Take Home Message
We analyzed the diagnostic value of a molecular urine test to detect recurrent disease in bladder cancer patients treated by (chemo)radiation and showed that a urine test has the potential to limit the number of cystoscopies and cytology specimens.
Keywords: Biological assay, Bladder cancer, Cytology, Radiation therapy, Recurrence, Progression, Urine specimen
Abstract
Background
Patients with muscle-invasive bladder cancer (MIBC) who receive radiotherapy with curative intent are followed by imaging, cystoscopy, and urine cytology. However, interpretation of cytology and cystoscopy is hampered by the impact of ionizing radiation on cells.
Objective
To assess the diagnostic performance of a genomic urine assay to detect urinary tract recurrences in patients with MIBC treated by (chemo)radiation.
Design, setting, and participants
Patients with nonmetastatic MIBC who underwent (chemo)radiation with curative intent from 2016 to 2020 were prospectively included. Follow-up consisted of cystoscopy and upper tract imaging. Prior to cystoscopy, a urine sample was analyzed to assess mutations in the genes FGFR3, HRAS, and TERT and methylation of OTX1, TWIST1, and ONECUT2. The treating physician was blinded for the assay result.
Outcome measurements and statistical analysis
The primary endpoint was a urinary tract recurrence. Cross-sectional sensitivity, specificity, and negative predictive value (NPV) were analyzed using a previously developed logistic regression model for the detection of bladder cancer with this assay. The secondary endpoint was the risk of a future urinary tract recurrence following a positive test and negative cystoscopy/imaging, using a time-dependent Cox proportional hazard analysis.
Results and limitations
A total of 143 patients were included, and 503 urine samples were analyzed. The median study duration was 20 mo (interquartile range [IQR] 10–33), and the median time to a recurrence was 16 mo (IQR 12–26). In 27 patients, 32 urinary tract recurrences were diagnosed, including three upper tract tumors. Of 32 recurrences, 18 (56%) had a concomitant urine test available. The diagnostic model had an area under the curve of 0.80 (95% confidence interval [CI] 0.69–0.90) with corresponding sensitivity, specificity, and NPV of 78 (95% CI 52–94), 77% (95% CI 73–81), and 99% (95% CI 97–100). When taking into account the anticipatory effect of the test, 28/32 (88%) recurrences were detected. A Cox regression analysis showed a hazard ratio of 14.8 for the development of a future recurrence (p < 0.001). A major limitation was the lack of a concomitant urine test result in 14/32 (44%) recurrences.
Conclusions
A genomic urine assay detected urinary tract recurrences after (chemo)radiation in patients with MIBC, and a positive test was strongly associated with future recurrences. Although validation in a large cohort is warranted, the test has the potential to limit frequent cystoscopies.
Patient summary
Radiotherapy is a bladder-sparing treatment in patients with bladder cancer. After treatment, these patients undergo visual inspection of the bladder by cystoscopy to detect possible recurrences. However, interpretation of cystoscopy is difficult due to the effects of radiation on the bladder lining. Hence, we analyzed the diagnostic value of a molecular urine test to detect recurrent disease in bladder cancer patients treated by radiotherapy, and we showed that the urine test has the potential to limit the number of cystoscopies.
1. Introduction
Bladder cancer (BC) is the tenth most common cancer worldwide, with ∼75% diagnosed as non–muscle-invasive (NMIBC) and ∼25% diagnosed as muscle-invasive (MIBC) bladder cancer at diagnosis [1]. Despite therapy, patients with nonmetastatic MIBC have a relatively poor prognosis with a 5-yr survival rate of ∼60% [1]. The recommended treatment of nonmetastatic MIBC patients consists of neoadjuvant chemotherapy followed by radical cystectomy (RC) with urinary diversion and bilateral pelvic lymphadenectomy [2]. RC is a surgical procedure that is associated with postoperative morbidity and even mortality [3], [4]. Furthermore, some patients with MIBC are unwilling or considered unfit to undergo an RC [5], [6]. These patients may be eligible for bladder-sparing treatment modalities, such as trimodality treatment (TMT). TMT consists of a transurethral resection of the bladder tumor (TURBT), external beam radiotherapy (RT), and concurrent chemotherapy. TMT is superior to RT without concurrent chemotherapy in terms of locoregional control and is recommended in major guidelines [2], [7]. Results of a meta-analysis of oncological outcomes following TMT showed that the 10-yr overall survival and disease-specific survival of TMT are comparable with those of RC and thus may be a good alternative treatment for MIBC patients [8].
