1. INTRODUCTION
Ocular pain has often been included as a component of dry eye (DE), a disease that affects 5-50% of the global population.1 As defined by the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.”2 Ocular pain is often characterized using descriptors such as “dryness,” “burning,” “aching,” and “throbbing,”3 and may have multiple contributors, including tear film instability, ocular surface inflammation, hyperosmolarity, neurosensory abnormalities, or a combination of these etiologies.4 These painful sensations can occur spontaneously or be triggered by wind, temperature, and light.5 Photophobia, or evoked pain to light, is a debilitating symptom which can severely impact an individual’s ability to carry out activities of daily living, even when wearing dark glasses.6 This may result in social impairment, unemployment, or school dropout.7 Given these significant stressors, an understanding of mechanisms that underlie photophobia in individuals with chronic ocular pain is needed to develop new treatments and improve quality of life.
Tinted lenses are one approach used to reduce symptom severity in individuals with photophobia, particularly lenses that block 480nm wavelengths (e.g., FL-41 tinted lenses which are rose colored in tint). In one study of 37 individuals with chronic migraine, optical notch filter lenses that maximally blocked 480nm wavelengths were found to improve Headache Impact Test (HIT-6) and photophobia symptom scores over a two week period.8 FL-41 lenses has also been used in individuals with photophobia associated with benign essential blepharospasm (BEB). Over a 2 week period, individuals with BEB reported improvements not only in photophobia (27%, n=8), but also in reading (31%, n=9) and blepharospasm frequency (27%, n=8) and severity (27%, n=8).9 These findings were replicated in a laboratory setting when 24 individuals with BEB were gradually exposed to increasing light intensity.10 After comparing seven different lens tints, 71% preferred FL-41 tinted lenses. Interestingly, a lens absorbing two ranges of wavelengths (<400nm and 500-600nm) allowed participants to tolerate higher light intensity compared to FL-41 (2406 vs. 1232 lux) but was not subjectively preferred. These data suggest that FL-41 tinted lenses are beneficial in individuals with photophobia across various conditions.
In addition to subjective measures of symptom severity, imaging tools such as functional magnetic resonance imaging (fMRI) have been used to objectively examine responses to light stimuli by using blood oxygen level dependent (BOLD) responses as an indirect measure of neural activity. We have studied neural mechanisms of photophobia in individuals with chronic ocular surface pain, and identified significantly greater light-evoked activation in pain-related areas within the trigeminal brainstem, primary somatosensory cortex (S1), anterior mid-cingulate cortex (aMCC), and insula in 8 cases compared to 8 controls.11 Others have studied light responses in individuals with migraine. One study found that while 17 cases and 19 controls had brainstem activity within the superior colliculi and spinal trigeminal nucleus when exposed to a rotating checkerboard visual, chronic migraineurs had significantly greater activity when compared to healthy controls.12 Other neuroimaging studies have demonstrated that the visual cortex and other areas of the cerebral cortex are more robustly activated by light in individuals with migraine compared to controls.13,14
Along with trigeminal pathway activation, fMRI studies have highlighted that other pathways, such as melanopsin associated circuitry may also contribute to light sensitivity. In a case study of a 39-year-old female with long-standing idiopathic photophobia, the presentation of a blue-white alternating checkerboard resulted in unpleasant sensations and activation in the bilateral pulvinar nuclei along with several other brain areas.15 When presented with an equally bright red-white alternating checkerboard, photophobia was not evoked. Collectively, this suggest that melanopsin pathways may have contributed to photophobia in this patient because 1) melanopsin containing intrinsically photosensitive retinal ganglion cells (ipRGC) respond to blue (and not red) light, 2) light activated the pulvinar nuclei, which receives input from ipRGCs16,17, and 3) and the patient reported that FL-41 tinted lenses managed her symptoms.
Missing from the literature is an investigation of the effects of FL-41 tinted lenses on subjective and objective metrics of photophobic responses in individuals with chronic ocular pain. As such, in this study we focus on the impact of FL-41 tinted lenses on light-evoked unpleasant sensations and neural circuity in individuals with chronic ocular pain, DE symptoms, and photophobia. This research is important as identifying brain regions associated with photophobia may facilitate the development of diagnostic tests and targeted treatments for individuals with this debilitating symptom.
2. METHODS
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Miami Veterans Affairs (VA) and the University of Miami Institutional Review Boards (IRB approval #3011.08 and #20190340). The study was conducted in accordance with the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants prior to any study activities.
2.2. Study Population
We recruited 25 subjects (8 females, 17 males, average age: 55.3±12.2 years old) who presented to the Miami VA eye clinic with chronic ocular pain (symptoms present ≥ 3 months, average pain rating over 1-week recall ≥ 1 on a 0-10 numerical rating scale (NRS)), DE symptoms (Dry Eye Questionnaire–5 (DEQ-5) score > 6)18, and photophobia (Ocular Surface Disease Index (OSDI), question #1 ≥ 1 and/or Neuropathic Pain Symptom Inventory modified for the Eye (NPSI-Eye), question #9 ≥ 1).19,20 Data on demographics and comorbidities were collected. Subjects were excluded from participation if they had ocular diseases that could confound photophobia (e.g., glaucoma, use of glaucoma medication, uveitis, iris transillumination defects, retinal degeneration, anatomic abnormalities of the cornea, conjunctiva, or eyelids, etc.) as the pathophysiology of ocular pain and photophobia in these individuals is likely different than in the group we aimed to study. We also excluded individuals with contraindications to fMRI scanning (e.g., pregnancy, pacemaker, implanted metal device).
2.3. Questionnaires
Subjects were administered questionnaires to collect demographic and supporting health information. Individuals filled out standardized questionnaires regarding ocular symptoms, including the DEQ-5 (range 0-22)18, OSDI (range 0-100)19, NRS for average ocular pain intensity during the past week (range 0-10), and NPSI-Eye (range 0-100).20 Individuals also completed standardized questionnaires regarding depression symptoms (Patient Health Questionnaire-9, PHQ-9, range 0-27).21 24 individuals completed all questionnaires (one individual did not fill out the entire NPSI-Eye questionnaire form).
2.4. Ocular Surface Evaluation
Subjects underwent an ocular surface evaluation, including tear breakup time (TBUT) (measured in seconds, with lower values indicating tear instability), fluorescein corneal staining (graded to the National Eye Institute (NEI) scale22 with higher values indicating a more irregular epithelium), and tear production using anesthetized Schirmer strips (measured in millimeters of wetting at 5 minutes, with lower values indicating less tear production). 24 individuals completed the ocular surface evaluation (one subject underwent fMRI scanning but not the ocular surface exam).
2.5. FL-41 Tinted Glasses Power Spectra and Transmittance
The FL-41 tinted glasses worn by each subject were manufactured by Axon Optics (Bountiful, Utah, USA) and were marketed for outdoor use. Power and transmittance spectra data were measured by the Ophthalmic Biophysics Center at Bascom Palmer Eye Institute (Figure 1).
Figure 1. Power and Transmittance Spectrum for FL-41 Lenses.
