Abstract
Introduction
Osteoporosis is a geriatric metabolic ailment distinguished by low bone mineral density (BMD) and strength with enhanced micro-architectural retrogression of the extracellular matrix, further increasing bone fragility risk. Osteoporotic fractures and associated complications become common in women and men after 55 and 65 years, respectively. The loss in BMD markedly enhances the risk of fracture, non-skeletal injury, and subsequent pain, adversely affecting the quality of life.
Methods
Data summarised in this review were sourced and summarised, including contributions from 2008 to 2023, online from scientific search engines, based on scientific inclusion and exclusion criteria.
Results
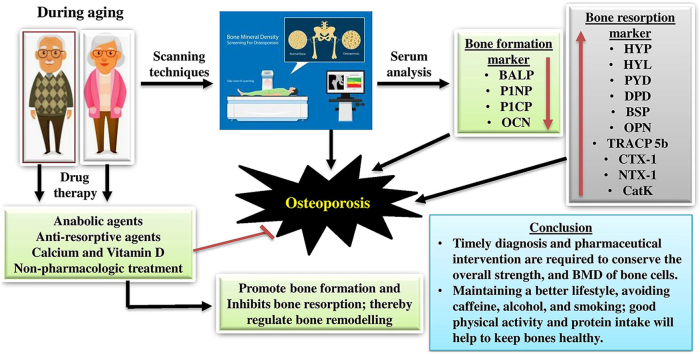
Biochemical serum markers such as BALP, collagen, osteocalcin, and cathepsin-K levels can reveal the osteoporotic status. DEXA scan techniques evaluate the whole body's BMD and bone mineral content (BMC), crucial in osteoporosis management. Anabolic and anti-osteoporotic agents are commonly used to enhance bone formation, minimize bone resorption, and regulate remodelling. The challenges and side effects of drug therapy can be overcome by combining the various drug moieties.
Conclusion
The current review discusses the management protocol for osteoporosis, ranging from lifestyle modification, including physical exercise, pharmaceutical approaches, drug delivery applications, and advanced therapeutic possibilities of AI and machine learning techniques to reduce osteoporosis complications and fracture risk.
Keywords: Artificial intelligence, Anabolic agents, Bisphosphonates, Bone mineral density, Calcium, Osteoporosis
Graphical abstract
1. Introduction
Osteoporosis (OP) is an inevitable chronic metabolic bone density ailment affecting human beings irrespective of sex. A typical feature of OP is severe bone loss without any symptoms or health issues; most probably, the first “symptom” is a broken bone in the patient, which further leads to other complicated health problems and even death in some cases. It is an inevitable old-age health issue, affecting 200 million people worldwide and representing a significant public health problem related to bone health.1,2 According to the International Osteoporosis Foundation (IOF), one new OP injury or fracture occurs every 3 seconds, with an average of nearly 8.9 million fractures yearly.3
As per the World Health Organization (WHO), BMD measurement is a mandatory prerequisite to monitoring bone strength. According to the IOF, even in European countries, the limited availability of bone densitometers, personal restrictions during screening procedures, public unawareness of BMD testing, and limited reimbursement make the treatment tedious.4 In the United States of America (USA), over 61 million men and women above 60 years of age are presently suffering from osteoporotic complications, and about 30% of all postmenopausal women (PMW) have osteoporosis. It is estimated that the treatment expense of osteoporotic fractures and associated morbidity of patients is between 5 and 6.5 trillion USD in Canada, the USA, and Europe.5 The percentage of aged people is increasing worldwide, which leads to increased fracture risk and OP-related disorders, both the incidence of osteoporotic fracture and its prevalence. Oden et al. (2015) reported that in 2010, nearly 158 million people were at high risk of osteoporotic fracture, and by 2040, it is estimated that the number will double due to the ageing population worldwide.6
Clinical surgeons can easily assess bone health status by checking the history of fractures, metabolic deficits, or complications incorporated with BMD, monitoring the bone scans, and evaluating the FRAX score and vertebral fracture assessment (VFA). An extensive preoperative bone health monitoring protocol is advised in osteoporotic patients before starting pharmacological aids to improve bone health.7 Current therapeutic strategies for OP patients involve supplementing calcium and vitamin D, inhibiting osteoclast proliferation and induction of osteoclast apoptosis by anti-resorptive agents, and administering anabolic agents that stimulate osteoblastic bone formation.8,9 The review provides an overall understanding of biochemical diagnostic parameters and scanning techniques to evaluate the osteoporotic status of patients. Proper diagnosis to access the BMD in OP patients with higher fracture possibility and availability of advanced technology, including absorptiometry criteria, is required to follow up the treatment options as a second-line treatment approach. A comprehensive review of drug efficacy, sequential and combined therapy advanced drug delivery, and AI therapeutic algorithms are required to explore the efficacy of nanoparticle encapsulated and aerosol-based treatment options for osteoporosis management and the limitations that need to be addressed in future research.
2. Methods
2.1. Data acquisition
Data summarised in this current review were gathered from various online scientific search platforms, including Google Scholar, Medline, Mendeley, PubMed, Research Gate, Scopus, and Springer Link, using keywords such as ‘Osteoporosis,’ ‘osteoporotic complications,’ ‘biochemical screening protocols for osteoporosis’, ‘scanning techniques for osteoporosis diagnosis’, ‘role of drugs in osteoporosis management’; ‘drug-induced osteoporotic complications,’ and ‘pharmacological and non-pharmacological treatment for osteoporosis; ‘role of AI in osteoporosis management, the role of physical exercise and habits in osteoporosis management’; ‘treatment protocol in osteoporosis’; ‘Role of anabolic agents in osteoporosis treatment.’ This review evaluated and cited 114 references published online between 2008 and 2023. The Inclusion and exclusion criteria used for the literature survey for the current comprehensive review are summarised as follows (Fig. 1).
Fig. 1.
Inclusion and exclusion criteria used for data acquisition for the manuscript; where; OP: Osteoporosis; WHO: World Health Organization.
3. Results
3.1. Aetiology of osteoporosis
Osteoporosis is a metabolic bone disease involving the loss of BMD, which deteriorates the bone tissues and results in porous bone, sequentially increasing the chance of fracture. In the old age population, the deficiency of calcium, phosphorus, and magnesium minerals enhances bone synthesis disorder and bone loss, which increases fracture possibility, especially hip and spine fractures. The hormones such as estrogen, testosterone, calcitonin, and parathyroid hormone are required for the homeostasis of bone health during ageing, and the deficiency of these hormones also leads to severe bone resorption and loss.10 The prevalence of osteoporotic fracture is significantly increasing due to higher life span, which directly affects the economic status of patients and leads to a financial burden.11 Various factors influence the BMD and quality of bone cells, such as hereditary, age, sex, health status, medication, and organ transplantation, thereby contributing to the risk of osteoporotic fracture. Table 1 summarizes the complete assessment of BMC and BMD is necessary to rule out the possibility of fracture risk.4,12
Table 1.
Factors increase the risk for osteoporosis and fracture risk include.
| Factors | Reasons |
|---|---|
| Sex | Women are more prone to osteoporotic fractures comparing to man due to lower peak BMD. |
| Age | As age progresses, bone resorption overcomes bone formation and increases the fracture risk. |
| Body size | Slim, lean-boned humanshave lower BMD, are at greater risk to osteoporosis |
| Race | Asian women, White men and women are at highest risk. African and American humans have a lower risk. |
| Family history | Risk for osteoporosis and fractures increase if one of your parents has a history of osteoporosis or hip fracture |
| Changes to hormones |
|
| Diet | Diet low in calcium and vitamin D increases the risk for osteoporotic fractures. Excessive dieting or poor protein intake also enhance the risk of bone loss and results in osteoporosis |
| Other diseases | Hormonal diseases (hypogonadism, hyperthyroidism, hyperparathyroidism, cushing's syndrome and early menopause), gastrointestinal diseases, rheumatoid arthritis, cancer, coeliac disease and inflammatory bowel disease increases the risk of osteoporotic fractures |
| Medications | Long-term use of glucocorticoids, adrenocorticotropic hormone, antiepileptic agents, cancer medications, proton pump inhibitors, and thiazolidinediones, can enhance the risk of fracture possibility and lead to osteoporosis. |
| Lifestyle | A healthy lifestyle is required for maintaining bones strength.
|
3.2. Biochemical markers for diagnostic protocols
Biochemical bone turnover markers are products released from osteoblasts, osteoclasts, or collagen breakdown pathways. Both osteoblast and osteoclast markers can be evaluated to understand the variations in bone remodelling.13 Other commonly used evaluation techniques for diagnosing OP and fracture risk may involve history-based treatment, including aetiology, taking the patient's hereditary pattern, physical examination, and biochemical marker study in serum and urine samples. The changes in serum and urine biochemical markers can be easily monitored for OP diagnosis and follow-up measures as prognosis. BFMs are the intermediate products released during various stages of osteoblastogenesis by active osteoblasts and reveal the status of osteoblast function, maturation, and differentiation (Table 2). Table 3 summarizes the BRMs, which are collagen degradation products produced during bone deterioration.13,14 Bone breakdown disorders, including osteoporosis, the BRMs were found at higher levels than standard conditions.14
Table 2.
