Abstract
Background
Anaplastic lymphoma kinase (ALK)-targeted tyrosine kinase inhibitors (TKIs) improve patient survival; however, some patients develop ALK-TKI resistance with unidentified mechanisms. We investigated ErbB family and c-MET expression in patients with ALK-positive non-small cell lung cancer (NSCLC) to understand their roles in the ALK-TKI response.
Methods
We studied 72 patients with advanced ALK-positive NSCLC with EML4-ALK fusion variant subtyping and immunostaining for c-MET, EGFR, HER2, and HER3 on tissue specimens both pre- (primary) and post-treatment (secondary) with ALK-TKI. We investigated the association of their expression with survival outcomes and assessed the effectiveness of combining ALK and EGFR inhibitors in ALK-positive NSCLC cell lines stimulated with the HER3-specific ligand HRG1.
Results
High expression of c-MET, EGFR, HER2, and HER3 was observed in 4.9%, 18.0%, 1.6%, and 25.8% of primary tumors, respectively, and 18.5%, 37.0%, 10.7%, and 35.7% of secondary tumors, respectively. HER3 overexpression in primary tumors showed inferior survival (P=0.132). In the subgroup with EML4-ALK variant 1/2 (V1/V2), HER3 overexpression was significantly associated with inferior survival in both primary and secondary tumors (P=0.022 and P=0.004, respectively). Combination treatment with lorlatinib and erlotinib significantly reduced HRG1-induced activation of RTK signaling in ALK-positive NSCLC cells.
Conclusions
HER3 overexpression has potential as a prognostic marker in ALK-positive NSCLCs, including ALK-TKI naïve and treated cases, especially those with EML4-ALK V1/V2. Assessing HER3 expression may be crucial for treatment planning and outcome prediction in these patients.
Keywords: Non-small cell lung cancer (NSCLC), EML4-ALK, EGFR, HER3, c-MET
Highlight box.
Key findings
• HER3 overexpression in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) was associated with a poor outcome in patients treated with ALK inhibitors.
• Combined lorlatinib and erlotinib treatment effectively reduced the HRG1-induced signaling activation in ALK-positive NSCLC cell lines.
What is known and what is new?
• Activation of ErbB family members, a recognized bypass pathway in EGFR-mutant NSCLC, has gained attention as a potential resistance mechanism in ALK-positive NSCLC.
• HER3 overexpression in ALK-tyrosine kinase inhibitor (TKI)-naïve (primary) NSCLCs indicated a tendency toward poor outcomes with ALK-TKIs, and it significantly correlated with poor overall survival in the EML4-ALK variant 1/2 subgroup in primary and secondary (post-ALK-TKI) tumors.
What is the implication, and what should change now?
• HER3 overexpression may serve as a prognostic marker in ALK-positive NSCLC and combining ALK inhibitor treatment with targeted inhibition of the ErbB family pathway can be an effective strategy for HER3-overexpressing ALK-positive NSCLCs.
Introduction
Anaplastic lymphoma kinase (ALK)-rearrangement has been detected in approximately 5% of patients with non-small cell lung cancer (NSCLC) (1). The most common fusion partner in ALK-rearranged NSCLC is EML4, and multiple variants of EML4-ALK fusion have been reported. As a driver mutation, EML4-ALK fusion induces aberrant activation of ALK kinase and its downstream signaling pathways (e.g., PI3K/AKT, RAS/ERK, and JAK/STAT) resulting in cellular proliferation and tumorigenesis (2). Following the introduction of the first-generation ALK-targeted tyrosine kinase inhibitor (TKI) crizotinib, several other ALK-TKIs, such as ceritinib, alectinib, brigatinib (second generation), and lorlatinib (third-generation), have been developed to improve overall survival (OS) (3,4). Nevertheless, it is important to note that the development of acquired resistance to ALK-TKIs remains a nearly inevitable challenge.
Several resistance mechanisms have been identified, including mutations within the ALK kinase domain and activation of ALK-independent bypass signaling pathways (5-8). While ALK-independent resistance mechanisms are still not fully understood, MET activation, a well-known bypass pathway in EGFR-mutant NSCLC, has recently gained attention as a potential resistance mechanism, especially in the context of ALK-positive NSCLC (9). In contrast to MET, the involvement of ErbB family members, such as EGFR, HER2, HER3, and HER4, in mediating resistance in ALK-positive NSCLC, remains relatively unexplored. Recent studies have shown that ALK inhibitor-resistant cell lines exhibit overexpression of phospho-EGFR or phospho-HER3, indicating their potential role in conferring resistance to ALK-TKIs (5,10). However, it remains unclear whether the activation of these alternative receptor tyrosine kinases (RTKs) in ALK-positive NSCLC affects the clinical outcomes of crizotinib, second-, or third-generation ALK-TKIs. In addition, multiple studies have focused on the activation status of RTKs after treatment with ALK inhibitors, whereas little is known about the prognostic significance of pre-existing activation of RTKs before ALK-TKI treatment.
Here, we have comprehensively evaluated the expression of multiple RTKs, including c-MET and ErbB family members, in NSCLC tumors (the primary tumor obtained at initial diagnosis and the secondary tumor after ALK-TKI treatment) and assessed their prognostic significance in patients with NSCLC treated with ALK inhibitors. To explore other therapeutic strategies, we further investigated the treatment efficacy of a combination of ALK and EGFR inhibitors in an in vitro system with ErbB family activation. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-804/rc).