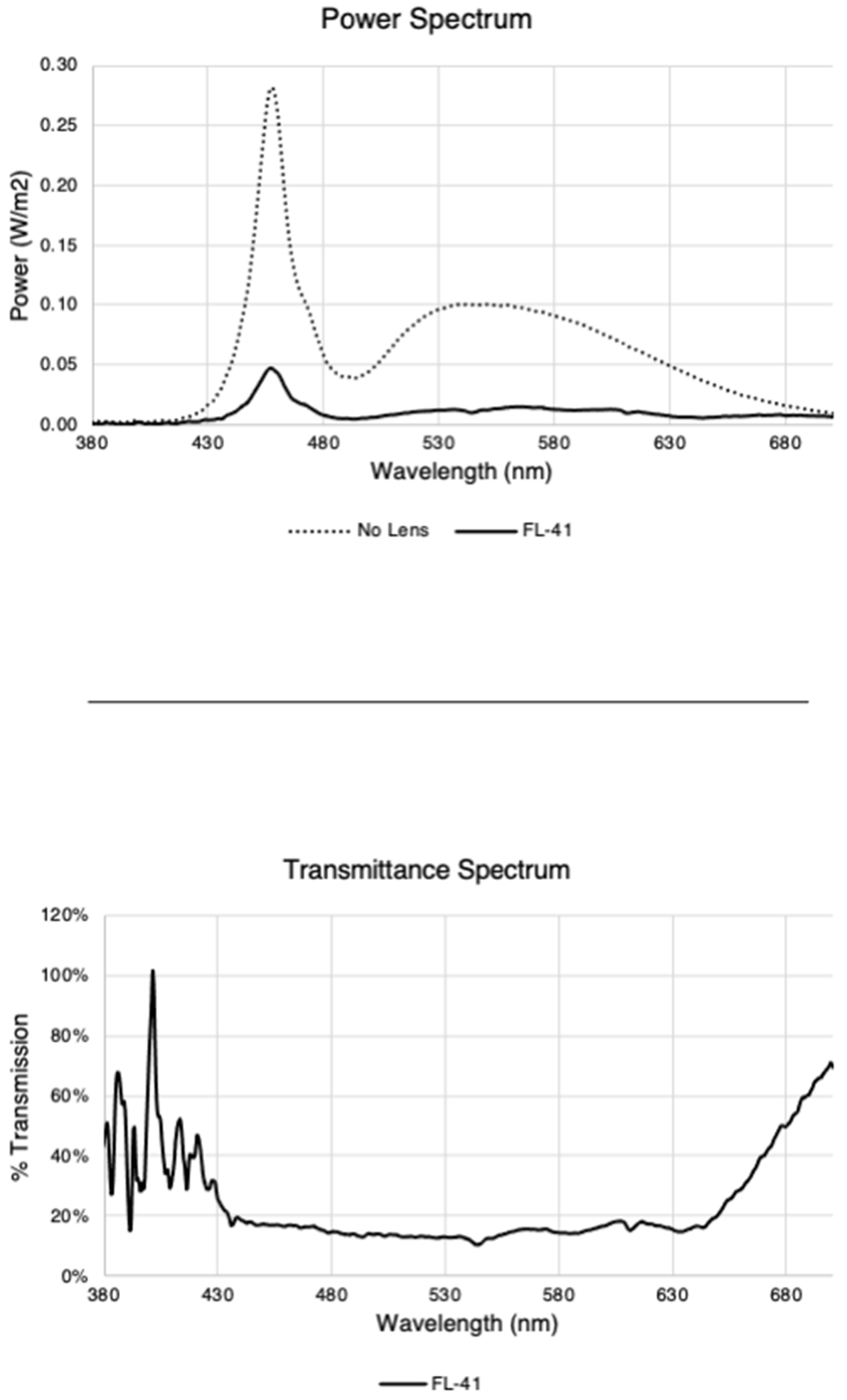
FL-41 tinted lenses attenuated a wide spectrum of wavelengths (approximately 430 – 630nm). Power and transmittance spectrum acquisition are described in Supplemental Text 1.
2.6. fMRI Protocol
The fMRI protocol was adopted and modified from prior studies on photophobia using visual stimuli to evoke pain.23 fMRI acquisition and pre-processing steps for whole brain analysis are included in Supplemental Text 2. In a single session, all individuals underwent two fMRI scans using the same protocol, first without FL-41 tinted glasses then a subsequent one with FL-41 tinted glasses. Prior to fMRI scanning, patients were instilled with an artificial tear eyedrop (Refresh Plus Lubricant Eye Drops, Allergan, Dublin, Ireland) to each eye. They were then presented with intermittent bright light in a darkened environment during each fMRI scan. The presentation consisted of two visual conditions: a black screen rest condition, which featured a white fixation cross on a black background (~0.5 lux); and a white screen stimulus condition, which featured a black fixation cross on a white background (~65 lux). Subjects were presented with 16 episodes of sustained bright light (white screen), each lasting 6 seconds. To avoid anticipatory processes, the inter-stimulus interval (ISI) varied between 26 and 34 seconds in 2-second increments. The scanner environment was kept dark during the entire experiment, with only a projector providing intermittent brief illumination. Subjects were instructed to keep their eyes open and blink normally throughout the duration of each scan.
2.7. fMRI Screen Condition Ratings
At the end of each scan, subjects were asked to rate the level of unpleasantness in their eyes evoked by each screen condition (i.e., black or white screen). Unpleasantness was explained to the participants as “something you do not like, that does not feel good, or that is uncomfortable in some way.” Subjects rated unpleasantness via a verbal NRS ranging from 0 (“not unpleasant at all”) to 100 (“the most unpleasant sensation imaginable”).
2.6. Statistical Analysis
Statistical analyses were performed using SPSS V.28.0 (SPSS, Chicago, Illinois, USA) statistical package. Paired t-tests were performed to detect mean differences in 1) black vs. white screen unpleasantness ratings in the no lens condition, 2) white screen unpleasantness ratings in the no lens vs. FL-41 glasses worn conditions, and 3) black screen unpleasantness ratings in the no lens vs. FL-41 worn conditions.
Next, we split the population into two groups based on their subjective response to FL-41 lenses. We defined responders as those who reported less unpleasantness to the white screen while wearing vs. not wearing the FL-41 lens while non-responders were defined as individuals whose unpleasantness rating increased or were not affected by FL-41 lenses. We then compared demographics, clinical factors, and tear parameters between the two groups, using independent t-tests or Chi-squared tests, as appropriate. fMRI findings were additionally compared between the two groups.
The statistical significance for whole brain group-level contrast analyses was set to a cluster-level threshold of P<0.05. Significant clusters were identified by region, and parameter estimate values from each subject were extracted from significant voxels within each region. For pain-related regions of interest (primary (S1) and secondary (S2) somatosensory, insular, temporal pole, paracingulate, precuneus, and anterior cingulate cortices), the parameter estimates across all significant cluster-based voxels of a given region were averaged for each subject. Except where otherwise indicated, means are reported with standard deviation (M±SD).
3. RESULTS
3.1. Subjects
A total of 28 subjects were initially enrolled into the study. Two subjects were determined to be poor activators (no BOLD responses in the visual cortex during light stimulus without FL-41 lens) and one subject reported no unpleasantness (score=0) upon light stimulus without FL-41 lens. Thus, a total of 25 subjects were included in the present analyses. Table 1 summarizes the demographics and co-morbidities for these participants while Table 2 summarizes their ocular symptoms and signs.
Table 1.