Bone formation markers.
| Bone formation markers | Tissue origin | Sample | Significance |
|---|---|---|---|
| Alkaline phosphatase (ALP) | Bone, Liver | Serum | Reliable bone marker in subjected to normal liver function |
| Bone alkaline phosphatase (BALP) | Bone | Serum | Sensitive and reliable marker of bone metabolic activity |
| Procollagen type 1 N-terminal propeptide (P1NP) | Bone, Skin | Serum | Sensitive biomarker of bone formation (Specific osteoblast and fibroblast derivative) |
| Procollagen type 1 C-terminal propeptide (P1CP) | Bone, skin, soft tissue | Serum | Indicator of COL1A1 synthesis in bone matrix formation |
| Osteocalcin | Bone, Thrombocytes | Serum | Late marker of bone formation |
Table 3.
Bone resorption biomarkers.
| Boneresorption markers | Tissue origin | Sample | Significance |
|---|---|---|---|
| Hydroxyproline (HYP) | Bone, skin, cartilage, soft tissue | Urine | Non-specific bone resorption biomarker of collagen turnover |
| Hydroxylysine (HYL) | Bone, Skin, Soft tissue and serum complement | Urine | Non-specific bone resorption biomarker of collagen turnover |
| Pyridinoline (PYD) | Bone, Tendon, Cartilage | Urine | Non-specific bone resorption biomarker |
| Deoxypyridinoline (DPD) | Bone, Dentine | Urine | Specific biomarker for bone resorption |
| Bone Sialoprotein (BSP) | Bone, calcified cartilage, Dentine, cementum | Serum | Resorption biomarker for osteoporosis assessment. |
| Osteopontin | Bone, Blood | Serum | BRM; Prognosis of PMO |
| Tartrate-resistant acid phosphatase 5b (TRACP 5b) | Bone, Blood | Plasma and serum | Enzyme marker for bone resorption |
| Carboxy-terminal crosslinkedtelopeptide of type 1 collagen (CTX-1) | Tissue contain COL1A1 | Urine, Serum | Bone resorption marker; Prognosis of PMO |
| Amino-terminal crosslinkedtelopeptide of type 1 collagen (NTX-1) | Tissue contain COL1A1 | Urine (α- and β-isoform), Serum (β-isoform) | Bone resorption marker; Prognosis of PMO |
| Cathepsin K (CatK) | Bone, Blood | Serum | Specific biomarker for bone resorption |
3.3. Scanning diagnosis criteria
Based on BMD and bone mineral content (BMC), OP has been clinically differentiated. Measuring the BMD values provides an exact idea regarding bone strength, mass, density, and overall bone health status. Scanning techniques with advanced imaging techniques will provide a clear-cut criterion to diagnose the fracture risk and help to monitor OP drug therapy's prognosis.15 The different scanning techniques available are as follows; The commonly used BMD test for diagnosing OP is a dual-energy X-ray absorptiometry (DEXA) scan. It is an invasive technique using an X-ray, which measures the BMD of the hip and lumbar spine as a densitometric diagnosis of OP.16 The signs and criteria for BMD measurements by DEXA scan are tabulated in Table 4.17 According to the WHO guidelines, a BMD value of 2.5 standard deviations or higher (T-score −2.5) lower than the mean value for young, healthy women, as quantified by DEXA, is considered an osteoporotic patient (Table 4). DEXA and T-score for BMD are recommended diagnostic criteria for osteoporotic patients.18
Table 4.
World Health Organization classifications for bone density levels.
| Health status | Hip BMD T-score (SD) |
|---|---|
| Normal | (+1 to −1) |
| Low bone mass (Osteopenia) | (-1 to −2.5). |
| Osteoporosis | (≤−2.5) |
| Severe osteoporosis | (≥- 2.5) |
Note: If the results of BMD test show the following T-score, chance for fracture is high and regular monitoring and doctor advice is necessary to take available therapy to prevent fractures.
The common WHO criterion does not apply to children and men younger than 50, and PMW, termed Z-score, is validated in those cases. A Z-score (based on age and sex) above −2.0 is expected in osteoporotic patients, according to the International Society for Clinical Densitometry (ISCD). Along with the Z-score, other bone density measurements are also used to diagnose osteoporosis in younger men, PMW, and children to avoid false positivity in treatment.19 Instead of these methods, vertebral fracture assessments (VFAs) and bone scans are also used to monitor the fracture risk during OP. Advanced chronic kidney disease (CKD) associated with osteoporosis exhibited an increased possibility of bone fractures. The complicated CKD patient's bone status can be regularly monitored due to fracture possibility by DEXA imaging.20 Peripheral DXA devices are small portable machines used to measure BMD on the periphery of the skeleton, especially the wrist, heel, or finger. The pDXA is associated with radiation exposure and cannot predict fracture risk in men.21
Quantitative computed tomography (QCT) is a BMD measurement technique in which the CT scanner results in a 3D image. The QCT is standardized using solid phantoms of calcium hydroxyapatite, a highly dynamic crystal entity used in medical imaging for quality control equipment standardization. After calibration, the Hounsfield unit (HU) is comparatively utilized to evaluate the radio density and employed for interpreting computed tomography (CT) images to BMD values.22 Compared to DEXA, the radiation exposure of CT is 100–300 millirem, with lower precision and accuracy but within the acceptable range. A non-invasive diagnostic method called quantitative ultrasound (QUS) densitometry uses broadband ultrasound attenuation and speed of sound at the heel, tibial plateau fracture, knee and other peripheral skeletal locations to measure BMD directly and estimate the bone mineral status. Due to its safe nature and lack of radiation exposure, it is commonly used in children. It can also be used in PMWand men in their 70s for validating the vertebral, hip, and overall fracture risk and non-vertebral fractures, respectively.23 QUS measurement and digital X-ray radiogrammetry (DXR) are used to determine BMD and the geometric measurement of bone dimensions. Both techniques have limitations, and biochemical marker analysis is preferred to confirm the osteoporotic status.
3.4. Treatment options and prevention
Thanks to improved research and clinical studies, effective treatments and supportive therapy have been introduced. The standard protocol used for osteoporosis diagnosis and treatment can be visualized in (Fig. 2). There are several classes of medications used to treat OP, and commonly used therapeutic and supportive therapies (Fig. 4) are classified as follows. (see Fig. 3)
Fig. 2.
Osteoporosis treatment protocol; where; DEXA: Dual x-ray absorptiometry; FRAX: Fracture Risk Assessment Tool; HRT: Hormone replacement therapy.
Fig. 4.
Osteoporosis management by therapeutic agents.
Fig. 3.
Mechanism of action of bisphosphonates in inhibiting bone resorption and regulating bone homeostasis. where; HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A; BMP: Bonemorphogenetic proteins; NO: Nitric oxide.
3.4.1. Non-pharmacologic treatment
Osteoporosis and associated problems can be regulated by improving lifestyle and diet and performing physical exercise to maintain body balance.24 Consuming a nutritious food rich in calcium (milk, cheese, and broccoli) and vitamin D (fatty fish, cereals, and milk), magnesium (lima beans, orange juice, legumes, whole grains, spinach), Potassium (dried fruits, nuts, spinach, oatmeal, banana, peanut butter), Vitamin K (green leafy vegetables, soybean), Fruits (avocado, banana, cantaloupe), vegetables and sufficient exposure to sunlight, is beneficial for maintaining good bone health. Avoiding the usage of sodium and caffeine will also enhance bone health.25 Various studies reported that a balanced diet rich in minerals and micronutrients helps to inhibit BMD loss in the elderly regardless of sex. Staying active by participating in physical activities such as weight-bearing exercises and walking and restricting the usage of alcohol and smoking is necessary to maintain bone health.26
During ageing, the total strength of bones and muscles reduces due to decreased physical activity and increased bone resorption.27 The cost-effective medical management of OP includes physical training schedules to prevent falls through improved balancing, locomotion, bone strength, and the proper supportive medication, as illustrated in Fig. 6. Studies reported that exercise at any age could achieve excellent osteo-positive effects and improve bone health and BMD. Various exercises, including weight training, trekking, climbing stairs, and walking, enhance the bones and muscles’ strength and increase bone health and density.28 The primary benefit of physical activity in osteoporotic patients may be enhancing muscle strength and overall coordination, decreasing the frequency of falls and improving gait.
Fig. 6.
Overall pharmacological and non-pharmacological strategies used for osteoporosis management. Where: Cat K: Cathepsin K; PTH peptides: Parathyroid hormone-related peptide; HRT:Hormone replacement therapy; SERMs: Selective estrogen receptor modulators. Instead of pharmacological treatment options; the non-pharmacological treatment options like kyphoplasty, vertebroplasty, hip protectors, and physical exercise, along with healthy diet and avoid smoking and alcohol usage for better osteoporosis management.
Vertebroplasty is an invasive procedure that injects cement into a cracked or broken spinal bone to eliminate pain caused by compression fractures. Percutaneous vertebroplasty has become widely accepted as a safe and minimally invasive protocol for treating painful vertebral body compression fractures by injecting polymethyl methacrylate cement.29 By injecting the cement, kyphoplasty controls back pain using an inflatable balloon tamp to stabilise the vertebral fracture and restore vertebral body anatomy. Vertebroplasty and kyphoplasty can effectively achieve height restoration.30 Vertebroplasty and kyphoplasty are minimally invasive approaches not proposed as first-line treatments for vertebral fractures. The long-term benefits of overall pain management of both the techniques are challenging and studies revealed that it also enhance the possibility of vertebral fractures in adjacent vertebrae.31 The significant limitations of both procedures are expensive and not affordable to the commoner, which results in a lack of long-term clinical data.18
Hip fractures are one of the significant causes of morbidity and complications in the aged population associated with osteoporosis. The hip fracture alone can cause a predicted global expense of 4.5 million USD by 2050.32 Hip protectors are usually polypropylene and minimize the chance of hip fractures associated with abnormal gait. The use of hip protectors in aged patients safeguard from fracture and minimize the constriction to the post-operative wound in a vulnerable age group.33 However, hip protectors may slightly increase the possibility of pelvic fractures in high risk popluation.34 In the old age population with acute vertebral compression fractures, vertebroplasty can quickly reduce pain and restore function and sagittal balance. As the vertebral compression progresses without checkpoints, the chances of vertebroplasty recovery are limited.35 Timely diagnosis using BMD screening, with proper medications, supportive aids, improving physical activity, lifestyle modification, and a healthy diet, can control and prevent osteoporosis complications and fracture risk. (see Fig. 5)
Fig. 5.
