Abstract
Sex-specific behaviors are critical for reproduction and species survival. The sex-specifically spliced transcription factor fruitless (fru) helps establish male courtship behaviors in invertebrates. Forcing male-specific fru (fruM) splicing in Drosophila melanogaster females produces male-typical behaviors while disrupting female-specific behaviors. However, whether fru’s joint role in specifying male and inhibiting female behaviors is conserved across species is unknown. We used CRISPR-Cas9 to force FruM expression in female Drosophila virilis, a species in which males and females produce sex-specific songs. In contrast to D. melanogaster, in which one fruM allele is sufficient to generate male behaviors in females, two alleles are needed in D. virilis females. D. virilis females expressing FruM maintain the ability to sing female-typical song as well as lay eggs, whereas D. melanogaster FruM females cannot lay eggs. These results reveal potential differences in fru function between divergent species and underscore the importance of studying diverse behaviors and species for understanding the genetic basis of sex differences.
Some functions of the highly conserved fruitless gene have diverged between a duetting and non-duetting Drosophila species.
INTRODUCTION
Sex-specific behaviors, such as reproduction, aggression, and parental care, are essential for species survival. Many of these innate behaviors are under genetic control in both vertebrates and invertebrates (1). In many insects, sex-specific splicing of two transcription factors called fruitless (fru) and doublesex are responsible for establishing sexually differentiated neural circuitry and somatic tissue (2–10). In Drosophila melanogaster, in which fru function was first described (11), splicing in the male pattern results in a functional protein called FruM in a subset (~2000, or ~2%) of male neurons, whereas splicing in the female pattern results in transcripts that are degraded, leading to no functional protein (3, 12, 13). Sex-specific fru splicing has since been found across many but not all insect species (14–22). Whether the role of fru in specifying sex-specific behaviors differs across species remains an open question.
Knocking out FruM expression in male D. melanogaster eliminates their ability to engage in courtship behaviors directed toward a female (3, 23), and this function appears to be conserved in insects (17, 24–27) [but see (28)]. Subsets of FruM-expressing neurons play distinct roles in producing male courtship behaviors in D. melanogaster (5, 29–33), and at least some of these neurons are conserved across Drosophila species despite divergence in courtship behaviors (23, 34, 35). A breakthrough in our understanding of fru function came from forcing male-specific fru splicing in female D. melanogaster (3, 6). This experiment was critical because fruM transcripts are also alternatively spliced at the 3′ end (Fig. 1A), giving rise to three isoforms differentially expressed across neurons: FruM-A, FruM-B, and FruM-C (3, 33, 36, 37). Females with male-specific splicing of fruM not only made FruM protein but also expressed the correct isoform in each cell type. Unexpectedly, these females engaged in male courtship behaviors, with disruptions in their ability to produce female-specific behaviors; hence, fru was considered a “switch gene” for specifying sexually dimorphic behaviors (3). Here, we use a similar strategy (via CRISPR-Cas9 gene editing) to force FruM expression in females of another Drosophila species and investigate whether this also results in male-typical courtship behaviors while impeding female-typical behaviors. In other words, does FruM expression in females result in different outcomes depending on the species context?
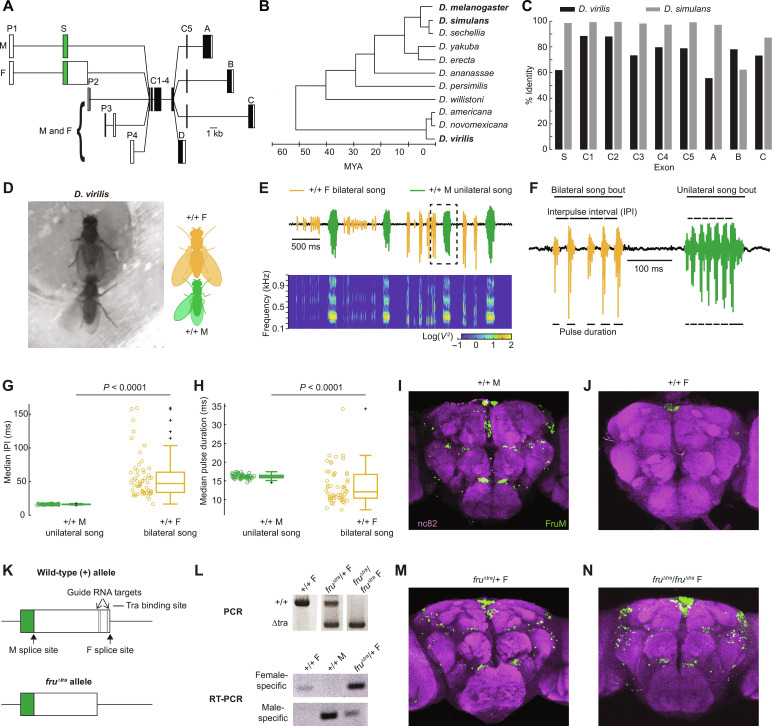
Fig. 1. CRISPR-Cas9 editing of the fruitless (fru) gene results in expression of male-specific FruM in D. virilis female brains.
(A) Transcripts resulting from alternative splicing of the fru gene. Filled and open boxes indicate coding and non-coding regions, respectively. P1 to P4 indicate promoters, S indicates the sex-specifically spliced exon, C1 to C5 indicate exons common to most fru transcripts, and A to D indicate alternative 3′ exons. Adapted from (3). (B) Drosophila phylogeny. Adapted from (39). (C) Comparison of the fru exon (coding regions only) nucleotide sequences. Percent identity is reported relative to D. melanogaster. (D) Video still (left) and schematic (right) of D. virilis courtship duets. Wild-type (+/+) D. virilis males and females sing using unilateral and bilateral wing vibration, respectively. (E) Microphone recording (top) and spectrogram (bottom) of an example duet. Songs were automatically segmented using a convolutional neural network (fig. S3; see Materials and Methods). (F) Close-up of the microphone recording outlined in (E). (G to H) IPI (G) and pulse durations (H) of unilateral and bilateral song. Each dot represents the median from one fly. Statistical tests were Wilcoxon rank sum tests with Bonferroni correction (n = 55 flies in both groups). (I and J) Antibody staining for FruM (green) and bruchpilot (nc82; magenta) in D. virilis +/+ male (I) and +/+ female (J) brains. The ocelli are FruM-immunopositive. (K) Schematic of the S-exon (top) and the result of CRISPR-Cas9–mediated removal of the transformer (Tra) binding sites (fruΔtra) (bottom). (L) Top: Polymerase chain reaction (PCR) genotyping of fruΔtra mutants using primers flanking the CRISPR guide RNA (gRNA) targets. Heterozygotes have both a +/+ and a shorter mutant allele (middle), whereas homozygous mutants have only the mutant allele (right). Bottom: Reverse transcription PCR (RT-PCR) using D. virilis female- and male-specific primers confirm that the brains of fruΔtra/+ females contain male fru transcripts. (M and N) FruM antibody staining in D. virilis fruΔtra/+ (M) and fruΔtra/fruΔtra (N) female brains. F, female; M, male.
We selected Drosophila virilis, which has male-specific FruM expression (22, 38) but which diverged from D. melanogaster almost 60 million years ago (Fig. 1B) (39) and shows only ~50 to 90% sequence identity (Fig. 1C). Importantly for our experiments, D. virilis produces markedly divergent courtship behaviors compared to D. melanogaster. Courtship in D. melanogaster consists of the male pursuing the female while tapping, licking, and singing to her with unilateral wing extensions (40). Females, in turn, arbitrate mating decisions by slowing down and allowing copulation when receptive and then laying eggs. In the vast majority of drosophilid species with courtship songs, it is the male who sings to the female (41). Females have been reported to sing back to males in just a few species, all within the D. virilis group (42). D. virilis males use unilateral wing vibration, while females use bilateral wing vibration to produce sex-specific pulse songs during acoustic duets (Fig. 1, D and E) (43). Males can also sing a female-like bilateral song on the infrequent occasions when they are courted by another male (43), demonstrating that song choice is context-dependent in males. In contrast, females do not naturally produce the male-typical unilateral song. Therefore, unilateral song is male-specific, whereas bilateral song appears to be sexually monomorphic in D. virilis.
Here, we analyze potential evolutionary divergence and conservation of the role of fru between D. virilis and D. melanogaster. Although FruM expression in D. virilis females results in male courtship behaviors, including unilateral song production, these effects require two alleles of fruM in D. virilis but just one allele in D. melanogaster (3). FruM expression in D. virilis females alters the amount and sound features of bilateral song produced while duetting with a male; such a function was not possible to query in D. melanogaster because those females do not sing. Similar to D. melanogaster, FruM expression reduced receptivity in D. virilis females, but, in contrast to D. melanogaster, FruM expression did not eliminate egg laying in D. virilis females. Whereas pairings between wild-type males and FruM females were dominated by courtship in D. melanogaster, D. virilis FruM females alternated between duetting and male-directed aggression. These results highlight the value of comparing diverse behaviors and species in evaluating the role of important “switch genes” involved in behavioral specification.
