 0.05) | 67 |
| Lung | Prospective | One hundred and twelve patients allocated in: -study group DEX 1 µg/kg (loading dose) then 0.3 µg/kg/h (continuous) (n = 59); -control group NaCl (n = 53) |
Increase in serum levels of IL-8, TNF-α but less than control group (p < 0.05) correlated to the decrease in miR-10a expression; increase in MDA; decrease in SOD | 54 |
| Lung | RCT | One hundred and thirty-two patients randomly allocated in: -study group DEX 2 µg/kg/h (loading dose) then 0.5 µg/kg/h (continuous) then 0.25 µg/kg/h POD1 (n = 33); -study group lidocaine (n = 33); -study group DEX + lidocaine (n = 33); -control group NaCl (n = 33) |
Decrease in the production of NETs, MMP-3, MMP-9, VEGF (p < 0.001), effects potentiated by lidocaine (p < 0.001); decrease in NK and IFN-γ/IL-4 but less than control group (p < 0.05) | 72 |
| Lung | Retrospective | After matching: -study group DEX (n = 251) (median 100 µg [57.47–140] i.v.); -control group NaCl (n = 251) |
Decreased OS (HR = 1.25, 95%CI [1.03–1.59], p = 0.024) | 49 |
| Lung | Meta-analysis | Eleven studies (RCTs) = 1,026 patients | Decrease in IL-6, IL-8 and TNF-α (p < 0.01) | 60 |
| Lung | RCT | One hundred and twenty patients randomly allocated in: -study group DEX 0.7 µg/kg (loading dose) then 0.3 µg/kg/h (continuous) (n = 60); -control group NaCl (n = 60) |
Increase in serum levels of IL-1β, IL-6, IL-10, TNF-α but less than control group (p < 0.001) | 68 |
| Lung | RCT | Forty patients randomly allocated in: -study group DEX 0.5 µg/kg/h (n = 20); -control group NaCl (n = 20) |
Increase in IL-8 but less than control group (p < 0.05) | 69 |
| Lung | RCT | One hundred and forty-three patients randomly allocated in: -study group DEX 0.5 µg/kg/h (n = 73); -control group NaCl (n = 70) |
Significant decrease in IL-8 (p = 0.02), IL-10 (p = 0.002), epinephrine and norepinephrine (p < 0.001) | 53 |
| Oral | RCT | Sixty-eight patients randomly allocated in: -study group DEX 0.5 µg/kg (loading dose) then 0.4 µg/kg/h (continuous) (n = 34); -control group NaCl (n = 34) |
Decrease in CD3+, CD4+, CD4+/CD8+, DCs but less than control group Significant decrease in MDSC (p < 0.05) |
75 |
| Ovarian | Retrospective | Three hundred and forty-three patients enrolled in: -study group DEX (n = 169) i.v.; -control group MDZ (n = 174) |
Decrease in serum levels of TNF-α and IL-6 (p < 0.05) | 58 |
| Prostate | RCT | One hundred and forty-six patients randomly allocated in: -study group DEX 0.2–0.7 µg/kg/h (n = 73); -control group without opioid (n = 71) |
No impact on RFS | 51 |
| Thyroid | Prospective | Ninety-six patients allocated in: -study group DEX 0.5 µg/kg (n = 49); -control group NaCl (n = 47) |
Decrease in MCP-1, ACTH, NE (p < 0.001) | 71 |
| Uterus | RCT | One hundred patients randomly allocated in: -study group DEX 0.4 µg/kg/h (continuous) then 0.15 µg/kg/h POD1 (n = 50); -control group NaCl (n = 50) |
Increase in IFN-γ (p = 0.003); no difference on NK cell activity, inflammatory response; no difference in recurrence, metastases or death | 52 |
| Uterus | RCT | Ninety patients randomly allocated in: -study group DEX 2 µg/kg + bupivacaine (local infiltration) (n = 30); -study group ketamine + bupivacaine (local infiltration) (n = 30); -control group bupivacaine (local infiltration) (n = 30) |
DEX + bupivacaine: opioid-sparing effect; no increase in cortisol, prolactin and glucose compared to the control group (p < 0.05) | 80 |
Abbreviations: ACTH, adrenocorticotropic hormone; AR, adrenoceptor; β-EP, β-enkephalin; CI, confidence interval; CRP, C-reactive protein; DC, dendritic cell; DEX, dexmedetomidine; HR, hazard ratio; IFN, interferon; IL, interleukin; i.v., intravenous; MCP-1, monocyte chemotactic protein-1; MDA, malondialdehyde; MDSC, myeloid-derived suppressor cells; MDZ, midazolam; MMP, matrix metalloproteinase; NA, non-applicable; NaCl, normal saline; NE, norepinephrine; NETs, neutrophil extracellular traps; NF-κB, nuclear factor kappa B; NK, natural killer cell; OS, overall survival; PFS, progression-free survival; POD, postoperative day; RCT, randomized controlled trial; RFS, recurrence-free survival; SOD, superoxide dismutase; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Ongoing studies
Completed trials
Eight RCTs and one observational prospective study evaluated whether DEX used during primary tumor resection could indirectly impact on oncological outcome. Three studies have specifically investigated if DEX modified the immune system. The number and the activity of leucocytes, in particular T-cells and NK cells collected from the peripheral blood, were compared prior and after the administration of intravenous DEX during breast and uterine cancer surgery (NCT01692210; NCT03109990; NCT02896413). Three trials are measuring whether DEX modifies the inflammation response and notably the secretion of the protumoral cytokine IL-6 after major oncological procedures generating high level of neuroendocrine and cytokine response such as HIPEC for colon malignancies, gastrectomy and thoracotomy (NCT03370588; NCT03960775; NCT04007341). Two trials are determining if the continuous administration of DEX could influence the inflammatory response during and after robotic or laparoscopic gastrectomy and hysterectomy by assessing various inflammatory molecules such as CRP, IL-6, IL-8, IL-10 and TNF-α (NCT03960775; NCT02896413). Interestingly, the study NCT06037135 aims to assess if DEX can decrease the release of catecholamines and preserve hemodynamic stability during and after the removal of active pheochromocytoma. The phase 3 trial NCT04148599 is designed to compare intravenous DEX, intravenous lidocaine and placebo in reduction of inflammatory markers (IL-6 and TNF-α) and stress reaction (insulin, lactate) after pelvi-abdominal cancer resection. Two pilot studies focus on the potential impact of DEX on recurrence-free and overall survival after breast cancer interventions and HIPEC (NCT03109990; NCT03370588). The NCT02739958 trial is also exploring whether continuous infusion of DEX with the intravenous hypnotic propofol can maintain the CD3+ T cells plasma level compared to the association of volatile hypnotic isoflurane with the opioid fentanyl during total laryngectomy surgery (Table 4). These completed trials focused on the potential impact of DEX on immune cells in particular in the context of minimally invasive procedures. In sum these results could lead to a reorientation of DEX toward specific protocols taking into consideration the level of surgical inflammation.