Demographics and Co-morbidities of Subjects.
| Cases (n=25) | |
|---|---|
| Demographics | |
| Age (mean ± SD; years) | 55.3 ± 12.2 |
| Sex, male % (n) | 68% (17) |
| Race, White % (n) | 68% (17) |
| Ethnicity, Hispanic % (n) | 48% (12) |
| Self-reported Comorbidities | |
| Diabetes mellitus % (n) | 4% (1) |
| PTSD % (n) | 44% (11) |
| Depression % (n) | 68% (17) |
| Arthritis % (n) | 44% (11) |
| Sleep apnea % (n) | 60% (15) |
| Migraine % (n) | 36% (9)* |
| Traumatic brain injury % (n) | 24% (6) |
| Past or current smoker % (n) | 60% (15) |
| Self-reported Medications | |
| Antidepressants % (n) | 64% (16) |
| Anxiolytics % (n) | 56% (14) |
| Gabapentin % (n) | 24% (6) |
| Pregabalin % (n) | 4% (1) |
| NSAIDs % (n) | 36% (9) |
n=number of subjects; SD=standard deviation; PTSD=post-traumatic stress disorder; NSAIDs=non-steroidal anti-inflammatory drugs
Two subjects (subjects #5 and #7 in Figure 2) reported having an active migraine episode while undergoing fMRI.
Table 2.
Ocular Symptoms and Signs of Subjects.
| Ocular symptoms assessed via questionnaires | |
| DEQ-5 (range 0-22), mean ± SD (n) | 14.4 ± 3.3 (25) |
| OSDI-1 (range 0-4), mean ± SD (n) | 2.6 ± 1.4 (25) |
| OSDI total (range 0-100), mean ± SD (n) | 52.3 ± 25.6 (25) |
| NPSI-Eye-9 (range 0-10), mean ± SD (n) | 5.7 ± 3.4 (25) |
| NPSI-Eye total (range 0-100), mean ± SD (n) | 39.0 ± 20.0 (24)* |
| Average pain rating over 1 week recall (range 0-10), mean ± SD (n) | 5.0 ± 3.0 (25) |
| Tear Parameters (value taken from the more abnormal eye) | |
| TBUT (mean ± SD; seconds) (n) | 6.1 ± 3.7 (24)* |
| Corneal staining (mean ± SD; range 0-15) (n) | 3.3 ± 3.0 (24)* |
| Schirmer’s (mean ± SD; mm) (n) | 9.6 ± 7.9 (24)* |
SD=standard deviation; n=number of subjects; DEQ-5=5 Item Dry Eye Questionnaire; OSDI=Ocular Surface Disease Index; OSDI-1=Ocular Surface Disease Index question #1 Have you experienced eyes that are sensitive to light during the last week?; NPSI-Eye=Neuropathic Pain Symptom Inventory modified for the Eye; NPSI-Eye-9=Neuropathic Pain Symptom Inventory modified for the Eye question #9 Is your pain provoked or increased by light during the past 24 hours?; TBUT=tear break-up time.
One patient did not complete the NPSI questionnaire and ocular surface exam.
3.2. Subjective Ratings During fMRI Scanning
In the no lens condition, unpleasantness ratings for the light stimulus (white screen) were significantly greater than for the rest condition (black screen) (52.5±32.7 vs. 20.9±30.2, paired t-test t(24)=−5.45, p<0.00001). When wearing FL-41 lenses, light-evoked unpleasantness significantly decreased relative to the no lens condition (52.5±32.7 vs. 39.7±33.6, paired t-test t(24)=2.21, p=0.04) (Figure 2). Unpleasantness during the rest condition was not significantly different when wearing FL-41 lenses compared to the no lens condition (20.9±30.2 vs. 20.9±24.6, paired t-test t(24)=0, p=1.0) (Figure 2). When separating subjects based on sex, males reported significantly lower unpleasantness scores to the light stimulus when wearing FL-41 lenses compared to females (26.0±29.3 vs. 68.6±22.2, paired t-test t(24)=−4.04, p<0.001). Overall, 19 subjects reported decreased unpleasantness ratings when wearing FL-41 lenses, 2 reported equivalent ratings, and 4 reported increased ratings to the light stimulus when compared to the no lens conditions (Figure 2).
Figure 2. FL-41 lenses reduce unpleasantness ratings while viewing a white, but not black, screen.
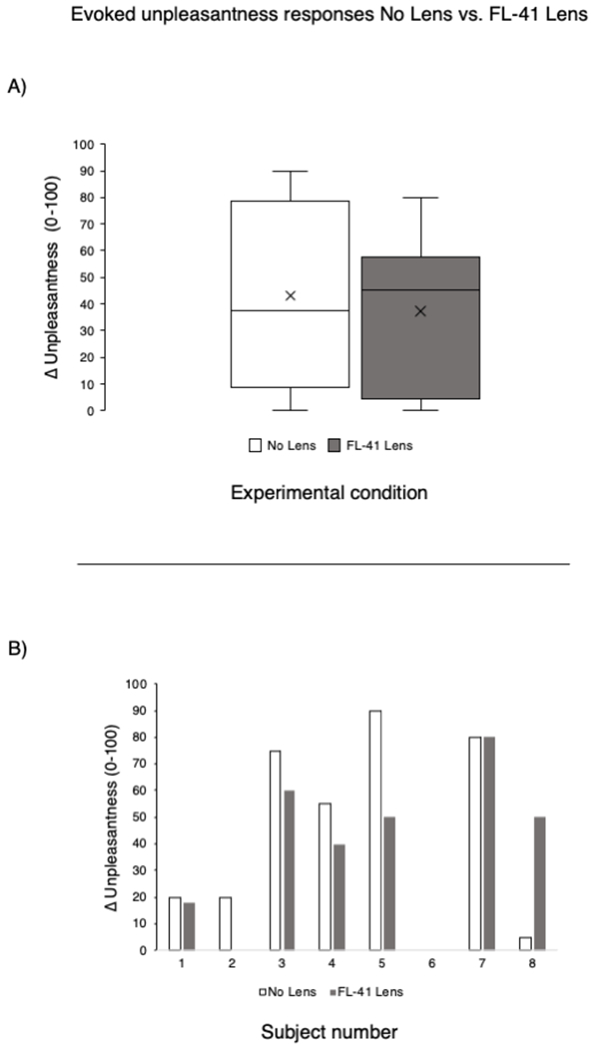
Top row: Unpleasantness ratings to the light stimulus (white screen) in the no lens and FL-41 lens conditions at the group level and at the individual subject level. Bottom row: Unpleasantness ratings to the rest condition (black screen) in the no lens and FL-41 lens conditions at the group level and at the individual level. Unpleasantness ratings were compared at the group level using paired t-test.
3.3. Light-Induced fMRI Activity in Subjects with Chronic Ocular Pain and Light Sensitivity With and Without FL-41 Tinted Lenses
Group analysis revealed functional differences in several brain structures when individuals with chronic ocular pain viewed a light stimulus (white screen) compared to the rest condition (black screen). Several brain regions showed significant BOLD responses to light stimuli in the no lens condition, including bilateral primary somatosensory (S1), bilateral secondary somatosensory (S2), bilateral insula, bilateral frontal pole, visual, precuneus, paracingulate, and anterior cingulate cortices (ACC) as well as cerebellar vermis, bilateral hemispheric lobule VI, bilateral crus I, and bilateral crus II (Figure 3A, No Lens). When wearing FL-41 lenses, significant BOLD responses to light stimulus were detected in the right trigeminal nucleus (SpV), visual, bilateral frontal pole, paracingulate, ACC, bilateral insula cortices, as well as bilateral cerebellar crus I, bilateral crus II, bilateral hemispheric lobule VI, and vermis (Figure 3B, FL-41). When statistically comparing activation with vs. without FL-41 lenses (Figure 3C, No FL-41>FL-41, and Figure 4), BOLD responses to light stimuli significantly decreased with FL-41 lenses in bilateral S1, bilateral S2, bilateral insular, right temporal pole, precuneus, ACC, and paracingulate cortices as well as bilateral cerebellar hemispheric lobule VI. In contrast, BOLD response to light stimuli was significantly increased with FL-41 lenses in the left angular gyrus, but not within regions associated with pain processing. Whole brain BOLD activity to light stimuli at the group level in the no lens, FL-41 lens, and contrast (no lens > FL-41 lens, FL-41 lens > no lens) conditions are found in Supplementary Table 1. In all regions, parameter estimates and unpleasantness ratings were not significantly correlated.