RESULTS
In D. virilis duets, two key features distinguish male-typical unilateral from female-typical bilateral song: the time between successive pulses, called interpulse intervals (IPIs), and the duration of each pulse (Fig. 1F). These features are stereotyped within and across males but are more variable in females (Fig. 1, G and H). In addition to unilateral song, D. virilis males can sing the female-typical bilateral song if they are courted by another male (fig. S1, A to C) (43), although male-directed courtship occurs less frequently than female-directed (fig. S1D). Male and female bilateral song is similar (fig. S1, E to G), suggesting that D. virilis may have two song circuits: one sexually monomorphic circuit for bilateral song and one dimorphic circuit for unilateral song. The role of fru in establishing either of these song circuits is unknown.
Transformer binding site removal results in FruM expression in D. virilis female brains
Similar to D. melanogaster, FruM expression in D. virilis is male-specific (Fig. 1, I and J) (22, 38). To understand the role FruM plays in specifying D. virilis courtship behaviors, we forced fruM splicing in female brains by removing the Transformer (Tra) binding sites from the sex-specifically spliced S exon (Fig. 1K) using CRISPR-Cas9, which in D. melanogaster results in splicing at the male site (3). This mutation is equivalent to the fruΔtra mutation in D. melanogaster (3). Polymerase chain reaction (PCR) (Fig. 1L, top) and sequencing confirmed that our mutagenesis resulted in removal of a portion of the S-exon containing the Tra binding sites. Reverse transcription PCR (RT-PCR) against female- and male-specific fru transcripts confirmed that fruΔtra/+ female brains contained both versions (Fig. 1L, bottom), demonstrating that Tra binding site removal was sufficient to produce male-specific fru transcripts. We then validated these results with antibody staining for FruM (see Materials and Methods) and found that females carrying the fruΔtra mutation had FruM expression in subsets of neurons (Fig. 1, M and N) that overall was consistent with the +/+ male expression pattern (Fig. 1I). In particular, we were able to identify all eight FruM+ neuron clusters described in the anterior central brain of D. melanogaster (fig. S2A) and at least four of the eight posterior clusters (fig. S2B). However, the relatively low resolution (20×) of these images prevented us from comparing the numbers of FruM+ cells across genotypes. Therefore, while the D. virilis fruΔtra alleles result in FruM expression in female brains, we are not able to assess potential differences in the level of FruM expression and/or number of FruM+ neurons.
One copy of fruΔtra is insufficient for male courtship behaviors in D. virilis females
The effects of FruM expression in D. melanogaster females requires only one copy of the fruΔtra allele (3), a pattern known as dominance. This suggests that the amount of FruM transcription factor resulting from one fruΔtra allele is sufficient to produce masculinized neural circuitry in females. To determine whether fruM is also dominant in D. virilis, we paired a single fruΔtra/+ female with a wild-type female (Fig. 2A) and quantified courtship behaviors. D. virilis courtship consists of the male orienting to the female, rubbing the underside of her abdomen with his front tarsi, licking her genitalia, and singing with unilateral wing extensions (43). We found that D. virilis fruΔtra/+ females exhibited very little male-specific courtship behavior (light blue in Fig. 2, B to D), and no unilateral song in pairings with wild-type females (Fig. 2D). These results differ from those in D. melanogaster females, in which one allele of fruΔtra leads to male courtship behaviors (3).
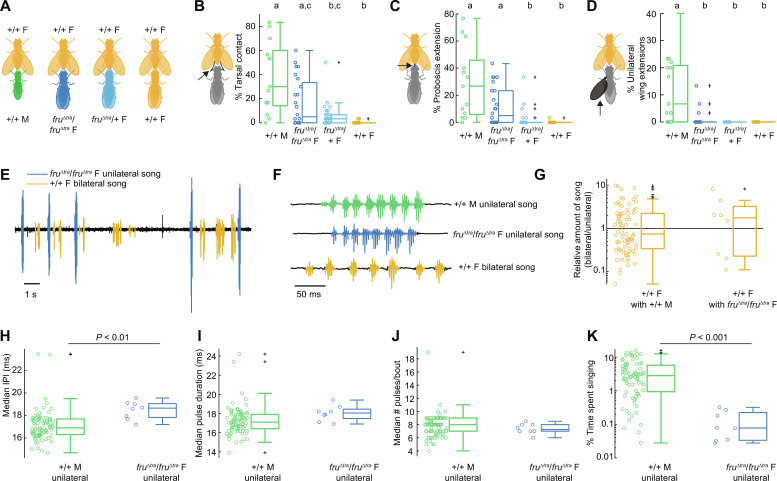
Fig. 2. D. virilis fruΔtra/fruΔtra females are capable of producing male-typical courtship behaviors directed toward +/+ females.
(A) To test whether FruM specifies male courtship behaviors in D. virilis, we paired single fruΔtra/fruΔtra and fruΔtra/+ females with a +/+ female. +/+ males and +/+ females (siblings to the fruΔtra females) each paired with a +/+ female served as controls. (B to D) % of manually scored bins containing instances where the experimental fly contacted the +/+ female with their front tarsi [(B), arrow in pictogram], extended their proboscis [(C), arrow], and extended wings unilaterally [(D), arrow]. Each dot represents the value for a single fly. Statistical significance was determined by Kruskal-Wallis tests followed by pairwise Wilcoxon rank sum tests with Bonferroni correction (n = 14, 24, 39, and 14 flies). (E) Audio recording (15 s) of a duet between a fruΔtra/fruΔtra female and a +/+ female. (F) Close-up of the waveforms of +/+ male unilateral, fruΔtra/fruΔtra female unilateral, and +/+ female bilateral songs. (G) Amount of bilateral song produced by the +/+ female normalized by the amount of unilateral song produced by the +/+ male (left) and fruΔtra/ fruΔtra female (right). Each dot represents one pair of flies. (H to K) Median IPI (H), median pulse duration (I), median number of pulses per bout (J), and percent time each fly spent singing unilateral song (K). Statistical tests were Wilcoxon rank sum tests with Bonferroni correction [n = 83 and 8 flies in (G) to (K)].
In contrast to D. melanogaster (3), D. virilis fruΔtra/+ females produced offspring after copulating with males; we found that fruΔtra/ fruΔtra females courted wild-type females (movie S1), with no significant differences in the amount of tarsal contact or proboscis extension compared to wild-type males (Fig. 2, B and C). We also observed unilateral wing extensions in a few fruΔtra/fruΔtra females (Fig. 2D), suggesting that fruΔtra/fruΔtra females may sing unilateral song when paired with a female.
To quantify song production, we built a new D. virilis song segmenter consisting of two convolutional neural networks (see Materials and Methods): one trained to distinguish among the four signal classes (unilateral song, bilateral song, overlap, and no song) (fig. S3A) and a second trained to distinguish between two signal classes (bilateral song and no song) (fig. S3B). Combining the output of the two networks (fig. S3C) resulted in high precision and sensitivity for detecting both unilateral and bilateral song, with equally good performance across genotypes (fig. S3, D to F). The performance of this segmenter is superior to that previously developed for D. virilis duets (43).
We found unilateral song in 8 of the 24 (33%) pairings between fruΔtra/fruΔtra D. virilis females and wild-type females. This song alternated with bilateral song from the wild-type female (Fig. 2E) and occurred in stereotyped bouts that looked similar to wild-type male unilateral song (Fig. 2F). We visually confirmed that the unilateral song occurred when the fruΔtra/fruΔtra female was performing unilateral wing extensions and that the bilateral song was produced solely by the wild-type female (movie S1). Wild-type females sang just as much bilateral song with fruΔtra/fruΔtra females as with wild-type males (Fig. 2G). fruΔtra/fruΔtra unilateral song had short IPIs in line with those of wild-type males, although the IPI was modestly but significantly increased by 1 to 2 ms (Fig. 2H). There was no difference in pulse duration (Fig. 2I) or the number of pulses per bout (Fig. 2J) between the unilateral song from fruΔtra/fruΔtra females and wild-type males. However, we found that fruΔtra/fruΔtra females produced almost an order of magnitude less unilateral song than wild-type males (Fig. 2K). Together, these results reveal that, while FruM arising from the fruΔtra allele specifies male-typical unilateral song in D. virilis, it is not sufficient (even with two alleles) to produce male-typical levels of courtship drive. Because FruM is a transcription factor involved in the development of sexually dimorphic neural circuitry, this finding suggests that, although fruΔtra/+ and fruΔtra/fruΔtra D. virilis females developed some FruM+ neurons (Fig. 1, M and N), there may be important differences in FruM expression levels and/or FruM+ neuron number, morphology, function, or connectivity patterns dependent on allele number.