Methods
Study cohort
We retrospectively collected consecutive cases with ALK-positive advanced-stage NSCLC (stage III or IV) between 2011 and 2021 in Asan Medical Center (AMC). All cases were pathologically confirmed as ALK-positive NSCLC by ALK D5F3 immunohistochemistry, ALK fluorescent in situ hybridization (FISH) analysis using a break-apart probe specific for the ALK locus (Vysis LSI ALK dual-color, break-apart rearrangement probe; Abbott Molecular, IL, USA), or clinically targeted next-generation sequencing (NGS) using the MiSeq platform (Illumina) with OncoPanel AMC version 3 (11-14). Medical records from the patients were reviewed and data regarding demographics, Eastern Cooperative Oncology Group (ECOG) performance status, smoking status, radiologic findings, pathologic diagnoses, molecular test results, treatment modality, and clinical follow-up were extracted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-1692). Informed consent was waived due to the retrospective nature of the study.
Tissue specimens and immunohistochemistry
We categorized the tissue specimens as primary tumors obtained at initial diagnosis and secondary tumors obtained after ALK-TKI treatment. All tumor cells in whole hematoxylin and eosin-stained slides were evaluated. Formalin-fixed, paraffin-embedded tissue sections (5 µm thick) were stained using an automatic device (Benchmark XT; Ventana Medical Systems, Tucson, AZ, USA) as per the manufacturer’s protocol (15). Immunohistochemistry (IHC) staining was performed on whole slides using a fully automated IHC assay on a Ventana BenchMark XT Autostainer (Ventana Medical Systems). The specimens were incubated with antibodies against c-MET (1:400; #257261, DAKO, Glostrup, Denmark), EGFR (1:200; 31G7, Zymed, South San Francisco, CA, USA), HER2 (1:200; 4B5, Ventana Medical System), and HER3 (1:50; D22C5, Cell Signaling Technology, Boston, MA, USA).
In the IHC analysis, we scored results based on both staining intensity and the proportion of stained cells. For c-MET and EGFR, we used the following scoring system: 0 indicated no membrane staining; 1+ represented faint and partial membrane staining in tumor cells; 2+ denoted weak and complete membrane staining in more than 10% of tumor cells; and 3+ signified intense and complete membrane staining in more than 10% of tumor cells. In this context, scores of 0, 1+, and 2+ were classified as low expression, while a score of 3+ was considered high expression (16,17).
For HER2 and HER3, the scoring was as follows: 0 for no staining or membrane staining in 10% or fewer tumor cells; 1+ for faint or barely perceptible membrane staining in more than 10% of tumor cells; 2+ for weak to moderate staining of the entire membrane in more than 10% of tumor cells; and 3+ for strong and complete membrane staining in more than 10% of tumor cells. In our study, we grouped scores of 0 or 1+ as low expression and scores of 2+ or 3+ as high expression. This categorization was based on the clinical significance of the HER2 score in various solid tumors, where a score above 2+ is generally considered clinically significant (18-20). Although there has been no established categorization for HER3, we followed the method developed for HER2.
Cell lines and cultures
The human ALK-positive NSCLC cell lines, H3122 (EML4-ALK variant 1; V1) and H2228 (EML4-ALK variant 3a/b; V3), were grown in RPMI 1640 (Invitrogen-GIBCO, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 50 µg penicillin/mL, and 100 µg streptomycin/mL at 37 ℃ in a 5% CO2 incubator. HRG1 was purchased from Prospec (cyt-733; Ness-Ziona, Israel) and stock solutions were prepared in DMSO. Lorlatinib (S7536) and erlotinib (S7786) were purchased from Selleckchem (Houston, TX, USA).
Western blot analyses
Cells were treated with either DMSO (10 nM HRG1 for 2 hours) followed by lorlatinib (3.12 nM), erlotinib (5 µM), or a combination of lorlatinib and erlotinib for 2 hours in the presence of HRG1. The selected doses of the drugs were the IC50 values determined by the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA) in H3122 cells. Whole-cell lysates were prepared in RIPA lysis buffer [50 mM Tris-HCL (pH 8.0), 150 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 0.1% NP-40, and 0.1% SDS] containing a protease inhibitor cocktail (BPI-9200, Tech & InnovationÔ, Bucheon, Korea) and a phosphatase inhibitor cocktail (45065; Santa Cruz, Santa Cruz, CA, USA). Proteins were separated on an 8% or 10% SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membranes using an iBlotÔ dry blotting system (Invitrogen). Immunoblotting analyses were performed with anti-phospho-ALK (Y1604) (3341; Cell Signaling Technology, Beverly, MA, USA), anti-ALK (3791; Cell Signaling Technology), anti-phospho-HER3 (Y1289) (4791; Cell Signaling Technology), anti-HER3 (M7297; DAKO), anti-phospho-HER2 (Y1248) (BS4090; Bioworld Technology, St. Louis Park, MN, USA), anti-HER2 (A0485; DAKO), anti-phospho-MET (Y1234/1235) (3077; Cell Signaling Technology), anti-MET (4560; Cell Signaling Technology), anti-phospho-AKT (S473) (9271; Cell Signaling Technology), anti-AKT (9272; Cell Signaling Technology), anti-phospho-Erk (T202/Y204) (9101; Cell Signaling Technology), anti-Erk (9102; Cell Signaling Technology), anti-phospho-Myc (S62) (ab185656; Abcam, Cambridge, UK), anti-Myc (ab39688; Abcam), and anti-β-actin (A5441; Sigma, St. Louis, MO, USA) antibodies and visualized using the SuperSignal West Pico Chemiluminescent Substrate (34080; Pierce, Rockford, IL, USA).