Table 4.
Completed trials investigating the role of dexmedetomidine on cancer outcome.
| Cancer | Design | Study type | Oncological endpoints | Potential endpoints related to cancer outcome | Phase | NCT number |
|---|---|---|---|---|---|---|
| Breast | DEX i.v. (1 bolus then continuous) | Obs | Pre- and postsurgical WBC count and function (NK cells) | NA | NCT01692210 | |
| Breast | DEX i.v. (1 bolus then continuous) vs placebo | RCT | RFS, OS | Number of CD3+, CD4+, CD8+, CD19+, NK cells | NA | NCT03109990 |
| Colon | DEX i.v. (continuous) vs placebo | RCT | Recurrence at 1 year | IL-6 level | NA | NCT03370588 |
| Gastric | DEX i.v. (continuous) vs placebo | RCT | CRP level, cytokine level (IFN-r, TNF-α, IL-6, IL-8, IL-10, HMGB1), WBC level | NA | NCT03960775 | |
| Larynx | DEX i.v. (continuous) + propofol vs isoflurane + fentanyl | RCT | CD3+ plasma level | NA | NCT02739958 | |
| Lung | DEX i.v. (1 bolus then continuous) vs placebo | RCT | IL-6 level | NA | NCT04007341 | |
| Pelvi-abdominal | DEX i.v. (1 bolus then continuous) vs lidocaine i.v. (continuous) vs placebo | RCT | Change in plasma levels of inflammatory mediators (IL-6, TNF-α), and stress markers (insulin, lactate) | 3 | NCT04148599 | |
| Pheochro-mocytoma | DEX i.v. (continuous) vs placebo | RCT | Level of catecholamines (epinephrine, norepinephrine) | NA | NCT06037135 | |
| Uterus | DEX i.v. (continuous) vs placebo | RCT | NK cell cytotoxicity; inflammatory response | NA | NCT02896413 |
Abbreviations: CRP, C-reactive protein; DEX, dexmedetomidine; HMGB1, high-mobility group box 1; IFN, interferon; IL, interleukin; i.v. intravenous; NA, non-applicable; NK, natural killer; Obs, observational; OS, overall survival; RCT, randomized controlled trial; RFS, recurrence-free survival; TNF, tumor necrosis factor; WBC, white blood cells.
Ongoing trials
Eight ongoing RCTs plan to investigate whether intraoperative DEX might improve the prognosis of cancer patient. Four studies are designed to evaluate whether DEX decreases the incidence of recurrences and enhances the survival after oncological surgery (NCT05742438; NCT04106999; NCT03012971; NCT06030804). Whereas these trials compare DEX to placebo, the study NCT05742438 investigates whether DEX might be superior to intravenous infusion of lidocaine or to intrathecal injection of morphine to decrease the relapses as well as protumor factors secreted during oncological procedures such as MMP-2, MMP-9, IL-6 and VEGF. In addition, the phase 2–3 study NCT04106999 investigates whether DEX reduces postoperative inflammatory markers in the peripheral blood such as CRP, erythrocyte sedimentation rate (ESR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), plasma viscosity and lactate on postoperative day 5. Three trials are exploring the surgical glucocorticoid stress generated by inflammatory pain during breast, pituitary, and uterine surgery (NCT02549768; NCT03524950; NCT03046238). These trials assess the plasma level of ACTH and cortisol prior and after the surgery and hypothesize that the intravenous DEX alone or DEX co-injected with the local anesthetic bupivacaine in pectoralis block might decrease the surgery-induced immunosuppressive stress. Moreover, the phase 1–2 NCT03024957 study investigates whether the combinations of DEX and bupivacaine or DEX and morphine injected through spinal anesthesia induces changes in cellular immunity and protumor inflammatory cytokine release immediately after and 1 day after surgery compared to the classically used association morphine and bupivacaine (Table 5). Of note, one retrospective study and two RCTs are evaluating whether DEX employed in an opioid-free anesthesia (OFA) protocol during primary solid tumor resection can minimize the secretion of circulating protumor factors, alleviate inflammatory and glucocorticoid stress, reduce the occurrence of relapses, and improve survival after surgery compared to the control groups receiving general anesthesia comprising opioids (NCT05448586; NCT04529135; NCT05172739) (Table 6). Some of these studies have protocols designed to mimic clinical practice by combining DEX with anti-tumor anesthetics such as lidocaine while avoiding the administration of opioids. Altogether, ongoing trials will yield additional data on the question whether the use of DEX could improve oncological outcomes by decreasing the incidence of recurrences.
Table 5.
Ongoing trials investigating the role of dexmedetomidine on cancer outcome.
| Cancer | Design | Study type | Oncological endpoints | Potential endpoints related to cancer outcome | Phase | Status | NCT number |
|---|---|---|---|---|---|---|---|
| Abdominal | DEX and bupivacaine i.t. vs DEX and morphine and bupivacaine i.t. vs morphine and bupivacaine i.t. | RCT | Change in cellular immunity (CD3+, CD4+, CD8+, CD16+, CD56+), change in cytokines (IL-1β, IL-6, IL-10, TNF) | 1–2 | Unknown | NCT03024957 | |
| Breast | DEX and bupivacaine in PECS block vs bupivacaine alone | RCT | Opioid consumption; pain; cortisol level | 3 | Unknown | NCT03046238 | |
| Colorectal | DEX (bolus then continuous) vs lidocaine i.v. (continuous) vs morphine i.t. | RCT | Recurrence | MMP-2, MMP-9, IL-6, VEGF Lymphocyte subset |
NA | Recruiting | NCT05742438 |
| Pituitary | DEX i.v. (bolus then continuous) vs placebo | RCT | Cortisol, ACTH Pain |
4 | Unknown | NCT02549768 | |
| Solid tumors | DEX i.v. (continuous) vs placebo | RCT | Recurrence | Inflammation (CRP, ESR, NLR, PLR, plasma viscosity, lactate) Opioid use |
2–3 | Not yet recruiting | NCT04106999 |
| Solid tumors | DEX i.v. (continuous) vs placebo | RCT | RFS, OS, cancer-specific survival; event-free survival | NA | Active, not yet recruiting | NCT03012971 | |
| Solid tumors | DEX i.v. (bolus then continuous) vs placebo | RCT | PFS, OS, cancer-specific survival; event-free survival | Pain | NA | Recruiting | NCT06030804 |
| Uterus | DEX i.v. (bolus then continuous) vs placebo | RCT | ACTH | 3 | Unknown | NCT03524950 |
Abbreviations: ACTH, adrenocorticotropic hormone; DEX, dexmedetomidine; ESR, erythrocyte sedimentation rate; IFN, interferon; IL, interleukin; i.t. intrathecal; i.v. intravenous; MMP, matrix metalloproteinase; NA, non-applicable; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PECS, pectoralis; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; RCT, randomized controlled trial; RFS, recurrence-free survival; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Table 6.