We next returned to D. melanogaster to reinvestigate fruM allele number and song production. We conducted single-pair courtship experiments using two D. melanogaster fruΔtra genotypes (fig. S4A): fruΔtra/+ to match the genotype of the D. virilis fruΔtra/+ females, and fruΔtra/fru4-40 (where fru4-40 is a fruM-null mutation) (12) to compare with previous experiments (3, 44). Both fruΔtra/+ (movie S2) and fruΔtra/fru4-40 D. melanogaster females courted wild-type females. Whereas both fruΔtra/+ and fruΔtra/fru4-40 females engaged in tapping and unilateral wing extensions directed toward a wild-type female (fig. S4, B to D), the amount of these behaviors were significantly less than those produced by wild-type males (fig. S4, C and D). Consistent with prior work (44), we found that, while fruΔtra females extended their wings, this did not produce bonafide song (fig. S4, E and F).
Together, our results reveal two key species differences in the role of FruM in specifying male courtship behaviors: (i) One copy of a fruΔtra allele can produce male courtship in D. melanogaster females but not in D. virilis females; (ii) two alleles of fruΔtra produce male-typical unilateral song in female D. virilis, whereas the effects of two alleles of fruM cannot be tested in female D. melanogaster as one allele renders females infertile (3). We are not able to rule out whether these species differences could be due to differences in FruM expression levels resulting from D. virilis versus D. melanogaster fruΔtra alleles.
Removing a fruM allele in D. virilis males has no effect on courtship behaviors
The requirement of two fruM alleles to produce unilateral song in D. virilis females raised the question of whether a similar pattern occurs in males. Wild-type males make (via splicing) two functional copies of FruM RNA in each cell, one from each allele. What happens to male behavior if we remove one of these copies? We generated males lacking one copy of fruM by removing the S-exon (fig. S5A) via CRISPR-Cas9 and confirming the removal with PCR (fig. S5, B and C) and sequencing. We refer to these males as −/+. We then paired −/+ males with wild-type females (fig. S5D) and found that −/+ males robustly courted females. There was a modest reduction in tarsal contact by −/+ males compared to wild-type males (fig. S5E) but no differences in the amount of proboscis extension (fig. S5F) or unilateral wing extension (fig. S5G). Wild-type females duetted with −/+ males, and the waveforms of −/+ male unilateral song appeared similar to wild-type male unilateral song (fig. S5, H to J). We found no differences in the quantitative parameters of −/+ unilateral song or in the amount or timing of unilateral song (fig. S5, K to O). −/+ males were also as likely to copulate as wild-type males (fig. S5P). Together, these results demonstrate that two copies of fruM are needed for the production of male courtship behaviors, including unilateral song, only in the female context in D. virilis.
FruM disrupts female receptivity but not egg laying in D. virilis
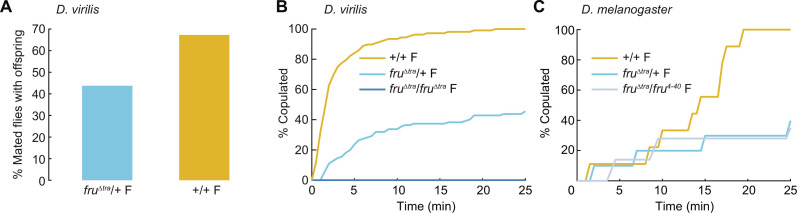
FruM expression in female D. melanogaster not only results in male-typical courtship behaviors but also interferes with female-typical courtship behaviors, such as egg laying and receptivity (3). In contrast to D. melanogaster, D. virilis fruΔtra/+ females maintained their ability to lay eggs, as almost 50% of fruΔtra/+ females that copulated produced offspring (Fig. 3A). In our single-pair courtship assays, the copulation rate of D. virilis fruΔtra/+ females was about 40% that of wild-type females (Fig. 3B). In a notable similarity between species, the copulation rate of D. melanogaster fruΔtra females was also 35 to 40% that of wild-type females (Fig. 3C) within our 25-min observation period. These results point to divergent effects of FruM expression on egg laying but conserved effects on female receptivity between the two species.
Fig. 3. Effects of FruM expression on female reproductive behaviors of D. virilis and D. melanogaster.
(A) Percentage of matings with +/+ males that resulted in larvae in D. virilis. n = 48 and 107 flies. (B) Cumulative percentage of copulated D. virilis females (paired with +/+ conspecific males) over the 25-min observation period. Curves are normalized to +/+ females. n = 118, 121, and 24 flies. (C) Same as (B) for D. melanogaster. n = 34, 38, and 54 flies.
Adding a second allele of fruΔtra to D. virilis females completely eliminated copulation (Fig. 3B) within our 25-min assays. This was not due to reduced attractiveness to males, as males directed equal amounts of courtship behaviors toward females of all genotypes (fig. S6, A to C). Therefore, FruM effects on female receptivity in D. virilis depend on the number of fruM alleles.
FruM alters female-typical bilateral song features and amount
The effects of FruM expression on female singing behavior has not previously been tested in any species. Because D. virilis bilateral song is sexually monomorphic (fig. S1) (43), we expected FruM expression in females to have no effect on bilateral song production. In pairings of fruΔtra/+ and fruΔtra/fruΔtra D. virilis females with wild-type males (Fig. 4A), fruΔtra females readily duetted with wild-type males (Fig. 4B and movie S3), with the female singing solely bilateral song and the male singing solely unilateral song. The waveform of fruΔtra bilateral song looked similar to wild-type female bilateral song (Fig. 4C). However, one allele of fruΔtra resulted in increased IPIs by about 10 ms relative to wild-type females (Fig. 4D). FruM expression also led to longer pulses (Fig. 4E) and shorter response times (Fig. 4F), regardless of the number of fruΔtra alleles. Unexpectedly, FruM expression markedly increased the amount of bilateral song, with fruΔtra/fruΔtra females singing almost an order of magnitude more bilateral song than wild-type females when paired with a wild-type male (fig. S6D). Because bilateral song is produced during a partnered duet, we wondered whether this increase in fruΔtra bilateral song simply reflected increased levels of courtship. However, wild-type males sang less unilateral song with a fruΔtra/fruΔtra female (fig. S6E) and directed equal amounts of other courtship behaviors toward fruΔtra and wild-type females (fig. S6, A to C). Accounting for differing amounts of unilateral song from the male revealed a significant increase in the amount of bilateral song produced by fruΔtra females (Fig. 4G). Increased bilateral song in fruΔtra females is consistent with the increased bilateral song produced by males relative to wild-type females when courted by another male (fig. S1H), suggesting that an up-regulation of bilateral song may be a consequence of FruM-induced masculinization. Together, our results show that, although bilateral song is sexually monomorphic, at least some of the underlying neural circuitry may be regulated by fru.
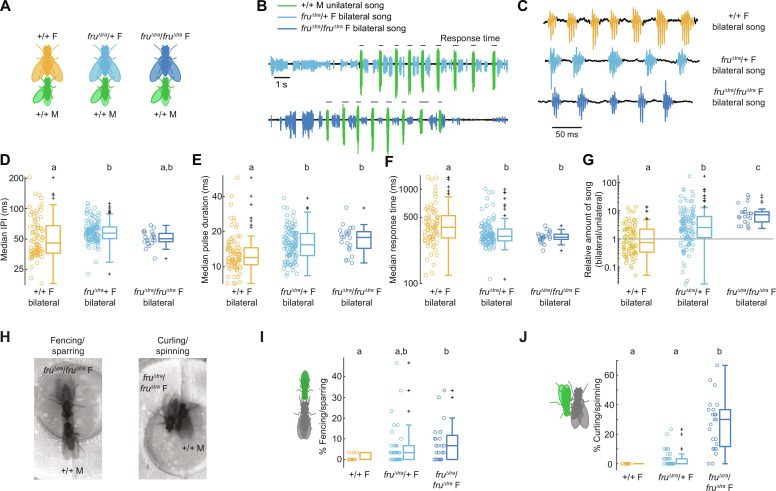
Fig. 4. FruM expression in D. virilis females has effects on bilateral song and aggression.
(A) To determine whether FruM expression affected bilateral song production, we paired single fruΔtra/+ and fruΔtra/fruΔtra D. virilis females with a +/+ male. Single +/+ females (siblings of the fruΔtra females) paired with a +/+ male served as controls. (B) Microphone recordings (15 s) of duets between fruΔtra females singing bilateral song and +/+ males singing unilateral song. In these pairings, unilateral song was produced solely by the +/+ male and bilateral song solely by the female. (C) Close-up of bilateral song waveforms. (D to G) Bilateral song median IPI (D), pulse duration (E), response time (delay between onset of unilateral bout and center of first following bilateral pulse) (F), and amount relative to unilateral song (G). Each dot represents one fly. n = 83, 114, and 22 flies. Statistical tests were Kruskal-Wallis tests followed by pairwise Wilcoxon rank sum tests with Bonferroni correction. (H) Video stills of fencing/sparring (left) and curling/spinning (right). (I to J) Percentage of bins with fencing/sparring (I) and curling/spinning (J). Each dot represents one fly. n = 13, 38, and 24 flies.