Statistical analysis
The Kaplan-Meier method was used to determine the 5-year OS and the survival difference was analyzed by the log-rank test. OS was calculated from the date of diagnosis to the date of death from any cause or the date of the last follow-up. Patients still alive at the time of data collection were censored. Expression levels of c-MET, EGFR, HER2, and HER3 in primary and secondary tumor cells, according to the EML4-ALK variants, were evaluated using the χ2 or Fisher’s exact tests. All statistical analyses were carried out using SPSS software package version 21.0.0 (SPSS Statistics software, IBM Corp, Armonk, NY, USA) and R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). P value <0.05 was considered significant.
Results
Baseline characteristics
A total of 104 patients were included in our study based on the inclusion criteria; however, 8 patients, who were lost to follow-up or not treated with ALK inhibitors, were excluded. Thus, 96 patients were finally enrolled and their baseline characteristics are summarized in Table 1. EML4-ALK variants were confirmed in 73 (76.0%) patients, with the majority of them having V1 (37.0%) or V3 (45.2%) variants. V2 (13.7%), V5 (2.7%), and V7 (1.4%) were also detected. Eight (8.3%) patients had prior surgery for the disease and subsequently received ALK inhibitor(s) for disease progression.
Table 1. Baseline characteristics of patients with ALK-positive advanced non-small cell lung cancer.
| Variables | Value |
|---|---|
| Age, years | 54.5 [53–78] |
| Sex | |
| Male | 47 (49.0) |
| Female | 49 (51.0) |
| Smoking history | |
| Current | 14 (14.6) |
| Former | 18 (18.8) |
| Never | 64 (66.7) |
| ECOG performance status | |
| 0 | 12 (16.4) |
| 1 | 59 (80.8) |
| 2 | 2 (2.7) |
| Pathologic diagnosis | |
| Adenocarcinoma | 93 (96.9) |
| Othera | 3 (3.1) |
| Brain metastasis | |
| Present | 59 (62.8) |
| Absent | 35 (37.2) |
| EML4-ALK fusion variant | |
| V1 | 27 (37.0) |
| V2 | 10 (13.7) |
| V3a/b | 33 (45.2) |
| V5 | 2 (2.7) |
| V7 | 1 (1.4) |
| Prior lines of therapy before ALK inhibitor | |
| 0 | 54 (56.3) |
| 1 | 34 (35.4) |
| 2 | 6 (6.3) |
| ≥3 | 2 (2.1) |
| Prior platinum-based therapy | |
| Present | 40 (41.7) |
| Absent | 56 (58.3) |
| Total | 96 (100.0)b |
Data are presented as median (range) or n [%]. a, includes large cell neuroendocrine carcinoma, squamous cell carcinoma, and non-small cell carcinoma, not-otherwise specified; b, some variables did not reach 96 due to the non-availability of data. ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group.
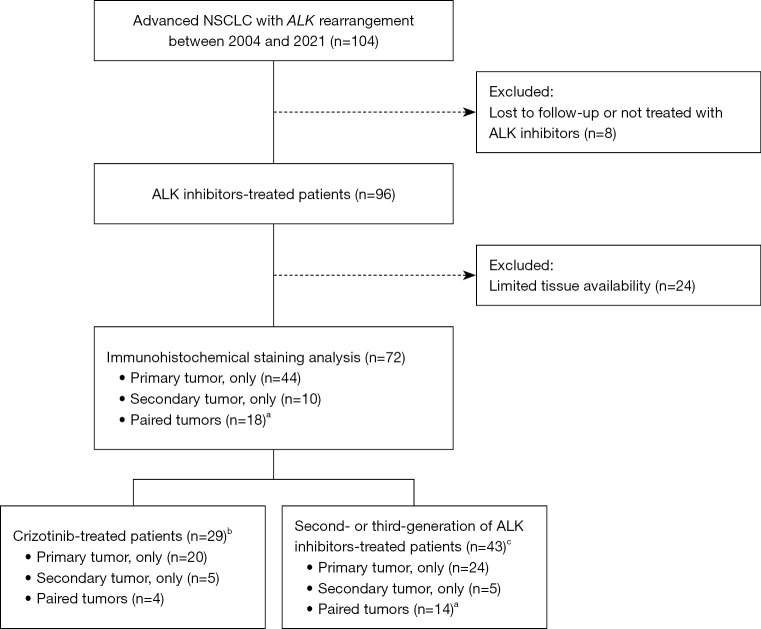
For the IHC analysis, 24 out of 96 cases (patients) were excluded due to limited tissue availability. Therefore, we performed the IHC analysis on the remaining 72 cases, and the number of primary and secondary tissue specimens analyzed is presented in Figure 1.
Figure 1.
Flow diagram of the cohort selection. a, one patient was additionally excluded due to limited tissue availability for the c-MET and EGFR analysis; b, patients who received crizotinib without further generations of ALK inhibitors during the follow-up; c, patients who received second- or third-generation ALK inhibitors as any line of therapy during the follow-up. NSCLC, non-small cell cancer; ALK, anaplastic lymphoma kinase.
At the last follow-up, among the 72 patients, 29 (40.3%) patients were receiving crizotinib, while second-generation ALK inhibitors (ceritinib, alectinib, or brigatinib) were being administered to 37 (51.4%) patients, while 6 (8.3%) patients received the third-generation inhibitor lorlatinib. Among the 43 patients not on crizotinib, 22 were receiving second-line ALK-TKIs after crizotinib, while the remaining patients were receiving them as first-line ALK-TKIs.