A limitation of bladder-sparing treatment with (chemo)radiation is the development of intravesical tumor recurrences [9]. Guidelines are inconclusive on an optimal surveillance schedule following (chemo)radiation [2], [7]. Hence, frequent cystoscopies and urine cytology are common practice in surveillance of MIBC patients who underwent bladder-sparing treatment [10]. Cystoscopies are invasive and uncomfortable, especially in patients with irradiated bladders, and may cause urinary tract infections or hematuria. Furthermore, both cystoscopy and urine cytology are less reliable after RT, resulting in false-positive findings and atypical cytology test results, possibly due to reactive changes of the bladder epithelium [11], [12], [13]. As a consequence, unnecessary biopsies are common, while TURBTs in irradiated necrotic tissue might even cause bladder fistulas. Genomic urine assays could therefore be useful to detect recurrences, sometimes even before the recurrences are diagnosed clinically, which is called the anticipatory effect, or urine tests may be used to rule out atypical abnormalities of patients with irradiated bladders.
BC-associated genomic alterations can be investigated in DNA isolated from voided urine, and these alterations can be used as biomarkers to detect urinary tract recurrences. Previously, we conducted a microsatellite analysis in the surveillance of irradiated BC patients [11]. The results of this albeit small study indicated that follow-up by urine testing could be feasible for these patients. We subsequently developed a urine test that combined mutation and methylation markers for the diagnosis and surveillance of BC [14], [15], [16], [17]. We prospectively validated this urine-based genomic assay, which detects mutations in the FGFR3, HRAS, and TERT genes, and methylation of OTX1, ONECUT2, and TWIST1. The assay was highly accurate in detecting BC in patients presenting with hematuria (area under the curve [AUC] 0.95) [17].
In the present study, we evaluated the diagnostic performance of our previously developed urine test to detect urinary tract recurrences after RT, because standard surveillance is impeded by a difficult assessment of irradiated bladders. We calculated the performance of the genomic assay and explored how the urine test could be a valuable replacement for standard diagnostic cystoscopies and urine cytology. Finally, we investigated whether a positive urine test in the absence of abnormalities by cystoscopy or imaging could predict future recurrences.
2. Patients and methods
2.1. Patients and samples
We performed a single-center prospective cohort study, which was approved by the Erasmus MC Medical Ethics Committee (MEC-2016-266). Written informed consent was obtained from all patients. Participants were prospectively enrolled in a consecutive series between August 2016 and July 2020 at the Erasmus MC Cancer Institute. The patient inclusion criteria were the following: nonmetastatic MIBC, clinical stage ≥T2 N0M0; bladder-sparing treatment with curative intent, which contained any type of RT; and more than zero urine samples collected during follow-up. All patients were discussed by a multidisciplinary team prior to inclusion, and the addition of chemotherapy to the treatment regimen was based upon patient performance and patient choice. Diagnostic transurethral resection of the bladder tumor specimens of the index tumors from all patients were reviewed by an expert uropathologist. Urine specimens were prospectively collected prior to cystoscopies. Clinical follow-up continued until July 2021. Patient data were collected retrospectively and blinded of assay results. Urinary test results were not reported to the treating physician, nor did it impact therapeutic decision-making. Details on RT, clinical follow-up, and treatment of recurrences are provided in the Supplementary material.
2.2. DNA isolation, mutation, and methylation analysis
Freshly voided urine samples were stored at 4°C, and cells were collected by centrifugation at 2500 rpm for 10 min. Pellets were resuspended in 900 µl phosphate-buffered saline and transferred into 1.5 ml Eppendorf tubes. Eppendorf tubes were centrifuged at 6000 rpm for 5 min. Supernatant was removed, and cell pellets were stored at –20°C until DNA isolation. DNA isolation was performed using the QIAamp DNA Mini Kit, according to the manufacturer’s protocol. DNA concentration was measured using the Qubit 2.0 Fluorometric Quantification. A SNaPshot mutation analysis for FGFR3, HRAS, and TERT was performed at the Erasmus MC pathology laboratory, as described in detail previously [15], [18]. Briefly, a polymerase chain reaction was performed to amplify the sites of interest. A mutation analysis was performed using the SNaPshot Multiplex Kit, to detect multiple mutation sites per gene [17]. A methylation analysis included the same genes (OTX1, TWIST1, and ONECUT2) as in previously published work [14], [17]. See the Supplementary material for additional information on mutation and methylation analyses.