Completed and ongoing trials investigating the role of OFA on cancer outcome.
| Tumor type | Design | OFA protocol | Study type | Oncological endpoints | Potential endpoints related to cancer outcome | Phase | NCT number |
|---|---|---|---|---|---|---|---|
| Breast Cervix Endometrial Ovarian | OFA vs standard GA (with opioids) | Dexmedetomidine +ketamine +lidocaine +propofol | Retrospective | Recurrence at 12 months Survival at 12 months |
Postoperative Systemic Inflammatory Response (CRP, WBC, platelets) | NA | NCT05448586 |
| Gastric | OFA vs standard GA (with opioids) | Dexmedetomidine +propofol | RCT | Readmission rate at 3 months | Pain, analgesic requirement | NA | NCT04529135 |
| Lung | OFA vs standard GA (with opioids) | Dexmedetomidine +hyoscine +ketamine +lidocaine +midazolam +pregabalin +propofol | RCT | Inflammation (LMR, NLR, PLR); surgical stress response (IL-1, IL-6, IL-8, IL-10, TNF-α, CRP, WBC, AVP, cortisol, HIF-1a, VEGF, NF-κB); pain | 4 | NCT05172739 |
Abbreviations: AVP, arginine-vasopressin; CRP, C-reactive protein; GA, general anesthesia; HIF, hypoxia-inducible factor; IL, interleukin; LMR, lymphocyte-to-monocyte ratio; NA, non-applicable; NK-κB, nuclear factor kappa B; NLR, neutrophil-to-lymphocyte ratio; OFA, opioid-free anesthesia; OS, overall survival; PLR, platelets-to-lymphocyte ratio; RCT, randomized controlled trial; RFS, recurrence-free survival; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; WBC, white blood cell.
Discussion
Previous basic research indicates that many analgesic agents used in clinical practice, such as local anesthetics, intravenous hypnotics or beta-blockers, might positively influence oncological outcomes.82–84 Here, we compiled evidence suggesting that the α2-AR agonist DEX, which is currently used in intensive and anesthetic care for its sedative and anxiolytic properties, exerts direct cytolytic effects on malignant cells and induces indirect anti-tumor immunity by reducing inflammatory pain with the consequent production of stress hormones and protumorigenic cytokines. Indeed, acute pain and inflammation favor oncogenesis by generating broad surgical stress responses. Nociception produced during the surgical resection of primary solid tumors, activates the surge of several stress hormones including ACTH, cortisol and catecholamines. ACTH decreases the synthesis of immunoglobulins, while cortisol and catecholamines alter the proliferation and activity of circulating leucocytes and negatively impact the infiltration of immune cells into the tumor microenvironment (TME).85,86 In addition, catecholamines directly stimulate the proliferation, invasiveness and survival of residual tumor cells by acting on α and β-ARs located on the plasma membrane surface of malignant cells, thus promoting the occurrence of secondary lesion in distant organs.87–95 DEX is well-known for efficiently decreasing stress responses and exerting immunoprotective effects.96,97 In rodent models of inflammation induced by injection of endotoxin, bone fracture or local pancreatic injury, DEX significantly reduced markers of inflammation such as IL-1β, IL-6 and TNF-α. In these models, DEX optimally controlled pain via the stimulation of the cholinergic anti-inflammatory pathway as indicated by the absence of additional beneficial effects of vagotomy or co-injection with acetylcholine receptors antagonists.9–11 In addition, DEX was described to minimize glucocorticoid stress by impairing the release of catecholamines and cortisol during major non-oncological surgeries98–100 leading to the hypothesis that DEX could promote similar effects during oncological procedures. In analogy to β-blockers, which mediate anti-tumor properties by directly acting on β-ARs expressed on malignant cells, DEX might interfere with procarcinogenic effects of epinephrine and norepinephrine acting on α-ARs on cancer cells.18,19,101–104 Based on these premises, further investigations are exploring the potential anti-tumor property of DEX. As veterinarians currently use DEX for sedation and general anesthesia during surgical procedures in a large variety of animals, the pharmacodynamics and pharmacokinetics of DEX are relatively well known, allowing impactful studies in rodent models. First in vitro and in vivo studies reported paradoxical results in thus far that DEX induced concentration-dependent and cell type-specific effects that often were divergent with respect to their oncological outcome.22,29 Nevertheless, when employed at clinically relevant concentrations through continuous infusion, DEX tends to promote antineoplastic effects and hence impairs the proliferation, invasiveness and survival of malignant cells.27–39,43,44 In addition, treatment of distinct types of tumors with DEX significantly limited inflammasome activation, thus decreasing the secretion of IL-1β that is mostly considered as a protumorigenic cytokine.105–107 Altogether, DEX alters the secretion of protumor inflammatory cytokines and alleviates surgical glucocorticoid stress, thus reducing tumor growth and limiting the spread of circulating tumor cells to distant organs. Published clinical research also revealed that DEX slows or halts surgery-induced immunosuppression.53,55,56,58,61–70,72,75,78,79 However, most studies reported a relatively moderate decrease in CD4+ T cells in DEX treated patients (as compared to CTLs), though did not investigate the true phenotype of CD4+ T cells, which also includes immunosuppressive regulatory T cells (Tregs). Two meta-analyses indicated that DEX administered during cancer surgery reduced the secretion of protumorigenic inflammatory cytokines such as IL-6 and TNF-α but increased the levels of circulating T cells. It should be noted that not all included RCTs have investigated DEX-mediated immunomodulation and its consequences on immunosurveillance59,60 (Figure 4). Very few RCTs aimed to evaluate the impact of DEX on recurrence-free survival or overall survival, yielding mostly inconclusive results. These studies were either retrospective, suffered from a lack of inclusions or were affected by confounding factors such as procedures afflicted by high morbidity and mortality.50 In addition, the study of Cata et al. involved important confusion biases such as aged patients with aggressive types of tumors and co-morbidities (ASA 3–4).50 Moreover, among the completed or ongoing studies evaluating the survival, NCT03370588 and NCT04106999 are including patients undergoing HIPEC surgery, which is a major procedure associated with high morbidity (22–50%) and mortality (2–5%), rendering difficult the interpretation of the results.108 Similarly, the trial NCT02739958 is enrolling patients operated for total laryngectomy, which is one of the most painful and inflammatory otorhinolaryngological procedures. None of these studies evaluated the possibility of synergistic effects of DEX associated with other agents.109–113 Interestingly, three ongoing trials are investigating the benefits of DEX combined with the local anesthetic, bupivacaine, administered through intrathecal, loco-regional or local injection, on the decrease in inflammatory stress markers and protumor factors such as MMP-2, MMP-9 and VEGF. Finally, three ongoing clinical trials focus on the OFA protocol combining DEX with local anesthetics, ketamine, magnesium, and propofol to reduce or avoid the requirement of opioid during surgery. Ample preclinical and clinical evidence indicates that local anesthetics and propofol mediate anti-tumor activity whereas opioids rather stimulate oncogenesis. Thus, OFA offers a possibility to potentiate the anticancer effects of DEX, while avoiding the use of protumorigenic agents. Of note, no clinical study using DEX with intravenous loading dose and continuous infusion, noticed severe side effects such as bradycardia or hypotension, which would have led to the termination of the trial. In sum, DEX appears as a safe and promising perioperative antitumor agent. Further data are expected to definitively prove the capacity of DEX to sustain anticancer immunity and to improve disease outcome after cancer surgery.