Overexpression of c-MET, EGFR, HER2, and HER3 before/after ALK inhibitor treatment
Of the tissue specimens collected from the 72 patients, 62 were primary tumors (44 from primary tumors only and 18 from paired tumors), and 28 were secondary tumors (10 from secondary tumors only and 18 from paired tumors) (Figure 1). Among the 62 primary specimens, 44 were small biopsies and 18 were resections. Among the 28 secondary tumor specimens, 24 were small biopsies and 4 were resections. One case each from the primary and secondary specimens was excluded from the c-MET and EGFR IHC analysis due to limited tissue availability. Consequently, 61 primary and 27 secondary tissues were assessed for c-MET and EGFR expression, while 62 primary and 28 secondary tissues were assessed for HER2 and HER3.
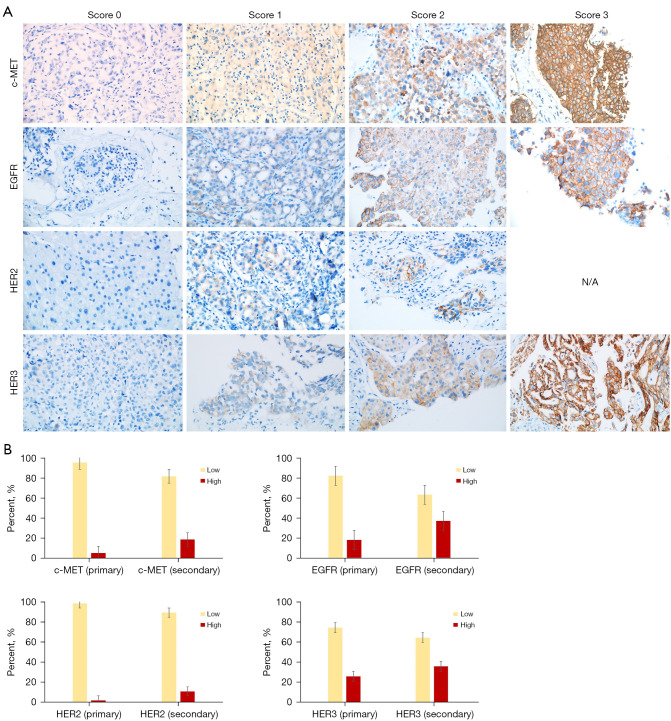
The IHC staining results, as depicted in Figure 2A, revealed that in primary tumors, 4.9% (3 of 61) exhibited high expression of c-MET, and 18.0% (11 of 61) showed high expression of EGFR. For HER2 and HER3, the proportions were 1.6% (1 of 62) and 25.8% (16 of 62), respectively. In secondary tumors, the rates of high expression were 18.5% for c-MET, 37.0% for EGFR, 10.7% for HER2, and 35.7% for HER3 (Figure 2B). It’s important to note that these percentages are not derived from paired case comparisons but instead from the total number of primary and secondary specimens. In cases of paired comparisons, among primary specimens with low expression, the rates of high expression in secondary specimens were 11.8% for c-MET, 40% for EGFR, 17.6% for HER2, and 21.4% for HER3. Due to the limited number of cases with high expression, paired comparisons did not reach statistical significance.
Figure 2.
Expression of c-MET, EGFR, HER2, and HER3 in ALK-positive non-small cell lung cancers. Representative immunohistochemistry images (magnification ×400) with scoring (A). For c-MET and EGFR, scores 0, 1, and 2 were low expression, while a score of 3 indicated high expression. For HER2 and HER3, scores 0 and 1 represented low expression, while scores 2 and 3 were high expression. Proportion of low and high expression in primary (n=61) and secondary (n=62) tumor specimens (B). High expression rates in primary tumors were 4.9% for c-MET, 18.0% for EGFR, 1.6% for HER2, and 25.8% for HER3. In secondary tumors, high expression rates were 18.5% for c-MET, 37.0% for EGFR, 10.7% for HER2, and 35.7% for HER3. ALK, anaplastic lymphoma kinase; N/A, not applicable.
Overall, secondary tumors showed higher RTK expression than ALK-naïve primary tumors. In particular, the proportion of tumors with c-MET overexpression increased approximately fourfold (4.9% to 18.5%) after ALK inhibitor treatment. We also found a specific subgroup of patients who had never been exposed to ALK inhibitors with high EGFR or HER3 expression in their primary tumors. These data suggest an initial heterogeneity of ALK-positive NSCLCs in terms of ErbB family expression.
Survival outcome with primary and secondary expression of c-MET, EGFR, HER2, and HER3
The median duration of follow-up was 30 months (range, 0.8–228 months) and the median 5-year OS was 52 months (95% confidence interval: 34.4–69.6). The median time interval between the initial diagnosis and the first application of ALK inhibitors was 2 months (range, 0–154 months). A secondary tumor specimen was obtained after the patient had received at least one prior course of ALK-TKI therapy, with a median time interval between the initiation of ALK-TKI treatment and the date of the second biopsy being 17 months (range, 3–71 months). Crizotinib has not been commonly used as first-line therapy since the introduction of second- and third-generation ALK inhibitors into clinical practice, so we differentiated the groups for the survival analysis based on the generation of ALK-TKIs. Patients were categorized into two groups: (I) those treated with second- or third-generation ALK-TKIs (n=43), and (II) those who had received only the first-generation ALK-TKI, crizotinib (n=29).