Mechanism of action of various therapeutic agents in controlling osteoporotic complications and regulating bone remodelling. Where: Macrophage colony-stimulating factor (M-CSF); Receptor activator of nuclear factor kappa beta (RANK); Osteoprotegerin (OPG), Osteoprotegerin ligand (RANKL); Parathyroid hormone 1 receptor (PTH1R), Cathepsin K (CatK). The balance of the OPG, RANKL and RANK maintains bone remodelling. OPG bind with RANK and inhibit the binding of RANKL and controls the bone resorption. Hemopoietic stem cell lineage differentiates into osteoclast (OC) and promotes bone resorption, the anti-resorptive agents such as bisphosphonates, calcitonin, HRT, SERMs, CatK act on OC and prevents bone resorption. Osteoprogenitor cell (mesenchymal stem cell) differentiates into osteoblast (OB), the anabolic agents such as teriparatide and strontium ranalete (SrRa) acts on OB and promote bone formation (SrRa inhibits bone resorption also). Abaloparatide promote bone formation through PTHR1 signalling pathway. Romosozumab act as sclerotin, a protein produced by osteocyte and promote bone formation. Denosumab inhibits the binding of RANKL and inhibits OC differentiation.
3.4.2. Pharmacologic treatment
Various effective drug therapies with diverse mechanisms of action are used for OP management. Calcium is an essential element for bone cell maturation and extracellular matrix mineralisation. The mineral phase constituting calcium and phosphorus joins with the cellular matrix to form calcium-phosphate crystals.36 The calcium hydroxyapatite crystals form the framework for the bone cells and provide impressive tensile strength. During OP, the total calcium pool of the body gets depleted and results in lower BMD, which further increases the chance of hip and spine fractures. Bone remodelling is well coordinated by the bone-forming (osteoblast) and bone resorption cells (osteoclast). The remodelling is vital for maintaining appropriate physiology, cellular homeostasis, and microarchitecture of the bone.37 CaSR is a calcium-sensitive transmembrane spanning extracellular protein which can regulate the recruitment, differentiation, and viability of bone-forming and bone resorption cells by activating multiple CaSR-mediated intracellular signalling. Biological research on CaSR and similar cation-sensing receptors in bone cells revealed the possibilities of these drug targets for OP management.38 Calcium supplementation is beneficial to reduce bone loss and strength in OVX experimental rodents and partially reimpose the level of estradiol, regulate lipid metabolism, induce the production of higher levels of glycerophospholipids, decreasing trabecular bone loss, and improve the BMD of the femur.39
Adequate calcium levels and optimal vitamin D concentrations have been vital for regulating well-balanced BMD and bone metabolism. Vitamin D reduces bone loss, increases overall BMD and lowers fracture risk, especially in the older population.40 The concentration of serum calcium level is strictly maintained at the physiological range by the synergetic action of calciotropic hormones such as parathyroid hormone (PTH), Fibroblast Growth Factor 23(FGF23), 1,25-dihydroxy vitamin D (calcitriol), and calcitonin. Calcitriol (active form of vitamin D) is approved for treating PMO at a dose of 0.25 μg twice daily.41 Calcitriol monotherapy can improve BMD in elderly osteoporotic Chinese patients by inhibiting bone resorption. Calcitriol can also regulate bone turnover markers (BTMs) and significantly improve muscle and bone strength. Calcitriol, combined with other therapeutic osteo-protective agents, was well endured and capable of improved bone-preserving effects compared with calcitriol alone in areas including BMD, BTMs, and fracture incidence.41,42 Different medications are available to treat osteoporosis are anti-resorptive agents include bisphosphonates (BPs), calcitonin, and estrogen; selective estrogen receptor modulators (SERMs), hormone replacement therapy (HRT), teriparatide, RANKL (receptor activator of nuclear factor κB ligand) inhibitors, and monoclonal antibodies such as denosumab, as summarised in Fig. 6. Anti-osteoporotic drugs slow down resorption, promote bone formation, and regulate the remodelling process by inhibiting osteoclast number and differentiation. However, anabolic agents such as PTH peptides increase the growth of bone cells and stimulate osteoblast activity to promote bone formation, thereby increasing the overall BMD. These pharmacologic agents’ commercial names, dosage, administration period, and mechanism of action are summarised (Table 5, Fig. 4).1,43, 44, 45
Table 5.
Regularly used anti-osteoporotic drugs for osteoporosis management.
| Drugs | Generic drugs | Men (dosage) | Women (dosage) |
|---|---|---|---|
| Bisphosphonates | |||
| Alendronate | Fosamax, Fosamax Plus D, Binosto | 5 mg; 10 mg daily | 5 mg; 10 mg daily |
| Ibandronate | Boniva | 3 mg IV every 3 months | 2.5 mg daily; 150 mg once monthly |
| Risedronate | Actonel, Atelvia | 5 mg daily or 35 mg once weekly | 5 mg daily or 35 mg once weekly |
| Zoledronic acid | Reclast | 5 mg IV once yearly | 5 mg IV once yearly |
| RANKL Inhibitor | |||
| Denosumab | Prolia | 60 mg/mL | 60 mg/mL |
| Estrogen Agonist/Antagonists | |||
| Raloxifene | Evista | – | 60 mg/day |
| Conjugated estrogens/bazedoxifene | Duavee (Pfizer) | – | 0.45–20 mg/day |
| Parathyroid Hormone Analogues | |||
| Recombinant human PTH 1-34 | Teriparatide (Forteo); Abaloparatide (Tymlos) | 20 μg/day IV for 2 years | 20 μg/day IV for 2 years |
| Recombinant human PTH 1-84 | 100 μg/day IV for 2 years | 100 μg/day IV for 2 years | |
| Calcitonin-Salmon | Generic | 200 IU/mL | 1 spray daily |
| Miacalcin (Novartis) | 200 IU/mL | 100 IU daily | |
Where; μg/day: Microgram/day; IU/mL: International Units Per Milliliter; IV: Intravenous; mg/ml:milligrams per milliliter.
The main target of anti-resorptive treatment is minimising bone microdamage and upgrading bone strength and structural integrity.46 Mechanism of action of these drugs is to reduce resorption or regulate bone remodelling, and an ideal anti-resorptive agent must have long-term biological efficacy with minimum side effects. BPs are analogues of inorganic pyrophosphate and are administered orally or intravenously, commonly used as a first-line therapeutic agent for osteoporosis treatment. BPs can directly inhibit bone resorption (BR) and promote osteoclast apoptosis.47 Due to the unique chemical structural similarity with pyrophosphate, BPs directly attach to hydroxyapatite binding sites (active resorption sites) on bone surfaces and inhibit BR. The extreme affinity toward hydroxyapatite has been exploited as radiolabelled BPs for bone scanning techniques. The different types of BPs used for OP treatment are the following: alendronate and risedronate (tablet), ibandronate (tablet or IV infusions), and zoledronic acid (IV infusion), which can enhance bone strength and minimize BMD loss.48
Alendronate (alendronate sodium) is an approved drug for treating postmenopausal osteoporosis (PMO). In PMO, daily alendronate administration has exhibited a notable reduction in vertebral, non-vertebral, and hip fractures. Bisphosphonate binds to hydroxyapatite crystals of the bone mineral matrix, reducing osteoclast-mediated BR or decreasing bone matrix breakdown. The dual mechanisms provide a well-regulated homeostasis for mineral reabsorption and bone turnover.49 Alendronate supplementation significantly increases BMD of the spine, hip, and total body and is used to cure OP in men. It is also utilized to control the complications associated with glucocorticoid-induced OP @ 5 mg/day.