Figure 4.
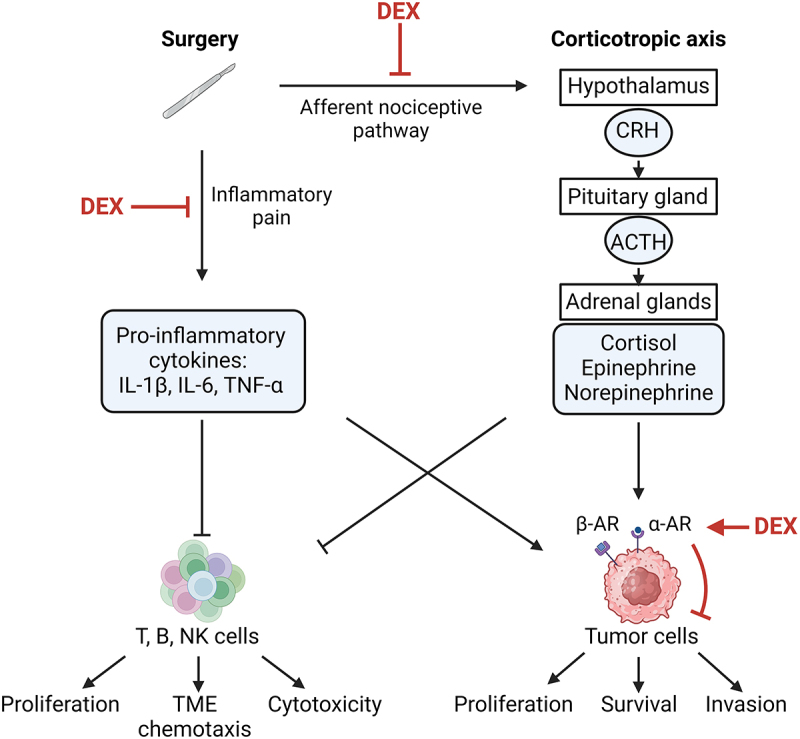
Scheme of central and peripheral actions of dexmedetomidine.
Surgery-induced inflammatory pain activates the corticotropic axis via the stimulation of afferent nociceptive pathways and promotes local production of protumor cytokines such as IL-1β, IL-6 and TNF-α. The hypothalamus produces corticotropin-releasing hormone (CRH), which stimulates the synthesis of adrenocorticotropic hormone (ACTH) by the pituitary gland. In response to ACTH, adrenal glands release cortisol and catecholamines (epinephrine and norepinephrine) into the systemic circulation. Catecholamines, potentiated by tumorigenic cytokines, act on α- and β-adrenoceptors (α-AR, β-AR) located on the surface of tumor cells to enhance their proliferation, survival and migration. These protumor molecules inhibit the chemotaxis and cytotoxicity of the immune effectors (T, B, NK cells) in the tumor bed and its microenvironment (TME). Dexmedetomidine (DEX) could alleviate both corticotropic axis activity and the release of protumor cytokines by optimally controlling inflammatory pain. Through its agonist effect on α-adrenoceptor, DEX might also impair the malignant properties of tumor cells directly. Created with https://www.BioRender.com
Conclusion
Accumulating preclinical and clinical evidence suggests that dexmedetomidine (DEX) exhibits anti-tumor effects, by directly acting on malignant cells to halt proliferation, invasion and survival, while indirectly reducing inflammatory stress responses produced by the surgical procedure, thus stimulating anticancer immunity. We must deplore the absence of prospective randomized trials investigating the impact of the administration of DEX on recurrence-free and overall survival, which would allow to recommend the broad clinical application of DEX in onco-anesthesia. Furthermore, we believe that additional trials should confirm the utility of DEX in opioid-free-anesthesia protocols that combine several anesthetic agents such as local anesthetics for the avoidance or reduction of adverse opioid effects as well as improved oncological outcome. Future clinical investigations will need to confirm such DEX-mediated anticancer effects.
Acknowledgments
KCLP receives funding from Agence Régionale en Santé (ARS) Ile de France Année-Recherche Pharmacie; OK receives funding from Institut National du Cancer (INCa) and Association pour la recherche sur le cancer (ARC); GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council (ICD-Cancer), European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; INCa; Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. LB receives funding from Société Française d’anesthésie-réanimation (SFAR); the Ligue contre le Cancer, and the Fondation Monahan.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Abbreviations
- Ach
Acetylcholine
- AChE
Acetylcholinesterase
- aHSC
Activated hepatic stellate cells
- AR
Adrenoceptor
- ASC
Adapter protein apoptosis speck-like protein containing a CARD
- AVP
Arginine-vasopressin
- CI
Confidence interval
- CPT1A
Carnitine palmitoyltransferase 1A
- DC
Dendritic cell
- DEX
Dexmedetomidine
- EGFR
Epidermal growth factor receptor
- ENT
Ear-Nose-Throat
- ERK
Extracellular regulated kinase
- ESR
Erythrocyte sedimentation rate
- GA
General anesthesia
- GABA
Gamma-aminobutyric acid
- HCC
Hepatocellular carcinoma
- HIF
Hypoxia-inducible factor
- HIPEC
Hyperthermic intraperitoneal chemotherapy
- HMGA2
High mobility group AT-hook 2
- HR
Hazard ratio
- IFN
Interferon
- IGF
Insulin-like growth factor
- IL
Interleukin
- IRS1
Insulin receptor substrate 1
- IV
Intravenous
- LMR
lymphocyte-to-monocyte ratio
- MALAT1
Metastasis associated lung adenocarcinoma transcript 1
- MMP
Matrix metalloproteinase
- NETs
Neutrophil extracellular traps
- NK
Natural killer
- NF-Κb
Nuclear factor kappa B
- NLR
neutrophil-to-lymphocyte ratio
- NLRP3
NOD-like receptor family pyrin domain containing 3
- OFA
Opioid-free-anesthesia
- OGD
Oxygen-glucose deprivation
- OS
overall survival
- PI3K/Akt
Phosphoinositide 3 kinase/Protein kinase B
- PLR
platelet-to-lymphocyte ratio
- RFS
Recurrence-free survival
- ROS
Reactive oxygen species
- SOX9
Sry (Sex determining region Y)-box 9
- STAT3
Signal transducer and activator of transcription 3
- TMPRSS2
Transmembrane protease serine 2
- TNF
Tumor necrosis factor
- TXNIP
Thioredoxin (TRX)-interacting protein
- VEGF
Vascular endothelial growth factor
- VLPN
Ventrolateral preoptic nucleus
- WBC
White blood cells
Disclosures statement
OK and GK have been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. OK is a scientific co-founder of Samsara Therapeutics. GK is in the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. GK’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflicts of interest.