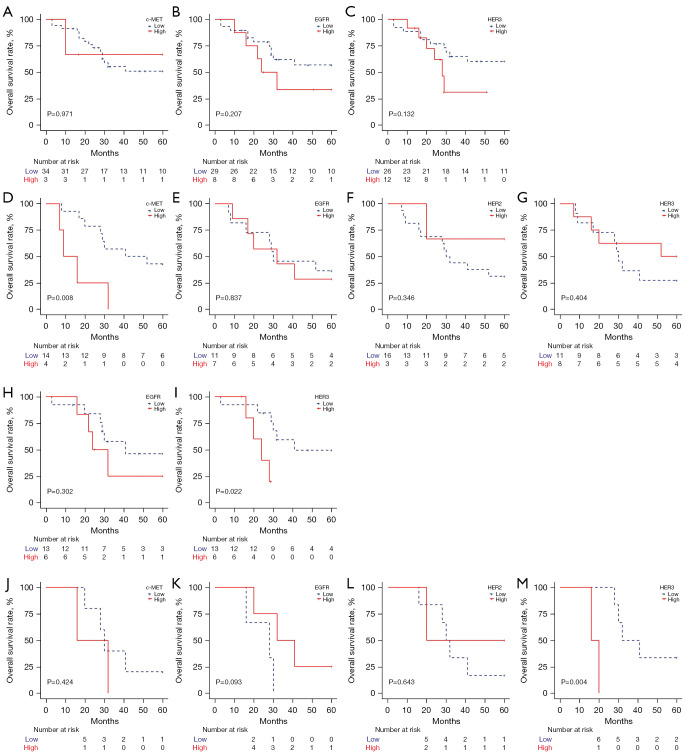
In the group treated with second- or third-generation ALK-TKIs, the primary c-MET analysis did not reveal a significant difference in survival by expression level (P=0.971; Figure 3A). However, the patients with high EGFR or HER3 expression in primary tumor cells showed a trend toward poorer outcomes, although these findings did not reach statistical significance (P=0.207 and 0.132, respectively; Figure 3B,3C). Survival analysis for patients with primary HER2 expression was not feasible due to the limited availability of cases; only one instance showed high expression (score 2+).
Figure 3.
Kaplan-Meier survival plots of 5-year overall survival in ALK-positive non-small cell lung cancer patients treated with second- or third-generation ALK inhibitors. The log-rank tests compared high and low expression levels of c-MET (A), EGFR (B), and HER3 (C) in primary tumors and those of c-MET (D), EGFR (E), HER2 (F), and HER3 (G) in secondary tumors. In the variant 1/2 subgroup, log-rank tests compared high and low expression levels of EGFR (H) and HER3 (I) in primary tumors and those of c-MET (J), EGFR (K), HER2 (L), and HER3 (M) in secondary tumors. ALK, anaplastic lymphoma kinase.
In secondary tumors, high c-MET expression was associated with inferior survival (P=0.008; Figure 3D). No significant difference in OS was observed between the high and low expression groups of EGFR, HER2, and HER3 in secondary tumors (Figure 3E-3G).
In the crizotinib-treated patients (n=29), the number of events in the high expression group was limited: c-MET (n=0), EGFR (n=1), HER2 (n=0), and HER3 (n=1) in the primary tumors and c-MET (n=1), EGFR (n=2), HER2 (n=0), and HER3 (n=1) in the secondary tumors. The survival difference was not significant.
We further examined the association of the expression of these RTKs with patient outcomes in terms of EML4-ALK variant status in the second- or third-generation ALK-TKI treated group. In the subgroup of V1 and V2, we observed a survival difference between high and low EGFR expression in primary tumors, but the difference did not reach statistical significance (Figure 3H). Notably, patients with high HER3 expression in primary tumors had a significantly worse OS compared to those with low expression (P=0.022; Figure 3I). Survival analysis of primary c-MET and HER2 expression in the V1/V2 group was not performed due to the limited number of cases with high expression. In secondary tumors, high expression of c-MET, EGFR, and HER2 did not show a significant association with survival (Figure 3J-3L). Interestingly, the V1/V2 group with high HER3 expression exhibited a significantly poor outcome (P=0.004; Figure 3M). In the EML4-ALK V3 subgroup, survival analysis was only conducted in the group with primary HER3 expression due to the limited number of cases and it did not reveal a significant difference in survival (P=0.811, data not shown). The distribution of RTK expression among the variants, including 1st to 3rd-generation ALK inhibitors, is summarized in Table 2. The proportions of low and high expression of RTK were not significantly different between V1/V2 and V3 in both primary and secondary tissues.
Table 2. EML4-ALK variants and immunohistochemical staining results of c-MET, EGFR, HER2, and HER3.
| Immunohistochemistry | Expression | EML4-ALK variant, n (%) | P | |
|---|---|---|---|---|
| V1/V2 | V3a/b | |||
| Primary tissue | ||||
| c-MET | High | 0 | 2 (100.0) | 0.079 |
| Low | 28 (62.2) | 17 (37.8) | ||
| EGFR | High | 8 (80.0) | 2 (20.0) | 0.138 |
| Low | 20 (54.1) | 17 (45.9) | ||
| HER2 | High | 1 (100.0) | 0 | 0.405 |
| Low | 27 (58.7) | 19 (41.3) | ||
| HER3 | High | 8 (53.3) | 7 (46.7) | 0.551 |
| Low | 20 (62.5) | 12 (37.5) | ||
| Total | 28 (100.0) | 19 (100.0) | ||
| Secondary tissue | ||||
| c-MET | High | 3 (75.0) | 1 (25.0) | 0.190 |
| Low | 7 (38.9) | 11(61.1) | ||
| EGFR | High | 4 (40.0) | 6 (60.0) | 0.639 |
| Low | 6 (50.0) | 6 (50.0) | ||
| HER2 | High | 2 (100.0) | 0 (0) | 0.122 |
| Low | 9 (42.9) | 12 (57.1) | ||
| HER3 | High | 3 (37.5) | 5 (62.5) | 0.469 |
| Low | 8 (53.3) | 7 (46.7) | ||
| Total | 10 (100.0)a | 12 (100.0) | ||
| 11 (100.0)b | ||||
a, c-MET and EGFR; b, HER2 and HER3. ALK, anaplastic lymphoma kinase.