Homozygous fruΔtra D. virilis females display male-directed aggression
We observed two types of male-directed aggressive behaviors from D. virilis fruΔtra females (Fig. 4H). Male-directed aggression was not reported in fruΔtra D. melanogaster females (3, 6), because these pairings are dominated by courtship from the male (45). In one behavior, the fruΔtra D. virilis female and male face each other and extend their front tarsi toward one another (movie S4), similar to previously described “low-posture fencing” (46) or “sparring” (47). In the second behavior, the fruΔtra female curls her abdomen toward the male’s head, similar to previously described curling (47, 48). While the female is curling, the male still tries to tap and lick her abdomen, which results in the two flies spinning together in a circle (movie S5). These aggressive behaviors were interspersed with duetting, with duetting often immediately preceding and/or following an aggressive bout. The amount of fencing/sparring and curling/spinning behaviors was dependent on the number of fruΔtra alleles in females (Fig. 4, I and J).
fruΔtra/fruΔtra D. virilis females were the most likely to produce aggression (Fig. 4, I and J) and were also least likely to copulate (Fig. 3B). However, we found no difference in the amount of aggressive behaviors in copulated versus non-copulated fruΔtra/+ females (fig. S6, F and G). These behaviors do not seem to be typical reactions to courtship of an unreceptive female, because non-copulating wild-type females did not engage in these behaviors (Fig. 4, I and J). We also did not observe aggressive behaviors in pairings between two wild-type males (dark green in fig. S5, Q to R), and only rarely between −/+ males and wild-type males (light green in fig. S5, Q to R), suggesting that these behaviors are dependent on the number of fruM alleles in D. virilis females but not males.
Although we and others did not observe aggression from D. melanogaster FruM females paired with wild-type males, likely because these pairings are dominated by female-directed courtship, fru does play a role in specifying sex-specific aggression in D. melanogaster males and females (45, 49–51). Together, these results illustrate an additional species difference: In D. melanogaster, pairings between wild-type males and fruΔtra females result in primarily female-directed courtship, whereas similar pairings in D. virilis result in alternations between female-directed courtship and male-directed aggression.
fruΔtra effects on courtship and aggression depend on allele number in female, but not male, D. virilis
So far, our results suggest that, in female D. virilis, FruM effects on both unilateral and bilateral song production as well as aggression depend on the number of fruΔtra alleles. To clarify the effects of FruM in both sexes, we performed principal components analysis (PCA) on courtship and aggressive behaviors exhibited by the same fly paired with a wild-type female versus a wild-type male (Fig. 5A). These results show that, compared to wild-type females, which do not produce any aggressive or male-typical courtship behaviors, adding one copy of fruΔtra to females caused movement primarily along principal component 2 (PC2; Fig. 5A), which correlated positively with both aggression (curling/spinning) and courtship (tarsal contact) directed toward a wild-type male. A second fruΔtra allele caused females to also move along PC1 (Fig. 5A), which correlated positively with courtship (tarsal contact and proboscis extension) directed toward a wild-type female. Only a few of the fruΔtra/fruΔtra females overlapped in PCA space with wild-type males, suggesting that FruM, while sufficient to produce male courtship behaviors including unilateral song in some D. virilis females, is not sufficient to fully masculinize females. In contrast to the effects of fruM allele number in females, removing one fruM allele in males had no effect on courtship or aggression (Fig. 5A). These results lend further support to the conclusion that fruM has allele number–dependent effects in D. virilis females but not males.
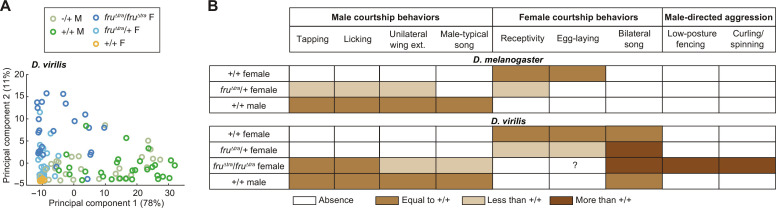
Fig. 5. FruM effects on D. virilis courtship and aggressive behaviors depend on allele number in females.
(A) Principal components analysis (PCA) on all scored courtship and aggressive behaviors exhibited by a single fly when paired with a +/+ female and (separately) a +/+ male. Principal component 1 (PC1) positively correlated with tarsal contact and proboscis extension directed toward a +/+ female, and PC2 positively correlated with curling/spinning and tarsal contact, both directed toward a +/+ male. Each dot represents one fly. n = 26 −/+ M, 29 +/+ M, 24 fruΔtra/fruΔtra F, 39 fruΔtra/+ F, and 14 +/+ F flies. (B) Table summarizing the effects of FruM expression in D. melanogaster (top) and D. virilis (bottom). Colors indicate the amount of each behavior.
DISCUSSION
Here, we provide evidence for both similarities and differences in the function of fru between two Drosophila species. We were able to make these comparisons by using CRISPR-Cas9 to force native male-specific fruM splicing in female D. virilis, which had previously been accomplished only in D. melanogaster. One of the notable species differences that we found is that D. virilis, but not D. melanogaster, females expressing FruM maintain their fecundity (Fig. 5B), enabling us to test the effects of one versus two alleles of fruΔtra on behaviors in D. virilis, but not in D. melanogaster. This revealed that fruM allele number in females (but not in males) affected the phenotypes observed. In contrast to D. melanogaster, in which one copy of fruΔtra is sufficient to produce some male courtship behaviors, two copies were needed in D. virilis (Fig. 5B). These male courtship behaviors were accompanied by male-typical song production in female D. virilis but not D. melanogaster (Fig. 5B) (3, 44). Therefore, while FruM plays a role in male-typical unilateral wing vibrations in both species, the extent to which the resulting song is fully masculinized is different across species. FruM expression in female D. virilis also had opposing effects on receptivity and bilateral (female-typical) song amount (Fig. 5B); effects on female song production could not be assayed in D. melanogaster because those females do not sing. That FruM expression does not inhibit egg laying or bilateral song production in D. virilis is different from the role of FruM in suppressing female behaviors in D. melanogaster and Aedes aegypti (3, 24). However, the disruption of female receptivity in FruM D. virilis females is in line with similar results in D. melanogaster. Last, D. virilis females expressing FruM exhibited male-directed aggression, whereas D. melanogaster females did not (Fig. 5B).
A role for FruM in both unilateral and bilateral song production in D. virilis
We found that FruM expression conferred some D. virilis females with the ability to produce male-specific (female-directed) unilateral song while up-regulating the amount of (male-directed) bilateral song. This suggests that the circuitry underlying each song type is susceptible to FruM expression and also raises the possibility that overlapping (instead of distinct) neural populations may contribute to unilateral and bilateral song in D. virilis males. Although D. melanogaster females do not naturally produce song, mutations of genes outside the sex determination cascade have caused females to produce unilateral wing extensions (52, 53). Artificial activation of fru+ ventral nerve cord circuitry in female D. melanogaster produced unilateral wing extensions with aberrations in pulse and sine song that were ameliorated by FruM expression (54). Activation of the dsx+ pC1 brain neuron cluster also produced male song in females (55). Together, these findings suggest that female D. melanogaster has latent circuitry capable of song production and that FruM unlocks the ability of this circuitry to produce male courtship behaviors, including wing extensions. It is tempting to speculate that the processes involved in producing male-specific behaviors in FruM female D. melanogaster might be similar to the processes involved in the up-regulation of bilateral song in FruM female D. virilis.
Differences between behaviors of fruΔtra/+ and fruΔtra/fruΔtra D. virilis females
In D. virilis, we found differences in the amount of male courtship behaviors, bilateral song, aggression, and receptivity between females that were heterozygous versus homozygous for fruΔtra. This pattern is suggestive of haploinsufficiency, in which one copy of a gene is insufficient for a particular phenotype. Haploinsufficiency is not uncommon for transcription factors. For instance, fruM is haploinsufficient for pheromone responses in Or47b olfactory receptor neurons in male D. melanogaster (56). In mice, haploinsufficiency of the transcription factor Six3 disrupts male reproduction by impeding development of the main olfactory epithelium (57). In human sex determination, multiple transcription factors display haploinsufficiency leading to sex reversals (58). Causes of haploinsufficiency are hypothesized to include insufficient amounts of protein product arising from a single gene copy, stoichiometric disruptions of protein complexes, and, more recently, a narrow range of tolerable protein expression levels (58, 59). However, homozygous fruΔtra D. virilis females were not fully masculinized, as only a few of these flies produced male-like levels of courtship behaviors. This result is likely due to the interplay between fru and other sex-determination genes, such as doublesex (see the next section).
Our finding that two copies of fruΔtra are needed for male courtship behaviors in female D. virilis is different from the results of similar experiments in D. melanogaster, in which one copy of fruΔtra produces male-specific behaviors (3). One cause of this apparent species difference could be that our D. virilis fruΔtra allele is hypomorphic for FruM, such that fruΔtra/+ D. virilis females express less FruM protein relative to wild-type males than fru∆tra/+ D. melanogaster females. This would mean that fruΔtra/+ D. virilis female brains express enough FruM to reduce female receptivity but not enough to disrupt egg laying or to display male courtship behaviors, suggesting potential dose-dependent effects of FruM in D. virilis females. Quantification of FruM protein levels in fruΔtra females of both species would be required to determine how FruM expression levels may relate to behavioral phenotypes.