References
- 1.DrugBank. Dexmedetomidine . 2024: https://go.drugbank.com/drugs/DB00633.
- 2.Virtanen R, Savola JM, Saano V, Nyman L.. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150(1–2):9–17. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 3.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76(6):948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Crit Care Clin. 2009;25(3):451–69. doi: 10.1016/j.ccc.2009.04.004. vii. [DOI] [PubMed] [Google Scholar]
- 6.Lee HG, Choi JI, Kim YO, Yoon MH. The role of alpha-2 adrenoceptor subtype in the antiallodynic effect of intraplantar dexmedetomidine in a rat spinal nerve ligation model. Neurosci Lett. 2013;557 (Pt B):118–122. doi: 10.1016/j.neulet.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Qi F, Zhang J, Ji Y, Zhang D, Shen Z, Lei W. Antinociceptive effects of dexmedetomidine via spinal substance P and CGRP. Transl Neurosci. 2015;6(1):259–64. doi: 10.1515/tnsci-2015-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 2012;529(1):70–4. doi: 10.1016/j.neulet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhu YJ, Peng K, Meng XW, Ji FH. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016;1644:1–8. doi: 10.1016/j.brainres.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 10.Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37(5):1763–70. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 11.Huang DY, Li Q, Shi CY, Hou CQ, Miao Y, Shen HB. Dexmedetomidine attenuates inflammation and pancreatic injury in a rat model of experimental severe acute pancreatitis via cholinergic anti-inflammatory pathway. Chin Med J. 2020;133(9):1073–9. doi: 10.1097/CM9.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of dexmedetomidine in neuropathic pain. Front Neurosci. 2020;14:330. doi: 10.3389/fnins.2020.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273(5276):801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 14.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–904. doi: 10.2147/JPR.S139387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd-Elshafy SK, Abdallal F, Kamel EZ, Edwar H, Allah EA, Maghraby HHM, Sayed JA, Ali MS, Elkhayat H, Mahran GSK. Paravertebral dexmedetomidine in video-assisted thoracic surgeries for acute and chronic pain prevention. Pain Physician. 2019;22(3):271–280. doi: 10.36076/ppj/2019.22.271. [DOI] [PubMed] [Google Scholar]
- 18.Xia M, Ji NN, Duan ML, Tong JH, Xu JG, Zhang YM, Wang SH. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20:3500–3506. [PubMed] [Google Scholar]
- 19.Wang C, Datoo T, Zhao H, Wu L, Date A, Jiang C, Sanders RD, Wang G, Bevan C, Ma D. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology. 2018;129(5):1000–14. doi: 10.1097/ALN.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Ding T, Yu L, Zhong Y, Dai H, Yan M. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J Pharm Pharmacol. 2012;64(1):120–127. doi: 10.1111/j.2042-7158.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen HY, Li GH, Tan GC, Liang H, Lai XH, Huang Q, Zhong JY. Dexmedetomidine enhances hypoxia-induced cancer cell progression. Exp Ther Med. 2019;18(6):4820–4828. doi: 10.3892/etm.2019.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang T, Lin L, Ye ZJ, Fang L, Shi S, Yu KD, Miao HH, Li TZ. Dexmedetomidine promotes angiogenesis and vasculogenic mimicry in human hepatocellular carcinoma through alpha (2)-AR/HIF-1alpha/VEGFA pathway. Biomed Environ Sci. 2022;35(10):931–942. doi: 10.3967/bes2022.120. [DOI] [PubMed] [Google Scholar]
- 23.Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Dexmedetomidine promotes breast cancer cell migration through Rab11-mediated secretion of exosomal TMPRSS2. Ann Transl Med. 2020;8(8):531. doi: 10.21037/atm.2020.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P, Luo X, Dai G, Jiang Y, Luo Y, Peng S, Wang H, Xie P, Qu C, Lin W. et al. Dexmedetomidine promotes the progression of hepatocellular carcinoma through hepatic stellate cell activation. Exp Mol Med. 2020;52(7):1062–74. doi: 10.1038/s12276-020-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, Elbaz E, Cata JP, Gottumukkala V, Ben-Eliyahu S. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–96. doi: 10.1016/j.bja.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su X, Fan Y, Yang L, Huang J, Qiao F, Fang Y, Wang J. Dexmedetomidine expands monocytic myeloid-derived suppressor cells and promotes tumour metastasis after lung cancer surgery. J Transl Med. 2018;16(1):347. doi: 10.1186/s12967-018-1727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian H, Hou L, Xiong Y, Cheng Q, Huang J. Effect of dexmedetomidine-mediated insulin-like growth factor 2 (IGF2) signal pathway on immune function and invasion and migration of cancer cells in rats with ovarian cancer. Med Sci Monit. 2019;25:4655–64. doi: 10.12659/MSM.915503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, He H, Bai Y, Liu W, Huang L. Dexmedetomidine suppresses the progression of esophageal cancer via miR-143-3p/epidermal growth factor receptor pathway substrate 8 axis. Anticancer Drugs. 2020;31(7):693–701. doi: 10.1097/CAD.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Chen Y, Yan H, Wu H. Effects of dexmedetomidine on glioma cells in the presence or absence of cisplatin. J Cell Biochem. 2020;121(1):723–34. doi: 10.1002/jcb.29318. [DOI] [PubMed] [Google Scholar]
- 30.Xu B, Qian Y, Hu C, Wang Y, Gao H, Yang J. Dexmedetomidine upregulates the expression of miR-493-5p, inhibiting growth and inducing the apoptosis of lung adenocarcinoma cells by targeting RASL11B. Biochem Cell Biol. 2021;99(4):457–464. doi: 10.1139/bcb-2020-0267. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Gu X, Liu Y. The effect of dexmedetomidine on biological behavior of osteosarcoma cells through miR-1307 expression. Am J Transl Res. 2021;13:4876–83. [PMC free article] [PubMed] [Google Scholar]
- 32.Tian H, Hou L, Xiong Y, Cheng Q. Dexmedetomidine upregulates microRNA-185 to suppress ovarian cancer growth via inhibiting the SOX9/Wnt/beta-catenin signaling pathway. Cell Cycle. 2021;20(8):765–780. doi: 10.1080/15384101.2021.1897270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan R, Jin S, Liu H, Le C, Gao J, Cheng J, Chen L, Li N. Dexmedetomidine inhibits cell malignancy in osteosarcoma cells via miR-520a-3p-YOD1 interactome. Biochem Bioph Res Co. 2021;543:56–64. doi: 10.1016/j.bbrc.2021.01.045. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Qiu LL, Zhao ZF, Long YX, Yang T. Dexmedetomidine represses proliferation and promotes apoptosis of esophageal cancer cells by regulating C-Myc gene expression via the ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(2):950–6. doi: 10.26355/eurrev_202101_24664. [DOI] [PubMed] [Google Scholar]