2.3. Definitions, endpoints, and statistics
The primary endpoint was a urinary tract recurrence, defined as (1) an intravesical biopsy-proven urothelial carcinoma; (2) a clinical suspicion of a urinary tract recurrence, which includes upper tract urothelial carcinomas (UTUCs) on computed tomography (CT) imaging; or (3) a tumor detected on cystoscopy in combination with a positive urinary cytology, according to The Paris System (TPS) 5 or 6. Cross-sectional, that is, based on a concomitant urine test and cystoscopy, sensitivity, specificity, and negative predictive value (NPV) were analyzed using a previously developed logistic regression model for the detection of BC with this assay [17]. The model’s intercept was –5.133, the mutation (y/n) coefficient was 4.111, and the methylation (0–3) coefficient was 0.857. The test was positive when at least a single mutation and/or two or more methylated genes were detected. Urine samples were excluded if results were incomplete (ie, all markers are needed). The net benefit was calculated based on a risk model decision analysis, as done previously [17]. The secondary endpoint was the longitudinal test characteristics, that is, taking into account the anticipatory effect, which is the risk of a future recurrence following a positive urine test, using a multivariable time-dependent Cox-proportional hazard model. The Kaplan-Meier method was used to estimate recurrence-free survival according to test results in relation to the presence or absence of a recurrence over time. The median study duration time was calculated as the time between study inclusion and last contact. Time to recurrence was measured as the time between the start of radiation and development of a recurrence. Progressive disease was defined as follows: (1) a histologically proven ≥T2 tumor in the urinary tract after RT, and (2) lymph node and/or distant metastatic disease as assessed by biopsy or CT imaging. Statistical significance was set at p < 0.05. R statistical software (v4.0.5) was used to perform the analyses and generate figures.
3. Results
3.1. Baseline study population and follow-up after RT
A total of 143 MIBC patients were included, and 503 urine samples were analyzed; the median follow-up was 20 mo (IQR 10–33). The median time between the last day of RT and the first urine test was 4 mo (IQR 3–10). In 27 patients, 36 urinary tract recurrences were diagnosed. The median time to recurrence was 16 (IQR 12–26) mo, while the median follow-up for patients without recurrences was 28 (IQR 16–47) mo. In eight patients, metastatic disease was diagnosed without a synchronous recurrence in the urinary tract. Therefore, these tumors were not included in the primary analysis on the diagnostic performance of the urine assay. In total, 108 patients (76%) remained disease free during follow-up. The baseline clinicopathological and follow-up data are summarized in Table 1, and the study design and overall outcome are illustrated in Supplementary Figure 1. Details on variant histology at diagnosis are provided in Supplementary Table 1. A swimmer’s plot for all patients with timing of tumor recurrences and outcome of urine tests and cystoscopies is depicted in Figure 1. Four recurrences had to be excluded from analyses as (1) no urine test was available prior to their second recurrence (R7, R71, and R83) and (2) an intravesical recurrence was diagnosed with recurrent disease in the upper urinary tract that was not surgically removed (R49). Hence, later test results could be influenced by shedding of tumor cells of the unresected ureter tumor. As a result, 32 tumors were included in the analysis (Fig. 1). Four recurrences were detected by CT imaging: three UTUCs (R49, R85, and R154) and one necrotic tumor extending into the seminal vesicles (R109; Fig. 1). Three tumors were not biopsied due to frailty, and diagnosis was based on high-grade (HG) cytology and a visible bladder tumor at cystoscopy (R18, R35, and R70). All other recurrent tumors were biopsy proven.
Table 1.
Baseline study characteristics of 143 muscle-invasive bladder cancer patients treated with (chemo)radiation of the bladder and who were under surveillance with urinary molecular diagnostics
| Characteristic | Characteristic | ||
|---|---|---|---|
| Age at diagnosis (yr) | Chemotherapy, n (%) | ||
| Median (IQR) | 76 (68–81) | Yes | 54 (38) |
| Sex, n (%) | Treatment, n (%) | ||
| Male | 115 (80) | Radiotherapy + chemotherapy | 89 (62) |
| Age | Radiotherapy + PC | 15 (11) | |
| Median (IQR) | 76 (68–88) | Radiotherapy alone | 39 (27) |
| Smoking, n (%) | Reason for radiotherapy, n (%) | ||
| Yes/stopped | 113 (79) | Contraindication for RC | 83 (58) |
| Missing | 9 (6) | Opted bladder-sparing therapy | 60 (42) |
| History of NMIBC, n (%) | Time to recurrence | ||
| Yes | 27 (19) | Median (IQR) | 16 (12–26) |
| Clinical T stage, n (%) | Patients with progression a,n (%) | ||
| cT2 | 112 (78) | Yes | 35 (24) |
| cT3 | 30 (21) | Time to progression | |
| cT4 | 1 (1) | Median (IQR) | 18 (12–26) |
| Tumor grade (1973), n (%) | Death, n (%) | ||
| G2 | 15 (11) | No | 85 (59) |
| G3 | 122 (85) | Yes (any cause) | 54 (38) |
| Non-UCC | 6 (4) | Yes (bladder cancer) | 23 (16) |
| Concomitant carcinoma in situ, n (%) | Missing | 4 (3) | |
| Yes | 45 (32) | Lost to follow-up, n (%) | |
| Variant histology, n (%) | Yes | 30 (21) | |
| Yes | 36 (25) | Study follow-up (mo) b | |
| Hydronephrosis at diagnosis, n (%) | Median (IQR) | 20 (10–33) | |
| Yes | 18 (13) | Treatment follow-up (mo) c | |
| Missing | 5 (3) | Median (IQR) | 30 (19–49) |
| Prior intravesical treatment, n (%) | |||
| Yes, chemotherapy | 6 (4) | ||
| Yes, BCG | 16 (11) |
BCG = bacillus Calmette-Guérin; CT = computed tomography; IQR = interquartile range; NMIBC = non–muscle-invasive bladder cancer; PC = partial cystectomy; RC = radical cystectomy. Please note: for variables with a positive or negative outcome, only the positive outcome is reported due to table size limitations.