Ibandronate is a specific and powerful nitrogen-containing bisphosphonate used to treat PMO.50 Ibandronate reduces bone resorption by decreasing osteoclast progenitor differentiation and maturation and promotes osteoclast cell death, which leads to an increased BMD. The mechanism of action of ibandronate on skeletal tissue is based on its affinity interactions with the hydroxyapatite crystal, which is part of the extracellular mineral matrix. Binding to the target site allows the drug moiety to interact with mature osteoclasts during resorption and potentiates selective osteoclast apoptosis. Daily oral ibandronate administration in PMO showed an anti-fracture efficacy for vertebral and non-vertebral fractures. BMD improved at the spine and hip, reducing BR to premenopausal levels.51 Monthly oral ibandronate administration can be a reliable and effective therapeutic approach in PMO patients with diabetic complications.52 Oral ibandronate therapy monthly or weekly BPs in PMO patients were more effective with lower baseline T scores and significantly lower risk of vertebral and hip fractures.53
Risedronate is another bisphosphonate drug that prevents bone breakdown and increases BMD. Risedronate is a pyridinyl bisphosphonate that specifically interacts with hydroxyapatite crystal residues and blocks osteoclast-directed BR. The bone turnover and BMD loss are minimized, while the bone formation and mineralisation are well-maintained. Risedronate is administered orally to treat PMO and osteoporosis in men to control vertebral and hip fracture possibility.54 Risedronate @ 5 mg daily administration significantly promote BMD, reduces vertebral and non-vertebral fractures and decreases the risk of hip and overall bone fractures in PMO. Risedronate and vitamin D3 are delivered together using deep lung targeted PAMAM-G5-NH2 dendrimers, which increases medication bioavailability by allowing absorption from the alveoli into the circulation and considerably raising serum calcium levels, phosphorus, and BMD in osteoporotic rats after 21 days of treatment.55 A recent study on risedronate sodium sublingual spray administration in glucocorticoid-induced models in rats exhibited a maximum drug release and better efficacy in combination with mucoadhesive polymers. It can be considered for novel drug delivery in managing OP.56
Zoledronic acid (ZA) is a nitrogen-containing, 3rd generation BPs utilized to treat PMO as an IV infusion. ZA is an anti-resorptive agent with a high affinity for mineralised bone, specifically bind and interact with high bone turnover sites.57 ZA can also modulate osteoblast differentiation and increase bone mineralisation and formation, which leads to an increase in overall BMD and BMC to prevent glucocorticoid-induced osteoporosis.58 In mineralised bone and cartilage, ZA blocks the osteoclastic resorption by binding to bone-forming cells, which includes the suppression of osteoclast differentiation via repressing RANKL/RANK pathway and by regulating the macrophage differentiation into osteoclasts, and promote the initiation of reactive oxygen species (ROS) activated signalling.59 In a Japanese epidemiological study, a drug combination of ZA and eldecalcitol (ELD) for 24 months significantly promotes BMD of the lumbar spine, total hip, tibial plateau and femoral neck in osteoporotic patients than ZA alone administration.60 The ZA and calcium carbonate combination nanoformulation inhibits osteoclast activity and significantly reverses bone mass loss in and downregulates TRACP in ovariectomised mice. This opens a novel nanoplatform targeting OCs as a therapeutic option for osteoporosis therapy.61
Pamidronic acid (PA) or pamidronate disodium pentahydrate is a nitrogen-containing bisphosphonate used to prevent OP.62 The mechanism of action of PA, the inorganic pyrophosphate (PPi), binds with high affinity to hydroxyapatite crystals found within areas of remodelling bone. A public health care study in Brazil showed that the intravenous PA administration in PMWs with a high fracture possibility exhibited a non-uniform hike in the spine BMD; nevertheless, phase trials and clinical randomised studies are mandatory to validate its anti-fracture activity.63 PA inhibits the FPP synthase-mediated mevalonate pathway due to the limitation of prenylated GTP-binding mediator proteins, which directly suppress osteoclast activity and promote osteoclast apoptosis, similar to ZA. Bisphosphonate therapy exhibited linear improvement in BMD and reduction in CTx and P1NP in the PMW group, irrespective of diabetes mellitus (DM) complications.64
Denosumab is a genetically engineered human monoclonal antibody that acts as an anti-resorptive agent with a potent affinity towards human RANKL, the master controller of osteoclastic mechanism.65 Denosumab inhibits the formation and binding of RANK, thereby reducing BR and promoting bone mass. It is commonly used to treat PMO and osteoporosis in men with high fracture risk patients. Denosumab is administered subcutaneously, 60–120 mg in the upper arm, upper thigh, or abdomen, depending upon the severity of OP. Per oral contraceptive clinical practice guidelines, denosumab is the first-line treatment option for preventing vertebral, hip, and non-vertebral fractures.66 Denosumab (Prolia) is available to men and women as an IV infusion every six months. Clinical studies have found that an increase in BMD can still be observed after 10 years of denosumab treatment, which is better than other anti-osteoporotic drugs. At the same time, clinical studies have reported that denosumab discontinuation results in a steep decline in BMD due to a rebound mechanism in osteoclasts, leading to an increased possibility of fracture risk.67 Clinicians suggested that if denosumab is tolerated with the patient without considering side effects, it can continue because, in the event of discontinuation, stepwise combination therapy with other anti-osteoporotic drugs should be considered to reduce the enhanced BMD loss and incidence of multiple vertebral fractures.64 Mochizuki et al. (2021) reported that romosozumab administration for three months is better than denosumab treatment in improving the BMD of the lumbar spine, with no adverse effect.68 Denosumab administration regulated BMD at the lumbar spine in osteoporotic patients after heart transplantation, and the fluctuations in serum calcium can be minimized by supplementing calcium regularly.69 BMD falls after a romosozumab dosing period of 12 months; in a comparative study, the findings revealed that denosumab could be a reliable, more effective drug candidate than ibandronate after the sequential withdrawal of romosozumab in PMO.70 The alternative and combined therapy using romosozumab and denosumab for 12 and 24 months, followed by romosozumab and alendronate for another 12 and 24 months, can minimize the frequency of new vertebral fractures and risk factors associated with future fragility fractures.71
HRT includes the supplementation of estrogen, testosterone, and androgen in osteoporotic patients to improve overall BMD. Estrogen therapy is commonly used in younger women and women who need to treat menopause symptoms.72 Estrogen is considered the master regulator of bone metabolism and can exert protective effects on micro-bone architecture. After menopause, the estrogen level drastically reduces, creating a significant gap between bone resorption and formation. The estrogen therapy and conjugated equine estrogen (CEE) increased bone density of the hip and spine and reduced bone turnover, with limited adverse effects.73 Studies revealed that the supplementation of medroxyprogesterone acetate, a CEE derivative (0.625 mg daily ±2.5 mg/day) can minimize the possibility of hip, vertebral and non-vertebral fractures in PMW. However, hormone therapy with oral CEE might have a higher risk of ischemic stroke than estradiol in postmenopausal Taiwanese women.74
Estrogen therapy regulates bone metabolism at the cellular level on osteoblasts, osteoclasts, and osteocytes, directing to maintenance of bone formation, reduced BR, and bone remodelling suppression, respectively. Estrogens act as anti-resorptive agents, reducing the activation of the bone remodelling cycle; the diverse therapeutic applications of estrogen combined with other hormones like progesterone or androgens lead to new approaches to prevent and treat PMO, as life-saving hormone therapy for treating OP.8 Testosterone administration can improve overall BMD in older adults. During early postmenopausal period administration of menopausal hormone therapy (MHT) effectively controls hot flashes and night sweats and regulates other complications associated with the genesis of osteoporosis and fracture risk, but the possibility of breast cancer exists. MHT should be considered in women for a short period with premature estrogen deficiency for the symptomatic treatment of menopause and enhanced risk of bone mass and mineral loss associated with osteoporotic fractures.75
SERMs are synthetic estrogen receptor ligands used to boost estrogen levels in the body and treat osteoporosis and PMO.76 Depending on the interactive sites in the target tissue, SERMs act as estrogen agonists and antagonists. The commonly used SERMs for PMO are raloxifene and bazedoxifene, which can induce osteoclast apoptosis, prevent bone loss, improve BMD, and decrease vertebral fracture risk. Barrionuevo et al. (2019) reviewed that the three available SERMs can significantly reduce non-vertebral, vertebral and spine fractures in PMW.77 All the SERMs are also found to be effective for breast cancer treatment. Due to the dual protective mechanism, they are considered good candidates for treating OP in women with a high possibility of fracture risk but without previous history of thromboembolic disease.78 Raloxifene, an FDA-approved second-generation SERM, has a positive effect of estrogens on the skeletal systems by increasing BMD and bone mass by decreasing BR. The overall mechanism of action of SERM is by regulating bone remodelling, structurally recovering BMD, and decreasing the risk of vertebral and spine fractures.79 Raloxifene is a widely used estrogen analogue to prevent and cure PMO @ one tablet daily for five years. Raloxifene decreases the risk of estrogen-dependent breast cancer by 65 per cent, minimising the chance of invasive breast cancer in women with or without OP.80
Mechanisms of action of anabolic agents are mainly by promoting bone formation rather than bone resorption, which is summarised in Fig. 5. The commonly used anabolic agents are as follows: Administration of PTH peptides have anabolic effects with increased bone formation by stimulating the different phases of the remodelling process. The human parathyroid hormone (hPTH) (1–34) and (1–84) amino acid fragments are novel bone anabolic agents capable of developing bone regeneration in osteoporotic patients. Teriparatide [PTH (1-34)] or PTH analogue, and abaloparatide [PTHrP (1-34)] or PTH-related protein analogue, are the FDA-approved anabolic medicines for the treatment of osteoporosis.81 Hence, anabolics can be a relevant alternative for patients with severe and glucocorticoid-induced osteoporosis. The synthetic PTHrP, abaloparatide (ABL) and teriparatide have an enhanced affinity to bind with the parathyroid hormone receptor 1 (PTHR1) on osteoblasts to promote bone formation. Along with teriparatide, ABL and romosozumab are also used as osteoanabolic therapeutic agents in the USA.82
ABL, a synthetic analogue of PTH-related peptide (PTHrP), can promote bone formation with less hypercalcemic issues when compared with PTH (1–34). The primary amino acid sequence of ABL, binds with greater affinity to RG conformation through amino acid position 22, and positively favours more cAMP responses in PTHR1-expressing cells, which directly influence the osteoanabolic effects of ABL. ABL can activate the parathyroid hormone 1 (PTH1) receptor signalling pathway, stimulate bone formation, and treat PMO.83 ABL treatment regulates BMD loss after ovariectomy and orchiectomy and enhance fracture healing; at the same time, continuing the treatment period of 18 months can promote BMD and minimize fracture risk in PMOW; it also increases spine and hip BMD and diminishes the possibility of vertebral and non-vertebral fractures. Recent studies showed that ABL significantly increased cortical thickness in femoral Gruen zones. Hence, ABL can optimise bone health before total hip arthroplasty (THA).84 ABL administration for 18 months significantly increased acetabular BMD and bone strength, thereby minimising the acetabular fragility fractures and providing better stability and longevity of acetabular cups in PMOW with OP.85
Calcitonin is a synthetic hormone approved by the FDA to treat OP in PMW after five years of menopause when other osteoporotic drugs, especially estrogen or bisphosphonates, are ineffective. The polypeptide hormone can slow bone breakdown and suppresses BR, increase BMD in the spine, minimize the possibility of spine and hip fractures.25 However, calcitonin is less effective than BPs in increasing BMD and reducing bone turnover; for these reasons, it is not commonly used as a frontline drug for treating PMO. Salmon calcitonin is a synthetic peptide form of calcitonin hormone used to inhibit BR, resulting in increased BMD. Miacalcin (calcitonin-salmon) is a synthetic form of a hormone utilized for PMO treatment.86 Similarly, fortical (calcitonin-salmon) nasal spray developed via recombinant DNA technology used to treat osteoporosis in women at least five years past menopause. Nowadays, physicians do not prefer nasal spray due to the severe allergic reactions and rapid heartbeat variations it causes.