Differences between behaviors of D. virilis males and fruΔtra females
We found that fruM allele–dependent effects were specific to the female context in D. virilis, because normal male courtship behavior was observed with just one allele of fruM in males (fig. S5). Another transcription factor called doublesex (dsx) is also sex-specifically spliced in D. melanogaster and, in contrast to fru, produces functional protein in males (DsxM) and females (DsxF) (2). Dsx is expressed in subsets of neurons that play key roles in both male- and female-specific behaviors (5, 60–63) and is co-expressed with FruM in some cell types in male brains (5, 36, 44, 64). Females generally have fewer numbers of dsx+ and fru+ neurons than males due to DsxF-dependent cell death (65) and FruM-dependent inhibition of cell death (66). Therefore, while the presence of FruM in female Drosophila brains sufficiently masculinizes some cell types (67), DsxF may act to limit the extent of this masculinization. For instance, although some fruΔtra females of both species produced male courtship behaviors, including singing, the overall levels of these behaviors were lower than those of wild-type males. Because of DsxF-mediated cell death (5), D. melanogaster FruM females lack the male-specific and DsxM+/FruM+ P1 neurons, which play a critical role in male courtship initiation and persistence (33, 68, 69). Therefore, the lower levels of male courtship produced by FruM females relative to males is in line with the absence of P1 neurons.
Role of FruM in D. virilis aggression
We found that, in pairings with wild-type males, fruΔtra D. virilis females alternate between playing the female-typical role in acoustic duets and directing aggression toward the male. We only saw these behaviors in fruΔtra females, raising the concern that these are aberrant behaviors caused by partial masculinization in a female background. Multiple lines of evidence argue against this possibility. First, curling and fencing/sparring have previously been reported in wild-type D. virilis (47, 48), suggesting that these are species-typical behaviors. It is possible that we did not provide sufficient conditions, such as a food patch or competing male, to provoke these behaviors in wild-type males. Second, although we did not observe male-directed aggression from D. melanogaster fruΔtra females, these females do show aggression in other contexts (45), and subsets of FruM+ neurons play a role in generating aggression in D. melanogaster (49–51). Therefore, a role for FruM in aggression in D. virilis is consistent with a similar role in D. melanogaster, although the species-specific types of aggressive behaviors may be different. The aggression of fruΔtra D. virilis females is unlikely to be a rejection of male courtship, as non-copulating females did not produce more of these behaviors than copulating females. Males also were not deterred by these behaviors and instead would often resume courtship immediately following an aggressive bout. In almost all instances, the fruΔtra female was the initiator of the aggression, and our interpretation of the spinning that accompanied the curling behavior is that the male was trying to reach the female’s abdomen and genitalia with his foretarsi and proboscis, respectively. As the female curled and spun around, the male followed. Together, these results suggest that FruM plays a role in producing species-typical aggression and that FruM expression in female D. virilis brains causes the female to be aggressive in situations where she otherwise would not be.
Importance of analyzing the role of switch genes across species
Findings in other insect species of sex-specific fru splicing and/or disruption of male courtship behaviors after fruM knockdown led to the hypothesis that fru’s role as a sex-determination switch gene was highly conserved (14, 15, 17, 18, 23, 24, 26, 27, 70). This hypothesis was also supported by findings that inserting fru genes from drosophilid species with divergent courtship behaviors into D. melanogaster males recapitulated D. melanogaster behaviors, instead of phenocopying each species’ own behaviors (71), which suggested that divergence in FruM downstream targets likely contributes to specifying species-specific behaviors. However, in most species previously studied, males and females engage in markedly different behaviors during courtship. By choosing a species in which the sexes produce a similar behavior, i.e., singing, we uncovered potential differences in some aspects of fru function, despite conservation of sex-specific FruM expression (Fig. 1, I and J) (22, 38). Additional sex-determination switch genes in D. melanogaster include dsx and three genes upstream of fru and dsx: sex-lethal (sxl), transformer (tra), and transformer-2 (tra2). Whereas dsx, sxl, and tra2 appear to be conserved at the sequence level in D. virilis (2, 72, 73), tra sequence comparisons suggest functional divergence in D. virilis and other Drosophila species (74, 75). The extent to which sequence divergence in sex determination genes contributes to species-specific behaviors remains to be determined. Quantifying how these genes are expressed in individual cell types will be critical to evaluating potential divergence versus conservation of gene function at the circuit level. Our findings here of divergent effects of FruM expression on sex-specific behaviors in D. virilis highlight the importance of going beyond sequence comparisons in carefully selected species for evaluating conservation versus divergence of switch gene function.
In summary, through gene editing and careful behavior quantification, we found evidence for both differences and similarities in fru function in divergent Drosophila species. Future work should investigate fru circuitry underlying sex-specific behaviors across species to understand the neural basis of behavioral divergence.
MATERIALS AND METHODS
Fly strains
We used D. virilis 15010-1051.47 (43) and D. melanogaster NM91 as wild-type strains. D. virilis was kept on standard medium at 20°C on a 16-hour:8-hour light:dark cycle (76) and aged 10 to 20 days, as this is the time required to reach sexual maturity (77). D. melanogaster was kept on standard medium at 25°C on a 12-hour:12-hour light:dark cycle and aged 3 to 7 days. Flies expected to produce male courtship behaviors [i.e., males and fruΔtra/fruΔtra, fruΔtra/+, and wild-type sibling (D. virilis) or control (D. melanogaster) females] were singly housed within 8 hours of eclosion, whereas courtship targets (wild-type females that were not siblings of fruΔtra females) were housed in groups of five to six flies.
Comparison of fruitless nucleotide sequences
We downloaded the following data in April and May 2023: D. melanogaster fru (FBgn0004652) exon sequences from ensembl.org; the D. virilis scaffold (scaffold_12855) containing fru and the Drosophila simulans chromosome (ch3R) containing most fru exons from the UC Santa Cruz Genome Browser (https://genome.ucsc.edu/); and the sequences containing the D. simulans B (accession number GI: 111258132) and C (accession number: KF005597) exons from GenBank (78). We used Geneious Prime 2023.1.2 to align the nucleotide sequence of each D. melanogaster exon to the relevant D. virilis and D. simulans sequences and recorded the % identity.
Generation of D. virilis fruitless mutants
fru Δtra
To examine the role of fruitless in D. virilis song production, we used CRISPR-Cas9 mutagenesis to remove the Tra binding sites from the S exon, similar to the fruΔtra mutation previously made in D. melanogaster (3). We identified the Tra binding sites in D. virilis based on sequence similarity with D. melanogaster (79). We then designed CRISPR guide RNAs (gRNAs) flanking the Tra binding sites using the CRISPR Optimal target finder (80). The gRNAs had a 20-nt target sequence and were flanked by a 3′ protospacer adjacent mofif (PAM) sequence (“NGG”) and a 5′ T7 RNA polymerase recognition sequence (“GG”). The target genomic region was sequenced using Sanger sequencing. gRNAs are listed below. The 5′ is the T7 promoter, bold indicates the gRNA target, and italics indicate the 3′ portion that overlaps with the reverse primer. The PAM is shown in parentheses.
L1: 5′ GAAATTAATACGACTCACTATAGGTGTCTATGCCTAGGACTT(AGG)GTTTTAGAGCTAGAAATAGC 3′
R1: 5′ GAAATTAATACGACTCACTATAGGCTAGAGGCACGTGAGTAG(TGG) GTTTTAGAGCTAGAAATAGC 3′
R2: 5′ GAAATTAATACGACTCACTATAGGAACTGCATACCGTGCGGCA(TGG) GTTTTAGAGCTAGAAATAGC 3′
The forward primer format and single guide RNA-reverse (sgRNA-R) primer sequences are based on (81). To generate the template for each sgRNA, we used the CRISPR forward and reverse 4 nmol Ultramer oligonucleotides (Integrated DNA Technologies). The full-length dsDNA template was amplified using Invitrogen Platinum PCR super mix high fidelity (catalog no.12532-016) and 0.5 μM forward and reverse primers. Reactions were carried on an Applied Biosystems 2720 Thermal Cycler, 95°C 2 m, 35 cycles of (95°C for 20 s, 60°C for 10 s, and 70°C for 10 s) and then purified with Ampure XP beads (A63880). In vitro transcription of 300 ng of sgRNA template DNA using T7 MEGAscript kit (Invitrogen, AM1333) was carried out at 37°C for 16 to 20 hours. Turbo deoxyribonuclease (Invitrogen, AM2239) was added for an additional 15 min at 37°C and then purified with Mega Clear Kit (Invitrogen, AM1908). The gRNA concentration and quality were checked with Agilent Bioanalyzer and frozen in small aliquots at −80°C for long-term storage. The CRISPR injection mixture contained recombinant Cas9 protein (300 ng/μl; PNA Bio CP01) and sgRNA (40 ng/ μl; per guide; we used one upstream L1 and two downstream R1 and R2 gRNA) and was injected into embryos of D. virilis wild-type strain 15010-1051.47. The Insect Transformation Facility at the University of Maryland performed all injections. We backcrossed the injected G0 flies to wild-type flies and selected lines in which germline mutagenesis was successful as determined by PCR genotyping (see below). PCR and sequencing confirmed that L1 and R2 successfully cut the DNA and removed 622 base pairs, including the Tra binding sites. We obtained 12 independent lines carrying the fruΔtra mutant allele and observed no differences in behaviors across lines.
fruM-null
The method to remove the S-exon of the fruitless gene followed the same general procedure described for the fruΔtra design, with the following changes. We designed two CRISPR target sites (L-1 and L-3) upstream of the D. virilis fru S-exon start codon. The target sites downstream of the S-exon were as described earlier (R1 and R2).