Progression was defined as the development of recurring biopsy-proven ≥T2 tumor, lymph node, and/or metastatic disease (using CT-scan imaging).
Time between study inclusion and last contact.
Time between start of radiotherapy and last contact.
Fig. 1.
Swimmer’s plot showing test results of a genomic urine assay from collected urine samples in 27 nonmetastatic muscle-invasive bladder cancer patients who developed 32 recurrences after treatment with (chemo)radiation of the bladder. Patients (R-number) are sorted by the length of follow-up. A horizontal line depicts the disease course of a single patient. If multiple cystoscopies took place after (chemo)radiation but before study inclusion, only the result of the cystoscopy prior to study inclusion is shown. CIS = carcinoma in situ; HG = high grade; UTUC = upper tract urothelial carcinoma.
3.2. Cross-sectional urine test sensitivity, specificity, and NPV
To analyze the diagnostic performance of the urine assay, we excluded 14 tumors that lacked a concomitant urine test, which included eight recurrences that occurred after urine collection had stopped (Fig. 1). One urine sample failed methylation analyses due to a low DNA yield (R7). Further details on urine collection, mutations, and methylation in all samples are summarized in Supplementary Table 2. Next, we determined whether our assay, which diagnosed BC efficiently in patients presenting with gross or microscopic hematuria, could also detect recurrent disease in patients after (chemo)radiation. The logistic regression model requires input from mutations (yes/no) and methylation count (1–3) [17]. Its application with prespecified coefficients and probability threshold (Pt) of 0.029 resulted in test performance with an AUC of 0.80 (95% CI 0.69–0.90). Associated test sensitivity was 78% (95% CI 52–94), specificity was 77% (95% CI 73–81), and NPV was 99% (95% CI 97–100). Albeit numbers were low, no differences were observed between patients with or without chemotherapy (data not shown). Interestingly, all three UTUCs (not possible to be diagnosed by cystoscopy) were detected by the urine test. However, four of the 18 recurrent tumors were missed. In R96, a T1HG tumor, the test was positive 6 mo prior to the clinically apparent recurrence, but in the concomitant analysis, only OTX1 was methylated (Fig. 1), which was insufficient for a positive test result. Four prior urine tests from patient R56 accurately detected two HG urothelial cell carcinomas (UCCs). The fifth test was negative, and interestingly, the pathology report after salvage cystectomy indicated a pT1 small cell neuroendocrine tumor in a diverticulum. All urine samples from R40 and R43 were negative, and as a result, two invasive UCCs (a T1HG and T2HG) were missed (Fig. 1).
3.3. Cytology performance after RT
Owing to the limited diagnostic performance of cytology after radiation, cytology was examined only in 17/32 (53%) urinary tract recurrences. As expected, urinary cytology had low sensitivity of 39% for HG cytology (TPS5). In only ten urinary tract recurrences, concomitant urine cytology and a urine test were available. In these tumors, urine cytology (TPS5) had 50% sensitivity and 74% specificity. The urine test was 80% sensitive and 69% specific. However, due to the low number of cases, these results should be treated with caution.
3.4. Net benefit analysis to compare urine testing with cystoscopies in all patients
Owing to the difficult interpretation of cystoscopies and cytology after radiation, and the high cross-sectional performance of the urine assay, we evaluated the usefulness of the urine test in a clinical setting by calculating its net benefit. Urologists need to reflect on how many cystoscopies would be acceptable to detect a single recurrence. Arguably, this could be between 0.025 (one positive cystoscopy per 40 performed) and 0.10 (one positive cystoscopy per ten performed). For this purpose, a decision curve analysis calculated the benefit of our model versus cystoscopies in all patients (Fig. 2). Based on these results, the urine test seemed preferable to a cystoscopy for all patients if a urologist would consider up to 36 cystoscopies to detect one recurrence. In other words, a cystoscopy is preferred if a urologist is willing to perform > 36 cystoscopies to find a single recurrence. Considering these high numbers, many cystoscopies may be preventable by implementation of a urine test.
Fig. 2.
Decision curve analysis of the genomic urine assay (red line) versus current best practice (cystoscopy in all patients, gray line). The net benefit risk threshold is 0.027, which corresponds to a cost-to-benefit ratio of 1:36.