Strontium ranelate (SrRn) is a novel drug used to treat PMO. SrRn can promote bone formation and, to a lesser extent, inhibit bone resorption, favour remodelling, and increase bone mass. Thus, SrRn has been validated to be a potent compound for chronic treatment of PMO, especially in women older than 70.87 SrRn is an anti-resorptive agent used as a second-line OP treatment option in case of intolerance or contraindication to bisphosphonates therapy. It is commonly used to treat PMO and old-age osteoporosis in men. Phase III trials have revealed that the quality of bone mineralisation is maintained in the long term, and bone microarchitecture is mitigated with increased bone strength. The mechanism of action of SrRn enhances osteoblast replication and differentiation, downregulates osteoclast differentiation, increases the OPG/RANKL ratio directly or via a calcium-sensing receptor (CaSR), and promotes osteoclast apoptosis.88 Reginster et al. (2012) reported that SrRn diminishes the chance of vertebral and non-vertebral associated fractures, including proximal femur fractures, and the anti-fracture efficacy over ten years with strontium ranelate in PMO.89 SrRn @ 2 g daily significantly decrease the risk of hip and vertebral fractures in PMO patients. A remarkable decrease in hip fracture was noticed in a post hoc analysis of 74 age-old women groups with a femoral neck BMD T-score below −2.4 SD. Due to the increased risk of myocardial infarction (MI) with SrRn use, this drug is used only in combination with other medications and only recommended for men and PMOWs more prone to fracture.90 SrRn, now used as a generic medication, provides a functional alternative pharmaceutical approach for high fracture-risk osteoporosis patients, with reported cardiovascular disturbances in some patients.91 A cardiovascular safety issue was reported by the European Pharmacovigilance Committee in 2012. Because of the enhanced possibility of MI in SrRn-administered patients, this drug is not preferred to treat severe OP in men and PMO.
Sclerostin is a small osteo-protein originated from osteocytes that helps to regulate bone metabolism by inhibiting bone formation. Romosozumab comes under sclerostin inhibitors and is considered an anabolic agent. The FDA has approved Romososumab-aqqg (Evenity) as an IV infusion for PMO patients with a high fracture risk. The drug binds sclerostin, enabling new bone formation and decreasing bone breakdown.92 In humans, another monoclonal antibody against sclerostin, namely blosozumab (LY2541546) and setrusumab (BPS804), have been evaluated in clinical trials in patient groups with more prone to fracture and associated with low BMD, including PMO and osteoporosis in men.93 Blosozumab, currently in phase III clinical trials, exhibited that blosozumab can enhance BMD and may open the possibilities of future OP treatment.94 A recent review showed that anti-sclerostin antibodies improved total hip and femoral neck BMD when compared with alendronate and teriparatide and can be considered a notable therapeutic option for osteoporosis treatment.95
Cathepsin K (CatK) is an bone resorbing enzyme from the family of cysteine proteases that can increase the degradation activity of the proteinaceous portion of the bone matrix, such as type one collagen and elastin and promote bone resorption.96 CatK is one of the most advanced and reliable targets for anti-osteoporotic drug moieties. Odanacatib is an oral, selective inhibitor of CatK with high medicinal value in patients with PMO. The development of ODN was ultimately discontinued during clinical trials due to cardio-cerebrovascular complications and adverse effects.97,98 Pharmacologic CatK inhibition continuously increases BMD for ≤5 years of treatment, reduces bone loss and enhances bone strength and BMD at the spine and hip.97 CatK is expressed explicitly by osteoclasts and precisely degrades type I and type II collagen, the prime and crucial matrix protein in cartilage, the significant component of the organic bone extracellular matrix, and is regulated by the RANKL-RANK signalling pathway of osteoclastogenesis.97 In osteoclast precursors, initiation of the RANKL-RANK pathway activates transcription of CatK by the pro-osteoclastogenic transcriptional factor NFATc1 (nuclear factor of activated T cells). Duong et al. (2016) reported that the pre-clinical studies showed that CatK inhibition increases BMD, improves osteo-cellular microarchitecture, and maintains structural integrity.99 Various CatK inhibitors, including balicatib and odanacatib, have entered clinical trials to develop osteoporotic agents for metabolic bone disorders, such as PMO, with decreased bone resorption. Balicatib qualifies for Phase 2 clinical trials and provides essential understanding into the clinical pharmacology and target-specific interaction of CatK inhibitory mechanism and the curative role of CatK in the bone turnover associated with the remodelling cycle and overall mineral homeostasis in osteoporosis recovery.100 Most CatK inhibitors cannot qualify for phase 3 clinical trials due to severe cardio-cerebrovascular incidents, so physicians do not nowadays prefer these inhibitors for OP management.
However, Sr-based prosthetic combinations and products are widely used for OP management. The titanium dioxide (TiO2) nanotubes act as a carrier implant surface incorporated with Sr and cobalt by anodic oxidation and hydrothermal reaction, which shows an excellent osteo-integration capability in osteoporotic rats. It can induce bone formation, accelerate osseointegration, and open a novel strategy for OP management rather than surgery.101 A recent study on Sr and fluoride-substituted hydroxyapatite microspheres can stimulate the proliferation of human osteoblast-like cells (MG-63) invitro and act as a relevant treatment option for OP and BMD defects.102
3.5. Challenges and side effects of drug therapy
Various studies reported that the continuous or long-term use of an osteoanabolic drug, such as hPTH) (1–34) and (1–84) amino acid fragments can result in hyperparathyroidism, which further leads to hypercalcemia and severe bone loss.103 Regular intake of oral osteoporotic drugs can cause gastrointestinal tract discomfort, which reduces the absorption of drugs to the target regions. The hazardous effect of anti-sclerostin antibodies increased the incidence of injection-site reactions more than other osteoporotic drugs.95 The continuous intake of drugs also results in circulating unwanted biological cellular fragments, which further results in severe health complications, so novel drug delivery approaches are required to treat osteoporosis.104 The SrRn, CatK inhibitors, Calcitonin spray and etidronate are not recommended for the treatment of OP due to severe contraindications and harmful effects during clinical trials. Osteonecrosis of the jaws (ONJ) occurs commonly in osteoporotic patients who continuously take oral BPs and denosumab without availing of any drug holiday (DH).105
DH is commonly advised to osteoporotic patients by physicians after a course of drug therapy (nearly 1–5 years) to check how long the patient can withstand the recovery period without the support of a drug. DH also maintains drug sensitivity and helps to reduce possible side effects of long-term usage of osteoporotic drugs. Therefore, the duration of medication and DH should also be considered once therapeutic support is initiated. Depending upon the patient's recovery, DH can be availed if the fracture possibility is less and the T-score ≤2.5 after five years of oral BP administration. If the BF risk remains, the treatment may be extended for five years or changed to another osteoanabolic agent after treatment.106 Using oral BPs for long periods is usually interspersed with DH every 1–2 years to prevent the possibility of atypical femur fractures. DH can effectively reduce the risk of antiresorptive medication-related osteonecrosis of the jaw development in patients with osteoporosis.107 Denosumab is a monoclonal antibody administered by subcutaneous injection every 6 months. Similarly, teriparatide and ABL are supplemented subcutaneously for up to 2 years. Subcutaneous injections of romosozumab, an anti-sclerostin monoclonal antibody, can stimulate bone formation and inhibit resorption for 1 year. Research revealed that anabolic agents produce more significant increases in BMD than anti-resorptive agents in terms of anti-fracture efficacy. The optimal treatment protocol for cycling osteo-anabolics and anti-resorptive agents with precisely regulated DH periods can dynamically regulate bone remodelling in osteoporotic patients.108 Instead, bone-targeted drug delivery and nanoparticles encapsulated delivery are also used to reduce systemic complications and side effects of osteoporotic drugs, which aid in delivering clinical doses directly to bone tissues, a promising approach for achieving a pinpoint treatment for osteoporosis.104
3.6. Role of AI and machine learning techniques in osteoporosis management
Artificial intelligence (AI) applications and development in all radiology and bone imaging areas are progressing quickly. AI tools for detecting vertebral fracture and evaluating BMD in osteoporotic patients have been successfully developed for CT and plain radiographs with advanced diagnostic approaches. AI-based techniques are commonly exploited for detecting musculoskeletal fractures and soft tissue injuries. Advanced research revealed that the physician or imaging expert can utilize the wider possibilities of AI technology for better diagnosis and prognosis, with the aid of radiological applications.109,110
Pinpoint evaluation of vertebral fractures in OP diagnosis is vital for selecting treatment options. A deep-learning protocol based on a convolutional neural network (CNN) has been gaining importance in the medical imaging sector, based on a multicenter clinical database, and effectively developed an automated system for evaluating minor and hairline vertebral fractures on magnetic resonance imaging (MRI).111 The intelligent module of the clinical decision support system is available at the Menopause and Osteoporosis Research Center, University of Ferrara. The current protocol uses machine learning techniques and the Ripper algorithm for deterministic rule-based classifier extraction and fracture possibility. It helps physicians to receive suggestions for treatment recommendations for osteoporotic patients.112 Considering the clinical safety and compliance review, our discussion provide a novel outlook on the development of next-generation sclerostin inhibitors based on scientific and experimental approaches, such as concomitant medication, AI-based strategy, druggability and target modification, and bispecific inhibitors strategy.113 AI algorithms have been designed based on radiology, DEXA scan, computed tomography, and MRI possibilities in osteoporotic care and management. These AI-based algorithms have been utilized for diverse applications, including automatically labelling vertebral positioning, assessing disc degenerative changes and stress fractures, detecting and classifying spine trauma, and identifying osseous lesions and hairline fractures.114
3.7. Strengths and limitations of the study
Osteoporosis is an inevitable metabolic old age disorder. We can delay and control old age complications by regulating bone mass through physical exercise and nutrient-rich foods. This review summarizes the osteoporosis diagnostic procedures, ranging from biochemical evaluation to bone scanning procedures, including treatment protocols ranging from pharmacological and non-pharmacological approaches along with lifestyle modifications and the advanced AI and machine learning techniques for Osteoporosis management. The current review opens a hot topic for discussion for physicians, pharmaceutical scientists and health experts related to osteoporosis management. Despite these strengths, some limitations to this current systemic review are as follows. Due to the expensive treatment options, more than 75% of osteoporotic patients are under-diagnosed in economically weaker populations. The women population believes that it is a part of ageing and has to live with these complications without proper diagnosis. Due to these issues, the availability of clinical data is very limited, and the researchers, along with health experts, have to resolve the issue with advanced research and affordable drugs. Detailed clinical trials are required for further research to reveal the exact physical and molecular mechanism that regulates osteoporotic complications and associated disorders. These limitations and gap areas in the current review can be addressed in future research.