- 35.Che J, Liu M, Lv H. Dexmedetomidine disrupts esophagus cancer tumorigenesis by modulating circ_0003340/miR-198/HMGA2 axis. Anticancer Drugs. 2022;33(5):448–58. doi: 10.1097/CAD.0000000000001284. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Zhang L, Cai XJ, Li D, Cao FJ, Zuo ZG, Song Y, Yu XJ, Liu S. Dexmedetomidine inhibits the growth and metastasis of esophageal cancer cells by down-regulation of lncRNA MALAT1. Kaohsiung J Med Sci. 2022;38(6):585–93. doi: 10.1002/kjm2.12506. [DOI] [PubMed] [Google Scholar]
- 37.Jun JH, Shim JK, Oh JE, Kim KS, Kwak YL, Soh S. Effects of dexmedetomidine on A549 non-small cell lung cancer growth in a clinically relevant surgical xenograft model. Sci Rep. 2023;13(1):12471. doi: 10.1038/s41598-023-39704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Wang XL. Dexmedetomidine promotes ferroptotic cell death in gastric cancer via hsa_circ_0008035/miR-302a/E2F7 axis. Kaohsiung J Med Sci. 2023;39(4):390–403. doi: 10.1002/kjm2.12650. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Qi Z, Fan P, Zhang N, Qian L, Chen C, Huang Y, Jin S. Dexmedetomidine provides type-specific tumour suppression without tumour-enhancing effects in syngeneic murine models. Br J Anaesth. 2023;130(2):142–53. doi: 10.1016/j.bja.2022.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Li J, Kang R, Tang D. Cell type-specific induction of ferroptosis to boost antitumor immunity. Oncoimmunology. 2023;12(1):2282252. doi: 10.1080/2162402X.2023.2282252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demuynck R, Efimova I, Catanzaro E, Krysko DV. Ferroptosis: friend or foe in cancer immunotherapy? Oncoimmunology. 2023;12(1):2182992. doi: 10.1080/2162402X.2023.2182992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kepp O, Kroemer G. Is ferroptosis immunogenic? The devil is in the details! Oncoimmunology. 2022;11(1):2127273. doi: 10.1080/2162402X.2022.2127273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suo L, Wang M. Dexmedetomidine attenuates oxygen-glucose deprivation/reperfusion-induced inflammation through the miR-17-5p/TLR4/NF-kappaB axis. BMC Anesthesiol. 2022;22(1):126. doi: 10.1186/s12871-022-01661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin S, Kim KJ, Hwang HJ, Noh S, Oh JE, Yoo YC. Immunomodulatory effects of perioperative dexmedetomidine in ovarian cancer: an in vitro and xenograft mouse model study. Front Oncol. 2021;11:722743. doi: 10.3389/fonc.2021.722743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kepp O, Liu P, Zitvogel L, Kroemer G. Tumor-infiltrating lymphocytes for melanoma immunotherapy. Oncoimmunology. 2023;12(1):2175506. doi: 10.1080/2162402X.2023.2175506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnestein R, Galland L, Kalfeist L, Ghiringhelli F, Ladoire S, Limagne E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology. 2022;11(1):2120676. doi: 10.1080/2162402X.2022.2120676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Sun J, Wu T, Lu X, Du Y, Duan H, Yu W, Su D, Lu J, Tian J. Effects of serum from breast cancer surgery patients receiving perioperative dexmedetomidine on breast cancer cell malignancy: a prospective randomized controlled trial. Cancer Med. 2019;8(18):7603–12. doi: 10.1002/cam4.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XH, Bai Q, Lv MM, Fu HG, Dong TL, Zhou Z. Effect of dexmedetomidine on immune function of patients undergoing radical mastectomy: a double blind and placebo control study. Eur Rev Med Pharmacol Sci. 2017;21:1112–6. [PubMed] [Google Scholar]
- 49.Cata JP, Singh V, Lee BM, Villarreal J, Mehran JR, Yu J, Gottumukkala V, Lavon H, Ben-Eliyahu S. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–23. doi: 10.4103/joacp.JOACP_299_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cata JP, Nguyen LT, Ifeanyi-Pillette IC, Van Meter A, Dangler LA, Feng L, Owusu-Agyemang P. An assessment of the survival impact of multimodal anesthesia/analgesia technique in adults undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a propensity score matched analysis. Int J Hyperthermia. 2019;36(1):368–374. doi: 10.1080/02656736.2019.1574985. [DOI] [PubMed] [Google Scholar]
- 51.Rangel FP, Auler JOC Jr., Carmona MJC, Cordeiro MD, Nahas WC, Coelho RF, Simoes CM. Opioids and premature biochemical recurrence of prostate cancer: a randomised prospective clinical trial. Br J Anaesth. 2021;126(5):931–9. doi: 10.1016/j.bja.2021.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Cho JS, Seon K, Kim MY, Kim SW, Yoo YC. Effects of perioperative dexmedetomidine on immunomodulation in uterine cancer surgery: a randomized, controlled trial. Front Oncol. 2021;11:749003. doi: 10.3389/fonc.2021.749003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG. Utilisation peropératoire de la dexmédétomidine pour la prévention de l’agitation au réveil et du delirium postopératoire en chirurgie thoracique: essai randomisé contrôlé. Can J Anesth/J Can Anesth. 2019;66(4):371–379. doi: 10.1007/s12630-019-01299-7. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y, Dong X, Zhang L. Dexmedetomidine can reduce the level of oxidative stress and serum miR-10a in patients with lung cancer after surgery. Thorac Cardiovasc Surg. 2023;71(3):197–205. doi: 10.1055/s-0041-1740558. [DOI] [PubMed] [Google Scholar]
- 55.Mao Y, Sun X, Si L, Chen L, Liu X, Zhang Z, Gu E. Perioperative dexmedetomidine fails to improve postoperative analgesic consumption and postoperative recovery in patients undergoing lateral thoracotomy for thoracic esophageal cancer: a randomized, double-blind, placebo-controlled trial. Pain Res Manag. 2020;2020:1–12. doi: 10.1155/2020/4145893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Xu X, Liu H, Ji F. Effects of dexmedetomidine on patients undergoing radical gastrectomy. J Surg Res. 2015;194(1):147–53. doi: 10.1016/j.jss.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Zheng W, Tian X, Fan J, Jiang X, He W. Application of dexmedetomidine in surgical anesthesia for Gastric cancer and its effects on IL-1beta, IL-6, TNF-alpha and CRP. Cell Mol Biol. 2023;69(3):177–181. doi: 10.14715/cmb/2023.69.3.26. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Yi Y, Zhao M. Effect of dexmedetomidine anesthesia on perioperative levels of TNF-alpha and IL-6 in patients with ovarian cancer. Oncol Lett. 2019;17(6):5517–5522. doi: 10.3892/ol.2019.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu W, Zheng Y, Suo Z, Fei K, Wang Y, Liu C, Li S, Zhang M, Zhang Y, Zheng Z. et al. Effect of dexmedetomidine on postoperative systemic inflammation and recovery in patients undergoing digest tract cancer surgery: a meta-analysis of randomized controlled trials. Front Oncol. 