3.5. The anticipatory effect of the urinary test
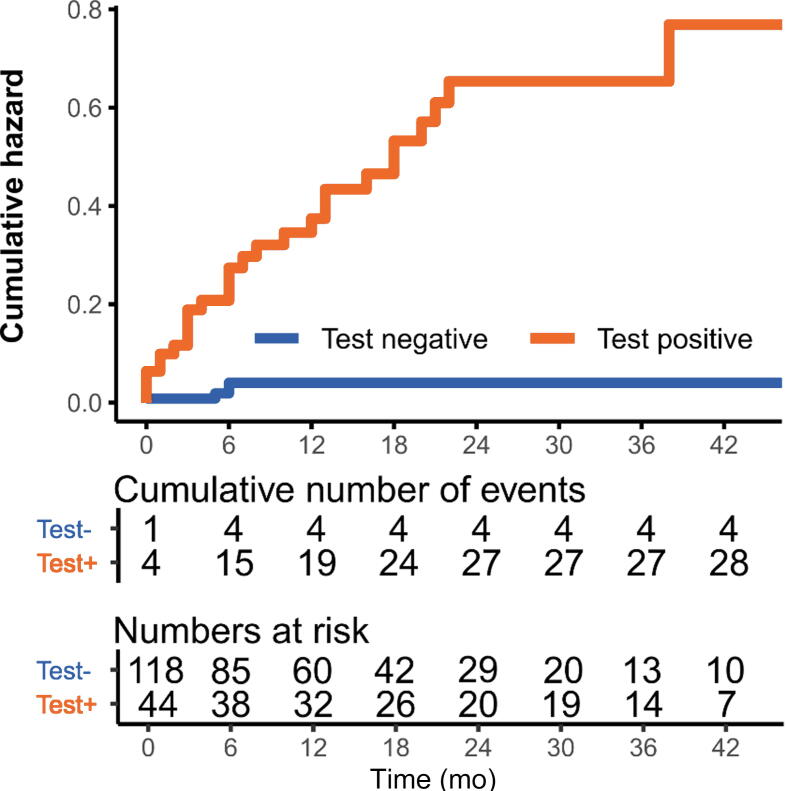
Urine tests have the advantage of detecting recurrences before the tumor is clinically diagnosed [19]. This phenomenon is called the anticipatory effect. In the present study, 28/32 (88%) tumors were detected following a positive urine test and negative cystoscopy (eg, R18 and R71 in Fig. 1) [19]. This could also be useful in the rare situation that a test fails, for instance, in the case of R7 (Fig. 1). A positive urine test was associated with a hazard ratio (HR) of 14.8 (4.95–42.5, p< 0 .001) for developing a recurrence over time (Fig. 3 and Supplementary Table 3). Although infrequent, a previous recurrence also led to a markedly increased risk of another recurrence (HR 8.69 [3.27–23.1], p < 0.001; Supplementary Table 2). No multivariable analysis was performed due to the low number of cases. In line with previous data, a positive urine test strongly corresponded with the risk of a future recurrence.
Fig. 3.
Kaplan-Meier cumulative hazard estimate depicting the risk of developing a urinary tract recurrence over time when a urine test was positive or negative in nonmetastatic muscle-invasive bladder cancer patients after treatment with (chemo)radiation of the bladder. At time = 0, patients started (chemo)radiation treatment. Of note: in several patients, multiple test periods per patient were analyzed; hence, the total number does not add up to N = 143.
3.6. Reducing the number of cystoscopies
In a randomized controlled interventional study, a negative urine test could be used to replace one or two of the next cystoscopies under the condition that the concomitant cystoscopy and imaging are unsuspicious and follow-up urine tests remain negative. When a urine test is positive, both the concomitant and the next cystoscopy are performed, to cover the anticipatory effect. In this scenario, only a single tumor would have been missed (R43) at the first possible opportunity. Interestingly, the total number of cystoscopies performed (n = 631) in our study would have been lowered by 139/631 (22%) to 183/631 (29%), depending on whether one or two cystoscopies were skipped. Furthermore, urine cytological evaluation could have been left behind altogether.
4. Discussion
Bladder-sparing multimodality treatment (MMT), which includes a TURBT and RT with or without concurrent chemotherapy, is a definitive first-line treatment for nonmetastatic MIBC [8], [20], [21]. A disadvantage of MMT is the risk of intravesical recurrences, warranting strict surveillance. In the Netherlands, an increasing number of patients undergo bladder-sparing MMT [22]. This can partly be explained by an aging population, in whom an RC procedure is considered not suitable [23]. However, a shift in focus to RT over RC can mostly be credited to the BC2001 trial, which showed 63% 5-yr locoregional disease-free survival for RT with concomitant chemotherapy [20]. Recently, a Dutch multicenter study demonstrated 76% locoregional disease-free survival for MIBC patients treated with MMT at 24 mo [22]. In our population, the intravesical recurrence rate was 18% at a median follow-up of 30 mo. This is on the low end, but can be explained by the fact that our study population is relatively old and many patients have died of other causes than BC. Nevertheless, it is recommended that surveillance of MIBC patients consists of an intensive 3-monthly schedule of cystoscopies and urine cytology [24]. Yet, both diagnostic modalities are less reliable due to the effects of RT, while cystoscopies in these patients may cause discomfort [11], [12], [13], [25]. Hence, there is a high clinical need to improve surveillance of irradiated BC patients.