3.8. Future recommendations
Although the available anti-resorptive and anabolic agents, along with hormone replacement therapy, are beneficial, they have serious drawbacks, i.e., long-term usage of estrogen and parathyroid hormone therapy increases the risks of cancer, uterine bleeding and bone deterioration. Finding new, safe and cost-effective drug moieties that can stimulate bone formation, reduce bone resorption, and regulate intact bone remodelling is desirable to overcome the wide range of side effects. AI-based machine tools and advanced imageing techniques can precisely evaluate the bone microcellular environment. Along with site-specific drug delivery and advanced diagnostic techniques we can minimize the complications related to osteoporosis treatment. However, further clinical trials are required to achieve new insights into their drug-cellular interactions and bone remodelling cycle and to enter a new era of AI-based therapeutic applications to delay and regulate osteoporotic complications. Finally, various pre-clinical and clinical studies are required to reveal the efficacy of long-acting drugs during longer duration. A large-scale randomised controlled trial with a primary endpoint and long-term exploratory research with sufficient sample size and study design is needed to evaluate the preliminary findings with detailed clinical information and fine-tune the treatment protocol according to each patient's need.
4. Conclusions
OP, an inevitable disorder of old age, due to the limited treatment options, unawareness of BMD testing, huge expenses and bystander fatigue, are creating a hectic hurdle for proper therapeutic and supportive therapy. Timely diagnosis and immediate pharmaceutical intervention are required to maintain the overall strength, BMD, and bone structure. Maintaining a better lifestyle with a good protein diet and physical activity and avoiding caffeine, alcohol, and smoking will help to keep bones healthy. Sufficient calcium intake and vitamin D should be included in the diet after age 50 for women and 60 for men to prevent bone loss and maintain the overall bone microarchitecture and integrity. The primary line of defence by anabolic agents, along with BPs, can regulate the overall BMD. HRT and estrogen supplementation can enhance bone mass, thereby preventing fracture risk and minimising the pathogenesis of osteoporosis. Despite all the complications and contraindications of medication in the long run, due to progressive research, we may hope better treatment options will be achieved soon with minimum side effects and maximum biological efficacy. However, a better understanding of the bone microenvironment's molecular mechanism and intercellular signalling is crucial for achieving this goal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Data availability
This article has no additional data.
Informed consent
Not applicable.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Funding statement
The authors declare no competing financial interests.
Guardian/patient's consent
Not applicable.
CRediT authorship contribution statement
Rajamohanan Jalaja Anish: Literature search, manuscript draft preparation, writing of the manuscript, Supervision, and, Conceptualization, design of the review. Aswathy Nair: Literature search, manuscript draft preparation and grammar modification, gave suggestions for the manuscript, literature search and review, preparation of the manuscript revision and editing, All the authors contributed to manuscript preparation, reviewed the article, edited, and approved the submitted manuscript.
Declaration of competing interest
Rajamohanan Jalaja Anish, and Aswathy Nair declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank the library facility of University of Kerala and Government Medical college, Trivandrum.
Contributor Information
Rajamohanan Jalaja Anish, Email: anishrj@keralauniversity.ac.in.
Aswathy Nair, Email: aswathykelpalm@gmail.com.
References
- 1.Barnsley J., Buckland G., Chan P.E., et al. Pathophysiology and treatment of osteoporosis: challenges for clinical practice in older people. Aging Clin Exp Res. 2021;33:759–773. doi: 10.1007/s40520-021-01817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firestein G.S., Budd R.C., Gabriel S.E., et al. Elsevier Health Sciences; 2016. Kelley and Firestein's Textbook of Rheumatology. [Google Scholar]
- 3.Sozen T., Özışık L., Başaran N.C. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgström F., Karlsson L., Ortsäter G., et al. Fragility fractures in Europe: burden, management and opportunities. Arch. Osteoporos. 2020;15:1–21. doi: 10.1007/s11657-020-0706-y. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemmak A.R., Rezapour A., Jahangiri R., et al. Economic burden of osteoporosis in the world: a systematic review. Med J Islam Repub Iran. 2020;34:154. doi: 10.34171/mjiri.34.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odén A., McCloskey E.V., Kanis J.A., et al. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26(9):2243–2248. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson P.A., Kadri A., Hare K.J., et al. Preoperative bone health assessment and optimization in spinesurgery. Neurosurg Focus. 2020;49(2):E2. doi: 10.3171/2020.5.FOCUS20255. [DOI] [PubMed] [Google Scholar]
- 8.Chen L.R., Ko N.Y., Chen K.H. Medical treatment for osteoporosis: from molecular to clinical opinions. Int J Mol Sci. 2019;20(9):2213. doi: 10.3390/ijms20092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ukon Y., Makino T., Kodama J., et al. Molecular-based treatment strategies for osteoporosis: a literature review. Int J Mol Sci. 2019;20(10):2557. doi: 10.3390/ijms20102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattarai H.K., Shrestha S., Rokka K., et al. Vitamin D, calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J Osteoporos. 2020 doi: 10.1155/2020/9324505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Y., Huang X., Wu J., et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990-2019. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Luo Y., Wang H., et al. Mechanistic advances in osteoporosis and anti-osteoporosis therapies. MedComm. 2023;4(3):e244. doi: 10.1002/mco2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cepelak I., Cvoriscec D. Biochemical markers of bone remodeling–review. Biochem Med. 2009;19(1):17–35. [Google Scholar]
- 14.Kuo T.R., Chen C.H. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5(1):1–9. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haseltine K.N., Chukir T., Smith P.J., et al. Bone mineral density: clinical relevance and quantitative assessment. J Nucl Med. 2021;62(4):446–454. doi: 10.2967/jnumed.120.256180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pezzuti I.L., Kakehasi A.M., Filgueiras M.T., et al. Imaging methods for bone mass evaluation during childhood and adolescence: an update. J Pediatr Endocrinol Metab. 2017;30(5):485–497. doi: 10.1515/jpem-2016-0252. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation . World Health Organization; 1994. WHO Study Group on Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group. No. 843-849) [PubMed] [Google Scholar]
- 18.Camacho P.M., Petak S.M., Binkley N., et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 19.Carey J.J., Delaney M.F., Love T.E., et al. Dual-energy X-ray absorptiometry diagnostic discordance between Z-scores and T-scores in young adults. J Clin Densitom. 2009;12(1):11–16. doi: 10.1016/j.jocd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg C., Ix J.H. Diagnosis and management of osteoporosis in advanced kidney disease: a review. Am J Kidney Dis. 2022;79(3):427–436. doi: 10.1053/j.ajkd.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Sung K.H., Choi Y., Cho G.H., et al. Peripheral DXA measurement around ankle joint to diagnose osteoporosis as assessed by central DXA measurement. Skeletal Radiol. 2018;47(8):1111–1117. doi: 10.1007/s00256-018-2876-x. [DOI] [PubMed] [Google Scholar]
- 22.Hong D., Lee S., Kim G.B., et al. Development of a CT imaging phantom of anthromorphic lung using fused deposition modeling 3D printing. Medicine. 2020;99(1) doi: 10.1097/MD.0000000000018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C., Sun J., Yu L. Diagnostic value of calcaneal quantitative ultrasound in the evaluation of osteoporosis in middle-aged and elderly patients. Medicine. 2022;101(2) doi: 10.1097/MD.0000000000028325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadopoulou S.K., Papadimitriou K., Voulgaridou G., et al. Exercise and nutrition impact on osteoporosis and sarcopenia-the incidence of osteosarcopenia: a narrative review. Nutrients. 2021;13(12):4499. doi: 10.3390/nu13124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu K.N., Lie J.D., Wan C.K., et al. Osteoporosis: a review of treatment options. Pharmacy and Therapeutics. 2018;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu C.Y., Huang C.Y., Hsieh C.H., et al. Regular exercise and weight-control behavior are protective factors against osteoporosis for general population: a propensity score-matched analysis from Taiwan biobank participants. Nutrients. 2022;14(3):641. doi: 10.3390/nu14030641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos L., Elliott-Sale K.J., Sale C. Exercise and bone health across the lifespan. Biogerontology. 2017;18:931–946. doi: 10.1007/s10522-017-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedetti M.G., Furlini G., Zati A., et al. The effectiveness of physical exercise on bone density in osteoporotic patients. BioMed Res Int. 2018 doi: 10.1155/2018/4840531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jay B., Ahn S.H. Vertebroplasty. Semin Intervent Radiol. 2013;30(3):297–306. doi: 10.1055/s-0033-1353483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J., Adams M.A., Dolan P. Vertebroplasty and kyphoplasty can restore normal spine mechanics following osteoporotic vertebral fracture. J Osteoporos. 2010;2010 doi: 10.4061/2010/729257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denaro V., Longo U.G., Maffulli N., et al. Vertebroplasty and kyphoplasty. Clin Cases Miner Bone Metab. 2009;6(2):125–130. [PMC free article] [PubMed] [Google Scholar]