2022;12:970557. doi: 10.3389/fonc.2022.970557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Y, Zhou Y, Maloney JD, Shan G. Effects of dexmedetomidine on inflammation and pulmonary function after thoracoscopic surgery for lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2023;15(6):3397–408. doi: 10.21037/jtd-23-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K, Li C. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-kappaB in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett. 2018;15(5):7153–7157. doi: 10.3892/ol.2018.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Liu G, Zhang F, Fang H, Zhang D, Liu S, Chen B, Xiao H. Analysis of postoperative cognitive dysfunction and influencing factors of dexmedetomidine anesthesia in elderly patients with colorectal cancer. Oncol Lett. 2019;18(3):3058–64. doi: 10.3892/ol.2019.10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong W, Chen MH, Yang YH, Zhang X, Huang MJ, Yang XJ, Wang HZ. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21:3510–5. [PubMed] [Google Scholar]
- 64.Ma XF, Lv SJ, Wei SQ, Mao BR, Zhao XX, Jiang XQ, Zeng F, Du XK. Influences of dexmedetomidine on stress responses and postoperative cognitive and coagulation functions in patients undergoing radical gastrectomy under general anesthesia. World J Gastrointest Surg. 2023;15(6):1169–77. doi: 10.4240/wjgs.v15.i6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Y, Jiang W, Zhao L, Wu Y, Xie H. Effect of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing radical resection of lung cancer. Int J Clin Exp Pathol. 2020;13:2544–53. [PMC free article] [PubMed] [Google Scholar]
- 66.Lai Y, Chen Q, Xiang C, Li G, Wei K. Comparison of the effects of dexmedetomidine and lidocaine on stress response and postoperative delirium of older patients undergoing thoracoscopic surgery: a randomized controlled trial. Clin Interv Aging. 2023;18:1275–83. doi: 10.2147/CIA.S419835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin H, Cao L, Zhao H, Yang Y. Effects of dexmedetomide, propofol and remifentanil on perioperative inflammatory response and lung function during lung cancer surgery. Am J Transl Res. 2021;13:2537–45. [PMC free article] [PubMed] [Google Scholar]
- 68.Ding J, Zhu M, Lv H, Zhang J, Chen W, Jan N. Application effect of dexmedetomidine and dezocine in patients undergoing lung cancer surgery under General Anesthesia and analysis of their roles in recovery time and cognitive function. Comput Math Method M. 2022;2022:1–8. doi: 10.1155/2022/9889534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng J, Lv Q, Yao J, Wang S, Yang K, Chen L. Effect of dexmedetomidine on postoperative lung injury during One-lung Ventilation in thoracoscopic surgery. Biomed Res Int. 2020;2020:1–8. doi: 10.1155/2020/4976205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Zheng L, Zhao J, Zheng L, Jing S, Wang X. Effect of dexmedetomidine on perioperative stress response and immune function in patients with tumors. Technol Cancer Res Treat. 2020;19:1533033820977542. doi: 10.1177/1533033820977542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du D, Qiao Q, Guan Z, Gao YF, Wang Q. Combined sevoflurane-dexmedetomidine and nerve blockade on post-surgical serum oxidative stress biomarker levels in thyroid cancer patients. World J Clin Cases. 2022;10(10):3027–34. doi: 10.12998/wjcc.v10.i10.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren B, Cheng M, Liu C, Zheng H, Zhang J, Chen W, Song J, Zhuang J, Liu T, Wang R. et al. Perioperative lidocaine and dexmedetomidine intravenous infusion reduce the serum levels of NETs and biomarkers of tumor metastasis in lung cancer patients: a prospective, single-center, double-blinded, randomized clinical trial. Front Oncol. 2023;13:1101449. doi: 10.3389/fonc.2023.1101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cedervall J, Herre M, Dragomir A, Rabelo-Melo F, Svensson A, Thalin C, Rosell A, Hjalmar V, Wallen H, Lindman H. et al. Neutrophil extracellular traps promote cancer-associated inflammation and myocardial stress. Oncoimmunology. 2022;11(1):2049487. doi: 10.1080/2162402X.2022.2049487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang ZZ, Peng ZP, Liu XC, Guo HF, Zhou MM, Jiang D, Ning WR, Huang YF, Zheng L, Wu Y. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. Oncoimmunology. 2022;11(1):2052418. doi: 10.1080/2162402X.2022.2052418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang L, Qin C, Wang L, Zhang T, Li J. Effects of dexmedetomidine on immune response in patients undergoing radical and reconstructive surgery for oral cancer. Oncol Lett. 2021;21(2):106. doi: 10.3892/ol.2020.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang Y, Zhou C, Li Q, Cheng X, Huang T, Li F, He L, Zhang B, Tu S. Targeting depletion of myeloid-derived suppressor cells potentiates PD-L1 blockade efficacy in gastric and colon cancers. Oncoimmunology. 2022;11(1):2131084. doi: 10.1080/2162402X.2022.2131084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kato T, Fukushima H, Furusawa A, Okada R, Wakiyama H, Furumoto H, Okuyama S, Takao S, Choyke PL, Kobayashi H. Selective depletion of polymorphonuclear myeloid derived suppressor cells in tumor beds with near infrared photoimmunotherapy enhances host immune response. Oncoimmunology. 2022;11(1):2152248. doi: 10.1080/2162402X.2022.2152248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu L, Lv H, Luo W, Jin S, Hang Y. Effects of dexmedetomidine on cellular immunity of perioperative period in children with brain neoplasms. Int J Clin Exp Med. 2015;8:2748–53. [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao L, Li Y. Application of dexmedetomidine combined with sufentanil in colon cancer resection and its effect on immune and coagulation function of patients. Oncol Lett. 2020;20(2):1288–94. doi: 10.3892/ol.2020.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohamed SA, Sayed DM, El Sherif FA, Abd El-Rahman AM. Effect of local wound infiltration with ketamine versus dexmedetomidine on postoperative pain and stress after abdominal hysterectomy, a randomized trial. Eur J Pain. 2018;22(5):951–60. doi: 10.1002/ejp.1181. [DOI] [PubMed] [Google Scholar]