To the best of our knowledge, this is the first prospective study that investigated a urine-based mutation/methylation assay to study recurring disease during surveillance of MIBC patients treated by RT. We found high cross-sectional sensitivity (78%) and specificity (77%), thereby demonstrating that the assay is efficacious. We confirmed a good AUC (0.80) using our published model. The test accurately identified 14 out of 18 intravesical recurrences. Consistent with previous work, the assay detected all three UTUCs during follow-up, which is of importance, as CT urography has limited sensitivity for the detection of UTUCs [26]. Limitations of the present study were the relatively low number of cases, a short study duration, and that the urine assay missed four tumors, which were all of HG and included a muscle-invasive tumor. This means that complete replacement of cystoscopies with the urine test is not possible. However, with conventional white-light cystoscopy, 10–20% of tumors are missed as well [25], [27]. Considering the inflammation and necrosis that frequently occur after RT, we speculate that this number is even higher in irradiated bladders [28]. In addition, the sensitivity of urine cytology was only 50% in this study. This suggests that the urine test presented here could be of considerable value during follow-up of this group of patients.
The genomic assay frequently detected recurrent tumors earlier than cystoscopy and had a very high anticipatory effect, with a longitudinal detection rate of 88% [16], [19], [29], [30]. A positive test result was associated with a highly increased incidence of recurrences over time. This finding indicates that increased vigilance is warranted during surveillance of patients with positive test results. The high NPV (99%) indicates that patients with a negative test result did not have a recurrence. Moreover, the net benefit analysis clearly showed that the urine test is superior to cystoscopies. Follow-up by cystoscopies at all follow-up visits were advantageous only if urologists were willing to perform >36 cystoscopies to find a single recurrence. The 99% NPV, the high anticipatory effect, and the net benefit of the test over cystoscopies in all patients can be used to introduce this assay to the clinic. Our scenario to limit cystoscopies with urine tests is encouraging and shows that in a randomized controlled interventional study, the number of cystoscopies could safely be lowered to 29%, while leaving out urine cytology altogether. Although prospective investigation is warranted, such a trial has high potential to cut costs and improve patient well-being.
There were several limitations in our study. Although the median study duration is relatively short, it is affected by dropout of patients with recurring disease, which is common in the first 24 mo after RT. The assay had frequent false positive findings. False positive findings can largely be explained by the high anticipatory effect: the test was often already positive some months prior to a clinically diagnosed recurrence. False negative test results could be caused by the fact that suspected lesions were not always followed by a biopsy or cytology, as these were judged to be due to radiation effects. In addition, 30/503 (6%) urine samples failed analyses. In future studies, the use of multiple urine samples at every time point may help overcome these limitations [31]. FGFR3 and HRAS mutations were rare, probably because these mutations are infrequent in MIBC as compared with NMIBC [32], [33], [34]. Removal of FGFR3 and HRAS may be considered in irradiated BC, as these markers were of no additional value. The addition of methylation enhanced sensitivity, without hampering specificity, possibly since invasive tumors are often hypermethylated [35]. Interestingly, all three UTUCs, known to frequently harbor FGFR3 alterations, were found hypermethylated for OTX, ONECUT2, and TWIST1, while FGFR3 mutations were absent. In the future, a new generation of tests, tailored to the genetic aberrations of a patients’ tumor, could be developed.
Finally, it would be of interest to investigate the performance of mRNA-based tests after radiation (eg, CxBladder Monitor and Xpert Bladder Cancer Monitor) [36], [37]. The mRNA tests showed promising results in detecting HG tumors with sensitivity of 95% and 73.7%, respectively, but as RT leads to reactive changes, it is not unlikely that this negatively impacts urine test results [11], [12]. However, apart from a single study investigating a microsatellite analysis in irradiated BC patients, we were unable to find other studies in the literature on alternatives for the follow-up of these patients [11].