- 32.Veronese N., Maggi S. Epidemiology and social costs of hip fracture. Injury. 2018;49(8):1458–1460. doi: 10.1016/j.injury.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Nolan P., Tiedt L., Ellanti P., et al. A description of novel uses of hip protectors in an elderly hip fracture population: a technical report. Cureus. 2022;14(1) doi: 10.7759/cureus.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santesso N., Carrasco-Labra A., Brignardello-Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev. 2014;3:CD001255. doi: 10.1002/14651858.CD001255.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chabert E., Hugonnet E., Kastler A., et al. Vertebroplasty versus bracing in acute vertebral compression fractures: a prospective randomized trial. Ann.Phys.Rehabil.Med. 2023;66(6) doi: 10.1016/j.rehab.2023.101746. [DOI] [PubMed] [Google Scholar]
- 36.Jeong J., Kim J.H., Shim J.H., et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23(4) doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenkre J.S., Bassett J.H. The bone remodelling cycle. Ann Clin Biochem. 2018;55:308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 38.Mao H., Wang W., Shi L., et al. Metabolomics and physiological analysis of the effect of calcium supplements on reducing bone loss in ovariectomized rats by increasing estradiol levels. Nutr Metab. 2021;18(1):76. doi: 10.1186/s12986-021-00602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cianferotti L., Gomes A.R., Fabbri S., et al. The calcium-sensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int. 2015;26:2055–2071. doi: 10.1007/s00198-015-3203-1. [DOI] [PubMed] [Google Scholar]
- 40.Veldurthy V., Wei R., Oz L., et al. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4(1):1–7. doi: 10.1038/boneres.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao E.Y., Zhang Z.L., Xia W.B., et al. Calcifediol (25-hydroxyvitamin D) improvement and calcium-phosphate metabolism of alendronate sodium/vitaminD3 combination in Chinese women with postmenopausal osteoporosis: a post hoc efficacy analysis and safety reappraisal. BMC MusculoskeletDisord. 2018;19(1):210. doi: 10.1186/s12891-018-2090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao R.X., Yu M., Jiang Y., et al. Management of osteoporosis with calcitriol in elderly Chinese patients: a systematic review. Clin Interv Aging. 2014;9:515–526. doi: 10.2147/CIA.S40465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong T.I.W., Lim L.L., Chan S.P., et al. A summary of the Malaysian Clinical Practice Guidelines on the management of postmenopausal osteoporosis 2022. Osteoporosis and Sarcopenia. 2023;9(2):60–69. doi: 10.1016/j.afos.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rey J.R., Cervino E.V., Rentero M.L., et al. Raloxifene: mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop J. 2009;3:14–21. doi: 10.2174/1874325000903010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid I.R., Billington E.O. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi: 10.1016/S0140-6736(21)02646-5. [DOI] [PubMed] [Google Scholar]
- 46.Tabatabaei-Malazy O., Salari P., Khashayar P., et al. New horizons in treatment of osteoporosis. DARU J. Pharm. Sci. 2017;25(1):1–6. doi: 10.1186/s40199-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewiecki E.M. Bisphosphonates for the treatment of osteoporosis: insights for clinicians. Ther Adv Chronic Dis. 2010;1(3):115–128. doi: 10.1177/2040622310374783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell K.B., Karpeisky A., Thamm D.H., et al. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018;9:47–60. doi: 10.1016/j.bonr.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris S.T., Reginster J.Y., Harley C., et al. Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone. 2009;44(5):758–765. doi: 10.1016/j.bone.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Cosman F. Treatment of osteoporosis and prevention of new fractures: role of intravenously administered bisphosphonates. Endocr Pract. 2009;15(5):483–493. doi: 10.4158/EP08306.ORR1. [DOI] [PubMed] [Google Scholar]
- 52.Kim J., Kim K.M., Lim S., et al. Efficacy of bisphosphonate therapy on postmenopausal osteoporotic women with and without diabetes: a prospective trial. BMC Endocr Disord. 2022;22(1):91–99. doi: 10.1186/s12902-022-01010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendes D., Penedones A., Alves C., et al. Ibandronate in the prevention of vertebral and nonvertebral osteoporotic fractures: a systematic review of experimental and observational studies. J Clin Rheumatol. 2023;29(2):78–83. doi: 10.1097/RHU.0000000000001902. [DOI] [PubMed] [Google Scholar]
- 54.Ohtori S., Akazawa T., Murata Y., et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17(2):209–213. doi: 10.1016/j.jocn.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Elsayyad N.M.E., Gomaa I., Salem M.A., et al. Efficient lung-targeted delivery of risedronate sodium/vitamin D3 conjugated PAMAM-G5 dendrimers for managing osteoporosis: pharmacodynamics, molecular pathways and metabolomics considerations. Life Sci. 2022;309 doi: 10.1016/j.lfs.2022.121001. [DOI] [PubMed] [Google Scholar]
- 56.Khandelwal M.K., Srinivasan B., Kumari K., et al. Peroral delivery of risedronate sodium for treatment of osteoporosis. Mater Today Proc. 2022;49:2404–2413. [Google Scholar]
- 57.Lambrinoudaki I., Vlachou S., Galapi F., et al. Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women. Clin Interv Aging. 2008;3(3):445–451. doi: 10.2147/cia.s2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook P.N., Roux C., Devogelaer J.P., et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone. 2012;50(1):289–295. doi: 10.1016/j.bone.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Fang D., Xu J., et al. Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: a brief review. BMC Cancer. 2020;20(1):1059. doi: 10.1186/s12885-020-07568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mochizuki T., Yano K., Ikari K., et al. Effects of romosozumab or denosumab treatment on the bone mineral density and disease activity for 6 months in patients with rheumatoid arthritis with severe osteoporosis: an open-label, randomized, pilot study. Osteoporosis and sarcopenia. 2021;7(3):110–114. doi: 10.1016/j.afos.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia F., Ruan L., Du C., et al. The nanoformula of zoledronic acid and calcium carbonate targets osteoclasts and reverses osteoporosis. Biomaterials. 2023;296 doi: 10.1016/j.biomaterials.2023.122059. [DOI] [PubMed] [Google Scholar]
- 62.Seifi M., Amdjadi P., Tayebi L. Biomaterials for Oral and Dental Tissue Engineering. 2017. Pharmacological agents for bone remodeling: an experimental approach; pp. 503–523. [Google Scholar]
- 63.Zanatta L.B., Marcatto C., Ramos C.S., et al. Use of pamidronate for osteoporosis treatment in public health care in Brazil. Rev Bras Reumatol Engl Ed. 2017;57(6):514–520. doi: 10.1016/j.rbre.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Bone H.G., Wagman R.B., Brandi M.L., et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 65.Hanley D.A., Adachi J.D., Bell A., et al. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66(12):1139–1146. doi: 10.1111/ijcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavone V., Testa G., Giardina S., et al. Pharmacological therapy of osteoporosis: a systematic current review of literature. Front Pharmacol. 2017;8:803. doi: 10.3389/fphar.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leder B.Z. Optimizing sequential and combined anabolic and antiresorptive osteoporosis therapy. J. Bone Miner. Res.Plus. 2018;2(2):62–68. doi: 10.1002/jbm4.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mochizuki T., Yano K., Ikari K., et al. Two-year effectiveness of zoledronic acid with or without eldecalcitol in Japanese patients with osteoporosis: a randomized prospective study. Osteoporosis and Sarcopenia. 2022;8(2):75–79. doi: 10.1016/j.afos.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uzquiano J.C., Méndez A.A., Bielsa Á.J., et al. Denosumab treatment for osteopenia or osteoporosis in heart transplant recipients: effects and safety. Transplant Rev. 2022;7(3) [Google Scholar]