Figure 3. Light-induced Activation in the Whole Brain is Decreased When Wearing FL-41 Lenses.
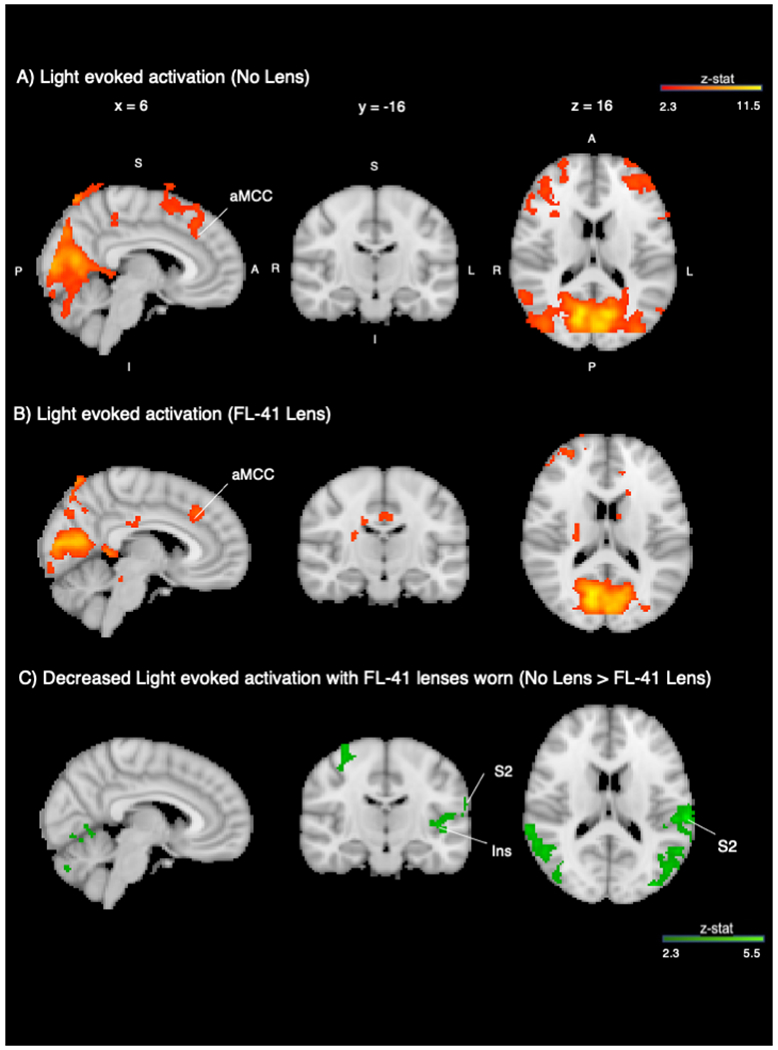
A) Group average activation during light stimulation vs. rest without FL-41 lenses (red-orange). B) Group average activation during light stimulation vs. rest while wearing FL-41 lenses (red-orange). C) Group contrast (No Lens > FL-41 Lens) displayed with MNI atlas underlay (dark green-light green). Both activation and contrast maps had an individual voxel threshold of z>2.3, and cluster-threshold of p<0.05. S=superior; I=inferior; A=anterior; P=posterior; R=right; L=left; ACC=anterior cingulate cortex; PCUN=precuneus; FP=frontal pole; CII=cerebellar crus II; INS=insula; S1=primary somatosensory cortex; S2=secondary somatosensory cortex; SpV=trigeminal nucleus.
Figure 4. Decreased Parameter Estimates of fMRI Activation With vs. Without FL-41 Lens Wear.
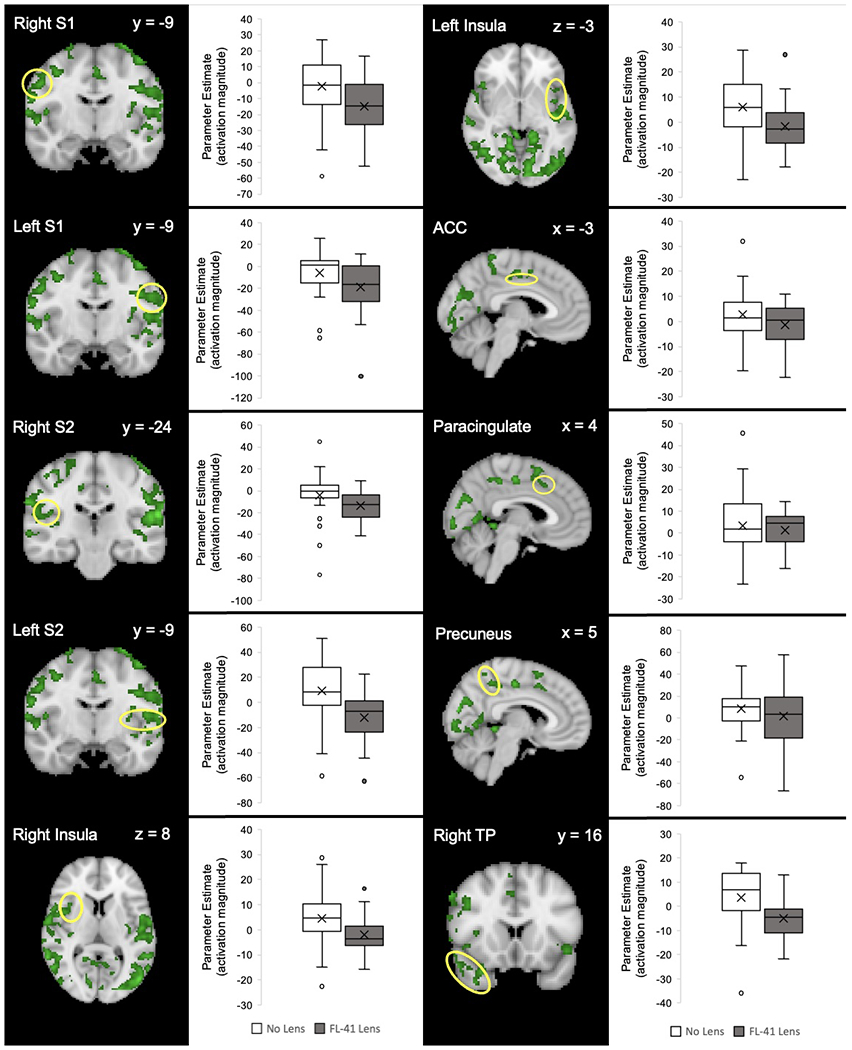
The Harvard-Oxford Subcortical and Cortical atlases were used to create anatomical masks of each region. BOLD signal activity in response to light for each region of interest is indicated by a yellow circle. Functional masks were created from group-level contrast maps to pull parameter estimates of BOLD signal responses to light using FEAT. S1=primary somatosensory cortex; S2=secondary somatosensory cortex; ACC=anterior cingulate cortex; TP=temporal pole.