L-1: GAAATTAATACGACTCACTATAGGAAACCTTTAAACGGAGAAT(TGG)GTTTTAGAGCTAGAAATAGC
L-3: GAAATTAATACGACTCACTATAGGACCAACTAGTGCTAGAT(CGG)GTTTTAGAGCTAGAAATAGC
The injection mix contained the L-1, L-3, R1, and R2 gRNA. We crossed injected flies to one another and used PCR genotyping of the offspring to identify lines with germline transmission. PCR and sequencing confirmed that the L-1 and R1 guides successfully removed the S-exon. We obtained seven independent lines in which the S-exon was removed.
PCR genotyping
We used PCR to identify the genotype of experimental flies. We extracted DNA from the whole fly or from just the body (saving the heads for immunostaining from a subset of flies) using a Quick-gDNA miniprep kit (Zymo Research, R1050). We designed primers upstream and downstream of the Tra binding sites as follows:
CRISPRcut F3: TACGTACACGAATAGCCTCTTG
CRISPRcut R1: TGCCCGATTGAGCAAAATGC
We designed an additional primer upstream of the S-exon to identify flies in which the S-exon was successfully removed.
CRISPRcut F1: TGAGAGTTGTGTGATGGCTTG
Reverse transcription polymerase chain reaction
We made total RNA from fly heads using a Quick RNA Microprep kit (Zymo Research, R1050). The reverse transcription reaction used the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814) and RNase inhibitor (Promega, N2515). We designed male-specific and female-specific forward RT-PCR primers and a primer from a downstream fru common exon based on sequence similarity with those used previously in D. melanogaster (8).
Female-specific primer 10136 Fwd: GCAAAAGGAAGAGAGC-CTCA
Male-specific primer 8200 Fwd: GATGGCCACCTCACAAGATT
Common primer C4 Rev: GCAGTCCATATTTCGAGACGA
Immunohistochemistry
We dissected brains in ice-cold phosphate-buffered saline (PBS) and then fixed in 4% paraformadehyde in PBSX1 (CellGro, 21-040) with 0.3% Triton X-100 (Sigma-Aldrich, X100) (abbreviated PBT3) for 45 min in the dark at room temperature. We blocked with 5% normal goat serum (Life Technologies, 16210-064) in PBT3 for two overnights at 4°C. We incubated brains with 1:5000 rabbit anti-FruM (36) (gift from S. Goodwin) and 1:20 mouse anti-nc82 (Developmental Studies Hybridoma Bank, Iowa City, Iowa) in blocking solution for three overnights at 4°C. We rinsed eight times for 20 min in PBT3 at room temperature before an overnight incubation in 1:500 goat anti-rabbit Alexa Fluor 633 (Invitrogen, A21070) and 1:500 goat anti-mouse Alexa Fluor 488 (Life Technologies, A11001) in blocking solution at 4°C. We rinsed four times for 20 min in PBT3 and then four times for 20 min in PBS at RT before leaving brains overnight in Vectashield (Vector Laboratories, H-1000-10) at 4°C. We mounted brains in Vectashield and imaged on a confocal microscope (Leica TCS SP8 X). Images were adjusted for brightness and contrast in ImageJ (National Institutes of Health).
Behavioral assays
Virgin males and females were used for behavior experiments. Video was captured at 60 frames/s with a Point Grey Flea 3 CMOS camera (FL3-U3-13E4C-C). Audio was recorded at 10 kHz on a 32-channel apparatus (43, 82). Each experimental fly was paired with a single homosexual +/+ partner on day 1 and with a single heterosexual +/+ partner on day 2. Each recording lasted 25 or 30 min. Experimental flies were singly housed to maintain their identity for PCR genotyping and/or checking for larvae. All assays occurred at ~22°C and between 0 and 4 hour of Zeitgeber time (ZT) (D. virilis) or 0 to 1.5 hours of ZT (D. melanogaster). Circular behavioral chambers were scaled for differences in fly body sizes between species: 11-mm diameter for D. melanogaster (82) and 20-mm diameter for D. virilis (43).
Behavior quantification
We uniformly sampled each video and manually scored 10-s bins spaced 1 min apart for the presence or absence of the following behaviors: tarsal contact (front tarsi contact with any part of the other fly); proboscis extension; unilateral wing extension; bilateral wing extension; wing-flicking (unilateral wing extension to ~45° that is quickly retracted without producing sound); fencing/sparring (flies face one another and extend their front tarsi toward each other while in a normal standing posture) (46); curling (fly curls abdomen laterally with the tip of the abdomen pointed toward the other fly) (47); and spinning (flies face in opposite directions and jointly move in a circle). Curling and spinning co-occurred so frequently that we did not attempt to score them separately. We report the percentage of bins containing at least one instance of the respective behavior in Figs. 2 (B to D) and 4 (I and J) and figs. S4 (C and D), S5 (E to G and Q to R), and S5 (A to C and F to G). We performed PCA on the amount of these seven behaviors each fly produced when paired with a +/+ male and a +/+ female using MATLAB 2019b. Copulation was defined as the time when the male first mounted the female and remained in the copulation position for at least 1 min in D. virilis (copulation duration, ~2 to 5 min) or 5 min in D. melanogaster (copulation duration, ~10 to 20 min) (83).
D. virilis song segmenter
Network structure
The D. virilis song segmenter consisted of two convolutional neural networks in Python 3.4 (fig. S2, A and B): one network for classifying each point in the microphone recording as unilateral song, bilateral song, overlap of the two song types, or no song; and a second network for classifying bilateral song versus no song. Using the four-class network alone led to many bilateral song false positives, as noises such as grooming, jumping, and rolling were often classified as bilateral song. Therefore, to improve the precision of bilateral song detection, we used a second, two-class network to classify bilateral versus no song. Both networks take the raw microphone recording as input. The four-class network used a window size of 4001 points (400.1 ms), batch size of 128, six epochs, 16 convolutional filters, 2 × 2 pooling, a convolutional kernel size of 9 convolutions and 4 padding, and trained on 10% of the data. The two-class network used a similar structure as the four-class network except it used a window size of 2001 points (200.1 ms) and three epochs during training.
Training data
A single observer used both audio and video to manually segment five 30-min recordings of +/+ males paired with +/+ females and one 30-min recording of a +/+ male paired with a fruΔtra/+ female. The fruΔtra/+ female recording was chosen because of the large amount of song from both flies. We then drew from these data to make training data for the two neural networks. Training data for the four-class network consisted of 31.6 total min (28 min from the fruΔtra/+ and +/+ male pairing and the remaining from the +/+ male and female pairs), resulting in a total of 4.9% unilateral song (585 unilateral bouts), 25.4% bilateral song (10,358 fruΔtra/+ bilateral pulses, 92 +/+ bilateral pulses), and 1.3% overlap between unilateral and bilateral. We found the best performance when data from different rounds of data collection were included in the training set. To make the training data for the two-class network, we removed unilateral and overlap portions from the training data for the four-class network and added 21.5 min of additional noise and quiet taken from recordings of +/+ female pairs.
Song segmentation
To combine the output of the two segmenters, we first applied a median filter (window size of 10 ms) to the output probabilities and then averaged the bilateral and no song probabilities from the four- and two-class networks. We ignored the output of the two-class network during portions identified as unilateral or no song by the four-class network (fig. S3C). The maximum probability at each timepoint was used to identify instances of song, with the following heuristics. To classify a point as bilateral song, we required the bilateral probability to be at least 1.25 times that of unilateral song, and, to classify a point as overlap, we required the overlap probability to be at least 0.85. We required bilateral song within 40 ms before or up to 10 ms after unilateral song to have a classification probability of 1; otherwise, it was assigned to unilateral song. We threw out segments predicted to be bilateral if they were shorter than 4 ms. We next defined unilateral bouts as contiguous unilateral predictions and bilateral pulses as contiguous bilateral predictions. To separate contiguous unilateral or bilateral song into pulses, we first calculated the difference of the upper and lower peak envelopes of the microphone signal with a width of 5 ms and then detected peaks in this signal. Minima on either side of these peaks were used to define where one pulse ended and the next started. We compared the segmenter output to ground truth data obtained by manually segmenting 22 recordings (fig. S3, D to F). Sensitivity, positive predictive value, and the harmonic mean (F) were calculated as previously described (82).