5. Conclusions
In conclusion, this is the first prospective trial that investigates the diagnostic performance of a urine-based assay in surveillance of MIBC patients treated with RT. We have shown that the DNA-based test can be used during surveillance and that previous treatment by RT does not negatively affect test in contrast to cystoscopy and cytology. The urine test uses robust and proven techniques, it is easy to implement in clinical practice, and the assay can be set up with limited effort in every diagnostic laboratory. The test was highly predictive of future recurrences and had a very high NPV. If negative, the urine assay may be useful for skipping cystoscopies. If positive, increased vigilance is warranted. Our findings may help guide us toward a more patient-tailored surveillance schedule, which should include a robust urine test and a safe reduction of the number of cystoscopies and TURBTs during surveillance after RT.
Author contributions: Joost L. Boormans had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: F.C. de Jong, Boormans, Zwarthoff.
Acquisition of data: F.C. de Jong, Iflé, J.J. de Jong, Van der Made, Kooper
Analysis and interpretation of data: F.C. de Jong, Iflé , J.J. de Jong, Boormans, Zwarthoff.
Drafting of the manuscript: F.C. de Jong, Iflé.
Critical revision of the manuscript for important intellectual content: F.C. de Jong, Incrocci, Franckena, J.J. de Jong, Zuiverloon, Boormans, Zwarthoff.
Statistical analysis: F.C. de Jong, J.J. de Jong, Iflé.
Obtaining funding: Boormans, Zwarthoff.
Administrative, technical, or material support: Zuiverloon, Van Criekinge, Incrocci, Franckena, Boormans, Zwarthoff.
Supervision: Boormans, Zwarthoff.
Other: None.
Financial disclosures: Joost L. Boormans certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Ellen C. Zwarthoff is an advisor for MDxHealth. Joost L. Boormans is a paid consultant for BMS, Astellas, Janssen, AstraZeneca, MSD, Merck, and Pfizer, all paid to Erasmus MC and not relevant to this work. Wim van Criekinge was the chief scientific officer for MDxHealth.
Funding/Support and role of the sponsor: This research was financially supported by Erasmus MC Cancer Institute (Pathology).
Acknowledgments: The authors would like to express their deep gratitude to Johan Vandersmissen for his help with the methylation assay.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2024.02.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Saginala K., et al. Epidemiology of bladder cancer. Med Sci. 2020;8:15. doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witjes J.A., et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Aziz A., et al. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur Urol. 2014;66:156–163. doi: 10.1016/j.eururo.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Mak K.S., et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2016;96:1028–1036. doi: 10.1016/j.ijrobp.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Novotny V., Zastrow S., Koch R., Wirth M.P. Radical cystectomy in patients over 70 years of age: impact of comorbidity on perioperative morbidity and mortality. World J Urol. 2012;30:769–776. doi: 10.1007/s00345-011-0782-0. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone E., et al. The effect of age and comorbidities on early postoperative complications after radical cystectomy: a contemporary population-based analysis. J Geriatr Oncol. 2019;10:623–631. doi: 10.1016/j.jgo.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Chang S.S., et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198:552–559. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahmy O., et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. 2018;36:43–53. doi: 10.1016/j.urolonc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Koga F., Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: a contemporary review. Int J Urol. 2012;19:388–401. doi: 10.1111/j.1442-2042.2012.02974.x. [DOI] [PubMed] [Google Scholar]
- 10.Sapre N., Anderson P., Foroudi F. Management of local recurrences in the irradiated bladder: a systematic review. BJU Int. 2012;110(Suppl 4):51–57. doi: 10.1111/j.1464-410X.2012.11476.x. [DOI] [PubMed] [Google Scholar]
- 11.van Rhijn B.W., et al. Surveillance with microsatellite analysis of urine in bladder cancer patients treated by radiotherapy. Eur Urol. 2003;43:369–373. doi: 10.1016/s0302-2838(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Beltran A., Luque R.J., Mazzucchelli R., Scarpelli M., Montironi R. Changes produced in the urothelium by traditional and newer therapeutic procedures for bladder cancer. J Clin Pathol. 2002;55:641–647. doi: 10.1136/jcp.55.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens C.L., VandenBussche C.J., Burroughs F.H., Rosenthal D.L. A review of reporting systems and terminology for urine cytology. Cancer Cytopathol. 2013;121:9–14. doi: 10.1002/cncy.21253. [DOI] [PubMed] [Google Scholar]
- 14.Kandimalla R., et al. A 3-plex methylation assay combined with the FGFR3 mutation assay sensitively detects recurrent bladder cancer in voided urine. Clin Cancer Res. 2013;19:4760–4769. doi: 10.1158/1078-0432.CCR-12-3276. [DOI] [PubMed] [Google Scholar]