3.4. Clinical and fMRI Findings in FL-41 Lens Responders and Non-Responders
Subjects were divided into two groups based on their unpleasantness reports to the light stimulus in the FL-41 vs no lens conditions. Individuals who reported a decrease in unpleasantness scores to light with FL-41 lenses were considered responders (unpleasantness to white screen with FL-41 lenses: 32.6±31.7 vs. no lens: 55.5±33.8, n=19, paired t-test t(18)=−4.31, p=0.0002). Non-responders comprised subjects who reported either no change or increased unpleasantness scores to light with FL-41 lenses (FL-41 lenses: 62.0±31.9 vs no lens: 42.8±29.4, n=6, paired t-test t(5)=2.13, p=0.04). No significant differences in demographics, co-morbidities, medication use, questionnaires, or tear film parameters were found between both groups.
When comparing BOLD responses between responders and non-responders, both groups showed a significant decrease in BOLD activity with FL-41 lens use in the right S1, right S2, right TP, and PCUN cortices (Figure 5 and Supplemental Figure 1). Several brain regions that were significantly decreased by FL-41 lens use in responders were not observed in non-responders, such as left S1, left S2, left TP, bilateral insula, ACC, and paracingulate cortices as well as bilateral cerebellar hemispheric lobule VI. Though a higher number of brain regions related to pain processing were modulated by FL-41 lenses in responders, no significant difference was detected in parameter estimate magnitude in overlapping brain regions between the two groups.
Figure 5. Responders had a Greater Number of Brain Regions Significantly Decreased by FL-41 Glasses Compared to Non-responders.
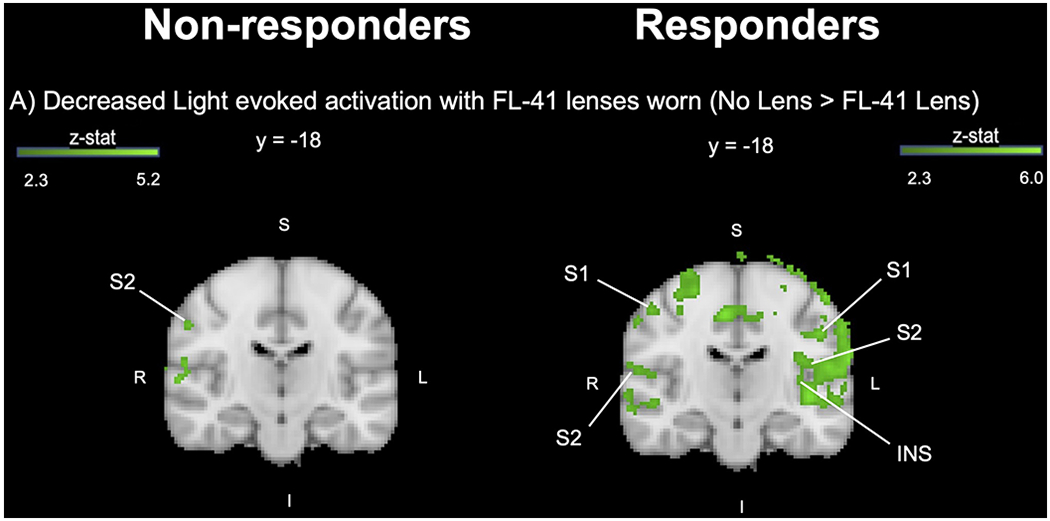
Left column: Non-responders. Right column: Responders. A) Group contrast (No Lens > FL-41 Lens) displayed with MNI atlas underlay (dark green-light green). Contrast maps had an individual voxel threshold of z>2.3, and cluster-threshold of p<0.05. S=superior; I=inferior; A=anterior; P=posterior; R=right; L=left; S1=primary somatosensory cortex; S2=secondary somatosensory cortex; INS=insula.
When further investigating the non-responder subgroup (n=4), PTSD frequency was higher in individuals whose unpleasantness scores to light increased with FL-41 tinted glasses compared to participants who reported no change or improved unpleasantness scores (100% versus 33%, X2 (1, n=25), p=0.03). No other significant differences were noted between the groups, including with regards to clinical examination findings and brain activity.
4. DISCUSSION
In this study of 25 individuals with chronic ocular pain, DE symptoms, and photophobia, we found that as a group, light-evoked unpleasantness ratings decreased with FL-41 tinted lenses. However, substantial inter-subject variability was noted, with certain individuals demonstrating greater improvement than others. Additionally, we found that FL-41 tinted lenses modulated light-evoked fMRI activity in brain regions associated with pain processing. When compared to the no lens condition, FL-41 lens use displayed significant reductions in light-evoked BOLD signals in bilateral S1, bilateral S2, bilateral insular, right temporal pole, precuneus, ACC, and paracingulate cortices as well as bilateral cerebellar hemispheric lobule VI, although the responses to light in pain processing regions of the brain were not completely eliminated.
Interestingly, we found that unpleasantness ratings were not correlated with change in BOLD activation while wearing FL-41 lenses. Similar findings have been noted in previous studies. In our prior studies using a similar fMRI protocol, pain/unpleasantness ratings after topical proparacaine placement and botulinum toxin-A (BoNT-A) injections did not relate to brain activity.11,24 Other studies have examined this question using thermal noxious stimuli with similar results.25 Intriguingly, the team that first suggested a relationship between pain ratings and FMRI activation26 published a more recent paper that contradicted their original findings.25 In 101 control subjects, the newer paper found no relationship between pain ratings evoked by a contact thermode and fMRI activation.25 Taken together, these findings suggesting that subjective pain assessments do not relate to the magnitude of brain activation.
4.1. Tinted Lenses Have Been Used as Treatment for Photophobia Related to Multiple Etiologies
In our study, 76% of patients with chronic ocular pain, DE symptoms, and photophobia reported clinically significant improvement in light-evoked unpleasantness ratings with FL-41 lens use. Prior reports have shown that tinted lenses improved photophobia symptoms in a variety of diseases including migraine, BEB, traumatic brain injury (TBI), and retinal disorders.10,27–30
Tinted lenses have been most robustly examined as a therapy in migraine. In a study of 20 children with migraine, individuals were randomized to wear blue-tinted or FL-41 glasses for at least eight hours a day over a four month period.27 Children in both groups reported decreased photophobia and glare symptoms with tinted lenses between migraine episodes but reported that the lenses did not improve photosensitivity during a migraine attack. Tinted lenses have also improved photophobia symptoms in adults with migraine. In a laboratory study of eleven adults with migraine and photophobia, visual discomfort scores (range 0-10) were recorded while viewing common migraine triggers through three lenses (individualized precision optical tint (POT) lenses that maximized visual comfort, grey lenses, and colored lenses (0.07 chromaticity difference from POT lenses).28 POT lenses were preferred by the group and reduced visual discomfort to a greater degree (70%) than colored (41%) and grey lenses (30%).