Song analysis
To calculate IPIs, we first calculated the difference of the upper and lower peak envelopes of the microphone signal with a width of 5 ms and then detected peaks in this signal. The difference in timing of these peaks was taken as the IPI. Remaining song analysis was largely based on a previous study of D. virilis duets (43). A unilateral bout was defined as a series of at least four unilateral pulses with IPIs of 60 ms or less. A bilateral bout was defined as any number of bilateral pulses with IPIs of 100 ms or less. Unilateral response times were defined as the delay between the offset of a bilateral pulse and the onset of the immediately following unilateral bout. Bilateral response times were defined as the time between the onset of a unilateral bout and the center of the following bilateral pulse. Only response times less than 1.5 s were included in our analysis.
For song feature measurements, we used only recordings with at least 10 unilateral and 10 bilateral pulses before copulation in copulating pairs or over the entire recording otherwise. We quantified the amount of unilateral and bilateral song by summing the total time of the recording classified as unilateral or bilateral and then dividing by the courtship time, defined as the time between the first and last pulse (of either song type) in the recording (for non-copulating pairs) or the last pulse of either song type before copulation (for copulating pairs).
Statistics
Because most measurements were not normally distributed according to Jarque-Bera tests, we used nonparametric tests throughout. All statistics were performed in MATLAB 2019b or 2022a.
Acknowledgments
We thank J. Clemens and T. Pereira for helpful discussions during the development of the D. virilis song segmenter; S. Goodwin and M. Perry for anti-FruM antibody; Y. Ding, M. Shahandeh, Z. Zhao, D. Mearns, and M. Aragon for comments on the manuscript; D. Parker for discussions of fruitless exons; and members of the Murthy lab for feedback on analyses.
Funding: This work was funded by the Jane Coffin Childs Memorial Foundation (to C.A.B.), NIH NINDS DP2 New Innovator Award (to M.M.), and HHMI Faculty Scholar Award (to M.M.).
Author contributions: Conceptualization: C.A.B., X.-J.G., and M.M. Methodology: C.A.B., X.-J.G., M.C., and M.M. Investigation: C.A.B. and X.-J.G. Visualization: C.A.B. Supervision: M.M. Writing—original draft: C.A.B., X.-J.G., and M.M. Writing—review and editing: C.A.B., X.J.G., M.C., and M.M.
Competing Interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All data and code are available in a publicly accessible digital repository (dataspace.princeton.edu; https://doi.org/10.34770/me8y-8k48).
Supplementary Materials
This PDF file includes:
Figs. S1 to S6
Legends for movies S1 to S5
References
Other Supplementary Material for this manuscript includes the following:
Movies S1 to S5
REFERENCES AND NOTES
- 1.Manoli D. S., Fan P., Fraser E. J., Shah N. M., Neural control of sexually dimorphic behaviors. Curr. Opin. Neurobiol. 23, 330–338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burtis K. C., Baker B. S., Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56, 997–1010 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Demir E., Dickson B. J., Fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Ito H., Fujitani K., Usui K., Shimizu-Nishikawa K., Tanaka S., Yamamoto D., Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. 93, 9687–9692 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura K., Hachiya T., Koganezawa M., Tazawa T., Yamamoto D., Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Manoli D. S., Foss M., Villella A., Taylor B. J., Hall J. C., Baker B. S., Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Peng Q., Chen J., Pan Y., From fruitless to sex: On the generation and diversification of an innate behavior. Genes Brain Behav. 20, e12772 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Ryner L. C., Goodwin S. F., Castrillon D. H., Anand A., Villella A., Baker B. S., Hall J. C., Taylor B. J., Wasserman S. A., Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Salvemini M., Polito C., Saccone G., Fruitless alternative splicing and sex behaviour in insects: An ancient and unforgettable love story? J. Genet. 89, 287–299 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Stockinger P., Kvitsiani D., Rotkopf S., Tirián L., Dickson B. J., Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Gill K. S., A mutation causing abnormal courtship and mating behavior in males of Drosophila melanogaster. Am. Zool.. 3, 507 (1963). [Google Scholar]
- 12.Lee G., Foss M., Goodwin S. F., Carlo T., Taylor B. J., Hall J. C., Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 43, 404–426 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Usui-Aoki K., Ito H., Ui-Tei K., Takahashi K., Lukacsovich T., Awano W., Nakata H., Piao Z. F., Nilsson E. E., Tomida J., Yamamoto D., Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat. Cell Biol. 2, 500–506 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Bertossa R. C., van de Zande L., Beukeboom L. W., The fruitless gene in Nasonia displays complex sex-specific splicing and contains new zinc finger domains. Mol. Biol. Evol. 26, 1557–1569 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Gailey D. A., Billeter J.-C., Liu J. H., Bauzon F., Allendorfer J. B., Goodwin S. F., Functional conservation of the fruitless male sex-determination gene across 250 Myr of insect evolution. Mol. Biol. Evol. 23, 633–643 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Laohakieat K., Isasawin S., Thanaphum S., The transformer-2 and fruitless characterisation with developmental expression profiles of sex-determining genes in Bactrocera dorsalis and B. correcta. Sci. Rep. 10, 17938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier N., Käppeli S. C., Niessen M. H., Billeter J.-C., Goodwin S. F., Bopp D., Genetic control of courtship behavior in the housefly: Evidence for a conserved bifurcation of the sex-determining pathway. PLOS ONE 8, e62476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvemini M., D’Amato R., Petrella V., Aceto S., Nimmo D., Neira M., Alphey L., Polito L. C., Saccone G., The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: Evolution of genomic organisation and alternative splicing. PLOS ONE 8, e48554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis T., Kurihara J., Yamamoto D., Genomic organisation and characterisation of the neural sex-determination gene fruitless (fru) in the Hawaiian species Drosophila heteroneura. Gene 246, 143–149 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Davis T., Kurihara J., Yoshino E., Yamamoto D., Genomic organisation of the Neural sex determination gene fruitless (Fuu) in the Hawaiian species Drosophila Siluestris and the conservation of the Fru BTB protein-protein-binding domain throughout evolution. Hereditas 132, 67–78 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T., Evolution of the neural sex-determination system in insects: Does fruitless homologue regulate neural sexual dimorphism in basal insects? Insect Mol. Biol. 28, 807–827 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto D., Usui-Aoki K., Shima S., Male-specific expression of the fruitless protein is not common to all Drosophila species. Genetica 120, 267–272 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Tanaka R., Higuchi T., Kohatsu S., Sato K., Yamamoto D., Optogenetic activation of the fruitless-labeled circuitry in Drosophila subobscura males induces mating motor acts. J. Neurosci. 37, 11662–11674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basrur N. S., De Obaldia M. E., Morita T., Herre M., von Heynitz R. K., Tsitohay Y. N., Vosshall L. B., Fruitless mutant male mosquitoes gain attraction to human odor. eLife 9, e63982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerjan B., Tobback J., Vandersmissen H. P., Huybrechts R., Schoofs L., Fruitless RNAi knockdown in the desert locust, Schistocerca gregaria, influences male fertility. J. Insect Physiol. 58, 265–269 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Boerjan B., Tobback J., De Loof A., Schoofs L., Huybrechts R., Fruitless RNAi knockdown in males interferes with copulation success in Schistocerca gregaria. Insect Biochem. Mol. Biol. 41, 340–347 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Clynen E., Ciudad L., Bellés X., Piulachs M.-D., Conservation of fruitless’ role as master regulator of male courtship behaviour from cockroaches to flies. Dev. Genes Evol. 221, 43–48 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Xu J., Liu W., Yang D., Chen S., Chen K., Liu Z., Yang X., Meng J., Zhu G., Dong S., Zhang Y., Zhan S., Wang G., Huang Y., Regulation of olfactory-based sex behaviors in the silkworm by genes in the sex-determination cascade. PLOS Genet. 16, e1008622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clowney E. J., Iguchi S., Bussell J. J., Scheer E., Ruta V., Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohl J., Ostrovsky A. D., Frechter S., Jefferis G. S. X. E., A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610–1623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro I. M. A., Drews M., Bahl A., Machacek C., Borst A., Dickson B. J., Visual projection neurons mediating directed courtship in Drosophila. Cell 174, 607–621.e18 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Ruta V., Datta S. R., Vasconcelos M. L., Freeland J., Looger L. L., Axel R., A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686–690 (2010). [DOI] [PubMed] [Google Scholar]