- 15.Beukers W., et al. FGFR3, TERT and OTX1 as a urinary biomarker combination for surveillance of patients with bladder cancer in a large prospective multicenter study. J Urol. 2017;197:1410–1418. doi: 10.1016/j.juro.2016.12.096. [DOI] [PubMed] [Google Scholar]
- 16.Zuiverloon T.C., et al. Combinations of urinary biomarkers for surveillance of patients with incident nonmuscle invasive bladder cancer: the European FP7 UROMOL project. J Urol. 2013;189:1945–1951. doi: 10.1016/j.juro.2012.11.115. [DOI] [PubMed] [Google Scholar]
- 17.van Kessel K.E.M., et al. A urine based genomic assay to triage patients with hematuria for cystoscopy. J Urol. 2020;204:50–57. doi: 10.1097/JU.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 18.Kompier L.C., et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuiverloon T.C., et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16:3011–3018. doi: 10.1158/1078-0432.CCR-09-3013. [DOI] [PubMed] [Google Scholar]
- 20.James N.D., et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 21.Royce T.J., et al. Trimodality therapy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2021;19:362–368. doi: 10.1016/j.clgc.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Ruiter B.M., et al. A multicenter retrospective cohort series of muscle-invasive bladder cancer patients treated with definitive concurrent chemoradiotherapy in daily practice. Eur Urol Open Sci. 2022;39:7–13. doi: 10.1016/j.euros.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verschoor N., Heemsbergen W.D., Boormans J.L., Franckena M. Bladder-sparing (chemo)radiotherapy in elderly patients with muscle-invasive bladder cancer: a retrospective cohort study. Acta Oncol. 2022;61:1019–1025. doi: 10.1080/0284186X.2022.2101381. [DOI] [PubMed] [Google Scholar]
- 24.Zuiverloon T.C.M., et al. Recommendations for follow-up of muscle-invasive bladder cancer patients: a consensus by the international bladder cancer network. Urol Oncol. 2018;36:423–431. doi: 10.1016/j.urolonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 25.van der Aa M.N., et al. Patients' perceived burden of cystoscopic and urinary surveillance of bladder cancer: a randomized comparison. BJU Int. 2008;101:1106–1110. doi: 10.1111/j.1464-410X.2007.07224.x. [DOI] [PubMed] [Google Scholar]
- 26.Albani J.M., Ciaschini M.W., Streem S.B., Herts B.R., Angermeier K.W. The role of computerized tomographic urography in the initial evaluation of hematuria. J Urol. 2007;177:644–648. doi: 10.1016/j.juro.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 27.Almallah Y.Z., Rennie C.D., Stone J., Lancashire M.J. Urinary tract infection and patient satisfaction after flexible cystoscopy and urodynamic evaluation. Urology. 2000;56:37–39. doi: 10.1016/s0090-4295(00)00555-0. [DOI] [PubMed] [Google Scholar]
- 28.Liberman D., Mehus B., Elliott S.P. Urinary adverse effects of pelvic radiotherapy. Transl Androl Urol. 2014;3:186–195. doi: 10.3978/j.issn.2223-4683.2014.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuiverloon T.C.M., de Jong F.C., Theodorescu D. Clinical decision making in surveillance of non-muscle-invasive bladder cancer: the evolving roles of urinary cytology and molecular markers. Oncology (Williston Park) 2017;31:855–862. [PubMed] [Google Scholar]
- 30.Gopalakrishna A., et al. Anticipatory positive urine tests for bladder cancer. Ann Surg Oncol. 2017;24:1747–1753. doi: 10.1245/s10434-016-5763-5. [DOI] [PubMed] [Google Scholar]
- 31.Zuiverloon T.C., et al. Optimization of nonmuscle invasive bladder cancer recurrence detection using a urine based FGFR3 mutation assay. J Urol. 2011;186:707–712. doi: 10.1016/j.juro.2011.03.141. [DOI] [PubMed] [Google Scholar]
- 32.van Rhijn B.W.G., et al. FGFR3 mutation status and FGFR3 expression in a large bladder cancer cohort treated by radical cystectomy: implications for anti-FGFR3 treatment? Eur Urol. 2020;78:682–687. doi: 10.1016/j.eururo.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Knowles M.A. FGFR3—a central player in bladder cancer pathogenesis? Bladder Cancer. 2020;6:403–423. [Google Scholar]
- 34.Knowles M.A., Williamson M. Mutation of H-ras is infrequent in bladder cancer: confirmation by single-strand conformation polymorphism analysis, designed restriction fragment length polymorphisms, and direct sequencing. Cancer Res. 1993;53:133–139. [PubMed] [Google Scholar]
- 35.Wolff E.M., et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010;70:8169–8178. doi: 10.1158/0008-5472.CAN-10-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavalieris L., et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J Urol. 2017;197:1419–1426. doi: 10.1016/j.juro.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Elsawy A.A., Awadalla A., Elsayed A., Abdullateef M., Abol-Enein H. Prospective validation of clinical usefulness of a novel mRNA-based urine test (Xpert® Bladder Cancer Monitor) for surveillance in non muscle invasive bladder cancer. Urol Oncol. 2021;39:77.e9-16. doi: 10.1016/j.urolonc.2020.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.