Other conditions found to benefit from tinted lenses include BEB, TBI, and retinal degeneration. Up to 80% of individuals with blepharospasm report that bright light exacerbates spasm.31 In a laboratory study that exposed 24 subjects with BEB to increasing light, 71% preferred FL-41 tinted lenses compared to 6 other chromatic tints.10 Similar findings were noted with respect to TBI. In a retrospective study of 62 individuals with mild TBI, 36% reported relief of photophobia with tinted lens use (further information on tint not specified).29 Other studies have found that blue tinted lenses were preferred in TBI. A study of 39 individuals with TBI exposed to a standard penlight found that blue tints reduced reported discomfort to the greatest degree (45%), followed by green (30%), red (27%), and purple (27%) tinted lenses.30 Finally, tinted contact lenses have been examined in individuals with retinal degeneration conditions such as achromatopsia or cone- and cone-rod dystrophy.32,33 In a retrospective case series, individuals with degenerative retinal diseases reported relief of photophobia immediately after placement of red-tinted contact lenses (n=23).32 This was also found over time in a prospective case series after wearing the red-tinted contact lenses for an average of 11 months (n=14).33 Taken together, these studies demonstrate that tinted lenses can improve photophobia symptoms stemming from a number of head and neck disorders, including chronic ocular pain.
4.2. Pathophysiological Mechanisms for Photophobia
Multiple light-evoked photophobia neural pathways have been described in both animal and human models. A pathway of particular interest in this study involves melanopsin, a photoactive pigment. Tinted lenses, including FL-41 lenses, have been shown to improve photophobia symptoms by preferentially blocking shorter wavelengths, particularly 480nm. This wavelength maximally activates melanopsin, which underlies the function of intrinsically photosensitive retinal ganglion cells (ipRGCs), named for their ability to detect light independent of traditional rod and cone photoreceptors.34 Upon activation, ipRGCs send signals directly to the posterior thalamic nuclei, such as the pulvinar and lateral posterior nucleus, which are involved in pain processing.35 Prior studies have examined whether melanopsin pathways are involved in photophobia.35,36 Studies in neonatal mice (whose ipRGCs but not rods and cones are responsive from birth) have found that during stimulation with bright-blue light emitting diodes (LED) (Jameco 183222, 468 nm λmax, 0.2 mW/cm2), mice displayed signs of photophobia and moved their bodies 180° away from the light source.36 The melanopsin pathway has also been implicated in photophobia in humans. In 20 blind individuals with chronic migraine (14 due to retinal rod and cone degeneration), individuals reported migraine exacerbation with light stimuli supporting activation of a non-image-forming pathway involving melanopsin.35 These findings support the notion that the ipRGC pathway is involved in photophobia and implicates melanopsin activity as a major component of light-evoked pain.
Theoretically, reduced melanopsin activation should decrease ipRGC phototransduction and signaling to pain-related networks in the brain. However, in our study, we did not see a reduction in posterior thalamus activation while wearing FL-41 lenses vs no lens conditions. This could be an imaging resolution issue or may indicate that other pathways, beyond the ipRGC system, were more strongly impacted by FL-41 lens wear in our population. Additionally, the light intensity we used may have impacted our findings. However, prior studies have found that light levels as low as 1 lux and 90 lux can influence melanopsin and ipRGC activation and mediate circadian rhythm control and pupillary constriction, respectively. However, the ipRGC threshold needed to evoke photophobia is unknown.37,38 Furthermore, melanopsin-containing cell populations have been found in the iris and cornea in animal models,39 and within neurons of the trigeminal ganglion in humans.40 Though controversial, photopigment found in these regions suggests that trigeminal activation may also be modulated by peripheral melanopsin-containing cell populations.39,40 In our study, reducing photoactivity in extraretinal melanopsin may have contributed to improved unpleasantness scores and reduced brain activation as FL-41 tinted glasses block 480nm light from reaching sites such as the iris and cornea as well as the retina.
The trigeminal nociceptive pathway is another neural circuit described in photophobia. In this circuit, signals originating from light-activated photoreceptors subsequently lead to parasympathetically-driven dilation of ocular blood vessels that are sensed by nearby trigeminal afferents.41 Through a chain of synapses, nociceptive signals are sent to the trigeminal nucleus caudalis (TNC), the posterior thalamus, and higher cortical centers. In the no lens condition of our study, we observed group level activation in a cortical center associated with this pathway (right S1), but not in the more proximal regions in the brainstem. However, with FL-41 lens use, significant group level light-evoked activation was observed in the SpV within the brainstem. Activation of the trigeminal nociceptive pathway with FL-41 lens wear was unexpected, especially as unpleasantness ratings to the light stimulus were decreased. As cutaneous allodynia is a common feature noted in individuals with chronic pain42, one explanation may be that this pattern of activation was due to allodynic periocular receptive fields making contact with the FL-41 frames.
4.3. Processing Beyond Trigeminal and Melanopsin Photophobia Neural Circuits
The sensation of pain is comprised of affective-motivational, sensory-discriminative, cognitive-evaluative, and pain-modulatory dimensions that are processed across intercommunicating brain regions.43 While the S2, insular, temporal pole, anterior cingulate, paracingulate cortices and cerebellar hemispheric lobule VI are more closely associated with the affective dimension of pain,44–47 the S1 cortex functions in the sensory-discriminative component of pain.46 In addition to processing the affective dimension of pain, lobule VI also functions in sensorimotor integration in response to pain.48 More recently, the precuneus cortex has been described to promote self-relevant information to consciousness, which highlights its role in pain processing as a salient sensory experience rather than a nociceptive one.49,50 In our study, FL-41 lens use was found to decrease brain region activity associated with multiple dimensions of pain processing, beyond melanopsin and trigeminal pathways. These findings highlight the global impact of FL-41 lens wear across different dimensions of pain processing evoked by light.
4.4. Comparing the Impact of Topical Anesthetic and FL-41 Tinted Glasses on Photophobia Symptoms and Neural Circuitry
We previously studied the impact of a short-term intervention (i.e. topical anesthetic) on a similar cohort of individuals with chronic ocular pain and photophobia (n=8) using fMRI.11 Overall, a higher proportion of individuals reported decreased light sensitivity with FL-41 lens compared to topical anesthetic (76% vs 50%). However, the overall intensity of light-evoked pain reduction was similar between the two treatment modalities with a decrease of 21% in the topical anesthesia group and 28% in the FL-41 tinted glasses group.
In examining the impact of topical anesthetic and FL-41 tinted lenses on light-evoked BOLD activity, some shared and some unique features were noted. Since topical anesthetic decreases pain signaling by targeting trigeminal peripheral nerve afferents in the cornea, the shared brain regions targeted by both proparacaine and FL-41 (anterior mid-cingulate and S1 cortices) may represent areas activated by corneal-mediated pain. The decreased activation of S1 and anterior cingulate cortices suggests that both topical anesthetic and FL-41 lenses may impact the sensory-discriminative (S1) and affective (anterior cingulate) dimensions of pain in individuals with photophobia. On the other hand, some brain regions (S2, insular, temporal pole, precuneus, and paracingulate cortices as well as cerebellar hemispheric lobule VI) had significantly decreased BOLD activity with FL-41 lens use but not proparacaine which may indicate areas more closely linked to light perception. Specifically, FL-41 tinted glasses likely have an impact on melanopsin-containing cells which have been found in the retina and perhaps the cornea and iris in animal models.34,39,40
While symptoms and some brain regions were similarly modulated by the two therapies, there are differences in their mechanisms of action. Proparacaine is instilled topically onto the eyes and acts by binding to voltage-gated sodium channels, subsequently inhibiting sodium ion influx and stabilizing the neuronal membrane.51 Blocking sodium influx, inhibits impulse conduction within neurons, reducing nociceptive afferent input to brain areas related to pain processing.51 In contrast, FL-41 lenses act as an optical blockade of light energy external to the eyes, preventing the 480nm wavelength (and other wavelengths of light) from activating melanopsin, and reducing melanopsin afferent input to the brain.34,35 Overall, the differing mechanisms of action and pathway targets may explain the varying therapeutic effect of each treatment modality in individuals with chronic ocular pain and photophobia. The finding that treatment responses were not uniform across subjects with both anesthetic and FL-41 lens wear points to heterogeneity within photophobia neural processing and points to the need for individualized therapies based on underlying mechanisms.