- 81.Russo M, Panini N, Fabbrizio P, Formenti L, Becchetti R, Matteo C, Meroni M, Nastasi C, Cappelleri A, Frapolli R. et al. Chemotherapy-induced neutropenia elicits metastasis formation in mice by promoting proliferation of disseminated tumor cells. Oncoimmunology. 2023;12(1):2239035. doi: 10.1080/2162402X.2023.2239035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bezu L, Wu Chuang A, Sauvat A, Humeau J, Xie W, Cerrato G, Liu P, Zhao L, Zhang S, Le Naour J. et al. Local anesthetics elicit immune-dependent anticancer effects. J Immunother Cancer. 2022;10(4):e004151. doi: 10.1136/jitc-2021-004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carnet Le Provost K, Kepp O, Kroemer G, Bezu L. Trial watch: beta-blockers in cancer therapy. Oncoimmunology. 2023;12(1):2284486. doi: 10.1080/2162402X.2023.2284486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bezu L, Kepp O, Kroemer G. Immunogenic stress induced by local anesthetics injected into neoplastic lesions. Oncoimmunology. 2022;11(1):2077897. doi: 10.1080/2162402X.2022.2077897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993;14(5):539–63. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y, Kroemer G. The cancer-immune dialogue in the context of stress. Nat Rev Immunol. 2023. doi: 10.1038/s41577-023-00949-8. [DOI] [PubMed] [Google Scholar]
- 87.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 88.Sacerdote P, Manfredi B, Bianchi M, Panerai AE. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun. 1994;8(3):251–60. doi: 10.1006/brbi.1994.1023. [DOI] [PubMed] [Google Scholar]
- 89.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54(1):21–8. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 90.Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F. Beta-endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977;197(4311):1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- 91.Baker GH, Irani MS, Byrom NA, Nagvekar NM, Wood RJ, Hobbs JR, Brewerton DA. Stress, cortisol concentrations, and lymphocyte subpopulations. BMJ. 1985;290(6479):1393. doi: 10.1136/bmj.290.6479.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M. et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25(9):1428–1441. doi: 10.1038/s41591-019-0566-4. [DOI] [PubMed] [Google Scholar]
- 93.Brownlie D, von Kries A, Valenzano G, Wild N, Yilmaz E, Safholm J, Al-Ameri M, Alici E, Ljunggren HG, Schliemann I. et al. Accumulation of tissue-resident natural killer cells, innate lymphoid cells, and CD8(+) T cells towards the center of human lung tumors. Oncoimmunology. 2023;12(1):2233402. doi: 10.1080/2162402X.2023.2233402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poinot H, Dupuychaffray E, Arnoux G, Alvarez M, Tachet J, Ezzar O, Moore J, Bejuy O, Olesti E, Visconti G. et al. Activation of endogenous glucocorticoids by HSD11B1 inhibits the antitumor immune response in renal cancer. Oncoimmunology. 2024;13(1):2286820. doi: 10.1080/2162402X.2023.2286820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yonekura S, Terrisse S, Alves Costa Silva C, Lafarge A, Iebba V, Ferrere G, Goubet AG, Fahrner JE, Lahmar I, Ueda K. et al. Cancer induces a stress ileopathy depending on beta-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 2022;12(4):1128–1151. doi: 10.1158/2159-8290.CD-21-0999. [DOI] [PubMed] [Google Scholar]
- 96.Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int Immunopharmacol. 2021;97:107709. doi: 10.1016/j.intimp.2021.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen R, Sun Y, Lv J, Dou X, Dai M, Sun S, Lin Y. Effects of dexmedetomidine on immune cells: a narrative review. Front Pharmacol. 2022;13:829951. doi: 10.3389/fphar.2022.829951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G, Ma D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123(6):777–94. doi: 10.1016/j.bja.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 99.Kim MH, Lee KY, Bae SJ, Jo M, Cho JS. Intraoperative dexmedetomidine attenuates stress responses in patients undergoing major spine surgery. Minerva Anestesiol. 2019;85(5):468–77. doi: 10.23736/S0375-9393.18.12992-0. [DOI] [PubMed] [Google Scholar]
- 100.Kang R, Jeong JS, Ko JS, Lee SY, Lee JH, Choi SJ, Cha S, Lee JJ. Intraoperative dexmedetomidine attenuates norepinephrine levels in patients undergoing transsphenoidal surgery: a randomized, placebo-controlled trial. BMC Anesthesiol. 2020;20(1):100. doi: 10.1186/s12871-020-01025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perez Pinero C, Bruzzone A, Sarappa MG, Castillo LF, Luthy IA. Involvement of alpha2- and beta2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166(2):721–736. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Castillo LF, Rivero EM, Goffin V, Luthy IA. Alpha(2)-adrenoceptor agonists trigger prolactin signaling in breast cancer cells. Cell Signal. 2017;34:76–85. doi: 10.1016/j.cellsig.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P. Beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93(3):279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- 104.Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepato-Gastroenterology. 2012;59(115):889–893. doi: 10.5754/hge11476. [DOI] [PubMed] [Google Scholar]
- 105.Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, Zhao W, Geng B. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. 2020;39(1):176. doi: 10.1186/s13046-020-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lasithiotaki I, Tsitoura E, Samara KD, Trachalaki A, Charalambous I, Tzanakis N, Antoniou KM, Ahmad A. NLRP3/Caspase-1 inflammasome activation is decreased in alveolar macrophages in patients with lung cancer. PloS One. 2018;13(10):e0205242. doi: 10.1371/journal.pone.0205242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, Moller A, Smyth MJ. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72(22):5721–5732. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 108.Huang CQ, Min Y, Wang SY, Yang XJ, Liu Y, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–83. doi: 10.18632/oncotarget.17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aranda F, Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology. 2013;2(12):e26621. doi: 10.4161/onci.26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology. 2013;2(2):e23082. doi: 10.4161/onci.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G. et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2(10):e25771. doi: 10.4161/onci.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Penetra M, Arnaut LG, Gomes-da-Silva LC. Trial watch: an update of clinical advances in photodynamic therapy and its immunoadjuvant properties for cancer treatment. Oncoimmunology. 2023;12(1):2226535. doi: 10.1080/2162402X.2023.2226535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Laureano RS, Sprooten J, Vanmeerbeerk I, Borras DM, Govaerts J, Naulaerts S, Berneman ZN, Beuselinck B, Bol KF, Borst J. et al. Trial watch: dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology. 2022;11(1):2096363. doi: 10.1080/2162402X.2022.2096363. [DOI] [PMC free article] [PubMed] [Google Scholar]