- 70.Kobayakawa T., Miyazaki A., Takahashi J., et al. Verification of efficacy and safety of ibandronate or denosumab for postmenopausal osteoporosis after 12-month treatment with romosozumab as sequential therapy: the prospective VICTOR study. Bone. 2022;162 doi: 10.1016/j.bone.2022.116480. [DOI] [PubMed] [Google Scholar]
- 71.Geusens P., Feldman R., Oates M., et al. Romosozumab reduces incidence of new vertebral fractures across severity grades among postmenopausal women with osteoporosis. Bone. 2022;54 doi: 10.1016/j.bone.2021.116209. [DOI] [PubMed] [Google Scholar]
- 72.Meczekalski B., Niwczyk O., Bala G., et al. Managing early onset osteoporosis: the impact of premature ovarian insufficiency on bone health. J Clin Med. 2023;12(12):4042. doi: 10.3390/jcm12124042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khosla S., Oursler M.J., Monroe D.G. Estrogen and the skeleton. Trends Endocrinol Metabol. 2012;23(11):576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang W.C., Wang J.H., Ding D.C. Conjugated equine estrogen used in postmenopausal women associated with a higher risk of stroke than estradiol. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-90357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kohn G.E., Rodriguez K.M., Hotaling J., et al. The history of estrogen therapy. Sex. Med. Rev. 2019;7(3):416–421. doi: 10.1016/j.sxmr.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.An K.C. Selective estrogen receptor modulators. Asian Spine J. 2016;10(4):787–791. doi: 10.4184/asj.2016.10.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrionuevo P., Kapoor E., Asi N., et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623–1630. doi: 10.1210/jc.2019-00192. [DOI] [PubMed] [Google Scholar]
- 78.D'Amelio P., Isaia G.C. The use of raloxifene in osteoporosis treatment. Expert OpinPharmacother. 2013;14(7):949–956. doi: 10.1517/14656566.2013.782002. [DOI] [PubMed] [Google Scholar]
- 79.Rey J.R., Cervino E.V., Rentero M.L., et al. Raloxifene: mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop J. 2009;3:14–21. doi: 10.2174/1874325000903010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sauter E.R. Breast cancer prevention: current approaches and future directions. Eur J Breast Health. 2018;14(2):64–71. doi: 10.5152/ejbh.2018.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haas A.V., LeBoff M.S. Osteoanabolic agents for osteoporosis. J Endocr Soc. 2018;2(8):922–932. doi: 10.1210/js.2018-00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brent M.B. Abaloparatide: a review of preclinical and clinical studies. Eur J Pharmacol. 2021;909 doi: 10.1016/j.ejphar.2021.174409. [DOI] [PubMed] [Google Scholar]
- 83.Hattersley G., Dean T., Corbin B.A., et al. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157(1):141–149. doi: 10.1210/en.2015-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sheth N.P., Winzenrieth R., Humbert L., et al. Abaloparatide increases bone mineral density in regions corresponding to gruen zones 1, 2, 6, and 7 in postmenopausal women with osteoporosis. J Clin Densitom. 2023;26(3) [Google Scholar]
- 85.Sheth N.P., Bostrom M.P., Winzenrieth R., et al. Effects of abaloparatide or placebo on bone mineral density in acetabular regions corresponding to DeLee and charnley zones in postmenopausal women with osteoporosis. J Clin Densitom. 2023;26(3) [Google Scholar]
- 86.Bandeira L., Lewiecki E.M., Bilezikian J.P. Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis. Expert Opin Drug MetabToxicol. 2016;12(6):681–689. doi: 10.1080/17425255.2016.1175436. [DOI] [PubMed] [Google Scholar]
- 87.Cianferotti L., D'Asta F., Brandi M.L. A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther Adv Musculoskelet Dis. 2013;5(3):127–139. doi: 10.1177/1759720X13483187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marx D., Yazdi A.R., Papini M., et al. A review of the latest insights into the mechanism of action of strontium in bone. BoneKEy Rep. 2020;12 doi: 10.1016/j.bonr.2020.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reginster J.Y., Kaufman J.M., Goemaere S., et al. Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int. 2012;23(3):1115–1122. doi: 10.1007/s00198-011-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta A., March L. Treating osteoporosis. Aust Prescr. 2016;39(2):40–46. doi: 10.18773/austprescr.2016.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curtis E.M., Cooper C., Harvey N.C. Cardiovascular safety of calcium, magnesium and strontium: what does the evidence say? Aging Clin Exp Res. 2021;33(3):479–494. doi: 10.1007/s40520-021-01799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cosman F., Crittenden D.B., Adachi J.D., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 93.Holdsworth G., Roberts S.J., Ke H.Z. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62(2):R167–R185. doi: 10.1530/JME-18-0176. [DOI] [PubMed] [Google Scholar]
- 94.Su Y., Wang W., Liu F., et al. Blosozumab in the treatment of postmenopausal women with osteoporosis: a systematic review and meta-analysis. Ann Palliat Med. 2022;11(10):3203–3212. doi: 10.21037/apm-22-998. [DOI] [PubMed] [Google Scholar]
- 95.Poutoglidou F., Samoladas E., Raikos N., et al. Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: a meta-analysis and systematic review. J Clin Densitom. 2022;25(3):401–415. doi: 10.1016/j.jocd.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Lemke C., Benysek J., Brajtenbach D., et al. An activity-based probe for cathepsin K imaging with excellent potency and selectivity. J Med Chem. 2021;64(18):13793–13806. doi: 10.1021/acs.jmedchem.1c01178. [DOI] [PubMed] [Google Scholar]
- 97.Dai R., Wu Z., Chu H.Y., et al. Cathepsin K: the action in and beyond bone. Front Cell Dev Biol. 2020;8:433. doi: 10.3389/fcell.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drake M.T., Clarke B.L., Oursler M.J., et al. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. 2017;38(4):325–350. doi: 10.1210/er.2015-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duong le T., Leung A.T., Langdahl B. Cathepsin K inhibition: a new mechanism for the treatment of osteoporosis. Calcif Tissue Int. 2016;98(4):381–397. doi: 10.1007/s00223-015-0051-0. [DOI] [PubMed] [Google Scholar]
- 100.Stone J.A., McCrea J.B., Witter R., et al. Clinical and translational pharmacology of the cathepsin K inhibitor odanacatib studied for osteoporosis. Br J Clin Pharmacol. 2019;85(6):1072–1083. doi: 10.1111/bcp.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y., Huang X., Zhang Q., et al. Effects of strontium and cobalt codoped nanotube implants on osseointegration in osteoporotic rats. Mater Today Commun. 2022;33 [Google Scholar]
- 102.Zhu Q.X., Nie Q.Y., Liu L., et al. Synthesis of fluoride-releasing strontium-substituted porous apatite microspheres for bone osteoporosis treatment. Ceram Int. 2023;49(9):14666–14672. [Google Scholar]
- 103.Bilezikian J.P., Bandeira L., Khan A., et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178. doi: 10.1016/S0140-6736(17)31430-7. [DOI] [PubMed] [Google Scholar]
- 104.Niu Y., Yang Y., Yang Z., et al. Aptamer-immobilized bone-targeting nanoparticles in situ reduce sclerostin for osteoporosis treatment. Nano Today. 2022;45 [Google Scholar]
- 105.Colella A., Yu E., Sambrook P., et al. What is the risk of developing osteonecrosis following dental extractions for patients on denosumab for osteoporosis? J Oral Maxillofac Surg. 2023;81(2):232–237. doi: 10.1016/j.joms.2022.10.014. [DOI] [PubMed] [Google Scholar]
- 106.Diab D.L., Watts N.B. Bisphosphonate drug holiday: who, when and how long. Ther. Adv. Musculoskelet. Dis. 2013;5(3):107–111. doi: 10.1177/1759720X13477714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu W.Y., Yang W.F., Wang L., et al. The effect of drug holiday on preventing medication-related osteonecrosis of the jaw in osteoporotic rat model. J.Orthop.Transl. 2023;39:55–62. doi: 10.1016/j.jot.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reid I.R., Billington E.O. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi: 10.1016/S0140-6736(21)02646-5. [DOI] [PubMed] [Google Scholar]
- 109.Owoyemi A., Owoyemi J., Osiyemi A., et al. Artificial intelligence for healthcare in Africa. Front.digit.Health. 2020;7(2):6. doi: 10.3389/fdgth.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ajmera P., Kharat A., Botchu R., et al. Real-world analysis of artificial intelligence in musculoskeletal trauma. J Clin Orthop Trauma. 2021;22 doi: 10.1016/j.jcot.2021.101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yabu A., Hoshino M., Tabuchi H., et al. Using artificial intelligence to diagnose fresh osteoporotic vertebral fractures on magnetic resonance images. J Spine. 2021;21(10):1652–1658. doi: 10.1016/j.spinee.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Bonaccorsi G., Giganti M., Nitsenko M., et al. Predictingtreatment recommendations in postmenopausal osteoporosis. J.Biomed.Inform. 2021;118 doi: 10.1016/j.jbi.2021.103780. [DOI] [PubMed] [Google Scholar]
- 113.Yu S., Li D., Zhang N., et al. Drug discovery of sclerostin inhibitors. Acta Pharm Sin B. 2022;12(5):2150–2170. doi: 10.1016/j.apsb.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martín-Noguerol T., Miranda M.O., Amrhein T.J., et al. The role of Artificial intelligence in the assessment of the spine and spinal cord. Eur J Radiol. 2023;161 doi: 10.1016/j.ejrad.2023.110726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.