4.5. Limitations
Our results need to be considered with respect to the study limitations. First, our FL-41 tinted lenses filtered a wide spectrum of wavelengths beyond 480nm, though this wavelength was included within our filtered spectrum. Beyond just melanopsin, FL-41 may simply be reducing light intensity across the visual spectrum, effectively reducing phototransduction across traditional visual pathways More studies with different lens types are needed to understand the impact of blocking non-specific and specific wavelengths on photophobia. In future studies, a neutral density filter could be used as a comparison lens to more specifically control for overall luminance. Second, potential order effects of scanning patients without FL-41 tinted glasses and then with may have influenced unpleasantness ratings and brain activity. Additionally, our findings are from a small sample of predominately male participants living in South Florida with a variety of comorbidities accompanying their ocular pain. Furthermore, there were significant differences in unpleasantness reports between sexes to our light stimulus while wearing FL-41 tinted glasses. Studies have similarly supported sex-specific differences in pain-related brain activity52–55 and the impact of sex on ocular pain will need to be further examined in future studies. Fortunately, age, race, and ethnicity have not been found to influence pain-related brain activity to the same extent.56,57 Furthermore, participants may have had a mix of nociceptive and neuropathic contributors to pain, given the heterogeneity of co-morbidities and ocular surface signs in our cohort. Specifically, some individuals reported a history of migraine (with two subject endorsing active migraine during fMRI), a condition in which fMRI abnormalities have been described.58 In addition, PTSD symptoms, which have also been shown to impact pain report59, may have contributed to the increased unpleasantness ratings noted in 4 individuals while wearing FL-41 tinted glasses. Other potential confounders include the use of medications as well as previous knowledge of therapeutic intent of FL-41 tinted glasses which may have influenced unpleasantness ratings and functional brain activation in our study participants. These realities highlight the need for validation studies in larger cohorts, taking into account the influence of potential co-morbidities.
4.6. Impact
Despite these limitations, this study provides evidence that FL-41 lenses can modulate photophobia and the way the brain processes light in patients with ocular pain, DE symptoms, and photophobia. Brain regions identified in our study may be key components in neuropathic mechanisms of ocular pain and an important first step in developing precision-based therapies in this patient population.
4.7. Conclusion
We demonstrated that FL-41 lenses can improve symptoms of photophobia in the majority but not all individuals with chronic ocular pain and can modulate functional brain activation in regions associated with both the sensory-discriminative and affective aspects of pain. Overall, the variable therapeutic efficacy of FL-41 lenses on unpleasantness ratings and brain activity suggests heterogeneity in neural mechanisms underlying photophobia. Future studies will need to build on our findings with the goal of developing precision-based therapies and personalized treatment plans for individuals with chronic ocular pain and photophobia.
Supplementary Material
Funding/support:
This study was supported by a research grant from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences (I01 CX002015).
Other support included: Rehabilitation R&D (RRD) I21 RX003883, Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Services (I01 BX004893), the Department of Defense Gulf War Illness Research Program (W81XWH-20-1-0579) and Vision Research Program (W81XWH-20-1-0820), the National Eye Institute (U01EY034686, R01EY026174, and R61EY032468), National Institutes of Health Core Grant (P30EY014801), and Research to Prevent Blindness Unrestricted Grant (GR004596).
No financial disclosures.
Other acknowledgements:
The authors would like to acknowledge Mireya Hernandez for patient recruitment and data collection, and Elizabet Reyes for technical assistance and acquiring data. The authors would also like to thank Dr. Athanasios Panorgias from the New England College of Optometry for assisting with refinement of the visual stimulation protocol. Additionally, the authors would like to thank Dr. Jean-Marie Patel and Alex Gonzalez for gathering spectroscopy measurements on our FL-41 tinted lenses. Lastly, the authors would like to thank Dana Cohen for providing MRI-compatible frames for the FL-41 lenses.
Footnotes
Supplemental Material available at AJO.com
References
- 1.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. Jul 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 2.Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. Sep 1 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalangara JP, Galor A, Levitt RC, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens. May 2017;43(3):192–198. doi: 10.1097/icl.0000000000000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. Jul 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Diel RJ, Mehra D, Kardon R, Buse DC, Moulton E, Galor A. Photophobia: shared pathophysiology underlying dry eye disease, migraine and traumatic brain injury leading to central neuroplasticity of the trigeminothalamic pathway. Br J Ophthalmol. Jun 2021;105(6):751–760. doi: 10.1136/bjophthalmol-2020-316417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. Jul-Aug 2016;61(4):466–77. doi: 10.1016/j.survophthal.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. Jul 2015;13(3):250–62. doi: 10.1016/j.jtos.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoggan RN, Subhash A, Blair S, et al. Thin-film optical notch filter spectacle coatings for the treatment of migraine and photophobia. J Clin Neurosci. Jun 2016;28:71–6. doi: 10.1016/j.jocn.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology. May 2009;116(5):997–1001. doi: 10.1016/j.ophtha.2008.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herz NL, Yen MT. Modulation of sensory photophobia in essential blepharospasm with chromatic lenses. Ophthalmology. Dec 2005;112(12):2208–11. doi: 10.1016/j.ophtha.2005.06.030 [DOI] [PubMed] [Google Scholar]
- 11.Choudhury A, Reyes N, Galor A, Mehra D, Felix E, Moulton EA. Clinical Neuroimaging of Photophobia in Individuals With Chronic Ocular Surface Pain. Am J Ophthalmol. Feb 2023;246:20–30. doi: 10.1016/j.ajo.2022.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulte LH, Allers A, May A. Visual stimulation leads to activation of the nociceptive trigeminal nucleus in chronic migraine. Neurology. May 29 2018;90(22):e1973–e1978. doi: 10.1212/wnl.0000000000005622 [DOI] [PubMed] [Google Scholar]
- 13.Martín H, Sánchez del Río M, de Silanes CL, Álvarez-Linera J, Hernández JA, Pareja JA. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache. Nov-Dec 2011;51(10):1520–8. doi: 10.1111/j.1526-4610.2011.02013.x [DOI] [PubMed] [Google Scholar]
- 14.Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J. Measurement of visual sensitivity in migraine: Validation of two scales and correlation with visual cortex activation. Cephalalgia. Jun 2015;35(7):585–92. doi: 10.1177/0333102414547782 [DOI] [PubMed] [Google Scholar]