HRG1-induced expression of RTKs in EML4-ALK variant-expressing cell lines
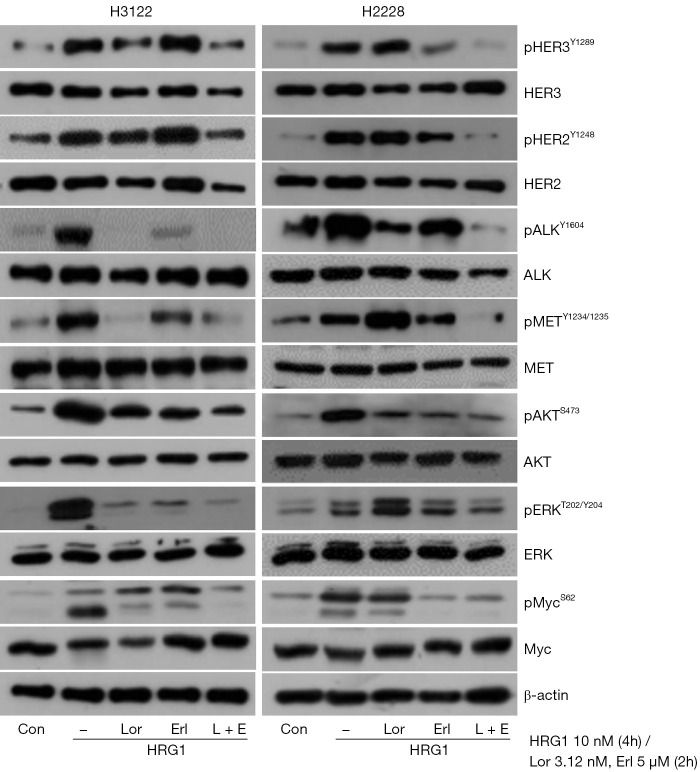
To evaluate whether HER3 expression plays a role in ALK-positive NSCLC cell lines, we investigated multiple RTK activations in H3122 (EML4-ALK V1) and H2228 (EML4-ALK V3) after treatment with a HER3 ligand, HRG1. HER3 activation significantly increased HER2 and c-MET activity together with their major downstream signaling pathways, such as MAPK/ERK and AKT. Unexpectedly, HER3 activation strongly induced ALK activation in both cell lines (Figure 4). Lorlatinib decreased the activity of AKT and its downstream effector, Myc, in those cells.
Figure 4.
The activation of multiple signaling pathways, including HER3, induced by HRG1 treatment, and the effects of ALK- or EGFR-tyrosine kinase inhibitors in H3122 (EML4-ALK variant 1) and H2228 (EML4-ALK variant 3a/b) cell lines. Cells were pre-treated with HRG1 (10 nM) for 4 hours followed by lorlatinib (Lor), Erlotinib (Erl), or a combination of both for 2 hours. ALK, anaplastic lymphoma kinase.
Considering that EML4-ALK V3 is significantly associated with a higher incidence of ALK resistance mutations, the failure of lorlatinib to suppress ERK, c-MET, HER2, and HER3 activity in H2228 cells is noticeable (21). Erlotinib, an EGFR-TKI, caused only a slight decrease in RTK activity and did not efficiently suppress ALK activity. However, the combination of lorlatinib and erlotinib significantly reduced the activities of these RTKs and Myc activity relative to lorlatinib or erlotinib alone.
Discussion
In this study, we conducted a comprehensive assessment of RTK expression, specifically c-MET and members of the ErbB family, in NSCLC tumors. Our analysis covered both primary tumors obtained at the initial diagnosis and secondary tumors following ALK-TKI therapy. Our findings revealed that within a subgroup of ALK-positive NSCLC patients, the presence of EGFR or HER3 overexpression in primary tumors was associated with a tendency toward poorer outcomes upon treatment with ALK inhibitors. We also observed that c-MET overexpression in secondary tumors was linked to a lower OS rate, consistent with previous reports (8,9). Moreover, HER3 overexpression emerged as a significant prognostic marker within a subgroup featuring EML4-ALK V1/V2 variants in both primary and secondary tumors. Cells carrying V1 or V3 exhibited activation of multiple RTKs, including ALK, upon exposure to the HER3 ligand (HRG1), and these activities were nearly completely suppressed when treated with a combination of ALK- and EGFR-inhibitors. These results indicate the potential of HER3 overexpression as a valuable prognostic marker in ALK-positive NSCLC. Early identification of patients exhibiting HER3 overexpression may be important in predicting the clinical benefits derived from ALK inhibitor therapy.
HER3, a member of the ErbB family, exhibits low kinase activity and functions by heterodimerizing with other ErbB family members, such as EGFR, HER2 and non-ErbB family receptors. This heterodimerization activates downstream pathways, including PI3K/AKT, MEK/ERK, and JAK/STAT, leading to oncogenesis (22-24). In our Western blot analysis, HRG1 significantly induced the expression of phospho-HER3, phospho-AKT, and phospho-ERK, confirming its role as a ligand for HER3 and its downstream activation. Regarding ALK-TKI resistance, several in vitro studies have reported increased HRG1 or HER3 expression in crizotinib- or alectinib-resistant NSCLC cell lines (5,8,25-28). Notably, our study found HER3 overexpression in 25.8% of primary tumors, correlating with an inferior survival tendency (P=0.132) in the second- or third-generation ALK-TKIs treated group. However, this phenomenon was not observed in the secondary HER3 overexpressing group. We attribute this difference in the ability of HER3 to signaling rebound kinetics in the initial response to ALK-TKIs.