- 33.von Philipsborn A. C., Liu T., Yu J. Y., Masser C., Bidaye S. S., Dickson B. J., Neuronal control of Drosophila courtship song. Neuron 69, 509–522 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Ding Y., Lillvis J. L., Cande J., Berman G. J., Arthur B. J., Long X., Xu M., Dickson B. J., Stern D. L., Neural evolution of context-dependent fly song. Curr. Biol. 29, 1089–1099.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Seeholzer L. F., Seppo M., Stern D. L., Ruta V., Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564–569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billeter J.-C., Villella A., Allendorfer J. B., Dornan A. J., Richardson M., Gailey D. A., Goodwin S. F., Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 16, 1063–1076 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Goodwin S. F., Taylor B. J., Villella A., Foss M., Ryner L. C., Baker B. S., Hall J. C., Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics 154, 725–745 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usui-Aoki K., Mikawa Y., Yamamoto D., Species-specific patterns of sexual dimorphism in the expression of fruitless protein, a neural musculinizing factor in Drosophila. J. Neurogenet. 19, 109–121 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Song X., Goicoechea J. L., Ammiraju J. S. S., Luo M., He R., Lin J., Lee S.-J., Sisneros N., Watts T., Kudrna D. A., Golser W., Ashley E., Collura K., Braidotti M., Yu Y., Matzkin L. M., McAllister B. F., Markow T. A., Wing R. A., The 19 genomes of Drosophila: A bac library resource for genus-wide and genome-scale comparative evolutionary research. Genetics 187, 1023–1030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spieth H. T., Courtship behavior in Drosophila. Annu. Rev. Entomol. 19, 385–405 (1974). [DOI] [PubMed] [Google Scholar]
- 41.A. Hoikkala, “Inheritance of male sound characteristics in Drosophila species” in Insect Sounds and Communication: Physiology, Behavior, Ecology and Evolution (CRC Press, 2005), pp. 167–177. [Google Scholar]
- 42.Donegan J., Ewing A. W., Duetting in Drosophila and Zaprionus species. Anim. Behav. 28, 1289 (1980). [Google Scholar]
- 43.LaRue K. M., Clemens J., Berman G. J., Murthy M., Acoustic duetting in Drosophila virilis relies on the integration of auditory and tactile signals. eLife 4, e07277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rideout E. J., Billeter J.-C., Goodwin S. F., The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr. Biol. 17, 1473–1478 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrontou E., Nilsen S. P., Demir E., Kravitz E. A., Dickson B. J., Fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9, 1469–1471 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Nilsen S. P., Chan Y.-B., Huber R., Kravitz E. A., Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. 101, 12342–12347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spieth H. T., Courtship behavior of endemic Hawaiian Drosophila. Univ. Tex. Publ. 6615, 245–313 (1966). [Google Scholar]
- 48.Spieth H. T., Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 99, 7 (1952). [Google Scholar]
- 49.Chan Y.-B., Kravitz E. A., Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 104, 19577–19582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Certel S. J., Savella M. G., Schlegel D. C. F., Kravitz E. A., Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. U.S.A. 104, 4706–4711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wohl M., Ishii K., Asahina K., Layered roles of fruitless isoforms in specification and function of male aggression-promoting neurons in Drosophila. eLife 9, e52702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook R., ‘Lesbian’ phenotype of Drosophila melanogaster? Nature 254, 241–242 (1975). [DOI] [PubMed] [Google Scholar]
- 53.Ditch L. M., Shirangi T., Pitman J. L., Latham K. L., Finley K. D., Edeen P. T., Taylor B. J., McKeown M., Drosophila retained/dead ringeris necessary for neuronal pathfinding, female receptivity and repression of fruitless independent male courtship behaviors. Development 132, 155–164 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clyne J. D., Miesenböck G., Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133, 354–363 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Rezával C., Pattnaik S., Pavlou H. J., Nojima T., Brüggemeier B., D’Souza L. A. D., Dweck H. K. M., Goodwin S. F., Activation of latent courtship circuitry in the brain of Drosophila females induces male-like behaviors. Curr. Biol. 26, 2508–2515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sethi S., Lin H.-H., Shepherd A. K., Volkan P. C., Su C.-Y., Wang J. W., Social context enhances hormonal modulation of pheromone detection in drosophila. Curr. Biol. 29, 3887–3898.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandolfi E. C., Hoffmann H. M., Schoeller E. L., Gorman M. R., Mellon P. L., Haploinsufficiency of SIX3 abolishes male reproductive behavior through disrupted olfactory development, and impairs female fertility through disrupted GnRH neuron migration. Mol. Neurobiol. 55, 8709–8727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veitia R. A., Exploring the etiology of haploinsufficiency. Bioessays 24, 175–184 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Morrill S. A., Amon A., Why haploinsufficiency persists. Proc. Natl. Acad. Sci. 116, 11866–11871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirangi T. R., Wong A. M., Truman J. W., Stern D. L., Doublesex regulates the connectivity of a neural circuit controlling Drosophila male courtship song. Dev. Cell 37, 533–544 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Kimura K., Sato C., Koganezawa M., Yamamoto D., Drosophila ovipositor extension in mating behavior and egg deposition involves distinct sets of brain interneurons. PLOS ONE 10, e0126445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou C., Pan Y., Robinett C. C., Meissner G. W., Baker B. S., Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Wang K., Wang F., Forknall N., Yang T., Patrick C., Parekh R., Dickson B. J., Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 589, 577–581 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F., Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanders L. E., Arbeitman M. N., Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol. 320, 378–390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura K.-I., Ote M., Tazawa T., Yamamoto D., Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229–233 (2005). [DOI] [PubMed] [Google Scholar]
- 67.von Philipsborn A. C., Jörchel S., Tirian L., Demir E., Morita T., Stern D. L., Dickson B. J., Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr. Biol. 24, 242–251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan Y., Meissner G. W., Baker B. S., Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc. Natl. Acad. Sci. 109, 10065–10070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.R. T. Coleman, I. Morantte, G. T. Koreman, M. L. Cheng, Y. Ding, V. Ruta, A modular circuit architecture coordinates the diversification of courtship strategies in Drosophila. bioRxiv. 2023. www.biorxiv.org/content/10.1101/2023.09.16.558080v1. [DOI] [PMC free article] [PubMed]
- 70.Petrella V., Aceto S., Colonna V., Saccone G., Sanges R., Polanska N., Volf P., Gradoni L., Bongiorno G., Salvemini M., Identification of sex determination genes and their evolution in Phlebotominae sand flies (Diptera, Nematocera). BMC Genomics 20, 522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cande J., Stern D. L., Morita T., Prud’homme B., Gompel N., Looking under the lamp post: Neither fruitless nor doublesex has evolved to generate divergent male courtship in Drosophila. Cell Rep. 8, 363–370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jinks T. M., Calhoun G., Schedl P., Functional conservation of the Sex-lethal sex determining promoter, Sxl-Pe, in Drosophila virilis. Dev. Genes Evol. 213, 155–165 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Chandler D., McGuffin M. E., Piskur J., Yao J., Baker B. S., Mattox W., Evolutionary conservation of regulatory strategies for the sex determination factor transformer-2. Mol. Cell. Biol. 17, 2908–2919 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neil M. T., Belote J. M., Interspecific comparison of the transformer gene of Drosophila reveals an unusually high degree of evolutionary divergence. Genetics 131, 113–128 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulathinal R. J., Skwarek L., Morton R. A., Singh R. S., Rapid evolution of the sex-determining gene, transformer: Structural diversity and rate heterogeneity among sibling species of Drosophila. Mol. Biol. Evol. 20, 441–452 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Suvanto L., Liimatainen J. O., Hoikkala A., Variability and evolvability of male song characters in Drosophila montana populations. Hereditas 130, 13–18 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Isoherranen E., Aspi J., Hoikkala A., Variation and consistency of female preferences for simulated courtship songs in Drosophila virilis. Anim. Behav. 57, 619–625 (1999). [DOI] [PubMed] [Google Scholar]
- 78.Parker D. J., Gardiner A., Neville M. C., Ritchie M. G., Goodwin S. F., The evolution of novelty in conserved genes; evidence of positive selection in the Drosophila fruitless gene is localised to alternatively spliced exons. Heredity 112, 300–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y., Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 252, 833–836 (1991). [DOI] [PubMed] [Google Scholar]
- 80.Gratz S. J., Ukken F. P., Rubinstein C. D., Thiede G., Donohue L. K., Cummings A. M., O’Connor-Giles K. M., Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kistler K. E., Vosshall L. B., Matthews B. J., Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 11, 51–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arthur B. J., Sunayama-Morita T., Coen P., Murthy M., Stern D. L., Multi-channel acoustic recording and automated analysis of Drosophila courtship songs. BMC Biol. 11, 11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto D., Jallon J.-M., Komatsu A., Genetic dissection of sexual behavior in Drosophila melanogaster. Annu. Rev. Entomol. 42, 551–585 (1997). [DOI] [PubMed] [Google Scholar]
- 84.Billeter J.-C., Goodwin S. F., Characterization of Drosophila fruitless-gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. J. Comp. Neurol. 475, 270–287 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S6
Legends for movies S1 to S5
References
Movies S1 to S5