- 15.Panorgias A, Lee D, Silva KE, Borsook D, Moulton EA. Blue light activates pulvinar nuclei in longstanding idiopathic photophobia: A case report. Neuroimage Clin. 2019;24:102096. doi: 10.1016/j.nicl.2019.102096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleki N, Becerra L, Upadhyay J, Burstein R, Borsook D. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: implications for photophobia. Hum Brain Mapp. Jan 2012;33(1):75–88. doi: 10.1002/hbm.21194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen AE, Procyk CA, Howarth M, Walmsley L, Brown TM. Visual input to the mouse lateral posterior and posterior thalamic nuclei: photoreceptive origins and retinotopic order. J Physiol. Apr 1 2016;594(7):1911–29. doi: 10.1113/jp271707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. Apr 2010;33(2):55–60. doi: 10.1016/j.clae.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 19.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. May 2000;118(5):615–21. doi: 10.1001/archopht.118.5.615 [DOI] [PubMed] [Google Scholar]
- 20.Farhangi M, Feuer W, Galor A, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain. Jul 2019;160(7):1541–1550. doi: 10.1097/j.pain.0000000000001552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. Sep 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. Apr 2007;5(2):108–52. doi: 10.1016/s1542-0124(12)70083-6 [DOI] [PubMed] [Google Scholar]
- 23.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. Oct 2009;145(3):358–363. doi: 10.1016/j.pain.2009.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes N, Huang JJ, Choudhury A, et al. Botulinum toxin A decreases neural activity in pain-related brain regions in individuals with chronic ocular pain and photophobia. Front Neurosci. 2023;17:1202341. doi: 10.3389/fnins.2023.1202341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeppli ME, Nahman-Averbuch H, Hinkle WA, et al. Dissociation between individual differences in self-reported pain intensity and underlying fMRI brain activation. Nat Commun. Jun 22 2022;13(1):3569. doi: 10.1038/s41467-022-31039-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. Jul 8 2003;100(14):8538–42. doi: 10.1073/pnas.1430684100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good PA, Taylor RH, Mortimer MJ. The use of tinted glasses in childhood migraine. Headache. Sep 1991;31(8):533–6. doi: 10.1111/j.1526-4610.1991.hed3108533.x [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia. Jun 2011;31(8):925–36. doi: 10.1177/0333102411409076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truong JQ, Ciuffreda KJ, Han MH, Suchoff IB. Photosensitivity in mild traumatic brain injury (mTBI): a retrospective analysis. Brain Inj. 2014;28(10):1283–7. doi: 10.3109/02699052.2014.915989 [DOI] [PubMed] [Google Scholar]
- 30.Clark J, Hasselfeld K, Bigsby K, Divine J. Colored Glasses to Mitigate Photophobia Symptoms Posttraumatic Brain Injury. J Athl Train. Aug 2017;52(8):725–729. doi: 10.4085/1062-6050-52.4.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson RL, Patel BC, Holds JB, Jordan DR. Blepharospasm: past, present, and future. Ophthalmic Plast Reconstr Surg. Sep 1998;14(5):305–17. [PubMed] [Google Scholar]
- 32.Park WL, Sunness JS. Red contact lenses for alleviation of photophobia in patients with cone disorders. Am J Ophthalmol. Apr 2004;137(4):774–5. doi: 10.1016/j.ajo.2003.09.061 [DOI] [PubMed] [Google Scholar]
- 33.Severinsky B, Yahalom C, Florescu Sebok T, Tzur V, Dotan S, Moulton EA. Red-Tinted Contact Lenses May Improve Quality of Life in Retinal Diseases. Optom Vis Sci. Apr 2016;93(4):445–50. doi: 10.1097/opx.0000000000000761 [DOI] [PubMed] [Google Scholar]
- 34.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. Feb 8 2002;295(5557):1070–3. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- 35.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. Feb 2010;13(2):239–45. doi: 10.1038/nn.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson J, Wu V, Donovan M, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. Oct 5 2010;107(40):17374–8. doi: 10.1073/pnas.1008533107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. Jan 2014;37(1):1–9. doi: 10.1016/j.tins.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooley JJ, Ho Mien I, Hilaire MA, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. Oct 10 2012;32(41):14242–53. doi: 10.1523/JNEUROSCI.1321-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delwig A, Chaney SY, Bertke AS, et al. Melanopsin expression in the cornea. Vis Neurosci. Jan 2018;35:E004. doi: 10.1017/s0952523817000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matynia A, Nguyen E, Sun X, et al. Peripheral Sensory Neurons Expressing Melanopsin Respond to Light. Front Neural Circuits. 2016;10:60. doi: 10.3389/fncir.2016.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. May 2010;149(2):235–242. doi: 10.1016/j.pain.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stucky CL, Mikesell AR. Cutaneous pain in disorders affecting peripheral nerves. Neurosci Lett. Nov 20 2021;765:136233. doi: 10.1016/j.neulet.2021.136233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. Aug 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 44.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. Jun 9 2000;288(5472):1769–72. doi: 10.1126/science.288.5472.1769 [DOI] [PubMed] [Google Scholar]
- 45.Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. Feb 2011;21(2):435–48. doi: 10.1093/cercor/bhq109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. Dec 12 2007;2(12):e1292. doi: 10.1371/journal.pone.0001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. May 15 2007;36(1):256–67. doi: 10.1016/j.neuroimage.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 48.Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. Mar 9 2011;31(10):3795–804. doi: 10.1523/jneurosci.6709-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Chen Q, Su Y, Meng J, Qiu J, Zheng W. Pain in the default mode network: a voxel-based morphometry study on thermal pain sensitivity. Neuroreport. Oct 7 2020;31(14):1030–1035. doi: 10.1097/wnr.0000000000001512 [DOI] [PubMed] [Google Scholar]
- 50.Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. Oct 1 2005;27(4):824–34. doi: 10.1016/j.neuroimage.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 51.National Center for Biotechnology Information. PubChem Compound Summary for CID 4935, Proparacaine. Updated 02/20/2023. https://pubchem.ncbi.nlm.nih.gov/compound/Proparacaine
- 52.Fauchon C, Meunier D, Rogachov A, et al. Sex differences in brain modular organization in chronic pain. Pain. Apr 1 2021;162(4):1188–1200. doi: 10.1097/j.pain.0000000000002104 [DOI] [PubMed] [Google Scholar]
- 53.Kim JA, Bosma RL, Hemington KS, et al. Sex-differences in network level brain dynamics associated with pain sensitivity and pain interference. Hum Brain Mapp. Feb 15 2021;42(3):598–614. doi: 10.1002/hbm.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. Aug 2006;291(2):R257–67. doi: 10.1152/ajpregu.00084.2006 [DOI] [PubMed] [Google Scholar]
- 55.Vincent K, Tracey I. Sex hormones and pain: the evidence from functional imaging. Curr Pain Headache Rep. Oct 2010;14(5):396–403. doi: 10.1007/s11916-010-0139-1 [DOI] [PubMed] [Google Scholar]
- 56.Losin EAR, Woo CW, Medina NA, Andrews-Hanna JR, Eisenbarth H, Wager TD. Neural and sociocultural mediators of ethnic differences in pain. Nat Hum Behav. May 2020;4(5):517–530. doi: 10.1038/s41562-020-0819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng MT, Chiang MC, Yazhuo K, Chao CC, Tseng WI, Hsieh ST. Effect of aging on the cerebral processing of thermal pain in the human brain. Pain. Oct 2013;154(10):2120–2129. doi: 10.1016/j.pain.2013.06.041 [DOI] [PubMed] [Google Scholar]
- 58.Schwedt TJ, Chiang CC, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol. Jan 2015;14(1):81–91. doi: 10.1016/s1474-4422(14)70193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor V, Rowland JA, Naylor JC, et al. Time doesn’t heal all: PTSD symptoms exacerbate the relationship between age and pain intensity. Front Psychiatry. 2023;14:1221762. doi: 10.3389/fpsyt.2023.1221762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