Several studies have reported that the EML4-ALK variant influences the benefit of ALK inhibitor treatment (29). Specifically, patients with EML4-ALK V3 have been found to be more prone to developing resistance mutations (particularly ALK G1202R) and have lower median progression-free survival compared to V1 when treated with second-generation ALK inhibitors (30-32). In our cohort, we found that patients with EML4-ALK V1/V2 who had HER3 overexpression in primary or secondary tumors showed significantly shorter OS compared to those without HER3 overexpression (P=0.022 and P=0.004, respectively). However, in the EML4-ALK V3 group, HER3 overexpression did not correlate with a poor outcome in primary tumors (secondary tumors were not analyzed). In the context of responsiveness to ALK inhibitors, the V3 group, known for its high kinase activity, structural stability and reduced sensitivity, may experience a lesser impact on prognosis from HER3 overexpression. Conversely, in the V1/V2 group, which is generally more sensitive to ALK inhibitors, HER3 overexpression could significantly contribute to oncogenic signaling, influencing rebound kinetics in response to ALK-TKI therapy and affecting treatment outcomes (33).
Our in vitro data revealed that HRG1 induced PI3K/AKT signaling activation and phosphorylation of ALK in ALK-positive NSCLC cell lines and a notable reduction of RTK expression when lorlatinib was combined with erlotinib, indicating that the combined use of ALK- and EGFR-inhibitors could be an effective strategy to overcome HER3 overexpression in ALK-positive cancers. While several studies demonstrated the effectiveness of combining crizotinib or lorlatinib with an EGFR inhibitor in inhibiting the growth of ALK inhibitor-resistant clones, the specific involvement of HER3 overexpression in this context has yet to be studied (10,34). Recent studies have reported therapeutic inhibition of the HRG1-HER3 interaction using HER3-directed antibodies, particularly bispecific antibodies targeting HER2/HER3 or HER3-directed antibody-drug conjugates in NRG1 (HRG1) fusion-positive solid tumors (35,36). While there is a need to explore the physiological roles of HRG1 expression in ALK-TKI resistant lung cancers further, the combination of a HER3-targeting antibody with ALK-TKI may be an efficient treatment strategy.
Despite the significant effectiveness of second- and third-generation ALK-TKIs, challenges persist due to on-target acquired resistance mutations in the ALK kinase domain and off-target resistance through parallel bypass pathways and reactivation of downstream signaling. In our cohort, seven cases exhibited secondary ALK resistance mutations (two G1269A, two L1196M, and three G1202R). However, in this limited number of cases, we did not identify any prognostic relevance of these mutations when compared to the non-ALK mutated group. We highlighted HER3 overexpression as a potential resistance mechanism, particularly observed in EML4-ALK V1/V2 variants. However, our study had limitations, such as a restricted number of cases and an uneven distribution of mutation types among patient groups. These factors impacted our ability to perform detailed subgroup analyses, including survival assessments. As an unavoidable feature of this retrospective analysis, another limitation arises from the inclusion of patients who received ALK-TKIs at various time points throughout their disease course. This implies that the OS data could be confounded by the frequency and duration of post-progression treatments, including treatment crossover in the crizotinib arm, potentially limiting the OS results. Our in vitro experiments primarily focused on evaluating the phosphorylation of RTK pathway proteins, lacking complementary evidence on cellular functions such as proliferation or survival. It is essential to consider that the activation of HER3/HER4 through heregulin-based mechanisms might not fully represent the significance of HER3 expression on the cell surface.
Conclusions
Our study investigated the expression of the ErbB family and c-MET in primary and secondary tumors of patients with ALK-positive NSCLC. We observed that HER3 overexpression commonly occurred in ALK-TKI naïve tumors and could serve as a potential prognostic marker of a poor outcome in primary tumors. Furthermore, HER3 overexpression was identified as a predictor of adverse outcomes in a specific subgroup of patients with EML4-ALK V1/V2. These findings indicate that evaluating HER3 expression status can be crucial for achieving improved clinical outcomes in ALK-positive NSCLC treated with ALK inhibitors.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant (No. 2020R1A2C2006815) funded by the Korean government (MSIT) and UK Research and Innovation Medical Research Council (No. MR/X008673/1 to R.B. and J.S.).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-1692). Informed consent was waived due to the retrospective nature of the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-804/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-804/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-804/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-804/coif). The authors have no conflicts of interest to declare.
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 2.Ducray SP, Natarajan K, Garland GD, et al. The Transcriptional Roles of ALK Fusion Proteins in Tumorigenesis. Cancers (Basel) 2019;11:1074. 10.3390/cancers11081074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabir SR, Yeoh S, Jackson G, et al. EML4-ALK Variants: Biological and Molecular Properties, and the Implications for Patients. Cancers (Basel) 2017;9:118. 10.3390/cancers9090118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. 10.1158/1078-0432.CCR-08-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong X, Fernandez-Salas E, Li E, et al. Elucidation of Resistance Mechanisms to Second-Generation ALK Inhibitors Alectinib and Ceritinib in Non-Small Cell Lung Cancer Cells. Neoplasia 2016;18:162-71. 10.1016/j.neo.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol 2022;19:499-514. 10.1038/s41571-022-00639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolle E, Taucher V, Lindenmann J, et al. Current Knowledge about Mechanisms of Drug Resistance against ALK Inhibitors in Non-Small Cell Lung Cancer. Cancers (Basel) 2021;13:699. 10.3390/cancers13040699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isozaki H, Ichihara E, Takigawa N, et al. Non-Small Cell Lung Cancer Cells Acquire Resistance to the ALK Inhibitor Alectinib by Activating Alternative Receptor Tyrosine Kinases. Cancer Res 2016;76:1506-16. 10.1158/0008-5472.CAN-15-1010 [DOI] [PubMed] [Google Scholar]
- 9.Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res 2020;26:2535-45. 10.1158/1078-0432.CCR-19-3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama Y, Yamada T, Tanimura K, et al. Adaptive resistance to lorlatinib via EGFR signaling in ALK-rearranged lung cancer. NPJ Precis Oncol 2023;7:12. 10.1038/s41698-023-00350-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol 2013;24:2589-93. 10.1093/annonc/mdt295 [DOI] [PubMed] [Google Scholar]
- 12.Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466-72. 10.1097/JTO.0b013e31820b82e8 [DOI] [PubMed] [Google Scholar]
- 13.Hwang HS, Kim D, Choi J. Distinct mutational profile and immune microenvironment in microsatellite-unstable and POLE-mutated tumors. J Immunother Cancer 2021;9:e002797. 10.1136/jitc-2021-002797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Yoon S, Lee DH, et al. Real-world utility of next-generation sequencing for targeted gene analysis and its application to treatment in lung adenocarcinoma. Cancer Med 2021;10:3197-204. 10.1002/cam4.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh YW, Hwang HS, Jung SJ, et al. Receptor tyrosine kinases MET and RON as prognostic factors in diffuse large B-cell lymphoma patients receiving R-CHOP. Cancer Sci 2013;104:1245-51. 10.1111/cas.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn 2008;10:242-8. 10.2353/jmoldx.2008.070178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuta K, Kozu Y, Mimae T, et al. c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol 2012;7:331-9. 10.1097/JTO.0b013e318241655f [DOI] [PubMed] [Google Scholar]
- 18.Cox G, Vyberg M, Melgaard B, et al. Herceptest: HER2 expression and gene amplification in non-small cell lung cancer. Int J Cancer 2001;92:480-3. 10.1002/ijc.1214 [DOI] [PubMed] [Google Scholar]
- 19.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012;25:637-50. 10.1038/modpathol.2011.198 [DOI] [PubMed] [Google Scholar]
- 20.Koeppen HK, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology 2001;38:96-104. 10.1046/j.1365-2559.2001.01084.x [DOI] [PubMed] [Google Scholar]
- 21.Christopoulos P, Kirchner M, Endris V, et al. EML4-ALK V3, treatment resistance, and survival: refining the diagnosis of ALK(+) NSCLC. J Thorac Dis 2018;10:S1989-91. 10.21037/jtd.2018.05.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 2010;107:7692-7. 10.1073/pnas.1002753107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jura N, Shan Y, Cao X, et al. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci U S A 2009;106:21608-13. 10.1073/pnas.0912101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra R, Patel H, Alanazi S, et al. HER3 signaling and targeted therapy in cancer. Oncol Rev 2018;12:355. 10.4081/oncol.2018.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanizaki J, Okamoto I, Okabe T, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res 2012;18:6219-26. 10.1158/1078-0432.CCR-12-0392 [DOI] [PubMed] [Google Scholar]
- 26.Tanimura K, Yamada T, Okada K, et al. HER3 activation contributes toward the emergence of ALK inhibitor-tolerant cells in ALK-rearranged lung cancer with mesenchymal features. NPJ Precis Oncol 2022;6:5. 10.1038/s41698-021-00250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015;27:397-408. 10.1016/j.ccell.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegde GV, de la Cruz CC, Chiu C, et al. Blocking NRG1 and other ligand-mediated Her4 signaling enhances the magnitude and duration of the chemotherapeutic response of non-small cell lung cancer. Sci Transl Med 2013;5:171ra18. 10.1126/scitranslmed.3004438 [DOI] [PubMed] [Google Scholar]
- 29.Elshatlawy M, Sampson J, Clarke K, et al. EML4-ALK biology and drug resistance in non-small cell lung cancer: a new phase of discoveries. Mol Oncol 2023;17:950-63. 10.1002/1878-0261.13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol 2017;28:791-7. 10.1093/annonc/mdw693 [DOI] [PubMed] [Google Scholar]
- 31.Tao H, Shi L, Zhou A, et al. Distribution of EML4-ALK fusion variants and clinical outcomes in patients with resected non-small cell lung cancer. Lung Cancer 2020;149:154-61. 10.1016/j.lungcan.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 32.Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol 2018;36:1199-206. 10.1200/JCO.2017.76.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bearz A, Martini JF, Jassem J, et al. Efficacy of Lorlatinib in Treatment-Naive Patients With ALK-Positive Advanced NSCLC in Relation to EML4::ALK Variant Type and ALK With or Without TP53 Mutations. J Thorac Oncol 2023;18:1581-93. 10.1016/j.jtho.2023.07.023 [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi N, Lucena-Araujo AR, Nakayama S, et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer 2014;83:37-43. 10.1016/j.lungcan.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uliano J, Corvaja C, Curigliano G, et al. Targeting HER3 for cancer treatment: a new horizon for an old target. ESMO Open 2023;8:100790. 10.1016/j.esmoop.2023.100790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schram AM, Odintsov I, Espinosa-Cotton M, et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov 2022;12:1233-47. 10.1158/2159-8290.CD-21-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as